This nonrandomized clinical trial evaluates the treatment outcomes and safety of mobocertinib treatment in patients with previously treated EGFR exon 20 insertion–positive metastatic non–small cell lung cancer.
Key Points
Question
Does mobocertinib have activity in patients with EGFR exon 20 insertion (EGFRex20ins)–positive metastatic non–small cell lung cancer (mNSCLC) previously treated with platinum-based chemotherapy?
Findings
In this nonrandomized clinical trial, mobocertinib showed antitumor activity in patients with platinum-pretreated EGFRex20ins-positive mNSCLC, with a confirmed objective response rate of 28%, median duration of response of 17.5 months, and median progression-free survival of 7.3 months by independent review committee assessments. The safety profile was characterized by gastrointestinal and cutaneous adverse events, which were largely manageable.
Meaning
Mobocertinib has a favorable risk-benefit profile in patients with previously treated EGFRex20ins-positive mNSCLC.
Abstract
Importance
Metastatic non–small cell lung cancer (mNSCLC) with EGFR exon 20 insertion (EGFRex20ins) mutations is associated with a poor prognosis. Mobocertinib is an oral tyrosine kinase inhibitor designed to selectively target EGFRex20ins mutations.
Objective
To evaluate treatment outcomes and safety of mobocertinib in patients with previously treated EGFRex20ins-positive mNSCLC.
Design, Setting, and Participants
This 3-part, open-label, phase 1/2 nonrandomized clinical trial with dose-escalation/dose-expansion cohorts (28 sites in the US) and a single-arm extension cohort (EXCLAIM; 39 sites in Asia, Europe, and North America) was conducted between June 2016 and November 2020 (data cutoff date). The primary analysis populations were the platinum-pretreated patients (PPP) cohort and the EXCLAIM cohort. The PPP cohort included 114 patients with platinum-pretreated EGFRex20ins-positive mNSCLC who received mobocertinib 160 mg once daily from the dose-escalation (n = 6), dose-expansion (n = 22), and EXCLAIM (n = 86) cohorts. The EXCLAIM cohort included 96 patients with previously treated EGFRex20ins-positive mNSCLC (10 were not platinum pretreated and thus were excluded from the PPP cohort).
Interventions
Mobocertinib 160 mg once daily.
Main Outcomes and Measures
The primary end point of the PPP and EXCLAIM cohorts was confirmed objective response rate (ORR) assessed by independent review committee (IRC). Secondary end points included confirmed ORR by investigator, duration of response, progression-free survival, overall survival, and safety.
Results
Among the PPP (n = 114) and EXCLAIM (n = 96) cohorts, the median (range) age was 60 (27-84) and 59 (27-80) years, respectively; most patients were women (75 [66%] and 62 [65%], respectively) and of Asian race (68 [60%] and 66 [69%], respectively). At data cutoff, median follow-up was 14.2 months in the PPP cohort (median 2 prior anticancer regimens; 40 [35%] had baseline brain metastases), with confirmed ORR of 28% (95% CI, 20%-37%) by IRC assessment and 35% (95% CI, 26%-45%) by investigator assessment; median duration of response by IRC assessment was 17.5 months (95% CI, 7.4-20.3). Median progression-free survival by IRC assessment was 7.3 months (95% CI, 5.5-9.2). Median overall survival was 24.0 months (95% CI, 14.6-28.8). In the EXCLAIM cohort, median follow-up was 13.0 months, with confirmed ORR by IRC assessment of 25% (95% CI, 17%-35%) and by investigator assessment of 32% (95% CI, 23%-43%). The most common treatment-related adverse events were diarrhea and rash.
Conclusions and Relevance
In this open-label, phase 1/2 nonrandomized clinical trial, mobocertinib was associated with clinically meaningful benefit in patients with previously treated EGFRex20ins-positive mNSCLC, with a manageable safety profile.
Trial Registration
ClinicalTrials.gov Identifier: NCT02716116
Introduction
EGFR exon 20 insertion (EGFRex20ins) mutations occur in 4% to 12% of EGFR-mutated non–small cell lung cancers (NSCLCs)1,2,3,4,5 and approximately 2% of all NSCLCs.4 Most patients with EGFRex20ins-positive metastatic NSCLC (mNSCLC) receive first-line platinum-based chemotherapy but typically develop progressive disease (PD) within 6 months.6,7,8 The EGFR TKIs, afatinib, erlotinib, and gefitinib, have limited activity in patients with EGFRex20ins-positive mNSCLC; in line-unspecified settings, their response rate is approximately 10% and median progression-free survival (PFS) is 1 to 3 months.9 Immune checkpoint inhibitors, alone or in combination with chemotherapy in a mixture of settings, have response rates of 0% to 25% and typical PFS of 2 to 3 months.8,10,11 Docetaxel, commonly used as second-line treatment for NSCLC, has a 14% response rate, median duration of response (DoR) of 5.6 to 6.2 months, and median PFS of 3 months in patients with mNSCLC (unspecified mutation) with PD following platinum-based therapy.12,13,14
Mobocertinib, a first-in-class, potent, oral, irreversible TKI designed to selectively target in-frame EGFRex20ins mutations in NSCLC, is approved in the US for patients with locally advanced or metastatic NSCLC with EGFRex20ins mutations whose disease has progressed on prior platinum-based chemotherapy.15 In an ongoing phase 1/2 clinical trial, its recommended phase 2 dose was determined to be 160 mg once daily.16 Among 28 patients with NSCLC with EGFRex20ins mutations treated at a dose of 160 mg daily in the phase 1/2 dose escalation and expansion, investigator-assessed confirmed objective response rate (ORR) as of January 27, 2020, was 43%, with median DoR of 13.9 months and median PFS of 7.3 months.16 These promising results led to an extension cohort (EXCLAIM) that evaluated mobocertinib, 160 mg once daily, in previously treated patients with EGFRex20ins-positive mNSCLC. Here, we report results of the primary analysis of EGFRex20ins-positive mNSCLC platinum-pretreated patients (PPP cohort) who received mobocertinib, 160 mg once daily, in the dose-escalation, expansion, or EXCLAIM extension cohort of the phase 1/2 trial.
Methods
Study Design
This 3-part, open-label, multicenter, phase 1/2 nonrandomized clinical trial (ClinicalTrials.gov identifier: NCT02716116; trial protocol in Supplement 1 and statistical analysis plan in Supplement 2) consists of a dose-escalation study in patients with advanced refractory NSCLC, an expansion study in 7 molecularly and histologically defined expansion cohorts (eFigure 1 in Supplement 3),16 and an extension cohort (EXCLAIM) evaluating activity of mobocertinib, 160 mg once daily, in patients with previously treated locally advanced or metastatic EGFRex20ins-positive NSCLC. The dose-escalation and expansion parts were conducted at 28 sites in the US. The EXCLAIM cohort enrolled patients at 39 sites in Asia (57 patients), North America (30 patients [1 not treated]), and Europe (10 patients).
The study protocol and all amendments were approved by appropriate institutional review boards or ethics committees. The study was conducted in accordance with the Declaration of Helsinki, International Council for Harmonisation Tripartite Guideline for Good Clinical Practice, and applicable local regulations. All patients provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
General eligibility criteria were reported previously.16 Patients (aged ≥18 years) had measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.117; Eastern Cooperative Oncology Group performance status of 0 or 1; adequate kidney, hepatic, and bone marrow function; and normal QT interval. Key exclusion criteria were spinal cord compression, leptomeningeal disease, interstitial lung disease, radiation pneumonitis requiring corticosteroids, drug-related pneumonitis, small-molecule anticancer therapy or radiotherapy within 14 days of initiating mobocertinib (except reversible EGFR TKIs [erlotinib, gefitinib], permitted up to 7 days prior), antineoplastic monoclonal antibody therapy within 28 days, and use of moderate or strong CYP3A inhibitors or inducers within 10 days.
Patients were eligible for the EXCLAIM cohort if they had NSCLC with documented in-frame EGFRex20ins mutation determined by a qualified local test or accredited laboratory, sufficient tumor tissue for central analysis, and 1 or 2 prior regimens of systemic anticancer chemotherapy for locally advanced or metastatic disease. Prior EGFR TKI treatment was allowed unless the patient had an objective response and subsequent disease progression during TKI treatment. Patients with active brain metastases (ie, either previously untreated or previously treated with documented progression following treatment) were excluded. Patients with brain metastases were allowed if their metastases were treated (surgery and/or radiation therapy) and stable (no corticosteroid therapy within 7 days of initiating mobocertinib) without evidence of new or enlarging brain metastases.
Procedures
Patients received mobocertinib, 160 mg once daily, and could continue treatment until PD requiring alternate therapy, intolerable adverse events (AEs) in the investigator’s opinion, or another protocol-specified discontinuation criterion was met. Mobocertinib could be continued after disease progression if the patient was experiencing clinical benefit per investigator assessment. Dose interruptions and reductions were implemented to manage AEs.
Disease assessment included imaging of the chest, abdomen, pelvis, and brain by computed tomography scans or magnetic resonance imaging with contrast (unless contraindicated) at screening, every 8 weeks through cycle 14 (28 days/cycle), and every 3 cycles thereafter. Investigators and a central independent review committee (IRC) assessed scans per RECIST version 1.1.17 Confirmed responses were responses that persisted at least 4 weeks after initial observation.
Formalin-fixed, paraffin-embedded tumor tissue samples collected at screening were analyzed by next-generation sequencing using Oncomine Dx Target Test (ThermoFisher Scientific) validated to detect EGFRex20ins mutations or TruSight Tumor 170 assay (Illumina, Inc). In cases of inadequate tissue quality or sequencing failure, the EGFRex20ins variant reported by local testing was used.
Adverse events were coded according to the Medical Dictionary for Regulatory Activities, version 23.0, and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, versions 4.0 and 5.0 (after Amendment 3). Race and ethnicity were either self-reported (in the US primarily) or investigator assessed (in Europe and Asia primarily) at screening and then recorded by investigators using Interactive Response Technology to allow identification of race- and ethnicity-associated treatment effects. Race was assigned to the following categories: American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or other Pacific Islander, White, or other. Ethnicity was assigned to the following categories: Hispanic or Latino and not Hispanic or Latino.
Outcomes
The primary end point of the PPP and EXCLAIM cohorts was confirmed ORR by IRC assessment per RECIST v1.1. Secondary end points included confirmed ORR by investigator assessment; DoR and time to response, per IRC and investigator assessment; disease control rate (percentage of patients with best response of complete response, partial response, or stable disease for at least 6 weeks), per IRC and investigator assessment; PFS, per IRC and investigator assessment; and overall survival (OS). Safety end points included AEs, laboratory values, vital signs, and physical examination findings. Patient-reported outcomes included the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life (QOL) Questionnaire-C30 (QLQ-C30) and EORTC QOL Questionnaire, lung cancer module (QLQ-LC13).
Statistical Analysis
The primary analysis populations were the PPP and EXCLAIM cohorts. The PPP cohort included platinum-pretreated patients with EGFRex20ins-positive mNSCLC who received mobocertinib, 160 mg once daily, in the dose-escalation part, expansion cohort 1, or EXCLAIM. For both the PPP and EXCLAIM cohorts, treatment outcomes and safety were evaluated in all patients who received at least 1 dose of mobocertinib. Primary analyses were planned to occur when all ongoing patients had completed their cycle 6 disease assessment. Two-sided exact 95% binomial CIs were computed for binary end points (eg, ORR). Time-to-event end points (eg, PFS), analyzed using Kaplan-Meier methods, are expressed as medians and corresponding 95% CIs. Sample size calculations, prespecified subgroup analyses, and definitions for time to event outcomes are described in eMethods in Supplement 3.
Core lung cancer symptoms (eg, dyspnea, cough, pain in chest) measured by EORTC QLQ-LC13 were analyzed in patients from the EXCLAIM cohort who had baseline and at least 1 postbaseline measurements. Changes from baseline in subscale scores of EORTC QLQ-LC13 were analyzed by linear mixed models including baseline score and visit as covariates.18,19 Missing data were not imputed. All statistical analyses were conducted using SAS, version 9.4 or later (SAS Institute).
Results
Patients
Between June 8, 2016, and November 1, 2020, 114 platinum-pretreated patients with EGFRex20ins-positive mNSCLC received mobocertinib, 160 mg once daily, in the dose-escalation part (n = 6), expansion cohort 1 (n = 22), and the EXCLAIM cohort (n = 86); the PPP cohort included these 114 patients (Figure 1). The EXCLAIM cohort included 96 patients (10 patients were not platinum pretreated and thus excluded from PPP cohort). Demographic and baseline characteristics were similar in the PPP and EXCLAIM cohorts (Table 1), with median (range) age of 60 (27-84) and 59 (27-80) years, respectively; most patients were women (75 [66%] and 62 [65%]) and never smokers (81 [71%] and 70 [73%]). For the PPP and EXCLAIM cohorts, respectively, 68 (60%) and 66 (69%) were of Asian race; 3 (3%) and 2 (2%) were of Black or African American race; 42 (37%) and 28 (29%) were of White race; and 1 (1%) and 0 did not have race reported; 113 (99%) and 95 (99%) reported Hispanic or Latino ethnicity, and 1 (1%) and 1 (1%) reported not Hispanic or Latino ethnicity. The median number of prior systemic anticancer regimens was 2 in the PPP cohort and 1 in the EXCLAIM cohort. Approximately one-third (40 [35%] and 33 [34%]) of patients had baseline brain metastases. At data cutoff, 26 patients (23%) in the PPP cohort and 25 patients (26%) in the EXCLAIM cohort remained on mobocertinib treatment, with median (range) time on treatment of 7.4 (0.0-34.0) months and 6.8 (0.0-18.8) months, respectively. Median (range) follow-up was 14.2 (0.7-35.8) months and 13.0 (0.7-18.8) months, respectively.
Figure 1. CONSORT Flow Diagram.
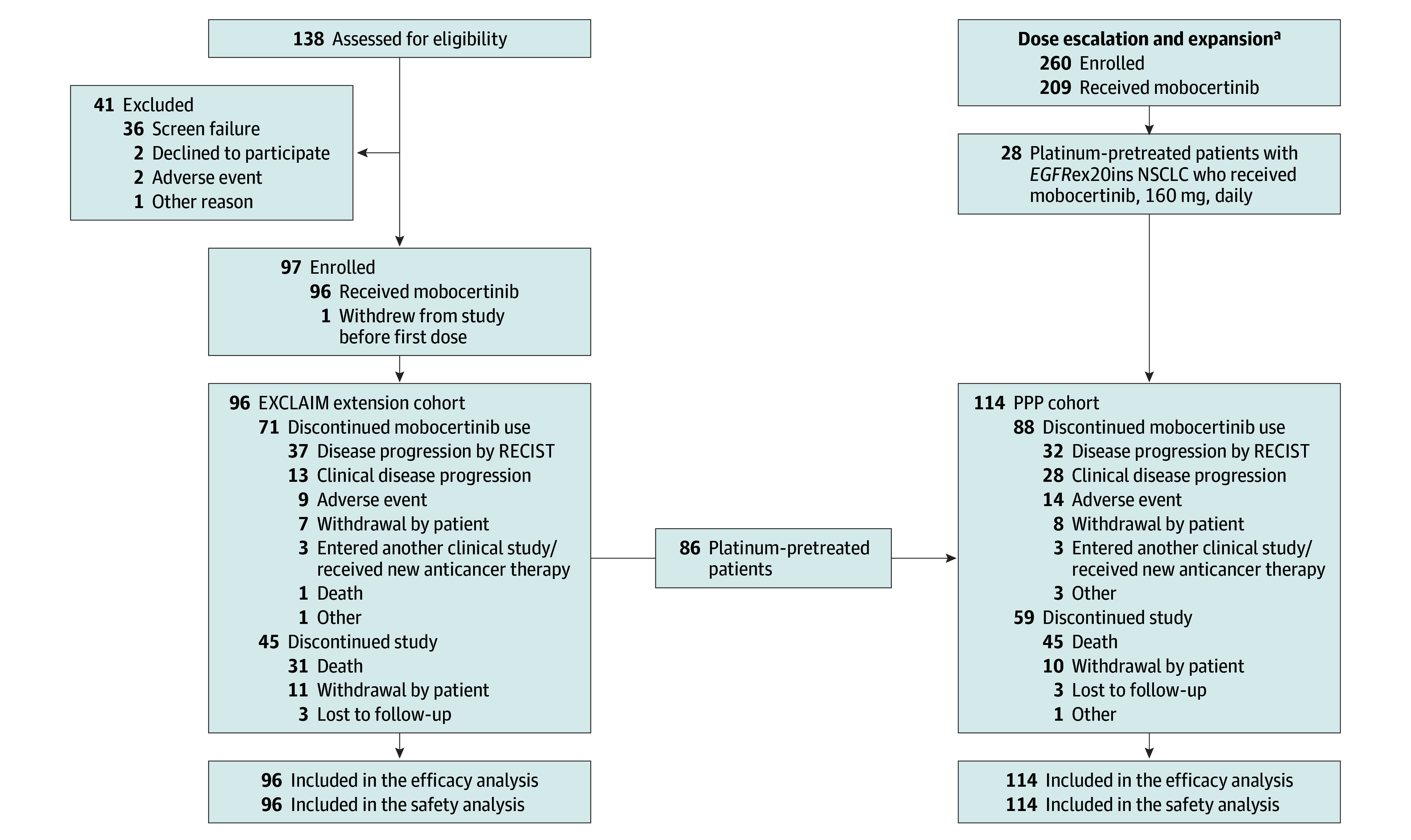
EGFRex20ins indicates EGFR exon 20 insertion; NSCLC, non–small cell lung cancer; PD, progressive disease; PPP, platinum-pretreated patients; RECIST, Response Evaluation Criteria in Solid Tumors.
aA CONSORT flow diagram for the dose escalation and expansion parts of the study has been published previously.16
Table 1. Patient Demographic and Baseline Characteristicsa.
| Characteristic | No. (%) | |
|---|---|---|
| PPP cohort (n = 114) | EXCLAIM cohort (n = 96) | |
| Age, median (range), y | 60 (27-84) | 59 (27-80) |
| Sex | ||
| Female | 75 (66) | 62 (65) |
| Male | 39 (34) | 34 (35) |
| Race | ||
| Asian | 68 (60) | 66 (69) |
| Black or African American | 3 (3) | 2 (2) |
| White | 42 (37) | 28 (29) |
| Not reported | 1 (1) | 0 |
| Ethnicity | ||
| Hispanic or Latino | 113 (99) | 95 (99) |
| Not Hispanic or Latino | 1 (1) | 1 (1) |
| Histologic type | ||
| Adenocarcinoma | 112 (98) | 95 (99) |
| Squamous | 1 (1) | 1 (1) |
| Large cell | 1 (1) | 0 |
| ECOG performance status | ||
| 0 | 29 (25) | 28 (29) |
| 1 | 85 (75) | 68 (71) |
| History of smoking | ||
| Never | 81 (71) | 70 (73) |
| Former | 31 (27) | 24 (25) |
| Current | 2 (2) | 2 (2) |
| No. of prior systemic anticancer regimens | ||
| 1 | 47 (41) | 49 (51) |
| 2 | 36 (32) | 30 (31) |
| ≥3 | 31 (27) | 17 (18) |
| Prior systemic anticancer therapyb | 114 (100) | 90 (94) |
| Platinum-based chemotherapy | 114 (100) | 86 (90) |
| Immunotherapy | 49 (43) | 33 (34) |
| EGFR TKI | 29 (25) | 30 (31) |
| Baseline brain metastases | 40 (35) | 33 (34) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; PPP, platinum-pretreated patients; TKI, tyrosine kinase inhibitor.
Percentages may not add up to 100% because of rounding.
Patients could have been counted in more than 1 category.
All patients had NSCLC with in-frame EGFRex20ins mutations documented by local testing at enrollment. The exact insertion was identified by retrospective central sequencing in 68 patients and available by local testing in 27 other patients. Among these 95 patients, 32 independent EGFRex20ins variants were identified (eTable in Supplement 3). The most frequent EGFRex20ins mutations were V769_D770insASV (ASV, 25 patients), D770_N771insSVD (SVD, 13 patients), and H773_V774insNPH (NPH, 9 patients), which together represented 41% of patients in the PPP cohort. Forty-eight patients (42%) had 29 distinct less frequent EGFRex20ins variants. Most patients (74%; 70 of 95) had near-loop insertions (positions 767-772), 25% (24 of 95) had far-loop insertions (positions 773-775), and 1 (1%; 1 of 95) had the FQEA insertion within the C-helix (A763_Y764insFQEA).
Treatment Outcomes
PPP Cohort
The confirmed ORR by IRC assessment was 28% (95% CI, 20%-37%) and by investigator assessment was 35% (95% CI, 26%-45%; Table 2). The confirmed disease control rate by IRC assessment was 78% (95% CI, 69%-85%). Best percentage change in target lesions and objective response by time on treatment are shown in Figure 2A and B, respectively. Ninety-six patients (84%) had a reduction from baseline in sum of target lesion diameters per IRC assessments. Median time to IRC-assessed confirmed response was 1.9 months (95% CI, 1.8-3.6) and median DoR was 17.5 months (95% CI, 7.4-20.3; Figure 2C). At data cutoff, 65 patients (57%) had events of IRC-assessed disease progression or death, with median PFS of 7.3 months (95% CI, 5.5-9.2; eFigure 2 in Supplement 3). Median investigator-assessed PFS was 7.3 months (95% CI, 5.6-8.8). Median OS was 24.0 months (95% CI, 14.6-28.8; Figure 2D).
Table 2. Antitumor Activity of Mobocertiniba.
| Outcome | No. (%) | |
|---|---|---|
| PPP cohort (n = 114) | EXCLAIM cohort (n = 96) | |
| IRC-assessed confirmed objective responseb | ||
| Patients, No. (%) [95% CI] | 32 (28) [20-37] | 24 (25) [17-35] |
| Complete response | 0 | 0 |
| Partial response | 32 (28) | 24 (25) |
| Stable diseasec | 57 (50) | 49 (51) |
| Not evaluable | 12 (11) | 10 (10) |
| Confirmed disease control rate, No. (%) [95% CI]d | 89 (78) [69-85] | 73 (76) [66-84] |
| Investigator-assessed confirmed objective response b | ||
| Patients, No. (%) [95% CI] | 40 (35) [26-45] | 31 (32) [23-43] |
| Complete response | 1 (<1) | 1 (1) |
| Partial response | 39 (34) | 30 (31) |
| Stable diseasec | 49 (43) | 41 (43) |
| Not evaluable | 11 (10) | 9 (9) |
| Confirmed disease control rate, No. (%) [95% CI]d | 89 (78) [69-85] | 72 (75) [65-83] |
| Duration of response in confirmed responderse | ||
| IRC-assessed | ||
| No. | 32 | 24 |
| Median (95% CI), mo | 17.5 (7.4-20.3) | NR (5.6-NR) |
| Investigator-assessed | ||
| No. | 40 | 31 |
| Median (95% CI), mo | 11.2 (5.6-NR) | 11.2 (7.0-NR) |
| Progression-free survival, median (95% CI), moe | ||
| No. | 114 | 96 |
| IRC-assessed | 7.3 (5.5-9.2) | 7.3 (5.5-9.1) |
| Investigator-assessed | 7.3 (5.6-8.8) | 7.3 (5.6-9.1) |
| Overall survival, median (95% CI), mo | ||
| No. | 114 | 96 |
| Median (95% CI), mo | 24.0 (14.6-28.8) | NR (13.1-NR) |
Abbreviations: IRC, independent review committee; NR, not reached; PPP, platinum-pretreated patients; RECIST, Response Evaluation Criteria in Solid Tumors.
Data cutoff date: November 1, 2020.
Objective response by RECIST version 1.1.
Stable disease observed 6 weeks or longer after first study drug administration.
Disease control rate is defined as the proportion of patients who have confirmed complete response or partial response, or best response of stable disease for 6 weeks or longer after initiation of study drug using RECIST version 1.1.
Duration of response, progression-free survival, and overall survival were estimated using Kaplan-Meier methods.
Figure 2. Mobocertinib Activity in Platinum-Pretreated Patients With EGFRex20ins Mutation–Positive Metastatic NSCLC (PPP Cohort).

A, Best percentage change from baseline in the sum of the longest diameters of target lesions per independent review committee (IRC) assessment in patients who underwent follow-up imaging and could be evaluated for a response (101 patients). The solid line at −30% indicates the threshold for partial response according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. B, Objective response per IRC assessments by time on treatment in confirmed responders (n = 32). C, Kaplan-Meier–estimated duration of confirmed response per IRC assessments. D, Kaplan-Meier estimates of overall survival (OS). Of the 114 patients in the PPP cohort, 46 (40%) died. Tick marks in Kaplan-Meier plots indicate censored data.
ASV indicates V769_D770insASV; CR, complete response; EGFRex20ins, EGFR exon 20 insertion; NPH, H773_V774insNPH; SVD, D770_N771insSVD; NSCLC, non–small cell lung cancer; ORR, objective response rate; PD, progressive disease; PPP, platinum-pretreated patients; PR, partial response; SD, stable disease.
aSpecific EGFRex20ins mutations were identified by central tumor sequencing in 68 patients, reported by local test in 27 patients, insertion unknown in 16 patients, and unconfirmed in 3 patients.
bThe total number of patients in the table in panel A includes 13 patients with data not shown in the waterfall plot because they could not be evaluated for tumor response (2 with ASV/SVD/NPH mutations, 7 with other EGFRex20ins mutations, 3 with insertion unknown, and 1 with unconfirmed EGFRex20ins mutation).
cUnconfirmed complete response.
Responses were observed in all prespecified subgroups, with no significant differences (overlapping 95% CIs) in ORR between subgroups (eFigure 3 in Supplement 3). Confirmed ORRs by IRC were: 24% (95% CI, 13%-39%) and 31% (95% CI, 20%-43%) in patients with and without prior immunotherapy, respectively; 21% (95% CI, 8%-40%) and 31% (95% CI, 21%-42%) in patients with and without prior EGFR TKI treatment, respectively, and 18% (95% CI, 7%-33%) and 34% (95% CI, 23%-46%) in patients with and without baseline brain metastases, respectively.
The IRC-assessed confirmed ORR was 32% (95% CI, 19%-47%) in patients with ASV, SVD, or NPH variants and 25% (95% CI, 14%-40%) in patients with less frequent variants. The median IRC-assessed PFS was 7.4 months (95% CI, 5.4-14.6) in patients with ASV, SVD, or NPH variants and 7.3 months (95% CI, 3.7-10.8) in patients with less frequent variants. Response rates were similar whether insertion mutations occurred in near-loop or far-loop positions, with IRC-assessed confirmed ORRs of 29% (95% CI, 18%-41%) and 25% (95%, 10%-47%), respectively. The 1 patient with FQEA C-helix insertion had a confirmed partial response.
EXCLAIM Cohort
The confirmed ORR in the EXCLAIM cohort was 25% (95% CI, 17%-35%) by IRC assessment and 32% (95% CI, 23%-43%) by investigators (Table 2). The median time to IRC-assessed confirmed response was 1.9 (95% CI, 1.8-3.6) months, with median DoR not estimable (95% CI, 5.6 months to not estimable). At data cutoff, IRC-assessed events of disease progression or death had occurred in 53 patients (55%), with median PFS of 7.3 (95% CI, 5.5-9.1) months. Median OS was not reached. Results of subgroup analyses of the EXCLAIM cohort were similar to those of the PPP cohort.20 The brain was the first site of investigator-assessed progression in 38% (22 of 58) of all patients with PD and 68% (17 of 25) of patients with baseline brain metastases who had PD. Median time on treatment beyond initial investigator-assessed disease progression was 1.6 (95% CI, −0.2 to 6.7) months in patients with first progression in the brain and 0.1 (95% CI, −1.0 to 10.0) months in patients with extracranial first sites of disease progression. Approximately 23% of patients (5 of 22) with first progression in the brain continued mobocertinib treatment for 3 months or longer after initial progression.
Safety
The most common any-grade treatment-related AEs in the PPP and EXCLAIM cohorts were diarrhea and rash (Table 3). Diarrhea was the only grade 3 or 4 treatment-related AE reported in greater than 10% of patients. Most gastrointestinal and skin events were grades 1 or 2 in severity, and all events were managed with supportive care, dose modification, and/or drug discontinuation.
Table 3. Safety Overview and Treatment-Related Adverse Events (AEs) of Any Grade Reported in 10% or More or Grade 3 or Higher AEs Reported in 3% or More Among All Patients in the EXCLAIM and PPP Cohorts.
| Adverse event | Patients, No. (%) | |||
|---|---|---|---|---|
| PPP cohort (n = 114) | EXCLAIM cohort (n = 96) | |||
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Overview of AEs | ||||
| Any | 114 (100) | 79 (69) | 96 (100) | 63 (66) |
| Any treatment-related | 113 (99) | 54 (47) | 95 (99) | 40 (42) |
| Serious | 56 (49) | 52 (46) | 45 (47) | 42 (44) |
| Leading to dose reduction | 29 (25) | NAa | 21 (22) | NAa |
| Leading to treatment discontinuation | 19 (17) | NAa | 10 (10) | NAa |
| Treatment-related AEs of any grade reported in ≥10% or of grade ≥3 reported in ≥3% of patients | ||||
| Diarrhea | 104 (91) | 24 (21) | 89 (93) | 15 (16) |
| Rash | 51 (45) | 0 | 43 (45) | 0 |
| Paronychia | 43 (38) | 1 (<1) | 37 (39) | 1 (1) |
| Decreased appetite | 40 (35) | 1 (<1) | 31 (32) | 1 (1) |
| Nausea | 39 (34) | 5 (4) | 29 (30) | 3 (3) |
| Dry skin | 35 (31) | 0 | 30 (31) | 0 |
| Vomiting | 34 (30) | 3 (3) | 25 (26) | 1 (1) |
| Blood creatinine increased | 29 (25) | 2 (2) | 27 (28) | 2 (2) |
| Stomatitis | 27 (24) | 5 (4) | 26 (27) | 3 (3) |
| Pruritus | 24 (21) | 1 (<1) | 19 (20) | 1 (1) |
| Lipase increased | 22 (19) | 4 (4) | 16 (17) | 2 (2) |
| Amylase increased | 21 (18) | 3 (3) | 19 (20) | 1 (1) |
| Dermatitis, acneiform | 21 (18) | 0 | 20 (21) | 1 (1) |
| Anemia | 20 (18) | 1 (<1) | 18 (19) | 1 (1) |
| Weight decreased | 15 (13) | 1 (<1) | 13 (14) | 0 |
| Alopecia | 17 (15) | 0 | 12 (13) | 0 |
| Fatigue | 16 (14) | 3 (3) | 12 (13) | 2 (2) |
| Rash, maculopapular | 16 (14) | 2 (2) | 10 (10) | 2 (2) |
| Gastroesophageal reflux disease | 14 (12) | 0 | 12 (13) | 0 |
| Mouth ulceration | 14 (12) | 0 | 14 (15) | 0 |
| Electrocardiogram QT prolonged | 12 (11) | 3 (3) | 8 (8) | 3 (3) |
| Rhinorrhea | 12 (11) | 0 | 11 (11) | 0 |
| Alanine aminotransferase increased | 9 (8) | 1 (<1) | 10 (10) | 1 (1) |
Abbreviations: AE, adverse event; NA, not applicable; PPP, platinum-pretreated patients.
AEs leading to dose reduction or discontinuation were not evaluated by AE grade.
In the PPP cohort, 19 of 114 patients (17%) discontinued treatment because of AEs. Adverse events leading to discontinuation in at least 2 patients were diarrhea (n = 5 [4%]), nausea (n = 4 [4%]), vomiting (n = 2 [2%]), decreased appetite (n = 2 [2%]), and stomatitis (n = 2 [2%]). Dose reductions due to an AE occurred in 29 patients (25%). Adverse events leading to dose reduction in more than 2 patients were diarrhea (n = 12 [11%]), nausea (n = 6 [5%]), fatigue (n = 3 [3%]), maculopapular rash (n = 3 [3%]), and vomiting (n = 3 [3%]). Response rates were numerically higher in the 85 patients without dose reduction due to AEs (ORR, IRC: 31% [95% CI, 21%-42%]) than in the 29 patients with dose reductions due to AEs (21% [95% CI, 8%-40%]). Twelve patients had AEs leading to death within 30 days after last dose; 1 event, cardiac failure, in a platinum-pretreated patient (from EXCLAIM) was deemed by the investigators to be treatment related.
In the EXCLAIM cohort, 10 of 96 patients (10%) discontinued treatment because of AEs. Adverse events leading to discontinuation in at least 2 patients were diarrhea (n = 2 [2%]) and nausea (n = 2 [2%]). Dose reductions due to an AE occurred in 21 patients (22%). Adverse events leading to dose reduction in more than 2 patients were diarrhea (n = 9 [9%]), fatigue (n = 3 [3%]), and nausea (n = 3 [3%]). Eight patients (8%) had AEs leading to death within 30 days of last dose; 1 event (the same patient with cardiac failure described previously for the PPP cohort) was considered treatment related.
Patient-Reported Outcomes
In the EXCLAIM cohort, improvements from baseline were observed in least squares mean scores on the EORTC QLQ-LC13 lung cancer core symptoms of dyspnea, coughing, and pain in chest from cycle 2 and were maintained throughout treatment (eFigure 4A-C in Supplement 3). The proportion of patients with clinically meaningful improvement (≥10-point decrease) from baseline in QLQ-LC13 core lung cancer symptoms was 54% for dyspnea (n = 49), 47% for coughing (n = 42), and 39% for pain in chest (n = 35). Least squares mean EORTC QLQ-C30 Global Health Status/QOL scores were maintained throughout the study (eFigure 5A in Supplement 3), despite worsening diarrhea scores during treatment (eFigure 5B in Supplement 3). Least squares mean EORTC QLQ-C30 diarrhea scores returned to baseline at 30 days after last dose. Other functioning subscales, including physical, role, emotional, cognitive, and social functioning scores, were maintained during treatment.21
Discussion
In patients with EGFRex20ins-positive mNSCLC pretreated with platinum-based chemotherapy (PPP cohort), mobocertinib demonstrated durable clinical benefit, with confirmed ORR of 28% per IRC assessment and 35% per investigators. The disease control rate was 78%, median DoR was 17.5 months, and median PFS was 7.3 months, all by IRC assessment. The median OS was 24.0 months. Response rates and DoR were better with mobocertinib compared with historical standard of care (second-line docetaxel; ORR, 14%; DoR, 5.6-6.2 months)12,13,14 and immuno-oncology therapy (ORR, 0%-25%; DoR, not reported).8,11 Investigator and IRC assessments of antitumor activity were consistent. Results were similar in the EXCLAIM extension cohort, with confirmed ORR of 25% by IRC assessment and 32% by investigators. Antitumor activity was associated with stable Global Health Status/QOL scores and improvements in lung cancer symptoms (dyspnea, coughing, and pain in chest).
Confirmed ORRs were similar in all evaluated subgroups, including patients with and without baseline brain metastases, prior EGFR TKI treatment, and prior immunotherapy. The observed EGFRex20ins variants reflected the expected molecular diversity of variants, with a preponderance of 3 frequently observed variants (ASV, SVD, and NPH).15,22 Responses occurred across EGFRex20ins mutation subtypes, regardless of mutation frequency or position, suggesting no clear genotype-activity correlation.
Because patients with baseline brain metastasis were required to have brain radiotherapy before enrollment, we did not evaluate intracranial activity using RECIST criteria. Therefore, we evaluated rates of first site of disease progression in the brain to assess intracranial activity. In the EXCLAIM cohort, 68% of patients with baseline brain metastasis and subsequent PD had first progression (per investigator assessment) in the brain, while 38% of all patients with PD had first progression in the brain. About 23% of patients remained on treatment at least 3 months after PD in the brain. These observations suggest that mobocertinib may have limited intracranial activity; however, patients may have systemic benefit from continuing mobocertinib treatment after progression in the brain.
Second- and third-generation EGFR TKIs have minimal activity for EGFRex20ins-positive mNSCLC. Poziotinib resulted in IRC-assessed ORRs of 15% to 19%, median PFS of 4.2 months, and median DoR of 7.4 months.23,24 Osimertinib demonstrated ORRs of 5% to 25%, median PFS of 3.6 to 9.7 months, and median DoR of 5.7 months, with the 25% response rate observed at a dose of 160 mg daily (twice the approved dose).25,26 Amivantamab, a human anti–EGFR-MET bispecific antibody, demonstrated clinical activity similar to mobocertinib, but the safety profiles of the 2 agents are different.27 The most common treatment-related AEs with amivantamab were cutaneous AEs, infusion-related reactions, and paronychia.27 The safety profile of mobocertinib was characterized mainly by gastrointestinal and cutaneous AEs, similar to the safety profile of other EGFR TKIs.28,29
Limitations
This study was a nonrandomized, open-label, phase 1/2 study that did not include a control arm; thus, data need to be interpreted in the context of historical comparison to current literature or real-world evidence in patients with EGFRex20ins-positive NSCLC. In addition, not all EGFRex20ins mutations were detectable by next-generation sequencing analysis, and tumor tissues were sometimes not of sufficient quality or quantity. Lastly, because patients with active or untreated brain metastases were excluded from the study and only approximately one-third of patients had baseline brain metastases, further study will be required to determine the activity of mobocertinib in this population.
Conclusions
In this phase 1/2 open-label nonrandomized clinical trial, mobocertinib, a first-in-class, once-daily oral EGFR TKI designed to target EGFRex20ins mutations, was associated with antitumor activity in platinum-pretreated patients with EGFRex20ins-positive mNSCLC. Confirmed responses were observed across various EGFRex20ins mutation subtypes. The AE profile was manageable and consistent with that of other EGFR TKIs. Antitumor activity and safety results were similar in the EXCLAIM cohort, which also demonstrated improved NSCLC symptom scores and stable Global Health Status/QOL scores. Mobocertinib appears to have a favorable risk-benefit profile in patients with previously treated EGFRex20ins-positive mNSCLC and may serve as a potential treatment option in this patient population, which has a high unmet medical need.
Trial Protocol
Statistical Analysis Plan
eMethods.
eTable. EGFR Exon 20 Insertion Mutations in the PPP Cohort
eFigure 1. Design and Patient Cohorts in Phases 1/2 and EXCLAIM
eFigure 2. PFS in the PPP Cohort
eFigure 3. Forest Plot of IRC-Assessed Confirmed ORR by Prespecified Subgroups in the PPP Cohort
eFigure 4. Change in European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire, Lung Cancer Module (QLQ-LC13) Core Lung Cancer Symptom Scores in the EXCLAIM Cohort
eFigure 5. Change in European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-C30 (QLQ-C30) in the EXCLAIM Cohort
Data Sharing Statement
References
- 1.Riess JW, Gandara DR, Frampton GM, et al. Diverse EGFR exon 20 insertions and co-occurring molecular alterations identified by comprehensive genomic profiling of NSCLC. J Thorac Oncol. 2018;13(10):1560-1568. doi: 10.1016/j.jtho.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220-229. doi: 10.1158/1535-7163.MCT-12-0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol. 2013;8(2):179-184. doi: 10.1097/JTO.0b013e3182779d18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang W, Huang Y, Hong S, et al. EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer. BMC Cancer. 2019;19(1):595. doi: 10.1186/s12885-019-5820-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13(1):e23-e31. doi: 10.1016/S1470-2045(11)70129-2 [DOI] [PubMed] [Google Scholar]
- 6.Byeon S, Kim Y, Lim SW, et al. Clinical outcomes of EGFR exon 20 insertion mutations in advanced non-small cell lung cancer in Korea. Cancer Res Treat. 2019;51(2):623-631. doi: 10.4143/crt.2018.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Yang G, Li J, et al. Real-world treatment outcome of advanced Chinese NSCLC EGFR exon 20 insertion patients [abstract]. J Clin Oncol. 2019;37(15)(suppl):9043. doi: 10.1200/JCO.2019.37.15_suppl.9043 [DOI] [Google Scholar]
- 8.Udagawa H, Matsumoto S, Ohe Y, et al. Clinical outcome of non-small cell lung cancer with EGFR/HER2 exon 20 insertions identified in the LCSCRUM-Japan [abstract OA07.03]. J Thorac Oncol. 2019;14(10)(suppl):S224. doi: 10.1016/j.jtho.2019.08.443 [DOI] [Google Scholar]
- 9.O’Kane GM, Bradbury PA, Feld R, et al. Uncommon EGFR mutations in advanced non-small cell lung cancer. Lung Cancer. 2017;109:137-144. doi: 10.1016/j.lungcan.2017.04.016 [DOI] [PubMed] [Google Scholar]
- 10.Yang G, Li J, Xu H, et al. EGFR exon 20 insertion mutations in Chinese advanced non-small cell lung cancer patients: molecular heterogeneity and treatment outcome from nationwide real-world study. Lung Cancer. 2020;145:186-194. doi: 10.1016/j.lungcan.2020.03.014 [DOI] [PubMed] [Google Scholar]
- 11.Negrao MV, Reuben A, Ponville Robichaux J, et al. Association of EGFR and HER-2 exon 20 mutations with distinct patterns of response to immune checkpoint blockade in non-small cell lung cancer [abstract]. J Clin Oncol. 2018;36(15)(suppl):9052. doi: 10.1200/JCO.2018.36.15_suppl.9052 [DOI] [Google Scholar]
- 12.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665-673. doi: 10.1016/S0140-6736(14)60845-X [DOI] [PubMed] [Google Scholar]
- 13.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalvez F, Vincent S, Baker TE, et al. Mobocertinib (TAK-788): a targeted inhibitor of EGFR exon 20 insertion mutants in non–small cell lung cancer. Cancer Discov. 2021;11(7):1672-1687. doi: 10.1158/2159-8290.CD-20-1683 [DOI] [PubMed] [Google Scholar]
- 16.Riely GJ, Neal JW, Camidge DR, et al. Activity and safety of mobocertinib (TAK-788) in previously treated non-small cell lung cancer with EGFR exon 20 insertion mutations from a phase I/II trial. Cancer Discov. 2021;11(7):1688-1699. doi: 10.1158/2159-8290.CD-20-1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 18.Blackhall F, Kim DW, Besse B, et al. Patient-reported outcomes and quality of life in PROFILE 1007: a randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9(11):1625-1633. doi: 10.1097/JTO.0000000000000318 [DOI] [PubMed] [Google Scholar]
- 19.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A; EORTC Quality of Life Group . The EORTC QLQ-C30 Scoring Manual. 3rd ed. European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 20.Ramalingam SS, Zhou C, Kim TM, et al. Mobocertinib (TAK-788) in EGFR exon 20 insertion (ex20ins)+ metastatic NSCLC (mNSCLC): additional results from platinum-pretreated patients (pts) and EXCLAIM cohort of phase 1/2 study [abstract]. J Clin Oncol. 2021;39(15)(suppl):9014. doi: 10.1200/JCO.2021.39.15_suppl.9014 [DOI] [Google Scholar]
- 21.Garcia Campelo MR, Zhou C, Ramalingam SS, et al. Mobocertinib (TAK-788) in EGFR exon 20 insertion+ metastatic NSCLC: patient-reported outcomes from EXCLAIM extension cohort [abstract FP09.02]. Presented at: 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vyse S, Huang PH. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther. 2019;4:5. doi: 10.1038/s41392-019-0038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le X, Goldman JW, Clarke JM, et al. Poziotinib shows activity and durability of responses in subgroups of previously treated EGFR exon 20 NSCLC patients [abstract]. J Clin Oncol. 2020;38(15)(suppl):9514. doi: 10.1200/JCO.2020.38.15_suppl.9514 [DOI] [Google Scholar]
- 24.Prelaj A, Bottiglieri A, Proto C, et al. Poziotinib in advanced NSCLC with EGFR or HER2 exon 20 insertion mutation: initial results from a single site expanded access program [abstract 1388P]. Ann Oncol. 2020;31(suppl 4):S882. doi: 10.1016/j.annonc.2020.08.1702 [DOI] [Google Scholar]
- 25.van Veggel B, Madeira R Santos JFV, Hashemi SMS, et al. Osimertinib treatment for patients with EGFR exon 20 mutation positive non-small cell lung cancer. Lung Cancer. 2020;141:9-13. doi: 10.1016/j.lungcan.2019.12.013 [DOI] [PubMed] [Google Scholar]
- 26.Piotrowska Z, Wang Y, Sequist LV, Ramalingam SS. ECOG-ACRIN 5162: a phase II study of osimertinib 160 mg in NSCLC with EGFR exon 20 insertions [abstract]. J Clin Oncol. 2020;38(15)(suppl):9513. doi: 10.1200/JCO.2020.38.15_suppl.9513 [DOI] [Google Scholar]
- 27.Sabari JK, Shu CA, Park K, et al. Amivantamab in post-platinum EGFR exon 20 insertion mutant non-small cell lung cancer [abstract OA04.04]. Presented at: Annual Meeting of the World Conference on Lung Cancer; January 28-31, 2021; Singapore. [Google Scholar]
- 28.Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6(10):803-812. doi: 10.1038/nrc1970 [DOI] [PubMed] [Google Scholar]
- 29.Hirsh V, Blais N, Burkes R, Verma S, Croitoru K. Management of diarrhea induced by epidermal growth factor receptor tyrosine kinase inhibitors. Curr Oncol. 2014;21(6):329-336. doi: 10.3747/co.21.2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods.
eTable. EGFR Exon 20 Insertion Mutations in the PPP Cohort
eFigure 1. Design and Patient Cohorts in Phases 1/2 and EXCLAIM
eFigure 2. PFS in the PPP Cohort
eFigure 3. Forest Plot of IRC-Assessed Confirmed ORR by Prespecified Subgroups in the PPP Cohort
eFigure 4. Change in European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire, Lung Cancer Module (QLQ-LC13) Core Lung Cancer Symptom Scores in the EXCLAIM Cohort
eFigure 5. Change in European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-C30 (QLQ-C30) in the EXCLAIM Cohort
Data Sharing Statement


