Abstract
Background
Bone remodeling is a lifelong process that ranges from orthodontic tooth movement/alignment to bone damage/healing, to overall bone health. Osteoprotegerin (OPG) and transforming growth factor β1 (TGF-β1) are secreted by osteoblasts and participate in bone remodeling. OPG promotes bone remineralization and stabilization prominent in post-mechanical repositioning of the teeth in the dental alveolus. TGF-β1 participates in regulatory processes to promote osteoblast and osteoclast equilibrium. In the context of orthodontic tooth movement, post-treatment fixation requires additional, exogenous, stabilization support. Recent research showcases supplementary solutions, in conjunction to standard tooth fixation techniques, such as OPG injections into gum and periodontal tissues to accelerate tooth anchorage; however, injections are prone to post-procedure complications and discomfort. This study utilizes noninvasive bioelectric stimulation (BES) to modulate OPG and TGF-β1 as a novel solution to regulate bone remineralization specifically in the context of post-orthodontic tooth movement.
Purpose
The aim of this study was to investigate a spectrum of BES parameters that would modulate OPG and TGF-β1 expression in osteoblasts.
Methods
Osteoblasts were cultured and stimulated using frequencies from 25 Hz to 3 MHz. RT-qPCR was used to quantify changes in OPG and TGFb-1 mRNA expression.
Results
OPG mRNA expression was significantly increased at frequencies above 10,000 Hz with a maximum expression increase of 332 ± 8% at 100 kHz. Conversely, OPG mRNA expression was downregulated at frequencies lower than 1000 Hz. TGF-β1 mRNA expression increased throughout all stimulation frequencies with a peak of 332 ± 72% at 250 kHz. Alizarin Red tests for calcium, indicated that mineralization of stimulated osteoblasts in vitro increased 28% after 6 weeks in culture.
Discussion
Results support the working hypothesis that OPG and TGF-β1 mRNA expression can be modulated through BES. Noninvasive BES approaches have the potential to accelerate bone remineralization by providing a novel tool to supplement the anchorage process, reduce complications, and promote patient compliance and reduce post-treatment relapse. Noninvasive BES may be applicable to other clinical applications as a novel therapeutic tool to modulate bone remodeling.
Keywords: Bone remodeling/regeneration, Orthodontic tooth movement, Electrophysiology, Gene expression, Osteoblast, Growth factor, Mineralization in vitro
Graphical abstract
Highlights
-
•
Post-orthodontic upkeep requires improvement to reduce likely relapse in patients.
-
•
Bioelectrical stimulation overexpresses specific biomolecules in bone.
-
•
Those biomolecules participate in bone deposition and mineralization.
-
•
Bioelectrical stimulation supports a new orthodontic biological control paradigm.
1. Introduction
Bioelectrical stimulation (BES) has been associated with tissue remodeling and repair within the body (Tyler, 2017) via distinct mechanisms (Levin, 2013) that make it attractive as a noninvasive therapy for many diseases. BES has the ability to produce specific tissue and cellular responses via manipulation of key electrical parameters like signal strength, frequency, pulse form, and duration, allowing for a broad depth of therapeutic possibilities.
Exogenous bioelectric stimulation has been implicated in the up- or down-regulation of various growth factors that, subsequently, elicit various cell-specific responses. This phenomenon, although still not clearly understood (Vander Molen et al., 2000), is particularly relevant in non-excitable cells where ion channels are highly involved in tissue homeostasis (Cervera et al., 2016). It has, therefore, been suggested by many clinical laboratories that BES may be a non-invasive approach for enhancing bone repair and reducing healing times following bone damage (Aleem et al., 2016; Bhavsar et al., 2019; Kuzyk and Schemitsch, 2009; Riwo Onibere, 2008).
One particular treatment in which bone remodeling is heavily involved is orthodontic tooth movement (OTM). In OTM, attached or removable dental appliances are used to apply mechanical forces to reposition teeth. Applying mechanical forces to the teeth initiates bone remodeling cascades in alveolar bone and periodontal tissues (Davidovitch, 1991; Krishnan and Davidovitch, 2006). These cascades induce osteoclastic activity along the leading (compression) edge of the tooth that instigate bone resorption (Yokoya et al., 1997). Similarly, osteoblastic activity is induced along the trailing (tension) edge of the tooth—promoting bone deposition or remineralization (Garlet et al., 2007). The interrelationship between osteoclastic and osteoblastic activities can be characterized by, and correlated to, observed changes in three specific bone-modulating growth factors—receptor activator of nuclear factor-kappa B (RANK), its ligand (RANKL), and osteoprotegerin (OPG) (Walsh and Choi, 2014; Yamaguchi, 2009).
OPG is one of the key factors responsible for maintaining bone homeostasis (Theoleyre et al., 2004). Its concentration in relation to RANK, RANKL, and other molecules influences bone metabolism and governs orthodontic tooth movement by determining bone deposition and resorption patterns (Ikebuchi et al., 2018). RANK is a type I homotrimer transmembrane protein expressed along osteoclast precursor membranes (Idriss and Naismith, 2000; Ito and Hata, 2004). When activated by RANKL, an osteoclastogenic factor is released by surrounding osteoblasts and these pre-osteoclastic cells differentiate into mature osteoclasts to promote bone resorption. OPG, a secreted osteoblast-derived RANK decoy receptor, can inhibit osteoclastogenesis by binding RANKL and preventing RANK activation (Baud’huin et al., 2013).
Transforming growth factor β1 (TGF-β1) is another molecule that is highly relevant and highly involved in regulating bone remodeling. TGF-β1 has multiple roles in bone formation. It enhances osteoblast proliferation (Kassem et al., 2000) and recruits osteoblastic precursors, or matrix-producing osteoblasts, to the region via chemotactic attraction (Lucas, 1989). During initial phases of osteoblastic differentiation, TGF-β1 enhances the production of extracellular bone matrix protein (Alliston et al., 2001) and cooperates with metalloproteins to regulate the differentiation of osteoblasts (Canalis et al., 2003).
While the exploration of TGF-β1 is still relatively nascent in OTM, the exploration of OPG has seen increased interest. For example, it has been found that the local injection of OPG directly into the gum and periodontal ligament can reduce molar movement and osteoclast numbers (Dunn et al., 2007). The injection delivery of OPG and its resultant tooth stabilizing effects, while not free from complications (Baxter et al., 2020), may provide novel pharmacological approaches to prevent undesired tooth relapse following appliance or aligner removal post-OTM (Dunn et al., 2007; Li and Tang, 2009).
BES provides an alternative, noninvasive approach to tooth stabilization. By delivering key electrical sequences within biological tissues, physiological responses are triggered that mimic, enhance, and/or modulate biomolecular processes. Several studies demonstrate the ability of BES to improve bone remodeling and regeneration (Hess et al., 2012; Srirussamee et al., 2019; Zhou et al., 2019b) or to modify cell growth patterns in vitro (Eischen-Loges et al., 2018; Spadari et al., 2017). In vitro, the mineralization process is active in cultured osteoblasts, and mineral plaques can be observed after 4 weeks in culture (Aboushady et al., 2018; Blair et al., 2017). This cellular physiological activity in vitro could be correlated with the mineralization in bone, and we hypothesize that it can be enhanced during the induction of expression of OPG during BES.
Considering its therapeutic potential, we hypothesize that BES can be successfully used in vitro to upregulate or modulate OPG and TGF-β1, and that it can be also applied in local periodontal tissues to modify gene expression. Successful modulation may lead to improved, noninvasive, and novel orthodontic treatments. Furthermore, we hypothesize that modulation would be dependent on both frequency and stimulation strength. BES protocols of varying frequencies and voltages were applied to osteoblasts in culture to determine the effects on OPG and TGF-β1 mRNA expression.
2. Materials and methods
2.1. Cell culture
Murine osteoblasts (MmOsteo; MC3T3-E1 Subclone 4) were acquired and cultured in alpha Minimum Essential Medium (αMEM; Gibco, Gaithersburg, MD, USA) supplemented with 10% FBS. Cells were maintained between six and nine total passages per supplier protocols (ATCC, Manassasm, VA, USA). Osteoblast dissociation was performed with Trypsin-0.25% EDTA (Caisson, Smithfield, USA) and cells were plated on Nunclon™ Delta 6-well MultiDishes (ThermoFisher, Waltham, MA, USA) with 5% Non-Essential Amino Acids (NEAA; Quality Biologicals, Gaithersburg, MD, USA) and 5% Penicillin-Streptomycin (Thermofisher, Waltham, MA, USA). All cultures were maintained in a 5% CO2 incubator at 37 °C.
2.2. Experimental design
Osteoblasts were grown on 6-well plates until they reached between 80% and 100% confluency. Cells were electrically stimulated for 30 min, immediately washed 3 times with PBS, and dissociated with trypsin for real-time polymerase chain reaction (RT-qPCR) analysis. After trypsinization, cells were collected, centrifuged, resuspended in PBS, and re-centrifuged. Supernatant was aspirated and the remaining cell pellet was immediately stored at -80 °C after each stimulation. OPG and TGF-β1 gene expression was quantified through qPCR (see Section 2.5).
2.3. Stimulation process
Bioelectric stimulation was applied to cultured osteoblasts in vitro using a commercially available constant voltage waveform generator RIGOL LXI 1022Z (Beaverton, OR, USA) via a 6-well stimulating plate interface (IONOPTIX, Westwood, MA, USA). To induce uniform electric fields in all stimulation chambers, 1.3 mL of DMEM solution was added to each well prior to BES signal application.
Fig. 1 shows the experimental setup for each BES stimulation experiment. Cells were plated in 6-well dishes with each well acting as a stimulation chamber. Each stimulation chamber consisted of a 30 mm-diameter well and a pair of 2.5 cm-wide carbon electrodes positioned opposite of each other across the dish. This arrangement provided a uniform electric field oriented in parallel to the bottom of the culture dish where the cells had been plated.
Fig. 1.
Bioelectric Stimulation System. Cells were plated in each dish and cultured to 80% - 100% confluency. Once confluent, cells were stimulated using an electrode array (shown at the top of panel A), which was inverted and introduced into the 6-well dish where cells were grown. Each well received uniform stimulation via a pair of carbon electrodes positioned at opposite sides (panel B).
Osteoblasts were stimulated for 30 min using a square, biphasic waveform at 50% duty. Frequency and voltages were fixed and set from 25 Hz to 3 MHz and 0.1 V, 1.0 V, or 2.0 V. The selection of test frequencies was based on other laboratories results (Genovese et al., 2009) where stimulation can last hours or only minutes (Krawczyk et al., 2020) and a patent that indicated at which frequencies and time of stimulation genetic expression could be achieved during orthodontic treatment (Howard et al., 2020). The majority of patents and works cited from other laboratories utilize stimulation frequencies below 500 Hz to increase gene expression. The use of higher frequencies, including ultrasound, have been used for nerve stimulation and blocking of electrical signals as well (Harmsen et al., 2019; Jamali et al., 2019; Kilgore and Bhadra, 2014). With these works in mind, it was deemed appropriate to pursue stimulating a large range of frequencies between low and high stimulation frequencies, as an exploratory search, and knowing that the stimuli that we are applying is not of drastically high intensity that would damage tissue (see Fig. 2).
Fig. 2.

Cell Growth Curves. Cell growth comparison between control (squares) and bioelectrically stimulated (circles) osteoblasts. Cells were counted 3 and 5 days after stimulation. No significant changes were observed in cell growth rates.
2.4. Cell viability and proliferation
To determine the effect of BES on cells, and to evaluate cell health, osteoblasts were plated, stimulated, and assessed for cell growth, shape, and viability. Density was controlled at 600,000 cells per well. Three wells received a 2.0 V, 500,000 Hz, biphasic stimulation at 50% duty for 30 min while the other three wells remained unstimulated as controls. After stimulation, micrographs were taken, and cells were observed for 24 h. After the observation period, cells were split using Trypsin-0.25% EDTA and 7000 cells were plated in 24-well plates. Cells were counted at 3 and 5 days after stimulation.
2.5. Gene expression
Gene expression was determined by extracting mRNA from osteoblasts and applying RT-qPCR. mRNA was extracted using Invitrogen PureLink RNA Mini Kit (Invitrogen, Carlsbad, CA, USA) to produce highly pure, intact mRNA. An ultraviolet absorbance ratio at 260:280 nm was measured using a NanoDrop (ThermoFisher, Waltham, MA USA) to ensure high-quality mRNA and achieve optimal reaction performance. mRNA was stored at -80 °C and good laboratory practices were followed to prevent degradation by exogenous ribonucleases prior to RT-qPCR analysis.
For complementary DNA (cDNA) generation, mRNA was reverse transcribed using ThermoFisher Scientific Maxima H Minus First Strand cDNA synthesis kit (ThermoFisher, Waltham, MA USA). Samples were processed following the manufacturer's guidelines. Genomic DNA was eliminated to ensure optimal gene expression profiling by incubating template RNA, 10× dsDNase Buffer, dsDNase, and nuclease-free water at 37 °C for 2 min. First-strand cDNA synthesis was performed by adding a mixture of oligo (dT) primer and random hexamer primer, 10 mM dNTP mix, nuclease-free water, 5× RT Buffer, and Maxima H Minus Enzyme mix into a reaction tube. The tube was incubated for 10 min at 25 °C followed by 15 min at 50 °C and 85 °C for 5 min. The synthesized cDNA was then stored on ice.
TaqMan qPCR primers and probes from Applied Biosystems® (Applied Biosystems, Foster City, CA, USA) for OPG and TGF-β1 (targets) and GAPDH (reference), were used to determine mRNA concentrations. GAPDH primers were chosen as endogenous controls based on relative expression levels (Chapman and Waldenström, 2015). The reaction mixture contained 2 μL of cDNA, 10 μL of TaqMan Fast Advanced Master Mix, 1.0 μL of TaqMan assay, and 7.0 μL of nuclease-free water. 2 μL of gene-specific primer were transferred into each well of a 384-well plate. 18 μL of the TaqMan assay mix were added into each cDNA-filled well. The PCR plate was then sealed with optical adhesive film, briefly centrifuged, and loaded into an Applied Biosystems QuantStudio3 Real-time PCR system (Applied Biosystems, Foster City, CA, USA). Denaturation took place at 95 °C for 10 min followed by 40 annealing cycles at 95 °C for 10s and an extension cycle of 60 °C for 20s. The measurement of fluorescence at each cycle allowed for direct detection of PCR products. Control experiments were performed and processed in parallel throughout the protocol and used within individual experiment sets to calculate change due to treatment (2-ΔΔCt). All RT-qPCR measurements were performed in triplicate and effectively used in analysis if the triplicate standard deviation was less than 0.50 Ct.
2.6. Mineralization
To determine mineralization changes in osteoblasts in vitro resulting from BES, we seeded (80% confluence) on 13 mm glass coverslips previously covered with collagen (US Biological, Collagen Type I, Rat C7510-18; 0.5 mg/mL in PBS) for 1 h and then washed out with PBS. Two coverslips were placed inside each well of a 6-well plate and three wells of the dish were used for control (not simulated). Alpha MEM complete media was replaced every 3-5 days for 6 weeks. After one week of seeding, cells were stimulated with 1000 kHz for 30 min. This stimulation was repeated, two and four weeks after seeding. At the end of 6 weeks, calcium deposits were quantified using Alizarin Red S, an anthraquinone derivative, used to identify calcium in tissue sections. Calcium forms an Alizarin Red S‑calcium complex in a chelation process, and the end-product is orange-red and birefringent.
Cells were fixed with alcohol 70% in deionized (DI) Water for 5 min. Control cells were not stimulated but followed the same feeding process for 6 weeks. Cells were washed with DI Water 3 times and Alizarin Red (2 g in 100 mL; pH 4.2) was added for 5 min. Coverslips were washed with Di Water, dried, and dehydrated with Acetone 100% Glass slides were placed in 24 well plates and covered with glycerin. Alizarin Red absorbance (405 nm) was measured for each coverslip using a Perkin Elmer EnSpire 2300 Multilabel reader.
2.7. Statistical analysis
A multiple factor polynomial regression was used to evaluate the main effect of frequency and voltage on log fold change in OPG and TGF-β1 expression due to treatment (2-ΔΔCt). Prior to gene expression analysis, outliers were identified and removed using scatter plot analyses. If significant, a one-sample t-test was performed to evaluate the log fold change due to treatment and determine its significance. The multiple comparisons were corrected using the Benjamini and Hochberg (1995) method. The generalized additive model was used to evaluate the modulation of the log fold change in OPG and TGF-β1 expression due to treatment (2-ΔΔCt) by either frequency or voltage and required cubic splines anchored by knots selected between data inflection points. All analyses were performed using RStudio (version 1.1.44, Boston, MA, USA) and R (version 3.4.4, Vienna, Austria) with packages: ggplot2, Rmisc, ggplot2, forcats, splines. Alpha was set at 0.05 for all statistical tests.
3. Results
3.1. Effect of bioelectrical stimulation on viability and proliferation of osteoblasts
Post-stimulation findings indicated that phenotype appeared to be unchanged, adhesion characteristics remained consistent with controls, and cell shape did not vary when compared to pre-experiment observations. Under identical growth conditions, no observable changes were noted in cell viability after 30 min of stimulation at 1 mV and 500 Hz. Growth rates were not significantly different after 3 and 5 days, post-cell stimulation (Fig. 2).
3.2. Effect of bioelectrical stimulation on OPG mRNA expression
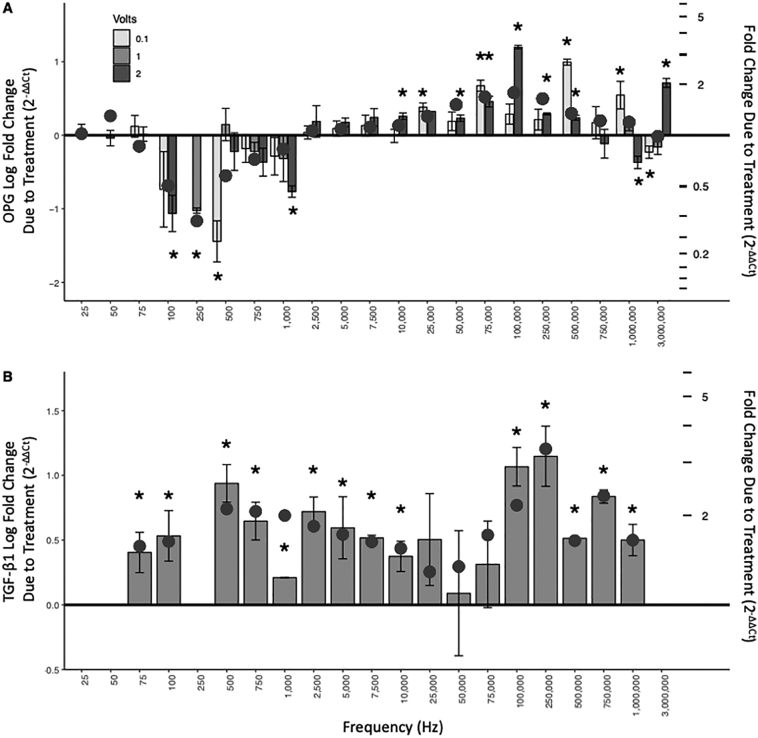
RT-qPCR analysis was used to determine changes in OPG expression via mRNA concentration analysis. OPG mRNA expression responded to changes in the frequency of electrical stimulations (4th order polynomial, p < 0.029) by exhibiting consistent upregulation or downregulation trends under controlled frequency conditions. Similar trends were not observed with voltage changes.
Fig. 3A shows mRNA fold change of OPG in response to specific stimulation signals. Fig. 3A (left axis) shows the logarithmic fold change of OPG. Values above zero reflect an increase in OPG mRNA expression compared to the basal expression level in control experiments while values below zero (an inverted bar element) reflect a decrease in mRNA expression. Fig. 3A (right axis) provides an alternative perspective by juxtaposing linear fold increases/decreases within the construct of the logarithmic scale. Circles along the curve indicate the predicted values for each frequency based on a general additive model with knots chosen between inflection points within the frequency domain defined at 20, 90, 250, 700, 2500 and 200,000 Hz (model fit adjusted R2 = 0.36, P < 0.001) for OPG mRNA expression.
Fig. 3.
Cumulative Results of OPG and TGF-β1 mRNA Expression in Osteoblasts Due to Bioelectric Stimulation. Panel A (OPG): Frequency-specific responses to BES produced two apparent regions of statistically significant influence for OPG. Low frequencies (between 100 Hz and 1000 Hz) resulted in a reduction of expression from baseline. Higher frequencies (between 10,000 Hz and 500,000 Hz) resulted in upregulation. Panel B (TGF-β1): Frequency-specific responses to BES produced two apparent regions of statistically significant influence for TGF-β1. Low frequencies (between 75 Hz and 10,000 Hz) resulted in an increase of expression from baseline. Higher frequencies (between 100,000 Hz and 1000,000 Hz) also resulted in upregulation. Left-y-axis is Log fold change due to treatment and tick marks on right-y-axis denote fold change due to treatment. Circles represent predicted values using a generalized additive model. * Indicates significantly different from no change, p < 0.05. OPG: Osteoprotegerin; TGF-β1: transforming growth factor β1.
OPG study outcomes, shown in Fig. 3A, can be broadly divided into two regions—frequencies that downregulate mRNA expression and those that upregulate it. OPG mRNA expression is reduced at frequencies between 100 Hz and 1000 Hz, with a negative peak close to 500 Hz. Frequencies between 10,000 Hz and 500,000 Hz increase OPG mRNA expression with a peak at 100,000 Hz. A cut score was defined at 2500 Hz (sensitivity: 0.67; specificity: 0.86; area under the curve 66%) where a transition point was identified between OPG mRNA up- and down-regulation. Mixed results were observed for stimulations at and above 750,00 Hz. It is worth noting that the x axis is not linear. Frequency selections were made to span the broad range of signals used in clinical practice and not on a strictly mathematical construct.
Voltages commonly applied in the clinical realm were chosen to determine the effect of voltage on OPG mRNA expression. Variability in OPG mRNA expression was observed, however, trends in upregulation and downregulation arising from different voltage applications showed no voltage dependence.
3.3. Effect of bioelectrical stimulation on TGF-β1 mRNA expression
RT-qPCR analysis was also used to determine changes in TGF-β1 expression via mRNA concentration analysis (Fig. 3B). TGF-β1 mRNA expression concomitantly responded to changes in signal frequency by, similarly, exhibiting consistent modulations in the upregulation throughout the frequency ranges tested (general additive model adjusted R2 = 0.29, p < 0.001, with inflection points, knots at 750, 2500, 75,000 and 500,000 Hz). In contrast to OPG expression, there are no frequencies in the test range that lead to a downregulation of mRNA (i.e., values below the zero line). Only results from 0.1 V stimulations were considered as it had been previously determined, during OPG experiments, that gene expression regulation was not voltage dependent.
TGF-β1 study outcomes, shown in Fig. 3B, can also be broadly divided into two regions, albeit slightly different than those observed with OPG. TGF-β1 mRNA expression is increased at frequencies between 75 Hz and 10,000 Hz, with a positive peak close to 500 Hz and again at higher frequencies between 100,000 Hz and 1000,000 Hz with a peak at 250,000 Hz thereby producing a positive double-peaked response to frequency changes. As with Fig. 3A, the x-axis of Fig. 3B is not linear and frequencies were selected to span the broad ranges used in clinical practice.
3.4. Effects of BES over mineralization
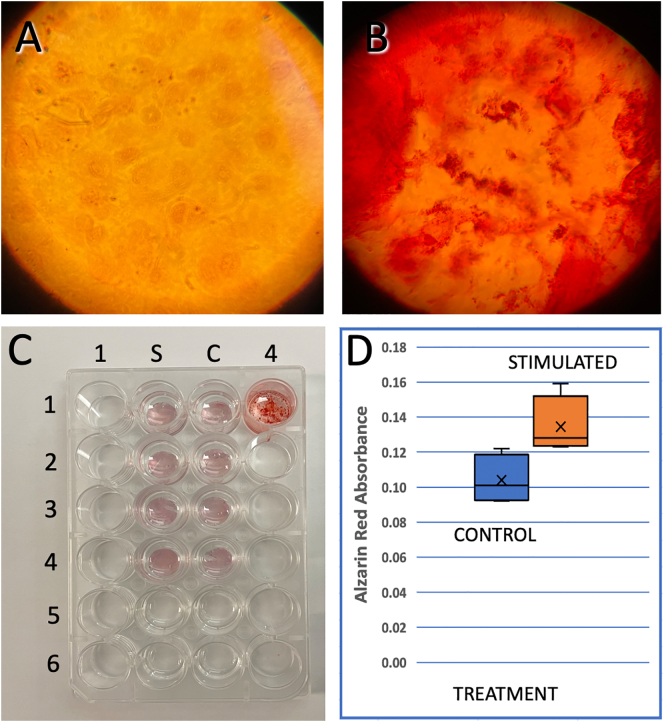
Mineralization is a common phenomenon observed after maintaining osteoblast in culture (Aboushady et al., 2018; Blair et al., 2017; Magloire and Joffre, 1979). These calcium deposits can be observed after two or three weeks (Fig. 4B). After stimulating the cells for 30 min with 2 V-100 kHz pulses, one week, two weeks and four weeks after plated, the absorbance of Alizarin Red for the stimulated cells (n = 4 cover slips) was 28% higher than in the control group after 6 weeks in culture (Fig. 4D) indicating an increase in calcium deposits. Unpaired t-student test indicates a significant difference with a confidence interval of 95%. (n = 4, df = 6 and t = 2.80, with a two-tailed P = 0.0310). As a control, calcium chloride and Alizarin red were added to well 1-4 to identify the red-orange reaction.
Fig. 4.
BES increases the mineralization rate of osteoblasts in culture. A) micrography of osteoblasts treated with Alizarin Red after 1 week. B. Osteoblasts treated with Alizarin Red after 4 weeks C. Coverslips from Stimulated (column S) and Control (Column C) at wells S5 and C5, we added a coverslip with cells not treated with Alizarin Red, and covered with glycerin, for optimization. D) Plot of the aggregated measurements of Alizarin absorbance at 405 nm. Notice that there is a significant increase in the amount of mineralization due to stimulation.
4. Discussion
There have been multiple studies investigating the effects of various OTM techniques that have resulted in the elucidation of several molecular mechanisms explaining the process and physiology of tooth movement (Huang et al., 2014). On the one hand, it has been clearly demonstrated that the expression of RANK and RANKL promote bone resorption via osteoclastic differentiation—initiating bone softening and subsequent tooth movement (Ikebuchi et al., 2018). On the other hand, the induction of tooth fixation, by way of increased OPG expression, stops bone softening by reducing osteoclastogenesis and promoting bone hardening (Baud’huin et al., 2013). Previous studies involving the injection of OPG directly into dental tissues have also proven successful (Baxter et al., 2020; Li et al., 2019; Li and Tang, 2009), although application can become stressful and painful to the patient during multiple treatments. This study documents that an alternative method, bioelectric stimulation, can increase the concentration of OPG and TGF-β1 mRNA expression within osteoblasts, leading to a potentially non-invasive alternative to increasing OPG expression without the associated risk and discomfort of repeat OPG injections. This developing approach may accelerate bone remineralization and mechanical tooth repositioning treatments that use attached or removable appliances.
Osteoblasts were stimulated in vitro with signals of varying voltage and frequency. Stimulated cells did not experience changes in growth rate nor were there significant alterations in cell morphology within 5 days (Fig. 2). This observation implies that BES targeted to bone remodeling specific pathways does not trigger deleterious effects such as programed cell death within stimulated the cells.
The results of this study demonstrate, for the first time, that OPG and TGFβ-1 expression, as measured by mRNA concentration, can be directly modulated by bioelectric stimulation. Upregulation for OPG was consistently and significantly induced at frequencies between 10,000 Hz and 500,000 Hz while downregulation was influenced by frequencies in the range of 100 Hz to 1000 Hz. Contrary to OPG, TGF-β1 displayed consistent upregulation where specific frequency ranges were more significant than others i.e., 75 Hz – 1000 Hz, 100,000 Hz – 1000,000 Hz. These consistent phenomena indicate a novel, and potentially more compliant, therapy for tooth movement/stabilization protocols.
Outcomes also demonstrated that these results are not a general initiation of cellular gene expression, but that there are specific frequencies that can be applied to osteoblasts whereby the expression of OPG and TGFβ-1 mRNA can be controlled, manipulated, and optimized. This novel finding implies that there are molecular pathways within osteoblasts, osteoclasts, and likely other cells that predictably respond to specific electrical stimuli.
Direct current stimulation has been shown to induce gene modulation (Mobini et al., 2017) where stimulation was provided for one to fourteen days. In this case, some molecules like Osteopontin and COL1A2 were upregulated. In other cases, bioelectrical stimulation of cultured cardiocytes induced changes in cellular phenotype (Genovese et al., 2008). Using microarray assays, multiple mRNAs were reported to be either upregulated or downregulated, but without distinguishing which genes were specifically involved in bone repair (Caputo et al., 2014). Comparatively, modulation of gene expression has also been reported after electromagnetic field stimulation (Chang et al., 2005; Hinsenkamp and Collard, 2011; Zhou et al., 2019a) with different results. More specifically, the release of insulin has been controlled through electrical stimulation in genetically engineered cells (Krawczyk et al., 2020) highlighting the therapeutic potential of bioelectric stimulation.
It is important to note the down regulation of OPG mRNA. Gene expression is primarily controlled at the level of transcription, largely because of binding of proteins to specific sites on DNA. Regulation of protein production is largely achieved by modulating access of RNA polymerase to the structural gene being transcribed.
At the DNA level, osteoblast gene expression can be upregulated by enhancers, which in turn are modulated by proteinic activators that increase the transcription of a particular gene expression (GM, 2000; Phillips, 2008). Specifically in osteoblasts, gene expression is regulated after differentiation by Runx2, the master regulator, as it can be regulated by phosphorylation, acetylation, and ubiquitination and some of its domains can mediate either transcriptional activation or repression through associations with co-activators and co-repressors (Jensen et al., 2010).
Runx2 is also regulated by parathyroid hormone, highly important in skeletal physiology(Bellido et al., 2003). Expression of mRNA for OPG can also be downregulated by testosterone and upregulated by estrogen. Therefore, there are multiple paths to follow for the reduction in expression of OPG as protein or its transcript mRNA.
There have been several attempts to describe the cellular mechanisms behind protein expression through BES. Calcium transmembrane relocation (Xu et al., 2009) and enzyme activation (e.g. alkaline phosphatase; (Caputo et al., 2014) can all be influenced by BES. Furthermore, bioelectric regulation of gene expression seems to be indicative of a multifactorial phenomenon that depends on the cell type and intracellular milieu, thus, requiring future cellular and molecular investigation (Caputo et al., 2014; Srirussamee et al., 2019; Thrivikraman et al., 2018; Wang et al., 2020).
Regardless of the underlying mechanisms, OPG and TGF-β1 mRNA expression, in response to BES, revealed a promising trend, namely, that BES can be used to consistently and significantly alter mRNA expression (in terms of both upregulation and downregulation) specifically in osteoblasts. Despite this general trend, some variability was observed, particularly regarding low voltages in OPG. Variability in the 0.1 V signals may be attributed to impedance within the stimulation system, which include connections, electrodes, and conductive media within each well. Such impedance likely reduced the signal to noise ratio of the stimulus that reached the cell monolayer. The standard error from sister control dishes ranged from 0.06 to 0.15 Log 2-ΔCt, which may be reflected in the final variability given that each sample was compared directly to its own control and not against the aggregate of all control values of the study.
Our results clearly indicate that bioelectric stimulation can induce both upregulation and downregulation of OPG mRNA expression and two upregulation regions of TGF-β1 mRNA expression in osteoblasts—mostly influenced by a signal frequency. This trend indicates that OPG and TGF-β1, both key bone remodeling and regulatory proteins, can be manipulated via bioelectric stimulation to potentially influence bone softening for teeth alignment and bone hardening for post-alignment tooth anchorage. In terms of mineralization, our mineralization data indicates that osteoblasts can respond to electrical stimulation with a physiological response resultant in more calcium deposits that directly instigate bone hardening in patients. Furthermore, this discovery opens an entirely new methodology for efficient, non-invasive, therapeutic treatment—particularly in orthodontic tooth movement.
In summary, this study demonstrated that BES selectively modulates mRNA expression of OPG and TGF-β1 in osteoblasts within a specific range of bioelectric frequency and voltage parameters. Frequency parameters produced predictable and consistent control of both upregulation and downregulation of OPG, and upregulation of TGF-β1 at low and high frequencies. It is feasible that such findings can be translated into clinical scenarios. Incorporating findings into clinical application could result in an effective, more aesthetic, and noninvasive method, leading to improved patient compliance in orthodontic treatment.
5. Conclusions
Orthodontic tooth movement involves long and painful periods of mechanical manipulation of teeth in patients’ mouths. The ability to modulate bone remodeling molecules like OPG and RANKL via BES creates new treatment paradigms to improve patients’ conditions by better ameliorating their stress, improving patient compliance, and reducing treatment time during orthodontic procedures.
Bioelectrical stimulation has been used in other tissues to induce gene expression. This manuscript presents significant data that points towards the controlled expression of OPG, an important molecule to strengthen bone, and TGFB-1 which participates in inflammation mechanisms. It is remarkable, that these two molecules have a different optimal pulse frequency to improve their expression. This goes in accordance to reported data that suggests that other biological gene/molecules possess their own optimal frequency for expression. The use of bioelectric signals is a remarkable improvement to reduce discomfort, accelerate mineralization, and improve tooth alignment.
6. Future studies
The possibility that the expression of TGF-β1 enhances the expression of OPG has been previously suggested (Yang et al., 2015). Nonetheless, in our cellular system, we indeed see that OPG, and TGF-β1 respond similarly to high frequencies, although they could be easily differentiated by several hundred Hertz in terms of response to the stimuli, suggesting that their expression may be interdependent. Conversely, the responses at low frequencies are totally opposite, strongly suggesting that TGF-β1 may not be directly involved in the expression of OPG in osteoblast in vitro, nonetheless, it would be necessary to explore the nature of possible interactions between the expression of these two molecules.
The clinical use of bioelectric stimulation will demand the discovery of electrical signature protocols that selectively induce the expression of distinct and specific molecules and growth factors. We have determined these electrical signatures for TGF-β1 and OPG in osteoblasts. However, such discovery warrants additional exploration in determining the expression changes of other mRNAs and proteins involved in bone remodeling process, such as the receptor activator of nuclear factor-kappa B (RANK) and bone morphogenic protein (BMP).
In addition to the clinical relevance of BES, another important goal of BES exploration is to determine the mechanism of action by which gene expression is influenced by specific electrical stimuli. Understanding the mechanism will allow better elucidation of different proteins and/or receptors that respond to electrical fields and intracellular pathways which lead to differential expression.
Lastly, the overarching influence of BES on physiological processes is necessary to understanding the clinical relevance of non-invasive growth factor modulation. Naturally, the functionality of the proteins expressed in response to bioelectrical stimulation must be explored to demonstrate that the genes are still functional. Bioassays will be used to demonstrate growth factor viability and to show that BES does not overexpress other genes that can compromise the function of a specific protein studied (e.g., OPG and TGFβ-1) (Table 1).
Table 1.
Fold and log fold change due to treatment (2-ΔΔCt) of mRNA expression from bioelectric stimulations.
| OPG |
TGF-β1 |
||||
|---|---|---|---|---|---|
| Frequency | Volts | Fold Change (2-ΔΔCt) | Log Fold Change (Log 2-ΔΔCt) | Fold Change (2-ΔΔCt) | Log Fold Change (Log 2-ΔΔCt) |
| 25 | 2.0 | 1.09 ± 0.07 | 0.08 ± 0.07 | ||
| 50 | 2.0 | 0.97 ± 0.10 | -0.04 ± 0.11 | ||
| 75 | 0.1 | 1.15 ± 0.17 | 0.12 ± 0.15 | 1.54 ± 0.25 | 0.41 ± 0.16⁎ |
| 75 | 2.0 | 1.04 ± 0.11 | 0.02 ± 0.10 | ||
| 100 | 0.1 | 0.76 ± 0.47 | -0.73 ± 0.51 | 1.77 ± 0.34 | 0.53 ± 0.20⁎ |
| 100 | 2.0 | 0.37 ± 0.09⁎ | -1.06 ± 0.25⁎ | ||
| 250 | 1.0 | 0.36 ± 0.01⁎ | -1.02 ± 0.04⁎ | ||
| 500 | 0.1 | 0.25 ± 0.07⁎ | -1.44 ± 0.28⁎ | 2.61 ± 0.40⁎ | 0.94 ± 0.15⁎ |
| 500 | 1.0 | 1.21 ± 0.24 | 0.15 ± 0.22 | ||
| 500 | 2.0 | 0.85 ± 0.21 | -0.22 ± 0.26 | ||
| 750 | 0.1 | 0.86 ± 0.15 | -0.18 ± 0.19 | 1.95 ± 0.30⁎ | 0.65 ± 0.15⁎ |
| 750 | 1.0 | 0.81 ± 0.10 | -0.22 ± 0.12 | ||
| 750 | 2.0 | 0.72 ± 0.12 | -0.37 ± 0.19 | ||
| 1000 | 0.1 | 0.78 ± 0.19 | -0.28 ± 0.25 | 1.24 ± 0.01⁎ | 0.21 ± 0.01⁎ |
| 1000 | 1.0 | 0.80 ± 0.25 | -0.32 ± 0.31 | ||
| 1000 | 2.0 | 0.47 ± 0.04⁎ | -0.77 ± 0.08⁎ | ||
| 2500 | 0.1 | 1.05 ± 0.09 | 0.04 ± 0.09 | 2.08 ± 0.22⁎ | 0.72 ± 0.11⁎ |
| 2500 | 2.0 | 1.26 ± 0.25 | 0.19 ± 0.22 | ||
| 5000 | 0.1 | 1.11 ± 0.11 | 0.09 ± 0.10 | 1.93 ± 0.50 | 0.6 ± 0.24⁎ |
| 5000 | 2.0 | 1.20 ± 0.07 | 0.18 ± 0.06 | ||
| 7500 | 0.1 | 1.16 ± 0.15 | 0.13 ± 0.14 | 1.68 ± 0.03⁎ | 0.52 ± 0.02⁎ |
| 7500 | 2.0 | 1.29 ± 0.16 | 0.24 ± 0.12 | ||
| 10,000 | 0.1 | 1.00 ± 0.09 | -0.01 ± 0.09 | 1.47 ± 0.17⁎ | 0.38 ± 0.12⁎ |
| 10,000 | 2.0 | 1.30 ± 0.06⁎ | 0.26 ± 0.05⁎ | ||
| 25,000 | 0.1 | 1.49 ± 0.09⁎ | 0.38 ± 0.06⁎ | 1.85 ± 0.52 | 0.51 ± 0.35 |
| 25,000 | 2.0 | 1.38 | 0.32 | ||
| 50,000 | 0.1 | 1.26 ± 0.16 | 0.19 ± 0.13 | 1.35 ± 0.55 | 0.09 ± 0.48 |
| 50,000 | 2.0 | 1.27 ± 0.06⁎ | 0.23 ± 0.04⁎ | ||
| 75,000 | 0.1 | 1.98 ± 0.14⁎ | 0.67 ± 0.08⁎ | 1.51 ± 0.42 | 0.31 ± 0.34 |
| 75,000 | 2.0 | 1.60 ± 0.12⁎ | 0.46 ± 0.07⁎ | ||
| 100,000 | 0.1 | 1.41 ± 0.24 | 0.29 ± 0.14 | 2.97 ± 0.44⁎ | 1.07 ± 0.15⁎ |
| 100,000 | 2.0 | 3.32 ± 0.08⁎ | 1.20 ± 0.02⁎ | ||
| 250,000 | 0.1 | 1.30 ± 0.16 | 0.21 ± 0.14 | 3.32 ± 0.72⁎ | 1.15 ± 0.23⁎ |
| 250,000 | 2.0 | 1.34 ± 0.02⁎ | 0.29 ± 0.01⁎ | ||
| 500,000 | 0.1 | 2.72 ± 0.11⁎ | 0.99 ± 0.04⁎ | 1.67 ± 0.01⁎ | 0.51 ± 0.01⁎ |
| 500,000 | 2.0 | 1.27 ± 0.04⁎ | 0.24 ± 0.03⁎ | ||
| 750,000 | 0.1 | 1.33 ± 0.26 | 0.17 ± 0.22 | 2.31 ± 0.12⁎ | 0.84 ± 0.05⁎ |
| 750,000 | 2.0 | 1.04 ± 0.21 | -0.12 ± 0.20 | ||
| 1000,000 | 0.1 | 2.06 ± 0.34⁎ | 0.55 ± 0.19⁎ | 1.68 ± 0.21⁎ | 0.50 ± 0.12⁎ |
| 1000,000 | 1.0 | 1.16 ± 0.10 | 0.14 ± 0.08 | ||
| 1000,000 | 2.0 | 0.70 ± 0.06⁎ | -0.37 ± 0.08⁎ | ||
| 3,000,000 | 0.1 | 0.81 ± 0.07⁎ | -0.23 ± 0.08⁎ | ||
| 3,000,000 | 1.0 | 0.87 ± 0.09 | -0.16 ± 0.10 | ||
| 3,000,000 | 2.0 | 2.04 ± 0.12⁎ | -0.71 ± 0.16⁎ | ||
Indicates significant change due to treatment, p < 0.05. OPG; Osteoprotegerin.
Credit authorship contribution statement
Chaudhari S.D., Conceptualization, Methodology, Investigation, Validation, Data Curation, Writing-Review & Editing. Sharma K.S, Conceptualization, Methodology, Writing-Review & Editing. Hydren R. H., Formal Analysis, Software, Data Curation, Writing-Review & Editing. Marchetto, J.J., Funding Acquisition, Data Curation, Clinical Methodology, Writing-Review & Editing Burton M.B., Conceptualization, Methodology, Writing-Review and Editing. Moreno A.P., Writing Original Draft, Methodology, Conceptualization, Data Curation, Investigation, Writing-Review & Editing.
All authors gave their final approval and agreed to be accountable for all aspects of the work.
Acknowledgments
The main source of funding was provided by a MedTech startup accelerator called Leonhardt Launchpads Utah, Inc. (Salt Lake City, Utah, USA), a subsidiary of Cal-X Stars Business Accelerator DBA Leonhardt's Launchpads based in Irvine, California, USA. Leonhardt's Launchpads, and its subsidiaries, focus on the development of regenerative MedTech devices and therapies via bioelectric stimulation. OrthodontiCell Inc. is a spin out entity from Leonhardt's Launchpads that is developing noninvasive technologies for improved orthodontic tooth movement via bioelectric stimulation. Both entities utilized laboratory space and equipment at the BioInnovations Gateway (BiG), a life-science incubator located in Salt Lake City, Utah, USA.
All authors are employees and/or consultants of Leonhardt's Launchpads Utah, Inc. (LLU) and OrthodontiCell Inc. (ODC). All authors declare no additional potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aboushady I.M., Salem Z.A., Sabry D., Mohamed A. Comparative study of the osteogenic potential of mesenchymal stem cells derived from different sources. J Clin Exp Dent. 2018;10(1):e7–e13. doi: 10.4317/jced.53957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleem I.S., Aleem I., Evaniew N., Busse J.W., Yaszemski M., Agarwal A., Einhorn T., Bhandari M. Efficacy of electrical stimulators for bone healing: a meta-analysis of randomized sham-controlled trials. Sci. Rep. 2016;6(1):31724. doi: 10.1038/srep31724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliston T., Choy L., Ducy P., Karsenty G., Derynck R. Tgf-beta-induced repression of cbfa1 by smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001;20(9):2254–2272. doi: 10.1093/emboj/20.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud’huin M., Duplomb L., Teletchea S., Lamoureux F., Ruiz-Velasco C., Maillasson M., Redini F., Heymann M.-F., Heymann D. Osteoprotegerin: multiple partners for multiple functions. Cytokine Growth Factor Rev. 2013;24(5):401–409. doi: 10.1016/j.cytogfr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Baxter S.J., Sydorak I., Ma P.X., Hatch N.E. Impact of pharmacologic inhibition of tooth movement on periodontal and tooth root tissues during orthodontic force application. Orthod. Craniofacial Res. 2020;23(1):35–43. doi: 10.1111/ocr.12350. [DOI] [PubMed] [Google Scholar]
- Bellido T., Ali A.A., Plotkin L.I., Fu Q., Gubrij I., Roberson P.K., Weinstein R.S., O'Brien C.A., Manolagas S.C., Jilka R.L. Proteasomal degradation of runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. a putative explanation for why intermittent administration is needed for bone anabolism. J. Biol. Chem. 2003;278(50):50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57(1):289–300. [Google Scholar]
- Bhavsar M.B., Leppik L., Oliveira K.M.C., Barker J.H. Electrical stimulation–fracture treatment: new insights into the underlying mechanisms. Bioelectron.Med. 2019;2(1):5–7. [Google Scholar]
- Blair H.C., Larrouture Q.C., Li Y., Lin H., Beer-Stoltz D., Liu L., Tuan R.S., Robinson L.J., Schlesinger P.H., Nelson D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. B Rev. 2017;23(3):268–280. doi: 10.1089/ten.teb.2016.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E., Economides A.N., Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 2003;24(2):218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- Caputo M., Zirpoli H., De Rosa M.C., Rescigno T., Chiadini F., Scaglione A., Stellato C., Giurato G., Weisz A., Tecce M.F. Effect of low frequency (lf) electric fields on gene expression of a bone human cell line. Electromagn. Biol. Med. 2014;33(4):289–295. doi: 10.3109/15368378.2013.822387. [DOI] [PubMed] [Google Scholar]
- Cervera J., Meseguer S., Mafe S. The interplay between genetic and bioelectrical signaling permits a spatial regionalisation of membrane potentials in model multicellular ensembles. Sci. Rep. 2016;6:35201. doi: 10.1038/srep35201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K., Chang W.H., Huang S., Huang S., Shih C. Pulsed electromagnetic fields stimulation affects osteoclast formation by modulation of osteoprotegerin, rank ligand and macrophage colony-stimulating factor. J. Orthop. Res. 2005;23(6):1308–1314. doi: 10.1016/j.orthres.2005.03.012.1100230611. [DOI] [PubMed] [Google Scholar]
- Chapman J.R., Waldenström J. With reference to reference genes: a systematic review of endogenous controls in gene expression studies. PLoS One. 2015;10(11):e0141853. doi: 10.1371/journal.pone.0141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovitch Z. Tooth movement. Crit. Rev. Oral Biol. Med. 1991;2(4):411–450. doi: 10.1177/10454411910020040101. [DOI] [PubMed] [Google Scholar]
- Dunn M.D., Park C.H., Kostenuik P.J., Kapila S., Giannobile W.V. Local delivery of osteoprotegerin inhibits mechanically mediated bone modeling in orthodontic tooth movement. Bone. 2007;41(3):446–455. doi: 10.1016/j.bone.2007.04.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eischen-Loges M., Oliveira K.M.C., Bhavsar M.B., Barker J.H., Leppik L. Pretreating mesenchymal stem cells with electrical stimulation causes sustained long-lasting pro-osteogenic effects. PeerJ. 2018;6:e4959. doi: 10.7717/peerj.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlet T.P., Coelho U., Silva J.S., Garlet G.P. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur. J. Oral Sci. 2007;115(5):355–362. doi: 10.1111/j.1600-0722.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- Genovese J.A., Spadaccio C., Langer J., Habe J., Jackson J., Patel A.N. Electrostimulation induces cardiomyocyte predifferentiation of fibroblasts. Biochem. Biophys. Res. Commun. 2008;370(3):450–455. doi: 10.1016/j.bbrc.2008.03.115. [DOI] [PubMed] [Google Scholar]
- Genovese J.A., Spadaccio C., Rivello H.G., Toyoda Y., Patel A.N. Electrostimulated bone marrow human mesenchymal stem cells produce follistatin. Cytotherapy. 2009;11(4):448–456. doi: 10.1080/14653240902960445. [DOI] [PubMed] [Google Scholar]
- GM C. The Cell: A molecular Approach. 2nd edition. Sinauer Associates; Sunderland (MA): 2000. Regulation of transcription in eukaryotes. [Google Scholar]
- Harmsen I.E., Lee D.J., Dallapiazza R.F., De Vloo P., Chen R., Fasano A., Kalia S.K., Hodaie M., Lozano A.M. Ultra-high-frequency deep brain stimulation at 10,000 hz improves motor function. Mov. Disord. 2019;34(1):146–148. doi: 10.1002/mds.27550. [DOI] [PubMed] [Google Scholar]
- Hess R., Jaeschke A., Neubert H., Hintze V., Moeller S., Schnabelrauch M., Wiesmann H.-P., Hart D.A., Scharnweber D. Synergistic effect of defined artificial extracellular matrices and pulsed electric fields on osteogenic differentiation of human mscs. Biomaterials. 2012;33(35):8975–8985. doi: 10.1016/j.biomaterials.2012.08.056. [DOI] [PubMed] [Google Scholar]
- Hinsenkamp M., Collard J.F. Bone morphogenic protein–mrna upregulation after exposure to low frequency electric field. Int. Orthop. 2011;35(10):1577–1581. doi: 10.1007/s00264-011-1215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard Leonhardt J., Genovese J., Marchetto J.J., Inventor, Calxstars Business Accelerator Inc, Assignee . 2020. Orthodontic Treatment. USA patent 10,695,563. [Google Scholar]
- Huang H., Williams R.C., Kyrkanides S. Accelerated orthodontic tooth movement: molecular mechanisms. Am. J. Orthod. Dentofac. Orthop. 2014;146(5):620–632. doi: 10.1016/j.ajodo.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Idriss H.T., Naismith J.H. Tnfα and the tnf receptor superfamily: structure-function relationship(s) Microsc. Res. Tech. 2000;50(3):184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ikebuchi Y., Aoki S., Honma M., Hayashi M., Sugamori Y., Khan M., Kariya Y., Kato G., Tabata Y., Penninger J.M. Coupling of bone resorption and formation by rankl reverse signalling. Nature. 2018;561(7722):195–200. doi: 10.1038/s41586-018-0482-7. [DOI] [PubMed] [Google Scholar]
- Ito S., Hata T. Vitamins & Hormones. Academic Press; 2004. Crystal structure of rank ligand involved in bone metabolism; pp. 19–33. [DOI] [PubMed] [Google Scholar]
- Jamali Y., Jamali M., Golshani M. bioRxiv; 2019. A New Method of Brain Stimulation at Ultra-high Frequency. 621771. [Google Scholar]
- Jensen E.D., Gopalakrishnan R., Westendorf J.J. Regulation of gene expression in osteoblasts. Biofactors. 2010;36(1):25–32. doi: 10.1002/biof.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem M., Kveiborg M., Eriksen E.F. Production and action of transforming growth factor-beta in human osteoblast cultures: dependence on cell differentiation and modulation by calcitriol. Eur. J. Clin. Investig. 2000;30(5):429–437. doi: 10.1046/j.1365-2362.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- Kilgore K.L., Bhadra N. Reversible nerve conduction block using kilohertz frequency alternating current. Neuromodulation. 2014;17(3):242–255. doi: 10.1111/ner.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk K., Xue S., Buchmann P., Charpin-El-Hamri G., Saxena P., Hussherr M.-D., Shao J., Ye H., Xie M., Fussenegger M. Electrogenetic cellular insulin release for real-time glycemic control in type 1 diabetic mice. Science. 2020;368(6494):993–1001. doi: 10.1126/science.aau7187. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Davidovitch Z.E. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006;129(4) doi: 10.1016/j.ajodo.2005.10.007. (469.e461-469.e432) [DOI] [PubMed] [Google Scholar]
- Kuzyk P.R., Schemitsch E.H. The science of electrical stimulation therapy for fracture healing. Indian J. Orthop. 2009;43(2):127–131. doi: 10.4103/0019-5413.50846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Reprogramming cells and tissue patterning via bioelectrical pathways: molecular mechanisms and biomedical opportunities. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013;5(6):657–676. doi: 10.1002/wsbm.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Tang L. Local delivery of osteoprotegerin may be a way of reinforcing orthodontic anchorage. Med. Hypotheses. 2009;72(2):178–179. doi: 10.1016/j.mehy.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Li C., Chung C.J., Hwang C.-J., Lee K.-J. Local injection of rankl facilitates tooth movement and alveolar bone remodelling. Oral Dis. 2019;25(2):550–560. doi: 10.1111/odi.13013. [DOI] [PubMed] [Google Scholar]
- Lucas P.A. Chemotactic response of osteoblast-like cells to transforming growth factor beta. Bone. 1989;10(6):459–463. doi: 10.1016/8756-3282(89)90079-3. [DOI] [PubMed] [Google Scholar]
- Magloire H., Joffre A. Fine structural observations of calcium storage in human dental pulp cells in primary culture. J. Biol. Buccale. 1979;7:307–320. [PubMed] [Google Scholar]
- Mobini S., Leppik L., Thottakkattumana Parameswaran V., Barker J.H. In vitro effect of direct current electrical stimulation on rat mesenchymal stem cells. PeerJ. 2017;5:e2821. doi: 10.7717/peerj.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TRotageieNE. Regulation of transcription and gene expression in eukaryotes. Nat. Educ. 2008;1(1):1. [Google Scholar]
- Riwo Onibere A.K. The role of electrical sitmulation in fracture healing. Internet J. Orthop. Surg. 2008;11(2):9. [Google Scholar]
- Spadari G.S., Zaniboni E., Vedovello S.A.S., Santamaria M.P., do Amaral M.E.C., dos Santos G.M.T., Esquisatto M.A.M., Mendonca F.A.S., Santamaria-Jr M. Electrical stimulation enhances tissue reorganization during orthodontic tooth movement in rats. Clin. Oral Investig. 2017;21(1):111–120. doi: 10.1007/s00784-016-1759-6. [DOI] [PubMed] [Google Scholar]
- Srirussamee K., Mobini S., Cassidy N.J., Cartmell S.H. Direct electrical stimulation enhances osteogenesis by inducing bmp2 and spp1 expressions from macrophages and preosteoblasts. Biotechnol. Bioeng. 2019;116(12):3421–3432. doi: 10.1002/bit.27142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoleyre S., Wittrant Y., Tat S.K., Fortun Y., Redini F., Heymann D. The molecular triad opg/rank/rankl: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15(6):457–475. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Thrivikraman G., Boda S.K., Basu B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: a tissue engineering perspective. Biomaterials. 2018;150:60–86. doi: 10.1016/j.biomaterials.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Tyler S.E.B. Nature's electric potential: a systematic review of the role of bioelectricity in wound healing and regenerative processes in animals, humans, and plants. Front. Physiol. 2017;8:627. doi: 10.3389/fphys.2017.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Molen M.A., Donahue H.J., Rubin C.T., McLeod K.J. Osteoblastic networks with deficient coupling: differential effects of magnetic and electric field exposure. Bone. 2000;27(2):227–231. doi: 10.1016/s8756-3282(00)00315-x. [DOI] [PubMed] [Google Scholar]
- Walsh M.C., Choi Y. Biology of the rankl-rank-opg system in immunity, bone, and beyond. Front. Immunol. 2014;5:511. doi: 10.3389/fimmu.2014.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Mit B., Han Z., John H.B. Effects of electrical stimulation on stem cells. Curr. Stem Cell Res. Ther. 2020;15:1–8. doi: 10.2174/1574888X15666200129154747. [DOI] [PubMed] [Google Scholar]
- Xu J., Wang W., Clark C.C., Brighton C.T. Signal transduction in electrically stimulated articular chondrocytes involves translocation of extracellular calcium through voltage-gated channels. Osteoarthr. Cartil. 2009;17(3):397–405. doi: 10.1016/j.joca.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M. Rank/rankl/opg during orthodontic tooth movement. Orthod. Craniofacial Res. 2009;12(2):113–119. doi: 10.1111/j.1601-6343.2009.01444.x. [DOI] [PubMed] [Google Scholar]
- Yang X., Wang Y., Han X., Shu R., Chen T., Zeng H., Xu X., Huang L., Ren A., Song J. Effects of tgf-β1 on opg/rankl expression of cementoblasts and osteoblasts are similar without stress but different with mechanical compressive stress. ScientificWorldJournal. 2015;2015:718180. doi: 10.1155/2015/718180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoya K., Sasaki T., Shibasaki Y. Distributional changes of osteoclasts and pre-osteoclastic cells in periodontal tissues during experimental tooth movement as revealed by quantitative immunohistochemistry of h+-atpase. J. Dent. Res. 1997;76(1):580–587. doi: 10.1177/00220345970760010901. [DOI] [PubMed] [Google Scholar]
- Zhou J., Gao Y.-H., Zhu B.-Y., Shao J.-L., Ma H.-P., Xian C.J., Chen K.-M. Sinusoidal electromagnetic fields increase peak bone mass in rats by activating wnt10b/β-catenin in primary cilia of osteoblasts. J. Bone Miner. Res. 2019;34(7) doi: 10.1002/jbmr.3704. [DOI] [PubMed] [Google Scholar]
- Zhou P., He F., Liu B., Wei S. Nerve electrical stimulation enhances osseointegration of implants in the beagle. Sci. Rep. 2019;9(1):4916. doi: 10.1038/s41598-019-41471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]