Abstract
Background:
Exposure to fine particulate matter (PM2.5), an ambient air pollutant with mass-based standards promulgated under the Clean Air Act, and black carbon (BC), a common component of PM2.5, are both associated with cardiovascular health effects.
Objectives:
To elucidate whether BC is associated with distinct, or stronger, cardiovascular responses compared to PM2.5, we conducted a systematic review. We evaluated the associations of short- and long-term BC, or the related component elemental carbon (EC), with cardiovascular endpoints including heart rate variability, heart rhythm, blood pressure and vascular function, ST segment depression, repolarization abnormalities, atherosclerosis and heart function, in the context of what is already known about PM2.5.
Data sources:
We conducted a stepwise systematic literature search of the PubMed, Web of Science and TOXLINE databases and applied Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines for reporting our results.
Study eligibility criteria:
Studies reporting effect estimates for the association of quantitative measurements of ambient BC (or EC) and PM2.5, with relevant cardiovascular endpoints (i.e. meeting inclusion criteria) were included in the review. Included studies were evaluated for risk of bias in study design and results.
Study appraisal and synthesis methods:
Risk of bias evaluations assessed aspects of internal validity of study findings based on study design, conduct, and reporting to identify potential issues related to confounding or other biases. Study results are presented to facilitate comparison of the consistency of associations with PM2.5 and BC within and across studies.
Results:
Our results demonstrate similar associations for BC (or EC) and PM2.5 with the cardiovascular endpoints examined. Across studies, associations for BC and PM2.5 varied in their magnitude and precision, and confidence intervals were generally overlapping within studies. Where differences in the magnitude of the association between BC or EC and PM2.5 within a study could be discerned, no consistent pattern across the studies examined was apparent.
Limitations:
We were unable to assess the independence of the effect of BC, relative the effect of PM2.5, on the cardiovascular system, nor was information available to understand the impact of differential exposure misclassification.
Conclusions:
Overall, the evidence indicates that both BC (or EC) and PM2.5 are associated with cardiovascular effects but the available evidence is not sufficient to distinguish the effect of BC (or EC) from that of PM2.5 mass.
Keywords: Black carbon, Fine particulate matter, Cardiovascular effects
1. Introduction
National Ambient Air Quality Standards (NAAQS) are promulgated for a set of “criteria” pollutants, including particulate matter (PM). PM is a heterogeneous mixture of particles and liquid droplets, comprising multiple components (e.g., organics, acids, metals, crustal material) and size fractions. The mass of particles measured in ambient air with a nominal mean aerodynamic diameter less than or equal to 2.5 μm (PM2.5) is one of the indicators used to determine compliance with the NAAQS. The choice of PM2.5 mass as an indicator for the standard is underpinned by evidence from numerous studies linking ambient exposure to PM2.5 with an array of cardiovascular effects, ranging from subtle subclinical measures (e.g., heart rate variability) to cardiovascular-related mortality (U.S. EPA, 2009).
Epidemiologic studies have found regional differences in the magnitude of the associations between PM2.5 and health effects that are not fully explained by variations in the concentration of ambient PM2.5 (U.S. EPA, 2009). Experimental studies focusing on individual components point to several components that may be highly toxic to the cardiovascular system motivating research designed to elucidate whether specific components of PM2.5 could explain the heterogeneity in PM2.5 risk estimates across the epidemiologic study findings (e.g., Baxter et al., 2012; Davis et al., 2011). The extent to which PM2.5 mass is the preferred indicator, and whether, or not, a particular component is more closely associated with health effects is of interest to policymakers.
Like exposure to PM2.5, exposure to black carbon (BC), a common component of PM2.5 and a potentially important contributor to total PM2.5 mass (Bell et al., 2007), is associated with cardiovascular effects (U.S. EPA, 2012). BC is generally present in submicron particles generated during combustion (e.g., transportation, biomass burning, residential heating and cooking, power plants, some industries). Although the evidence linking BC to subclinical cardiovascular endpoints is more limited than that for PM2.5, BC is of interest from a health perspective because multiple studies report associations between combustion-related air pollution and health effects (U.S. EPA, 2009, 2012; WHO, 2012) and some research suggests that combustion-related particles may have a greater relative toxicity on a per mass basis than PM2.5 (Janssen et al., 2011).
The U.S. EPA Integrated Science Assessment for PM (U.S. EPA, 2009) and the subsequent Report to Congress on Black Carbon (U.S. EPA, 2012) concluded that there was limited evidence to indicate BC or other sources and components would be a better predictor of health effects than PM2.5, or that the associations between health effects and BC concentrations observed in epidemiologic studies are independent of the associations with PM2.5. Reviews focusing on studies that applied source apportionment methods to examine health effects with source categories that include BC also substantiate the conclusion that, based on the current evidence, health effects are attributed to the mixture containing BC as opposed to any individual component such as BC (Stanek et al., 2011; Vedal et al., 2013; Thurston et al., 2016). By contrast, a report by the World Health Organization (WHO) concluded that BC is a better indicator of harmful PM exposure than mass concentration (WHO, 2012). The authors of the WHO report used increment-based, rather than interquartile range (IQR)-based standardization, which largely explained the different interpretations of evidence across reports (Luben et al., 2017).
The epidemiologic evidence linking BC to cardiovascular effects is expanding, and to our knowledge, has not been systematically reviewed. This systematic review aims to examine the strength and trends in the relationships between PM2.5 and BC and selected cardiovascular endpoints. We build on the analysis of Luben et al. (2017) who conducted a systematic review to examine the extent to which the current evidence supported an independent effect of BC, separate from PM2.5, on hospital admissions, emergency department visits, and mortality from cardiovascular causes. Luben et al. (2017) found exposures to both PM2.5 and BC were positively associated with cardiovascular outcomes and that the associations for both PM2.5 and BC were generally similar in magnitude. They further concluded that there was insufficient evidence to distinguish the observed effects of BC from those of PM2.5. The current study applies similar methods to that of Luben et al. (2017), but focuses on changes in cardiovascular responses that can be measured prior to the manifestation of clinical disease. These endpoints can provide key evidence that supports the biological plausibility for air pollution exposure to affect the cardiovascular system and result in more severe (and more commonly studied) outcomes, like hospital admissions and mortality. Like Luben et al. (2017) our evaluation of BC-associated cardiovascular effects is considered in the context of what is already understood about the health effects of PM2.5.
Several pathways by which short- and long-term exposure to PM2.5 can lead to overt cardiovascular disease and mortality have been postulated (U.S. EPA, 2009; Brook et al., 2010). Activation of the sensory nerves in the lung can lead to changes in heart rate, heart rate variability (HRV) and effects on vascular function followed by increased blood pressure, which is a risk factors for cardiovascular disease. In addition, exposure to PM2.5 can result in pulmonary inflammation, which can then induce systemic inflammation. Circulating inflammatory cytokines can stimulate the liver to release additional proteins and coagulation factors that can alter hemostasis and increase the potential for thrombosis. In addition to its effect on hemostasis, systemic inflammation can induce vascular dysfunction leading to further plaque development and rupture of existing atherosclerotic plaques, obstruction of blood flow to the heart (i.e., ischemic heart disease), and decreased cardiac output (i.e., heart failure). Although the mechanism of toxicity for BC is not as well understood as that for PM2.5, the plausibility that BC may affect the cardiovascular system through pathways involving inflammation and oxidative stress, leading to endothelial dysfunction has been demonstrated in some studies (Niranjan and Thakur, 2017). Thus, a systematic evaluation of the associations between BC (or EC) and PM2.5 and cardiovascular endpoints on these pathways could elucidate whether the observed effects can be attributed to the mixture containing BC (i.e., PM2.5) as opposed to individual components of PM2.5, including BC.
This systematic review uses the following Population, Exposure, Comparison, Outcome, Study Design (PECOS) statement: In any population of adults (ages 18+), including subgroups of susceptible individuals (P), what is the association of BC or PM2.5 (E) per unit (μg/m3) increase equal to the interquartile range (C) with cardiovascular endpoints (i.e., heart rate (HR) and HRV, preclinical atherosclerosis, electrocardiogram (ECG) changes, and blood pressure) (O) observed in panel (for short-term exposure) or cohort (for long-term exposures) epidemiologic studies (S)?
2. Methods
2.1. Definition of black carbon and elemental carbon
This systematic review was developed as part of a series of manuscripts and its rationale as it relates to exposure metrics is described in detail elsewhere (Luben et al., 2017; Nichols et al., 2013). Briefly, both BC (a carbonaceous or “sooty” material) and elemental carbon (EC) are included in this systematic review with the understanding that they have fundamentally different operational definitions (Arnott et al., 2005) and are measured using different analytical techniques. EC is composed of carbon that is not bound to other elements and for which the natural carbon structure may or may not be preserved, while BC contains EC but refers generally to the dark, components of aerosols that absorb light (WHO, 2012; US EPA, 2012). The terms BC and EC are often used interchangeably in the epidemiologic literature because they are both indicators of carbon-rich combustion sources and are strongly correlated with each other when measured by filter-based methods (U.S. EPA, 2012; Arnott et al., 2005). Epidemiologic studies of less precisely defined carbonaceous materials such as soot and black smoke are not included in this review. When comparing associations with BC to those of PM2.5 we scale to IQR increments because the use of a standard increment does not account for the fact that mass-based concentration of BC is often substantially lower than that of PM2.5 and, thus, may not reflect real-world exposure to BC.
2.2. Search strategy
A comprehensive search of the scientific literature compiled in the PubMed, Web of Science and TOXLINE databases through December 31, 2017 was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Search strings, inclusion and exclusion criteria are summarized in Fig. 1. The initial PubMed search string was developed to identify publications on BC, EC and PM2.5. The second PubMed search string was used to identify studies of cardiovascular health effects. The references were manually screened for relevance to the review (initially by author JN and updated by EFK). Titles and abstracts were subsequently screened using SWIFT-ActiveScreener (SWIFT-AS), a software application employing machine learning in real-time based on inclusions and exclusion screening decisions to predict relevant references (by EFK). Using this method, references were queued based on predicted relevance, until a 95% threshold was reached (Cohen et al., 2006; Howard et al., 2016). The full-text of the potentially relevant publications was reviewed against inclusion and exclusion criteria (by EFK) to identify studies for a subsequent risk of bias evaluation described below.
Fig. 1.
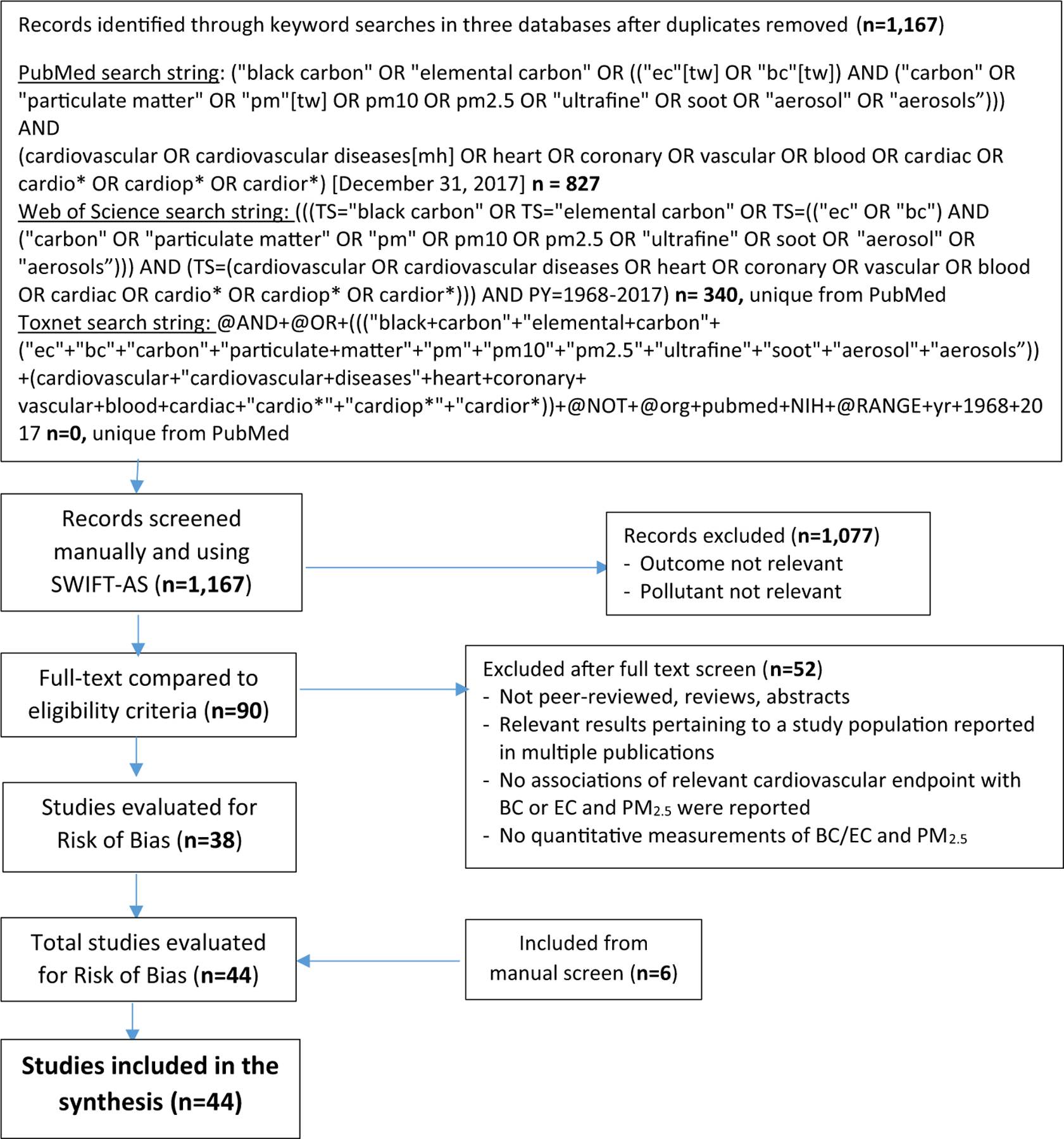
Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) flow diagram summarizing the systematic literature search, inclusion and exclusion criteria for studies of cardiovascular responses included in this review. Note: mh = MeSH headings; n = number of records; tw = text words.
Except for the endpoints examined, study inclusion criteria listed below are similar to those described by Luben et al. (2017):
Original peer-reviewed research article;
Published in the English language;
Quantitative measurement of BC or EC and PM2.5 to characterize exposure to these pollutants in outdoor air;
Associations of BC or EC and PM2.5 concentrations with inter-mediate cardiovascular endpoints reported.
The cardiovascular endpoints typically measured in short-term exposure studies (hours to days) that were included in this review were indicators of autonomic nervous system tone (i.e., heart rate variability [HRV]), heart rhythm, blood pressure and vascular function, ST segment depression, and repolarization abnormalities. Cardiovascular endpoints evaluated in long-term exposure studies were HRV, atherosclerosis and heart function. The studies of short-term exposures are discussed separately from studies of long-term exposure (months to years).
2.3. Risk of bias evaluation
Epidemiologic studies meeting inclusion criteria were evaluated for risk of bias in results and study design. Published sources on systematic review were considered when developing a risk of bias framework for use in this review (Agency for Healthcare and Quality, 2012; Higgins and Green, 2011; Rooney et al., 2014). Epidemiologic studies were evaluated by two study authors (EFK primary and AB or TJL secondary) for evidence of confounding bias, exposure misclassification, selection bias, detection bias, disease misclassification, and selective reporting. Discrepancies between authors were reconciled after the independent evaluations were completed. These study aspects could be rated as “high”, “probably high”, “probably low”, or “low” depending on the standardized evaluation criteria. In short, the methods applied to the risk of bias evaluation were adapted from the Office of Health Assessment and Translation (OHAT) risk-of-bias tool described in Rooney et al. (2014) and modified for application to the observational studies included in this review. Specifically, questions pertaining to randomization, treatment allocation groups or blinding were not used. Rather, more general questions that applied to observational studies were used and additional criteria were developed to facilitate consistent evaluation of the studies (see Supplemental text and Table 1).
2.4. Data extraction and synthesis
Study details and relevant results from epidemiologic studies that met inclusion criteria were initially extracted (EFK) and validated by a second author (TJL). Inconsistencies between the two reviewers were discussed for clarification and agreement on final reporting. Epidemiologic studies of cardiovascular responses to air pollution often examine several sub-daily, daily or longer exposure periods in relation to the endpoint measured, which may be measured continuously, in real time (e.g., ECG or ICD recordings). Because the biologically relevant timing and duration of air pollution exposure on these intermediate endpoints is not certain, we selected effect estimates to maximize comparability across studies. If results for both sub-daily and daily exposures were presented, we selected results for the 24 h average exposure because the majority of the data on PM2.5 exposures and health effects encompasses this averaging time. Otherwise, the longest duration sub-daily exposure metric was selected. If only associations with multi-day average exposures were reported, the shortest duration exposure metric was selected. Although exposures were lagged in some studies such that a defined period prior to the outcome measurement was not considered in the analysis, exposures measured concurrently relative to the outcome were preferentially selected if available. Effect estimates from fully adjusted models were extracted for display in forest plots to reflect an increase in exposure concentration equal to the IQR as reported in the study.
As noted previously, our evaluation of BC-associated health effects is considered in the context of what is already understood about the health effects of exposure to PM2.5. The scientific evidence for long- and short-term exposure to ambient PM2.5 was systematically synthesized and characterized in the Integrated Science Assessment for PM (U.S. EPA, 2009). The approach used in the Integrated Science Assessment (ISA) relies upon integration of the scientific evidence both within and across scientific disciplines (i.e. epidemiologic, experimental animal and controlled human exposure studies), and draws upon the Hill criteria for causation (Hill, 1965) emphasizing biological plausibility, consistency within lines of evidence, and coherence across lines of evidence to draw conclusions (Owens et al., 2017; U.S. EPA, 2015). Based on the application of this approach, the 2009 PM ISA concluded that there was a causal relationship between short- and long-term exposure to PM2.5 and cardiovascular effects (U.S. EPA, 2009). Although the evidence base is more limited, we apply a similar approach to BC in this study, briefly characterizing the biological plausibility and the experimental evidence that informs coherence between the epidemiologic and experimental results. Our emphasis, however, is on elucidating the similarities and/or inconsistences of the results and discernable trends, both within and across the epidemiologic studies in order to consider whether BC is a more strongly associated with cardiovascular responses than PM2.5. Results presented in plots were ordered by type of population (i.e. healthy adults, older adults, pre-existing disease).
3. Results
3.1. Search strategy and risk of bias evaluation
The PRISMA search of the PubMed, Web of Science and TOXLINE databases returned 1167 records. Ninety of these records, plus an addition 6 records identified through manual screening, were selected for a full text review after screening the title and abstract, and 40 short-(< 30-day exposure) and 4 long-term exposure (months to years) epidemiologic studies were confirmed to meet the inclusion criteria defined by the PECOS statement after the full text review. These results are summarized in the PRISMA flow diagram in Fig. 1.
The risk of bias framework developed for this series of reviews addresses relevant biases encountered in the field of epidemiology. Overall, the risk of bias assigned to studies evaluated in this review was either “low” or “probably low” (Fig. 2). The methods and study designs applied by various investigators were similar across studies and study attributes that would constitute “high” or “probably high” risk of bias, were not identified. The majority of differences in risk of bias evaluations across studies stemmed from different exposure assessment methods. We distinguished methods that derived exposure estimates by averaging concentrations from centrally located monitors from those that better characterized spatial and temporal variability of PM2.5 and BC. Additional information regarding the specific questions used to evaluate these biases, criteria for assigning a risk of bias category, and the evaluation of results for all studies are provided in Supplemental materials (Risk of Bias Evaluation Summary and Supplemental Table 1.) Study design is also noted in the text of the supplement and in Supplemental Table 1.
Fig. 2.
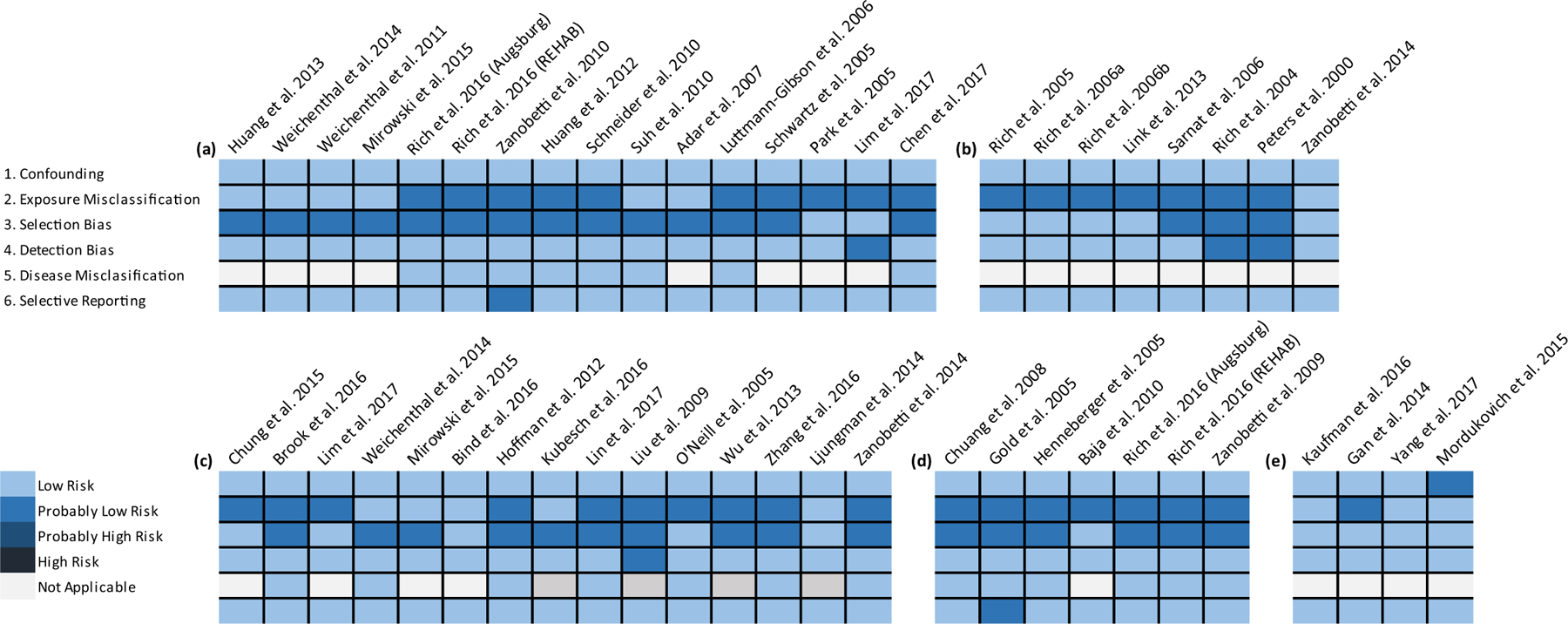
Risk of bias evaluation for epidemiologic studies of subclinical cardiovascular endpoints (a) Heart Rate Variability; (b) Arrhythmia; (c) Blood Pressure and Vascular Function; (d) Ischemia and Repolarization Abnormalities (e) Long-term Exposure. Note: Rich et al., 2016 report includes findings for the Augsburg Germany and REHAB (Rochester, NY) studies.
3.2. Cardiovascular responses associated with short-term exposure to BC (or EC) and PM2.5
3.2.1. Autonomic nervous system tone
HRV provides a noninvasive marker of cardiac autonomic nervous system tone. The variation in the intervals between heartbeats can be quantified in either the time domain or the frequency domain (TFESC and NASPE, 1996). Common time domain measures of HRV include the standard deviation of all normal to-normal intervals (SDNN, an index of total HRV), the root mean-square of successive differences (rMSSD, an index influenced mainly by the parasympathetic nervous system) and pNN50 (proportion of NN50 divided by the total number of NN (R-R) intervals). In the frequency domain, HRV is usually divided into the high frequency (HF [generally reflecting parasympathetic activity]) and low frequency (LF [generally reflecting sympathetic activity]) components, as well as the ratio of the LF to HF components (LFHFR) (TFESC and NASPE, 1996). Decreases in indices of HRV have been associated with increased risk of cardiovascular events in prospective cohort studies (La Rovere et al., 2003; Kikuya et al., 2000; Tsuji et al., 1994, 1996). Overall reduction in HRV is a powerful predictor of adverse cardiac events in individuals with pre-existing heart disease and the general population (Zulfiqar et al., 2010; La Rovere et al., 2003; Kleiger et al., 1987), although it is recognized that acute changes in HRV in response to pollutant exposure may not be persistent (Rowan et al., 2007).
A total of 16 studies of short-term exposure to BC/EC or PM2.5 with HRV meeting the inclusion criteria were identified for this review. Characteristics of the studies highlighted below and including Chen et al. (2017), Huang et al. (2013) and Schneider et al. (2010), are compiled in Supplemental Table 2. The most commonly reported metrics of HRV were SDNN, rMSSD, pNN50, LF, HF and LFHFR, but most studies did not present associations for each of these metrics. Study design varied with panel, repeated measures, prescribed exposures, and cross-sectional analyses available for inclusion in this review.
Plots comparing selected results from studies that present associations for BC/EC and PM2.5 and time domain measures of HRV are presented in Fig. 3. Results are organized by type of population (i.e. health adults, pre-existing disease, and older adults). Overall, associations with BC/EC were similar to associations with PM2.5 for most studies and HRV metrics. Where point estimates were less similar, confidence intervals were generally relatively wide and overlapping. An exception is Luttmann-Gibson et al. (2006) who reported a larger decrease in SDNN associated with PM2.5. By contrast, Suh and Zanobetti (2010) examined the impact of differential exposure misclassification between PM2.5 and BC reporting markedly stronger magnitude associations of personal exposure to BC with decreased SDNN (shown in Fig. 3) and relatively weak or null associations with 24-hour ambient exposure to BC or PM2.5 (not shown in Fig. 3).
Fig. 3.
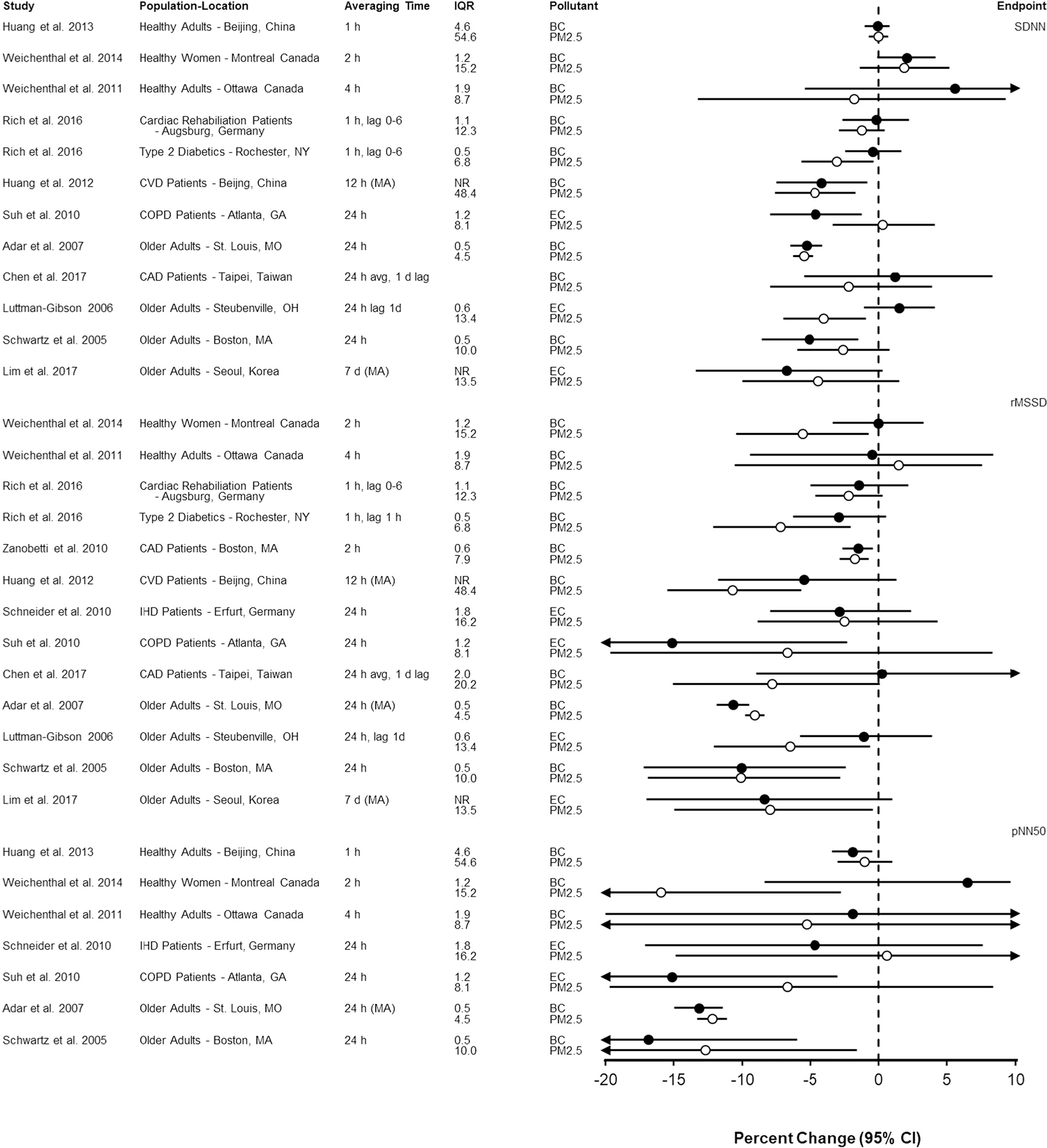
Association of short-term exposure to black carbon or elemental carbon (black circles) and particulate matter < 2.5 μm in diameter (open circles) with time domain measures of heart rate variability per interquartile range increase in mean (or median) pollutant concentration (in μg/m3). Studies are organized by endpoint (SDNN, rMSSD, pNN50), population type (i.e., healthy, pre-existing disease, older adults) and averaging time (i.e., shortest averaging time first). Circles represent point estimates and horizontal lines represent 95% confidence intervals. BC = black carbon; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CVD = cardiovascular disease; d = day; EC = elemental carbon; h = hour; IQR = interquartile range; MA = moving average; pNN50 = proportion of successive NNs that differ by > 50 ms; rMSSD = root mean-square of successive differences; SD: standard deviation; SDNN = standard deviation of all normal to-normal intervals.
Results of several studies included in Supplemental Table 2 are not pictured in Fig. 3 because they could not be standardized to the IQR or were reported only in figures. Mirowsky et al. (2015) reported that decreased rMSSD was associated with EC but not PM2.5 in their case cross-over study of healthy adults walking along roadways in New Jersey. Park et al. (2005) found both PM2.5 and BC were associated with decreases in SDNN of similar magnitude with wide, largely overlapping confidence intervals. Zanobetti et al. (2010) presented figures showing that BC was associated with reduced SDNN while PM2.5 was not associated with SDNN. Findings for HF were similar across PM2.5 and BC for moving averages up to 2 h but only BC was associated with HF for longer moving averages in this study.
A generally similar pattern of results for frequency domain measure of HRV is apparent in Fig. 4. Luttmann-Gibson et al. (2006) and Huang et al. (2012) reported larger decreases of LF and HF, respectively, in association with PM2.5 compared to BC. In a study using centrally located monitors to characterize exposure, Schwartz et al. (2005) reported a larger increase in LFHFR in association with EC compared to PM2.5. Adar et al. (2007) measured exposure using real time monitors while study participants were riding a bus and observed a larger decrease in HF and LFHFR in association with BC than PM2.5.
Fig. 4.
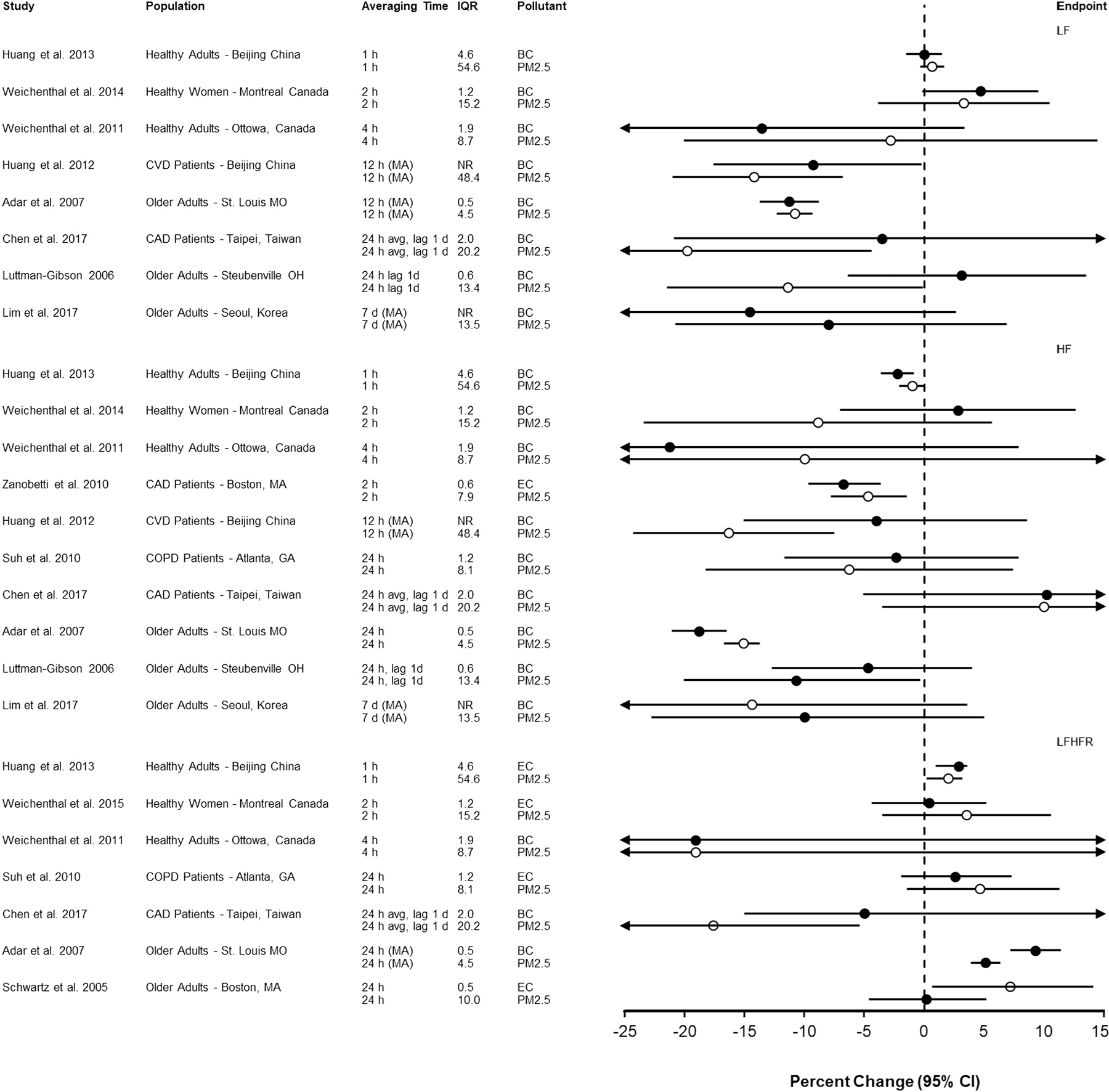
Association of short-term exposure to black carbon or elemental carbon (black circles) and particulate matter < 2.5 μm in diameter (open circles) with frequency domain measures of heart rate variability per interquartile range increase in mean (or median) pollutant concentration (in μg/m3). Studies are organized by endpoint (LF, HF, LFHFR), population type (i.e., healthy, pre-existing disease, older adults) and averaging time. Circles represent point estimates and horizontal lines represent 95% confidence intervals. BC = black carbon; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; CVD = cardiovascular disease; d = day; EC = elemental carbon; h = hour; HF = high frequency; IQR = interquartile range; LF = low frequency; LFHFR = low frequency high frequency ratio; MA = moving average.
3.2.2. Heart rhythm abnormalities
Heart rhythm abnormalities or arrhythmias can originate in the upper (atria) or lower (ventricles) chambers of the heart and range in severity. Ectopy (supraventricular ectopy [SVE] and ventricular ectopy [VE]), or premature heartbeats, may indicate risk for severe arrhythmias. Atrial fibrillation (AF) is the most common sustained arrhythmia and is associated with various forms of cardiovascular disease and mortality while ventricular arrhythmia (VA) is a well-known cause of sudden death (Laupacis et al., 1994; Prystowsky et al., 1996; Roy et al., 2009; Kannel et al., 1983). Arrhythmia is ascertained by examining output from implantable cardioverter defibrillator (ICD) devices or ECG recordings.
A total of 8 studies of heart rhythm abnormalities or arrhythmias meeting our inclusion criteria were identified. Findings from these studies are organized by type in Fig. 5 (and Supplemental Table 3, which includes the studies highlighted below and results for Rich et al. (2005), Rich et al. (2006a), Rich et al. (2006b) and Sarnat et al. (2006)). Overall, point estimates are similar for BC or EC and PM2.5 and confidence intervals are overlapping in all of these studies. Most were repeated measures or case-crossover designs and all relied on central site monitors to estimate exposure to BC and PM2.5. One notable exception is Zanobetti et al. (2014a, 2014b) in which authors estimated BC and PM2.5 concentrations using methods to maximize spatial and temporal resolution. These authors found similar magnitude associations for 24 h average BC and PM2.5 concentrations with arrhythmia episode. ICD discharge results for Rich et al. (2004), which were null for both BC and PM2.5, are not included in Fig. 5 because quantitative results were not available.
Fig. 5.
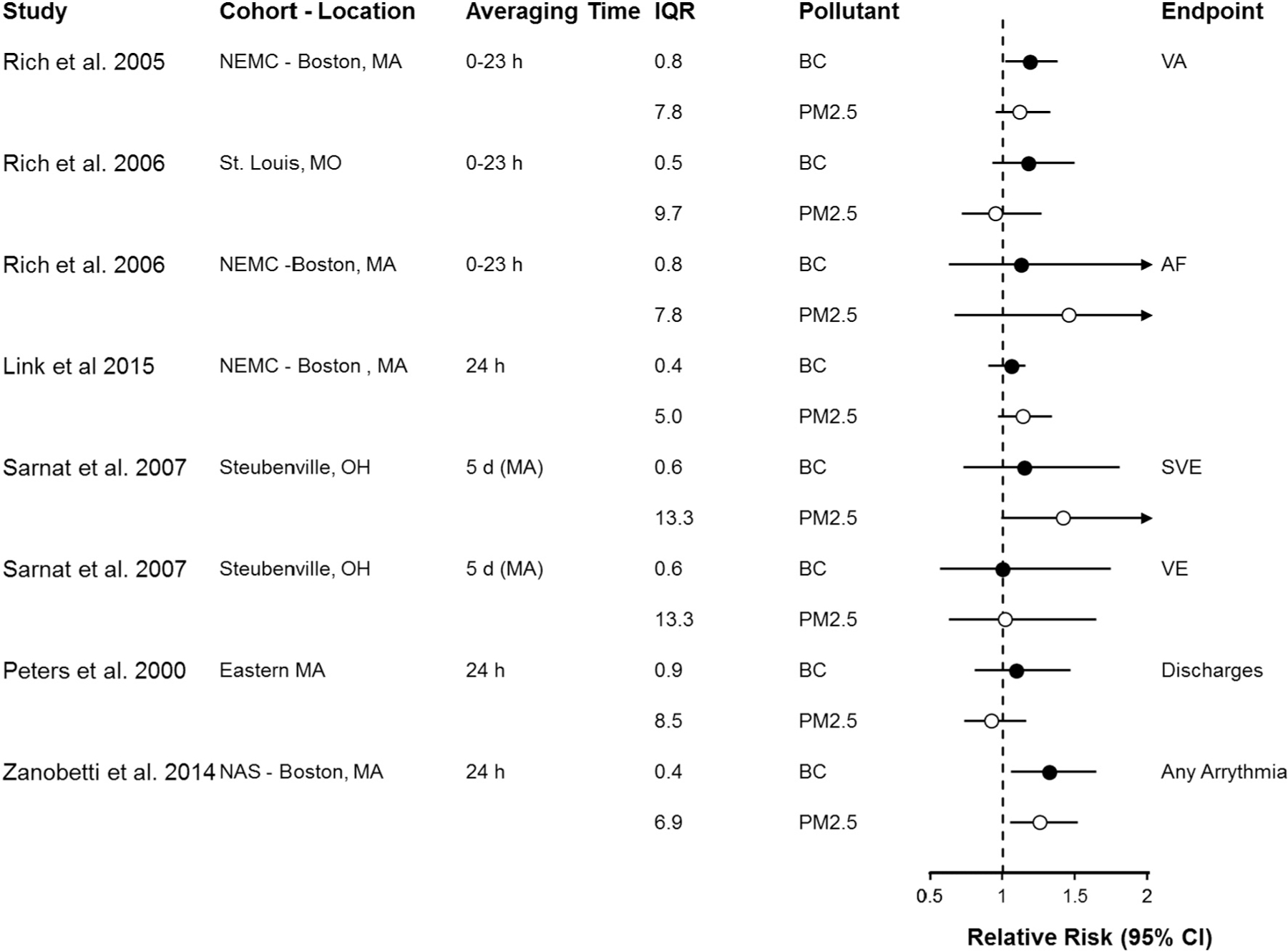
Association of short-term exposure to black carbon or elemental carbon (black circles) and particulate matter < 2.5 μm in diameter (open circles) with arrhythmia recorded on implantable cardioverter defibrillator or electrocardiogram, per interquartile range increase in mean (or median) pollutant concentration (in μg/m3). Studies are organized by endpoint (VA, AF, SVE, discharges, any arrhythmia), population type (i.e., healthy, pre-existing disease, older adults) and averaging time. Circles represent point estimates and horizontal lines represent 95% confidence intervals. BC = black carbon; EC = elemental carbon; h = hour; IQR = interquartile range; MA = moving average; PM2.5 = particulate matter < 2.5 μm in diameter; VA = ventricular arrhythmia; AF = atrial fibrillation; SVE = supraventricular ectopy; VE = ventricular ectopy.
3.2.3. Blood pressure and vascular function
The vascular endothelium plays a fundamental role in the maintenance of vascular tone and the regulation of blood pressure and blood flow. Studies of blood pressure report several metrics including systolic blood pressure (SBP), diastolic blood pressure (SBP), mean arterial pressure (MAP) and pulse pressure (PP). Endothelium dependent vascular reactivity is measured through a variety of clinical tests that ascertain the extent to which blood flow, vessel tone and diameter respond to hyperemia or nitroglycerin. The epidemiologic studies available to evaluate the association of BC and PM2.5 with blood pressure included 14 studies. In addition, six studies of vascular reactivity endpoints including flow mediated dilation (FMD) or nitroglycerin (nitroglycerin mediated dilation [NMD]) reactive hyperemia index (RHI), and peripheral arterial tonometry (PAT) ratio were identified (Supplemental Table 4).
Associations of BC and PM2.5 with SBP and DBP were not consistently observed across studies and, where associations were reported, confidence intervals for PM2.5 and BC were generally overlapping (Chung et al., 2015; Brook et al., 2016; Lim et al., 2017; Weichenthal et al., 2014; Hoffmann et al., 2012; Kubesch et al., 2015; Lin et al., 2017; Wu et al., 2013). Although strong associations of both BC and PM2.5 with FMD and NMD were observed by O’Neill et al. (2005) among diabetics, confidence intervals were overlapping. A study of healthy women cycling in high and low traffic routes did not provide evidence that PM2.5 or BC were associated with changes in RHI (Weichenthal et al., 2014). Ljungman et al. (2014) reported associations of both BC and PM2.5 with baseline pulse amplitude but not PAT ratio, with little evidence to support a difference between BC and PM2.5 in this study.
There were some exceptions where differences in the magnitude of the association between BC and PM2.5 could be discerned. Bind et al. (2016) reported a larger association of BC than PM2.5 with SBP. Mirowsky et al. (2015) found larger changes in blood pressure (SBP, DBP, PP, MAP) in association with EC compared to PM2.5 in their study of healthy adults walking along roadways in New Jersey. Zhang et al. (2016) reported a somewhat larger magnitude association between BC and RHI than between PM2.5 and RHI. Zanobetti et al. (2014a, 2014b) found a stronger magnitude association between ambient BC and NMD than between PM2.5 and NMD. Associations of both pollutants with FMD were similar in magnitude but imprecise; however, Liu et al. (2009) reported significant associations of BC with SBP, DBP and BAD while PM2.5 was only associated with DBP.
3.2.4. Ischemia and repolarization abnormalities
Seven studies of ST segment depression or repolarization abnormalities were identified for this review including those discussed below and including Baja et al. (2010) (Supplemental Table 5.) The ST segment of the ECG represents the interval between ventricular depolarization and repolarization and is often used as a nonspecific measure of myocardial ischemia. The QT interval and T Wave complexity provide ECG markers of ventricular repolarization. Prolongation and increased variability of the QT interval are associated with increased risk of life-threatening ventricular arrhythmias (Castro-Torres et al., 2015; Brook et al., 2004).
Chuang et al. (2008) and Gold et al. (2005) found small (0.02–0.08 mm) changes in ST segment depression in association with both BC and PM2.5. Associations of similar magnitudes and with overlapping confidence intervals were also reported by Henneberger et al. (2005) who examined several repolarization abnormalities (i.e., QTc Interval, T wave complexity, T wave variability, T wave amplitude). Rich et al. (2016) conducted a factor analysis to reduce the number of ECG outcomes included in the analysis and focused on T wave complexity. These authors did not find consistent changes in T wave complexity across the Augsburg or Rochester panel studies. Zanobetti et al. (2009) reported a stronger association of T wave alternans ≥26 μV with BC than with PM2.5. Overall, the limited number of studies evaluated point to an effect of both BC and PM2.5 on ST segment depression while there was no consistent trend for the relationship of these pollutants with repolarizations abnormalities.
3.3. Cardiovascular responses associated with long-term exposure to BC (or EC) and PM2.5
Four studies that examined the association between long-term exposure to BC and PM2.5 with intermediate cardiovascular endpoints that met our inclusion criteria were identified (Supplemental Table 6). Kaufman et al. (2016) reported increased coronary artery calcification (CAC) progression during the follow-up period, in association with PM2.5 but not in association with BC. These analyses of the Multi Ethnic Study of Atherosclerosis (MESA) cohort did not support an effect of either BC or PM2.5 on increased carotid intima media thickness (cIMT). Similarly, Gan et al. (2014) reported little evidence of an effect of BC or PM2.5 on cIMT progression (or progression of other measures of atherosclerosis, Supplemental Table 6). In another study, Yang et al. (2017) examined left ventricular structure and function in relation to long-term exposure to BC and PM2.5. Although these analyses supported effects on left ventricular function assessed by ejection fraction and left atrial volume index (as well as additional metrics not included in Supplemental Table 5), the magnitude of the associations with BC and PM2.5 were similar. Finally, Mordukhovich et al. (2015) reported associations of long-term exposure to BC and PM2.5 with HRV (SDNN, LF, HF, LFHFR) that were imprecise, often spanning the null value, with generally overlapping confidence intervals.
4. Discussion
As discussed previously, our evaluation of BC-associated health effects is considered in the context of what is already understood about the health effects of PM2.5 with the objective of understanding whether or not BC (or EC), which are components of PM2.5, have stronger associations with cardiovascular effects or distinct cardiovascular responses, compared to PM2.5 as a whole. In addition, understanding the available evidence pertaining to the cardiovascular responses included in this review may elucidate the extent to which exposure to BC or EC and PM2.5 perturb specific disease pathways and lead to various cardiovascular diseases. With these objectives in mind, we chose to standardize the estimates from each study to an increase in concentration equal to the IQR for each pollutant. Scaling to the IQR de-emphasizes the evaluation of the relative toxicity of BC (or EC) versus PM2.5 in favor of an evaluation of observed associations due to real-world ambient exposures, where EC typically represents < 10% of PM2.5 mass (Bell et al., 2007).
Application of PRISMA guidelines and systematic evaluation of risk of bias allowed us to avoid the selective inclusion of studies as well as to critically evaluate each of the included studies according to the same criteria. We used a combination of approaches including SWIFT-AS, which relies on machine learning to identify and predict relevant references, and manual screening of titles and abstracts to identify potentially relevant references. Overall, we observed very little difference across studies regarding risk of bias because the study designs and methods were similar (Supplemental Table 1). All the studies identified in the literature search were deemed of sufficient quality for inclusion in the review.
Specific study results were selected for extraction to maximize the number of studies that could be compared. For example, associations with the most common averaging time (i.e. concurrent 24-hour exposures) were selected if available. If 24-hour concurrent exposure was not examined in a particular study, the longest sub-daily or shortest daily lag was selected. As a result of this practical decision, we could not consider timing of exposure in our analysis, although multiple studies presented associations for multiple averaging and lag times. Despite some uncertainty, the evidence for short-term PM2.5 exposure and cardiovascular effects generally supports an immediate effect in the range of lag 0 to 1 day, however (U.S. EPA, 2009).
A small number of the studies included in this review examined cardiovascular responses in healthy populations during prescribed exposures (e.g. riding a bike near a busy road or other source of exposure), while other studies examined cardiovascular responses in people with preexisting disease or among older adults. The heterogeneity of the populations studied may limit the generalizability of the specific studies and, in addition, limit the extent to which findings can be compared across studies. Hence, our emphasis is on comparison of findings within studies and across studies of similar populations.
The results of most studies included in our systematic review support a similar risk or overlapping confidence intervals for the associations of BC or EC and PM2.5 with indicators of autonomic nervous system tone (i.e., heart rate variability), heart rhythm, blood pressure and vascular function, ST segment depression, repolarization abnormalities, atherosclerosis and heart function. Although there were some differences in the magnitude of the single pollutant associations across pollutants within studies, most noticeably in the studies of blood pressure and vascular function, no consistent pattern was discernable across the group of studies we evaluated. This finding is consistent with previous reviews focusing on hospital admissions and emergency department visits for cardiovascular disease (Luben et al., 2017) and populations with pre-existing disease (Nichols et al., 2013). Our results are also coherent with experimental studies of animals and humans that demonstrate effects on cardiovascular endpoints following exposures used to mimic ambient pollution such as concentrated ambient particles (CAPs) and diesel exhaust (Lippmann and Chen, 2009; Lippmann, 2009). For example, Gong et al. (2003) conducted a series of experimental studies in humans and found that CAPs exposures resulted in changes in HR and HRV. Campen et al. (2010) and Bai et al. (2011) reported diesel exhaust-related changes in metrics related to atherosclerosis in ApoE−/− mice.
To fully explore whether BC or EC are more closely associated with cardiovascular effects than PM2.5, the extent to which the effect of each is statistically independent is an important consideration. Several experimental studies in humans, dogs, and rats included both PM2.5 and BC or EC simultaneously in multiple regression models. This set of studies did not provide consistent evidence that changes in cardiovascular endpoints including heart rate variability and endothelial dysfunction were more consistently demonstrated following exposure to either PM2.5 or BC/EC (Urch et al., 2004; Kamal et al., 2011; Gong et al., 2003, 2008; Clarke et al., 2000; Bartoli et al., 2009; Ghelfi et al., 2008).
This review identified a limited body of epidemiologic studies that were designed to examine whether associations observed with BC or EC were statistically independent of the effect of PM2.5 or vice versa. For example, Huang et al. (2012) reported similar magnitude reductions in SDNN and LF in association with both PM2.5 and BC. In two pollutant models these associations with PM2.5 persisted after adjustment for BC. Lim et al. (2017) reported associations of EC that were adjusted for PM2.5 mass, which were similar to the single pollutant associations, although less precise. Chuang et al. (2008) reported results from two-pollutant models showing attenuation in the association of ST segment depression with PM2.5 while the association with BC remained after adjustment. Overall, no consistent pattern of results emerged from the evaluation of this set of studies.
There are complications involved with distinguishing the effects of PM2.5 on health from those of its components. Mostofsky et al. (2012) noted that the use of two-pollutant models, such as those described above, may result in an over-adjustment or model convergence issues due to collinearity if pollutants are highly correlated. Most correlations between PM2.5 and BC reported in short-term exposure studies were moderate to high (i.e., r > 0.5 in most studies reporting this parameter) suggesting the potential for confounding. The correlations in the limited number of long-term exposure studies included in this review were more variable and reported correlations ranging from 0.13 (Gan et al., 2014) to 0.89 (Kaufman et al., 2016).
The potential for differential exposure measurement error (i.e. exposure measurement error that is different for PM2.5 versus its components) also has implications for the interpretation of our findings. Studies using centrally located monitors for exposure assessment were assigned a risk of bias score of “probably low” in order to maintain consistency with the approach taken in our previous systematic review (Luben et al., 2017). However, it is known that BC concentrations are more spatially variable than PM2.5 concentrations (Clougherty et al., 2008). Consequently, we recognize the potential for the exposure error for PM2.5 to be different than the exposure error for BC/EC, particularly in studies that rely upon monitors located at various distances from subjects’ homes or clinic locations to estimate exposure. In fact, it has been suggested that attenuation of the association between local pollutants such as BC and health effects in epidemiologic studies using centrally located monitors may be substantial (Dionisio et al., 2014).
Overall, the studies included in this review did not provide information needed to assess the use of centrally located monitors in a particular study was appropriate to characterize BC or EC exposure. Many studies reported the distance from the monitor to the medical clinic or participants’ residence (Supplemental Tables 2–5) in an effort to provide some information about the extent to which the centrally located monitor was appropriate exposure metric, but the appropriate distance is not known. Link et al. (2013) conducted a sensitivity analysis limiting the analysis to participants residing within 26 km as opposed to farther from the monitoring station, reporting that the associations of PM2.5 and BC with AF persisted but were less precise. Further, time activity patterns that determine exposure to ambient air pollutants were not examined in the studies included in this review.
Several studies applied methods designed to reduce exposure measurement error. Suh and Zanobetti (2010) examined the effect of exposure error by comparing the associations observed for HRV with EC, PM2.5 and other pollutants depending on whether personal or ambient exposure metrics were used in the analysis. Authors report larger decreases across all HRV metrics with personal exposure to BC compared to ambient BC exposure. No similar pattern was observed for PM2.5, and associations with PM2.5 were generally weaker in magnitude than those for EC. Stronger magnitude associations between BC or EC and cardiovascular endpoints (compared to associations with PM2.5) are not consistently observed in studies that employ exposure assessment methods designed to achieve greater characterization of the spatial variability of the pollutant concentrations (Adar et al., 2007; Mirowsky et al., 2015; Weichenthal et al., 2014; Weichenthal et al., 2011; Zanobetti et al., 2014a, 2014b).
5. Conclusion
Overall, we found generally similar magnitude associations of BC (or EC) and PM2.5 with the cardiovascular endpoints examined in the majority of studies. The evidence did not suggest that BC would be a better indicator of cardiovascular effects than PM2.5. Confidence intervals for the associations of BC (or EC) and PM2.5 were overlapping in most studies. Where differences in the magnitude of the single pollutant associations were observed across pollutants within a study, no consistent pattern was discernable across the literature as a whole. Although timing of exposure was an important consideration in many studies, we were limited in our ability to consider exposure lags. There was a general lack of consistency in the lags reported across studies and, consequently an insufficient number of results to compare. Further, only a limited number of studies attempted to distinguish the independent effect of BC or EC from that of PM2.5 or examine the effect of exposure measurement error on their results. The interplay between these two factors can lead to possibly differential bias in the observed associations and future studies would benefit from applying methods designed to address these issues.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Rebecca Nachman and Dr. Aimen Farraj for comments on drafts of this work and Beth Gatling and Ryan Jones for their oversight and implementation of the literature search. This work is supported in part by an appointment to the Internship/Research Participation Program at Office of Research and Development (National Center for Environmental Assessment), U.S. Environmental Protection Agency, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and EPA.
Footnotes
Disclaimer
This manuscript has been reviewed by the U.S. Environmental Protection Agency and approved for publication. The views expressed in this manuscript are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Financial interests’ declaration
The authors declare that they have no actual or potential competing financial interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2019.02.027.
References
- Adar SD, Gold DR, Coull BA, Schwartz J, Stone PH, Suh H, 2007. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology 18, 95–103. [DOI] [PubMed] [Google Scholar]
- Agency for Healthcare Research and Quality, 2012. Grading the Strength of a Body of Evidence When Assessing Health Care Interventions–AHRQ and the Effective Health Care Program: An Update: Draft Report Agency for Healthcare Research and Quality, Rockville, MD. http://effectivehealthcare.ahrq.gov/ehc/products/457/1163/gradingthestrengthofevidence_draftmethodschapter_20120625.pdf. [PubMed] [Google Scholar]
- Arnott WP, Zielinska B, Rogers CF, Sagebiel J, Park K, Chow J, Moosmuller H, Watson JG, Kelly K, Wagner D, Sarofim A, Lighty J, Palmer G, 2005. Evaluation of 1047-nm photoacoustic instruments and photoelectric aerosol sensors in source-sampling of black carbon aerosol and particle-bound PAHs from gasoline and diesel powered vehicles. Environ. Sci. Technol 39, 5398–5406. [DOI] [PubMed] [Google Scholar]
- Bai N, Kido T, Suzuki H, Yang G, Kavanagh TJ, Kaufman JD, Rosenfeld ME, van Breemen C, Eeden SF, 2011. Changes in atherosclerotic plaques induced by inhalation of diesel exhaust. Atherosclerosis 216 (2), 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baja ES, Schwartz JD, Wellenius GA, Coull BA, Zanobetti A, Vokonas PS, Suh HH, 2010. Traffic-related air pollution and QT interval: modification by diabetes, obesity, and oxidative stress gene polymorphisms in the Normative Aging Study. Environ. Health Perspect 118, 840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli CR, Wellenius GA, Diaz EA, Lawrence J, Coull BA, Akiyama I, Lee LM, Okabe K, Verrier RL, Godleski JJ, 2009. Mechanisms of inhaled fine particulate air pollution-induced arterial blood pressure changes. Environ. Health Perspect 117 (3), 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LK, Duvall RM, Sacks J, 2012. Examining the effects of air pollution composition on within region differences in PM2.5 mortality risk estimates. J. Expo. Sci. Environ. Epidemiol 23 (5), 457–465. [DOI] [PubMed] [Google Scholar]
- Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM, 2007. Spatial and temporal variation in PM(2.5) chemical composition in the United States for health effects studies. Environ. Health Perspect 115 (7), 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bind MA, Peters A, Koutrakis P, Coull B, Vokonas P, Schwartz J, 2016. Quantile regression analysis of the distributional effects of air pollution on blood pressure, heart rate variability, blood lipids and biomarkers of inflammation in elderly American men: the Normative Aging Study. Environ. Health Perspect 124, 1189–1198. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC Jr., Tager I, 2004. Air pollution and cardiovascular disease: a statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation 109, 2655–2671. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr., Whitsel L, Kaufman JD, 2010. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378. [DOI] [PubMed] [Google Scholar]
- Brook RD, Sun Z, Brook JR, Zhao X, Ruan Y, Yan J, Mukherjee B, Rao X, Duan F, Sun L, Liang R, Lian H, Zhang S, Fang Q, Gu D, Sun Q, Fan Z, Rajagopalan S, 2016. Extreme air pollution conditions adversely affect blood pressure and insulin resistance the air pollution and cardiometabolic disease study. Hypertension 67, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen MJ, Lund AK, Knuckles TL, Conklin DJ, Bishop B, Young D, Seilkop S, Seagrave J, Reed MD, Mcdonald JD, 2010. Inhaled diesel emissions alter atherosclerotic plaque composition in ApoE(-/-) mice. Toxicol. Appl. Pharmacol 242 (3), 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Torres Y, Carmona-Puerta R, Katholi RE, 2015. Ventricular repolarization markers for predicting malignant arrhythmias in clinical practice. World J. Clin. Cases 3 (8), 705–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Chan CC, Su TC, 2017. Particulate and gaseous pollutants on inflammation, thrombosis, and autonomic imbalance in subjects at risk for cardiovascular disease. Environ. Pollut 223, 403–408. . [DOI] [PubMed] [Google Scholar]
- Chuang KJ, Coull BA, Zanobetti A, Suh H, Schwartz J, Stone PH, Litonjua A, Speizer FE, Gold DR, 2008. Particulate air pollution as a risk factor for ST-segment depression in patients with coronary artery disease. Circulation 118, 1314–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M, Wang DD, Rizzo AM, Gachette D, Delnord M, Parambi R, Kang CM, Brugge D, 2015. Association of PNC, BC, and PM2.5 measured at a central monitoring site with blood pressure in a predominantly near highway population. Int. J. Environ. Res. Public Health 12, 2765–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RW, Coull B, Reinisch U, Catalano P, Killingsworth CR, Koutrakis P, Kavouras I, Murthy GGK, Lawrence J, Lovett E, Wolfson JM, Verrier RL, Godleski JJ, 2000. Inhaled concentrated ambient particles are associated with hematologic and bronchoalveolar lavage changes in canines. Inhal. Toxicol 108 (12), 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty JE, Wright RJ, Baxter LK, Levy JI, 2008. Land use regression modeling of intra-urban residential variability in multiple traffic-related air pollutants. Environ. Health 7, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AM, Hersh WR, Peterson K, Yen PY, 2006. Reducing workload in systematic review preparation using automated citation classification. J. Am. Med. Inform. Assoc 13, 206–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Meng Q, Sacks JD, Dutton SJ, Wilson WE, Pinto JP, 2011. Regional variations in particulate matter composition and the ability of monitoring data to represent population exposures. Sci. Total Environ 409, 5129–5135. [DOI] [PubMed] [Google Scholar]
- Dionisio KL, Baxter LK, Chang HH, 2014. An empirical assessment of exposure measurement error and effect attenuation in bipollutant epidemiologic models. Environ. Health Perspect 122, 1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan WQ, Allen RW, Brauer M, Davies HW, Mancini GB, Lear SA, 2014. Long-term exposure to traffic-related air pollution and progression of carotid artery atherosclerosis: a prospective cohort study. Br. Med. J. Open 4, e004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelfi E, Rhoden CR, Wellenius GA, Lawrence J, Gonzalez-Flecha B, 2008. Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated ambient particles are mediated by TRP-dependent pulmonary reflexes. Toxicol. Sci 102 (2), 328–336. [DOI] [PubMed] [Google Scholar]
- Gold DR, Litonjua AA, Zanobetti A, Coull BA, Schwartz J, Maccallum G, Verrier RL, Nearing BD, Canner MJ, Suh H, Stone PH, 2005. Air pollution and ST-segment depression in elderly subjects. Environ. Health Perspect 113, 883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H Jr., Linn WS, Sioutas C, Terrell SL, Clark KW, Anderson KR, Terrell LL, 2003. Controlled exposures of healthy and asthmatic volunteers to concentrated ambient fine particles in Los Angeles. Inhal. Toxicol 15 (4), 305–325. [DOI] [PubMed] [Google Scholar]
- Gong H Jr., Linn WS, Clark KW, Anderson KR, Sioutas C, Alexis NE, Cascio WE, Devlin RB, 2008. Exposures of healthy and asthmatic volunteers to concentrated ambient ultrafine particles in Los Angeles. Inhal. Toxicol 20 (6), 533–545. [DOI] [PubMed] [Google Scholar]
- Henneberger A, Zareba W, Ibald-Mulli A, Rückerl R, Cyrys J, Couderc JP, Mykins B, Woelke G, Wichmann HE, Peters A, 2005. Repolarization changes induced by air pollution in ischemic heart disease patients. Environ. Health Perspect 113, 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S, 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration; Available from. www.cochrane-handbook.org. [Google Scholar]
- Hill AB, 1965. The environment and disease: association or causation? Proc. R. Soc. Med 58 (5), 295–300. [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Luttmann-Gibson H, Cohen A, et al. , 2012. Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environ. Health Perspect 120, 241–246. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard BE, Phillips J, Miller K, Tandon A, Mav D, Shah MR, Holmgren S, Pelch KE, Walker V, Rooney AA, MaCleod M, Shah RR, Thayer K, 2016. SWIFT-Review: a text-mining workbench for systematic review. Syst. Rev 5, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhu T, Pan X, Hu M, Lu SE, Lin Y, Wang T, Zhang Y, Tang X, 2012. Air pollution and autonomic and vascular dysfunction in patients with cardiovascular disease: interactions of systemic inflammation, overweight, and gender. Am. J. Epidemiol 176, 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Deng F, Wu S, Lu H, Hao Y, Guo X, 2013. The impacts of short-term exposure to noise and traffic-related air pollution on heart rate variability in young healthy adults. J. Expo. Sci. Environ. Epidemiol 23, 559–564. [DOI] [PubMed] [Google Scholar]
- Janssen NA, Hoek G, Simic-Lawson M, Fischer P, van Bree L, Ten Brink H, Keuken M, Atkinson RW, Anderson HR, Brunekreef B, Cassee FR, 2011. Black carbon as an additional Indicator of the adverse health effects of airborne particles compared with PM10 and PM2.5. Environ. Health Perspect 119, 1691–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal AS, Rohr AC, Mukherjee B, Morishita M, Keeler GJ, Harkema JR, Wagner JG, 2011. PM2.5-induced changes in cardiac function of hypertensive rats depend on wind direction and specific sources in Steubenville, Ohio. Inhal. Toxicol 23 (7), 417–430. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Abbott RD, Savage DD, McNamara PM, 1983. Coronary heart disease and atrial fibrillation: the Framingham Study. Am. Heart J 106, 389–396. [DOI] [PubMed] [Google Scholar]
- Kaufman JP, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, Diez Roux AV, Gassett AJ, Jacobs DR Jr., Kronmal R, Larson TV, Navas-Acien A, Olives C, Sampson PD, Sheppard L, Siscovick DS, Stein JH, Szpiro AA, Watson KE, 2016. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet 388, 696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y, 2000. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension 36, 901–906. [DOI] [PubMed] [Google Scholar]
- Kleiger RE, Miller JP, Bigger JT Jr., Moss AJ, Multicenter Post-Infarction Research Group, 1987. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol 59, 256–262 [DOI] [PubMed] [Google Scholar]
- Kubesch N, De Nazelle A, Guerra S, et al. , 2015. Arterial blood pressure responses to short-term exposure to low and high traffic-related air pollution with and without moderate physical activity. Eur. J. Prev. Cardiol 2015 (22), 548–557. . [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C, Riccardi PG, Traversi E, Cobelli F, 2003. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 107, 565–570. [DOI] [PubMed] [Google Scholar]
- Laupacis A, Boysen G, Connolly S, 1994. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch. Intern. Med 154, 1449–1457. [PubMed] [Google Scholar]
- Lim YH, Bae HJ, Yi SM, Park E, Lee BE, Hong YC, 2017. Vascular and cardiac autonomic function and PM2.5 constituents among the elderly: a longitudinal study. Sci. Total Environ 607–608, 847–854. [DOI] [PubMed] [Google Scholar]
- Lin Z, Niu Y, Chen R, et al. , 2017. Fine particulate matter constituents and blood pressure in patients with chronic obstructive pulmonary disease: a panel study in Shanghai, China. Environ. Res 159, 291–296. . [DOI] [PubMed] [Google Scholar]
- Link MS, Luttmann-Gibson H, Schwartz J, Mittleman MA, Wessler B, Gold DR, Dockery DW, Laden F, 2013. Acute exposure to air pollution triggers atrial fibrillation. J. Am. Coll. Cardiol 62, 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M, 2009. Semi-continuous speciation analyses for ambient air particulate matter: an urgent need for health effects studies. J. Expo. Sci. Environ. Epidemiol 19, 235–247. [DOI] [PubMed] [Google Scholar]
- Lippmann M, Chen LC, 2009. Health effects of concentrated ambient air particulate matter (CAPs) and its components. Crit. Rev. Toxicol 39, 895–913. [DOI] [PubMed] [Google Scholar]
- Liu L, Ruddy T, Dalipaj M, et al. , 2009. Effects of indoor, outdoor, and personal exposure to particulate air pollution on cardiovascular physiology and systemic mediators in seniors. J. Occup. Environ. Med 51, 1088–1098. 19701101. [DOI] [PubMed] [Google Scholar]
- Ljungman PL, Wilker EH, Rice MB, Schwartz J, Gold DR, Koutrakis P, Vita JA, Mitchell GF, Vasan RS, Benjamin EJ, Mittleman MA, Hamburg NM, 2014. Short-term exposure to air pollution and digital vascular function. Am. J. Epidemiol 180, 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luben TJ, Nichols JL, Dutton SJ, Kirrane E, Owens EO, Datko-Williams L, Madden M, Sacks JD, 2017. A systematic review of cardiovascular emergency department visits, hospital admissions and mortality associated with ambient black carbon. Environ. Int 107, 154–162. (Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttmann-Gibson H, Suh HH, Coull BA, Dockery DW, Sarnet SE, Schwartz J, Stone PH, Gold DR, 2006. Short-term effects of air pollution on heart rate variability in senior adults in Steubenville, Ohio. J. Occup. Environ. Med 48, 780–788. [DOI] [PubMed] [Google Scholar]
- Mirowsky JE, Peltier RE, Lippmann M, Thurston G, Chen LC, Neas L, Diaz-Sanchez D, Laumbach R, Carter JD, Gordon T, 2015. Repeated measures of inflammation, blood pressure, and heart rate variability associated with traffic exposures in healthy adults. Environ. Health 14, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordukhovich I, Coull B, Kloog I, Koutrakis P, Vokonas P, Schwartz J, 2015. Exposure to sub-chronic and long-term particulate air pollution and heart rate variability in an elderly cohort: the Normative Aging Study. Environ. Health 14, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky E, Schwartz J, Coull BA, Koutrakis P, Wellenius GA, Suh HH, Gold DR, Mittleman MA, 2012. Modeling the association between particle constituents of air pollution and health outcomes. Am. J. Epidemiol 176, 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JL, Owens EO, Dutton SJ, Luben TJ, 2013. Systematic review of the effects of black carbon on cardiovascular disease among individuals with pre-existing disease. Int. J. Public Health 58, 707–724 (Review). [DOI] [PubMed] [Google Scholar]
- Niranjan R, Thakur AK, 2017. The toxicological mechanisms of environmental soot (black carbon) and carbon black: focus on oxidative stress and inflammatory pathways. Front. Immunol 8, 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MS, Veves A, Zanobetti A, et al. , 2005. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation 111, 2913–2920. . [DOI] [PubMed] [Google Scholar]
- Owens EO, et al. , 2017. Framework for assessing causality of air pollution-related health effects for reviews of the National Ambient Air Quality Standards. Regul. Toxicol. Pharmacol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, O’Neill MS, Vokonas PS, Sparrow D, Schwartz J, 2005. Effects of air pollution on heart rate variability: the VA Normative Aging Study. Environ. Health Perspect 113, 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prystowsky EN, Benson DW Jr., Fuster V, Hart RG, Kay GN, Myerburg RJ, Naccarelli GV, Wyse DG, 1996. Management of patients with atrial fibrillation. In: A Statement for Healthcare Professionals. From the Subcommittee on Electrocardiography and Electrophysiology American Heart Association. [DOI] [PubMed] [Google Scholar]
- Rich KE, Petkau J, Vedal S, Brauer M, 2004. A case-crossover analysis of particulate air pollution and cardiac arrhythmia in patients with implantable cardioverter defibrillators. Inhal. Toxicol 16, 363–372. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Schwartz J, Mittleman MA, Link M, Luttmann-Gibson H, Catalano PJ, Speizer FE, Dockery DW, 2005. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am. J. Epidemiol 161, 1123–1132. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Kim MH, Turner JR, Mittleman MA, Schwartz J, Catalano PJ, Dockery DW, 2006a. Association of ventricular arrhythmias detected by implantable cardioverter defibrillator and ambient air pollutants in the St Louis, Missouri metropolitan area. Occup. Environ. Med 63, 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Mittleman MA, Link MS, Schwartz J, Luttmann-Gibson H, Catalano PJ, Speizer FE, Gold DR, Dockery DW, 2006b. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ. Health Perspect 114, 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Peters A, Schneider A, Wojciech Z, Breiner S, Oakes D, Wiltshire J, Kane C, Framtpon MW, Hampel R, Hopke PK, Cyrys J, Utell MJ, 2016. Ambient and Controlled Particle Exposures as Triggers for Acute ECG Changes (186) Health Effects Institute. [HEI], Boston, MA. [PubMed] [Google Scholar]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA, 2014. Systematic review and evidence integration for literature-based environmental health science assessments. Environ. Health Perspect 122, 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan WHIII, Campen MJ, Wichers LB, Watkinson WP, 2007. Heart rate variability in rodents: uses and caveats in toxicological studies. Cardiovasc. Toxicol 7, 28–51. [DOI] [PubMed] [Google Scholar]
- Roy D, Talajic M, Dubuc M, Thibault B, Guerra P, Macle L, Khairy P, 2009. Atrial fibrillation and congestive heart failure. Curr. Opin. Cardiol 44, 29. [DOI] [PubMed] [Google Scholar]
- Sarnat SE, Suh HH, Coull BA, Schwartz J, Stone PH, Gold DR, 2006. Ambient particulate air pollution and cardiac arrhythmia in a panel of older adults in Steubenville, Ohio. Occup. Environ. Med 63, 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Hampel R, Ibald-Mulli A, Zareba W, Schmidt G, Schneider R, Rückerl R, Couderc JP, Mykins B, Oberdörster G, Wölke G, Pitz M, Wichmann HE, Peters A, 2010. Changes in deceleration capacity of heart rate and heart rate variability induced by ambient air pollution in individuals with coronary artery disease. Part. Fibre Toxicol 7, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, Nearing B, Verrier R, Stone P, Maccallum G, Speizer FE, Gold DR, 2005. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax 60, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek LW, Sacks JD, Dutton SJ, Dubois JJB, 2011. Attributing health effects to apportioned components and sources of particulate matter: an evaluation of collective results. Atmos. Environ 45, 5655–5663. [Google Scholar]
- Suh HH, Zanobetti A, 2010. Exposure error masks the relationship between traffic-related air pollution and heart rate variability. J. Occup. Environ. Med 52, 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TFESC NASPE, 1996. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93, 1043–1065. [PubMed] [Google Scholar]
- Thurston GD, Burnett RT, Turner MC, Shi Y, Krewski D, Lall R, Ito K, Jerrett M, Gapstur SM, Diver WR, Pope CA III, 2016. Ischemic heart disease mortality and long-term exposure to source-related components of U.S. fine particle air pollution. Environ. Health Perspect 124, 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H, Venditti FJ, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D, 1994. Reduced heart rate variability and mortality risk in an elderly cohort: the Framingham Heart Study. Circulation 90, 878–883. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Larson MG, Venditti FJ Jr., Manders ES, Evans JC, Feldman CL, Levy D, 1996. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 94, 2850–2855. [DOI] [PubMed] [Google Scholar]
- U.S. EPA, 2009. Integrated Science Assessment for Particulate Matter (EPA/600/R-08/139F) U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment-RTP Division, Research Triangle Park, NC. [Google Scholar]
- U.S. EPA, 2012. Report to Congress on Black Carbon (EPA-450/R-12–001). [EPA Report] U.S. Environmental Protection Agency. http://www.epa.gov/blackcarbon/2012report/fullreport.pdf. [Google Scholar]
- U.S. EPA, 2015. EPA/600/R-15/067. [EPA Report] National Center for Environmental Assessment, Office of Research and Development, Research Triangle Park, NC. [Google Scholar]
- Urch B, Brook JR, Wasserstein D, Brook RD, Rajagopalan S, Corey P, Silverman F, 2004. Relative contributions of PM2.5 chemical constituents to acute arterial vasoconstriction in humans. Inhal. Toxicol 6–7 (16), 345–352. [DOI] [PubMed] [Google Scholar]
- Vedal S, Campen MJ, Mcdonald JD, Larson TV, Sampson PD, Sheppard L, Simpson CD, Szpiro AA, 2013. National Particle Component Toxicity (NPACT) initiative report on cardiovascular effects. In: Research Report 178. Health Effects Institute, pp. 5–8. [PubMed] [Google Scholar]
- Weichenthal S, Kulka R, Dubeau A, Martin C, Wang D, Dales R, 2011. Traffic-related air pollution and acute changes in heart rate variability and respiratory function in urban cyclists. Environ. Health Perspect 119, 1373–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal S, Hatzopoulou M, Goldberg MS, 2014. Exposure to traffic-related air pollution during physical activity and acute changes in blood pressure, autonomic and micro-vascular function in women: a cross-over study. Part. Fibre Toxicol 11, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2012. In: Janssen NAH, Gerlofs-Niljand ME, Lanki T, Salonen RO, Cassee F, Hoek G, Fischer P, Brunekreef B, Kryzanowski M (Eds.), Health Effects of Black Carbon, ISBN 978 92 890 0265 3 http://www.euro.who.int/en/publications/abstracts/health-effects-of-black-carbon-2012. [Google Scholar]
- Wu S, Deng F, Huang J, et al. , 2013. Blood pressure changes and chemical constituents of particulate air pollution: results from the healthy volunteer natural relocation (HVNR) study. Environ. Health Perspect 121, 66–72. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WY, Zhang ZY, Thijs L, Bijnens EM, Janssen BG, Vanpoucke C, Lefebvre W, Cauwenberghs N, Wei FF, Luttun A, Verhamme P, Van Hecke E, Kuznetsova T, D’hooge J, Nawrot TS, Staessen JA, 2017. Left ventricular function in relation to chronic residential air pollution in a general population. Eur. J. Prev. Cardiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Stone PH, Speizer FE, Schwartz JD, Coull BA, Suh HH, Nearing BD, Mittleman MA, Verrier RL, Gold DR, 2009. T-wave alternans, air pollution and traffic in high-risk subjects. Am. J. Cardiol 104, 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Gold DR, Stone PH, Suh HH, Schwartz J, Coull BA, Speizer FE, 2010. Reduction in heart rate variability with traffic and air pollution in patients with coronary artery disease. Environ. Health Perspect 118, 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Coull BA, Gryparis A, Kloog I, Sparrow D, Vokonas PS, Wright RO, Gold DR, Schwartz J, 2014a. Associations between arrhythmia episodes and temporally and spatially resolved black carbon and particulate matter in elderly patients. Occup. Environ. Med 71, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Luttmann-Gibson H, Horton ES, Cohen A, Coull BA, Hoffmann B, Schwartz JD, Mittleman MA, Li Y, Stone PH, de Souza C, Lamparello B, Koutrakis P, Gold DR, 2014b. Brachial artery responses to ambient pollution, temperature, and humidity in people with type 2 diabetes: a repeated-measures study. Environ. Health Perspect 122, 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Staimer N, Tjoa T, Gillen DL, Schauer JJ, Shafer MM, Hasheminassab S, Pakbin P, Longhurst J, Sioutas C, Delfino RJ, 2016. Associations between microvascular function and short-term exposure to traffic-related air pollution and particulate matter oxidative potential. Environ. Health 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulfiqar U, Jurivich DA, Gao W, Singer DH, 2010. Relation of high heart rate variability to healthy longevity. Am. J. Cardiol 105 (8), 1181–1185. (Epub 2010 Feb 20). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


