Summary
Background
Psoriasis is a serious and chronic noncommunicable disease. However, the fundamental measure of disease occurrence, the incidence, has been scarcely reported globally. There are no previous studies of psoriasis incidence in Latin America.
Aim
To estimate the incidence rates of psoriasis in Chile during 2016 and 2017 using an administrative database, the Waiting List Repository.
Methods
We examined referrals of psoriasis at onset, made by physicians to dermatologists, evaluated the agreement of diagnosis, and estimated the incidence of the disease considering the eligible population at risk.
Results
In most cases, the referrals corresponded to incident cases of psoriasis (73.3%; 95% CI: 66.6–79.2). The national incidence rates of psoriasis were 22.1 (95% CI: 21.1–23.1) and 22.7 (95% CI: 21.8–23.6) per 100 000 person‐years in 2016 and 2017, respectively. The most common type of psoriasis was the late‐onset type. We observed a high variation in the figures throughout the country, with a range from 0.75 (95% CI: 0.3–1.5) per 100 000 person‐years in the Metropolitan region to 164.9 (95% CI: 138.6–195.1) per 100 000 person‐years in the Aysen region.
Conclusion
We describe for the first time the incidence of psoriasis in a Latin American country. Our findings could potentially guide collaborations to improve our global understanding of psoriasis in Latin America.
Introduction
Psoriasis is a common chronic inflammatory skin disease that entails both a physical and psychological burden, and is associated with multiple comorbidities. The available epidemiological information on psoriasis varies depending on the geographical region assessed. Only 19% of the countries of the world report information on the epidemiology of psoriasis, according to the latest available systematic analysis. 1 The majority of information comes from the USA and Europe, and focuses on disease prevalence.
Incidence studies of psoriasis are limited in number and there are no established epidemiological criteria for the definition of a new psoriasis case. To our knowledge, only 10 countries have reported on the incidence of psoriasis at a population level, with studies coming from Canada, Denmark, Germany, Italy, Israel, Russia, Taiwan, the Netherlands, the UK and the USA. 1 The incidence rates reported in these studies range between 22 and 521 cases per 100 000 person‐years. 2 , 3 This broad range is due in part to different criteria being used, and the data show significant geographical variations.
A recent systematic analysis reported an estimated prevalence of psoriasis of 0.87% (95% uncertainty interval 0.29%–2.41%) for high‐income southern Latin America. 1 There are no population‐level reports of the incidence of psoriasis from the region, a finding that was also corroborated by a regional systematic review. 4
The aim of the present study was to examine the incidence of psoriasis in Chile. We show the development and validation of an algorithm that detects new cases of psoriasis, and then demonstrate its use by estimating annual incidence rates of psoriasis in Chile during 2016–2017, using an administrative database, the Waiting List Repository (WLR). This database records the first contact of a patient requiring specialist care for a determined problem within the public healthcare system. A detailed description of the Chilean healthcare system, the database and its processing can be found in Data S1 (Appendix; section headed ‘The Chilean Public Healthcare System’).
Methods
As de‐identified data were provided, the study did not require ethics committee approval as the information is public.
Study design
We performed an open cohort study using the WLR. Access to the WLR was obtained by request through the Chilean transparency law to the 29 health services of the country from January 2017 to December 2018.
In Chile, primary care physicians must refer all patients with psoriasis (even those with mild disease) according to the requirement expressed in the national medical curriculum; this requirement is assessed by a mandatory test before PCPs are allowed to provide medical care in the country. 5 Therefore, our definition of an incident case was the record of the first referral due to psoriasis in the WLR. To validate this selection of cases, namely, to determine if the referral corresponded to the first diagnosis of psoriasis, we examined the commentaries made by the dermatologists after care was provided, and the positive predictive value (PPV) was calculated. A comprehensive description of the validation process can be found in Data S1 (Appendix; ‘Validation’) and Table S1.
The population at risk of developing the disease was defined as a dynamic population, taking into account beneficiaries of the public health system at mid‐year.
Statistical analysis
Crude and direct standardized incidence rates were calculated, and adjusted by age and sex with respect to the most recently observed population, FONASA‐2017 (see Data S1). 95% CI of each incidence rate was computed as previously reported. 6
Results
The PPV of the algorithm was 73.3% (95% CI: 66.6–79.2). The flowchart representing the steps required to process the database and to validate the referrals as incident cases can be seen in Fig. 1. The detailed figures and characteristics of the study population are presented in Data S1 (Appendix; ‘Characteristics of the study population’), Figure S1, and Tables S2 and S3.
Figure 1.
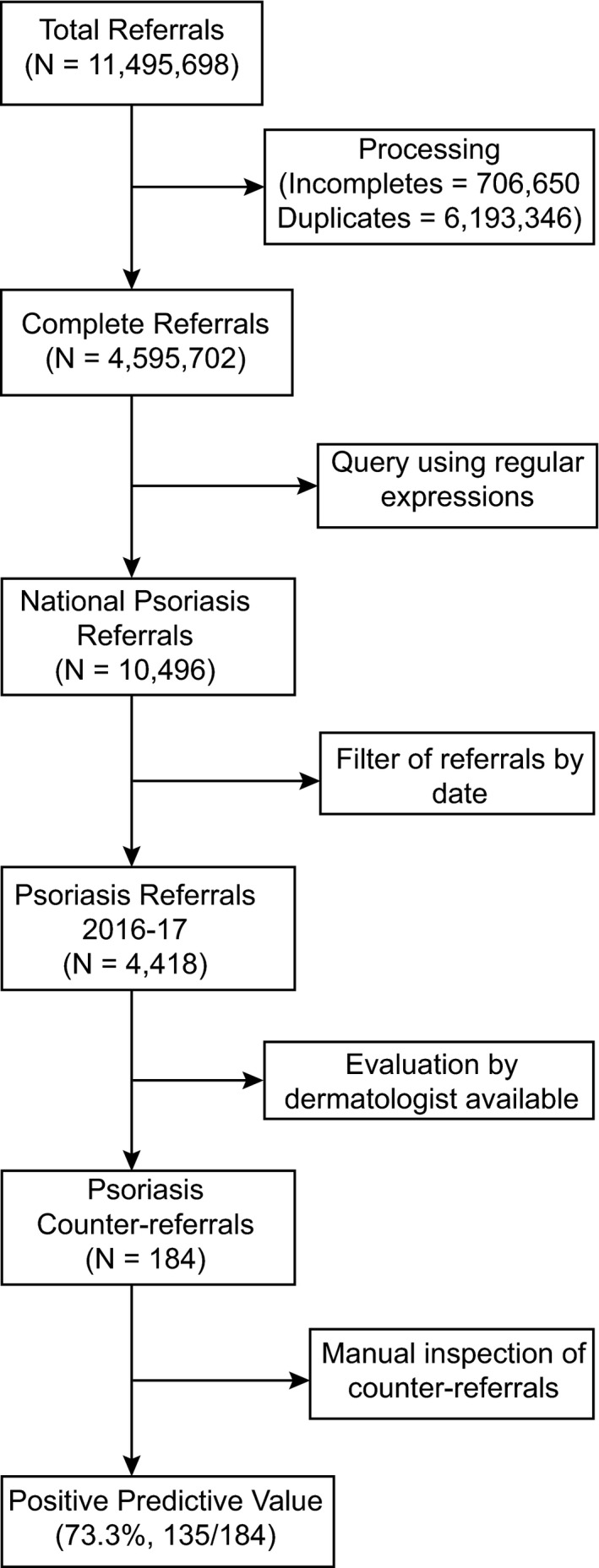
Flowchart of the validation algorithm. Raw data requested through the Chilean transparency law were termed ‘total referrals’, and data after processing were termed ‘complete referrals’. Within this data subset, a query using regular expressions was performed, obtaining the national psoriasis referrals. Referrals were filtered by the time window of this study, thus obtaining psoriasis referrals for 2016–17. Data were also available for specialist evaluation of the referral (i.e. the counter‐referral) for one of the largest health services in the country, the South‐East Metropolitan Health Service of Santiago, and with these data we computed the positive predictive value, with 135 of 184 cases established as incident cases for 2017.
Standardized incidence rates of psoriasis
The national standardized annual incidence rates of psoriasis were 22.1 (95% CI: 21.1–23.1) and 22.7 (95% CI: 21.8–23.6) per 100 000 person‐years for 2016 and 2017, respectively.
Incidence rates by age
Cases, population and estimates were stratified by age groups (Table 1). The incidence rates of psoriasis were similar for both years, with the minimum incidence found in the demographic age group 0–5 years: 6.4 (95% CI: 4.5–8.9) and 4.9 (95% CI: 3.4–6.9) per 100 000 person‐years for 2016 and 2017, respectively. The maximum incidence rate was found in the age group 55–65 years, corresponding to 37.1 (95% CI: 33.5–41.1) and 39.4 (95% CI: 35.9–43.1) cases per 100 000 person‐years for 2016 and 2017, respectively. The incidence then dropped for the age group > 75 years, corresponding to 14.6 (95% CI: 11.5–18.5) and 16.5 (95% CI: 13.4–20.1) cases per 100 000 person‐years, for 2016 and 2017.
Table 1.
Cases, population at risk and standardized incidence rates of psoriasis in Chile by sex and age groups during 2016 and 2017.
| Year | Age group, years | Females | Males | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Population | Rates a | Cases | Population | Rates a | Cases | Population | Rates a | ||
| 2016 | 0–5 | 18 | 283 119 | 6.36 (3.77–10.05) | 19 | 292 799 | 6.49 (3.91–10.13) | 37 | 575 918 | 6.42 (4.52–8.86) |
| 6–15 | 97 | 604 166 | 16.06 (13.02–19.59) | 74 | 630 304 | 11.74 (9.22–14.74) | 171 | 1 234 471 | 13.85 (11.85–16.09) | |
| 16–25 | 129 | 694 412 | 18.58 (15.51–22.07) | 77 | 651 947 | 11.81 (9.32–14.76) | 206 | 1 346 360 | 15.3 (13.28–17.54) | |
| 26–35 | 139 | 707 114 | 19.66 (16.53–23.21) | 68 | 558 925 | 12.17 (9.45–15.42) | 207 | 1 266 040 | 16.35 (14.2–18.74) | |
| 36–45 | 160 | 638 966 | 25.04 (21.31–29.24) | 144 | 510 687 | 28.2 (23.78–33.2) | 304 | 1 149 653 | 26.44 (23.55–29.59) | |
| 46–55 | 225 | 671 469 | 33.51 (29.27–38.18) | 190 | 566 988 | 33.51 (28.91–38.63) | 415 | 1 238 456 | 33.51 (30.36–36.89) | |
| 56–65 | 194 | 549 084 | 35.33 (30.53–40.67) | 183 | 465 528 | 39.31 (33.82–45.44) | 377 | 1 014 612 | 37.16 (33.5–41.1) | |
| 66–75 | 91 | 385 615 | 23.6 (19–28.97) | 113 | 313 298 | 36.07 (29.73–43.36) | 204 | 698 914 | 29.19 (25.32–33.48) | |
| > 75 | 40 | 309 648 | 12.92 (9.23–17.59) | 33 | 187 352 | 17.61 (12.12–24.74) | 73 | 497 000 | 14.69 (11.51–18.47) | |
| Total | 1093 | 4 843 594 | 22.45 (21.14–23.82) | 901 | 4 177 828 | 21.88 (20.47–23.36) | 1994 | 9 021 422 | 22.11 (21.15–23.11) | |
| 2017 | 0–5 | 18 | 327 163 | 5.5 (3.26–8.7) | 15 | 337 766 | 4.44 (2.49–7.32) | 33 | 664 928 | 4.96 (3.42–6.97) |
| 6–15 | 119 | 703 262 | 16.92 (14.02–20.25) | 75 | 733 812 | 10.22 (8.04–12.81) | 194 | 1 437 074 | 13.5 (11.67–15.54) | |
| 16–25 | 121 | 800 246 | 15.12 (12.55–18.07) | 84 | 756 794 | 11.1 (8.85–13.74) | 205 | 1 557 040 | 13.17 (11.43–15.1) | |
| 26–35 | 192 | 851 014 | 22.56 (19.48–25.99) | 102 | 685 162 | 14.89 (12.14–18.07) | 294 | 1 536 176 | 19.14 (17.01–21.46) | |
| 36–45 | 191 | 751 090 | 25.43 (21.95–29.3) | 131 | 611 941 | 21.41 (17.9–25.4) | 322 | 1 363 030 | 23.62 (21.11–26.35) | |
| 46–55 | 278 | 780 939 | 35.6 (31.54–40.04) | 220 | 666 854 | 32.99 (28.78–37.65) | 498 | 1 447 792 | 34.4 (31.44–37.56) | |
| 56–65 | 235 | 659 038 | 35.66 (31.24–40.52) | 248 | 566 468 | 43.78 (38.5–49.58) | 483 | 1 225 506 | 39.41 (35.98–43.09) | |
| 66–75 | 137 | 448 116 | 30.57 (25.67–36.14) | 159 | 369 742 | 43 (36.58–50.23) | 296 | 817 858 | 36.19 (32.19–40.56) | |
| > 75 | 48 | 371 264 | 12.93 (9.53–17.14) | 51 | 229 514 | 22.22 (16.54–29.22) | 99 | 600 779 | 16.48 (13.39–20.06) | |
| Total | 1339 | 5 692 133 | 23.33 (22.09–24.62) | 1085 | 4 958 052 | 22.19 (20.89–23.56) | 2424 | 10 650 184 | 22.72 (21.82–23.64) | |
Cases per 100 000 person‐years (95% CI).
Incidence rates by sex
Cases, population and estimates were also stratified by sex (Table 1) and were found to vary differently throughout life depending on sex (Fig. 2). In women there were two peaks of incidence, one during childhood and adolescence (5–15 years) and a second in middle age (from 45–65 years), whereas the incidence for men showed a single later peak (56–75 years). For both sexes, the incidence rates decreased from the age of 75 years.
Figure 2.
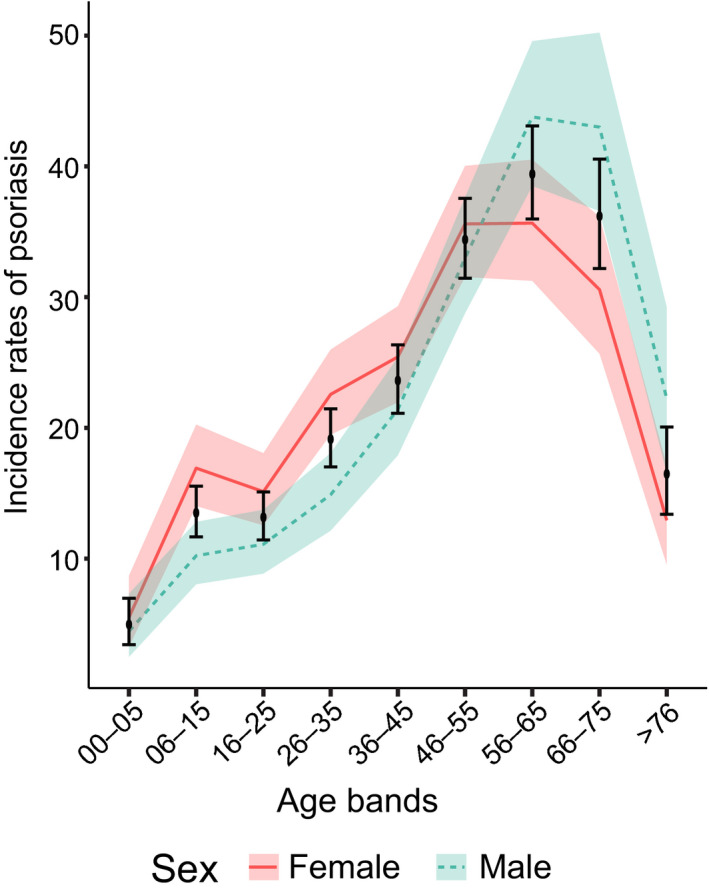
Incidence rates of psoriasis by age of onset and sex in Chile, 2017. Continuous red line indicates females, dashed blue line represents males, bars represent both sexes (upper and lower bounds of the CI intervals for the national estimates of psoriasis incidence rates and shaded regions correspond to the CIs of the incidence rates.
Incidence rates by region
To examine the occurrence of psoriasis throughout Chile, we plotted the incidence rates on a choropleth map (Fig. 3a). The rates fluctuated from a minimum of 0.75 (95% CI: 0.3–1.5) cases per 100 000 person‐years in the Metropolitan South Health Service (Santiago; central zone, 33.4°S), to a maximum of 164.9 (95% CI: 138.6–195.1) cases per 100 000 person‐years in the Aysen Health Service (below southern region; 46°S). The median of the observed figures across the health services was 20.9, with 75% of the observations having an incidence of < 49 cases per 100 000 person‐years (Fig. 3b).
Figure 3.
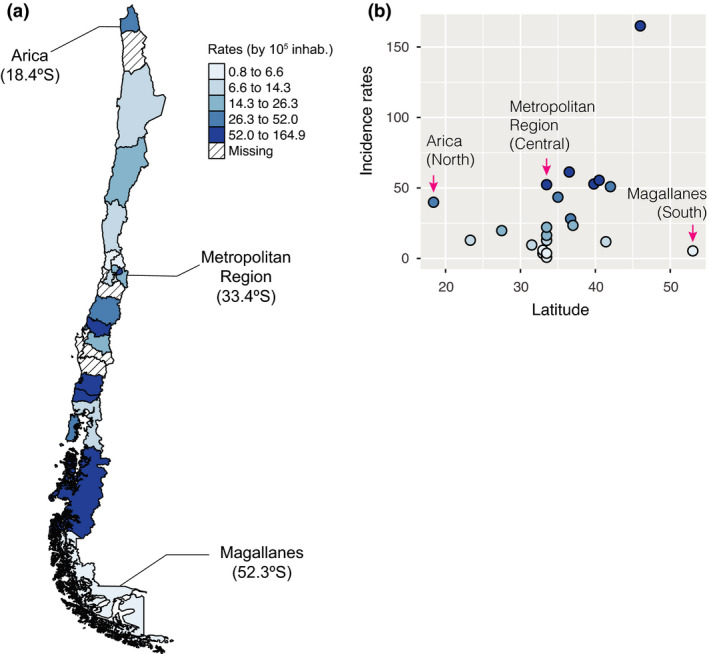
(a,b) Incidence rates of psoriasis in Chile in 2017, stratified by health service. (a) Choropleth map of the health services in Chile. The blue scale represents the value of the incidence rates of psoriasis, with darker tones indicating higher rates. The scale is categorized into five quantiles of data distribution. (b) Psoriasis incidence rates in Chile showing variations between health services located in the same administrative region and along the geographical latitude spectrum.
Discussion
We report the first population‐based cohort study examining the incidence of psoriasis in Chile, and indeed the first in Latin America. We used the WLR to perform the study. Unusually, and different from European results, we observed that most new cases of psoriasis corresponded to the late‐onset type (occurring for the first time in people aged > 40 years), with earlier presentation in women and a slight predominance of new male cases in adulthood and old age. We also found geographical variation in the incidence of psoriasis across the country. We believe these variations can be explained by the different levels of complexity in the Chilean healthcare system, i.e. the different capabilities of the 29 health services to adequately manage referrals to dermatology specialists. As in other countries, the distribution of dermatologists is not uniform in Chile and is concentrated in large conurbations. 7
A strength of this study is that it was conducted both on a national scale and in individual geographical areas. This high coverage of patients allowed us to depict the geographical heterogeneity in psoriasis incidence in Chile. Another strength was our use of WLR referrals to detect incident cases. This novel method allowed the use of semistructured data as cases were detected by searching for character strings rather than structured diagnostic codes. According to a recent systematic review, case detection using regular expressions is as good as that with rule‐based or probabilistic algorithms, with the advantage that its implementation is simple and understandable. 8 The WLR method differs from previous work calculating incidence rates of psoriasis using administrative data 2 , 3 , 9 , 10 by the fact that the referral is an explicit representation of a new episode of disease. A final strength was the high value of the PPV that we found during the validation. Our results are similar to those presented by Basarab et al., 11 who reported that the accuracy of a diagnosis of psoriasis made by general practitioners is 78%.
One of the main limitations of this study relates to the data‐generating process. We believe that the psoriasis incidence rates estimated using this repository demonstrate a phenomenon known as the inverse care law. 12 This is a public health problem, and means that even though the most deprived populations have greater health problems (both in severity and frequency), they usually receive less care in comparison with wealthier populations. Although it is stipulated in Chile’s professional regulations that all cases of psoriasis should be referred, 5 it seems unlikely that this actually happens. For example, we observed that in the Metropolitan Region (approximately 40% of the Chilean population), the psoriasis incidence rates were higher in the east side of Santiago, in areas where average deprivation is lower or they have easier access to dermatologists (Fig. 3a). Consequently, the figures presented here should be interpreted with caution, as our estimates refer only to the population who consulted healthcare professionals, thus there is a potential issue of underestimating the true incidence of the disease. This is a limitation common to the majority of studies using population‐based databases. As noted previously, 13 the computation of incidence from first contact with healthcare is accurate if the disease is severe and the data from the healthcare systems are centralized. However, Chile has a mixed healthcare system and patients can access dermatologists directly through the private system, which may contribute to a bias toward underestimation.
Our findings add to the existing literature from other regions that have used routinely collected data for the estimation of incidence of psoriasis at a national level, such as in North America, Europe and Asia. 2 , 3 , 6 , 9 , 10 , 14 , 15 , 16 , 17 , 18 , 19 , 20 The only previous work that studied the incidence of psoriasis in Latin America at a population level was a survey performed in the 1960s by Convit, and Farber and Nall, in Bolivia, Ecuador, Peru and Venezuela; they did not find any cases in the region. 21 , 22 A more recent cross‐sectional survey performed in Brazil reported a psoriasis prevalence of 1.3% (95% CI: 1.1%–1.5%). 23 The range of our incidence rates across Chile is very wide, with the lower range being similar to the disease frequency in China 10 and the upper range being similar to that in the UK. 6 The median was similar to a German study 3 of 2012, which reported rates of 17–26 and 19–26 cases per 100 000 person‐years for males and females, respectively; however, these figures are at variance with other estimates from Germany, which reported a rate of 521.1 per 100 000 person‐years. 2 This discordance demonstrates that even for the same country, using the same database and assessing across very similar periods of observation, the estimates can vary widely depending on the methodology applied.
As previously reported in the literature, the incidence rates of psoriasis in our study showed evidence of different disease onset by age group and sex, 24 with the highest rates observed in age group of 46–65 years in women, and 56–75 years in men, which is similar to results from Canada, Denmark and the USA. 9 , 15 , 17 Overall, our study showed that the most common type of psoriasis was the late‐onset type. We also observed a bimodal presentation in women, which was less pronounced than in other studies, but we did not see a bimodal onset in men. As summarized in a recent systematic review, our study was concordant with an earlier onset of psoriasis in women than in men, and an increasing trend of incidence until 70 years, after which it decreased. 24
Conclusion
We provide for the first time a core measure of epidemiological information about psoriasis not previously available in Latin America. Specifically, we have proposed a novel methodology that uses an information resource, the WLR, not previously considered as a means to identify new cases of psoriasis at the population level. We believe the variations observed could be due to (i) awareness of the disease, meaning that better medical care could produce more referrals to dermatologists in a more timely manner; (ii) administrative and economic reasons, related to the number of available physicians in the geographical zones, and that wealthy patients can self‐refer directly to a dermatologist; (iii) environmental factors, such as latitude, solar irradiance and pollution; and (iv) genetic admixtures of the populations, as psoriasis has an important genetic component in its aetiopathogenesis. Our findings have important consequences for health policy in the region, as they show that incidence rates vary throughout the country, with areas in which psoriasis is as frequent as in Europe and the USA.
Conflict of interest
CL has participated in clinical trials sponsored by Pfizer and Novartis. CD has served as speaker and principal investigator to Lilly, Pfizer, AbbVie, Janssen, Novartis and Amgen. JTM has served as advisor and/or received speaking fees and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, BMS, Celgene, Eli Lilly, LEO Pharma, Janssen‐Cilag, MSD, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi and UCB. CEMG reports receiving honorariums or research grants from AbbVie, Almirall, Celgene, Eli Lilly, Galderma, Janssen, LEO Pharma, Novartis, Pfizer, Sandoz, Sanofi and UCB Pharma. DMA reports research grants from AbbVie, Almirall, Celgene, Eli Lilly, Novartis, UCB and the Leo Foundation. The remaining authors state they have no conflict of interest. The Global Psoriasis Atlas (GPA) project is delivered by the academic project staff based at the University of Manchester and University Medical Center Hamburg‐Eppendorf. The GPA is a collaboration between three leading international organizations in the world of dermatology: the International Federation of Psoriasis Associations (IFPA), The International League of Dermatological Societies (ILDS) and the International Psoriasis Council (IPC). The GPA has been supported by grants and sponsorship from the Leo Foundation, AbbVie, Eli Lilly UK, Novartis Pharma AG, UCB and Almirall. All decisions concerning analysis, interpretation and publication are made independently of any industrial contribution. DMA and CEMG are funded in part by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre. JD and FV are funded by CMM‐Basal AFB170001, supported by Cost Center 570111 – CIMT‐CORFO and Project U‐INICIA VID 2019 UI‐004/19. SH is funded by ICM P09–015‐F, DAAD 57220037 and 57168868, CORFO 16CTTS‐66390, Fondecyt 1181823, FONDEF 19I10334 and ANID COVID0733. JMT is an employee of USZ and holds a ‘Filling the GAP’ scholarship.
Supporting information
Figure S1. Population pyramids of the study, database and source population in 2017.
Data S1. Appendix.
Table S1. Misclassification bias analysis of referrals of psoriasis using the inverse matrix method. Note that as the predictive values of the classification schemes are modified, the estimates of the algorithm are biased towards or away of the null.
Table S2. Study population (WLR), database population (FONASA), and source population (national) during the time window of the study.
Table S3. Regression analysis of genetic ancestry and incidence rates of psoriasis in Chile.
References
- 1. Parisi R, Iskandar IYK, Kontopantelis E et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ 2020; 369: m1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacob C, Meier F, Neidhardt KS et al. Epidemiology and costs of psoriasis in Germany – a retrospective claims data analysis. Value Health 2016; 19: A566. [Google Scholar]
- 3. Sewerin P, Brinks R, Schneider M et al. Prevalence and incidence of psoriasis and psoriatic arthritis. Ann Rheum Dis 2019; 78: 286–7. [DOI] [PubMed] [Google Scholar]
- 4. Hernández‐Vásquez A, Molinari L, Larrea N, Ciapponi A. Psoriasis in Latin America and the Caribbean: a systematic review. J Eur Acad Dermatol Venereol 2017; 31: 1991–8. [DOI] [PubMed] [Google Scholar]
- 5. Association of Faculties of Medicine of Chile . Profile of Knowledge EUNACOM. Single National Exam of Medicine Knowledge (in Spanish). 2010. Available at: https://www.eunacom.cl/contenidos/PerfilNew.pdf (accessed 28 May 2021).
- 6. Springate DA, Parisi R, Kontopantelis E et al. Incidence, prevalence and mortality of patients with psoriasis: a U.K. population‐based cohort study. Br J Dermatol 2017; 176: 650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chilean Ministry of Health . Study determining the gaps of general practitioners and specialists according to the methodology of rates of use of medical and specialized benefits in Chile (in Spanish). 2017. Available at: https://www.minsal.cl/wp‐content/uploads/2018/03/Estudio‐determinaci%C3%B3n‐de‐brechas‐m%C3%A9dicos.pdf (accessed 28 May 2021).
- 8. Ford E, Carroll JA, Smith HE et al. Extracting information from the text of electronic medical records to improve case detection: a systematic review. J Am Med Inform Assoc 2016; 23: 1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eder L, Widdifield J, Rosen CF et al. Trends in the prevalence and incidence of psoriasis and psoriatic arthritis in Ontario, Canada: a population‐based study. Arthritis Care Res 2019; 71: 1084–91. [DOI] [PubMed] [Google Scholar]
- 10. Wei JC, Shi LH, Huang JY et al. Epidemiology and medication pattern change of psoriatic diseases in Taiwan from 2000 to 2013: a nationwide, population‐based cohort study. J Rheumatol 2018; 45: 385–92. [DOI] [PubMed] [Google Scholar]
- 11. Basarab T, Munn SE, Jones RR. Diagnostic accuracy and appropriateness of general practitioner referrals to a dermatology out‐patient clinic. Br J Dermatol 1996; 135: 70–3. [PubMed] [Google Scholar]
- 12. Cookson R, Doran T, Asaria M et al. The inverse care law re‐examined: a global perspective. Lancet 2021; 397: 828–38. [DOI] [PubMed] [Google Scholar]
- 13. Bagley SC, Altman RB. Computing disease incidence, prevalence and comorbidity from electronic medical records. J Biomed Inform 2016; 63: 108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang Y‐T, Chen T‐J, Liu P‐C et al. Epidemiological study of psoriasis in the national health insurance database in Taiwan. Acta Derm Venereol 2009; 89: 262–6. [DOI] [PubMed] [Google Scholar]
- 15. Egeberg A, Skov L, Gislason GH et al. Incidence and prevalence of psoriasis in Denmark. Acta Derm Venereol 2017; 97: 808–12. [DOI] [PubMed] [Google Scholar]
- 16. Huerta C, Rivero E, Rodríguez LAG. Incidence and risk factors for psoriasis in the general population. Arch Dermatol 2007; 143: 1559–65. [DOI] [PubMed] [Google Scholar]
- 17. Icen M, Crowson CS, McEvoy MT et al. Trends in incidence of adult‐onset psoriasis over three decades: a population‐based study. J Am Acad Dermatol 2009; 60: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schonmann Y, Ashcroft DM, Iskandar IYK et al. Incidence and prevalence of psoriasis in Israel between 2011 and 2017. J Eur Acad Dermatol Venereol 2019; 33: 2075–81. [DOI] [PubMed] [Google Scholar]
- 19. Shalom G, Zisman D, Babaev M et al. Psoriasis in Israel: demographic, epidemiology, and healthcare services utilization. Int J Dermatol 2018; 57: 1068–74. [DOI] [PubMed] [Google Scholar]
- 20. Vena GA, Altomare G, Ayala F et al. Incidence of psoriasis and association with comorbidities in Italy: a 5‐year observational study from a national primary care database. Eur J Dermatol 2010; 20: 593–8. [DOI] [PubMed] [Google Scholar]
- 21. Convit J. Investigation of the incidence of psoriasis among Latin American Indians. In: Proceedings of the 13th Congress on Dermatology. Amsterdam: Excerpta Medica, 1962; 196. [Google Scholar]
- 22. Farber EM, Nall L. Psoriasis in the tropics. Epidemiologic, genetic, clinical, and therapeutic aspects. Dermatol Clin 1994; 12: 805–16. [PubMed] [Google Scholar]
- 23. Romiti R, Amone M, Menter A, Miot HA. Prevalence of psoriasis in Brazil – a geographical survey. Int J Dermatol 2017; 56: e167–8. [DOI] [PubMed] [Google Scholar]
- 24. Iskandar IYK, Parisi R, Griffiths CEM, Ashcroft DM. Systematic review examining changes over time and variation in the incidence and prevalence of psoriasis by age and gender. Br J Dermatol 2021; 184: 243–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Population pyramids of the study, database and source population in 2017.
Data S1. Appendix.
Table S1. Misclassification bias analysis of referrals of psoriasis using the inverse matrix method. Note that as the predictive values of the classification schemes are modified, the estimates of the algorithm are biased towards or away of the null.
Table S2. Study population (WLR), database population (FONASA), and source population (national) during the time window of the study.
Table S3. Regression analysis of genetic ancestry and incidence rates of psoriasis in Chile.


