Abstract
Indocyanine green (ICG) fluorescence imaging is widely used in abdominal surgery. The implementation of minimally invasive rectal surgery using new methods like robotics or a transanal approach required improvement of optical systems. In that setting, ICG fluorescence optimizes intraoperative vision of anatomical structures by improving blood and lymphatic flow. The purpose of this review was to summarize all potential applications of this upcoming technology in rectal cancer surgery. Each type of use has been separately addressed and the evidence was investigated. During rectal resection, ICG fluorescence angiography is mainly used to evaluate the perfusion of the colonic stump in order to reduce the risk of anastomotic leaks. In addition, ICG fluorescence imaging allows easy visualization of organs such as the ureter or urethra to protect them from injury. This intraoperative technology is a valuable tool for conducting lymph node dissection along the iliac lymphatic chain or to better identifying the rectal dissection planes when a transanal approach is performed. This is an overview of the applications of ICG fluorescence imaging in current surgical practice and a synthesis of the results obtained from the literature. Although further studies are need to investigate the real clinical benefits, these findings may enhance use of ICG fluorescence in current clinical practice and stimulate future research on new applications.
Keywords: Indocyanine green, Fluorescence imaging, Near infrared, Rectal cancer, Total mesorectal excision, Anastomotic leakage
Core Tip: There is growing interest in real-time fluorescence-guided surgery. The intraoperative use of indocyanine green (ICG) during rectal cancer surgery has found many applications over time. Given the wide availability in current practice, it is important for clinicians to be aware of all potential uses of ICG fluorescence technology in order to facilitate the procedures, limit injuries, and improve outcomes. Herein, we provide a concise overview of the literature regarding the use of ICG fluorescence imaging in this setting.
INTRODUCTION
Colorectal carcinoma is the third most common cancer for both men and women and the second leading cause of cancer-related deaths[1]. Primary rectal localization occurs in 35% of cases[2]. Although multidisciplinary management of rectal cancer is the standard of care, surgery remains the cornerstone of curative treatment. Total mesorectal excision (TME), described for the first time by Heald et al[3,4], should now be performed routinely in cases of middle and lower rectal tumors after neoadjuvant therapy for locally advanced cancer (T3-4 and/or N+)[5]. Respect for the principles of surgical oncology, including complete TME, and negative distal and circumferential resection margins is mandatory to achieve improved survival. Also, much effort has been made to achieve faster and enhanced recovery[6,7] and better quality of life after rectal cancer surgery[8] over time. Advances in oncology research[9] have resulted in increasing roles for the latest technologies in surgery. Enhanced video/camera systems[10], robotic technology[11], powered staplers[12], and specialized operating platforms for natural orifice transluminal endoscopic surgery[13] are just a few examples of surgical innovations in colorectal surgery introduced in the last decades. In this setting, laser fluorescence using indocyanine green (ICG) dye is a promising and widespread real-time technology because of its easy accessibility, accuracy and cost-effectiveness.
The concept of ICG intraoperative angiography is based on the ability of ICG to absorb near-infrared light (NIR) at 800 nm and to emit fluorescence at a wavelength of 830 nm. Albumin is the most important intravascular binding-protein for ICG so that tissue microperfusion is revealed by the presence of fluorescence. In brief, a bolus of ICG is injected into the patient intravenously, NIR light is then absorbed by ICG in the tissue, and the resulting fluorescence is a reflection of perfusion. After the introduction of ICG angiography in clinical practice in 1989 to evaluate choroidal circulation[14], fluorescence imaging technology has been used in hepatobiliary[15,16], gastric cancer[17], gynecologic cancer[18], breast cancer[19] and transplantation surgery[20]. Furthermore, many colorectal surgeons routinely use ICG imaging to assess bowel viability during colorectal anastomosis. However, several applications of ICG angiography have been described in colorectal surgery with increasing interest for rectal cancer resection. In fact, the implementation of minimally invasive rectal surgery using new approaches (e.g., robotic, and transanal) required the improvement of optical systems. In that regard, ICG fluorescence has optimized the intraoperative vision of anatomical structures by the enhancement of blood and lymph flow. The aim of this review is to identify and synthesize data from original research evaluating any possible application of ICG fluorescence imaging in rectal cancer surgery. In the last 5 years, the use of ICG fluorescence in rectal cancer surgery has attracted great interest. The items regarding different intraoperative applications are summarized below.
ASSESSMENT OF VASCULAR PERFUSION AT THE ANASTOMOTIC SITE
Anastomotic leakage (AL) is the most feared complication after TME because it is associated with increased mortality, reoperation, and definitive stoma formation[21,22]. Additionally, the relationship between AL and local recurrence has been found to lead to significant differences in long-term outcomes[23]. It has a significant impact on postoperative functional outcome[24] and increases the economic burden of public health systems[25]. That easily explains the importance of developing prevention strategies in order to reduce the amount of related complications affecting survival and quality of life. The AL rate after anterior rectal resection ranges from 3% to 23%[26,27]. AL is defined by the International Study Group of Rectal cancer as a defect at the level of the anastomotic site that allows communication between the intraluminal and extraluminal compartments identified through clinical evaluation with digital rectal exploration, endoscopic examination, computed tomography radiological evidence of contrast leakage through the suture gap or the presence of perianastomotic hydro-aerial collection. It is also classified as grade A if it does not affect the postoperative course, grade B when conservative management antibiotic therapy or percutaneous/transanal drainage is required, and grace C when surgical revision is required[26,28].
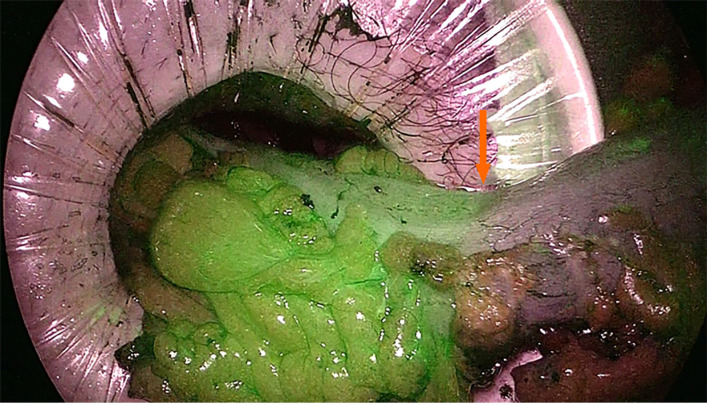
Various risk factors are associated with AL, but insufficient blood perfusion is generally considered as one of the main causes[29,30]. ICG is a water soluble, tricarbocyanine dye that provides real-time visualization of vascular structures by emitting fluorescence when stimulated by polarized light[31]. The evaluation of perfusion of the supposed proximal section line starts within 60 s of an intravenous bolus injection of ICG. An NIR camera detects the fluorescence of the microcirculation of the colonic wall receiving adequate vascularization. Therefore, ICG fluorescence reflects the colonic stump perfusion allowing the choice of the most appropriate site for the final section and then for the anastomosis (Figure 1). A second check can be also performed after the anastomosis construction both by injecting a second bolus of ICG to verify fluorescence of the stumps and by transanal endoscopic suture line visualization. Some limitations of the technique are related to its being, once again, a subjective evaluation by the surgeon of the intensity of the emitted infrared light. Furthermore, it is not a completely standardized technique as there are variables related to the dose of injected ICG (0.013-0.89 mg/kg), the proximity of the laparoscope to the colic wall, the number and type of checks performed, and differences in the equipment available on the market[32-34].
Figure 1.
Evaluation of intestinal perfusion after intravenous injection of indocyanine green during total mesorectal excision allows identification of the section line before anastomosis construction (orange arrow).
Kudszus was the first to show the usefulness of ICG fluorescence angiography (FA) in colorectal surgery[35]. The study reported that ICG FA led to a change in the location of the planned proximal resection line in 13.9% of patients. ICG FA significantly reduced AL by 4% compared with the control group. Furthermore, Jafari et al[36] assessed the utility of ICG FA in left colectomy and anterior resection in the PILLAR II study. The incidence of AL in their study was 1.4%, with a change in the surgical strategy in 8% of patients. Boni et al[37] reported that ICG FA could be safely and effectively performed in rectal surgery. The use of IGC-FA changed the surgical plan in 4.7% of their patients and AL did not occur in the ICG group, compared with an incidence of 5.2% in the control group. However, the differences were not statistically significant. The studies agree on the safety and feasibility of the technique and demonstrate its usefulness in the assessment of tissue perfusion.
Several comparative retrospective and prospective studies investigated the relationship between the use of ICG and the 30-d AL rate as the primary outcome[31,33,38-40]. They showed a statistically significant correlation between ICG FA and reduction in the risk of AL associated with the modification of the proximal section line. In a propensity score-matched analysis[41], AL rates of Clavien-Dindo grade ≥ II and ≥ III were 10.4% (22/211) and 9.5% (20/211) in the non-ICG FA group and 4.7% (10/211) and 2.8% (6/211) in the ICG FA group, respectively. Similarly, Foo et al[42] demonstrated that the use of ICG FA was significantly associated with a lower AL rate in TME (4.7% vs 11.6%; P = 0.043) but not non-TME resections. However, not all studies agree on the results. Two propensity score-matched studies failed to demonstrate a statistically significant difference in the rate of AL between the two compared groups, although both reported a change in the proximal section after ICG injection of 27.1% and 18.18%, respectively[43,44]. Most of the studies had limitations such as a small sample size or their retrospective nature. Only two randomized controlled trials (RCTs) were published. In the first one[45], differences in AL or reoperation rates between ICG and the control group (5% vs 9% and 6.7% vs 6.5%) were not significant. Although the authors confirmed the efficacy of ICG in bowel viability assessment during left colectomy or anterior resection, a real advantage related to AL was not demonstrated. The FLAG trial[46] involved 377 patients who underwent anterior rectal resection, 187 in the ICG FA group and 190 in the control group. The results showed that changes in the transection line were performed in almost 20% of patients. A decrease in AL was achieved using ICG FA, but the difference was statistically significant only in cases with low rectal anastomosis (14.4% with ICG FA vs 25.7% without ICG FA; P = 0.04). Two recent meta-analyses assessed the role of ICG FA imaging on the incidence of AL after rectal cancer surgery[34,47]. In a pooled analysis of 2088 patients, the AL rate in the ICG group was significantly lower than that in the control group, and the intraoperative use of ICG was associated with a decreased overall complication rate and reduced reoperation rate. However, both analyses suffer from the same limitations as the studies taken into consideration and the lack of RCTs in the analysis.
Finally, evaluation of bowel perfusion by ICG FA was reported exclusively after transanal total mesorectal excision (TaTME) by Mizrahi et al[48]. In their retrospective cohort of 54 patients who received a very low anastomosis, a check was performed before proximal transection and at the completion of anastomosis. In 18.5% of the cases the surgeon changed the proximal resection margin because of impaired fluorescence. All those anastomoses were shown to be successful at the second control. Two patients (3.7%) suffered from AL, and in neither of them was the splenic flexure mobilized. Furthermore, ICG FA improved clinical outcomes also after robotic sphincter-saving rectal resections[49]. In conclusion, the use of fluorescent angiography with ICG injection has proven to be a safe and feasible method to evaluate bowel perfusion whatever the surgical approach for rectal resection. At the time of the construction of the anastomosis, ICG FA can influence decision making by reconsidering the resection line. Several studies have found a significant decrease in the rate of AL after ICG FA imaging, and that has had a large impact on recovery. Future high quality trials should confirm the impact on AL rates and standardize the technique.
URETHRA VISUALIZATION DURING TRANSANAL TME
TaTME is a relatively new procedure[50] for the curative resection of rectal tumors. It was developed to overcome the difficult dissection at the lower third of the rectum, especially in obese male patients and/or bulky tumors. Some retrospective series have reported that enhanced visualization of the dissection plane allowed better nerve preservation, improved resection margins, and improved functional outcomes compared with laparoscopic TME[51,52]. However, the bottom-up transanal approach is not without complications. Incidence of iatrogenic urethral injuries has been reported, ranging from 1% to 6.7% during TaTME procedures[53]. An international inquiry reported 34 urethral injuries from 32 surgical teams worldwide between 2010 and 2017, resulting in a significant postoperative morbidity rate of 26%[50]. However, there is still concern that urologic injuries during TaTME may be underreported and that their incidence might be related to surgeon experience. Therefore, enhancement of urethral visualization should be considered useful and advantageous in the early learning experience. Several bioimaging modalities exist that can improve urethral identification, including ICG NIR fluorescence[54]. Different systems have been successfully used to detect the urethra by ICG fluorescence imaging such as the IRIS ureteral kit (Stryker, Kalamazoo, MI, United States)[55] or the PINPOINT laparoscopic system (Stryker, United States)[56] with intraurethral ICG injection or infiltration adjacent to the catheter in the urethra, respectively. Experimental studies demonstrated that direct ICG instillation into the urethra or through a urine catheter for NIR fluorescence imaging seem to be easily applicable and clinically reproducible during TaTME[55-57]. Although an open-label clinical feasibility study (NCT03204201) with intraoperative direct instillation of ICG into the urethra for low rectal cancers was terminated because of technique failures, implementation of the technique has been described by some authors. Barnes et al[58] evaluated the efficacy of two novel methods in cadaveric models. In the first, ICG mixed with silicone was infiltrated into 10-Fr one-way Foley catheter and allowed to set for 1 wk. In the second, new preclinical IRDye 800BK (LI-COR Biosciences®, Lincoln, Nebraska, United States) was infiltrated directly into the urethra via the urethral meatus prior to dissection. Both methods were effective in identifying the fluorescence located only within the urethra. IRDye 800BK provided a greater depth of penetration than the ICG-silicone mix, suggesting it could be a more satisfactory alternative to ICG. In addition, Barberio et al[59] demonstrated the superior brightness of near-infrared coating of equipment (NICE) coated catheter compared with ICG-based solutions in cadaveric experiments by exhibiting a higher fluorescence intensity than urinary catheters filled with ICG. In conclusion, no final specific recommendations can be drawn from the clinical use of ICG fluorescence imaging to identify and prevent urethral injuries during TaTME procedures because of the very limited data available. However, future studies will have to take into account that fluorescence technology plays a major role in this setting.
URETER IDENTIFICATION
The incidence of iatrogenic urethral injuries (IUI) ranges from 0.24% to 1.95% in colorectal surgery, and rectal cancer is considered a risk factor for IUI because of the close proximity of the ureters to the dissection plane[60], similar to the risk with deep pelvic endometriosis[61,62]. Despite its low incidence, IUI significantly affects postoperative morbidity, mortality, length of stay and hospital charges[60]. Visualization of the ureters is thus advocated during pelvic surgery by the visible peristalsis that occurs when the ureter is gently pressed (Kelly’s sign). However, adhesions, obesity, and an incorrect plane of dissection contribute to the lack of or incorrect recognition of the ureter, which can jeopardize its integrity. For that reason, a selective use of prophylactic urethral stents in high risk procedures is commonly accepted, but there is no sufficient evidence to support a decrease in IUI or intraoperative identification[63,64]. In that setting, interest in fluorescence imaging has been increasing over time. While contrasting results were found for intravenous administration of methylene blue dye to urethral detection in colorectal surgery[65,66], ICG FA proved to be a viable alternative to real-time ureter identification and IUI prevention. Before surgery, a 6-Fr catheter is placed into the urethral orifice by cystoscopy. As ICG binds to the proteins of the ureteric epithelium[67], a retrograde injection of 5 mg ICG diluted in 2 mL of distilled water is made, and infrared emission is captured by the filtered lens system and electronically converted into green color visualizing ureter location. The technique has proven to be safe and helpful to identify the ureter in several small case-series who underwent minimally invasive pelvic surgery[68-72]. As a catheter insertion of only 1 cm is required, there is a lower risk of IUI during catheterization than during conventional endoscopic stenting procedures, thus avoiding additional cystoscopies to remove the catheter. Furthermore, ICG urethral instillation is less expensive than other fluorescence-based systems such as illuminated catheters[68].
White et al[73] recently evaluated the safety and efficacy of intraurethral ICG FA along with any potential benefit related to the technique during colorectal robotic surgery. In their experience involving 16 patients, there were short procedure times, low morbidity, and reliable urethral identification and avoidance. The United States Food and Drug Administration approval of ICG is limited to intravenous use[74].Therefore, disclosure of intraurethral off-label use would be needed. In contrast, new intravenous fluorescent dyes with renal clearance, such as fluorescein sodium[75] and IRDye® 800-BK[76] have been used in experimental models to test the penetration of fluorescence in the ureters, with promising results for surgical practice. Additionally, the formulation of ICG in a liposome-based delivery system allows its excretion in urine in animal models and seems a promising fluorophore solution[77,78]. In conclusion, evidence supporting the use of intraurethral ICG instillation in order to improve intraoperative ureter detection is based on few noncomparative feasibility studies involving mixed pelvic surgeries. Despite the efficacy demonstrated in ordinary or complex situations, no study exclusively focused on rectal cancer resections exists to date. It remains to be proven whether this innovation significantly affects surgical procedures and provides clinical benefits by reducing IUI.
LYMPH NODE MAPPING
Lateral pelvic lymph node dissection (LLND) allows the removal of the nodal compartment along the common iliac, internal iliac, and obturator arteries. The lymphatic stations are considered a major cause of locoregional recurrence in rectal cancer and are treated with preoperative chemoradiotherapy and curative resection[79]. While it is widely accepted to perform LLND in selected patients with rectal cancer and lateral lymph nodes that are clinically positive[5], the Japanese Society for Cancer of the Colon and Rectum guidelines[80] recommend LLND even when lateral lymph node metastasis is not detected by preoperative or intraoperative diagnosis. Indeed, LLND is associated with a lower rate of local recurrence compared with TME alone despite no significant differences in either overall survival or local recurrence-free survival[81].
As ICG fluorescence imaging has proven to be a useful tool for identifying lymphatic drainage in colorectal surgery[82,83], ICG-enhanced NIR fluorescence-guided imaging has been used to improve the accuracy and the completeness of LLND. In such cases, ICG fluorescence imaging is carried out the injection of ICG dye into the submucosal layer on the distal side of the tumor through the anus immediately before surgery. In a comparative retrospective series of 42 mid and low rectal cancer patients, the ICG group experienced a significantly lower intraoperative blood loss and a larger number of harvested lateral pelvic lymph nodes[84]. The use of ICG may improve the safety of LLND that is affected by the technical difficulties of the procedure, complicated pelvic wall anatomy, and the effects of preoperative radiation on the tissues. In that setting, real-time identification of lateral pelvic nodes could help to distinguish lymphatic tissue from vascular and nervous structures, thus avoiding postoperative genitourinary dysfunction and providing better surgical staging. However, evidence is still limited[85-87] and additional studies are needed to address the real clinical advantages and standardization of this technique.
A sentinel node (SN) is defined as the first node in the regional peritumoral area that drains the tumor. SN biopsy, in addition to conventional resection, may add clinically significant prognostic information in colorectal surgery[88-90]. NIR laparoscopy with ICG mapping allowed easy intraoperative identification of mesocolic lymphatic drainage and SN during colorectal oncologic resections[91]. Similarly Noura et al[92] described the detection of SN by ICG with an NIR system in 25 patients who had no preoperative diagnosis of metastatic lateral pelvic lymph nodes. The success rate of detecting the lateral SN was 92%, and 100% concordance was observed between SN and dissected lateral lymph nodes status. That preliminary study highlighted the feasibility and reliability of lateral SN biopsy as a potential discriminator to perform LLND, but the sensitivity may be compromised by preoperative neoadjuvant chemoradiotherapy[93,94].
TUMOR LOCALIZATION
Several reports have described the intraoperative identification of colonic tumors by NIR with ICG fluorescence imaging, with satisfactory results[95-98]. Accurate identification of the location of colorectal tumors is crucial in minimally invasive surgery because of the lack of tactile perception, especially for cancer at an early stage because of its small size or location on a movable part of the colon. As for rectal cancer, precise tumor site localization allows achieving a clear and safe distal resection margin, which may affect not only oncological outcomes but also bowel function and quality of life. In that setting, endoscopic tattooing of rectal tumors, both with a high-definition fluorescence imaging system (Karl Storz GmbH & Co. KG, Tuttlingen, Germany)[93] and the PINPOINT® endoscopic fluorescence imaging system (PINPOINT system; Novadaq Technologies Inc., Mississauga, ON, Canada)[99], is feasible and has clinical advantages. In a comparative retrospective series, 342 patients scheduled for laparoscopic colorectal resection were enrolled after propensity score matching[100]. The tumor was tattooed in 114 patients. In a subgroup analysis of 160 patients who underwent anterior resection, the tattooed group had a significantly shorter operative time (unlike right and left colectomy), less blood loss, and a shorter hospital stay than the non-tattooed group. In addition, Goo et al[101] compared 200 tattooed colorectal cancer patients (44 rectal cancers) with 879 non-tattooed patients (300 rectal cancers) to evaluate the effect of preoperative colonoscopic tattooing with ICG on adequate lymph node harvest in colorectal cancer. They found that preoperative tattooing in T1 colorectal cancer significantly improved adequate lymph node harvest, with a higher number of retrieved lymph nodes in rectal cancer than in colon cancer.
Fluorescence technology to localize rectal tumors has been developed not only in the field of imaging systems[99], but also by ICG formulation. Fenestrated peritumoral capillaries and impaired lymphatic drainage delay the washout of large molecules from tumors, which has been described as the enhanced permeability and retention effect[102]. The formulation of ICG as a liposome-based delivery system improved tumor-specific localization in experimental models, with the advantages of intravenous injection and better results than free ICG[103,104]. Finally, there are limited data on the role of ICG in the detection of peritoneal carcinomatosis of colorectal origin. Cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy is the only potentially curative option in patients with limited peritoneal metastases[105]. Intraoperative injection of ICG seems a useful tool to identify peritoneal metastases and detect additional subclinical malignant peritoneal nodules, resulting in modification of the planned surgery in 29% of patients[106]. However, further investigations are required to draw firm conclusions.
OTHER USES
Peritumoral injection of ICG may help the surgeon to perform an adequate dissection along the embryological surgical planes and visualize the relationship with surrounding structures during TaTME[107], conventional laparoscopic TME[108], and abdominoperineal resection (APR)[109]. Omentoplasty is a well-known method to fill the pelvic cavity after APR or in case of complications after rectal cancer surgery. A pilot study of the intraoperative value of NIR fluorescence imaging with ICG to assess omental perfusion after the creation of a pedicled omentoplasty found that a change in decision making occurred in 80% of the cases[110], and a positive impact on the nonhealing rates of patients undergoing salvage surgery for chronic pelvic sepsis was also observed[111].
CONCLUSION
The adoption of ICG fluorescence imaging in rectal cancer surgery and its multiple applications has increased over time. The major field of application is the evaluation of bowel perfusion at the time of anastomosis construction along with a better intraoperative identification of anatomical structures such as the ureter, urethra, lymph nodes, and tumor location. These objectives are relevant because they aim to improve patient safety by avoiding or reducing the risk of complications. However, further investigations are needed to assess the impact of intraoperative ICG fluorescence imaging on clinical, oncological and cost-effective outcomes.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Unsolicited manuscript
Peer-review started: April 18, 2021
First decision: June 30, 2021
Article in press: August 18, 2021
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu A S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Xing YX
Contributor Information
Roberto Peltrini, Department of Public Health, University of Naples Federico II, Napoli 80131, Italy. roberto.peltrini@gmail.com.
Mauro Podda, Department of Emergency Surgery, Cagliari University Hospital "Duilio Casula", Azienda Ospedaliero-Universitaria di Cagliari, Cagliari 09100, Italy.
Simone Castiglioni, Department of Medical, Oral and Biotechnological Sciences, University G. D’Annunzio Chieti-Pescara, Pescara 65100, Italy.
Maria Michela Di Nuzzo, Department of Public Health, University of Naples Federico II, Napoli 80131, Italy.
Michele D'Ambra, Department of Public Health, University of Naples Federico II, Napoli 80131, Italy.
Ruggero Lionetti, Department of Public Health, University of Naples Federico II, Napoli 80131, Italy.
Maurizio Sodo, Department of Public Health, University of Naples Federico II, Napoli 80131, Italy.
Gaetano Luglio, Department of Public Health, University of Naples Federico II, Napoli 80131, Italy.
Felice Mucilli, Department of Medical, Oral and Biotechnological Sciences, University G. D’Annunzio Chieti-Pescara, Pescara 65100, Italy.
Salomone Di Saverio, Department of General Surgery, University of Insubria, ASST Sette Laghi, Varese 21100, Italy.
Umberto Bracale, Department of Public Health, University of Naples Federico II, Napoli 80131, Italy.
Francesco Corcione, Department of Public Health, University of Naples Federico II, Napoli 80131, Italy.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22–iv40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 3.Heald RJ. A new approach to rectal cancer. Br J Hosp Med. 1979;22:277–281. [PubMed] [Google Scholar]
- 4.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 5.You YN, Hardiman KM, Bafford A, Poylin V, Francone TD, Davis K, Paquette IM, Steele SR, Feingold DL On Behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis Colon Rectum. 2020;63:1191–1222. doi: 10.1097/DCR.0000000000001762. [DOI] [PubMed] [Google Scholar]
- 6.EuroSurg Collaborative. Safety and efficacy of non-steroidal anti-inflammatory drugs to reduce ileus after colorectal surgery. Br J Surg. 2020;107:e161–e169. doi: 10.1002/bjs.11326. [DOI] [PubMed] [Google Scholar]
- 7.Peltrini R, Cantoni V, Green R, Greco PA, Calabria M, Bucci L, Corcione F. Efficacy of transversus abdominis plane (TAP) block in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol. 2020;24:787–802. doi: 10.1007/s10151-020-02206-9. [DOI] [PubMed] [Google Scholar]
- 8.Peltrini R, Luglio G, Cassese G, Amendola A, Caruso E, Sacco M, Pagano G, Sollazzo V, Tufano A, Giglio MC, Bucci L, Palma GD. Oncological Outcomes and Quality of Life After Rectal Cancer Surgery. Open Med (Wars) 2019;14:653–662. doi: 10.1515/med-2019-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Palma FDE, Luglio G, Tropeano FP, Pagano G, D'Armiento M, Kroemer G, Maiuri MC, De Palma GD. The Role of Micro-RNAs and Circulating Tumor Markers as Predictors of Response to Neoadjuvant Therapy in Locally Advanced Rectal Cancer. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21197040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corcione F, Silvestri V, Merola G, Dambra M, Lionetti R, Pirozzi N, Peltrini R, Pontecorvi E, Bracale U. Use of the ORBEYETM Exoscope in General Surgery: The Advent of Video-Assisted Open Surgery. Surg Innov. 2021;28:79–84. doi: 10.1177/1553350620965344. [DOI] [PubMed] [Google Scholar]
- 11.Tejedor P, Sagias F, Khan JS. The Use of Enhanced Technologies in Robotic Surgery and Its Impact on Outcomes in Rectal Cancer: A Systematic Review. Surg Innov. 2020;27:384–391. doi: 10.1177/1553350620928277. [DOI] [PubMed] [Google Scholar]
- 12.Herzig DO, Ogilvie JW, Chudzinski A, Ferrara A, Ashraf SQ, Jimenez-Rodriguez RM, Van der Speeten K, Kinross J, Schimmelpenning H, Sagar PM, Cannon JA, Schwiers ML, Singleton DW, Waggoner JR, Fryrear R 2nd, Sylla P. Assessment of a circular powered stapler for creation of anastomosis in left-sided colorectal surgery: A prospective cohort study. Int J Surg. 2020;84:140–146. doi: 10.1016/j.ijsu.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Karimyan V, Sodergren M, Clark J, Yang GZ, Darzi A. Navigation systems and platforms in natural orifice translumenal endoscopic surgery (NOTES) Int J Surg. 2009;7:297–304. doi: 10.1016/j.ijsu.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Destro M, Puliafito CA. Indocyanine green videoangiography of choroidal neovascularization. Ophthalmology. 1989;96:846–853. doi: 10.1016/s0161-6420(89)32826-0. [DOI] [PubMed] [Google Scholar]
- 15.Dip F, LoMenzo E, Sarotto L, Phillips E, Todeschini H, Nahmod M, Alle L, Schneider S, Kaja L, Boni L, Ferraina P, Carus T, Kokudo N, Ishizawa T, Walsh M, Simpfendorfer C, Mayank R, White K, Rosenthal RJ. Randomized Trial of Near-infrared Incisionless Fluorescent Cholangiography. Ann Surg. 2019;270:992–999. doi: 10.1097/SLA.0000000000003178. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Teh CSC, Ishizawa T, Aoki T, Cavallucci D, Lee SY, Panganiban KM, Perini MV, Shah SR, Wang H, Xu Y, Suh KS, Kokudo N. Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery. Ann Surg. 2021;274:97–106. doi: 10.1097/SLA.0000000000004718. [DOI] [PubMed] [Google Scholar]
- 17.He M, Jiang Z, Wang C, Hao Z, An J, Shen J. Diagnostic value of near-infrared or fluorescent indocyanine green guided sentinel lymph node mapping in gastric cancer: A systematic review and meta-analysis. J Surg Oncol. 2018;118:1243–1256. doi: 10.1002/jso.25285. [DOI] [PubMed] [Google Scholar]
- 18.Koual M, Benoit L, Nguyen-Xuan HT, Bentivegna E, Azaïs H, Bats AS. Diagnostic value of indocyanine green fluorescence guided sentinel lymph node biopsy in vulvar cancer: A systematic review. Gynecol Oncol. 2021;161:436–441. doi: 10.1016/j.ygyno.2021.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Goonawardena J, Yong C, Law M. Use of indocyanine green fluorescence compared to radioisotope for sentinel lymph node biopsy in early-stage breast cancer: systematic review and meta-analysis. Am J Surg. 2020;220:665–676. doi: 10.1016/j.amjsurg.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Gerken ALH, Nowak K, Meyer A, Weiss C, Krüger B, Nawroth N, Karampinis I, Heller K, Apel H, Reissfelder C, Schwenke K, Keese M, Lang W, Rother U. Quantitative Assessment of Intraoperative Laser Fluorescence Angiography with Indocyanine Green Predicts Early Graft Function after Kidney Transplantation. Ann Surg. 2020:Publish Ahead of Print. doi: 10.1097/SLA.0000000000004529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peeters KC, Tollenaar RA, Marijnen CA, Klein Kranenbarg E, Steup WH, Wiggers T, Rutten HJ, van de Velde CJ Dutch Colorectal Cancer Group. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg. 2005;92:211–216. doi: 10.1002/bjs.4806. [DOI] [PubMed] [Google Scholar]
- 22.Matthiessen P, Hallböök O, Andersson M, Rutegård J, Sjödahl R. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis. 2004;6:462–469. doi: 10.1111/j.1463-1318.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 23.Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253:890–899. doi: 10.1097/SLA.0b013e3182128929. [DOI] [PubMed] [Google Scholar]
- 24.Kverneng Hultberg D, Svensson J, Jutesten H, Rutegård J, Matthiessen P, Lydrup ML, Rutegård M. The Impact of Anastomotic Leakage on Long-term Function After Anterior Resection for Rectal Cancer. Dis Colon Rectum. 2020;63:619–628. doi: 10.1097/DCR.0000000000001613. [DOI] [PubMed] [Google Scholar]
- 25.Ashraf SQ, Burns EM, Jani A, Altman S, Young JD, Cunningham C, Faiz O, Mortensen NJ. The economic impact of anastomotic leakage after anterior resections in English NHS hospitals: are we adequately remunerating them? Colorectal Dis. 2013;15:e190–e198. doi: 10.1111/codi.12125. [DOI] [PubMed] [Google Scholar]
- 26.Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Peltrini R, Imperatore N, Carannante F, Cuccurullo D, Capolupo GT, Bracale U, Caricato M, Corcione F. Age and comorbidities do not affect short-term outcomes after laparoscopic rectal cancer resection in elderly patients. A multi-institutional cohort study in 287 patients. Updates Surg. 2021;73:527–537. doi: 10.1007/s13304-021-00990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulu Y, Ulrich A, Bruckner T, Contin P, Welsch T, Rahbari NN, Büchler MW, Weitz J International Study Group of Rectal Cancer. Validation of the International Study Group of Rectal Cancer definition and severity grading of anastomotic leakage. Surgery. 2013;153:753–761. doi: 10.1016/j.surg.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Attard JA, Raval MJ, Martin GR, Kolb J, Afrouzian M, Buie WD, Sigalet DL. The effects of systemic hypoxia on colon anastomotic healing: an animal model. Dis Colon Rectum. 2005;48:1460–1470. doi: 10.1007/s10350-005-0047-3. [DOI] [PubMed] [Google Scholar]
- 30.Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V. Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum. 2000;43:76–82. doi: 10.1007/BF02237248. [DOI] [PubMed] [Google Scholar]
- 31.Impellizzeri HG, Pulvirenti A, Inama M, Bacchion M, Marrano E, Creciun M, Casaril A, Moretto G. Near-infrared fluorescence angiography for colorectal surgery is associated with a reduction of anastomotic leak rate. Updates Surg. 2020;72:991–998. doi: 10.1007/s13304-020-00758-x. [DOI] [PubMed] [Google Scholar]
- 32.Boni L, David G, Dionigi G, Rausei S, Cassinotti E, Fingerhut A. Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg Endosc. 2016;30:2736–2742. doi: 10.1007/s00464-015-4540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Losurdo P, Mis TC, Cosola D, Bonadio L, Giudici F, Casagranda B, Bortul M, de Manzini N. Anastomosis Leak: Is There Still a Place for Indocyanine Green Fluorescence Imaging in Colon-Rectal Surgery? Surg Innov. 2020:1553350620975258. doi: 10.1177/1553350620975258. [DOI] [PubMed] [Google Scholar]
- 34.Shen Y, Yang T, Yang J, Meng W, Wang Z. Intraoperative indocyanine green fluorescence angiography to prevent anastomotic leak after low anterior resection for rectal cancer: a meta-analysis. ANZ J Surg. 2020;90:2193–2200. doi: 10.1111/ans.15809. [DOI] [PubMed] [Google Scholar]
- 35.Kudszus S, Roesel C, Schachtrupp A, Höer JJ. Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch Surg. 2010;395:1025–1030. doi: 10.1007/s00423-010-0699-x. [DOI] [PubMed] [Google Scholar]
- 36.Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DA, Lee SW, Senagore AJ, Phelan MJ, Stamos MJ. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg. 2015;220:82–92.e1. doi: 10.1016/j.jamcollsurg.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E. Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc. 2017;31:1836–1840. doi: 10.1007/s00464-016-5181-6. [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa H, Tsukada Y, Wakabayashi M, Nomura S, Sasaki T, Nishizawa Y, Ikeda K, Akimoto T, Ito M. Impact of intraoperative indocyanine green fluorescence angiography on anastomotic leakage after laparoscopic sphincter-sparing surgery for malignant rectal tumors. Int J Colorectal Dis. 2020;35:471–480. doi: 10.1007/s00384-019-03490-0. [DOI] [PubMed] [Google Scholar]
- 39.Otero-Piñeiro AM, de Lacy FB, Van Laarhoven JJ, Martín-Perez B, Valverde S, Bravo R, Lacy AM. The impact of fluorescence angiography on anastomotic leak rate following transanal total mesorectal excision for rectal cancer: a comparative study. Surg Endosc. 2021;35:754–762. doi: 10.1007/s00464-020-07442-6. [DOI] [PubMed] [Google Scholar]
- 40.Benčurik V, Škrovina M, Martínek L, Bartoš J, Macháčková M, Dosoudil M, Štěpánová E, Přibylová L, Briš R, Vomáčková K. Intraoperative fluorescence angiography and risk factors of anastomotic leakage in mini-invasive low rectal resections. Surg Endosc . 2021;35:5015–5023. doi: 10.1007/s00464-020-07982-x. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe J, Ishibe A, Suwa Y, Suwa H, Ota M, Kunisaki C, Endo I. Indocyanine green fluorescence imaging to reduce the risk of anastomotic leakage in laparoscopic low anterior resection for rectal cancer: a propensity score-matched cohort study. Surg Endosc. 2020;34:202–208. doi: 10.1007/s00464-019-06751-9. [DOI] [PubMed] [Google Scholar]
- 42.Foo CC, Ng KK, Tsang J, Wei R, Chow F, Chan TY, Lo O, Law WL. Colonic perfusion assessment with indocyanine-green fluorescence imaging in anterior resections: a propensity score-matched analysis. Tech Coloproctol. 2020;24:935–942. doi: 10.1007/s10151-020-02232-7. [DOI] [PubMed] [Google Scholar]
- 43.Bonadio L, Iacuzzo C, Cosola D, Cipolat Mis T, Giudici F, Casagranda B, Biloslavo A, de Manzini N. Indocyanine green-enhanced fluorangiography (ICGf) in laparoscopic extraperitoneal rectal cancer resection. Updates Surg. 2020;72:477–482. doi: 10.1007/s13304-020-00725-6. [DOI] [PubMed] [Google Scholar]
- 44.Wada T, Kawada K, Hoshino N, Inamoto S, Yoshitomi M, Hida K, Sakai Y. The effects of intraoperative ICG fluorescence angiography in laparoscopic low anterior resection: a propensity score-matched study. Int J Clin Oncol. 2019;24:394–402. doi: 10.1007/s10147-018-1365-5. [DOI] [PubMed] [Google Scholar]
- 45.De Nardi P, Elmore U, Maggi G, Maggiore R, Boni L, Cassinotti E, Fumagalli U, Gardani M, De Pascale S, Parise P, Vignali A, Rosati R. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: results of a multicenter randomized controlled trial. Surg Endosc. 2020;34:53–60. doi: 10.1007/s00464-019-06730-0. [DOI] [PubMed] [Google Scholar]
- 46.Alekseev M, Rybakov E, Shelygin Y, Chernyshov S, Zarodnyuk I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: results of the FLAG randomized trial. Colorectal Dis. 2020;22:1147–1153. doi: 10.1111/codi.15037. [DOI] [PubMed] [Google Scholar]
- 47.Song M, Liu J, Xia D, Yao H, Tian G, Chen X, Liu Y, Jiang Y, Li Z. Assessment of intraoperative use of indocyanine green fluorescence imaging on the incidence of anastomotic leakage after rectal cancer surgery: a PRISMA-compliant systematic review and meta-analysis. Tech Coloproctol. 2021;25:49–58. doi: 10.1007/s10151-020-02335-1. [DOI] [PubMed] [Google Scholar]
- 48.Mizrahi I, de Lacy FB, Abu-Gazala M, Fernandez LM, Otero A, Sands DR, Lacy AM, Wexner SD. Transanal total mesorectal excision for rectal cancer with indocyanine green fluorescence angiography. Tech Coloproctol. 2018;22:785–791. doi: 10.1007/s10151-018-1869-z. [DOI] [PubMed] [Google Scholar]
- 49.Kim JC, Lee JL, Park SH. Interpretative Guidelines and Possible Indications for Indocyanine Green Fluorescence Imaging in Robot-Assisted Sphincter-Saving Operations. Dis Colon Rectum. 2017;60:376–384. doi: 10.1097/DCR.0000000000000782. [DOI] [PubMed] [Google Scholar]
- 50.Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24:1205–1210. doi: 10.1007/s00464-010-0965-6. [DOI] [PubMed] [Google Scholar]
- 51.de'Angelis N, Portigliotti L, Azoulay D, Brunetti F. Transanal total mesorectal excision for rectal cancer: a single center experience and systematic review of the literature. Langenbecks Arch Surg. 2015;400:945–959. doi: 10.1007/s00423-015-1350-7. [DOI] [PubMed] [Google Scholar]
- 52.Chen CC, Lai YL, Jiang JK, Chu CH, Huang IP, Chen WS, Cheng AY, Yang SH. Transanal Total Mesorectal Excision Versus Laparoscopic Surgery for Rectal Cancer Receiving Neoadjuvant Chemoradiation: A Matched Case-Control Study. Ann Surg Oncol. 2016;23:1169–1176. doi: 10.1245/s10434-015-4997-y. [DOI] [PubMed] [Google Scholar]
- 53.Al-Taher M, Knapen B, Barberio M, Felli E, Gioux S, Bouvy ND, Stassen LPS, Marescaux J, Diana M. Near infrared fluorescence imaging of the urethra: a systematic review of the literature. Minim Invasive Ther Allied Technol. 2020:1–8. doi: 10.1080/13645706.2020.1826974. [DOI] [PubMed] [Google Scholar]
- 54.Atallah S, Mabardy A, Volpato AP, Chin T, Sneider J, Monson JRT. Surgery beyond the visible light spectrum: theoretical and applied methods for localization of the male urethra during transanal total mesorectal excision. Tech Coloproctol. 2017;21:413–424. doi: 10.1007/s10151-017-1641-9. [DOI] [PubMed] [Google Scholar]
- 55.Nitta T, Tanaka K, Kataoka J, Ohta M, Ishii M, Ishibashi T, Okuda J. Novel technique with the IRIS U kit to prevent urethral injury in patients undergoing transanal total mesorectal excision. Ann Med Surg (Lond) 2019;46:1–3. doi: 10.1016/j.amsu.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barnes TG, Penna M, Hompes R, Cunningham C. Fluorescence to highlight the urethra: a human cadaveric study. Tech Coloproctol. 2017;21:439–444. doi: 10.1007/s10151-017-1615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohta S, Nishi M, Tokunaga T, Yoshikawa K, Higashijima J, Miyatani T, Kashihara H, Takasu C, Ishikawa D, Shimada M. Usefulness of an ICG fluorescence catheter system in TaTME for avoiding intraoperative urethral injury. J Med Invest. 2020;67:285–288. doi: 10.2152/jmi.67.285. [DOI] [PubMed] [Google Scholar]
- 58.Barnes TG, Volpi D, Cunningham C, Vojnovic B, Hompes R. Improved urethral fluorescence during low rectal surgery: a new dye and a new method. Tech Coloproctol. 2018;22:115–119. doi: 10.1007/s10151-018-1757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barberio M, Al-Taher M, Forgione A, Hoskere Ashoka A, Felli E, Agnus V, Marescaux J, Klymchenko A, Diana M. A novel method for near-infrared fluorescence imaging of the urethra during perineal and transanal surgery: demonstration in a cadaveric model. Colorectal Dis. 2020;22:1749–1753. doi: 10.1111/codi.15156. [DOI] [PubMed] [Google Scholar]
- 60.Halabi WJ, Jafari MD, Nguyen VQ, Carmichael JC, Mills S, Pigazzi A, Stamos MJ. Ureteral injuries in colorectal surgery: an analysis of trends, outcomes, and risk factors over a 10-year period in the United States. Dis Colon Rectum. 2014;57:179–186. doi: 10.1097/DCR.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 61.Azioni G, Bracale U, Scala A, Capobianco F, Barone M, Rosati M, Pignata G. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010;19:292–297. doi: 10.3109/13645706.2010.507345. [DOI] [PubMed] [Google Scholar]
- 62.Bracale U, Azioni G, Rosati M, Barone M, Pignata G. Deep pelvic endometriosis (Adamyan IV stage): multidisciplinary laparoscopic treatments. Acta Chir Iugosl. 2009;56:41–46. doi: 10.2298/aci0901041b. [DOI] [PubMed] [Google Scholar]
- 63.Croghan SM, Zaborowski A, Mohan HM, Mulvin D, McGuire BB, Murphy M, Galvin DJ, Lennon G, Quinlan D, Winter DC. The sentinel stent? Int J Colorectal Dis. 2019;34:1161–1178. doi: 10.1007/s00384-019-03314-1. [DOI] [PubMed] [Google Scholar]
- 64.Speicher PJ, Goldsmith ZG, Nussbaum DP, Turley RS, Peterson AC, Mantyh CR. Ureteral stenting in laparoscopic colorectal surgery. J Surg Res. 2014;190:98–103. doi: 10.1016/j.jss.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 65.Yeung TM, Volpi D, Tullis ID, Nicholson GA, Buchs N, Cunningham C, Guy R, Lindsey I, George B, Jones O, Wang LM, Hompes R, Vojnovic B, Hamdy F, Mortensen NJ. Identifying Ureters In Situ Under Fluorescence During Laparoscopic and Open Colorectal Surgery. Ann Surg. 2016;263:e1–e2. doi: 10.1097/SLA.0000000000001513. [DOI] [PubMed] [Google Scholar]
- 66.Al-Taher M, van den Bos J, Schols RM, Bouvy ND, Stassen LP. Fluorescence Ureteral Visualization in Human Laparoscopic Colorectal Surgery Using Methylene Blue. J Laparoendosc Adv Surg Tech A. 2016;26:870–875. doi: 10.1089/lap.2016.0264. [DOI] [PubMed] [Google Scholar]
- 67.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 68.Mandovra P, Kalikar V, Patankar RV. Real-Time Visualization of Ureters Using Indocyanine Green During Laparoscopic Surgeries: Can We Make Surgery Safer? Surg Innov. 2019;26:464–468. doi: 10.1177/1553350619827152. [DOI] [PubMed] [Google Scholar]
- 69.Siddighi S, Yune JJ, Hardesty J. Indocyanine green for intraoperative localization of ureter. Am J Obstet Gynecol. 2014;211:436.e1–436.e2. doi: 10.1016/j.ajog.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 70.Foppa C, Spinelli A. Ureteric identification with indocyanine green fluorescence in laparoscopic redo pouch surgery. Tech Coloproctol. 2018;22:627–628. doi: 10.1007/s10151-018-1838-6. [DOI] [PubMed] [Google Scholar]
- 71.Kanabur P, Chai C, Taylor J. Use of Indocyanine Green for Intraoperative Ureteral Identification in Nonurologic Surgery. JAMA Surg. 2020;155:520–521. doi: 10.1001/jamasurg.2020.0094. [DOI] [PubMed] [Google Scholar]
- 72.Lee Z, Moore B, Giusto L, Eun DD. Use of indocyanine green during robot-assisted ureteral reconstructions. Eur Urol. 2015;67:291–298. doi: 10.1016/j.eururo.2014.08.057. [DOI] [PubMed] [Google Scholar]
- 73.White LA, Joseph JP, Yang DY, Kelley SR, Mathis KL, Behm K, Viers BR. Intraureteral indocyanine green augments ureteral identification and avoidance during complex robotic-assisted colorectal surgery. Colorectal Dis. 2021;23:718–723. doi: 10.1111/codi.15407. [DOI] [PubMed] [Google Scholar]
- 74.Yellinek S, Krizzuk D, J Nogueras J, D Wexner S. Ureteral Injury During Colorectal Surgery: Two Case Reports and a Literature Review. J Anus Rectum Colon. 2018;2:71–76. doi: 10.23922/jarc.2017-052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dip FD, Nahmod M, Anzorena FS, Moreira A, Sarotto L, Ampudia C, Kalaskar SN, Ferraina P, Rosenthal RJ, Wexner SD. Novel technique for identification of ureters using sodium fluorescein. Surg Endosc. 2014;28:2730–2733. doi: 10.1007/s00464-014-3519-5. [DOI] [PubMed] [Google Scholar]
- 76.Al-Taher M, van den Bos J, Schols RM, Kubat B, Bouvy ND, Stassen LPS. Evaluation of a novel dye for near-infrared fluorescence delineation of the ureters during laparoscopy. BJS Open. 2018;2:254–261. doi: 10.1002/bjs5.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Portnoy E, Nizri E, Golenser J, Shmuel M, Magdassi S, Eyal S. Imaging the urinary pathways in mice by liposomal indocyanine green. Nanomedicine. 2015;11:1057–1064. doi: 10.1016/j.nano.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 78.Friedman-Levi Y, Larush L, Diana M, Marchegiani F, Marescaux J, Goder N, Lahat G, Klausner J, Eyal S, Magdassi S, Nizri E. Optimization of liposomal indocyanine green for imaging of the urinary pathways and a proof of concept in a pig model. Surg Endosc. 2018;32:963–970. doi: 10.1007/s00464-017-5773-9. [DOI] [PubMed] [Google Scholar]
- 79.Kim TH, Jeong SY, Choi DH, Kim DY, Jung KH, Moon SH, Chang HJ, Lim SB, Choi HS, Park JG. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol. 2008;15:729–737. doi: 10.1245/s10434-007-9696-x. [DOI] [PubMed] [Google Scholar]
- 80.Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1–42. doi: 10.1007/s10147-019-01485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, Yamaguchi T, Bandou H, Katsumata K, Murata K, Akagi Y, Takiguchi N, Saida Y, Nakamura K, Fukuda H, Akasu T, Moriya Y Colorectal Cancer Study Group of Japan Clinical Oncology Group. Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg. 2017;266:201–207. doi: 10.1097/SLA.0000000000002212. [DOI] [PubMed] [Google Scholar]
- 82.Watanabe J, Ota M, Suwa Y, Ishibe A, Masui H, Nagahori K. Real-Time Indocyanine Green Fluorescence Imaging-Guided Complete Mesocolic Excision in Laparoscopic Flexural Colon Cancer Surgery. Dis Colon Rectum. 2016;59:701–705. doi: 10.1097/DCR.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 83.Park SY, Park JS, Kim HJ, Woo IT, Park IK, Choi GS. Indocyanine Green Fluorescence Imaging-Guided Laparoscopic Surgery Could Achieve Radical D3 Dissection in Patients With Advanced Right-Sided Colon Cancer. Dis Colon Rectum. 2020;63:441–449. doi: 10.1097/DCR.0000000000001597. [DOI] [PubMed] [Google Scholar]
- 84.Zhou SC, Tian YT, Wang XW, Zhao CD, Ma S, Jiang J, Li EN, Zhou HT, Liu Q, Liang JW, Zhou ZX, Wang XS. Application of indocyanine green-enhanced near-infrared fluorescence-guided imaging in laparoscopic lateral pelvic lymph node dissection for middle-low rectal cancer. World J Gastroenterol. 2019;25:4502–4511. doi: 10.3748/wjg.v25.i31.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim HJ, Park JS, Choi GS, Park SY, Lee HJ. Fluorescence-guided Robotic Total Mesorectal Excision with Lateral Pelvic Lymph Node Dissection in Locally Advanced Rectal Cancer: A Video Presentation. Dis Colon Rectum. 2017;60:1332–1333. doi: 10.1097/DCR.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 86.Kawada K, Yoshitomi M, Inamoto S, Sakai Y. Indocyanine Green Fluorescence-Guided Laparoscopic Lateral Lymph Node Dissection for Rectal Cancer. Dis Colon Rectum. 2019;62:1401. doi: 10.1097/DCR.0000000000001475. [DOI] [PubMed] [Google Scholar]
- 87.Kazanowski M, Al Furajii H, Cahill RA. Near-infrared laparoscopic fluorescence for pelvic side wall delta mapping in patients with rectal cancer--'PINPOINT' nodal assessment. Colorectal Dis. 2015;17 Suppl 3:32–35. doi: 10.1111/codi.13030. [DOI] [PubMed] [Google Scholar]
- 88.van der Pas MH, Meijer S, Hoekstra OS, Riphagen II, de Vet HC, Knol DL, van Grieken NC, Meijerink WJ. Sentinel-lymph-node procedure in colon and rectal cancer: a systematic review and meta-analysis. Lancet Oncol. 2011;12:540–550. doi: 10.1016/S1470-2045(11)70075-4. [DOI] [PubMed] [Google Scholar]
- 89.Joosten JJ, Strobbe LJ, Wauters CA, Pruszczynski M, Wobbes T, Ruers TJ. Intraoperative lymphatic mapping and the sentinel node concept in colorectal carcinoma. Br J Surg. 1999;86:482–486. doi: 10.1046/j.1365-2168.1999.01051.x. [DOI] [PubMed] [Google Scholar]
- 90.Nastro P, Sodo M, Dodaro CA, Gargiulo S, Acampa W, Bracale U, Renda A. Intraoperative radiochromoguided mapping of sentinel lymph node in colon cancer. Tumori. 2002;88:352–353. doi: 10.1177/030089160208800426. [DOI] [PubMed] [Google Scholar]
- 91.Cahill RA, Anderson M, Wang LM, Lindsey I, Cunningham C, Mortensen NJ. Near-infrared (NIR) laparoscopy for intraoperative lymphatic road-mapping and sentinel node identification during definitive surgical resection of early-stage colorectal neoplasia. Surg Endosc. 2012;26:197–204. doi: 10.1007/s00464-011-1854-3. [DOI] [PubMed] [Google Scholar]
- 92.Noura S, Ohue M, Seki Y, Tanaka K, Motoori M, Kishi K, Miyashiro I, Ohigashi H, Yano M, Ishikawa O, Miyamoto Y. Feasibility of a lateral region sentinel node biopsy of lower rectal cancer guided by indocyanine green using a near-infrared camera system. Ann Surg Oncol. 2010;17:144–151. doi: 10.1245/s10434-009-0711-2. [DOI] [PubMed] [Google Scholar]
- 93.Handgraaf HJ, Boogerd LS, Verbeek FP, Tummers QR, Hardwick JC, Baeten CI, Frangioni JV, van de Velde CJ, Vahrmeijer AL. Intraoperative fluorescence imaging to localize tumors and sentinel lymph nodes in rectal cancer. Minim Invasive Ther Allied Technol. 2016;25:48–53. doi: 10.3109/13645706.2015.1042389. [DOI] [PubMed] [Google Scholar]
- 94.Braat AE, Oosterhuis JW, Moll FC, de Vries JE, Wiggers T. Sentinel node detection after preoperative short-course radiotherapy in rectal carcinoma is not reliable. Br J Surg. 2005;92:1533–1538. doi: 10.1002/bjs.5169. [DOI] [PubMed] [Google Scholar]
- 95.Nagata J, Fukunaga Y, Akiyoshi T, Konishi T, Fujimoto Y, Nagayama S, Yamamoto N, Ueno M. Colonic Marking With Near-Infrared, Light-Emitting, Diode-Activated Indocyanine Green for Laparoscopic Colorectal Surgery. Dis Colon Rectum. 2016;59:e14–e18. doi: 10.1097/DCR.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 96.Zako T, Ito M, Hyodo H, Yoshimoto M, Watanabe M, Takemura H, Kishimoto H, Kaneko K, Soga K, Maeda M. Extra-luminal detection of assumed colonic tumor site by near-infrared laparoscopy. Surg Endosc. 2016;30:4153–4159. doi: 10.1007/s00464-015-4669-9. [DOI] [PubMed] [Google Scholar]
- 97.Watanabe M, Tsunoda A, Narita K, Kusano M, Miwa M. Colonic tattooing using fluorescence imaging with light-emitting diode-activated indocyanine green: a feasibility study. Surg Today. 2009;39:214–218. doi: 10.1007/s00595-008-3849-9. [DOI] [PubMed] [Google Scholar]
- 98.Miyoshi N, Ohue M, Noura S, Yano M, Sasaki Y, Kishi K, Yamada T, Miyashiro I, Ohigashi H, Iishi H, Ishikawa O, Imaoka S. Surgical usefulness of indocyanine green as an alternative to India ink for endoscopic marking. Surg Endosc. 2009;23:347–351. doi: 10.1007/s00464-008-9938-4. [DOI] [PubMed] [Google Scholar]
- 99.Watanabe M, Murakami M, Ozawa Y, Yoshizawa S, Matsui N, Aoki T. Intraoperative Identification of Colonic Tumor Sites Using a Near-Infrared Fluorescence Endoscopic Imaging System and Indocyanine Green. Dig Surg. 2017;34:495–501. doi: 10.1159/000458450. [DOI] [PubMed] [Google Scholar]
- 100.Park JH, Moon HS, Kwon IS, Yun GY, Lee SH, Park DH, Kim JS, Kang SH, Lee ES, Kim SH, Sung JK, Lee BS, Jeong HY. Usefulness of colonic tattooing using indocyanine green in patients with colorectal tumors. World J Clin Cases. 2018;6:632–640. doi: 10.12998/wjcc.v6.i13.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goo JJ, Ryu DG, Kim HW, Park SB, Kang DH, Choi CW, Kim SJ, Nam HS, Kim HS, Son GM, Park BS. Efficacy of preoperative colonoscopic tattooing with indocyanine green on lymph node harvest and factors associated with inadequate lymph node harvest in colorectal cancer. Scand J Gastroenterol. 2019;54:666–672. doi: 10.1080/00365521.2019.1612940. [DOI] [PubMed] [Google Scholar]
- 102.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 103.Magdassi S, Bar-David S, Friedman-Levi Y, Zigmond E, Varol C, Lahat G, Klausner J, Eyal S, Nizri E. Intraoperative Localization of Rectal Tumors Using Liposomal Indocyanine Green. Surg Innov. 2017;24:139–144. doi: 10.1177/1553350617690310. [DOI] [PubMed] [Google Scholar]
- 104.Bar-David S, Larush L, Goder N, Aizic A, Zigmond E, Varol C, Klausner J, Magdassi S, Nizri E. Size and lipid modification determine liposomal Indocyanine green performance for tumor imaging in a model of rectal cancer. Sci Rep. 2019;9:8566. doi: 10.1038/s41598-019-45038-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Glockzin G, Zeman F, Croner RS, Königsrainer A, Pelz J, Ströhlein MA, Rau B, Arnold D, Koller M, Schlitt HJ, Piso P. Perioperative Systemic Chemotherapy, Cytoreductive Surgery, and Hyperthermic Intraperitoneal Chemotherapy in Patients With Colorectal Peritoneal Metastasis: Results of the Prospective Multicenter Phase 2 COMBATAC Trial. Clin Colorectal Cancer. 2018;17:285–296. doi: 10.1016/j.clcc.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 106.Liberale G, Vankerckhove S, Caldon MG, Ahmed B, Moreau M, Nakadi IE, Larsimont D, Donckier V, Bourgeois P Group R&D for the Clinical Application of Fluorescence Imaging of the Jules Bordetʼs Institute. Fluorescence Imaging After Indocyanine Green Injection for Detection of Peritoneal Metastases in Patients Undergoing Cytoreductive Surgery for Peritoneal Carcinomatosis From Colorectal Cancer: A Pilot Study. Ann Surg. 2016;264:1110–1115. doi: 10.1097/SLA.0000000000001618. [DOI] [PubMed] [Google Scholar]
- 107.Dapri G, Cahill R, Bourgeois P, Liberale G, Galdon Gomez M, Cadière GB. Peritumoural injection of indocyanine green fluorescence during transanal total mesorectal excision to identify the plane of dissection - a video vignette. Colorectal Dis. 2017;19:599–600. doi: 10.1111/codi.13698. [DOI] [PubMed] [Google Scholar]
- 108.Ismael G, Al Furajji H, Cahill RA. Near-infrared laparoscopic fluorescence to guide fascial plane identification in total mesorectal excision for rectal cancer: A Video Vignette. Colorectal Dis. 2015;17 Suppl 3:36. doi: 10.1111/codi.13089. [DOI] [PubMed] [Google Scholar]
- 109.Kawada K, Hida K, Yoshitomi M, Sakai Y. A novel use of indocyanine green to identify the plane of dissection during abdominoperineal resection by the transperineal approach - a video vignette. Colorectal Dis. 2018;20:455–456. doi: 10.1111/codi.14065. [DOI] [PubMed] [Google Scholar]
- 110.Slooter MD, Blok RD, Wisselink DD, Buskens CJ, Bemelman WA, Tanis PJ, Hompes R. Near-infrared fluorescence angiography for intra-operative assessment of pedicled omentoplasty for filling of a pelvic cavity: a pilot study. Tech Coloproctol. 2019;23:723–728. doi: 10.1007/s10151-019-02048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Slooter MD, Blok RD, de Krom MA, Buskens CJ, Bemelman WA, Tanis PJ, Hompes R. Optimizing omentoplasty for management of chronic pelvic sepsis by intra-operative fluorescence angiography: a comparative cohort study. Colorectal Dis. 2020;22:2252–2259. doi: 10.1111/codi.15276. [DOI] [PMC free article] [PubMed] [Google Scholar]