Abstract
BACKGROUND
Stereotactic radiosurgery (SRS) is increasingly used for management of perioptic meningiomas.
OBJECTIVE
To study the safety and effectiveness of SRS for perioptic meningiomas.
METHODS
From 12 institutions participating in the International Radiosurgery Research Foundation (IRRF), we retrospectively assessed treatment parameters and outcomes following SRS for meningiomas located within 3 mm of the optic apparatus.
RESULTS
A total of 438 patients (median age 51 yr) underwent SRS for histologically confirmed (29%) or radiologically suspected (71%) perioptic meningiomas. Median treatment volume was 8.01 cm3. Median prescription dose was 12 Gy, and median dose to the optic apparatus was 8.50 Gy. A total of 405 patients (93%) underwent single-fraction SRS and 33 patients (7%) underwent hypofractionated SRS. During median imaging follow-up of 55.6 mo (range: 3.15-239 mo), 33 (8%) patients experienced tumor progression. Actuarial 5-yr and 10-yr progression-free survival was 96% and 89%, respectively. Prescription dose of ≥12 Gy (HR: 0.310; 95% CI [0.141-0.679], P = .003) and single-fraction SRS (HR: 0.078; 95% CI [0.016-0.395], P = .002) were associated with improved tumor control. A total of 31 (10%) patients experienced visual decline, with actuarial 5-yr and 10-yr post-SRS visual decline rates of 9% and 21%, respectively. Maximum dose to the optic apparatus ≥10 Gy (HR = 2.370; 95% CI [1.086-5.172], P = .03) and tumor progression (HR = 4.340; 95% CI [2.070-9.097], P < .001) were independent predictors of post-SRS visual decline.
CONCLUSION
SRS provides durable tumor control and quite acceptable rates of vision preservation in perioptic meningiomas. Margin dose of ≥12 Gy is associated with improved tumor control, while a dose to the optic apparatus of ≥10 Gy and tumor progression are associated with post-SRS visual decline.
Keywords: Perioptic meningioma, Stereotactic radiosurgery, Gamma Knife, Outcomes, Progression-free survival, Visual outcomes
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- BED
biologically effective dose
- CI
confidence interval
- EBRT
External Beam Radiation Therapy
- HR
hazard ratio
- MRI
magnetic resonance imaging
- PFS
progression-free survival
- SRS
stereotactic radiosurgery
Management of meningiomas residing in close spatial proximity to the optic apparatus (ie, perioptic meningiomas) remains challenging.1-5 Microsurgical and/or endoscopic tumor resection and decompression of the optic apparatus have been traditionally considered the primary treatment option for most perioptic meningiomas.1,6-8 However, due to close proximity to the optic nerves, infiltrative growth, and invasion of the skull base dura and cavernous sinus, resection of perioptic lesions can be a technically challenging procedure even in experienced hands, and is associated with high risk of visual impairment.2,6,9-12
Radiosurgery is often considered for treatment of residual skull base meningiomas.13,14 Radiosurgery is also increasingly used as upfront treatment for patients who are not optimal surgical candidates, perhaps due to advanced age and/or serious medical comorbidities. Stereotactic radiosurgery (SRS) is associated with high and sustained local tumor control rates of skull base meningiomas that often exceed 90%.15,16 However, sensitivity of the anterior visual pathway to radiation is the major limiting factor that often precludes safe delivery of radiosurgery to lesions located in close spatial proximity (usually within 3 mm) to the optic nerves. The risk for developing optic neuropathy after radiosurgery is dose-dependent, and cumulative radiation dose to the optic nerves and chiasm should not exceed 8 to 12 Gy in a single fraction.17-19 Fractionated SRS allows an increase in the radiation tolerance of the cumulative dosing to the optic apparatus.7,13,20 Single-fraction SRS has an established effectiveness and safety profile for management of perioptic tumors, including pituitary adenomas,21 optic pathway and hypothalamic gliomas,22 orbital apex tumors,23 and clinoid, sellar, and parasellar meningiomas.24,25 Hence, the aim of this multicentered study was to evaluate safety and effectiveness of SRS for the management of perioptic meningiomas.
METHODS
Patients and Setting
A total of 438 patients were identified from 12 institutions affiliated with the International Radiosurgery Research Foundation (IRRF). The study inclusion criteria were diagnosis of meningiomas based on magnetic resonance imaging (MRI) or histological examination, meningiomas located 3 mm or closer to the optic nerve or chiasm, and treatment using single-session or hypofractionated SRS. Patients with histories of fractionated radiation therapy for an index lesion were allowed in the study. Data collection was approved by institutional review boards at each of the participating centers. Due to retrospective design, informed consents were not obtained. MRI imaging features of meningiomas included extra-axial location, dural involvement, and avid enhancement after gadolinium administration.
Clinical Assessment
We gathered information regarding patient gender, presenting symptoms, age at diagnosis, pre-SRS functional status, ophthalmological function, therapies preceding SRS, WHO grade, and imaging characteristics of perioptic meningiomas.
Stereotactic Radiosurgery Technique
SRS was performed following standard techniques using Gamma Knife units (Elekta AB, Stockholm, Sweden) using frame-based or frameless approach for hypofractionated SRS or when stereotactic frame application was not technically possible. The decision to use single-fraction or hypofractionated SRS techniques was made at the discretion of the treating team. Biologically effective dose (BED) was calculated for proper comparison of different fractionation regimens using α/β ratio of 3.26,27
Clinical and Radiographic Follow-up
Imaging and clinical follow-up was performed at 3- to 6-mo intervals for the first 2 yr after SRS, with annual follow-up thereafter. Volume of perioptic meningiomas at latest imaging follow-up was compared to pre-SRS imaging data and was categorized as stable (within 20% change), regression (>20% decrease), or progression (≥20% increase).28,29 Time to tumor volume change and death were recorded.
Visual follow-up was obtained through a combination of ophthalmic visual field examination and outpatient clinic visits. Formal visual field testing was performed as indicated and per the protocol of the individual sites. Visual status change at last follow-up was categorized by the treating team as not changed, improved, or declined. SRS-related adverse events were categorized according to the Radiation Therapy Oncology Group Central Nervous System toxicity criteria.30
Statistical Analyses
Statistical analyses were performed with the IBM Statistical Package for the Social Sciences Statistics for Windows, version 25.0 (IBM Corp, Armonk, New York). Progression-free survival (PFS) was defined as interval (in months) from SRS for perioptic meningioma to last imaging follow-up or MRI documented progression of an index tumor, whichever occurred first. Time to visual decline was defined as interval (in months) from SRS for perioptic meningioma to deterioration of visual function (as deemed by the treating team) on formal ophthalmologic examination or clinical examination or last follow-up. The association of clinical and SRS factors with PFS and time to visual change was first investigated using the Kaplan-Meier method, and univariate and multivariate Cox regression analyses, with significant predictors in univariate analyses being entered in multivariate Cox regression analysis models. BED, margin dose, and maximal dose to optic apparatus were considered separately in regression models because they are inter-related. Results of Cox regression analysis are presented as hazard ratio (HR), 95% CI, and P value.
RESULTS
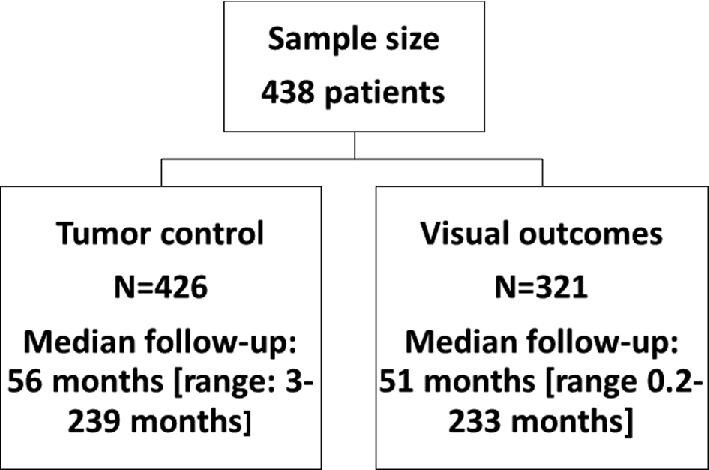
A total of 438 patients underwent SRS for perioptic meningiomas (Table 1; Figure 1). The majority of SRS-treated tumors were clinoidal (31%) and tuberculum sella (31%) meningiomas. A total of 153 patients (35%) had histories of at least 1 resection surgery of the perioptic meningioma. Pathology reports were available for 126 patients: 124 patients were diagnosed with WHO grade I meningiomas and 2 with WHO grade II meningiomas.
TABLE 1.
Demographic and Clinical Characteristics of the Study Patients (n = 438)
| Gender, n (%) | |
| Men | 99 (23%) |
| Women | 339 (77%) |
| Age (yr) | |
| Median [range]; mean ± SD | 51 [15-83]; 51.13 ± 12.28 |
| Karnofsky Performance Index before SRS (score) | |
| Median [range]; mean ± SD | 90 [50-100]; 85.31 ± 13.97 |
| Data not reported | 244 (56%) |
| Pre-SRS visual deficit, n (%) | |
| Yes | 257 (59%) |
| No | 181 (41%) |
| Other presenting symptoms, na | |
| Headache | 85 |
| Diplopia | 43 |
| Ptosis | 41 |
| Incidental | 35 |
| Seizure | 18 |
| Other | 62 |
| Duration of symptoms (mo) | |
| Median [range]; mean ± SD | 10 [0-240]; 19.60 ± 30.23 |
| Unknown or data not available | 71 (16%) |
| Pre-SRS endocrinopathy, n (%) | |
| None | 412 (94%) |
| Hypothyroidism | 15 (3%) |
| Estrogen/testosterone deficiency | 3 (1%) |
| Diabetes insipidus | 1 (0%) |
| Not reported | 7 (2%) |
| Nearest distance to the optic apparatus (mm) | |
| Median [range]; mean ± SD | 0 [0-2.3]; 0.36 ± 0.67 |
| In direct contact with optic pathway | 328 (75%) |
| Meningioma principal location, n (%) | |
| Clinoid | 191 (44%) |
| Tuberculum sella | 136 (31%) |
| Sphenoid wing | 31 (7%) |
| Planum sphenoidale | 17 (4%) |
| Clival/petroclival | 15 (3%) |
| Parasellar | 10 (2%) |
| Suprasellar | 8 (2%) |
| Intraorbital | 4 (1%) |
| Intrasellar | 2 (1%) |
| Optic sheath | 1 (0%) |
| Temporal pole | 1 (0%) |
| Frontobasal | 1 (0%) |
| Optic nerve sheath | 1 (0%) |
| Not specified/other | 8 (2%) |
| Other meningiomas, n (%) | 77 (8%) |
| Index meningioma surgery before SRS, n (%) | 153 (35%) |
| Number of prior resections, n (%) | |
| 1 | 121 (79%) |
| 2 | 28 (18%) |
| 3 | 3 (2%) |
| 4 | 1 (1%) |
| Type of prior resection, n (%) | |
| Gross total resection | 21 (14%) |
| Subtotal resection | 120 (79%) |
| Biopsy | 9 (6%) |
| Data not available | 2 (1%) |
| Interval between surgery and SRS (mo) | |
| Median [range]; mean ± SD | 9 [1-246]; 28.32 ± 44.57 |
| Meningioma WHO grade, n (%) | |
| I | 124 (28%) |
| II | 2 (1%) |
| No pre-SRS surgery or data not available | 312 (71%) |
| Histological type of index meningioma, n (%) | |
| Meningothelial | 30 (7%) |
| Transitional | 28 (6%) |
| Psammomatous | 10 (2%) |
| Fibroblastic | 5 (1%) |
| Mixed transitional and fibroblastic | 5 (1%) |
| Angiomatous | 1 (0.2%) |
| Microcystic | 1 (0.2%) |
| Secretory | 1 (0.2%) |
| Atypical | 1 (0.2%) |
| No pre-SRS surgery or data not available | 356 (81%) |
| Pre-SRS fractionated radiation therapy, n (%) | 10 (2%) |
| Pre-SRS radiation therapy dose (Gy) | |
| Median [range]; mean ± SD | 45 [13-60]; 40.42 ± 18.15 |
| Data not available | 4 (40%) |
| Pre-SRS radiation therapy number of fractions | |
| Median [range]; mean ± SD | 17 [1-30]; 15.67 ± 12.21 |
| Data not available | 4 (40%) |
SD, standard deviation.
aTotal number does not add up to 438 because some patients had more than 1 presenting symptom.
FIGURE 1.
Flowchart.
SRS Characteristics
The majority of patients underwent single-fraction SRS (92.5%; Table 2). Treatment volumes and shortest distance to the optic apparatus were similar in patients treated using single-session vs hypofractionated SRS (P values ≥.65). The median treatment volume was 8.01 cm3 (range: 0.130-57.3 cm3). Median marginal prescription dose for single-fraction SRS was 12 Gy (range: 7-18 Gy). Median maximal radiation dose to any portion of the optic apparatus was 8.50 Gy (range: 2-23 Gy). In total, 3 out of 4 patients who received maximal radiation dose to the optic apparatus of >16 Gy were treated using hypofractionated approach and 1 patient was blind on the ipsilateral eye before SRS. Patients who received ≥10 Gy vs <10 Gy to the optic apparatus had smaller tumor volume (7.22 ± 5.51 cm3 vs 10.48 ± 9.02 cm3, P = .001) but similar distance to the optic apparatus (P = .63).
TABLE 2.
SRS Characteristics of the Study Patients
| Parameters | |
|---|---|
| Number of SRS fractions | |
| Single | 405 (92.5%) |
| 2 | 1 (0.2%) |
| 3 | 6 (1.4%) |
| 4 | 15 (3.4%) |
| 5 | 10 (2.3%) |
| Data not available | 1 (0.2%) |
| Treatment volume (cm3) | |
| Median [range]; mean ± SD | 8.01 [0.130-57.3]; 9.78 ± 8.53 |
| Number of isocenters | |
| Median [range]; mean ± SD | 14 [1-54]; 16.55 ± 9.60 |
| Margin tumor dose for single-fraction SRS (Gy) | |
| Median [range]; mean ± SD | 12 [7-18]; 11.94 ± 1.33 |
| BED (Gy) | |
| Median [range]; mean ± SD | 60 [23.3-101.3]; 58.20 ± 12.17 |
| Maximal tumor dose (Gy) | |
| Median [range]; mean ± SD | 24 [8-48]; 23.49 ± 4.52 |
| Maximal BED to optic apparatus (Gy) | |
| Median [range]; mean ± SD | 36 [5.3-101.3]; 34.07 ± 13.26 |
| Maximal dose to optic apparatus | |
| Median [range]; mean ± SD | 8.50 [2-23]; 8.63 ± 2.08 |
| Maximal dose to optic nerve (Gy) | |
| Median [range]; mean ± SD | 8.30 [2-19]; 8.25 ± 1.99 |
| Maximal dose to optic chiasm (Gy) | |
| Median [range]; mean ± SD | 7.90 [1-21]; 7.49 ± 2.06 |
| Maximal dose to optic tract (Gy) | |
| Median [range]; mean ± SD | 6.30 [0-15]; 6.00 ± 2.02 |
SD, standard deviation.
Tumor Control
Post-SRS imaging follow-up was available for 426 patients and median duration was 56 mo (range: 3-239 months) (Table 3). The majority of perioptic meningiomas remained stable or regressed, and 33 patients (8%) experienced radiological progression at median interval from SRS to progression of 94 mo (range: 12-233 mo).
TABLE 3.
Imaging Follow-up
| Characteristic | |
|---|---|
| Available data, n (%) | 426 |
| Imaging follow-up duration (mo) | |
| Median [range]; mean ± SD | 55.6 [3.15-239]; 65.19 ± 43.02 |
| Imaging outcomes at last follow-up, n (%) | |
| Stable | 215 (51%) |
| Regression | 178 (42%) |
| Progression | 33 (8%) |
| Time to progression (mo) | |
| Median [range]; mean ± SD | 94.07 [11.74-233.34]; 103.74 ± 56.34 |
| Time to regression (mo) | |
| Median [range]; mean ± SD | 64.85 [3.18-191.18]; 70.24 ± 40.87 |
SD, standard deviation.
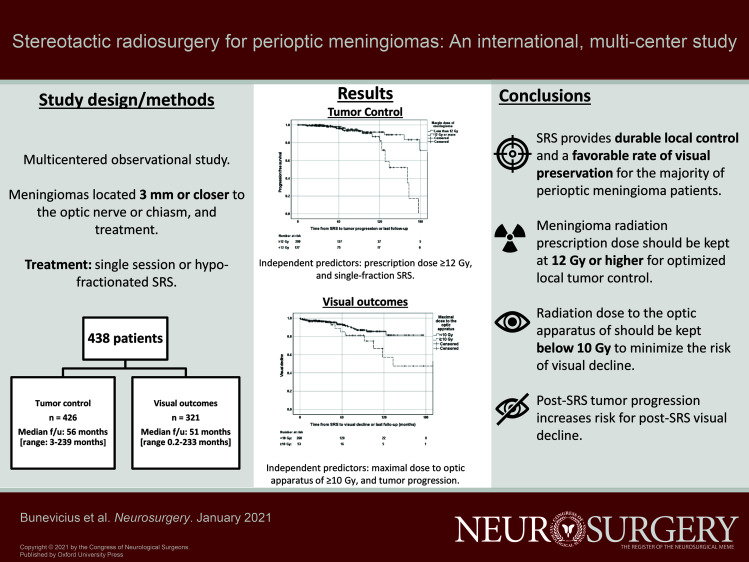
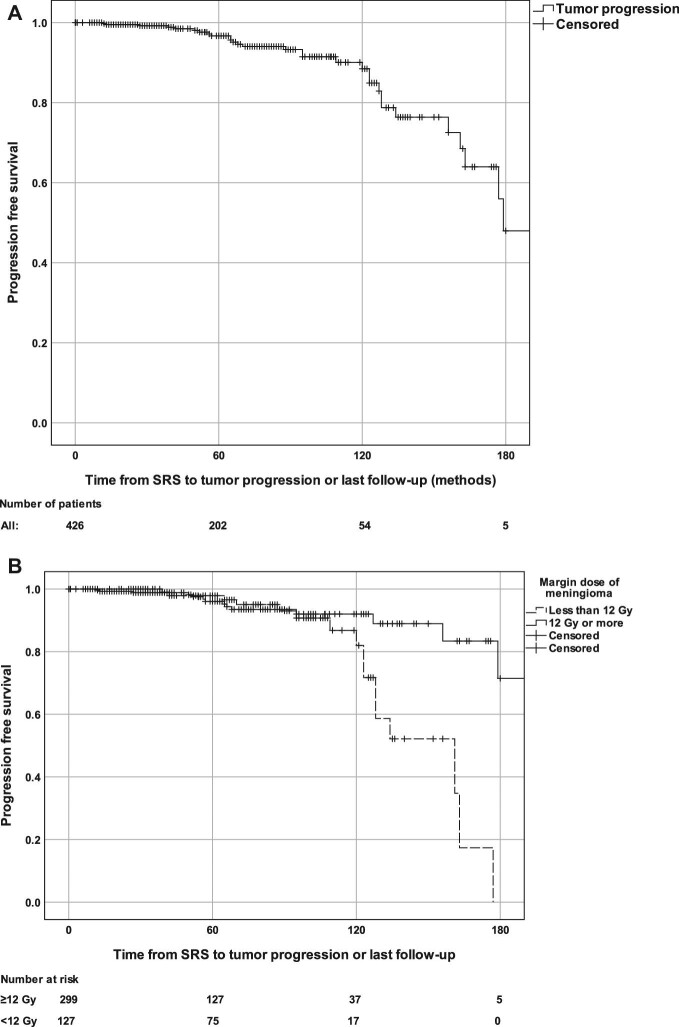
Actuarial PFS rates at 5 and 10 yr after the SRS were 96% and 89%, respectively (Figure 2A). PFS was significantly shorter in patients treated with a tumor prescription dose of >12 Gy when compared to ≥12 Gy (P = .024; Figure 2B). In univariate Cox regression analysis, tumor prescription dose of ≥12 Gy (vs <12 Gy) (P = .003), BED (P = .002), the use of single-fraction SRS (vs hypofractionated SRS) (P = .048), and a greater maximal tumor radiation dose (P = .015) were associated with decreased risk of tumor progression, while pre-SRS radiation therapy (P = .021) and pre-SRS visual deficit (P = .031) were associated with increased perioptic meningioma progression risk (Table 4). In multivariate Cox regression analysis, prescription dose of ≥12 Gy (HR: 0.310 95% CI [0.141-0.679], P = .003), BED of ≥60 Gy (HR: 0.310 95% CI [0.141-0.679], P = .003), and single-fraction SRS (HR: 0.078 95% CI [0.016-0.395], P = .002) remained associated with lower risk for post-SRS progression. When considering only patients who underwent single-session radiosurgery (n = 405), in multivariate Cox regression analysis prescription dose of ≥12 Gy (HR: 0.323 95% CI [0.147-0.708], P = .005), BED of ≥60 Gy (HR: 0.323 95% CI [0.147-0.708], P = .005), and previous radiotherapy (HR: 24.603 95% CI [2.735-221.3], P = .004) remained associated with lower risk for tumor progression.
FIGURE 2.
Kaplan-Meier analysis of progression-free survival in the total cohort A and stratified by margin dose of 12 Gy B.
TABLE 4.
Predictors of Progression-Free Survival (n = 429)
| Predictors | Univariate | Multivariate |
|---|---|---|
| Margin tumor prescription dose ≥12 Gy (vs <12 Gy) | HR = 0.306 95% CI [0.140-0.670], P = .003 | HR = 0.310 95% CI [0.141-0.679], P = .003a |
| BED ≥60 Gy (vs <60 Gy) | HR = 0.291 95% CI [0.135-0.624], P = .002 | HR = 0.310 95% CI [0.141-0.679], P = .003a |
| Single-fraction SRS (vs hypofractionated) | HR = 0.148 95% CI [0.032-0.692], P = .048 | HR = 0.078 95% CI [0.016-0.395], P = .002 |
| Maximal tumor dose (Gy) | HR = 0.909 95% CI [0.842-0.981], P = .015 | P = .076 |
| Dose to optic apparatus (Gy) | P = .231 | – |
| Distance to optic apparatus (mm) | P = .755 | – |
| Tumor volume (cm3) | P = .854 | – |
| Pre-SRS radiation therapy | HR = 11.222, 95% CI [1.1430-88.060], P = .021 | P = .063 |
| Pre-SRS surgery | P = .371 | – |
| Pre-SRS visual deficit | HR = 2.560 [1.087-6.028], P = .031 | P = .133 |
| Age (yr) | P = .741 | – |
| Gender | P = .793 | – |
SD, standard deviation.
a– seprate multivariate Cox regression models adjusted for signifficant predictors in univariate analyses.
Visual Outcomes
Post-SRS visual outcomes were evaluated in 321 patients, with median duration of post-SRS visual follow-up of 51 mo (range: 0.2-233 mo) (Table 5). In the majority of patients visual status at last follow-up visit was not changed (60%) or improved (30%) when compared to pre-SRS function. In total, 4 (1%) patients with pre-SRS visual decline experienced new blindness in the ipsilateral eye after the SRS. A total of 31 (10%) patients experienced visual function decline after SRS at median interval from SRS of 52 mo (range: 3-152 mo). A total of 13 of those patients (42%) experienced tumor radiological progression while the remaining did not. Pre-SRS visual impairment (absent vs present) was associated with visual function stabilization (94% vs 45%), improvement (0% vs 44%), and decline (13% vs 11%) at last post-SRS follow-up (P < .001).
TABLE 5.
Visual Outcomes
| Visual follow-up | |
|---|---|
| N | 321 |
| Follow-up duration (mo) | |
| Median [range]; mean ± SD | 50.86 [0.2-233.34]; 60.23 ± 4.58 |
| Outcome at last follow up, n (%) | |
| All patients (n = 321) | |
| No change | 196 (61%) |
| Improved | 94 (29%) |
| Declined | 31 (10%) |
| Pre-SRS visual impairment (n = 212) | |
| No change | 95 (45%) |
| Improved | 93 (44%) |
| Declined | 24 (11%) |
| No pre-SRS visual impairment (n = 109) | |
| No change | 102 (94%) |
| Improved | 0 (0%) |
| Declined | 7 (13%) |
| Time to visual declinea | |
| Median [range]; mean ± SD | 52.00 [0.2-133.0]; 49.92 ± 39.42 |
| Time to visual improvementa | |
| Median [range]; mean ± SD | 54.62 [3-151.7]; 62.34 ± 37.47 |
SD, standard deviation.
aTime to vision change or last formal visual field testing or clinical follow-up.
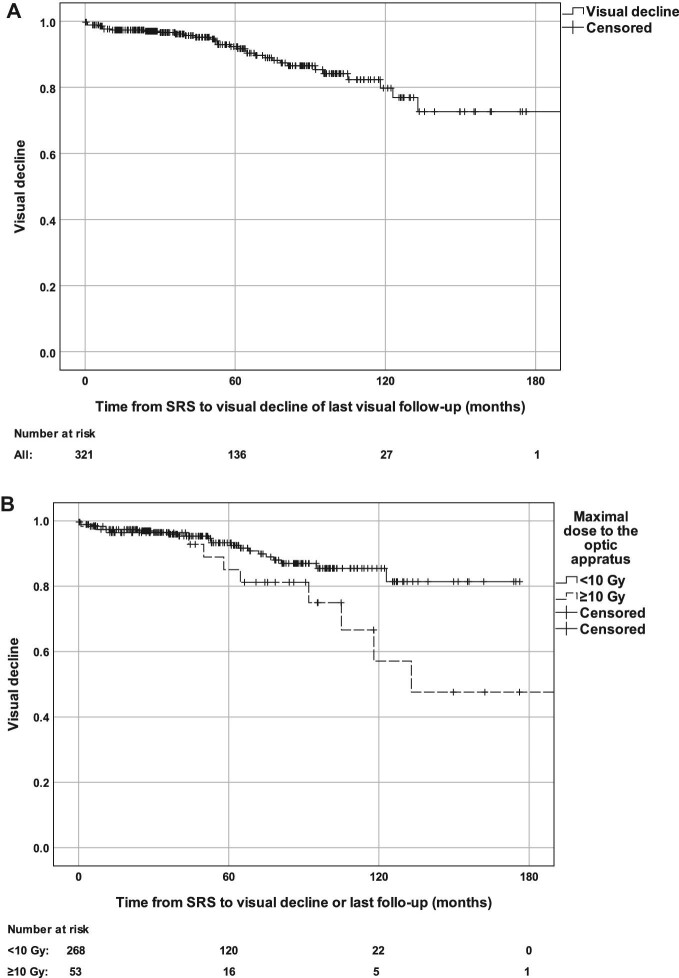
Actuarial rates of visual decline at 5 and 10 yr following SRS were 9% and 21%, respectively (Figure 3A). Patients who received a maximal dose of ≥10 Gy vs <10 Gy to the optic apparatus had greater actuarial visual decline rates at 5 yr (18% vs 7%) and 10 yr (46% vs 15%) after the SRS (P = .034) (Figure 3B). Maximal radiation dose to the optic apparatus of ≥10 Gy (P = .03), maximal BED of >36 Gy (P = .03), and tumor progression (P < .001) were associated with greater risk of visual decline (Table 6). In multivariate Cox regression, maximal dose to optic apparatus of ≥10 Gy (HR = 2.370 95% CI [1.086-5.172], P = .03), BED of >36 Gy (HR = 2.370 95% CI [1.086-5.172], P = .03), and tumor progression (HR = 4.340 95% CI [2.070-9.097], P < .001) were associate with greater risk for post-SRS visual decline. In patients who underwent single-session SRS (n = 304), maximal dose to the optic apparatus of ≥10 Gy (HR = 2.318 95% CI [1.062-5.058], P = .04), BED to the optic apparatus of >36 Gy (HR = 2.318 95% CI [1.062-5.058], P = .04), and tumor progression (HR = 2.322 95% CI [2.062-9.061], P < .001) remained associated with visual decline.
FIGURE 3.
Visual decline rate in the total cohort A and stratified by maximal dose to the optic apparatus of 10 Gy B.
TABLE 6.
Predictors of Visual Outcomes
| Univariate | Multivariate | |
|---|---|---|
| Visual decline | ||
| Maximal dose to optic apparatus ≥10 Gy (vs <10 Gy) | HR = 2.268 95% CI [1.044-4.927], P = .039 | HR = 2.370 95% CI [1.086-5.172], P = .03a |
| Maximal BED to the optic apparatus >36 Gy (vs ≤36 Gy) | HR = 2.373 95% CI [1.086-5.181], P = .030 | HR = 2.370 95% CI [1.086-5.172], P = .03a |
| Single-fraction SRS (vs hypofractionated) | P = .564 | – |
| Nearest distance to optic apparatus (mm) | P = .860 | – |
| In contact with optic apparatus | P = .857 | – |
| Pre-SRS surgery | P = .140 | – |
| Pre-SRS radiation therapy | P = .144 | – |
| Pre-SRS visual impairment | P = .119 | – |
| Treatment volume (cm3) | P = .332 | – |
| Tumor radiological progression | HR = 4.186 95% CI [2.012-8.709], P < .001 | HR = 4.340 95% CI [2.070-9.097], P < .001 |
SD, standard deviation.
aSeprate multivariate Cox regression models adjusted for signifficant predictors in univariate analyses.
DISCUSSION
Key Results
Treatment with SRS was associated with durable control of perioptic meningiomas in the majority of patients. Marginal prescription dose of 12 Gy or greater and the use of single-fraction SRS were associated with better control of perioptic meningiomas. Visual status remained stable or improved in the vast majority of patients. Visual decline was uncommon after SRS, and was associated with a maximal dose to the optic apparatus of 10 Gy or greater and radiological tumor progression.
Interpretation
During a median post-SRS imaging follow-up of 56 mo, 8% of patients experienced radiological progression. In the majority of patients, perioptic meningiomas remained stable or regressed. Long-term control rate of perioptic meningiomas in our multi-institutional series was comparable to previously published SRS experiences for perioptic meningiomas.7,13 CyberKnife (Accuray) cooperative study that included 167 perioptic meningiomas treated with multisession SRS (25 Gy in 5 fractions) reported 8-yr PFS of 90%.7 Adler et al treated 49 perioptic tumors (27 meningiomas) with multisession CyberKnife SRS, and 94% of patients experienced disease stabilization or regression during a median imaging follow-up of 45 mo, with 2 meningiomas progressing close to or within the treatment field.13 Long-term local control rates of perioptic meningiomas treated with SRS appears to be similar to local control rates of intracranial meningiomas of other anatomic locations that often exceed 85%.25,31 SRS offers reasonable long-term local control of perioptic meningiomas and should be considered for tumors residing in this surgically challenging anatomic location.
Single-session SRS and tumor prescription dose of 12 Gy or more were independently associated with better local control rate of perioptic meningiomas, underscoring the importance of adequate tumor dose prescription to achieve long-term tumor control. Indeed, adequate radiation dose is important for optimized local control of intracranial meningiomas.32-34 Commonly used margin doses for intracranial meningiomas are from 12 Gy to 16 Gy for WHO grade I meningiomas, 16 Gy to 20 Gy for WHO grade II meningiomas, and 18 Gy to 24 Gy for WHO grade III tumors.31,35-37 In our series, pathology results were available for 29% of the patients and a vast majority of meningiomas with available pathology results were WHO grade I tumors. Prescribed radiation dose of at least 12 Gy to the tumor margin should be attempted for perioptic meningioma, as long as doses to the optic apparatus remain below 10 Gy.
Differences in cell response to different irradiation doses have been documented.38,39 In our series, single-session SRS was associated with superior tumor control when compared to hypofractionated (2-5 fractions) SRS. Hypofractionated SRS allows preservation of surrounding normal tissues13,40,41 and excellent local tumor control with comparable safety profile.7,13,36,42,13 However, the number of patients treated with hypofractionated SRS in our cohort was small (n = 32) thus limiting generalizability of our findings to hypofractionated SRS.
Visual function remained stable or improved in the majority of patients, and only 10% of patients experienced post-SRS visual decline at median interval of 52 mo after the SRS. Visual outcomes in our multi-institutional cohort were comparable to previously reported visual outcomes of perioptic meningiomas treated using multisession SRS.7,13 Fractionated External Beam Radiation Therapy (EBRT) is traditionally consider for management of lesions in close contact with the optic apparatus,43 and allows good local control rates of intracranial meningiomas that exceed 84%.44-46 However, treatment plans of EBRT are usually less conformal when compared to the SRS, and can be therefore association with irradiation of the optic apparatus and other surrounding brain and intracranial structures, which can subsequently increase risk for long-term adverse events.47-51 In our series, the vast majority of perioptic meningiomas were treated using single-session SRS, indicating that it is the preferred treatment approach in the majority of participating centers. We did not find an association of SRS fractionation schedule with visual outcomes; however, hypofractionated SRS was associated with inferior local tumor control rate, suggesting that single-session SRS can potentially allow superior local tumor control without increasing risk of optic neuropathy, and should be therefore considered in the context of tumor volume, pre-SRS visual function and other relevant tumor and patient related parameters.
Maximal radiation dose to the optic apparatus of 10 Gy or more and tumor progression emerged as important independent predictors of visual deterioration. These findings underscore the importance of careful SRS dose planning and radiation dose consideration for perioptic meningiomas and adjacent optic structures because inadequate tumor prescription dose (<12 Gy) can be associated with suboptimal long-term local tumor control that can subsequently impair visual function. Our findings confirm findings from prior studies showing that irradiation of the optic nerve with doses less than 10 Gy is safe and associated with minimal risk of optic neuropathy, with exponential increasing risk with radiation doses exceeding 10 Gy and 12 Gy.18,19,52 Tumor progression emerged as another important predictor of visual decline underscoring the importance of adequate tumor control in order to preserve visual function. Careful selection of radiation planning strategies (eg, use of curved posts) is imperative to optimize radiation dose to perioptic meningiomas and adjacent optic apparatus.
Limitations
Limitations of this study should be acknowledged. Approximately 2/3 of our patients did not have pathological confirmation and were diagnosed based on imaging findings. The possible impact of technological improvements in GK device and planning software, and learning curve is limited because all patients were managed at high-volume SRS centers and according to the prevailing guidelines.53 BED was calculated using α/β ratio of 3 Gy, which is historically used for late responding tissues such as meningiomas; even small variations of α/β ratio could have significant effect on BED.26,27,54 Objective assessment of visual function was not made in all patients at all times, thereby potentially limiting the validity of this component of the analysis. On the other hand, large sample size and long imaging and visual follow-up fortify reliability of our findings.7,13
Generalizability
The majority of our patients were treated with a single-session SRS using GKRS thus limiting generalizability of our experience to other radiotherapy techniques.
CONCLUSION
SRS provides durable local control and a favorable rate of visual preservation for the majority of perioptic meningioma patients. Margin meningioma radiation dose of ≥12 Gy and single-fraction SRS are associated with improved tumor control, while radiation dose to the optic apparatus of ≥10 Gy and tumor progression are associated with increased risk for post-SRS visual decline.
Funding
This study did not receive any funding or financial support.
Disclosures
Dr Liscak is a consultant of Elekta. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Cifarelli receives speaking honoraria from Carl Zeiss Meditech AG.
Contributor Information
Adomas Bunevicius, Department of Neurosurgery, University of Virginia, Charlottesville, Virginia.
Rithika Kormath Anand, Department of Neurosurgery, University of Virginia, Charlottesville, Virginia.
Mohanad Suleiman, Department of Neurosurgery, University of Virginia, Charlottesville, Virginia.
Ahmed M Nabeel, Gamma Knife Center Cairo, Nasser Institute Hospital, Cairo, Egypt; Neurosurgery Department, Benha University, Qalubya, Egypt.
Wael A Reda, Gamma Knife Center Cairo, Nasser Institute Hospital, Cairo, Egypt; Neurosurgery Department, Ain Shams University, Cairo, Egypt.
Sameh R Tawadros, Gamma Knife Center Cairo, Nasser Institute Hospital, Cairo, Egypt; Neurosurgery Department, Ain Shams University, Cairo, Egypt.
Khaled Abdelkarim, Gamma Knife Center Cairo, Nasser Institute Hospital, Cairo, Egypt; Clinical Oncology Department, Ain Shams University, Cairo, Egypt.
Amr M N El-Shehaby, Gamma Knife Center Cairo, Nasser Institute Hospital, Cairo, Egypt; Neurosurgery Department, Ain Shams University, Cairo, Egypt.
Reem M Emad, Gamma Knife Center Cairo, Nasser Institute Hospital, Cairo, Egypt; Radiation Oncology Department, National Cancer Institute, Cairo University, Giza, Egypt.
Tomas Chytka, Stereotactic and Radiation Neurosurgery Department, Na Homolce Hospital, Prague, Czech Republic.
Roman Liscak, Stereotactic and Radiation Neurosurgery Department, Na Homolce Hospital, Prague, Czech Republic.
Kimball Sheehan, Department of Neurosurgery, University of Virginia, Charlottesville, Virginia.
Darrah Sheehan, Department of Neurosurgery, University of Virginia, Charlottesville, Virginia.
Marco Perez Caceres, Department of Neurosurgery, Université de Sherbrooke, Centre de recherche du CHUS, Sherbrooke, Québec, Canada.
David Mathieu, Department of Neurosurgery, Université de Sherbrooke, Centre de recherche du CHUS, Sherbrooke, Québec, Canada.
Cheng-chia Lee, Department of Neurosurgery, Neurological Institute, Taipei Veteran General Hospital, Taipei, Taiwan; School of Medicine, National Yang-Ming University, Taipei, Taiwan.
Huai-che Yang, Department of Neurosurgery, Neurological Institute, Taipei Veteran General Hospital, Taipei, Taiwan; School of Medicine, National Yang-Ming University, Taipei, Taiwan.
Piero Picozzi, Department of Neurosurgery, Humanitas Clinical and Research Center – IRCCS, Milan, Italy.
Andrea Franzini, Department of Neurosurgery, Humanitas Clinical and Research Center – IRCCS, Milan, Italy.
Luca Attuati, Department of Neurosurgery, Humanitas Clinical and Research Center – IRCCS, Milan, Italy.
Herwin Speckter, Centro Gamma Knife Dominicano and CEDIMAT Radiology Department, Santo Domingo, Dominican Republic.
Jeremy Olivo, Centro Gamma Knife Dominicano and CEDIMAT Radiology Department, Santo Domingo, Dominican Republic.
Samir Patel, Division of Radiation Oncology, Department of Oncology, University of Alberta, Edmonton, Canada.
Christopher P Cifarelli, Department of Neurosurgery, West Virginia University, Morgantown, West Virginia; Department of Radiation Oncology, West Virginia University, Morgantown, West Virginia.
Daniel T Cifarelli, Department of Neurosurgery, West Virginia University, Morgantown, West Virginia.
Joshua D Hack, Department of Radiation Oncology, West Virginia University, Morgantown, West Virginia.
Ben A Strickland, Department of Neurosurgery, University of Southern California, Los Angeles, California.
Gabriel Zada, Department of Neurosurgery, University of Southern California, Los Angeles, California.
Eric L Chang, Department of Radiation Oncology, University of Southern California, Los Angeles, California.
Kareem R Fakhoury, Department of Radiation Oncology, University of Colorado, Denver, Colorado.
Chad G Rusthoven, Department of Radiation Oncology, University of Colorado, Denver, Colorado.
Ronald E Warnick, Gamma Knife Center, Jewish Hospital, Mayfield Clinic, Cincinnati, Ohio.
Jason Sheehan, Department of Neurosurgery, University of Virginia, Charlottesville, Virginia.
Neurosurgery Speaks! Audio abstracts available for this article at www.neurosurgery-online.com.
REFERENCES
- 1.Eddleman CS, Liu JK. Optic nerve sheath meningioma: current diagnosis and treatment. Neurosurg Focus. 2007;23(5):E4. [DOI] [PubMed] [Google Scholar]
- 2.Risi P, Uske A, de Tribolet N. Meningiomas involving the anterior clinoid process. Br J Neurosurg. 1994;8(3):295-305. [DOI] [PubMed] [Google Scholar]
- 3.Hayhurst C, Teo C. Tuberculum sella meningioma. Otolaryngol Clin North Am. 2011;44(4):953-963. [DOI] [PubMed] [Google Scholar]
- 4.Al-Mefty O. Clinoidal meningiomas. J Neurosurg. 1990;73(6):840-849. [DOI] [PubMed] [Google Scholar]
- 5.Talacchi A, Hasanbelliu A, D’Amico Aet al. Long-term follow-up after surgical removal of meningioma of the inner third of the sphenoidal wing: outcome determinants and different strategies. Neurosurg Rev. 2020;43(1):109-117. [DOI] [PubMed] [Google Scholar]
- 6.Magill ST, Morshed RA, Lucas C-HGet al. Tuberculum sellae meningiomas: grading scale to assess surgical outcomes using the transcranial versus transsphenoidal approach. Neurosurg Focus. 2018;44(4):E9. [DOI] [PubMed] [Google Scholar]
- 7.Marchetti M, Conti A, Beltramo Get al. Multisession radiosurgery for perioptic meningiomas: medium-to-long term results from a CyberKnife cooperative study. J Neurooncol. 2019;143(3):597-604. [DOI] [PubMed] [Google Scholar]
- 8.Pechlivanis I, Wawrzyniak S, Engelhardt M, Schmieder K. Evidence level in the treatment of meningioma with focus on the comparison between surgery versus radiotherapy. A review. J Neurosurg Sci. 2011;55(4):319-328. [PubMed] [Google Scholar]
- 9.Schick U, Dott U, Hassler W. Surgical management of meningiomas involving the optic nerve sheath. J Neurosurg. 2004;101(6):951-959. [DOI] [PubMed] [Google Scholar]
- 10.Bassiouni H, Asgari S, Sandalcioglu IE, Seifert V, Stolke D, Marquardt G. Anterior clinoidal meningiomas: functional outcome after microsurgical resection in a consecutive series of 106 patients: clinical article. J Neurosurg. 2009;111(5):1078-1090. [DOI] [PubMed] [Google Scholar]
- 11.Giammattei L, Starnoni D, Levivier M, Messerer M, Daniel RT. Surgery for clinoidal meningiomas: case series and meta-analysis of outcomes and complications. World Neurosurg. 2019;129:e700-e717. [DOI] [PubMed] [Google Scholar]
- 12.Taha ANM, Erkmen K, Dunn IF, Pravdenkova S, Al-Mefty O. Meningiomas involving the optic canal: pattern of involvement and implications for surgical technique. Neurosurg Focus. 2011;30(5):E12. [DOI] [PubMed] [Google Scholar]
- 13.Adler JR, Gibbs IC, Puataweepong P, Chang SD. Visual field preservation after multisession CyberKnife radiosurgery for perioptic lesions. Neurosurgery. 2006;59(2):244-254; discussion 244-254. [DOI] [PubMed] [Google Scholar]
- 14.Dufour H, Muracciole X, Métellus P, Régis J, Chinot O, Grisoli F. Long-term tumor control and functional outcome in patients with cavernous sinus meningiomas treated by radiotherapy with or without previous surgery: is there an alternative to aggressive tumor removal? Neurosurgery. 2001;48(2):285-294; discussion 294-296. [DOI] [PubMed] [Google Scholar]
- 15.Cohen-Inbar O, Lee C, Schlesinger D, Xu Z, Sheehan JP. Long-term results of stereotactic radiosurgery for skull base meningiomas. Neurosurgery. 2016;79(1):58-68. [DOI] [PubMed] [Google Scholar]
- 16.Kondziolka D, Mathieu D, Lunsford LDet al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62(1):53-60; discussion 58-60. [DOI] [PubMed] [Google Scholar]
- 17.Minniti G, Amichetti M, Enrici RM. Radiotherapy and radiosurgery for benign skull base meningiomas. Radiat Oncol. 2009;4(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stafford SL, Pollock BE, Leavitt JAet al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55(5):1177-1181. [DOI] [PubMed] [Google Scholar]
- 19.Milano MT, Grimm J, Soltys SGet al. Single- and multi-fraction stereotactic radiosurgery dose tolerances of the optic pathways. Int J Radiat Oncol Biol Phys. published online: 2018. (doi:10.1016/j.ijrobp.2018.01.053). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conti A, Pontoriero A, Midili Fet al. CyberKnife multisession stereotactic radiosurgery and hypofractionated stereotactic radiotherapy for perioptic meningiomas: intermediate-term results and radiobiological considerations. Springerplus. 2015;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bunevicius A, Kano H, Lee C-Cet al. Early versus late Gamma Knife radiosurgery for Cushing's disease after prior resection: results of an international, multicenter study. J Neurosurg. published online: February 21, 2020. (doi:10.3171/2019.12.JNS192836). [DOI] [PubMed] [Google Scholar]
- 22.El-Shehaby AMN, Reda WA, Abdel Karim KM, Emad Eldin RM, Nabeel AM. Single-session Gamma Knife radiosurgery for optic pathway/hypothalamic gliomas. J Neurosurg. 2016;125(Suppl_1):50-57. [DOI] [PubMed] [Google Scholar]
- 23.Kim M-S, Park K, Kim JH, Kim Y-D, Lee J-I. Gamma Knife radiosurgery for orbital tumors. Clin Neurol Neurosurg. 2008;110(10):1003-1007. [DOI] [PubMed] [Google Scholar]
- 24.Akyoldaş G, Hergünsel ÖB, Yılmaz M, Şengöz M, Peker S. Gamma Knife radiosurgery for anterior clinoid process meningiomas: a series of 61 consecutive patients. World Neurosurg. 2020;133:e529-e534. [DOI] [PubMed] [Google Scholar]
- 25.Sheehan JP, Starke RM, Kano Het al. Gamma Knife radiosurgery for sellar and parasellar meningiomas: a multicenter study. J Neurosurg. 2014;120(6):1268-1277. [DOI] [PubMed] [Google Scholar]
- 26.Vernimmen F, Slabbert JP.. Assessment of the alpha/beta ratios for arteriovenous malformations, meningiomas, acoustic neuromas, and the optic chiasma. Int J Radiat Biol. 2010;86(6):486-498. [DOI] [PubMed] [Google Scholar]
- 27.Shrieve DC, Hazard L, Boucher K, Jensen RL. Dose fractionation in stereotactic radiotherapy for parasellar meningiomas: radiobiological considerations of efficacy and optic nerve tolerance. J Neurosurg. 2004;101(Suppl 3):390-395. [PubMed] [Google Scholar]
- 28.Yang D-Y, Sheehan J, Liu Y-Set al. Analysis of factors associated with volumetric data errors in Gamma Knife radiosurgery. Stereotact Funct Neurosurg. 2009;87(1):1-7. [DOI] [PubMed] [Google Scholar]
- 29.Chukwueke UN, Wen PY.. Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. 2019;8(1):CNS28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw E, Scott C, Souhami Let al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291-298. [DOI] [PubMed] [Google Scholar]
- 31.Sheehan JP, Williams BJ, Yen CP. Stereotactic radiosurgery for WHO grade I meningiomas. J Neurooncol. 2010;99(3):407-416. [DOI] [PubMed] [Google Scholar]
- 32.Ganz JC, Backlund E-O, Thorsen FA. The results of Gamma Knife surgery of meningiomas, related to size of tumor and dose. Stereotact Funct Neurosurg. 1993;61(Suppl 1):23-29. [DOI] [PubMed] [Google Scholar]
- 33.Meniai-Merzouki F, Bernier-Chastagner V, Geffrelot Jet al. Hypofractionated Stereotactic Radiotherapy for patients with intracranial meningiomas: impact of radiotherapy regimen on local control. Sci Rep. 2018;8(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vera E, Iorgulescu JB, Raper DMSet al. A review of stereotactic radiosurgery practice in the management of skull base meningiomas. J Neurol Surg B Skull Base. 2014;75(3):152-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee C-C, Trifiletti DM, Sahgal Aet al. Stereotactic Radiosurgery for benign (World Health Organization grade I) cavernous sinus meningiomas—International Stereotactic Radiosurgery Society (ISRS) practice guideline: a systematic review. Neurosurgery. 2018;83(6):1128-1142. [DOI] [PubMed] [Google Scholar]
- 36.Cohen-Inbar O, Lee C-C, Sheehan JP. The contemporary role of stereotactic radiosurgery in the treatment of meningiomas. Neurosurg Clin N Am. 2016;27(2):215-228. [DOI] [PubMed] [Google Scholar]
- 37.Ding D, Starke RM, Hantzmon J, Yen C-P, Williams BJ, Sheehan JP. The role of radiosurgery in the management of WHO grade II and III intracranial meningiomas. Neurosurg Focus. 2013;35(6):E16. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Barros M, Paris F, Cordon-Cardo Cet al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155-1159. [DOI] [PubMed] [Google Scholar]
- 39.Nagle PW, Hosper NA, Barazzuol Let al. Lack of DNA damage response at low radiation doses in adult stem cells contributes to organ dysfunction. Clin Cancer Res. 2018;24(24):6583-6593. [DOI] [PubMed] [Google Scholar]
- 40.Barnett GH, Linskey ME, Adler JRet al. Stereotactic radiosurgery—an organized neurosurgery-sanctioned definition. J Neurosurg. 2007;106(1):1-5. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen JH, Chen C-J, Lee C-Cet al. Multisession Gamma Knife radiosurgery: a preliminary experience with a noninvasive, relocatable frame. World Neurosurg. 2014;82(6):1256-1263. [DOI] [PubMed] [Google Scholar]
- 42.Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18(4):215-222. [DOI] [PubMed] [Google Scholar]
- 43.Bloch O, Sun M, Kaur G, Barani IJ, Parsa AT. Fractionated radiotherapy for optic nerve sheath meningiomas. J Clin Neurosci. 2012;19(9):1210-1215. [DOI] [PubMed] [Google Scholar]
- 44.Onodera S, Aoyama H, Katoh Net al. Long-term outcomes of fractionated stereotactic radiotherapy for intracranial skull base benign meningiomas in single institution. Jpn J Clin Oncol. 2011;41(4):462-468. [DOI] [PubMed] [Google Scholar]
- 45.Milker-Zabel S, Zabel A, Schulz-Ertner D, Schlegel W, Wannenmacher M, Debus J. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 2005;61(3):809-816. [DOI] [PubMed] [Google Scholar]
- 46.Soldà F, Wharram B, De Ieso PB, Bonner J, Ashley S, Brada M. Long-term efficacy of fractionated radiotherapy for benign meningiomas. Radiother Oncol. 2013;109(2):330-334. [DOI] [PubMed] [Google Scholar]
- 47.Kiehna EN, Mulhern RK, Li C, Xiong X, Merchant TE. Changes in attentional performance of children and young adults with localized primary brain tumors after conformal radiation therapy. J Clin Oncol. 2006;24(33):5283-5290. [DOI] [PubMed] [Google Scholar]
- 48.Jalali R, Gupta T, Goda JSet al. Efficacy of stereotactic conformal radiotherapy vs conventional radiotherapy on benign and low-grade brain tumors: a randomized clinical trial. JAMA Oncol. 2017;3(10):1368-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haddy N, Mousannif A, Tukenova Met al. Relationship between the brain radiation dose for the treatment of childhood cancer and the risk of long-term cerebrovascular mortality. Brain. 2011;134(5):1362-1372. [DOI] [PubMed] [Google Scholar]
- 50.Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OMet al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(8):2330-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung CS, Yock TI, Nelson K, Xu Y, Keating NL, Tarbell NJ. Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys. 2013;87(1):46-52. [DOI] [PubMed] [Google Scholar]
- 52.Leavitt JA, Stafford SL, Link MJ, Pollock BE. Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2013;87(3):524-527. [DOI] [PubMed] [Google Scholar]
- 53.Bunevicius A, Sheehan D, Lee Vance M, Schlesinger D, Sheehan JP. Outcomes of Cushing's disease following Gamma Knife radiosurgery: effect of a center's growing experience and era of treatment. J Neurosurg. published online:January 31, 2020. (doi:10.3171/2019.12.JNS192743). [DOI] [PubMed] [Google Scholar]
- 54.Speckter H, Santana J, Miches Iet al. Assessment of the alpha/beta ratio of the optic pathway to adjust hypofractionated stereotactic radiosurgery regimens for perioptic lesions. J Radiat Oncol. 2019;8(3):279-289. [Google Scholar]