Abstract
The fate of malachite green and its main metabolite leucomalachite green during thermal treatment was examined in seafood (brook trout and white shrimp) using non-target analysis. Samples were extracted using QuEChERS and analyzed using liquid chromatography coupled with quadruple time of flight mass spectrometry. Malachite green levels were reduced in meat during boiling (∼40%), microwaving (64%), and canning (96%). Only microwaving was successful in significantly decreasing leucomalachite green levels in brook trout. The reduction percentages of the two target analytes were not significantly different in shrimp (mean fat content = 0.8 ± 0.3%) and in brook trout (mean fat content = 3.5 ± 1.7%), suggesting that a higher fat content may not affect the reduction of the more lipophilic leucomalachite green in these two matrices. Three transformation products were tentatively identified in the cooked tissues, resulting from the cleavage of the conjugated structure or through demethylation. Further research is needed to determine possible adverse health effects. The findings of this study show how non-target analysis can complement targeted methodologies in identifying and evaluating risks to human health.
Keywords: Non-target analysis, Veterinary drugs, Cooking, Transformation products, HRMS
Graphical abstract
Highlights
-
•
Non-target analysis was applied to study the fate of malachite/leucomalachite green.
-
•
Thermal processing significantly reduced malachite green in seafood by up to 96%.
-
•
Leucomalachite green levels were significantly reduced only after microwaving.
-
•
Three transformation products were tentatively identified.
1. Introduction
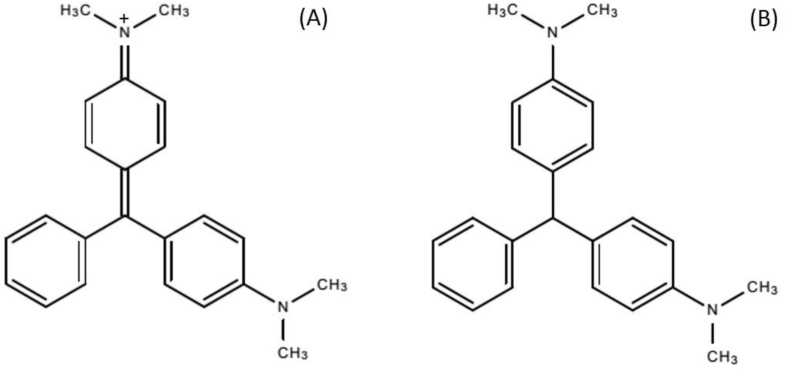
Malachite green (MG, Fig. 1), despite its ban in food producing animals due to possible carcinogenicity of its main metabolite in fish, i.e., leucomalachite green (LMG) (Le Curieux et al., 2021), continues to be detected in seafood, as it is a widely-available, highly effective and low cost anti-fungal (Dinh et al., 2020; EFSA, 2016; Love et al., 2011). Furthermore, MG is also used as an industrial dye and its presence in the aquatic biota can be also linked to release of industrial wastewater (Schuetze et al., 2008). Current regulatory limits have set action levels (i.e. levels above which the products could be considered non-compliant), at 1 and 0.5 ng g−1 for the sum of MG and LMG, in Canada and Europe, respectively (EU Commission, Regulation, 2019/1871; Health Canada, 2017). Seafood products are likely to be eaten cooked rather than raw and studies have shown reduction in contaminants levels after cooking for compounds such as persistent organic pollutants (POPs) in salmon (Bayen et al., 2005). Studying the fate of chemical contaminants following cooking is important to assess dietary exposure that is more representative of the actual levels to which consumers are exposed (WHO, 2009) and to identify other adverse health effects to human health as some newly formed products may still pose a risk (Nguyen et al., 2015). These products, often referred to as transformation products (TPs) can be formed following hydrolysis, conjugation, demethylation and hydroxylation reactions (Bletsou et al., 2015). In the case of banned chemical contaminants, like MG, elucidation of TPs after cooking may be used to identify markers of food contamination, especially when the parent compound is completely degraded in the tissues or is below the limit of detection of analysis methods.
Fig. 1.
Structures of MG (A) and LMG (B).
Targeted approaches used for quantification of parent compounds are insufficient for the identification of TPs, as this workflow is based on use of standards of known compounds and the mass analyzers do not offer the high mass accuracy needed for formula generation (Knolhoff and Croley, 2016). In this context, identification of TPs can be achieved using non-targeted approaches based on high-resolution mass spectrometry (HRMS).Non-target analysis (NTA) is based on simple, unspecific, sample preparation methods that can extract as many compounds as possible (Fu et al., 2017). Analysis with HRMS can provide accurate mass information, mass to charge ratio, isotopic distribution and fragmentation patterns, generating possible chemical formulas. The data can be screened through available chemical databases to confirm the identity of compounds of interest (Fu et al., 2017). This workflow is often referred to as suspect screening. “True” non-target analysis refers to the identification of compounds for which there is no information available, e.g., new metabolites, TPs. Although this is more challenging, tentative identification is still possible using fragmentation information (Fu et al., 2017). NTA has been successfully used to identify TPs of veterinary drugs in seafood and honey (Tian and Bayen, 2018; von Eyken and Bayen, 2020).
Regarding MG and LMG, NTA has been used to identify other MG metabolites in raw seafood muscle (Dubreil et al., 2019; Baesu et al., 2021). However, there is currently limited information on its fate during thermal processing, with only target analysis used so far to quantify MG and LMG levels in seafood muscle after cooking. MG levels were reduced in carp and tilapia muscle after baking, boiling, microwaving and frying by more than 50% depending on the type and duration of treatment (Mitrowska et al., 2007; Shalaby et al., 2016). In carp, LMG was reduced by 40% only after microwaving, with boiling and baking achieving less than 5% reduction. On the other hand, in tilapia, baking and frying, under similar temperatures and cooking time, reduced LMG levels by 26 and 35% respectively (Shalaby et al., 2016). The studies were limited to only quantifying the parent compounds and no possible thermal TPs were proposed. LMG is more lipophilic (log Kow 5.72) compared to MG (log Kow 0.62) (National Library of Medicine, 2020). Therefore, one possible reason for the reduction in LMG observed in tilapia but not carp for baking for example, may be the influence of the food matrix composition, like fat content. Based on nutritional information listed in the USDA Food Composition Database (USDAa), raw carp muscle has a fat content of 5.6% compared to 1.7% for raw tilapia muscle. In another thermal transformation study, the food matrix had an effect on the stability of chlortetracycline during cooking of eggs whites and yolks, presumably because of the binding of the antibiotic to egg white proteins (Alaboudi et al., 2013). Differences in reduction rates between different matrices or cooking treatments could perhaps lead to the formation of different TPs. Indeed, this has been observed in the case of tylosin A where different compounds were detected in honey compared to water (von Eyken and Bayen, 2020). Therefore, the outcome of the food safety risk assessment for specific chemical residues could be different for different types of processed foods.
To the best of our knowledge, no study has investigated the fate of MG and LMG after cooking in other seafood matrices beside carp or tilapia nor qualified any thermal TPs of the two compounds in seafood. Therefore, the aim of this study was twofold: (i) compare the percent reduction rate in MG and LMG levels in water and two food matrices: pacific white shrimp (Litopenaeus vannamei) i.e., low-fat matrix and brook trout (Salvenilus fontinalis), i.e., high-fat matrix with the hypothesis that a higher reduction of LMG would be observed in shrimp compared to trout, (ii) apply a non-target data treatment workflow to identify thermal TPs. The novel aspects of this study are the assessment of the stability of MG and LMG in two previously unstudied matrices, brook trout and pacific white shrimp, and the application of NTA to identify thermal TPs in muscle tissues following cooking.
2. Materials and methods
2.1. Chemicals
MG chloride (>96.0%), LMG (>98.0%), d5-LMG (>98.0%) analytical standards were obtained from Sigma Aldrich (St Louis, MO, USA). Labelled injection internal standards, d3-diphenhydramine and d3-6-acetylmorphine, 13C6-propylparaben were purchased from Cerilliant (Round Rock, TX, USA) and Sigma Aldrich respectively. HPLC grade acetonitrile, methanol and water, as well as LC-MS grade formic acid, acetic acid and ammonium acetate were obtained from Fisher Chemical (Pittsburgh, PA, USA). Anhydrous magnesium sulfate and sodium acetate were purchased from Sigma Aldrich. Primary secondary amine (PSA) sorbent was purchased from Agilent (Santa Clara, CA, USA). All glassware used was baked in an oven at 320 °C for 4 h and rinsed with methanol before use. Labelled internal standard solution of 0.4 μg mL−1 was prepared in methanol and stored at −20 °C in amber vials. MG, LMG and d5-LMG standards of 1 mg mL−1 and working standards of 10 μg mL−1 were prepared in methanol and stored at −20 °C in amber vials. All standards were prepared fresh every 6 months (Andersen et al., 2006). Six calibration standards, ranging from 2 to 40 ng mL−1, were prepared in water (0.1% formic acid), before analysis.
2.2. Sample preparation
Incurred shrimp and brook trout samples were obtained from a controlled exposure experiment (Baesu et al., 2021). In total, 10 individual shrimp and trout exposed to MG were used for the cooking treatments. Ten non-exposed individuals were used as control samples. The extraction method was chosen based on criteria used in NTA, as described previously (Baesu et al., 2021). Briefly, 1.0 g of homogenized muscle sample was weighed in a centrifuge tube, 100 μL of a 10 μg mL−1 d5-LMG solution was added and allowed to equilibrate for 10 min. Solvent, 5 mL (84:16 v/v) acetonitrile/water with 1% acetic acid was added and vortexed for 1 min. To each sample, 1.0 g of MgSO4 and 0.30 g sodium acetate were added, vortexed for 1 min followed by centrifugation (Eppendorf, Hamburg, Germany) at 4400 rpm (3000×g, 25 °C) for 5 min. Two mL of supernatant was transferred to clean tubes containing 0.24 g MgSO4 and 25 mg PSA, vortexed for 1 min and centrifuged for 5 min at 4400 rpm. Extracts were filtered using a 0.22 μm PTFE filter and stored in amber vials at −20 °C in the dark. Procedural blanks were prepared similarly. Five quality control (QC) samples were prepared by pooling 20 μL of each replicate extract and blank. Prior to LC-MS analysis, extracts were diluted (1/10) with water and 50 μL of a 0.4 μg mL−1 solution of the labelled internal standards was added. Labelled standards were added to monitor instrument performance and were used for further assessment of matrix effect.
For recovery experiments, control raw and cooked samples were spiked with 40 μL of a working MG and LMG standard solution of 10 μg mL−1 (target concentration in muscle 400 ng g−1) and allowed to equilibrate for 10 min for extraction.
2.3. Fat analysis
Determination of fat in raw shrimp and trout muscle was based on USDA method for fat analysis (USDAb, 2009). Shrimp and trout muscle were freeze-dried at −90 °C (Martin Christ Gamma 1–16 LSC freeze-dryer, Germany). Then, 0.3 g of freeze-dried sample (n = 5) was extracted with 200 mL hexane using a Soxhlet apparatus for 4 h. Solvent was evaporated using a rotary evaporator (Büchi, Switzerland) and the lipid residues were measured gravimetrically.
2.4. Thermal treatments
2.4.1. Water
Aqueous standard solutions of 10 ng mL−1 MG and LMG (n = 6) were dispensed into 2 mL amber vials (500 μL) and placed in a water bath at 100 °C. Vials were removed at 10, 30 and 120 min and allowed to cool at room temperature. Water samples were heated for up to 2 h to represent extreme conditions that could generate transformation products.
2.4.2. Boiling
Approximately 2.5 g of shrimp and 5 g of trout were placed in 40 mL amber vials (n = 10), capped and transferred to a water bath at 100 °C. Vials were removed at 10 and 30 min and allowed to cool at room temperature. Boiling for 10 min had been reported in the literature to decrease MG levels by 43%, and further increasing cooking time by 5 min lead to a total decrease of 54% (Mitrowska et al., 2007). In this study, trout were further heating for a total of 30 min to mimic more extreme cooking conditions and ensure formation of transformation products. Shrimp were only boiled for 10 min, as a higher cooking time led to too much breakdown of the muscle. Any juices present in the vials were collected and analyzed as well. Boiling was chosen as the cooking procedure for shrimp, in order to test the hypothesis that LMG would be reduced in a low-fat matrix. From previous thermal transformation studies, LMG should be stable in high-fat fish muscle, e.g., carp, during boiling. Hence, if fat content would affect the behaviour of LMG, boiling should lead to a reduction in the lower fat shrimp but not brook trout.
2.4.3. Microwaving
Approximately 5 g of trout muscle was placed in beakers, covered with parafilm and microwaved (Sylvania, 1300W) for 1.5 min. A longer microwaving time led to too much drying and at times burning of the muscle; therefore, a time of 1.5 min was deemed acceptable. Beakers were removed and allowed to cool at room temperature. No juices were observed after cooking.
2.4.4. Canning
An Instant Pot Max 9-in-1 (Instant Brands, Ottawa, Canada) with the canning option selected was used for canning trout muscle. Briefly, 50 g of trout was added along with 100 mL of water to 120 mL glass jars and capped with metal covers. Trout was heated for 50 min at 121 °C. No other studies were found in the literature that evaluated changes in MG and LMG contents during canning. Recommended canning time using home pressure cookers at 15 PSI is 100 min for pint jars (USDAc, 2015). Based on the smaller sample and jar size used in this study and the hypothesis that MG would behave similarly during boiling and canning treatment (i.e., at least 50% reduction should be achieved) a shorter canning time of 50 min was chosen.
2.5. Instrumental analysis
Samples were analyzed using an Agilent UHPLC 1290 coupled with an Agilent 6545 QTOF-ESI-MS, in both positive and negative ionization modes. In positive mode, mobile phases were (A) H2O with 0.1% formic acid and (B) acetonitrile, and in negative mode, mobile phases used were (A) 0.05 M ammonium acetate and (B) acetonitrile. The same gradient elution was used for both positive and negative modes, increasing from 5% B at 1 min to 100% B after 15 min, then maintained at 100% B from 15 to 20 min, and then re-equilibrated at 5% B for 5 min at the end. An InfinityLab Poroshell 120 (Pheny-Hexyl, 3.0 × 100 mm, 2.7 μm, Agilent Technologies) with a Poroshell (4.6 mm) Phenyl Hexyl pre-column was used. Flow rate was set at 0.2 mL/min, injection volume was 2 μL and column temperature was 20 °C. The MS parameters were as follows: sheath gas temperature 275 °C, drying gas temperature 325 °C, drying gas flow 5 L/min, sheath gas flow 12 L/min, nebulizer pressure 20 psi, capillary voltage 4000 V, nozzle voltage 2000 V, fragmentor voltage 175 V, skimmer voltage 65 V. All ion MS/MS mode at collision energies of 0, 10, 20 and 40 V was used. Data was collected between 100 and 1700 m/z at a rate of 3 spectra/s. Samples were kept at 4 °C in the multi sampler compartment.
2.6. Data treatment
2.6.1. Quantification
SPSS Statistics software (v.26) (IBM, NY, USA) was used to for statistical analysis, such as comparison of reduction percentages between trout and shrimp, with level of significance set at 0.05. Concentrations were computed using Agilent Mass Hunter Quantitative Analysis B.10.0, using a mass extraction window of ±20.0 ppm and retention time window of ±0.30 min. The most abundant ions were used for quantification: [M]+ of 329.2017 for MG and [M+H]+ of 331.2168 for LMG.
Method detection limit (MDL) and limit of quantification (LOQ) were calculated as 3σ and 10σ, respectively, where σ is the standard deviation, of the procedural blanks integrated at the retention time of the target compounds.
Recoveries and matrix effects (n = 6) were calculated as described in Matuszewski et al. (2003), using external calibration. Matrix effect (ME) was calculated by comparing the analyte response (A) spiked post-extraction with the response in pure solvent (B), where ME = A/B×100. Levels above 100% show ion enhancement while values < 100% indicate ion suppression. Recoveries (RE) were calculated by comparing the analyte response spiked pre-extraction (C) with the response in pure solvent, where RE = C/A×100.
Matrix effect for labelled injection standards was calculated using the same formula as above, by comparing the mean area across the six calibration standards with the peak area of the internal standard in control raw and cooked sample replicates.
In raw and cooked samples, concentrations for LMG were calculated following the internal standard method using its deuterated surrogate and the relative response factor (RRF) (equation (1)) (USEPA, 2007).
| (1) |
For MG, matrix matched external calibration using control samples was prepared at six levels (10, 20, 35, 60, 125 and 280 ng g−1) by spiking MG and LMG standards onto post-extraction control samples. Only 1 g of the total muscle cooked was used for extraction, therefore concentrations were also adjusted to account for the weight loss during cooking. In addition, the computed concentrations in raw and cooked incurred samples were corrected for recovery.
2.6.2. Identification of thermal TPs
Data alignment and feature extraction were conducted using Agilent Mass Hunter Profinder software B.10.0 using the following parameters: peak filter height 200 counts, retention time window ±0.30 min, mass window ±10.00 ppm, post-processing peak absolute height 1000 counts, MFE score 80. Selection of data processing parameters has been discussed previously (Baesu et al., 2021).
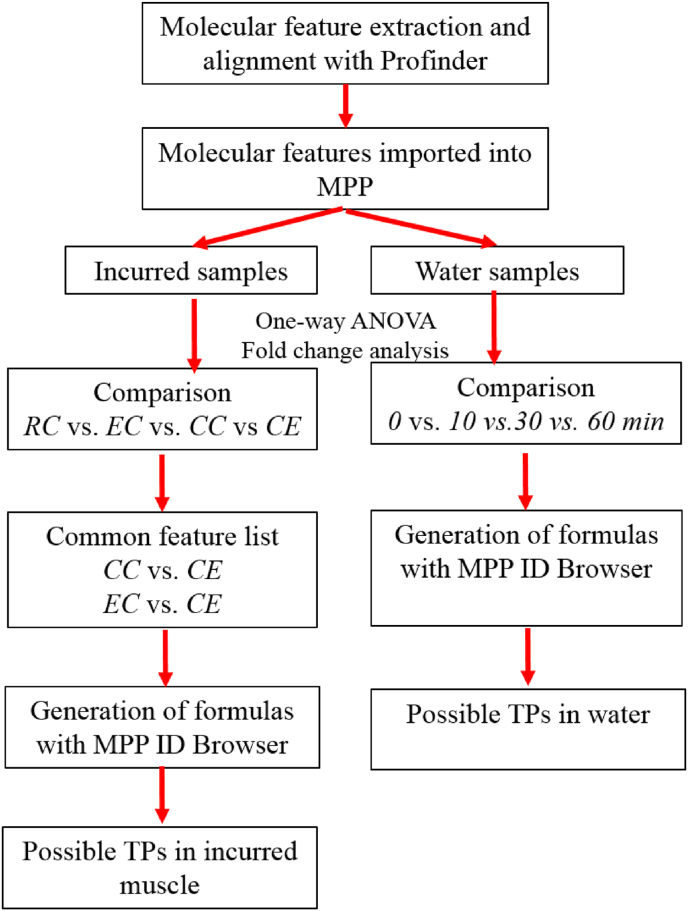
The workflow for the identification of possible TPs is presented in Fig. 2. Briefly, molecular features were exported as.pfa files and imported into Mass Profiler Professional (v 15.0) with a percentile shift (75.0) normalization. Trout and shrimp samples were grouped into raw control (RC), raw exposed (EC), cooked control (CC) and cooked exposed (CE). Fold change analysis (>2) along with one-way ANOVA (p < 0.05) with Benjamini-Hochberg correction and Tukey post-hoc test were applied to identify which compounds were present at statistically significant higher levels in specific groups. Fold change analysis along with statistical tests have been applied to study the fate of some food contaminants such as pharmaceuticals during thermal or photo-transformation (Lege et al., 2020; von Eyken and Bayen, 2020). Formula for molecular features of interest were generated through the ID browser analysis, with mass accuracy set at 5 ppm, in Mass Profiler Professional and compounds that had a score >70% were considered. This matching score is typically used in compound identification in non-target analysis (Du et al., 2017; von Eyken and Bayen, 2020). These features of interest were re-run in Targeted MS/MS mode, confirming fragment information, which enabled a search through ChemSpider (Royal Society of Chemistry, 2020) using Agilent Molecular Structure Correlator (MSC). Targeted molecular features were extracted using Agilent Qualitative Analysis B.10.0 and exported as.cef files. These files were then imported into MSC with features ran through Chemspider (Royal Society of Chemistry, 2020) as well as Agilent Metlin (30,232 compounds) databases.
Fig. 2.
Workflow for the identification of thermal TPs of MG and LMG.
3. Results and discussion
3.1. Method validation
The suitability of the method for extraction of incurred samples within a NTA context, e.g., number of features extracted has been covered elsewhere (Baesu et al., 2021). In addition, mass measurement errors (Table S1) for MG and LMG in raw and cooked samples were calculated according to Brenton and Godfrey (2010) and were significantly different between the raw and cook matrices (p < 0.0005). For both MG and LMG, mass measurement errors were below the 5 ppm threshold usually set in non-target analysis (Ponce-Robles et al., 2018).
For fortified samples, recoveries above 60% were obtained for MG and LMG in raw and cooked muscle (Table S2). For example, in trout, MG recoveries were 67 ± 10%, 111 ± 6%, 105 ± 3%, 62 ± 12% and 71 ± 10% in raw, boiled (10 and 30 min), microwaved and canned muscle respectively. Generally, lower recoveries for MG have been reported compared to LMG (Bergwerff and Scherpenisse, 2003; Chen and Miao, 2010; Mitrowska et al., 2007). López-Gutiérrez et al. (2012) determined recoveries for a similar QuEChERS extraction but with sorbent clean-up step omitted, were between 48 and 81% for MG and between 63 and 102% for LMG in shrimp and trout, depending on the spiking level. Parameters that can improve the recovery of MG from fortified samples include the incubation time between spiking and extraction, and a longer extraction time (Hall, Hopley and O'Connor, 2008). For example, Bergwerff and Scherpenisse (2003) found that in fortified turbot and trout, recoveries decreased from 81% to 63% when the incubation time between moment of spiking and extraction increased from 1 to 15 min. The incubation time in this study was 10 min. It is important to note that recoveries from fortified samples may not reflect the extraction efficiency from incurred samples. Indeed, the inclusion of a heated sonication step improved the extraction of MG from incurred samples compared to fortified samples (Eich et al., 2020).
Little matrix effect, between 89 and 121%, was observed for MG across the two different matrices both raw and cooked (Table S2). In the case of LMG, ion suppression was only observed in shrimp with matrix effects of 54 and 64% in raw and cooked muscle, respectively. Shrimp tissues also induced a pronounced matrix effect for the negative labelled internal standard, 13C-propylparaben (Table S3).
Significant difference (p < 0.0005) was found for the calculated recoveries and matrix effects of MG and LMG between shrimp and trout, both raw and cooked. Inter-day precision, calculated as the relative standard deviation (RSD) across all six replicates was generally below 20%.
Adequate instrument linearity (R2 > 0.99) was achieved. For trout, MDLs of 0.9 and 0.5 ng g−1 were obtained for MG and LMG respectively, while LOQs of 3.1 and 1.6 ng g−1 were obtained for MG and LMG. In shrimp, MDLs of 0.7 and 0.3 ng g−1 were obtained for MG and LMG respectively, while LOQs 2.2 and 1.1 ng g−1 were obtained for MG and LMG. Overall, acceptable recoveries, low MDLs/LOQs. and high mass accuracy were obtained, confirming that the methods used were adequate.
3.2. Fat analysis
Fat content (wet weight) was found to be significantly higher in trout (3.5 ± 1.7%) compared to shrimp (0.8 ± 0.3%) (p = 0.024). Although diet and habitat may have an effect on nutritional content of fish, the results obtained in this study are comparable to fat content of 2.7% determined in wild caught brook trout (Tidball et al., 2017). Fat content in white shrimp was also consistent with the general reported range of 0.5–1% (USDAa, 2019).
3.3. Stability of MG and LMG during thermal treatment
In water at 100 °C, the maximum reduction rate for MG was 19.9 ± 4.8% after 120 min, and the concentrations were significantly different only after 120 min (p = 0.001). For LMG, a similar reduction rate of 21.1 ± 3.3% was observed in water, with statistically significant differences across all four heating times (p < 0.0005). These results are comparable with those previously reported, with less than 20% decrease of the compound levels during water heating (Mitrowska et al., 2007).
In food matrices, MG was significantly reduced in both shrimp and trout muscles for all three types of thermal processing (Table 1). MG was not detected in any sampled juices or canned water samples, therefore the reduction in MG levels is not due to leaching from muscle into juices. Reduction percentage of MG was similar in boiled shrimp (36 ± 49%) and boiled trout muscle (32 ± 18%) (p = 0.828). These reduction rates were similar to those (43% decrease after boiling for 10 min) reported for carp muscle (Mitrowska et al., 2007). The larger variability observed for the quantification of MG in shrimp (standard deviation of ±49) was due to the presence of an outlier sample (doubled concentration after cooking). Without the outlier sample, the reduction of MG would be 47 ± 24%. The most efficient treatment in significantly reducing MG levels was canning, with more than 90% decrease after 50 min (see Table 1).
Table 1.
Effect of thermal treatments on MG and LMG levels in brook trout and shrimp.
| Sample | Treatment | Time (min) | Average MG concentration in musclea (ng g−1) | Average MG % reduction rateb | Average LMG concentration in musclea (ng g−1) | Average LMG % reduction rateb | Mass balancec |
|---|---|---|---|---|---|---|---|
| Trout | Boiling | 0 | 815 ± 215 | 0 | 1376 ± 432 | 0 | N/A |
| 10 | 531 ± 142 | −32 ± 18 * | 1463 ± 449 | 9 ± 23 | 0.92 ± 0.20 | ||
| 30 | 410 ± 110 | −49 ± 12 * | 1777 ± 448 | 35 ± 32 | 1.02 ± 0.22 | ||
| Canning | 0 | 757 ± 186 | 0 | 1227 ± 359 | 0 | N/A | |
| 50 | 28 ± 9 | −96 ± 2 * | 1527 ± 499 | 29 ± 39 | 0.81 ± 0.27 | ||
| Microwave | 0 | 759 ± 159 | 0 | 1099 ± 299 | 0 | N/A | |
| 1.5 | 274 ± 80 | −64 ± 9 * | 716 ± 240 | −34 ± 18 * | 0.54 ± 0.13 | ||
| Shrimp | Boiling | 0 | 175 ± 98 | 0 | 486 ± 130 | 0 | N/A |
| 10 | 91 ± 52 | −36 ± 49 * | 586 ± 173 | 20 ± 40 | 1.11 ± 0.42 |
* Statistically significant at p < 0.05.
Expressed as the mean concentrations across all ten replicates ± standard deviation.
Expressed as the mean reduction rate across all ten replicates ± standard deviation.
Expressed as the [(MG concentration + LMG concentration in cook muscle)/(MG concentration + LMG concentration in raw muscle)] ± standard deviation.
LMG levels increased, except for microwaving, during the thermal processing in both matrices. In shrimp, levels increased by 20 ± 40% (p=0.194) with increases of 9 ± 23% (p = 1.000), 35 ± 32% (p = 0.056) and 29 ± 39% (p = 0.080) in boiled and canned trout respectively. This increase may be due to reduction of MG into LMG occurring during cooking, or a possible increased efficiency of LMG extraction from cooked samples. For example, LMG along with its demethylated forms have been reported to be produced during fungal biotransformation of MG (Cha et al., 2001). Photo-transformation of an aqueous solution of MG also produced LMG along with its demethylated and hydroxylated forms (Perez-Estrada et al., 2008). Generally, chemicals like ascorbic acid, N,N,N,N-tetramethyl-1,4-phenylenediamine hydrochloride or hydroxylamine have also been added during sample extraction as they can prevent demethylation of MG or reduction to LMG (López-Gutiérrez et al., 2013). However, as the goal of this study was to identify TPs of MG and LMG, these chemicals were not used in the extraction procedure. Their omission may also influence the results obtained and the increase in LMG levels. A mass balance (Table 1) was calculated for the two compounds to investigate the possibility of MG reduction into LMG. For boiled shrimp, boiled trout and canned trout, calculated mass balances were 1.11, 0.92 and 0.82 respectively. Therefore, it is likely that the increase in LMG levels after cooking, and the variability observed, is due to the reduction of MG. There was no significant difference between the increase percentage for LMG observed in trout (9 ± 23%) and shrimp (20 ± 40%) boiled for 10 min (p = 0.457). Although raw shrimp were found to have a much lower fat content, LMG levels did not decrease. Hence, in this study, fat content did not seem to influence the fate of LMG in muscle during cooking.
The only treatment that did lead to a significant (p = 0.001) decrease of 34 ± 18% in LMG levels was microwaving. This is consistent with the observations made for carp and tilapia muscles, as LMG levels were observed to be reduced by 40% after microwaving for 1 min (Mitrowska et al., 2007; Shalaby et al., 2016). Consequently, the decrease of the LMG levels is not necessarily due to temperature during treatment, but as it has been suggested, it is rather due to the presence of electromagnetic waves generated during microwaving (Mitrowska et al., 2007). This similar behaviour has been observed for other veterinary drugs such as nitroimidazoles or penicillin G, where the compounds were stable during boiling treatment but levels were reduced during microwaving in chicken and cattle muscle (Rose et al., 1997; Rose et al., 1999). The drugs were considered stable during boiling as the residues lost from the muscle were accounted for in the surrounding fluids. Reduction in drug levels during microwaving may also have been due to their transfer into juices, however very low volume or absence of juices were observed as they had likely evaporated (Rose et al., 1999). Although no juices were observed during microwaving in the current study, based on the mass balance calculated (0.55), it is likely that the corresponding reduction rate is not only due to leaching into juices and their subsequent evaporation, but that there is indeed some transformation of LMG.
3.4. Identification of thermal TPs
Compounds that may be considered as possible TPs of MG and LMG, based on fold change and ANOVA statistical analysis, are listed in Tables 2, S4 and S5. In boiled and canned trout, no molecular features of interest were identified in negative ionization mode based on the data treatment criteria (fold change and statistical analysis). Some compounds had molecular weight higher than the parent compounds, indicating possible reactions with matrix components. A search through the Chemspider and Metlin databases did not yield any possible structural match.
Table 2.
Features identified in positive ionization mode in cooked exposed (CE) trout and shrimp based on fold change and statistical analysis √: increasing in CE (with fold change >2 compared to CC and EC) and statistically significant (p < 0.05), ↑: increasing in CE (with fold change >2 compared to CC and EC) but not statistically significant (p > 0.05), = : detected in CE but fold change <2 compared to CC and RE) and not statistically significant (p > 0.05), ND: not detected.
| Compound | Rt (min) | Mass | Formula (score) | Mass measurement error (ppm) | Trout |
Shrimp |
Water |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Boiling 10min | Boiling 30min | Canning | Microwave | Boiling 10 min | 100 °C 120 min | |||||
| C1 | 3.5 | 135.0548 | C5H5N5 (80) | −1.25 | ND | ND | ND | ND | ✓ | ND |
| C2 | 13.5 | 210.0927 | C8H18O4S (71) | 0.69 | = | = | = | ↑ | = | ND |
| C3 | 12.6 | 211.0997 | C14H13NO (87) | 1.83 | = | = | = | ↑ | = | ND |
| C4 | 11.7 | 268.2883 | C17H36N2 (85) | 1.79 | ND | ND | ND | ND | ↑ | ND |
| C5 | 10.3 | 301.1709 | C21H21N2 (94) | 1.38 | = | = | ✓ | = | = | ND |
| C6 | 9.8 | 302.1771 | C21H22N2 (80) | −4.05 | ND | ND | ND | ✓ | = | ND |
| C7 | 11.4 | 303.1622 | C13H25N3O3S (71) | 1.62 | ND | ND | ✓ | ND | ND | ND |
| C8 | 16.0 | 304.2406 | C20H32O2 (85) | 1.29 | = | = | = | ND | ↑ | ND |
| C9 | 8.5 | 310.2410 | C18H34N2S (99) | −1.80 | = | = | ND | ND | ↑ | ND |
| C10 | 12.9 | 314.2060 | No formula generated | ND | ND | ND | ND | ND | ✓ | |
| C11 | 7.6 | 346.1609 | C13H26N6OS2 (81) | −0.71 | = | ↑ | ND | ND | ND | ND |
| C12 | 14.9 | 376.2614 | C23H36O4 (81) | −2.12 | ND | ND | = | ND | ✓ | ND |
| C13 | 15.0 | 385.2590 | C21H37O6 (98) | −1.37 | ND | ND | ND | ND | ✓ | ND |
| C14 | 7.6 | 449.1086 | C18H24ClNO10 (82) | −0.54 | ✓ | ✓ | ND | ND | ND | ND |
| C15 | 12.6 | 458.3257 | C26H42N4O3 (97) | 0.69 | ND | ND | ND | ND | ✓ | ND |
| C16 | 15.6 | 475.3633 | C25H53N3OS2 (76) | 0.70 | ND | ✓ | ND | ND | ND | ND |
| C17 | 8.5 | 505.2361 | No Formula generated | ✓ | ✓ | ND | ND | ND | ND | |
| C18 | 13.0 | 533.2872 | C33H35N5O2 (94) | 0.49 | ND | ND | ND | ✓ | ND | ND |
| C19 | 13.7 | 547.3635 | C32H51O7 (97) | 0.10 | ND | ND | ND | ND | ✓ | ND |
| C20 | 11.2 | 552.3179 | C29H36N12 (75) | −1.26 | ND | ND | ✓ | ND | ND | ND |
| C21 | 16.5 | 572.1387 | C32H30NO3S3 (73) | −0.45 | ND | ND | ↑ | ND | ND | ND |
| C22 | 13.4 | 659.3180 | C34H47N2O11 (91) | 0.06 | ND | ✓ | ND | ND | ND | ND |
| C23 | 16.7 | 829.5609 | C35H71N15O8 (97) | −0.10 | ND | ND | ✓ | ND | ND | ND |
| C24 | 16.2 | 853.5621 | C45H83N5O4S3 (88) | 0.79 | ND | ND | ND | ✓ | ND | ND |
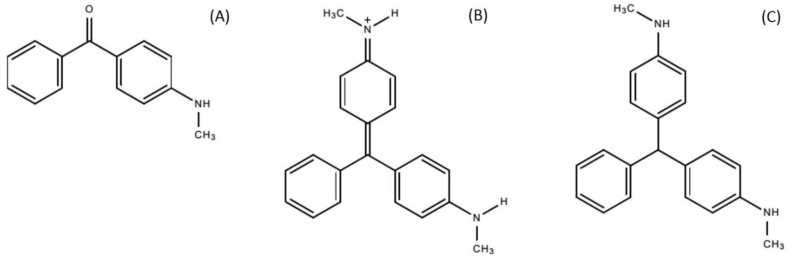
Photo-transformation or metabolomic studies have identified other TPs of MG and LMG (Table S6). The masses included in the list were manually screened for in raw and cooked incurred samples. Only three compounds (Fig. 3) were tentatively identified as TPs of MG and LMG in this study. C3 (Fig. 3, Fig. 4) was proposed as a possible TP of MG in microwaved trout. This compound has previously been identified as a photo-TP (Perez-Estrada et al., 2008) formed through hydroxyl radical attack and cleavage/demethylation of the parent trimethylmethane structure. Hydroxyl radicals can be formed during cooking and may cause oxidation of macronutrients, like proteins (Soladoye et al., 2015). These radicals may be responsible for the oxidation of MG and detection of the benzophenone derivative as a possible TP. C5 was tentatively identified as 2-desmethylated MG, which has also been described as a photo-TP (Perez-Estrada et al., 2008). Although it was found in the other cooked tissues, e.g., boiled trout it was present with a fold change<2 and not statistically significant. One possibility for this may be variability in the extraction efficiency between raw and cooked samples. 2-desmethylated MG was however found at statistically significant higher abundance in canned trout (Figure S1); in this context, this compound can therefore be regarded as a transformation of MG during heating. C6 (Fig. 4) was tentatively identified as 2-desmethylated LMG, which has been proposed as a fungal biotransformation product (Cha et al., 2001) as well as a metabolite in catfish muscle (Doerge et al., 1998). C6 was detected at a statistically significant higher abundance in microwaved trout. This is in line with the fact that microwaving was the only thermal treatment that reduced LMG levels.
Fig. 3.
Structures of tentative TPs of MG and LMG: C3 (A), C5 (B), C6 (C).
Fig. 4.
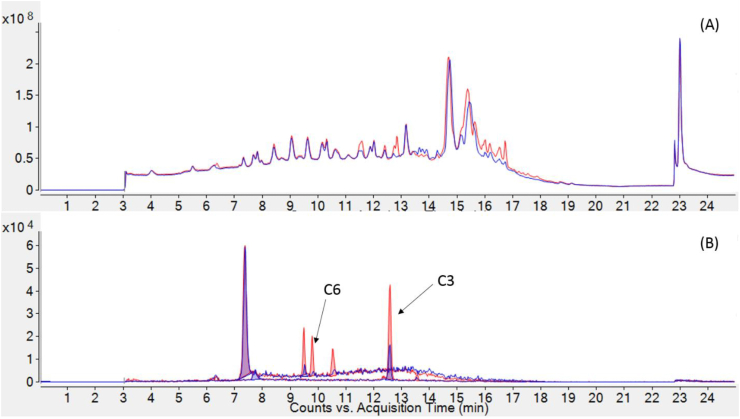
(A) Total Ion Chromatogram (TIC) for raw exposed trout (blue) and microwaved exposed trout (red); (B) Extracted Ion Chromatogram (EIC) for C3 and C6 at m/z 212.1069 and 303.1845 respectively (±20 ppm). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Implications of the present findings
The results obtained in this study, using two previously un-studied seafood matrices, are in line with previous investigations into the losses of MG and LMG during cooking, which showed that cooking procedures are not sufficient in reducing the levels of the two compounds. Canning, which has been used as a cooking treatment to evaluate MG and LMG for the first time in this study, was able to achieve the almost complete reduction (∼96%) of MG levels. However, the canning treatment applied in this study simulated home canning rather than more industrial canning. Depending on can sizes, recommended treatment duration at a retort temperature of 121 °C may vary from 33 to 187 min (Featherstone, 2016). Currently, there is limited information in the literature on the detection of MG and LMG in canned seafood sampled from local markets for example. As part of a total diet study, Tittlemier et al. (2007) sampled and analyzed canned tuna purchased from the Canadian market, but did not find any MG or LMG. MG and LMG were detected in canned fried dace fish at levels below 10 ng g−1 (Hong Kong Center for Food Safety, 2016; Andersen et al., 2018). Therefore, canned seafood should also be analyzed as part of monitoring MG and LMG levels, especially since LMG appears to be more persistent in muscle during canning treatment compared to MG.
Different possible TPs were detected across all the thermal treatments and in all matrices. This study, along with previously reported thermal studies of food contaminants (Tian and Bayen, 2018; von Eyken and Bayen, 2020) reinforces the conclusion that some compounds do not have the same behaviour or follow the same transformation mechanisms across different food matrices and thermal treatments. One possibility for this may be the different degree of oxidation occurring during different cooking procedures. For example, Khan et al. (2015) found that cholesterol underwent more oxidation during microwaving as a larger amount of cholesterol oxidation products (COPs) were detected in meats compared to other cooking procedures, e.g., roasting. Furthermore, depending on the meat, i.e., bacon vs. sausage, different COPs were detected for the same cooking procedure.
The two tentatively identified TPs 2-desmethylated MG and LMG, have been detected as possible metabolites in the livers of rats that were fed the two compounds through their diet (Culp et al., 1999). The subsequent oxidation of these compounds may contribute to the formation of DNA adducts, which have been detected in tissues of rats (Culp et al., 1999). There are of course some limitations to the current findings. First, the MG and LMG levels in incurred samples are much higher than levels encountered in market samples, which are usually below 10 ng g−1 (EU RASFF Portal, 2020). Hence, the study can be replicated using real-market samples contaminated with MG and LMG to determine if these tentatively proposed TPs are detected at lower parent compound concentrations. Second, currently there are no standards available for confident identification and quantification of these TPs. In this context, a semi-quantification approach using the parent compounds (von Eyken and Bayen, 2020) combined with a threshold of toxicological concern approach should be explored as a first screening tool to assess if TPs are priority compounds for further toxicological testing.
4. Conclusion
In this study, non-target analysis using high resolution mass spectrometry was applied for the first time to study the fate of MG and LMG in brook trout and crustaceans. Even though shrimp was found to have a lower fat content, this matrix did not impact the concentration changes for the more lipophilic LMG, with only microwaving achieving a significant reduction of the metabolite. Three compounds, resulting from the possible demethylation and cleavage of the conjugated structures have been proposed as possible TPs. Their identity could not be confirmed due to the lack of available analytical standards of the pure compounds and further research is needed to determine possible toxicity. More research, such as extraction of molecular features filtered on mass defect, is needed to try and identify the high-mass features identified in cooked compared to raw samples. The findings of this study show the importance of integrating analysis of processed, e.g., canned seafood when assessing the human exposure and possible health risk associated with MG and LMG, as these processing treatments are not adequate in reducing residues present in muscle.
CRediT authorship contribution statement
Anca Baesu: Conceptualization, Formal analysis, Investigation, Methodology, Data curation, Writing – original draft, Writing – review & editing. Céline Audet: Methodology, Resources, Writing – original draft, Writing – review & editing. Stéphane Bayen: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors report no conflict of interest for the following manuscript submitted for your review (Original Article):
Acknowledgements
The authors would like to thank Regina Basumatary and Dr. Hosahalli S. Ramaswamy for their technical support for the Instant Pot canning experiment. We wish to acknowledge the financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC) (RGPIN/04800–2017) and the Canada Foundation for Innovation/John R. Evans Leaders Fund grant (Project #35318) research grants to S. Bayen.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2021.09.010.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alaboudi A., Basha E.A., Musallam I. Chlortetracycline and sulfanilamide residues in table eggs: prevalence, distribution between yolk and white and effect of refrigeration and heat treatment. Food Contr. 2013;33(1):281–286. doi: 10.1016/j.foodcont.2013.03.014. [DOI] [Google Scholar]
- Andersen W.C., Turnipseed S.B., Roybal J.E. Quantitative and confirmatory analyses of malachite green and leucomalachite green residues in fish and shrimp. J. Agric. Food Chem. 2006;54(13):4517–4523. doi: 10.1021/jf0532258. [DOI] [PubMed] [Google Scholar]
- Andersen W.C., Casey C.R., Nickel T.J., Young S.L., Turnipseed S.B. Dye residue analysis in raw and processed aquaculture products: matrix extension of AOAC INTERNATIONAL official method 2012.25. J. AOAC Int. 2018;101(6):1927–1939. doi: 10.5740/jaoacint.18-0015. [DOI] [PubMed] [Google Scholar]
- Baesu A., Audet C., Bayen S. Evaluation of different extractions for the metabolite identification of malachite green in brook trout and shrimp. Food Chem. 2021 doi: 10.1016/j.foodchem.2021.130567. [DOI] [PubMed] [Google Scholar]
- Bayen S., Barlow P., Lee H.K., Obbard J.P. Effect of cooking on the loss of persistent organic pollutants from salmon. J. Toxicol. Environ. Health. 2005;68(4):253–265. doi: 10.1080/15287390590895126. [DOI] [PubMed] [Google Scholar]
- Bergwerff A.A., Scherpenisse P. Determination of residues of malachite green in aquatic animals. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2003;788(2):351–359. doi: 10.1016/s1570-0232(03)00042-4. [DOI] [PubMed] [Google Scholar]
- Bletsou A.A., Jeon J., Hollender J., Archontaki E., Thomaidis N.S. Targeted and non-targeted liquid chromatography-mass spectrometric workflows for identification of transformation products of emerging pollutants in the aquatic environment. Trac. Trends Anal. Chem. 2015;66:32–44. doi: 10.1016/j.trac.2014.11.009. [DOI] [Google Scholar]
- Brenton A.G., Godfrey A.R. Accurate mass measurement: terminology and treatment of data. J. Am. Soc. Mass Spectrom. 2010;21(11):1821–1835. doi: 10.1016/j.jasms.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Cha C.J., Doerge D.R., Cerniglia C.E. Biotransformation of malachite green by the fungus cunninghamella elegans. Appl. Environ. Microbiol. 2001;67(9):4358–4360. doi: 10.1128/aem.67.9.4358-4360.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Miao S. HPLC determination and MS confirmation of malachite green, gentian violet, and their leuco metabolite residues in channel catfish muscle. J. Agric. Food Chem. 2010;58(12):7109–7114. doi: 10.1021/jf9043925. [DOI] [PubMed] [Google Scholar]
- Culp S.J., Blankenship L.R., Kusewitt D.F., Doerge D.R., Mulligan L.T., Beland F.A. Toxicity and metabolism of malachite green and leucomalachite green during short-term feeding to Fischer 344 rats and B6C3F1 mice. Chem. Biol. Interact. 1999;122(3):153–170. doi: 10.1016/S0009-2797(99)00119-2. [DOI] [PubMed] [Google Scholar]
- Dinh Q.T., Munoz G., Vo Duy S., Tien Do D., Bayen S., Sauvé S. Analysis of sulfonamides, fluoroquinolones, tetracyclines, triphenylmethane dyes and other veterinary drug residues in cultured and wild seafood sold in Montreal, Canada. J. Food Compos. Anal. 2020;94 doi: 10.1016/j.jfca.2020.103630. [DOI] [Google Scholar]
- Doerge D.R., Churchwell M.I., Gehring T.A., Pu Y.M., Plakas S.M. Analysis of malachite green and metabolites in fish using liquid chromatography atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1998;12(21):1625–1634. doi: 10.1002/(SICI)1097-0231(19981115)12:21<1625::AID-RCM373>3.0.CO;2-I. 10.1002/(SICI)1097-0231(19981115)12:21<1625::AID-RCM373>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Du B., Lofton J.M., Peter K.T., Gipe A.D., James C.A., McIntyre J.K., Kolodziej E.P. Development of suspect and non-target screening methods for detection of organic contaminants in highway runoff and fish tissue with high-resolution time-of-flight mass spectrometry. Environ Sci Process Impacts. 2017;19(9):1185–1196. doi: 10.1039/c7em00243b. [DOI] [PubMed] [Google Scholar]
- Dubreil E., Mompelat S., Kromer V., Guitton Y., Danion M., Morin T., Verdon E. Dye residues in aquaculture products: targeted and metabolomics mass spectrometric approaches to track their abuse. Food Chem. 2019;294:355–367. doi: 10.1016/j.foodchem.2019.05.056. [DOI] [PubMed] [Google Scholar]
- Efsa Scientific opinion on malachite green in food. EFSA Journal. 2016;14(7):4530–4580. doi: 10.2903/j.efsa.2016.4530. [DOI] [Google Scholar]
- Eich J., Bohm D.A., Holzkamp D., Mankertz J. Validation of a method for the determination of triphenylmethane dyes in trout and shrimp with superior extraction efficiency. Food Addit. Contam. Part A Chem Anal Control Expo Risk Assess. 2020;37(1):84–93. doi: 10.1080/19440049.2019.1671611. [DOI] [PubMed] [Google Scholar]
- EU Commission Regulation 2019/1871 on reference points for action for non-allowed pharmacologically active substances present in food of animal origin and repealing Decision 2005/34/EC. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R1871&from=EN
- EU RASFF Portal. 2020. Available from https://webgate.ec.europa.eu/rasff-window/screen/search.
- Featherstone S. In: A Complete Course in Canning and Related Processes. Fourteenth Edition. Featherstone S., editor. Woodhead Publishing; 2016. 6 - Canning of fish and seafood; pp. 231–265. [Google Scholar]
- Fu Y., Zhao C., Lu X., Xu G. Nontargeted screening of chemical contaminants and illegal additives in food based on liquid chromatography–high resolution mass spectrometry. Trac. Trends Anal. Chem. 2017;96:89–98. doi: 10.1016/j.trac.2017.07.014. [DOI] [Google Scholar]
- Hall Z., Hopley C., O'Connor G. High accuracy determination of malachite green and leucomalachite green in salmon tissue by exact matching isotope dilution mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;874(1–2):95–100. doi: 10.1016/j.jchromb.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Health Canada Malachite green: questions and answers. 2017. https://inspection.canada.ca/food-safety-for-consumers/fact-sheets/specific-products-and-risks/chemical-hazards/malachite-green/eng/1332268890141/1332268947157
- Hong Kong Center for Food Safety Press release. Malachite green in canned fried dace samples. 2016. https://www.cfs.gov.hk/english/press/20161121_0686.html
- Khan M.I., Min J.S., Lee S.O., Yim D.G., Seol K.H., Lee M., Jo C. Cooking, storage, and reheating effect on the formation of cholesterol oxidation products in processed meat products. Lipids Health Dis. 2015;14:89. doi: 10.1186/s12944-015-0091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolhoff A.M., Croley T.R. Non-targeted screening approaches for contaminants and adulterants in food using liquid chromatography hyphenated to high resolution mass spectrometry. J. Chromatogr. A. 2016;1428:86–96. doi: 10.1016/j.chroma.2015.08.059. [DOI] [PubMed] [Google Scholar]
- Le Curieux F., Gohlke J.M., Pronk A., Andersen W.C., Chen G., Fang J.-L.…Schubauer-Berigan M.K. Carcinogenicity of gentian violet, leucogentian violet, malachite green, leucomalachite green, and CI Direct Blue 218. Lancet Oncol. 2021;22(5):585–586. doi: 10.1016/S1470-2045(21)00178-9. [DOI] [PubMed] [Google Scholar]
- Lege S., Sorwat J., Yanez Heras J.E., Zwiener C. Abiotic and biotic transformation of torasemide - occurrence of degradation products in the aquatic environment. Water Res. 2020;177 doi: 10.1016/j.watres.2020.115753. [DOI] [PubMed] [Google Scholar]
- López-Gutiérrez N., Romero-González R., Plaza-Bolaños P., Martínez-Vidal J.L., Garrido-Frenich A. Simultaneous and fast determination of malachite green, leucomalachite green, crystal violet, and brilliant green in seafood by ultrahigh performance liquid chromatography–tandem mass spectrometry. Food Analytical Methods. 2012;6(2):406–414. doi: 10.1007/s12161-012-9456-9. [DOI] [Google Scholar]
- López-Gutiérrez N., Romero-González R., Martínez Vidal J.L., Frenich A.G. Analysis of triphenylmethane dyes in seafood products: a review of extraction methods and determination by liquid chromatography coupled to mass spectrometry. Analytical Methods. 2013;5(14):3434. doi: 10.1039/c3ay40485d. [DOI] [Google Scholar]
- Love D.C., Rodman S., Neff R.A., Nachman K.E. Veterinary drug residues in seafood inspected by the European Union, United States, Canada, and Japan from 2000 to 2009. Environ. Sci. Technol. 2011;45(17):7232–7240. doi: 10.1021/es201608q. [DOI] [PubMed] [Google Scholar]
- Matuszewski B.K., Constanzer M.L., Chavez-Eng C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC−MS/MS. Anal. Chem. 2003;75(13):3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- Mitrowska K., Posyniak A., Zmudzki J. The effects of cooking on residues of malachite green and leucomalachite green in carp muscles. Anal. Chim. Acta. 2007;586(1–2):420–425. doi: 10.1016/j.aca.2007.01.042. [DOI] [PubMed] [Google Scholar]
- National Libraty of Medicine . 2020. Pubchem Database.https://pubchem.ncbi.nlm.nih.gov/ [Google Scholar]
- Nguyen V., Nguyen V., Li C.B., Zhou G.H. The degradation of oxytetracycline during thermal treatments of chicken and pig meat and the toxic effects of degradation products of oxytetracycline on rats. Journal of Food Science and Technology-Mysore. 2015;52(5):2842–2850. doi: 10.1007/s13197-014-1306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Estrada L.A., Aguera A., Hernando M.D., Malato S., Fernandez-Alba A.R. Photodegradation of malachite green under natural sunlight irradiation: kinetic and toxicity of the transformation products. Chemosphere. 2008;70(11):2068–2075. doi: 10.1016/j.chemosphere.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Ponce-Robles L., Oller I., Agüera A., Trinidad-Lozano M.J., Yuste F.J., Malato S., Perez-Estrada L.A. Application of a multivariate analysis method for non-target screening detection of persistent transformation products during the cork boiling wastewater treatment. Sci. Total Environ. 2018;633:508–517. doi: 10.1016/j.scitotenv.2018.03.179. [DOI] [PubMed] [Google Scholar]
- Rose M.D., Bygrave J., Farrington W.H.H. The effect of cooking on veterinary drug residues in food.Part 8. Benzylpenicillin†. Analyst. 1997;122(10):1095–1099. doi: 10.1039/A702771K. [DOI] [PubMed] [Google Scholar]
- Rose M.D., Bygrave J., Sharman M. Effect of cooking on veterinary drug residues in food Part 9.† Nitroimidazoles. Analyst. 1999;124(3):289–294. doi: 10.1039/A809062I. [DOI] [PubMed] [Google Scholar]
- Royal Society of Chemistry . 2020. Chemspider Database.http://www.chemspider.com/Default.aspx [Google Scholar]
- Schuetze A., Heberer T., Juergensen S. Occurrence of residues of the veterinary drug malachite green in eels caught downstream from municipal sewage treatment plants. Chemosphere. 2008;72(11):1664–1670. doi: 10.1016/j.chemosphere.2008.05.036. [DOI] [PubMed] [Google Scholar]
- Shalaby A.R., Abdelmagui N.M., Emam W.H. Impact of cooking on malachite green and leucomalachite green residues existing in Tilapia fish. Am. J. Food Technol. 2016;12(1):60–65. doi: 10.3923/ajft.2017.60.65. [DOI] [Google Scholar]
- Soladoye O.P., Juárez M.L., Aalhus J.L., Shand P., Estévez M. Protein oxidation in processed meat: mechanisms and potential implications on human health. Compr. Rev. Food Sci. Food Saf. 2015;14(2):106–122. doi: 10.1111/1541-4337.12127. [DOI] [PubMed] [Google Scholar]
- Tian L., Bayen S. Thermal degradation of chloramphenicol in model solutions, spiked tissues and incurred samples. Food Chem. 2018;248:230–237. doi: 10.1016/j.foodchem.2017.12.043. [DOI] [PubMed] [Google Scholar]
- Tidball M.M., Exler J., Somanchi M., Williams J., Kraft C., Curtis P., Tidball K.G. Addressing information gaps in wild-caught foods in the US: brook trout nutritional analysis for inclusion into the USDA national nutrient database for standard reference. J. Food Compos. Anal. 2017;60:57–63. doi: 10.1016/j.jfca.2017.03.004. [DOI] [Google Scholar]
- Tittlemier S.A., Van de Riet J., Burns G., Potter R., Murphy C., Rourke W.…Dufresne G. Analysis of veterinary drug residues in fish and shrimp composites collected during the Canadian Total Diet Study, 1993-2004. Food Addit. Contam. 2007;24(1):14–20. doi: 10.1080/02652030600932937. [DOI] [PubMed] [Google Scholar]
- USDAa . 2019. Agricultural Research Service. (Food data central fdc.nal.usda.gov) [Google Scholar]
- USDAb . vol. 3. 2009. (Determination of Fat CLG-FAT). [Google Scholar]
- USDAc . 2015. Guide 5 Preparing and Canning Poultry, Red Meats and Seafood.https://nchfp.uga.edu/publications/usda/GUIDE05_HomeCan_rev0715.pdf [Google Scholar]
- USEPA . 2007. Method 1694: Pharmaceuticals and Personal Care Products in Water. soil, sediment, and biosolids by HPLC/MS/MS EPA-821-R-08-002. [Google Scholar]
- von Eyken A., Bayen S. Non-targeted study of the thermal degradation of tylosin in honey, water and water:honey mixtures. Food Addit. Contam. Part A Chem Anal Control Expo Risk Assess. 2020;37(3):421–437. doi: 10.1080/19440049.2019.1704442. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization; 2009. Principles and Methods for the Risk Assessment of Chemicals in Food. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.