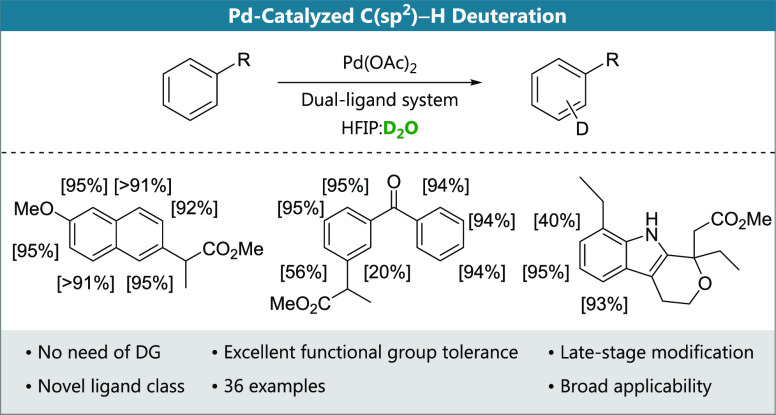
Abstract
We describe a palladium-catalyzed nondirected late-stage deuteration of arenes. Key aspects include the use of D2O as a convenient and easily available deuterium source and the discovery of highly active N,N-bidentate ligands containing an N-acylsulfonamide group. The reported protocol enables high degrees of deuterium incorporation via a reversible C–H activation step and features extraordinary functional group tolerance, allowing for the deuteration of complex substrates. This is exemplified by the late-stage isotopic labeling of various pharmaceutically relevant motifs and related scaffolds. We expect that this method, among other applications, will prove useful as a tool in drug development processes and for mechanistic studies.
Over the last decades the incorporation of hydrogen atom isotopes into organic molecules has received considerable attention and remains a key research goal in both academic and industrial research.1 Isotopically labeled compounds feature a broad range of applications, starting from their use in the elucidation of reaction mechanisms2 or as internal standards in mass spectrometry studies.3 Isotopically labeled analogues of bioactive molecules play a critical role in drug discovery processes, for example in absorption, distribution, metabolism, and excretion (ADME) studies to gain insights into their metabolic profile and toxicity.4 In an increasing number of cases, deuterated molecules are marketed as new pharmaceuticals,5 often characterized by improved pharmacokinetic and pharmacodynamic properties. These diverse applications have spurred continued interest in the development of convenient and robust synthetic methods to incorporate deuterium into complex aromatic scaffolds, which occur in many bioactive molecules and related compounds.1d,1e
Methods such as the de novo synthesis of complex deuterated analogues and the introduction of D/T in prefunctionalized positions often prove to be time-consuming and cost-intensive.6 Efforts have thus been made to establish methods for direct hydrogen isotope exchange (HIE) of aromatic C–H bonds that could in principle enable efficient postsynthetic incorporation of hydrogen isotopes into bioactive molecules.1d,1e,7
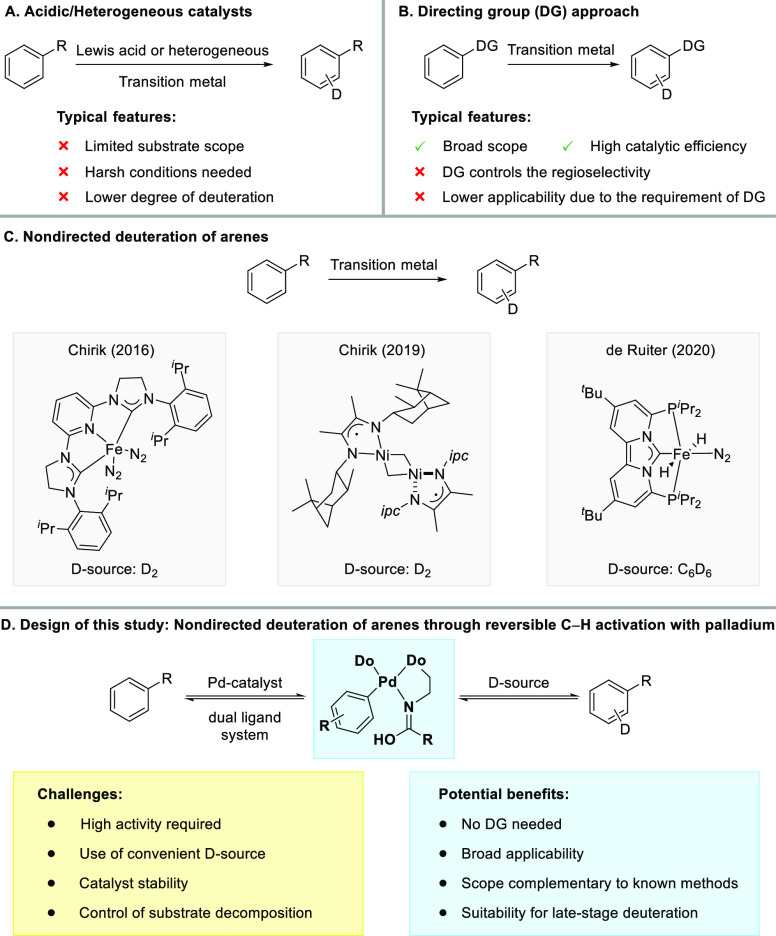
Traditional methods for direct H/D exchange of arenes include pH-dependent methods (Scheme 1A), where the incorporation of deuterium is achieved by the use of Brønsted/Lewis acids, mostly via an SEAr-type mechanism.8 Examples of base-mediated HIE reactions of arenes are also known.9 Owing to the typically harsh reaction conditions, these methods are usually employed for simple arenes. Heterogenous methods for HIE of arenes are well-developed, and high activity can be achieved with many transition metals.3e,10 This approach offers technical advantages like simple purification11 but faces challenges such as undesired side reactions.12
Scheme 1. Approaches toward the Deuteration of Arenes.
The potential to achieve high selectivities for HIE under comparably mild conditions, thus enabling a broader functional group tolerance, has spurred research toward homogeneously catalyzed methods.1b,1d,1e,13 In this context, the use of directing groups (DGs) has proven to be highly useful.14 Methods based on various transition metals have been established and feature high efficiencies and broad functional group tolerances (Scheme 1B).15 While DGs usually lead to selective deuteration at the ortho position, specialized DGs to achieve meta deuteration have also been described.16
Recent studies have focused on the use of native functional groups rather than designed DGs to enable directed late-stage C–H deuteration.17
These directed protocols are complemented by nondirected approaches,18 which offer the possibility to address unbiased C–H bonds without requiring a DG on the substrate, thus potentially enabling H/D exchange on a substantially broader range of substrates. Nondirected methods for the deuteration of simple arenes are well-established,19 but catalysts that enable nondirected HIE of drug molecules and other similarly complex scaffolds have only recently been described (Scheme 1C).20 Chirik and co-workers introduced an iron catalyst capable of inducing HIE with a variety of pharmaceuticals using D2 as the deuterium source.20a The same group later described a Ni-based catalyst that delivered deuterated and tritiated drug molecules efficiently using D2 and T2 as the sources of deuterium and tritium, respectively.20c Recently, de Ruiter et al. described an Fe–PCP-pincer complex that proved to be highly active for the nondirected H/D exchange of arenes using C6D6 as the deuterium source and tolerated a considerable range of functional groups.20e These catalysts provided substantial progress toward the mild and efficient HIE of complex molecules and raised interest in the development of complementary methods.1e,20f
Our group has recently developed Pd-catalysts for nondirected late-stage functionalization of complex (hetero)arenes.21,22 An extensive mechanistic investigation of our dual-ligand-based catalyst system23 showed that the C–H activation step is reversible (Scheme 1D). We envisioned that a highly active catalyst for the reversible C–H activation of arenes using our dual ligand design could enable a homogeneous nondirected method for Pd-catalyzed late-stage HIE with the potential to complement existing methods based on 3d metals with regard to the substrate scope and/or deuterium source used.
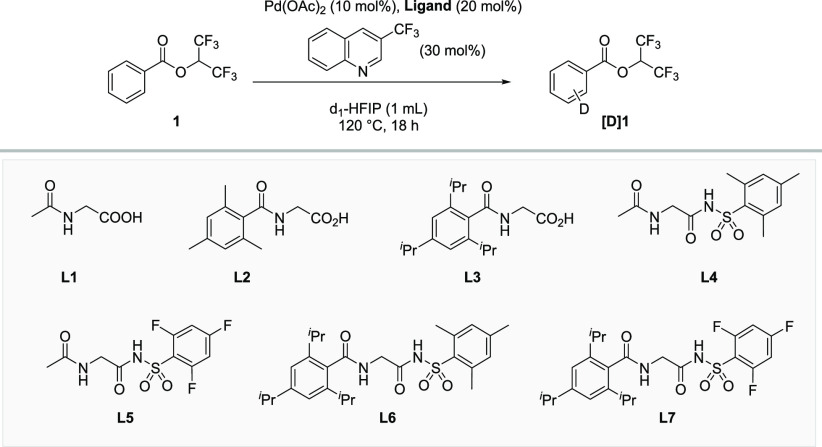
On the basis of these considerations, we engaged in detailed optimization studies.24Table 1 shows the deuteration of model substrate 1 using different bidentate ligands in 1,1,1,3,3,3-hexafluoropropan-2-ol-d1 (d1-HFIP). N-Acetylglycine (L1) as the ligand resulted in moderate H/D-exchange (entry 1). Building upon our recent finding that bulky arylamides as concerted metalation–deprotonation (CMD)-promoting groups in ethylenediamine ligands show superior activity in HIE,24 we synthesized the analogous glycine derivatives L2 and L3 (entries 2 and 3). These α-amino acid-derived ligands lead to a significant improvement in deuterium incorporation. An extensive search for novel ligand classes with improved properties regarding activity and regioselectivity led us to discover N,N-bidentate ligands that feature N-acylsulfonamide groups. Interestingly, introducing this motif instead of the carboxylic acid moiety offers additional potential for ligand diversification by introducing further variable positions. Using mesityl-substituted ligand L4 gave similar results as L1, albeit with less deuteration at the ortho position, whereas L5 led to decreased values (entries 4 and 5). A significant improvement resulted when the two structural variations were combined in L6 and L7 (entries 6 and 7).
Table 1. Optimization of the Ligand Structurea,b.
| D content
(%) |
||||||
|---|---|---|---|---|---|---|
| entry | ligand | yield (%) | ortho | meta | para | total D content |
| 1 | L1 | 99 | 11 | 50 | 23 | 1.66 |
| 2 | L2 | 95 | 22 | 73 | 41 | 2.42 |
| 3 | L3 | 97 | 24.5 | 79 | 47 | 2.65 |
| 4 | L4 | 98 | 4 | 46 | 23 | 1.27 |
| 5 | L5 | 98 | 7 | 35 | 21 | 1.05 |
| 6 | L6 | 97 | 5 | 72 | 46 | 2.08 |
| 7 | L7 | 97 | 17 | 90 | 74 | 2.87 |
| 8c | L7 | 95 | 39 | 95 | 84 | 3.51 |
| 9c,d | L7 | 99 | 34 | 60 | 32 | 2.15 |
| 10c | no L7 | 98 | 0 | 0 | 0 | 0 |
| 11c,e | L7 | 94 | 62 | 95 | 95 | 4.05 |
Reactions were performed on a 0.1 mmol scale.
Yields and degrees of deuteration were determined by 1H NMR spectroscopy using mesitylene as an internal standard. The total deuterium content was determined by mass spectrometry.
The reaction was performed with D2O/HFIP (7:3) as the solvent. Since D2O is used as part of the solvent system, this corresponds to an excess of approximately 390 equiv.
No 3-trifluoromethylquinoline.
The reaction was performed with a reaction time of 48 h.
An investigation of alternative, more convenient deuterium sources showed that improved results are obtained with a 7:3 D2O/HFIP mixture as the solvent (Table 1, entry 8). This is particularly attractive since d1-HFIP, which is comparably costly or needs to be synthesized, can be replaced by a cheap and convenient deuterium source. Control experiments at this stage revealed that both ligands are indeed required to obtain optimal results (entries 9 and 10). Finally, nearly complete deuteration of the meta and para positions was observed when L7 was used with an increased reaction time (entry 11).
Interestingly, the seemingly sterically most hindered ligand enables the highest deuteration at the ortho position. This can be explained by two factors. First, the steric bulk does not point toward the substrate in the key C–H activation step,23 and second, the ligand enables the highest overall activities, such that even the least reactive site on the substrate is deuterated. However, it remains substantially slower than the other positions (entries 8 and 11). Since the conditions developed in Table 1 (conditions B in Scheme 2) were found using a particularly challenging electron-poor substrate, we hypothesized that more electron-rich substrates might be deuterated under milder conditions. A reoptimization (see the Supporting Information (SI) for details) delivered a second set of reaction conditions using L3 with AgF as an additive at lower temperatures (conditions A in Scheme 2)
Scheme 2. Reaction Scope,
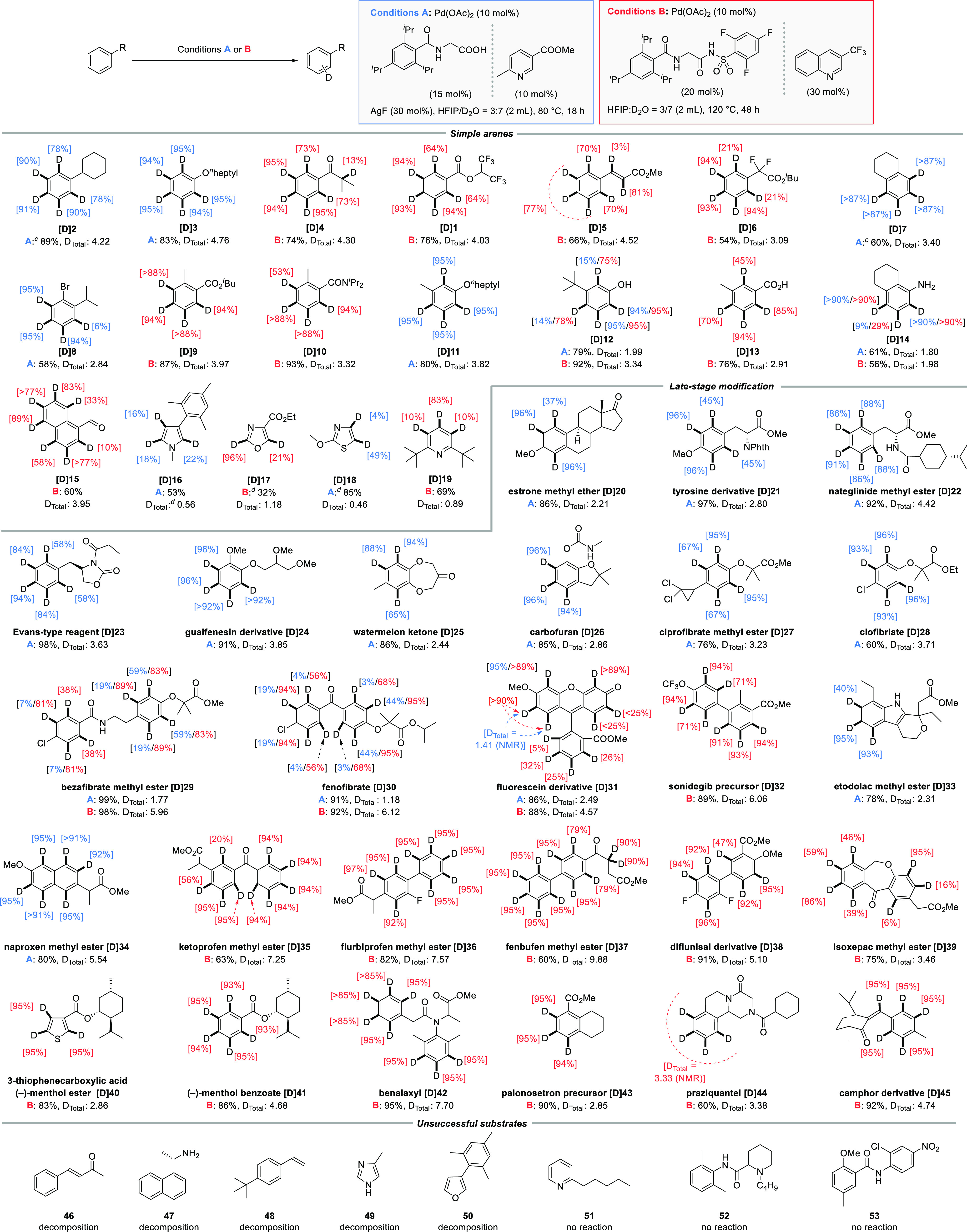
Reactions were performed on a 0.2 mmol scale.
Positions with less than 10% D incorporation are typically not depicted explicitly but are reflected in the DTotal value (for experimental details, see the SI).
The reaction was performed at 40 °C for 72 h.
Determined by 1H NMR spectroscopy.
Having established two sets of conditions, we evaluated the scope of our method (Scheme 2). We initiated our investigation by using simple arenes to assess the general functional group tolerance when applying our catalyst systems. The yields of the reisolated substrates were in general good to excellent. The use of alkylated arene 2 under very mild conditions resulted in high H/D exchange in the arene moiety (DTotal = 4.22). Excellent degrees of deuteration were also observed for anisole derivative 3. Notably, our protocol tolerates ketones (4), a functional group that occurs in a wide range of bioactive molecules, but is challenging for many literature methods. Using conditions B, in addition to the deuteration on the arene core, butyrophenone 4 underwent little but measurable isotope exchange in the relatively acidic α-position, presumably via an acid–base mechanism. The electron-poor arenes 1, 5, and 6 were likewise subjected to conditions B, leading to very high degrees of deuteration, especially at the meta and para positions. Dialkyl-substituted substrate 7 smoothly underwent H/D exchange in the arene moiety. Interestingly, halogenated arene 8 was well-tolerated under conditions A, giving the reisolated substrate in good yield with a high overall degree of deuteration. Further disubstituted arenes containing ester, amide, and ether, as well as free hydroxy and acid groups (9–13) gave high levels of deuterium incorporation (up to DTotal = 3.97). Aniline derivatives (14) as well as aldehydes and extended π systems (15) are likewise tolerated under the reaction conditions. Finally, we probed whether our protocol can be used for heterocycles. The comparably electron-rich heteroarenes pyrrole 16, oxazole 17, and thiazole 18 could be deuterated in moderate to good yields with appreciable levels of deuterium incorporation. The deuteration of pyridine derivative 19 confirmed that this substrate class is in principle amenable if the N atom is sufficiently shielded to avoid catalyst poisoning.
We proceeded to evaluate the suitability of our method for the late-stage deuteration of bioactive molecules and related scaffolds. Subjecting estrone derivative 20 to conditions A delivered the deuterated compound [D]20 in very good yield with a high degree of deuteration on the arene moiety. Interestingly, the sterically most congested position underwent H/D exchange to a reduced extent. Similarly, with tyrosine derivative 21 the deuterium incorporation at the sterically more hindered position was lower than at the position ortho to the methoxy group. Furthermore, nateglinide methyl ester 22, the Evans-type reagent 23, guaifenesin derivative 24, watermelon ketone (25), and carbofuran (26) were subjected to conditions A, leading to almost complete deuterium incorporation into the respective arene moieties, thereby demonstrating functional group tolerance toward amides, esters, ethers, and carbamates.
Representatives of the fibrate class such as cipofibrate methyl ester (27), clofibrate (28), bezafibrate methyl ester (29), and fenofibrate (30) were efficiently deuterated. Because of the presence of an electron-poor and a rather electron-rich arene moiety, substrates 29 and 30 were subjected to both conditions A and B. With the milder reaction conditions A, a good degree of deuteration on the electron-rich arene was observed, while with conditions B both arene moieties were efficiently deuterated.
Fluorescein derivative 31 was also subjected to both catalyst systems. With conditions A, the electron-rich positions underwent efficient H/D exchange (DTotal = 2.49) exclusively, whereas conditions B led to substantially increased overall deuterium incorporation (DTotal = 4.57). Nearly complete deuteration of the arene moieties occurred with sonidegib precursor 32. Etodolac methyl ester (33), which contains an indole substructure, likewise underwent efficient H/D exchange using conditions A.
Methyl ester derivatives of naproxen (34), ketoprofen (35), and flurbiprofen (36), as representatives of the profen class of medications, were almost completely deuterated at the arene position (up to DTotal = 7.57). Fenbufen derivative 37 could likewise be deuterated. It should be noted that besides the aromatic core, the α-keto position underwent almost complete deuteration, presumably via an acid/base-type mechanism.
Derivatives of diflunisal (38) and isoxepac (39) gave high degrees of deuteration under conditions B. (−)-Menthol esters of 3-thiophenecarboxylic acid (40) and benzoic acid (41) could both be deuterated efficiently. Finally, subjecting benalaxyl (42), palonosetron precursor 43, praziquantel (44), and camphor derivative 45 to our catalyst led to nearly complete deuterium incorporation in the arene moieties as well as the olefinic position of 45.
Finally, Scheme 2 depicts a number of substrates that could not be deuterated using our method because of either substrate decomposition (46–50) or an absence of reactivity that presumably originates from catalyst poisoning by the substrate (51–53).
As evidenced by the above scope studies, we have developed a broadly applicable protocol for the nondirected late-stage deuteration of arenes using dual ligand-based palladium catalysts. Enabled by the development of a novel ligand class, a variety of bioactive molecules and related structures could be isotopically labeled using D2O as a cheap and convenient deuterium source. This method is applicable to both electron-rich and electron-poor arenes and tolerates a wide range of functional groups, rendering it complementary to established protocols. We expect that our catalysts will prove useful for isotopic labeling in various fields, with potential applications ranging from mechanistic studies to drug development.
Acknowledgments
We thank all of the members of our MS and NMR department for their excellent service, A. Uttry for helpful scientific discussions, and Prof. Dr. Frank Glorius for his generous support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c08233.
Optimization of the reaction conditions, preparative procedures, and analytical data for the compounds (PDF)
Author Contributions
† M.F. and A.M. contributed equally.
Author Contributions
‡ S.M. and F.D. contributed equally.
We acknowledge generous financial support by the DFG (Emmy Noether Programme) and WWU Münster. Additionally, this project received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme (Grant Agreement 946044).
The authors declare no competing financial interest.
Supplementary Material
References
- a Junk T.; Catallo W. J. Hydrogen isotope exchange reactions involving C–H (D, T) bonds. Chem. Soc. Rev. 1997, 26, 401–406. 10.1039/CS9972600401. [DOI] [Google Scholar]; b Atzrodt J.; Derdau V.; Fey T.; Zimmermann J. The renaissance of H/D exchange. Angew. Chem., Int. Ed. 2007, 46, 7744–7765. 10.1002/anie.200700039. [DOI] [PubMed] [Google Scholar]; c Atzrodt J.; Derdau V.; Kerr W. J.; Reid M. Deuterium- and Tritium-Labelled Compounds: Applications in the Life Sciences. Angew. Chem., Int. Ed. 2018, 57, 1758–1784. 10.1002/anie.201704146. [DOI] [PubMed] [Google Scholar]; d Atzrodt J.; Derdau V.; Kerr W. J.; Reid M. C-H. Functionalisation for Hydrogen Isotope Exchange. Angew. Chem., Int. Ed. 2018, 57, 3022–3047. 10.1002/anie.201708903. [DOI] [PubMed] [Google Scholar]; e Kang Q.-K.; Shi H. Catalytic Hydrogen Isotope Exchange Reactions in Late-Stage Functionalization. Synlett 2021, 10.1055/a-1354-0367. [DOI] [Google Scholar]
- Simmons E. M.; Hartwig J. F. On the interpretation of deuterium kinetic isotope effects in C–H bond functionalizations by transition-metal complexes. Angew. Chem., Int. Ed. 2012, 51, 3066–3072. 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
- a Wehmeyer K. R.; Knight P. M.; Parry R. C. Evaluation of a benchtop ion trap gas chromatographic-tandem mass spectrometric instrument for the analysis of a model drug, tebufelone, in plasma using a stable-isotope internal standard. J. Chromatogr., Biomed. Appl. 1996, 676, 53–59. 10.1016/0378-4347(95)00417-3. [DOI] [PubMed] [Google Scholar]; b Kao C.-Y.; Giese R. W. Measurement of N7-(2’-hydroxyethyl)guanine in human DNA by gas chromatography electron capture mass spectrometry. Chem. Res. Toxicol. 2005, 18, 70–75. 10.1021/tx049854d. [DOI] [PubMed] [Google Scholar]; c Stokvis E.; Rosing H.; Beijnen J. H. Stable isotopically labeled internal standards in quantitative bioanalysis using liquid chromatography/mass spectrometry: necessity or not?. Rapid Commun. Mass Spectrom. 2005, 19, 401–407. 10.1002/rcm.1790. [DOI] [PubMed] [Google Scholar]; d Mutlib A. E. Application of stable isotope-labeled compounds in metabolism and in metabolism-mediated toxicity studies. Chem. Res. Toxicol. 2008, 21, 1672–1689. 10.1021/tx800139z. [DOI] [PubMed] [Google Scholar]; e Atzrodt J.; Derdau V. Pd- and Pt-catalyzed H/D exchange methods and their application for internal MS standard preparation from a Sanofi-Aventis perspective. J. Labelled Compd. Radiopharm. 2010, 53, 674–685. 10.1002/jlcr.1818. [DOI] [Google Scholar]
- a Marathe P. H.; Shyu W. C.; Humphreys W. G. The use of radiolabeled compounds for ADME studies in discovery and exploratory development. Curr. Pharm. Des. 2004, 10, 2991–3008. 10.2174/1381612043383494. [DOI] [PubMed] [Google Scholar]; b Harbeson S. L.; Tung R. D. Deuterium in Drug Discovery and Development. Annu. Rep. Med. Chem. 2011, 46, 403–417. 10.1016/B978-0-12-386009-5.00003-5. [DOI] [Google Scholar]; c Isin E. M.; Elmore C. S.; Nilsson G. N.; Thompson R. A.; Weidolf L. Use of radiolabeled compounds in drug metabolism and pharmacokinetic studies. Chem. Res. Toxicol. 2012, 25, 532–542. 10.1021/tx2005212. [DOI] [PubMed] [Google Scholar]; d Gant T. G. Using deuterium in drug discovery: leaving the label in the drug. J. Med. Chem. 2014, 57, 3595–3611. 10.1021/jm4007998. [DOI] [PubMed] [Google Scholar]; e Elmore C. S.; Bragg R. A. Isotope chemistry; a useful tool in the drug discovery arsenal. Bioorg. Med. Chem. Lett. 2015, 25, 167–171. 10.1016/j.bmcl.2014.11.051. [DOI] [PubMed] [Google Scholar]
- a Mullard A. FDA approves first deuterated drug. Nat. Rev. Drug Discovery 2017, 16, 305. 10.1038/nrd.2017.89. [DOI] [PubMed] [Google Scholar]; b Pirali T.; Serafini M.; Cargnin S.; Genazzani A. A. Applications of Deuterium in Medicinal Chemistry. J. Med. Chem. 2019, 62, 5276–5297. 10.1021/acs.jmedchem.8b01808. [DOI] [PubMed] [Google Scholar]
- a Thomas A. F.Deuterium Labeling in Organic Chemistry; Appleton-Century-Crofts, 1971. [Google Scholar]; b Chapelle M. R.; Kent B. B.; Jones J. R.; Lu S.-Y.; Morgan A. D. Development of combined microwave-enhanced labelling procedures for maximising deuterium incorporation. Tetrahedron Lett. 2002, 43, 5117–5118. 10.1016/S0040-4039(02)00986-3. [DOI] [Google Scholar]; c Alonso F.; Beletskaya I. P.; Yus M. Metal-mediated reductive hydrodehalogenation of organic halides. Chem. Rev. 2002, 102, 4009–4091. 10.1021/cr0102967. [DOI] [PubMed] [Google Scholar]; d Guaragna A.; Pedatella S.; Pinto V.; Palumbo G. Synthesis of C-Protected 2,2-Dideutero β 3 -Amino Acids. Synthesis 2006, 2006, 4013–4016. 10.1055/s-2006-950314. [DOI] [Google Scholar]; e Fontana E.; Venegoni S. Syntheses of (R,S)-naproxen and its 6-O-desmethylated metabolite labelled with 2H. J. Labelled Compd. Radiopharm. 2008, 51, 239–241. 10.1002/jlcr.1511. [DOI] [Google Scholar]; f Kallepalli V. A.; Gore K. A.; Shi F.; Sanchez L.; Chotana G. A.; Miller S. L.; Maleczka R. E.; Smith M. R. Harnessing C-H Borylation/Deborylation for Selective Deuteration, Synthesis of Boronate Esters, and Late Stage Functionalization. J. Org. Chem. 2015, 80, 8341–8353. 10.1021/acs.joc.5b01588. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Upshur M. A.; Chase H. M.; Strick B. F.; Ebben C. J.; Fu L.; Wang H.; Thomson R. J.; Geiger F. M. Vibrational Mode Assignment of α-Pinene by Isotope Editing: One Down, Seventy-One To Go. J. Phys. Chem. A 2016, 120, 2684–2690. 10.1021/acs.jpca.6b01995. [DOI] [PubMed] [Google Scholar]; h Neumann K. T.; Lindhardt A. T.; Bang-Andersen B.; Skrydstrup T. Synthesis and selective 2 H-, 13 C-, and 15 N-labeling of the Tau protein binder THK-523. J. Labelled Compd. Radiopharm. 2017, 60, 30–35. 10.1002/jlcr.3470. [DOI] [PubMed] [Google Scholar]
- Voges R.; Heys J. R.; Moenius T.. Preparation of Compounds Labeled with Tritium and Carbon-14; Wiley, 2009. [Google Scholar]
- a Garnett J. L.; Long M. A.; Vining R. F. W.; Mole T. New simple method for rapid, selective aromatic deuteration using organoaluminum dihalide catalysts. J. Am. Chem. Soc. 1972, 94, 5913–5914. 10.1021/ja00771a073. [DOI] [Google Scholar]; b Long M. A.; Garnett J. L.; Vining R. F. W. Rapid deuteriation and tritiation of organic compounds using organometallic and elemental halides as catalysts. J. Chem. Soc., Perkin Trans. 2 1975, 1298. 10.1039/p29750001298. [DOI] [Google Scholar]; c Seibles J. C.; Bollinger D. M.; Orchin M. Synthesis of Perdeuteriobenzo[a] pyrene. Angew. Chem., Int. Ed. Engl. 1977, 16, 656–657. 10.1002/anie.197706561. [DOI] [Google Scholar]; d Branch C. S.; Barron A. R. Arene-mercury complexes stabilized by gallium chloride: relative rates of H/D and arene exchange. J. Am. Chem. Soc. 2002, 124, 14156–14161. 10.1021/ja0206590. [DOI] [PubMed] [Google Scholar]; e Hakala U.; Wähälä K. Expedient deuterolabeling of polyphenols in ionic liquids-DCl/D2O under microwave irradiation. J. Org. Chem. 2007, 72, 5817–5819. 10.1021/jo070231p. [DOI] [PubMed] [Google Scholar]; f Martins A.; Lautens M. A simple, cost-effective method for the regioselective deuteration of anilines Org. Org. Lett. 2008, 10, 4351–4353. 10.1021/ol801763j. [DOI] [PubMed] [Google Scholar]; g Mačková M.; Himl M.; Minářová L.; Lang J.; Lhoták P. Regioselective deuteration of 25,27-dialkoxycalix[4]arenes. Tetrahedron Lett. 2011, 52, 2543–2546. 10.1016/j.tetlet.2011.03.030. [DOI] [Google Scholar]; h Zhou L.; Bian X.; Yang S.; Mu B. A two-step synthesis of deuterium labeled 8, 8, 9, 9-d4-hexadecane from nonanoic acid. J. Labelled Compd. Radiopharm. 2012, 55, 158–160. 10.1002/jlcr.1962. [DOI] [Google Scholar]; i Murai Y.; Wang L.; Masuda K.; Sakihama Y.; Hashidoko Y.; Hatanaka Y.; Hashimoto M. Rapid and Controllable Hydrogen/Deuterium Exchange on Aromatic Rings of α-Amino Acids and Peptides. Eur. J. Org. Chem. 2013, 2013, 5111–5116. 10.1002/ejoc.201300405. [DOI] [Google Scholar]; j Müller K.; Seubert A. Synthesis of deuterium-labelled fluorobenzoic acids to be used as internal standards in isotope dilution mass spectrometry. Isot. Environ. Health Stud. 2014, 50, 88–93. 10.1080/10256016.2013.830612. [DOI] [PubMed] [Google Scholar]; k Wang L.; Murai Y.; Yoshida T.; Okamoto M.; Masuda K.; Sakihama Y.; Hashidoko Y.; Hatanaka Y.; Hashimoto M. Hydrogen/deuterium exchange of cross-linkable α-amino acid derivatives in deuterated triflic acid Biosci. Biosci., Biotechnol., Biochem. 2014, 78, 1129–1134. 10.1080/09168451.2014.917267. [DOI] [PubMed] [Google Scholar]; l Munz D.; Webster-Gardiner M.; Fu R.; Strassner T.; Goddard W. A.; Gunnoe T. B. Proton or Metal? The H/D Exchange of Arenes in Acidic Solvents. ACS Catal. 2015, 5, 769–775. 10.1021/cs501620f. [DOI] [Google Scholar]; m Fischer O.; Hubert A.; Heinrich M. R. Shifted Selectivity in Protonation Enables the Mild Deuteration of Arenes Through Catalytic Amounts of Bronsted Acids in Deuterated Methanol. J. Org. Chem. 2020, 85, 11856–11866. 10.1021/acs.joc.0c01604. [DOI] [PubMed] [Google Scholar]
- a Beak P.; Brown R. A. The tertiary amide as an effective director of ortho lithiation. J. Org. Chem. 1982, 47, 34–46. 10.1021/jo00340a008. [DOI] [Google Scholar]; b Clayden J.; Pink J. H.; Westlund N.; Wilson F. X. Controlling the regioselectivity of lithiation using kinetic isotope effects: Deuterium as a protecting group for carbon. Tetrahedron Lett. 1998, 39, 8377–8380. 10.1016/S0040-4039(98)01930-3. [DOI] [Google Scholar]; c Ahmed A.; Clayden J.; Rowley M. Anion translocation in organolithiums: A mechanism for the lithiation and cyclisation of tertiary naphthamides. Tetrahedron Lett. 1998, 39, 6103–6106. 10.1016/S0040-4039(98)01291-X. [DOI] [Google Scholar]; d Zhan M.; Xu R.; Tian Y.; Jiang H.; Zhao L.; Xie Y.; Chen Y. A Simple and Cost-Effective Method for the Regioselective Deuteration of Phenols. Eur. J. Org. Chem. 2015, 2015, 3370–3373. 10.1002/ejoc.201500192. [DOI] [Google Scholar]; e Salamanca V.; Albéniz A. C. Deuterium Exchange between Arenes and Deuterated Solvents in the Absence of a Transition Metal: Synthesis of D-Labeled Fluoroarenes. Eur. J. Org. Chem. 2020, 3206–3212. 10.1002/ejoc.202000284. [DOI] [Google Scholar]
- a Fraser R. R.; Renaud R. N. The Steric Effect in the Platinum-Catalyzed Exchange Reaction between Aromatic Ring Protons and Deuterium Oxide. J. Am. Chem. Soc. 1966, 88, 4365–4370. 10.1021/ja00971a011. [DOI] [Google Scholar]; b Matsubara S.; Yokota Y.; Oshima K. Palladium-catalyzed decarboxylation and decarbonylation under hydrothermal conditions: decarboxylative deuteration. Org. Lett. 2004, 6, 2071–2073. 10.1021/ol0492602. [DOI] [PubMed] [Google Scholar]; c Yamamoto M.; Yokota Y.; Oshima K.; Matsubara S. H-D exchange reaction on benzene ring of polystyrene in hydrothermal deuterium oxide with platinum(IV) oxide catalyst. Chem. Commun. 2004, 1714–1715. 10.1039/B405063K. [DOI] [PubMed] [Google Scholar]; d Yamamoto M.; Oshima K.; Matsubara S. Platinum(IV) oxide catalyzed H-D exchange reactions in arylsilanes. Org. Lett. 2004, 6, 5015–5017. 10.1021/ol047738w. [DOI] [PubMed] [Google Scholar]; e Derdau V.; Atzrodt J.; Zimmermann J.; Kroll C.; Brückner F. Hydrogen-deuterium exchange reactions of aromatic compounds and heterocycles by NaBD4-activated rhodium, platinum and palladium catalysts. Chem. - Eur. J. 2009, 15, 10397–10404. 10.1002/chem.200901107. [DOI] [PubMed] [Google Scholar]; f Sajiki H. Development of deuterium labeling method based on the heterogeneous platinum group metal-catalyzed C-H activation. Yakugaku Zasshi 2013, 133, 1177–1193. 10.1248/yakushi.13-00218. [DOI] [PubMed] [Google Scholar]; g Pieters G.; Taglang C.; Bonnefille E.; Gutmann T.; Puente C.; Berthet J.-C.; Dugave C.; Chaudret B.; Rousseau B. Regioselective and stereospecific deuteration of bioactive aza compounds by the use of ruthenium nanoparticles. Angew. Chem., Int. Ed. 2014, 53, 230–234. 10.1002/anie.201307930. [DOI] [PubMed] [Google Scholar]; h Bresó-Femenia E.; Godard C.; Claver C.; Chaudret B.; Castillón S. Selective catalytic deuteration of phosphorus ligands using ruthenium nanoparticles: a new approach to gain information on ligand coordination. Chem. Commun. 2015, 51, 16342–16345. 10.1039/C5CC06984J. [DOI] [PubMed] [Google Scholar]; i Sawama Y.; Nakano A.; Matsuda T.; Kawajiri T.; Yamada T.; Sajiki H. H. –D Exchange Deuteration of Arenes at Room Temperature. Org. Process Res. Dev. 2019, 23, 648–653. 10.1021/acs.oprd.8b00383. [DOI] [Google Scholar]; j Park K.; Ito N.; Yamada T.; Sajiki H. Efficient Continuous-Flow H–D Exchange Reaction of Aromatic Nuclei in D2O/2-PrOH Mixed Solvent in a Catalyst Cartridge Packed with Platinum on Carbon Beads. Bull. Chem. Soc. Jpn. 2021, 94, 600–605. 10.1246/bcsj.20200325. [DOI] [Google Scholar]
- Smith G. V.; Notheisz F.. Heterogeneous Catalysis in Organic Chemistry; Academic Press, 1999. [Google Scholar]
- Beller M.; Bolm C.. Transition Metals for Organic Synthesis: Building Blocks and Fine Chemicals, 2nd rev. enl. ed.; Wiley-VCH, 2004. [Google Scholar]
- a Feng Y.; Lail M.; Foley N. A.; Gunnoe T. B.; Barakat K. A.; Cundari T. R.; Petersen J. L. Hydrogen-deuterium exchange between TpRu(PMe3)(L)X (L = PMe3 and X = OH, OPh, Me, Ph, or NHPh; L = NCMe and X = Ph) and deuterated arene solvents: evidence for metal-mediated processes. J. Am. Chem. Soc. 2006, 128, 7982–7994. 10.1021/ja0615775. [DOI] [PubMed] [Google Scholar]; b Allen P. H.; Hickey M. J.; Kingston L. P.; Wilkinson D. J. Metal-catalysed isotopic exchange labelling: 30 years of experience in pharmaceutical R&D. J. Labelled Compd. Radiopharm. 2010, 53, 731–738. 10.1002/jlcr.1825. [DOI] [Google Scholar]; c Lockley W. J. S.; Heys J. R. Metal-catalysed hydrogen isotope exchange labelling: a brief overview. J. Labelled Compd. Radiopharm. 2010, 53, 635–644. 10.1002/jlcr.1851. [DOI] [Google Scholar]; d Di Giuseppe A.; Castarlenas R.; Oro L. A. Mechanistic considerations on catalytic H/D exchange mediated by organometallic transition metal complexes. C. R. Chim. 2015, 18, 713–741. 10.1016/j.crci.2015.02.006. [DOI] [Google Scholar]
- a Whisler M. C.; MacNeil S.; Snieckus V.; Beak P. Beyond thermodynamic acidity: a perspective on the complex-induced proximity effect (CIPE) in deprotonation reactions. Angew. Chem., Int. Ed. 2004, 43, 2206–2225. 10.1002/anie.200300590. [DOI] [PubMed] [Google Scholar]; b Sambiagio C.; Schönbauer D.; Blieck R.; Dao-Huy T.; Pototschnig G.; Schaaf P.; Wiesinger T.; Zia M. F.; Wencel-Delord J.; Besset T.; Maes B. U. W.; Schnürch M. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. 10.1039/C8CS00201K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Nilsson G. N.; Kerr W. J. The development and use of novel iridium complexes as catalysts for ortho-directed hydrogen isotope exchange reactions. J. Labelled Compd. Radiopharm. 2010, 53, 662–667. 10.1002/jlcr.1817. [DOI] [Google Scholar]; b Brown J. A.; Cochrane A. R.; Irvine S.; Kerr W. J.; Mondal B.; Parkinson J. A.; Paterson L. C.; Reid M.; Tuttle T.; Andersson S.; Nilsson G. N. The Synthesis of Highly Active Iridium(I) Complexes and their Application in Catalytic Hydrogen Isotope Exchange. Adv. Synth. Catal. 2014, 356, 3551–3562. 10.1002/adsc.201400730. [DOI] [Google Scholar]; c Ma S.; Villa G.; Thuy-Boun P. S.; Homs A.; Yu J.-Q. Palladium-catalyzed ortho-selective C–H deuteration of arenes: evidence for superior reactivity of weakly coordinated palladacycles. Angew. Chem., Int. Ed. 2014, 53, 734–737. 10.1002/anie.201305388. [DOI] [PubMed] [Google Scholar]; d Giles R.; Ahn G.; Jung K. W. H–D exchange in deuterated trifluoroacetic acid via ligand-directed NHC-palladium catalysis: a powerful method for deuteration of aromatic ketones, amides, and amino acids. Tetrahedron Lett. 2015, 56, 6231–6235. 10.1016/j.tetlet.2015.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Kerr W. J.; Lindsay D. M.; Reid M.; Atzrodt J.; Derdau V.; Rojahn P.; Weck R. Iridium-catalysed ortho-H/D and -H/T exchange under basic conditions: C–H activation of unprotected tetrazoles. Chem. Commun. 2016, 52, 6669–6672. 10.1039/C6CC02137A. [DOI] [PubMed] [Google Scholar]; f Valero M.; Kruissink T.; Blass J.; Weck R.; Güssregen S.; Plowright A. T.; Derdau V. C–H Functionalization-Prediction of Selectivity in Iridium(I)-Catalyzed Hydrogen Isotope Exchange Competition Reactions. Angew. Chem., Int. Ed. 2020, 59, 5626–5631. 10.1002/anie.201914220. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Hu G.-Q.; Li E.-C.; Zhang H.-H.; Huang W. Ag(i)-Mediated hydrogen isotope exchange of mono-fluorinated (hetero)arenes. Org. Biomol. Chem. 2020, 18, 6627–6633. 10.1039/D0OB01273D. [DOI] [PubMed] [Google Scholar]; h Kerr W. J.; Knox G. J.; Paterson L. C. Recent advances in iridium(I) catalysis towards directed hydrogen isotope exchange. J. Labelled Compd. Radiopharm. 2020, 63, 281–295. 10.1002/jlcr.3812. [DOI] [PubMed] [Google Scholar]
- a Bag S.; Petzold M.; Sur A.; Bhowmick S.; Werz D. B.; Maiti D. Palladium-Catalyzed Selective meta-C–H Deuteration of Arenes: Reaction Design and Applications. Chem. - Eur. J. 2019, 25, 9433–9437. 10.1002/chem.201901317. [DOI] [PubMed] [Google Scholar]; b Xu H.; Liu M.; Li L.-J.; Cao Y.-F.; Yu J.-Q.; Dai H.-X. Palladium-Catalyzed Remote meta-C–H Bond Deuteration of Arenes Using a Pyridine Template. Org. Lett. 2019, 21, 4887–4891. 10.1021/acs.orglett.9b01784. [DOI] [PubMed] [Google Scholar]
- a Burhop A.; Weck R.; Atzrodt J.; Derdau V. Hydrogen-Isotope Exchange (HIE) Reactions of Secondary and Tertiary Sulfonamides and Sulfonylureas with Iridium(I) Catalysts. Eur. J. Org. Chem. 2017, 2017, 1418–1424. 10.1002/ejoc.201601599. [DOI] [Google Scholar]; b Jess K.; Derdau V.; Weck R.; Atzrodt J.; Freytag M.; Jones P. G.; Tamm M. Hydrogen Isotope Exchange with Iridium(I) Complexes Supported by Phosphine-Imidazolin-2-imine P,N Ligands. Adv. Synth. Catal. 2017, 359, 629–638. 10.1002/adsc.201601291. [DOI] [Google Scholar]; c Valero M.; Becker D.; Jess K.; Weck R.; Atzrodt J.; Bannenberg T.; Derdau V.; Tamm M. Directed Iridium-Catalyzed Hydrogen Isotope Exchange Reactions of Phenylacetic Acid Esters and Amides. Chem. - Eur. J. 2019, 25, 6517–6522. 10.1002/chem.201901449. [DOI] [PubMed] [Google Scholar]; d Müller V.; Weck R.; Derdau V.; Ackermann L. Ruthenium(II)-Catalyzed Hydrogen Isotope Exchange of Pharmaceutical Drugs by C–H Deuteration and C–H Tritiation. ChemCatChem 2020, 12, 100–104. 10.1002/cctc.201902051. [DOI] [Google Scholar]; e Valero M.; Bouzouita D.; Palazzolo A.; Atzrodt J.; Dugave C.; Tricard S.; Feuillastre S.; Pieters G.; Chaudret B.; Derdau V. NHC-Stabilized Iridium Nanoparticles as Catalysts in Hydrogen Isotope Exchange Reactions of Anilines. Angew. Chem., Int. Ed. 2020, 59, 3517–3522. 10.1002/anie.201914369. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Daniel-Bertrand M.; Garcia-Argote S.; Palazzolo A.; Mustieles Marin I.; Fazzini P.-F.; Tricard S.; Chaudret B.; Derdau V.; Feuillastre S.; Pieters G. Multiple Site Hydrogen Isotope Labelling of Pharmaceuticals. Angew. Chem., Int. Ed. 2020, 59, 21114–21120. 10.1002/anie.202008519. [DOI] [PubMed] [Google Scholar]
- a Kuhl N.; Hopkinson M. N.; Wencel-Delord J.; Glorius F. Beyond directing groups: transition-metal-catalyzed C–H activation of simple arenes. Angew. Chem., Int. Ed. 2012, 51, 10236–10254. 10.1002/anie.201203269. [DOI] [PubMed] [Google Scholar]; b Hartwig J. F.; Larsen M. A. Undirected, Homogeneous C–H Bond Functionalization: Challenges and Opportunities. ACS Cent. Sci. 2016, 2, 281–292. 10.1021/acscentsci.6b00032. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wedi P.; van Gemmeren M. Arene-Limited Nondirected C-H Activation of Arenes. Angew. Chem., Int. Ed. 2018, 57, 13016–13027. 10.1002/anie.201804727. [DOI] [PubMed] [Google Scholar]; d Mondal A.; Wedi P.; van Gemmeren M.. The Non-directed Distal C(sp2)–H Functionalization of Arenes. In Remote C–H Bond Functionalizations; Maiti D., Guin S., Eds.; Wiley-VCH, 2021; pp 191–219. [Google Scholar]
- a Kański R.; Kańska M. Deuteriation of hydroxybenzoic acids in the presence of homogeneous platinum catalyst. J. Radioanal. Nucl. Chem. 2003, 257, 385–390. 10.1023/A:1024752300898. [DOI] [Google Scholar]; b Hanson S. K.; Heinekey D. M.; Goldberg K. I. C–H Bond Activation by Rhodium(I) Phenoxide and Acetate Complexes: Mechanism of H–D Exchange between Arenes and Water. Organometallics 2008, 27, 1454–1463. 10.1021/om7012259. [DOI] [Google Scholar]; c Hickman A. J.; Villalobos J. M.; Sanford M. S. Quantitative Assay for the Direct Comparison of Platinum Catalysts in Benzene H/D Exchange. Organometallics 2009, 28, 5316–5322. 10.1021/om900495n. [DOI] [Google Scholar]; d Emmert M. H.; Gary J. B.; Villalobos J. M.; Sanford M. S. Platinum and palladium complexes containing cationic ligands as catalysts for arene H/D exchange and oxidation. Angew. Chem., Int. Ed. 2010, 49, 5884–5886. 10.1002/anie.201002351. [DOI] [PubMed] [Google Scholar]; e Rhinehart J. L.; Manbeck K. A.; Buzak S. K.; Lippa G. M.; Brennessel W. W.; Goldberg K. I.; Jones W. D. Catalytic Arene H/D Exchange with Novel Rhodium and Iridium Complexes. Organometallics 2012, 31, 1943–1952. 10.1021/om2012419. [DOI] [Google Scholar]; f Iluc V. M.; Fedorov A.; Grubbs R. H. H/D Exchange Processes Catalyzed by an Iridium-Pincer Complex. Organometallics 2012, 31, 39–41. 10.1021/om201049p. [DOI] [Google Scholar]; g Ibañez S.; Poyatos M.; Peris E. Mono and dimetallic pyrene-imidazolylidene complexes of iridium(iii) for the deuteration of organic substrates and the C-C coupling of alcohols. Dalton. Trans. 2016, 45, 14154–14159. 10.1039/C6DT02942F. [DOI] [PubMed] [Google Scholar]; h Li E.-C.; Hu G.-Q.; Zhu Y.-X.; Zhang H.-H.; Shen K.; Hang X.-C.; Zhang C.; Huang W. Ag2CO3-Catalyzed H/D Exchange of Five-Membered Heteroarenes at Ambient Temperature. Org. Lett. 2019, 21, 6745–6749. 10.1021/acs.orglett.9b02369. [DOI] [PubMed] [Google Scholar]; i Lassalle S.; Jabbour R.; Schiltz P.; Berruyer P.; Todorova T. K.; Veyre L.; Gajan D.; Lesage A.; Thieuleux C.; Camp C. Metal-Metal Synergy in Well-Defined Surface Tantalum-Iridium Heterobimetallic Catalysts for H/D Exchange Reactions. J. Am. Chem. Soc. 2019, 141, 19321–19335. 10.1021/jacs.9b08311. [DOI] [PubMed] [Google Scholar]; j Smith J. D.; Durrant G.; Ess D. H.; Gelfand B. S.; Piers W. E. H/D exchange under mild conditions in arenes and unactivated alkanes with C6D6 and D2O using rigid, electron-rich iridium PCP pincer complexes. Chem. Sci. 2020, 11, 10705–10717. 10.1039/D0SC02694H. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Martin J.; Eyselein J.; Grams S.; Harder S. Hydrogen Isotope Exchange with Superbulky Alkaline Earth Metal Amide Catalysts. ACS Catal. 2020, 10, 7792–7799. 10.1021/acscatal.0c01359. [DOI] [Google Scholar]; l Dong B.; Cong X.; Hao N. Silver-catalyzed regioselective deuteration of (hetero)arenes and α-deuteration of 2-alkyl azaarenes. RSC Adv. 2020, 10, 25475–25479. 10.1039/D0RA02358B. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Tlahuext-Aca A.; Hartwig J. F. Site-Selective Silver-Catalyzed C–H Bond Deuteration of Five-Membered Aromatic Heterocycles and Pharmaceuticals. ACS Catal. 2021, 11, 1119–1127. 10.1021/acscatal.0c04917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Pony Yu R.; Hesk D.; Rivera N.; Pelczer I.; Chirik P. J. Iron-catalysed tritiation of pharmaceuticals. Nature 2016, 529, 195–199. 10.1038/nature16464. [DOI] [PubMed] [Google Scholar]; b Yang H.; Zarate C.; Palmer W. N.; Rivera N.; Hesk D.; Chirik P. J. Site-Selective Nickel-Catalyzed Hydrogen Isotope Exchange in N -Heterocycles and Its Application to the Tritiation of Pharmaceuticals. ACS Catal. 2018, 8, 10210–10218. 10.1021/acscatal.8b03717. [DOI] [Google Scholar]; c Zarate C.; Yang H.; Bezdek M. J.; Hesk D.; Chirik P. J. Ni(I)-X Complexes Bearing a Bulky α-Diimine Ligand: Synthesis, Structure, and Superior Catalytic Performance in the Hydrogen Isotope Exchange in Pharmaceuticals. J. Am. Chem. Soc. 2019, 141, 5034–5044. 10.1021/jacs.9b00939. [DOI] [PubMed] [Google Scholar]; d Corpas J.; Viereck P.; Chirik P. J. C(sp2)–H Activation with Pyridine Dicarbene Iron Dialkyl Complexes: Hydrogen Isotope Exchange of Arenes Using Benzene- d6 as a Deuterium Source. ACS Catal. 2020, 10, 8640–8647. 10.1021/acscatal.0c01714. [DOI] [Google Scholar]; e Garhwal S.; Kaushansky A.; Fridman N.; Shimon L. J. W.; Ruiter G. de. Facile H/D Exchange at (Hetero)Aromatic Hydrocarbons Catalyzed by a Stable Trans-Dihydride N-Heterocyclic Carbene (NHC) Iron Complex. J. Am. Chem. Soc. 2020, 142, 17131–17139. 10.1021/jacs.0c07689. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Yang H.; Hesk D. Base metal-catalyzed hydrogen isotope exchange. J. Labelled Compd. Radiopharm. 2020, 63, 296–307. 10.1002/jlcr.3826. [DOI] [PubMed] [Google Scholar]
- a Chen H.; Wedi P.; Meyer T.; Tavakoli G.; van Gemmeren M. Dual Ligand-Enabled Nondirected C–H Olefination of Arenes. Angew. Chem., Int. Ed. 2018, 57, 2497–2501. 10.1002/anie.201712235. [DOI] [PubMed] [Google Scholar]; b Chen H.; Mondal A.; Wedi P.; van Gemmeren M. Dual Ligand-Enabled Nondirected C–H Cyanation of Arenes. ACS Catal. 2019, 9, 1979–1984. 10.1021/acscatal.8b04639. [DOI] [Google Scholar]; c Mondal A.; Chen H.; Flämig L.; Wedi P.; van Gemmeren M. Sterically Controlled Late-Stage C–H Alkynylation of Arenes. J. Am. Chem. Soc. 2019, 141, 18662–18667. 10.1021/jacs.9b10868. [DOI] [PubMed] [Google Scholar]; d Chen H.; Farizyan M.; Ghiringhelli F.; van Gemmeren M. Sterically Controlled C–H Olefination of Heteroarenes. Angew. Chem., Int. Ed. 2020, 59, 12213–12220. 10.1002/anie.202004521. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Mondal A.; van Gemmeren M. Catalyst-Controlled Regiodivergent C–H Alkynylation of Thiophenes. Angew. Chem., Int. Ed. 2021, 60, 742–746. 10.1002/anie.202012103. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Santiago C.; Chen H.; Mondal A.; van Gemmeren M. Dual Ligand-Enabled Late-Stage Fujiwara–Moritani Reactions. Synlett 2021, 10.1055/s-0040-1706014. [DOI] [Google Scholar]
- For selected examples of arene-limited nondirected C–H activations with palladium catalysts, see:; a Wang P.; Verma P.; Xia G.; Shi J.; Qiao J. X.; Tao S.; Cheng P. T. W.; Poss M. A.; Farmer M. E.; Yeung K.-S.; Yu J.-Q. Ligand-accelerated non-directed C–H functionalization of arenes. Nature 2017, 551, 489–493. 10.1038/nature24632. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Naksomboon K.; Valderas C.; Gómez-Martínez M.; Álvarez-Casao Y.; Fernández-Ibáñez M. Á. S. O-Ligand-Promoted Palladium-Catalyzed C–H Functionalization Reactions of Nondirected Arenes. ACS Catal. 2017, 7, 6342–6346. 10.1021/acscatal.7b02356. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Naksomboon K.; Poater J.; Bickelhaupt F. M.; Fernández-Ibáñez M. Á. para-Selective C–H Olefination of Aniline Derivatives via Pd/S,O-Ligand Catalysis. J. Am. Chem. Soc. 2019, 141, 6719–6725. 10.1021/jacs.9b01908. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zhao Da; Xu P.; Ritter T. Palladium-Catalyzed Late-Stage Direct Arene Cyanation. Chem. 2019, 5, 97–107. 10.1016/j.chempr.2018.09.027. [DOI] [Google Scholar]; e Liu L.-Y.; Yeung K.-S.; Yu J.-Q. Ligand-Promoted Non-Directed C–H Cyanation of Arenes. Chem. - Eur. J. 2019, 25, 2199–2202. 10.1002/chem.201805772. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Liu L.-Y.; Qiao J. X.; Yeung K.-S.; Ewing W. R.; Yu J.-Q. meta-Selective C–H Arylation of Fluoroarenes and Simple Arenes. Angew. Chem., Int. Ed. 2020, 59, 13831–13835. 10.1002/anie.202002865. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Dhankhar J.; González-Fernández E.; Dong C.-C.; Mukhopadhyay T. K.; Linden A.; Čorić I. Spatial Anion Control on Palladium for Mild C-H Arylation of Arenes. J. Am. Chem. Soc. 2020, 142, 19040–19046. 10.1021/jacs.0c09611. [DOI] [PubMed] [Google Scholar]; h Yin B.; Fu M.; Wang L.; Liu J.; Zhu Q. Dual ligand-promoted palladium-catalyzed nondirected C–H alkenylation of aryl ethers. Chem. Commun. 2020, 56, 3293–3296. 10.1039/D0CC00940G. [DOI] [PubMed] [Google Scholar]
- Wedi P.; Farizyan M.; Bergander K.; Mück-Lichtenfeld C.; van Gemmeren M. Mechanism of the Arene-Limited Nondirected C–H Activation of Arenes with Palladium. Angew. Chem., Int. Ed. 2021, 60, 15641–15649. 10.1002/anie.202105092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The initial optimization studies were conducted using the reverse dedeuteration with deuterated HFIP-benzoate [D]1 as a model substrate. See:Uttry A.; Mal S.; van Gemmeren M. Late-Stage β-C(sp3)–H Deuteration of Carboxylic Acids. J. Am. Chem. Soc. 2021, 143, 10895–10901. 10.1021/jacs.1c06474. [DOI] [PubMed] [Google Scholar]; See the SI for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.