Abstract
Neutrophil-derived microvesicles (NDMVs) are liberated by neutrophils upon cell activation by molecules. Once activated, neutrophils are primarily involved in acute inflammation; however, the microvesicles they produce are largely anti-inflammatory. NDMVs have been shown to protect cartilage during inflammatory arthritis. They exert these effects by inhibiting or affecting the function of target cells, including macrophages. NDMVs have the potential to act as disease-modifying agents, especially for inflammatory diseases. This protocol describes a method using differential centrifugation to separate neutrophils from whole human blood. Subsequently, neutrophils are identified by cytospin and Wright’s staining, and then the NDMVs are isolated using differential centrifugation.
Keywords: Microvesicles, Neutrophils, Isolation, Differential centrifugation, Cytospin
Background
Microvesicles (MVs) are a type of extracellular vesicle that can be between 100 to 1,000 nm in size ( Leblanc et al., 2017 ; Zhan et al., 2021 ). They are produced by exocytic budding from the plasma membrane and can be shed from diverse cell types as a result of events such as cell activation by stimuli or apoptosis ( Lima et al., 2011 ). Stimuli activating these events increase intracellular calcium, leading to cytoskeletal rearrangement and eventually microvesicle budding ( Distler et al., 2005 ). MVs can carry specific sets of secreted molecules between cells, such as functional proteins, lipids, and nucleic acids including mRNAs and microRNAs, making them an important mode of intercellular communication ( Leblanc et al., 2017 ). Through horizontal transfer of these molecules, MVs have the ability to transcriptionally regulate target cells ( Greening et al., 2017 ). This ability, coupled with their association with a multitude of different diseases, including rheumatoid arthritis, vasculitis, and various cancers, makes them interesting targets for disease modification. MVs also exhibit superior potential as non-invasive biomarkers for certain diseases, as they are detectable in all biological fluids, including blood (both serum and plasma), urine, and saliva ( Leblanc et al., 2017 ). Moreover, surface proteins differ based on the type of cell they are shed from ( Lima et al., 2011 ), and both the inducer and severity of disease can alter what set of molecules MVs carry ( Ridger et al., 2017 ).
Neutrophil-derived microvesicles (NDMVs) are liberated by neutrophils upon cell activation by molecules. Activators include N-formyl peptides, which are proteins produced by bacteria or eukaryotic mitochondria containing N-formylmethionine, and tumor necrosis factor-α (TNF-α) ( Mantovani et al., 2011 ). When stimulated, neutrophils play a crucial role in acute inflammation; however, the microvesicles they generate are essentially anti-inflammatory ( Trongtorsak et al., 2018 ; Zhan et al., 2021 ). In fact, neutrophils are part of a small group of cells whose MVs are known to promote tissue regeneration and, in some cases, repair ( Rhys et al., 2018 ). For example, polymorphonuclear neutrophil-derived MVs (NDMVs) can protect cartilage during inflammatory arthritis ( Headland et al., 2015 ; Topping et al., 2017 ). They exert these effects by inhibiting or affecting the function of target cells, including macrophages and fibroblast-like synoviocytes, which can be modified by NDMVs to display a more anti-inflammatory phenotype ( Rhys et al., 2018 ; Zhan et al., 2021 ). These characteristics suggest NMDVs have potential disease-modifying effects, especially for inflammatory diseases.
In this protocol, we describe a method using differential centrifugation to separate polymorphonuclear neutrophils from whole blood. TNF-α is added to activate these neutrophils and stimulate MV release. Finally, the MVs are isolated using differential centrifugation, and their size distribution and concentration are characterized using Nanoparticle Tracking Analysis (NTA).
Materials and Reagents
Safety blood collection set with Luer adapter (Greiner Bio-One, Vacuette®, catalog number: 450081)
4 ml NH sodium heparin blood collection tubes (Greiner Bio-One, Vacuette®, catalog number: 454051)
50 ml polypropylene centrifuge tube (Labcon, PerformRTM, catalog number: 3186-345)
15 ml polypropylene centrifuge tubes (Labcon, PerformRTM, catalog number: 3136-345)
1.5 ml polypropylene microcentrifuge tubes (Labcon, SuperSpin®, catalog number: 3016-870)
Ultracentrifuge tube (Beckman Coulter, Quick-Seal Polypropylene Tube, bell-top, catalog number: 365470)
Foil
1 ml syringe (Nipro, catalog number: JD+01D2238)
Syringe filter (Whatman, Anotop 10 syringe filter, pore size 0.02 μm, catalog number: Z747637)
ddH2O (purified by Thermo Fisher Scientific Water Purification System)
Whole human blood collected in accordance with institutional biosafety and ethics guidelines
PolymorphprepTM (Axis-Shield, catalog number: 1114683)
RPMI 1640 (Gibco, catalog number: 11530586)
Red Blood Cell Lysis Buffer (Roche, catalog number: 11 814 389 001)
Human AB serum (Thermo Fisher Scientific, Coring Human AB serum 100 ml, catalog number: MT35060CI)
Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: 60004)
Glass slide (Tharmac, catalog number: JC311-100)
Filter cards (Tharmac, catalog number: JC305-200)
Cytospin funnel (Tharmac, Single Cellfunnel, catalog number: JC304-12)
Cytoclip (Tharmac, Accessories for Cellspin, catalog number: JC302-1)
Wright’s Solution (Merck, Wright’s eosin methylene blue solution, catalog number: 101383)
Recombinant Human Tumor Necrosis Factor-α (rhTNF-α) (Biolegend, catalog number: 717904)
NaCl (Vivantis, catalog number: PC0914-1kg)
KH2PO4 (Sigma-Aldrich, catalog number: 7758-11-4)
KCl (Sigma-Aldrich, catalog number: 7447-40-7)
Na2HPO4 (Sigma-Aldrich, catalog number: 7558-79-4)
HCl (VWR International, catalog number: 7647-01-0)
Phosphate buffer saline (PBS) (see Recipes)
Equipment
Water Purification System (Thermo Scientific, Barnstead Pacific RO, catalog number: 50132389)
Pipettes: 0.5-10 μl, 10-100 μl, and 100-1,000 μl (Eppendorf, Eppendorf Research plus, catalog numbers: 3120000020, 3120000046, 3120000060)
Balance (Fisher Scientific, model: SartoriusTM MSE125P100DU)
Universal 320R centrifuge equipped with a swing-out rotor (6-place) and fixed angle rotor (24- place) (Hettich, model: UNIVERSAL 320R), Centrifuge Rotor (Hettich, model: Rotor 1619); Centrifuge Accessary (Hettich, model: accessory 1462-A); Centrifuge Rotor (Hettich, model: Rotor 1420B)
Ultracentrifuge equipped with type 100Ti fixed-angle rotor (Beckman Coulter, model: OptimaTM XPN-100), Ultracentrifuge rotor (Beckman Coulter, model: Type 100Ti Fixed angle rotor, catalog number: 363013)
Water bath equipment (Memmert, Waterbath WNB 22)
Hemocytometer (Hausser Scientific, Bright-Line Hemacytometer, catalog number: 3120)
Cytospin device (Tharmac, Cellspin I)
Light Microscope (Nikon, Upright Microscope Eclip 50i)
NanoSight NS300 (Malvern Panalytical, O-ring top-plate, 405 nm laser)
Autoclave (Tomy, catalog number: LSX-500)
Refrigerator (4°C) (Samsung, 21 cu. ft. Capacity Top Freezer Refrigerator with FlexZone)
Refrigerator (-80°C) (Thermo, standard performance ultra-low freezers)
Software
Nanosight NTA 3.1 Software Build 3.1.54 (Malvern Panalytical)
Procedure
-
Isolation of neutrophils from whole blood
Collect approximately 40 ml of whole human blood into 10 × 4 ml tubes with heparin anticoagulant by phlebotomy in accordance with institutional biosafety and ethics guidelines.
Add 6 ml of PolymorphprepTM to 6 × 15-ml centrifuge tubes.
Add 6 ml of whole human blood into these tubes (Polymorphprep:whole blood v/v= 1:1). Be sure to slowly add so the blood sits on top of the PolymorphprepTM and the two layers do not mix.
Centrifuge at 500 × g (Hettich 320R) for 35 min (room temperature) to separate the layers (Figure 1).
Remove plasma and peripheral blood mononuclear cell layers from solution using a 1,000-μl pipette.
Collect the lower PMN layer and place into 3 × 15-ml centrifuge tubes. Avoid the red blood cell layer at the bottom.
-
Add 1-2 ml RPMI 1640 to each tube.
Note: Add some extra RPMI 1640 as needed to equalize the volumes in all tubes. This will ensure the centrifuge is balanced.
Centrifuge at 500 × g (Hettich 320R) for 5 min (room temperature) to wash the PMN layer and remove any residual PolymorphprepTM.
Remove supernatant and add 9 ml of Red Blood Cell Lysis Buffer along with 1 ml of RPMI 1640 (v/v ratio 9:1) to resuspend pellet. The lysis buffer will remove any contaminating erythrocytes (RBCs) by hypotonic cell lysis. Incubate at room temperature for 3 min.
Centrifuge at 400 × g (Hettich 320R) for 3 min (room temperature). The pellet produced will contain neutrophils.
Remove supernatant and resuspend the neutrophil pellet in 1 ml of RPMI 1640. Count the number of cells using a hemocytometer.
Adjust neutrophil concentration to 20 × 106 cells/ml by adding the appropriate amount of RPMI 1640 (approximately 200-600 μl).
-
Microscopic observation of isolated neutrophils: cytospin and Wright's staining
Add 5 μl of neutrophil solution containing 105 neutrophils from Step A12 into 200 μl of PBS with 10 mM EDTA and pipette mixture gently.
Align and assemble cytospin funnel, filters card, glass slide, and cytoclip, then put them into cytospin slots of cytospin device.
Transfer all neutrophil mixture into cytospin funnel rapidly and centrifuge at 32 × g for 5 min at room temperature.
Unlock cytoclip and remove cytospin funnel and filter card from glass slides to avoid touching the cell smear.
Air-dry the glass slides for 2 min.
Add 1 ml of Wright’s solution to each glass slide and incubate for 3 min at room temperature.
Add 1 m of purified water (l) to the Wright’s solution on each glass slide and incubate for 5 min.
Rinse glass slides with ddH2O for 1 min and repeat this step twice.
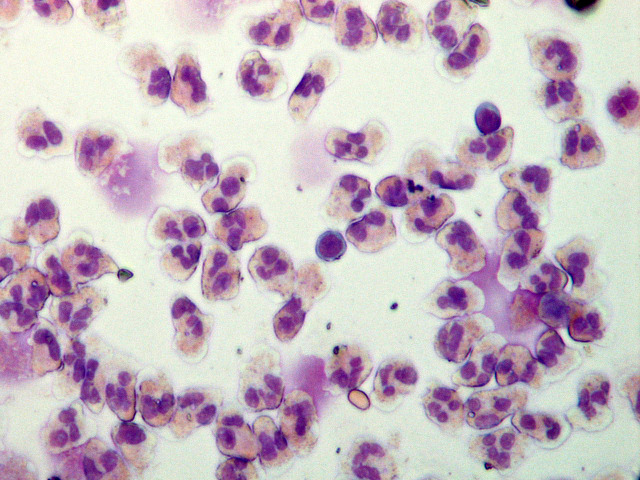
Observe the glass slides with a light microscope and count three fields of view (approximately 300-400 cells in one view). A representative image is shown in Figure 2.
-
Microvesicle release from treated neutrophils
Supplement the neutrophil solutions from Step A12 with human AB serum (approximately 50-150 μl) to be 5% of the volume.
Add the appropriate volume of rhTNF-α (400-600 μl) to achieve a final concentration of 50 ng/ml to activate neutrophils, promoting microvesicle release.
Incubate in a water bath at 37°C for 20 min to simulate body temperature and roll the tube gently and periodically. This prevents the neutrophils from dying.
-
Isolation of microvesicles
Move the solution from Step C3 to two freshly sterilized 1.5 ml microcentrifuge tubes.
Centrifuge at 4,400 × g (Hettich 320R) for 15 min at 4°C to remove any large cell bodies.
Collect the supernatant, which contains the microvesicles, carefully to not disturb the pellet and transfer to two freshly sterilized 1.5 ml microcentrifuge tubes.
Centrifuge at 13,000 × g (Hettich 320R) for 2 min at 4°C to remove platelets.
Collect supernatant and transfer into two freshly sterilized 1.5 ml microcentrifuge tubes.
-
Place the supernatant into an ultracentrifuge tube that is sealed to prevent evaporation of the solution.
Note: This will be ultracentrifuged; however, only one tube will be used, so a balance tube is needed. We used an equivalent tube weighing 5.76 g. To balance the tubes, add RPMI 1640 until the tube containing supernatant is of equal weight to your balance tube.
Ultracentrifuge at 100,000 × g (Optima XPN-100) for 60 min at 4°C. The pellet produced will contain neutrophil-derived microvesicles.
-
Carefully remove the supernatant (Figure 3) and resuspend the pellet in 200 μl of PBS. Transfer the pellet to a freshly sterilized 1.5 ml microcentrifuge tube and cover it with foil to keep it dark.
Note: Freshly isolated microvesicles can be used immediately to test microvesicle uptake by other cells. Microvesicles stored at -80°C should be used within one month for analysis of protein, RNA, lipid, or other component contents.
-
Nanoparticle Tracking Analysis (NTA)
Set up NanoSight NS300 hardware and software using the following parameters: 60 s length videos for 5 times with a highly sensitive camera level, 15 at 25°C, detective threshold 3, “water” viscosity, “automatic” blur size, and “automatic” max jump distance.
Dilute fresh NDMVs from Step D8 1,000× in particle-free PBS to achieve a density range of 106-109 particles/ml.
Aspirate the diluted NDMV solution into a 1 ml syringe, avoiding air bubbles.
Insert the syringe bore into the inlet port of the O-ring top-plate of the laser module and slowly inject 1 ml of NDMV solution over 15 to fill the chamber, avoiding air bubble production.
Mount the laser module into the main instrument housing and connect the power adaptor insider.
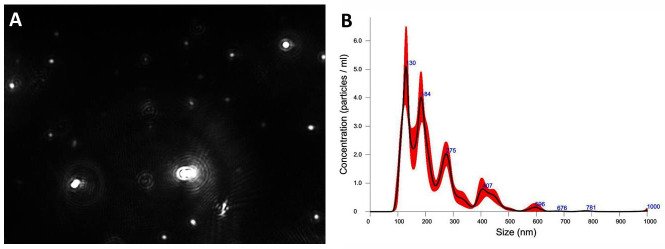
Start the NTA software to record NDMVs Brownian motion and analyze the nanoparticle motion automatically (Figure 4).
Figure 1. Separated layers from bottom to top.
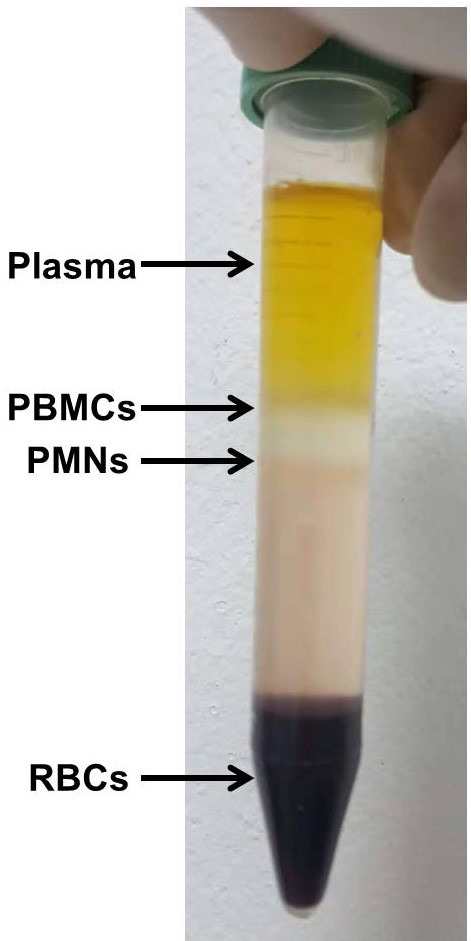
Red blood cells (RBCs), polymorphonuclear layer (PMNs), peripheral blood mononuclear cells (PBMCs), plasma. PolymorphprepTM is used to isolate a pure PMN layer.
Figure 2. Isolated neutrophils stained with Wright’s solution and visualized under a light microscopy.
Neutrophil nuclei stained with methylene blue are subdivided into 2-5 lobes, and their cytoplasms contain fine granules stained by eosin red. The purity of neutrophils isolated by PolymorphprepTM is approximately 95%-99%. 100× magnification.
Figure 3. Isolated neutrophil-derived microvesicles (NDMVs) at the bottom of an ultracentrifugation tube.
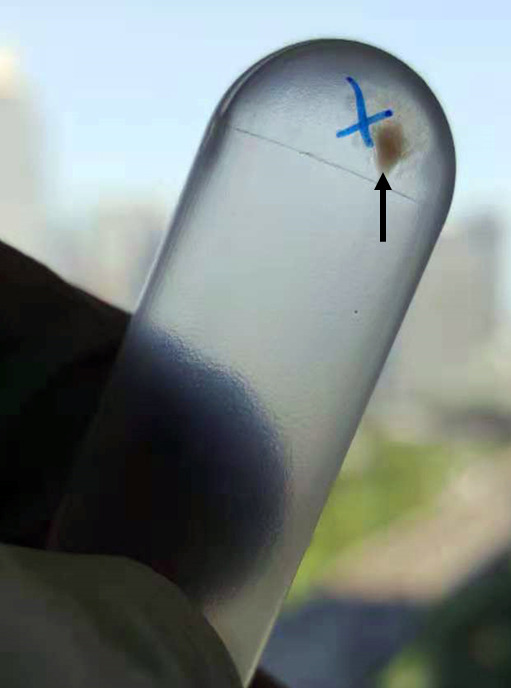
The black arrow indicates the white microvesicle pellet.
Figure 4. Nanoparticle tracking analysis (NTA) results for neutrophil-derived microvesicles (NDMVs).
A. Highlighted white particles are NDMVs captured in an image from a recorded video. B. The graph illustrates the distribution of NDMV sizes from 100 to 1,000 nm and their concentrations. NTA showed that NDMV concentration was 5.80 × 108 ± 2.34 × 107 particles/ml (mean ± standard deviation).
Recipes
-
Phosphate-buffered saline (PBS)
9 g/L NaCl
0.24 g/L KH2PO4
0.2 g/L KCl
1.42 g/L Na2HPO4
Adjust the pH to 7.4 with HCl
Note: All PBS used for experiments should be passed through a syringe filter to obtain particle-free PBS.
Acknowledgments
This work was supported by the Ratchadapiseksompotch Fund, Chulalongkorn University, grant number CUGR63953002, and Osteoarthritis and Musculoskeleton Research Unit. Dong Zhan was supported by a grant from the 100th anniversary Chulalongkorn University Fund and China Scholarship Council for Doctoral Scholarship and Overseas Research Experience Scholarship for Graduate Student.
Competing interests
The authors declare no conflict of interest.
Ethics
The study protocol conformed to the ethical standards outlined in the Declaration of Helsinki and was approved by the Ethical Committee on Human Research of the Faculty of Medicine, Chulalongkorn University (No. 565/59). All study subjects were fully informed regarding the study protocol and procedures prior to participating in the study. Written informed consent was obtained from all participants.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Distler J. H. W., Pisetsky D. S., Huber L. C., Kalden J. R., Gay S. and Distler O.(2005). Microparticles as regulators of inflammation: Novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum 52(11): 3337-3348. [DOI] [PubMed] [Google Scholar]
- 2. Greening D. W., Xu R., Gopal S. K., Rai A. and Simpson R. J.(2017). Proteomic insights into extracellular vesicle biology– defining exosomes and shed microvesicles. Expert Rev Proteomic 14(1): 69-95. [DOI] [PubMed] [Google Scholar]
- 3. Headland S. E., Jones H. R., Norling L. V., Kim A., Souza P. R., Corsiero E., Gil C. D., Nerviani A., F. Dell'Accio, Pitzalis C., et al.(2015). Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med 7(315): 315ra190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leblanc P., Arellano-Anaya Z. E., Bernard E., Gallay L., Provansal M., Lehmann S., Schaeffer L., Raposo G. and Vilette D.(2017). Isolation of exosomes and microvesicles from cell culture systems to study prion transmission. Methods Mol Biol 1545: 153-176. [DOI] [PubMed] [Google Scholar]
- 5. Lima L. G., Oliveira A. S., Campos L. C., Bonamino M., Chammas R., Werneck C., Vicente C. P., Barcinski M. A., Petersen L. C. and Monteiro R. Q.(2011). Malignant transformation in melanocytes is associated with increased production of procoagulant microvesicles. Thromb Haemost 106(4): 712-723. [DOI] [PubMed] [Google Scholar]
- 6. Mantovani A., Cassatella M. A., Costantini C. and Jaillon S.(2011). Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11(8): 519-531. [DOI] [PubMed] [Google Scholar]
- 7. Rhys H. I., F. Dell'Accio, Pitzalis C., Moore A., Norling L. V. and Perretti M.(2018). Neutrophil microvesicles from healthy control and rheumatoid arthritis patients prevent the inflammatory activation of macrophages. EBioMedicine 29: 60-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ridger V. C., Boulanger C. M., Angelillo-Scherrer A., Badimon L., Blanc-Brude O., Bochaton-Piallat M. L., Boilard E., Buzas E. I., Caporali A., Dignat-George F., et al.(2017). Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology(ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb Haemost 117(7): 1296-1316. [DOI] [PubMed] [Google Scholar]
- 9. Topping L., Rhys H., Norling L. and Nissim A.(2017). FRI0011 Targeting neutrophil microvesicles to damaged cartilage using antibodies to post translationally modified collagen ii. Ann the Rheum Dis 2): 483-484. [Google Scholar]
- 10. Trongtorsak P., Olankijanunt W., Trongtorsak A., Intamaso U.(2018). Antidepressant and antiinflammatory effects of a combined fluoxetine and celecoxib treatment in a rat model of depression. Chula Med J 62(4): 653-665. [Google Scholar]
- 11. Zhan D., Cross A., Wright H. L., Moots R. J., Edwards S. W. and Honsawek S.(2021). Internalization of Neutrophil-Derived Microvesicles Modulates TNFα-Stimulated Proinflammatory Cytokine Production in Human Fibroblast-Like Synoviocytes. Int J Mol Sci 22(14):7409. [DOI] [PMC free article] [PubMed] [Google Scholar]