Abstract
Aim
To evaluate the effectiveness of early visual training and environmental adaptation on visual function and neurological development in infants with visual impairment.
Method
This was a pilot intervention clinical trial study. Thirty infants (mean age 5.9mo, SD 2.1mo, range 4–11mo; 16 males, 14 females) with peripheral visual impairment (PVI, n=15) or cerebral visual impairment (CVI, n=15) participated in a 6‐month visual intervention programme. Thirty matched infants (mean age 6mo, SD 1.4mo, range 4–9mo; 18 males, 12 females) served as a comparison group. Primary outcome measures were visual acuity, contrast sensitivity, and qualitative ocular motor functions. Secondary outcomes were scores on the Griffiths Mental Developmental Scales (GMDS).
Results
The treatment group showed a significant improvement in all the primary outcomes (p<0.01). The comparison group improved only in visual acuity and contrast sensitivity (p<0.01). The treatment group showed greater improvement than the comparison group in visual fixation (p=0.033) and smooth pursuit (p<0.01). The CVI subgroup showed greater improvement in visual acuity than the PVI subgroup (p<0.01). GMDS subscales of hand–eye coordination (p=0.01) and performance (p<0.01) increased in the treatment group, while the total score of the comparison group decreased, driven by language (p=0.039) and hand–eye coordination (p=0.025) subscales.
Interpretation
Results suggest that, in infants with visual impairment, visual function and certain developmental outcomes improve in response to early visual training and environmental adaptation, in an interactive context.
What this paper adds.
Early visual training and environmental adaptation are associated with enhanced visual acuity and smooth pursuit.
Early visual training and environmental adaptation are associated with an improvement of neurological developmental outcome.
Performance, hand–eye coordination, and language scores in Griffiths Mental Developmental Scales increase after visual training.
After training, visual acuity improves more in infants with cerebral rather than anterior visual impairment.
Type and complexity of visual impairment contribute to determine infants’ response to training.
What this paper adds
Early visual training and environmental adaptation are associated with enhanced visual acuity and smooth pursuit.
Early visual training and environmental adaptation are associated with an improvement of neurological developmental outcome.
Performance, hand–eye coordination, and language scores in Griffiths Mental Developmental Scales increase after visual training.
After training, visual acuity improves more in infants with cerebral rather than anterior visual impairment.
Type and complexity of visual impairment contribute to determine infants’ response to training.
This article's abstract has been translated into Spanish and Portuguese.
Follow the links from the abstract to view the translations.
Entrenamiento visual temprano y adaptación ambiental para bebés con discapacidad visual
Objetivo
Evaluar la efectividad del entrenamiento visual temprano y la adaptación ambiental en la función visual y el desarrollo neurológico en bebés con discapacidad visual.
Método
Este fue un estudio piloto de intervención clínica. Treinta lactantes (edad media 5,9 meses, DE 2,1 meses, rango de 4 a 11 meses; 16 varones, 14 mujeres) con discapacidad visual periférica (PVI, n = 15) o discapacidad visual cerebral (CVI, n = 15) participaron en un programa de intervención visual que duro 6 meses. Treinta lactantes emparejados (edad media 6 meses, DE 1,4 meses, rango 4‐9 meses; 18 varones, 12 mujeres) sirvieron como grupo de comparación. Las medidas de resultado primarias fueron la agudeza visual, la sensibilidad al contraste y las funciones motoras oculares cualitativas. Los resultados secundarios fueron puntuaciones en las Escalas de desarrollo mental de Griffiths (GMDS).
Resultados
El grupo de tratamiento mostró una mejora significativa en todos los resultados primarios (p <0,01). El grupo de comparación mejoró solo en agudeza visual y sensibilidad al contraste (p <0.01). El grupo de tratamiento mostró una mayor mejora en la fijación visual (p = 0,033) y la búsqueda suave (p <0,01). El subgrupo CVI mostró una mayor mejora en la agudeza visual que el subgrupo PVI (p <0,01). Las subescalas del GMDS de coordinación ojo‐mano (p = 0,01) y rendimiento (p <0,01) aumentaron en el grupo de tratamiento, mientras que la puntuación total del grupo de comparación disminuyó, impulsada por el lenguaje (p = 0,039) y la coordinación ojo‐mano (p = 0,025) subescalas.
Interpretación
Los resultados sugieren que, en los bebés con discapacidad visual, la función visual y ciertos resultados del desarrollo mejoran en respuesta al entrenamiento visual temprano y la adaptación ambiental, y en un contexto interactivo.
Treinamento visual e adaptação ambiental precoces para lactentes com deficiência visual
Objetivo
Avaliar a efetividade do treino e adaptação ambiental precoces sobre a função motora e desenvolvimento neurológicos para lactentes com deficiência visual.
Método
Este foi um estudo piloto de intervenção clínica. Trinta lactentes (média de idade 5,9m, DP 2,1m, variação 4–11m; 16 do sexo masculino, 14 do sexo feminino) com deficiência visual periférica (DVP, n=15) ou deficiência visual cerebral (DVC, n=15) participaram de um programa de intervenção visual de 6 meses. Trinta lactentes pareados (média de idade 6m, DP 1,4m, variação 4–9m; 18 do sexo masculino, 12 do sexo feminino) serviram como grupo de comparação. As medidas de desfecho primárias foram a acuidade visual, a sensibilidade a contrastes, e funções óculo‐motoras qualitativas. Desfechos secundários foram as pontuações na Escala de Desenvolvimento Mental de Griffiths (EDMG).
Resultados
O grupo tratado mostrou melhora significativas em todos os desfechos primários (p<0,01). O grupo de comparação melhorou apenas na acuidade visual e sensibilidade a contrastes (p<0,01). O grupo tratado mostrou mais melhora na fixação visual (p=0,033) e acompanhamento suave (p<0,01). O subgrupo DVC mostrou mais melhora na acuidade visual do que o grupo DVP (p<0,01). As subescalas de coordenação olho‐mão (p=0,01) e desempenho (p<0,01)da EGMG aumentaram no grupo tratado, enquanto a pontuação total do grupo de comparação diminuiu, guiadas pelas subescalas de linguagem (p=0,039) e coordenação olho‐mão (p=0,025).
Interpretação
Os resultados sugerem que, em lactentes com deficiência visual, a função visual e certos resultados desenvolvimentais melhoram em reposta ao treinamento visual e adaptações ambientais precoces, e em um contexto interativo.
Abbreviations
- CVI
Cerebral visual impairment
- GMDS
Griffiths Mental Developmental Scales
- PVI
Peripheral visual impairment
According to the World Health Organization, an estimated 19 million infants are visually impaired worldwide, of whom 1.4 million are considered blind. 1 Visual impairment is defined as an ‘impairment of visual capacity (in terms of visual responses to light and structured stimuli) caused by congenital or acquired pathologies of the eye and/or optical pathways involving the cerebral visual cortex. 2 The disorders of the visual system can be divided into two main groups: peripheral visual impairment (PVI) or cerebral visual impairment (CVI). The first group includes all pregeniculate ophthalmological disorders of the ocular globe, retina, and from the anterior optic nerve to the optic chiasm. 2 Most congenital PVIs are caused by genetic defects, such as hereditary retinal dystrophies. 2 , 3 Other general common causes include cataracts and retinopathy of prematurity. 2 , 3 The second group involves damage or malfunction of post‐geniculate visual pathways, including the optic radiations, occipital cortex, and visual associative areas. 4 , 5 , 6 The combination of increasing survival rates of infants with brain injuries and greater success in managing eye‐related disorders has contributed to the fact that CVI is the most frequent taxonomic category of congenital visual impairment in infants in industrialized countries. 7 CVI is caused by brain injury arising, for example, from birth hypoxia/ischemia, head injury/trauma, infection (e.g. encephalitis, meningitis), seizure disorder, genetic disorder, and metabolic disorder. 8 , 9
Vision plays a central role in infant development, 10 , 11 and early and severe visual impairment may affect the behaviour and development of motor skills, 12 , 13 cognitive functions, 14 social‐communicative abilities, and formation of social relationships. 15 Vision can also be considered as ‘a window to the brain’ 16 since numerous cerebral areas and pathways participate in the processing of visual information and many neurological developmental disorders are often associated with visual impairments. Studies involving infants and young children with PVI have investigated the effects of visual impairment on early developmental outcomes, with the greatest delays observed in infants with profound blindness. 2 , 8 , 13 , 14 , 17 , 18 Specifically, infants with profound visual impairment (i.e. no form vision or light perception) seem to be vulnerable to developmental delays and cognitive deficits/regression in the early years of life, whereas infants with severe visual impairment (i.e. within the low vision range) do not seem to be as severely affected. 2 , 14 The presence of (albeit limited) form vision in the first months of life seems to exert a protective effect on early neurological developmental functions. 14 The development of the immature visual system is particularly responsive to an enriched surrounding environment at very early stages, 19 and vision training in a social context may modulate early neuroplastic changes involving local and global functional connectivity networks. 20
Infants with congenital visual deficits are thus recognized as being a highly vulnerable clinical population, 2 which leads to two important questions. First, what is the vulnerability of the developing visual system itself (and in the context of both PVI‐ and CVI‐related impairment)? Second, what is the potential benefit of vision training to improve vision and minimize vision disability and its potential disabling associations?
Evaluating visual function is important for establishing a child’s level of vision and creating a baseline assessment, and for measuring progress in response to a visual training/early intervention programme. 21
In typically developing infants and young children, vision seems to develop rapidly during the first 6 months of life followed by slower, continuous development during preschool age. 18 , 22 In children affected by congenital visual impairment, the possibility and the speed of visual development can be disrupted, depending on the type of visual impairment. Compared with PVI, CVI may be associated with a greater generalized vulnerability implicating numerous key pathways supporting the developing visual system. 23 , 24 Advanced neuroimaging studies in CVI have documented that the development of key white matter tracts implicated in visual processing seems to be markedly reduced in participants with CVI compared with both PVI and sighted comparison individuals. 25 While sensory experience and environmental enrichment are known to be important for development, 19 we also do not know their impact with respect to the type of congenital visual impairment (i.e. PVI compared with CVI).
Visual function needs to be promoted as early as possible, given that neuroplasticity is considered maximal within the first 2 years of age. 26 Early exposure to a rich visual environment along with multisensorial objects combined with social interactions that encourage natural explorative behaviours have been shown to improve not only visual functions but also cognitive skills, physical activity, and social interaction. 27 Environmental enrichment, which promotes early sensory function and experiences, profoundly affects the central nervous system at functional, anatomical, and molecular levels. 28 In the early stages of brain development, environmental enrichment has been shown to trigger a marked acceleration in the maturation of the visual system, with maternal behaviour acting as a fundamental mediator. Although these factors have acquired experimental support (particularly from animal studies), there is a lack of robust practical clinical application; moreover, clinical studies demonstrating the effectiveness of early visual intervention programmes remain inconclusive. 29
In the 1960s, Natalie Barraga 30 promoted the concept of vision rehabilitation, which eventually became common practice in early intervention programmes and teaching for infants with visual impairments. These rehabilitation programmes replaced the prevailing ‘sight saving’ view, as it became apparent that vision would not be further impaired through continued use. 31 Vervloed et al. 32 defined visual rehabilitation as interventions aimed at improving and recovering visual function, and emphasized the distinctions between interventions of vision stimulation and visual training. The former aimed at improving vision by enhancing and changing anatomy and physiology, while the latter was concerned with stimulating the development of children with visual impairments by means of visual materials, with the goal of improving visual function and behaviour. According to Vervloed et al., 32 vision stimulation is characterized by the exposure to strong visual stimuli such as flashing lights and brightly coloured materials, without a direct link to the behaviour of the infant and to the timing and intensity of the stimulation (‘non‐contingent stimuli’). It is most often applied to children who show minimal responses to normal visual impressions. In contrast, visual training teaches infants to make functional use of their sense of sight through physical, tactile, or verbal guidance to improve skills by changing visual behaviour. 32 Visual training that is behaviourally relevant and adapted to the infant’s needs should improve visual functions including acuity 33 and contrast sensitivity, 34 attention, and spontaneous visual curiosity. 35 , 36 However, little is known about the type, duration, and age of visual training, 33 which still remains only weakly recommended according to a recent meta‐analysis. 34 As Elsman et al. 29 have suggested, the lack of high‐quality, well‐designed, and adequately reported research limits the conclusions that can be drawn about the effectiveness of these interventions. Furthermore, there is still no consensus about the most suitable methods or instruments required to measure the outcomes of these interventions.
Taking these issues into consideration, we performed a pilot intervention study of a visual training programme (lasting 6mo) within an interactive and social context, and in an intensive and family‐oriented manner. We incorporated key features of clinical trial study design, allowing the assessment of the effectiveness of early visual training combined with environmental adaptations in infants with PVI or CVI, with a primary focus on the development of visual functions and secondarily on general development. Our initial hypothesis was that early, intensive, and family‐oriented visual training in a social context would improve visual and neurological developmental outcomes compared with a group not participating in the structured treatment programme. Second, we hypothesized that infants would respond differently to treatment on the basis of the cause (i.e. PVI compared with CVI) and the degree of visual impairment.
METHOD
Study design
We conducted a pilot intervention clinical trial following a prospective two‐group, parallel, single‐blinded assessment design to evaluate the effectiveness of an early visual training programme combined with environmental adaptations on visual function and neurological development in infants with congenital visual impairment: PVI and CVI (Fig. S1, online supporting information). The study was conducted in accordance with the ethical guidelines established by the Declaration of Helsinki, and was approved by the Ethics Committee of Brescia. Written informed consent was obtained from all participants and/or their parents before data collection.
Participants
A convenience sample was identified on the basis of infants with visual impairment, and sourced from the entire national territory who were subsequently referred to our Neuro‐ophthalmological Tertiary Centre, Child Neurology and Psychiatry Unit, ASST Spedali Civili of Brescia, from May 2014 to May 2016.
The eligibility criteria for participation were as follows. (1) A diagnosis of PVI or CVI. The diagnosis was made by a multidisciplinary team comprising a child neuropsychiatrist, ophthalmologist, orthoptist, and child therapist specializing in visual function and neurological development. (2) Age at first assessment of less than 12 months corrected for gestational age. Excluded from participation were infants living with parents who did not have a sufficient level of understanding of the Italian language to follow instructions related to the study.
During the period of convenience sampling, 213 infants (97 with PVI, 116 with CVI) were found to meet inclusion criteria for the study. The families of 30 infants were excluded because they did not meet the criterion of sufficient proficiency in the Italian language. Eight eligible families chose not to participate in either the treatment or comparison groups for personal reasons. Among the 175 eligible families, 30 children with PVI and CVI were then recruited to participate in the treatment group. These families attended the training programme in its entirety and no data were lost because of attrition. Among the remaining 145 families, we selected 30 infants to serve as the comparison group by sequential matching to infants in the treatment group (1:1 ratio) on the basis of the PVI and CVI subgroups, diagnosis, gestational age (±6mo), chronological age (±6mo), and sex. If more than one individual in the comparison group matched a single treated participant, the individual was selected on the basis of random sampling. For two female participants, we were unable to find a sex‐matched control. All the infants in the treatment group were represented equally with visual impairments due to PVI (e.g. Leber congenital amaurosis and ocular albinism) or CVI (e.g. preterm birth associated with intraventricular haemorrhage or periventricular leukomalacia) and participated in an early visual training programme for 6 months and with monthly follow‐ups (see below for further details). The infants in the comparison group did not undergo the structured visual training programme and attended only monthly clinical follow‐up. During the same 6‐month period, the infants of the comparison group received other types of routine treatment from their local healthcare providers such as proper holding, handling, and motor function, but no specific visual training was given. The parents in both the treatment and comparison groups received some general information and advice on activities for stimulating children, including suggestions on how to create an adapted environment in a very quiet and distraction‐free room. Parents were aware of which group they were assigned to as well as differences related to the intervention between the two study arms.
None of the recruited families were offered financial compensation for participation, and no specific data relating to socioeconomic status were collected. Two families in the treatment group and three families in the comparison group self‐identified as members of ethnic minorities. Families who lived close to or further away from the clinical centre were similar in terms of clinical diagnosis and types of routine treatment.
The demographic and clinical findings for the participating infants are outlined in Table 1.
Table 1.
Demographic and clinical features of the study sample
| Treatment group (n=30) | PVI treatment subgroup (n=15) | CVI treatment subgroup (n=15) | Comparison group (n=30) | PVI comparison subgroup (n=15) | CVI comparison subgroup (n=15) | |
|---|---|---|---|---|---|---|
| Mean age±SD (range), mo a | 5.9±2.1 (4–11) | 6.0±2.0 (4–11) | 5.5±1.5 (4–8) | 6.0±1.4 (4–9) | 6.0±1.0 (4–9) | 6.5±1.2 (5–8) |
| Male/female distribution | 16/14 | 5/10 | 11/4 | 18/12 | 8/7 | 10/5 |
| Mean gestational age±SD (range), wks | 39.3±1.3 (24.2–41.2) | 39.3±1.3 (37–41.2) | 29±3.4 (24–35.6) | 39.1±1.1 (25.4–41) | 39.1±1.1 (37.2–41) | 29.2±3.7 (25.4–36.6) |
| Mean birthweight±SD (range), g | 2377±1074 (520–3730) | 3194±333 (2720–3730) | 1352±524 (520–2400) | 2581±914 (587–4210) | 3217±448 (2410–4210) | 1483±165 (587–2690) |
| Apgar score >8, n | 20 | 15 | 5 | 19 | 15 | 4 |
| Neurological examination, n | ||||||
| Typical | 19 | 11 | 8 | 23 | 12 | 9 |
| Atypical | 11 | 4 | 7 | 7 | 1 | 6 |
| Causes of visual impairment, n | ||||||
| Leber congenital amaurosis | 5 | 5 | 0 | 5 | 5 | 0 |
| Albinism | 5 | 5 | 0 | 5 | 5 | 0 |
| Ocular malformation | 5 | 5 | 0 | 5 | 5 | 0 |
| Preterm birth, n | 15 | 0 | 15 | 15 | 0 | 15 |
| With IVH | 5 | 0 | 5 | 5 | 0 | 5 |
| With PVL | 5 | 0 | 5 | 5 | 0 | 5 |
| With negative neuroimaging | 5 | 0 | 5 | 5 | 0 | 5 |
Corrected age according to expected birth date for infants born ≤37wks. PVI, peripheral visual impairment; CVI, cerebral visual impairment; IVH, intraventricular haemorrhage; PVL, periventricular leukomalacia.
Assessments
All infants participated in a detailed collection of medical history data, neurological examination, visual assessment, and video‐recorded evaluation, as well as developmental assessment, to obtain a developmental quotient 37 , 38 , 39 at baseline (T 0: before starting the visual training programme) and after 6 months (T 1: at the end of the visual training programme). Neurological examination was performed according to the Amiel‐Tison protocol 40 and the neurological examination was defined as atypical in the presence of one or more neurological signs, and typical in the absence of any neurological signs.
Visual assessment included ophthalmological evaluation (i.e. cycloplegic refraction, anterior segment, and ocular fundus examination), basic visual function (i.e. visual acuity and contrast sensitivity), and ocular motor functions (i.e. fixation, smooth pursuit, and reactive saccades). Visual field assessment was not performed because of the technical difficulty of formally evaluating this function in infants. With regard to ocular motor functions, fixation was defined as present (score 1: stable for more than 3s), mildly impaired (score 2: unstable for less than 3s), or severely impaired (score 3: i.e. not elicited). Smooth pursuit was defined as present (score 1: continuous), mildly impaired (score 2: discontinuous), or severely impaired (score 3: difficult to elicit). Reactive saccadic eye movements were defined as present (score 1: both latency and amplitude of saccade were normal), normometric with increased latency (score 2: amplitude was normal), dysmetric but with normal latency (score 3: altered amplitude as multiple saccades were necessary to reach the target), dysmetric with increased latency (score 4: amplitude was altered and increased latency owing to impaired suppression of fixation and generating saccades), or absent (score 5). 5 , 39 Visual acuity was evaluated under full refractive correction using Teller Acuity Cards 41 and expressed in cycles per degree. Teller Acuity Cards were used as they provide a quantitative measure of grating acuity based on preferential looking, and thus do not require a verbal response. Contrast sensitivity was evaluated using the Hiding Heidi Low Contrast Face Test by Lea Test Ltd. 42 To assess developmental skills, the Griffiths Mental Developmental Scales 38 (GMDS) were used. The developmental quotient for the total scores and the standard index (motor, language, personal–social, hand–eye coordination, and performance subscales) were all measured with ranges defined as follows: typical, >85; borderline, between 75 and 85; deficient, <70. We modified the GMDS according to previous studies and incorporated necessary accommodations for each item. 43 Accommodations were made in administration conditions and success criteria (response rate and time). This was done mainly for items that required hand–eye coordination. Accommodations included the enhancement of outlines, isolation of a visual stimulus, and contrast enhancement (such as the use of visual targets with a more vivid colour). The accommodations in administration conditions also included better lighting in the work area. Tactile exploration of the material before performing the task was permitted. A trained expert child neuropsychiatrist performed the neurological assessment, supported by a child therapist who conducted the video recording. An ophthalmologist performed the ophthalmology evaluation. A trained neuropsychologist, who was blind to the group assignment, assessed the developmental quotient.
Training procedure
The visual training programme implemented in this study was characterized as early (applied when participants were aged between 4 and 12mo corrected age), intensive (at least three 45‐min sessions per week for 6mo), and family‐oriented (parents were present during the training sessions and engaged in the rehabilitation programme). See Appendix S1 (online supporting information) for additional details about training procedures.
Outcome measurements
Outcomes were assessed at two time points: baseline (T 0) and 6 months (T 1). The primary clinical outcome was the visual function profile (including visual acuity, contrast sensitivity, visual fixation, smooth pursuit, and reactive saccades). In both the treatment and comparison groups, participants were stratified according to the type of visual deficit (i.e. PVI or CVI). Secondary clinical outcomes were measures of neurological development profile as assessed by the GMDS, and included developmental quotient, motor, language, personal–social, hand–eye coordination, and performance subscales.
Statistical analysis
Data trends were described using means, standard deviation, and range for quantitative variables, and counts for categorical variables. All quantitative outcomes were analysed as continuous variables assuming normal parametric distribution (verified using residual plots). A generalized estimation equation approach was used to estimate robust standard errors accounting for repeated measures. As all outcomes (excluding visual acuity) were censored (i.e. values under a certain threshold were set to a fixed value), data were analysed using censored regression models. Results are reported as estimated mean values and with 95% confidence intervals. Categorical outcomes (visual fixation, smooth pursuit, and reactive saccades) were treated as ordinal variables and modelled using Cumulative Link Mixed Models with logit link. All analyses were performed using R statistical package (version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria) and the statistical significance threshold was set at 5%.
RESULTS
Demographic data, clinical features, causes of visual impairment, and ocular/ocular motor findings are shown in Tables 1 and S1 (online supporting information). The treatment and comparison groups were very similar in terms of baseline characteristics. This included no statistically significant differences between the groups in baseline visual acuity (p=0.64), contrast sensitivity (p=0.55), visual fixation (p=0.58), smooth pursuit (p=0.26), reactive saccades (p=0.73), and GMDS profile subscales (motor [p=0.94], language [p=0.76], personal–social [p=0.39], hand–eye coordination [p=0.65], performance [p=0.59], and developmental quotient [p=0.92]) (Table 2).
Table 2.
Neurovisual and developmental profile of the samples at T 0 and T 1
| Primary outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment group (n=30) | T 1 vs T 0 | Comparison group (n=30) | T 1 vs T 0 | Treatment vs comparison group at T 0 | Interaction time×group | ||||
| T 0 | T 1 |
Δ time (95% CI) p |
T 0 | T 1 |
Δ time (95% CI) p |
Δ treatment (95% CI) p |
ΔΔ (95% CI) p |
||
| Visual acuity (cycles per degree) | 2.36 (1.57–3.16) | 4.16 (3.18–5.15) |
1.80 (0.94–2.66) <0.01 |
2.63 (1.84–3.42) | 3.84 (3.10–4.59) |
1.21 (0.64–1.79) <0.01 |
−0.26 (−1.38 to 0.86) 0.645 |
0.58 (−0.45 to 1.62) 0.267 |
|
| Contrast sensitivity a (%) | 15.15 (8.29–27.68) | 4.05 (2.28–7.20) |
0.27 (0.16–0.46) <0.01 |
11.75 (6.59–20.94) | 4.40 (2.44–7.97) |
0.38 (0.23 to 0.61) <0.01 |
0.37 (−0.82 to 1.56) 0.546 |
−0.49 (−1.52 to 0.54) 0.353 |
|
| Visual fixation b | 2.00 (1.96–2.04) | 1.14 (0.88–1.40) |
−0.86 (−1.10 to −0.62) <0.01 |
1.98 (1.92–2.04) | 1.66 (1.20–2.12) |
−0.32 (−0.75 to 0.10) 0.138 |
0.02 (−0.05 to 0.08) 0.583 |
−0.53 (−1.02 to −0.04) 0.033 |
|
| Smooth pursuit c | 2.24 (1.89–2.59) | 1.53 (1.08–1.99) |
−0.71 (−1.10 to −0.32) <0.01 |
2.04 (1.93–2.14) | 1.90 (1.72–2.08) |
−0.14 (−0.30 to 0.03) 0.098 |
0.21 (−0.15 to 0.56) 0.258 |
−0.57 (−0.96 to −0.18) <0.01 |
|
| Saccades d | 3.87 (3.47–4.27) | 2.77 (2.24–3.29) |
−1.10 (−1.76 to −0.44) <0.01 |
3.77 (3.35–4.19) | 3.50 (3.05–3.95) |
−0.27 (−0.88 to 0.35) 0.396 |
0.10 (−0.48 to 0.68) 0.735 |
−0.83 (−1.74 to 0.07) 0.071 |
|
| Secondary outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| T 0 | T 1 |
Δ time (CI) p |
T 0 | T 1 |
Δ time (95% CI) p |
Δ treatment (95% CI) p |
ΔΔ (95% CI) p |
||
| Developmental quotient GMDS (SS) | 83.35 (77.03–89.68) | 87.43 (80.35–94.51) |
4.08 (−1.23 to 9.39) 0.131 |
84.03 (77.11–90.95) | 73.63 (65.84–81.42) |
−10.40 (−17.44 to −3.35) <0.01 |
−0.67 (−14.49 to 13.14) 0.924 |
14.48 (5.78–23.17) <0.01 |
|
| Motor | 81.44 (72.97–89.90) | 82.82 (75.67–89.97) |
1.38 (−5.95 to 8.72) 0.710 |
80.81 (73.66–87.97) | 74.19 (64.87–83.51) |
−6.63 (−14.51 to 1.26) 0.099 |
0.62 (−15.01 to 16.25) 0.938 |
8.01 (−2.61 to 18.62) 0.139 |
|
| Personal–social | 88.49 (80.60–96.38) | 91.06 (81.82–100.31) |
2.57 (−4.20 to 9.34) 0.453 |
81.79 (74.15–89.43) | 76.97 (67.93–86.01) |
−4.83 (−15.51 to 5.86) 0.372 |
6.70 (−8.61 to 22.01) 0.391 |
7.40 (−5.10 to 19.89) 0.246 |
|
| Language | 89.75 (83.31–96.18) | 90.66 (83.83–97.49) |
0.91 (−5.73 to 7.56) 0.786 |
92.17 (84.56–99.78) | 74.35 (64.51–84.20) |
−17.81 (−27.63 to −8.00) <0.01 |
−2.42 (−17.99 to 13.15) 0.761 |
18.73 (6.98–30.47) <0.01 |
|
| H–E GMDS (SS) | 75.18 (67.91–82.45) | 82.99 (75.26–90.72) |
7.81 (1.81–13.80) 0.011 |
78.68 (70.72–86.64) | 70.63 (61.54–79.72) |
−8.05 (−15.67 to −0.43) 0.038 |
3.50 (−18.61 to 11.62) 0.651 |
15.86 (6.23–25.48) <0.01 |
|
| Performance GMDS (SS) | 80.51 (73.89–87.14) | 89.53 (81.81–97.25) |
9.01 (3.08–14.95) <0.01 |
84.50 (77.93–91.07) | 76.29 (68.45–84.13) |
−8.21 (−15.36 to −1.07) 0.025 |
−3.99 (−18.45 to 10.48) 0.589 |
17.23 (8.01–26.44) <0.01 |
|
Bold type indicates statistically significant p‐values. Data are mean (95% CI), unless otherwise indicated.
Contrast sensitivity was analysed on a log scale and therefore the effect is reported as a ratio.
Visual fixation was categorized in ordinal scores: 1, stable; 2, mildly impaired; 3, severely impaired.
Smooth pursuit was categorized in ordinal scores: 1, present; 2, mildly impaired; 3, severely impaired.
Reactive saccades were categorized in ordinal scores: 1, present; 2, normometric with increased latency; 3 dysmetric but with normal latency; 4, dysmetric with increased latency; 5, absent. T 0, developmental quotient at baseline; T 1, developmental quotient after 6mo; Δ, change; CI, confidence interval; ΔΔ, interaction estimate, that is (T1 vs T0)treatment–(T1 vs T0); GMDS, Griffiths Mental Developmental Scales; SS, standard scores; H–E, hand–eye coordination.
Primary outcomes: visual function profile
After the 6‐month treatment period, we observed a significant improvement in visual function (i.e. visual acuity and contrast sensitivity) in both groups (p<0.01; Table 2). With regard to ocular motor function, visual fixation, smooth pursuit, and reactive saccades, all improved significantly in the treatment group (p<0.01). However, the comparison group did not show significant improvement in these same functions (visual fixation p=0.14, smooth pursuit p=0.1, reactive saccades p=0.4). Interestingly, the comparison between the two groups over time (interaction effect) showed that variation from T 0 to T 1 (i.e. before and after the treatment period) was significantly different between the groups for visual fixation scores (−0.86 treatment vs −0.32 comparison, p=0.033) and for smooth pursuit scores (−0.71 treatment vs −0.14 comparison, p<0.01).
A stratified analysis according to the type of visual impairment (PVI and CVI) was also performed (results summarized in Tables 3 and 4). Visual acuity did not improve in either subgroup and contrast sensitivity showed a similar improvement in both treatment and comparison PVI subgroups (p<0.01). Visual fixation (p=0.01), smooth pursuit (p<0.01), and reactive saccades (p<0.01) improved in the PVI treatment subgroup. The PVI comparison subgroup did not show a significant improvement (visual fixation p=0.29; smooth pursuit p=0.34; reactive saccades p=0.24) (Fig. 1). Variation from T 0 to T 1 was significantly different between the PVI treatment and comparison subgroups for visual fixation (−0.74 treatment vs −0.08 comparison, p<0.03) and for smooth pursuit (−1.08 treatment vs −0.09 comparison, p<0.01). For the CVI subgroup, visual acuity and contrast sensitivity significantly improved in both CVI treatment and comparison subgroups (visual acuity: p<0.01 in both subgroups; contrast sensitivity: p<0.01 on treatment subgroup; p=0.02 in the comparison subgroup). With regard to ocular motor functions, only the CVI treatment subgroup showed significant improvement (visual fixation p<0.01; smooth pursuit p<0.01; reactive saccades p=0.02).
Table 3.
Neurovisual and developmental profile of the PVI subgroup at T 0 and T 1
| Primary outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|
| PVI treatment subgroup (15) | T 1 vs T 0 | PVI comparison subgroup (15) | T 1 vs T 0 | Treatment vs comparison group at T 0 | Δ time treatment vs comparison group | |||
| T 0 | T 1 |
Δ (95% CI) p |
T 0 | T 1 |
Δ (95% CI) p |
Δ (95% CI) p |
ΔΔ (95% CI) p |
|
| Visual acuity (cycles per degree) | 1.85 (0.56–3.15) | 2.32 (1.48–3.17) |
0.47 (−0.62 to 1.55) 0.397 |
2.02 (1.15–2.89) | 3.26 (2.31–4.21) |
1.24 (0.53–1.95) 0.07 |
−0.17 (−1.73 to 1.40) 0.836 |
−0.77 (−2.07 to 0.52) 0.242 |
| Contrast sensitivity a (%) | 21.68 (9.53–49.33) | 6.18 (2.75–13.87) |
0.28 (0.15–0.53) <0.01 |
14.20 (6.76–29.82) | 4.18 (2.02–8.65) |
0.29 (0.15–0.58) <0.01 |
1.53 (0.50–4.62) 0.45 |
0.97 (0.39–2.43) 0.943 |
| Visual fixation b | 2.06 (1.86–2.26) | 1.32 (0.67–1.98) |
−0.74 (−1.31 to −0.16) 0.012 |
2.02 (1.93–2.12) | 1.94 (1.76–2.12) |
−0.08 (−0.24 to 0.07) 0.295 |
0.04 (−0.17 to 0.24) 0.707 |
−0.65 (−1.25 to −0.06) 0.030 |
| Smooth pursuit c | 2.91 (2.61–3.21) | 1.83 (1.39–2.27) |
−1.08 (−1.43 to −0.73) <0.01 |
2.08 (1.85–2.31) | 1.99 (1.91–2.06) |
−0.09 (−0.29 to 0.10) 0.341 |
0.83 (0.44–1.22) <0.01 |
−0.98 (−1.38 to −0.59) <0.01 |
| Saccades d | 4.07 (3.72–4.41) | 3.20 (2.64–3.76) |
−0.87 (−1.52 to −0.21) <0.01 |
4.13 (3.88–4.39) | 3.87 (3.50–4.23) |
−0.27 (−0.71 to 0.18) 0.238 |
−0.07 (−0.49 to 0.36) 0.759 |
−0.60 (−1.39 to 0.19) 0.138 |
| Secondary outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|
| T 0 | T 1 |
Δ (95% CI) p |
Δ (95% CI) p |
Δ (95% CI) p |
ΔΔ (95% CI) p |
|||
| Developmental quotient GMDS (SS) | 87.67 (80.94–94.40) | 93.13 (80.32–105.94) |
5.46 (−3.60 to 14.53) 0.235 |
82.75 (69.44–96.06) | 71.26 (55.10–87.42) |
−11.49 (−25.91 to 2.93) 0.117 |
4.91 (−9.96 to 19.79) 0.514 |
16.95 (−0.01 to 33.91) 0.050 |
| Motor | 91.75 (80.78–102.72) | 92.83 (83.82–101.84) |
1.08 (−8.64 to 10.80) 0.826 |
82.65 (72.21–93.09) | 77.88 (61.60–94.17) |
−4.76 (−17.21 to 7.69) 0.450 |
9.10 (−6.04 to 24.24) 0.236 |
5.85 (−9.92 to 21.62) 0.464 |
| Personal–social | 92.17 (83.45–100.88) | 99.08 (82.99–115.16) |
6.91 (−6.01 to 19.83) 0.291 |
80.59 (65.94–95.23) | 77.26 (59.94–94.58) |
−3.33 (−23.99 to 17.33) 0.750 |
11.58 (−5.40 to 28.56) 0.179 |
10.24 (−14.12 to 34.60) 0.406 |
| Language | 95.08 (89.61–100.55) | 99.08 (88.67–109.50) |
4.00 (−4.06 to 12.06) 0.327 |
91.39 (76.20–106.57) | 73.49 (55.74–91.25) |
−17.90 (−34.68 to −1.12) 0.037 |
3.69 (−12.44 to 19.83) 0.651 |
21.90 (3.28 to 40.51) 0.022 |
| H–E GMDS (SS) | 77.22 (66.89–87.55) | 85.35 (71.23–99.47) |
8.13 (−2.07 to 18.32) 0.117 |
73.34 (58.03–88.65) | 65.16 (47.31–83.01) |
−8.18 (−22.45 to 6.10) 0.258 |
3.88 (−14.39 to 22.15) 0.674 |
16.30 (−1.25 to 33.85) 0.068 |
| Performance GMDS (SS) | 83.00 (68.98–97.03) | 94.41 (80.86–107.95) |
11.40 (1.07–21.73) 0.031 |
86.43 (74.83–98.04) | 74.38 (59.70–89.07) |
−12.05 (−24.06 to −0.04) 0.049 |
−3.43 (−21.43 to 14.58) 0.706 |
23.45 (7.54–39.36) <0.01 |
Bold type indicates statistically significant p‐values. Data are mean (95% CI), unless otherwise indicated.
Contrast sensitivity was analysed on a log scale and therefore the effect is reported as a ratio.
Visual fixation was categorized in ordinal scores: 1, stable; 2, mildly impaired; 3, severely impaired.
Smooth pursuit was categorized in ordinal scores: 1, present; 2, mildly impaired; 3, severely impaired.
Reactive saccades were categorized in ordinal scores: 1, present; 2, normometric with increased latency; 3, dysmetric but with normal latency; 4, dysmetric with increased latency; 5, absent. PVI, peripheral visual impairment; T 0, developmental quotient at baseline; T 1, developmental quotient after 6mo; Δ, change; CI, confidence interval; ΔΔ, interaction estimate, that is (T1 vs T0)treatment–(T1 vs T0); GMDS, Griffiths Mental Developmental Scales; SS, standard scores; H–E, hand–eye coordination.
Table 4.
Neurovisual and developmental profile of the CVI subgroup at T 0 and T 1
| Primary outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|
| CVI treatment subgroup (15) | T 1 vs T 0 | CVI comparison subgroup (15) | T 1 vs T 0 | Treatment vs comparison group at T 0 | Δ time treatment vs comparison group | |||
| T 0 | T 1 |
Δ (95% CI) p |
T 0 | T 1 |
Δ (95% CI) p |
Δ (95% CI) p |
ΔΔ (95% CI) p |
|
| Visual acuity (cycles per degree) | 2.88 (2.02–3.73) | 6.01 (4.81–7.20) |
3.13 (2.19–4.07) <0.01 |
3.24 (2.00–4.47) | 4.43 (3.35–5.51) |
1.19 (0.29–2.09) <0.01 |
−0.36 (−1.87 to 1.14) 0.637 |
1.94 (0.64–3.24) <0.01 |
| Contrast sensitivity a (%) | 11.10 (4.79–25.71) | 2.81 (1.31–6.03) |
0.25 (0.11–0.59) <0.01 |
9.97 (4.23–23.51) | 4.62 (1.86–11.46) |
0.46 (0.24–0.90) 0.023 |
1.11 (0.34–3.70) 0.86 |
0.55 (0.19–1.60) 0.268 |
| Visual fixation b | 1.90 (1.63–2.18) | 1.04 (0.90–1.18) |
−0.86 (−1.11 to −0.61) <0.01 |
1.84 (1.41–2.27) | 1.19 (0.71–1.67) |
−0.65 (−1.09 to −0.21) 0.07 |
0.07 (−0.43 to 0.56) 0.789 |
−0.21 (−0.66 to 0.23) 0.350 |
| Smooth pursuit c | 2.01 (1.95–2.08) | 1.28 (0.71–1.85) |
−0.74 (−1.26 to −0.21) <0.01 |
2.00 (1.94–2.05) | 1.78 (1.28–2.27) |
−0.22 (−0.69 to 0.24) 0.346 |
0.02 (−0.07 to 0.10) 0.734 |
−0.51 (−1.22 to 0.19) 0.151 |
| Saccades d | 3.67 (2.96–4.37) | 2.33 (1.49–3.17) |
−1.33 (−2.43 to −0.23) 0.017 |
3.40 (2.64–4.16) | 3.13 (2.35–3.91) |
−0.27 (−1.35 to 0.82) 0.631 |
0.27 (−0.77 to 1.30) 0.614 |
−1.07 (−2.61 to 0.48) 0.176 |
| Secondary outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|
| T 0 | T 1 |
Δ (95% CI) p |
T 0 | T 1 |
Δ (95% CI) p |
Δ (95% CI) p |
ΔΔ (95% CI) p |
|
| Developmental quotient GMDS (SS) | 79.80 (64.75–94.85) | 82.77 (65.61–99.93) |
2.97 (−3.01 to 8.95) 0.327 |
85.22 (68.02–102.41) | 75.79 (59.03–92.55) |
−9.43 (−14.03 to −4.82) <0.01 |
−5.41 (−27.73 to 16.90) 0.631 |
12.39 (4.90–19.89) <0.01 |
| Motor | 72.73 (54.02–91.44) | 74.39 (56.53–92.25) |
1.66 (−9.60 to 12.91) 0.771 |
79.33 (60.37–98.30) | 71.14 (51.01–91.28) |
−8.19 (−17.92 to 1.55) 0.098 |
−6.60 (−32.59 to 19.39) 0.616 |
9.84 (−5.01 to 24.70) 0.192 |
| Personal–social | 85.48 (67.57–103.39) | 84.48 (64.54–104.42) |
−1.00 (−6.50 to 4.50) 0.719 |
82.94 (65.40–100.47) | 76.81 (57.66–95.96) |
−6.13 (−14.50 to 2.24) 0.149 |
2.54 (−22.03 to 27.12) 0.838 |
5.13 (−4.88 to 15.14) 0.312 |
| Language | 85.37 (68.66–102.08) | 83.66 (66.30–101.01) |
−1.71 (−11.82 to 8.39) 0.737 |
92.94 (73.04–112.83) | 75.28 (54.86–95.70) |
−17.66 (−29.01 to −6.31) <0.01 |
−7.57 (−33.00 to 17.87) 0.556 |
15.94 (0.88–31.01) 0.038 |
| H–E GMDS (SS) | 73.56 (57.03–90.09) | 81.11 (64.08–98.14) |
7.55 (0.54–14.56) 0.035 |
83.42 (66.24–100.60) | 75.42 (57.73–93.11) |
−8.00 (−15.87 to −0.13) 0.046 |
−9.86 (−32.99 to 13.27) 0.400 |
15.55 (5.05–26.04) <0.01 |
| Performance GMDS (SS) | 78.51 (62.47–94.56) | 85.53 (66.92–104.13) |
7.01 (0.47–13.56) 0.036 |
82.80 (65.95–99.64) | 78.03 (59.90–96.15) |
−4.77 (−12.62 to 3.08) 0.231 |
−4.28 (−26.68 to 18.11) 0.705 |
11.78 (1.55–22.01) 0.024 |
Bold type indicates statistically significant p‐values. Data are mean (95% CI), unless otherwise indicated.
Contrast sensitivity was analysed on a log scale and therefore the effect is reported as a ratio.
Visual fixation was categorized in ordinal scores: 1, stable; 2, mildly impaired; 3, severely impaired.
Smooth pursuit was categorized in ordinal scores: 1, present; 2, mildly impaired; 3, severely impaired.
Reactive saccades were categorized in ordinal scores: 1, present; 2, normometric with increased latency; 3, dysmetric but with normal latency; 4, dysmetric with increased latency; 5, absent. CVI, cerebral visual impairment; T 0, developmental quotient at baseline; T 1, developmental quotient after 6mo; Δ, change; CI, confidence interval; ΔΔ, interaction estimate, that is (T1 vs T0)treatment–(T1 vs T0); GMDS, Griffiths Mental Developmental Scales; SS, standard scores; H–E, hand–eye coordination.
Figure 1.
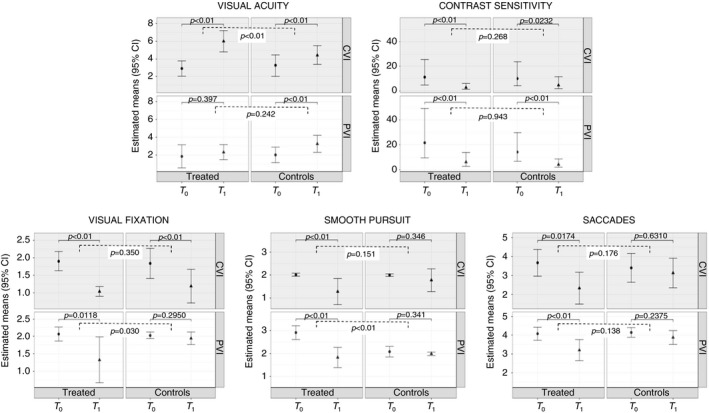
Primary outcomes.
Variation from T 0 to T 1 was significantly different between the CVI treatment and comparison subgroups for visual acuity (3.13 treatment vs 1.19 comparison, p<0.01). Moreover, the CVI subgroups showed significantly greater improvement over time in visual acuity compared with the variation in T 0 to T 1 in the PVI subgroups (p<0.01).
Secondary outcomes: neurological developmental profile
Assessment of the neurological developmental profile before and after the training period is summarized in Tables 2, 3, 4. After 6 months, the treatment group showed increased scores according to the GMDS in developmental quotient and for all subscales. Hand–eye coordination and performance subscales reached statistical significance (developmental quotient: rate of increase 4.08 standard scores, p=0.13; motor: rate of increase 1.38 standard scores, p=0.71; personal–social: rate of increase 2.57 standard scores, p=0.45; language: rate of increase 0.91 standard scores, p=0.79; hand–eye coordination: rate of increase 7.81 standard scores, p=0.01; performance: rate of increase 9.01 standard scores, p<0.01). In the comparison group, GMDS scores decreased in developmental quotient and in all the subscales, reaching statistical significance in the developmental quotient and language subscales (developmental quotient: rate of decrease 10.4 standard scores, p<0.01; motor: rate of decrease 6.63 standard scores, p=0.1; personal–social: rate of decrease 4.83 standard scores, p=0.37; language: rate of decrease 17.8 standard scores, p<0.01; hand–eye coordination: rate of decrease 8.05 standard scores, p=0.04; and performance: rate of decrease −8.21 standard scores, p=0.02) (Fig. 2). The comparison of the two groups over time (interaction effect) showed that variation from T 0 to T 1 was significantly different between treatment and comparison groups for the developmental quotient (4.08 treatment vs −10.4 controls, p<0.01) and for language (0.91 treatment vs −17.81 controls, p<0.01), hand–eye coordination (7.81 treatment vs −8.05 controls, p<0.01), and performance (9.01 treatment vs −8.21 controls, p<0.01) subscales. Stratified analysis of secondary outcomes was performed according to the type of visual impairment (PVI and CVI); the results are presented in Tables 3 and 4. The PVI treatment subgroup improved in performance scores (p=0.03). The CVI treatment subgroup confirmed the results of the total treatment group and showed a significant improvement in hand–eye coordination (p=0.03) and performance (p=0.04) scores. The PVI and CVI comparison subgroups both showed a statistically significant decrease in all the scores for developmental quotient (p<0.01), and language (p<0.01) and hand–eye coordination (p=0.05) subscales in the CVI comparison subgroup; in language (p=0.04) and performance (p=0.05) subscales in the PVI comparison subgroup.
Figure 2.
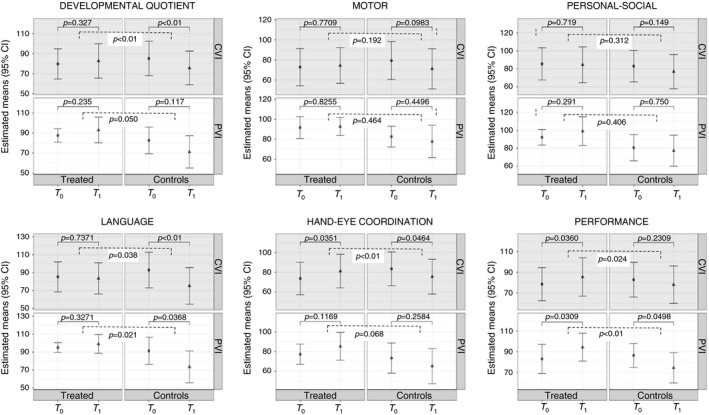
Secondary outcomes.
DISCUSSION
The results of this pilot intervention study suggest that a 6‐month early, intensive, and family‐oriented visual training programme (including environmental adaptations and the promotion of social interactions) can improve visual function and specific aspects of neurological development in infants with visual impairment. Specifically, we found that the treatment group showed a greater improvement than the comparison group for visual fixation, smooth pursuit, and in specific subscales of the GMDS (performance, hand–eye coordination, and language). Moreover, the CVI subgroup showed a significantly greater improvement over time in visual acuity than the PVI subgroup.
Although the results should still be considered preliminary, they are promising in supporting the effectiveness of early visual training in visually impaired children within a context of social interaction. Our vision training programme can be considered as intensive, highly structured, individualized, and with a high degree of social interaction, all serving as key features. More specifically, visual stimuli were delivered to the infant according to their responses. Their attention was continuously sustained by an adult through communication in a dynamic socially interactive context. The adult was also responding to the child’s own focus of interest, intentions, and desires, and thus was encouraged to socially interact and play with the child throughout the session.
Recent reviews have discussed the fact that studies implementing early visual intervention training programmes have provided promising, but often inconclusive, results in visually impaired children. 29 , 33 , 34 Several contributing factors need to be considered including the quality, design, and reporting of results. For example, the nature of the intervention programme proposed (e.g. visual stimulation compared with visual training, the timing and duration of the intervention, and outcome measurements used) as well as the incorporation of a comparison group, 44 blinded assessment, 33 and method of treatment allocation 36 are all design features that need to be clearly described and considered. In this study, we provide extensive details of the intervention programme (although it is important to consider participants were not randomized to treatment), and we implemented single‐blinded assessment of infants (the neuropsychologist, who assessed the developmental quotient, was blind to the group assignment) before and after treatment with a comparison group matched for corrected age, sex, and diagnosis. The incorporation of these study design features allows a more robust characterization of the effect of our intervention programme on visual function and specific aspects of neurological development.
Regarding visual functions, we observed that, after the 6‐month period, visual acuity and contrast sensitivity improved significantly in both the treatment and comparison groups. It has been reported in the literature that both visual acuity and contrast sensitivity progressively improve between birth and 48 months of age 45 as a consequence of postnatal maturation of the visual system and, in particular, the development of foveal cones and the refinement of the retinal and cortical architecture. 45 Grating visual acuity also improves rapidly in the first 6 months of life in response to visual experience. 46
Furthermore, we found differences in relation to the type of visual impairment between the PVI and CVI subgroups. In our study, visual acuity showed greater improvement in infants with CVI compared with PVI, in agreement with previous clinical studies. 36 , 47 , 48 , 49 The CVI treatment subgroup included infants born preterm and it is interesting to note that this cohort showed the greatest improvement after early visual training, especially with regard to visual acuity. Early exposure to an unnatural postnatal environment, together with early and unexpected removal from a protective milieu, are exclusive and peculiar factors of preterm birth that interfere with the typical development of the visual system in infants born preterm. 50 Retinopathy and CVI (secondary to brain lesions affecting the central visual pathway) are often associated findings in infants born preterm. In this context, myelination acts as a protective factor and may be useful for predicting visual outcome. Indeed, the immature brain can ‘amplify’ visual function through neuroplastic changes involving local and global functional connectivity networks by activating, modulating, and strengthening residual visual signals. 20 The development of the immature brain, in particular the visual system, is particularly responsive to a surrounding enriched environment at very early stages. 19
An early enriched environment, defined as ‘a combination of complex inanimate objects and social stimulation’, 51 plays an important role in the maturation of visual pathways and structures, triggering a sort of ‘reactive plasticity’ (a form of activity‐dependent plasticity) after chronic sensory deprivation or brain injury. 28 Environmental enrichment is known to have positive effects on the maturation of the visual system 52 and can induce long‐term changes in visual neural circuits with enduring modifications in brain structure and visual function. 19 In our study, we focused particularly on ensuring environmental adaptations according to the skills of the infants with visual impairment. For example, we introduced brightly coloured and/or multi‐sensorial toys that were easy to reach and handle and engaged parents/caregivers in social interaction and in accordance with the principle of the usefulness of the enriched environment. 27 We also found that, after visual training, the CVI subgroup showed a greater improvement in visual acuity compared with the PVI subgroup. It is important to note that at the start of the study, most of the infants in the PVI subgroup presented with a visual acuity lower than 1 cycle per degree (owing to damage to the ocular globe, retina, and/or the optic nerve). Thus, severely reduced visual acuity in the PVI subgroup at baseline may be a factor that contributed to less of an improvement after the training programme. Future studies should match all groups on the basis of entering visual acuities to investigate and clarify the potential effect of this factor with respect to response to treatment.
Ocular motor functions characterized by visual fixation, smooth pursuit, and reactive saccades also significantly improved in the treatment group. These functions are considered to be extremely immature at birth, but improve with age. 22 Fixation seems more stable from 4 to 15 years of age, when the duration of fixation increases and the number of intruding saccades decreases. 22 Smooth pursuit, even if it undergoes significant improvement during the first year of life, 53 seems more accurate during childhood and adolescence (the speed of slow eye movements gets close to that of the moving target because of an increase in ability to predict the movement of the target). It has also been found that saccade accuracy seems to reach adult‐level performance by 8 years of age, and saccade latency stabilizes at approximately 14 to 15 years of age. 22 These age‐related changes in eye movement control are probably related to maturation in brain anatomy and function owing to the integration of long‐range brain circuits encompassing cortical, subcortical, and cerebellar structures and to the increase in speed of information processing supported by synaptic pruning and myelination. 22 Indeed, several neural structures and pathways have been implicated in the generation of ocular motor functions including frontal eye fields, superior colliculus, basal ganglia, thalamus, and cerebellum. 22
Beyond the timing of natural development of the ocular motor system, our visual training programme seems to have a positive effect on developmental trajectories of fixation, smooth pursuit, and reactive saccades. In our study, the treatment group showed a better rate of improvement, expressed as a greater number of infants whose visual fixation, smooth pursuit, and reactive saccades more frequently shifted from severe to mild impairment or even typical functioning. As previous literature suggests, visual experience as well as sensory‐motor learning can improve ocular motor functions and, in particular, reactive saccades (saccadic adaptation) in infants within the first year of development. 54 , 55 Adaptation ability thus seems to be influenced by training and experience within this period, given that the basic neural circuitry involved in reactive saccadic adaptation already exists in infancy, while the brain continues to undergo structural changes in grey matter and white matter throughout childhood and adolescence. 55
The improvement of visual skills and, in particular, ocular motor functions could have a significant impact on object handling because visual feedback is essential for the control of goal‐directed arm movements 56 and is a prerequisite for the attentive capacity to predict where and when an object is moving. In our study, it seems that the visual training programme improved not only visual functions but also specific aspects of neurological development. 57 Visual training had a positive effect on specific subscales as assessed by the GMDS. Specifically, developmental quotient, hand–eye coordination, and performance subscales significantly improved after 6 months of training in the treatment group, while all the subscales worsened in the comparison group.
The benefit of the visual training for specific neurological developmental functions could be explained by the strong relationship between vision and infant neurological development 5 and between early intervention and the development of mental skills. 58 On the other hand, the comparison group’s decreasing GMDS scores might be related to the fact that visual impairment, as has long been recognized, is disruptive to early neurological development, with delays being observed in neuromotor, cognitive, language, and social domains. 14 With particular regard to language scores (which increased slightly in the treatment group and were significantly decreased in the comparison group), vision is implicated in general language development, as visually driven joint attention experiences in early childhood provide a framework for language learning. From the first months of life, infants use visual behaviours such as eye contact, gaze following, and joint attention to establish and sustain communication, and to learn about the behaviours and intentions of others especially during the pre‐linguistic stage. During the training sessions, caregivers in the treatment group may have learned to interpret and respond sensitively to their infants’ signals, thus indirectly promoting their infants’ communication strategies. On the other hand, the lack of direct suggestions and structured activities aimed at supporting social interaction in the families belonging to the comparison group probably led to poorer joint attention engagement and, thus, a lack of opportunities for enhanced communication. 59 , 60 , 61
The type of visual impairment seems to be insignificant in determining neurological developmental function after visual training. We found that, in the treated group, infants with PVI and CVI showed the same level of developmental quotient at baseline, infants in both groups improved their developmental quotient significantly according to the performance subscale, and those in the CVI subgroup improved in hand–eye coordination. An early, intensive, and personalized intervention has the potential of maximum effect in the promotion of specific aspects of neurological developmental functions 58 , 62 and can encourage compensatory neuroplastic reorganization both in infants with PVI 23 , 63 and those with CVI. 64 This seems to be possible despite the view that the brain develops and adapts differently in the context of damage to visual cerebral structures compared with damage to the eye, 23 and the fact that widespread neuronal damage (often found in children with CVI) could be very detrimental to a child’s development and learning. 65 Our study emphasizes the potential role of early intervention for modulating the specific aspects of neurological developmental trajectory of infants with visual impairment exposed to a carefully designed and structured early visual training programme. These findings also provide a more optimistic perspective about the potential of improved visual function and developmental outcomes, particularly in infants with CVI, as well as the importance of early diagnosis and intervention within the setting of early‐onset neurological injury.
Regarding potential limitations associated with this study, we need to consider the heterogeneous nature of the visual impairments of the participants. Despite the fact that groups were closely matched with respect to several developmental variables, including disease aetiology as well as other potential variables of interest such as age and sex (however, see the comment above about visual acuity), it is important to realize that all these factors could also be potential covariates. The heterogeneity of visual impairment aetiology is particularly evident in infants belonging to the PVI subgroup, consisting mainly of infants affected by Leber congenital amaurosis and ocular albinism. These eye disorders are characterized by different visual outcomes. Specifically, children with Leber congenital amaurosis (and other retinal disorders) show relatively slow progression and most remain with severe visual impairment as they grow older, regardless of their level of cognitive ability. In contrast, ocular albinism has very slow visual development in the first months of life but then rapid progression, and by 1 to 2 years many children will have moderate visual impairment.
Another limitation is related to the methodology of our evaluations. For example, ocular motor outcomes lacked quantitative accuracy. To quantify ocular motor functions, researchers typically use invasive and uncomfortable techniques such as scleral search coils and electro‐oculography, or less intrusive eye tracking technology. Therefore, the level of compliance to task instructions, the presence of glasses or contact lenses, and eye physiology are all relevant factors for collecting high‐quality eye tracking data. For these reasons, these instruments could not be easily applied in this study, and, furthermore, are not typically used in clinical practice for infants with visual impairment aged less than 12 months. 66 Furthermore, the use of Teller Acuity Cards for measuring visual acuity in infants and young children with visual impairment may have led to an evaluation bias since about half of the children with severe–profound visual impairment had an acuity below the largest stripes used, and thus were unable to see the gratings target. Finally, it is important to note that while the GMDS was used to evaluate the developmental quotient, it is not validated for assessing development in infants and young children with visual impairment. Our choice of using the modified GMDS to assess the developmental quotient was driven by the necessity to apply the same outcome measure to all the study participants.
Regarding the training programme, the high frequency of training sessions (at least three sessions per week) can be considered time consuming and costly, and thus impractical for some families. Finally, there remains the issue of not controlling the activities and environmental adaptations of the infants in the comparison group. Even though suggestions about activities aimed at promoting general and visual functions were provided to the families in the comparison group as well, it is likely that a similar structured and intensive procedure was followed as with the treatment group (e.g. environmental adaptations and infant–parent interactions). This may also be a source of experimental variability. Further research should consider alternative means to verify the effects of visual rehabilitation programmes such as remote assessment by telerehabilitation to further enhance the quality of data capture.
In summary, the preliminary results from this pilot intervention clinical trial suggest that early visual training (along with environmental adaptations and high social engagement) can improve vision‐related performance and specific aspects of neurological development outcomes in infants with visual impairment; both in the case of impairment of anterior pathways (PVI) as well as CVI. While our study demonstrates the efficacy of this intervention, other alternative models need to be considered to explain these observed improvements of visual function in infants with visual impairment. 63 , 67 Finally, further research is required to understand whether these improvements in visual function and neurological development after visual training can be sustained over a longer period. Further evidence about the clinical effectiveness of these visual training interventions is thus needed. 29 , 33 , 34
Supporting information
Figure S1: Flow chart of the pilot study protocol.
Appendix S1: Training procedures.
Table S1: Neurovisual profile of the study sample.
Acknowledgements
Members of the Early Visual Intervention Study Group are as follows:
Patrizia Accorsi and Anna Alessandrini (Unit of Child Neurology and Psychiatry, ASST Spedali Civili of Brescia, Brescia), Alice Bertoletti (Eye Clinic Department of Neurological Science and Vision, ASST Spedali Civili, Brescia), Elena Campostrini (Department of Clinical and Experimental Sciences, University of Brescia, Brescia), Nicole D’Adda (Unit of Child Neurology and Psychiatry, ASST Spedali Civili of Brescia, Brescia), Alessandra Franzoni (Eye Clinic Department of Neurological Science and Vision, ASST Spedali Civili, Brescia), Elisa Fumagalli (Department of Clinical and Experimental Sciences, University of Brescia, Brescia), Erica Grassi Scalvini, Paola Martelli, Melissa Marras, and Anna Molinaro (Unit of Child Neurology and Psychiatry, ASST Spedali Civili of Brescia, Brescia), Mario Motta (Neonatal Intensive Care Unit, ASST Spedali Civili of Brescia, Brescia), Nadia Pasini (Eye Clinic Department of Neurological Science and Vision, ASST Spedali Civili, Brescia), Lorenzo Pinelli (Neuroradiology Unit, Pediatric Neuroradiology Section, ASST Spedali Civili, Brescia), and Francesco Semeraro (Eye Clinic, Department of Neurological and Vision Sciences, University of Brescia, Brescia, Italy).
The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Gothwal VK, Sumalini R, Bharani S. Assessing the effectiveness of low Vision Rehabilitation in children: An observational study. Invest Ophthalmol Vis Sci 2015; 56: 3355–60. [DOI] [PubMed] [Google Scholar]
- 2. Dale N, Sakkalou E, O’Reilly M, Springall C, De Haan M, Salt A. Functional vision and cognition in infants with congenital disorders of the peripheral visual system. Dev Med Child Neurol 2017; 59: 725–31. [DOI] [PubMed] [Google Scholar]
- 3. Rahi JS, Cable N. British Childhood Visual Impairment Study Group. Severe visual impairment and blindness in children in the UK. Lancet 2003; 362: 1359–65. [DOI] [PubMed] [Google Scholar]
- 4. Dutton GN, Jacobson LK. Cerebral visual impairment in children. Semin Neonatol 2001; 6: 477–85. [DOI] [PubMed] [Google Scholar]
- 5. Fazzi E, Signorini SG, Bova SM, et al. Spectrum of visual disorders in children with cerebral visual impairment. J Child Neurol 2007; 22: 294–301. [DOI] [PubMed] [Google Scholar]
- 6. Sakki HEA, Dale NJ, Sargent J, Perez‐Roche T, Bowman R. Is there consensus in defining childhood cerebral visual impairment? A systematic review of terminology and definitions. Br J Ophthalmol 2018; 102: 424–32. [DOI] [PubMed] [Google Scholar]
- 7. Kong L, Fry M, Al‐Samarraie M, Gilbert C, Steinkuller PG. An update on progress and the changing epidemiology of causes of childhood blindness worldwide. J AAPOS 2012; 16: 501–7. [DOI] [PubMed] [Google Scholar]
- 8. Sonksen PM, Dale N. Visual impairment in infancy: impact on neurodevelopmental and neurobiological processes. Dev Med Child Neurol 2002; 44: 782–91. [DOI] [PubMed] [Google Scholar]
- 9. Bennett CR, Bauer CM, Bailin ES, Merabet LB. Neuroplasticity in cerebral visual impairment (CVI): assessing functional vision and the neurophysiological correlates of dorsal stream dysfunction. Neurosci Biobehav Rev 2020; 108: 171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fraiberg S, Adelson E. Self‐representation in language and play: observations of blind children. Psychoanal Q 1973; 42: 539–62. [PubMed] [Google Scholar]
- 11. Lueck AH, Dutton G. Vision and the Brain: Understanding Cerebral Visual Impairment in Children. New York, NY: American Foundation for the Blind Press, 2015. [Google Scholar]
- 12. Tröster H, Brambring M, Beelmann A. Prevalence and situational causes of stereotyped behaviors in blind infants and preschoolers. J Abnorm Child Psychol 1991; 19: 569–90. [DOI] [PubMed] [Google Scholar]
- 13. Fazzi E, Josée L, Oreste F‐G, et al. Gross motor development and reach on sound as critical tools for the development of the blind child. Brain Dev 2002; 24: 269–75. [DOI] [PubMed] [Google Scholar]
- 14. Dale N, Sonksen P. Developmental outcome, including setback, in young children with severe visual impairment. Dev Med Child Neurol 2002; 44: 613–22. [DOI] [PubMed] [Google Scholar]
- 15. Tröster H, Brambring M. Early social‐emotional development in blind infants. Child Care Health Dev 1992; 18: 207–27. [DOI] [PubMed] [Google Scholar]
- 16. Shaikh AG, Zee DS. Eye Movement research in the twenty‐first century—a window to the brain, mind, and more. Cerebellum 2018; 17: 252–8. [DOI] [PubMed] [Google Scholar]
- 17. Fazzi E, Signorini SG, Bomba M, Luparia A, Lanners J, Balottin U. Reach on sound: a key to object permanence in visually impaired children. Early Hum Dev 2011; 87: 289–96. [DOI] [PubMed] [Google Scholar]
- 18. Sakki H, Bowman R, Sargent J, Kukadia R, Dale N. Visual function subtyping in children with early‐onset cerebral visual impairment. Dev Med Child Neurol 2021; 63: 303–12. [DOI] [PubMed] [Google Scholar]
- 19. Baroncelli L, Braschi C, Spolidoro M, Begenisic T, Sale A, Maffei L. Nurturing brain plasticity: impact of environmental enrichment. Cell Death Differ 2010; 17: 1092–103. [DOI] [PubMed] [Google Scholar]
- 20. Sabel BA, Flammer J, Merabet LB. Residual vision activation and the brain‐eye‐vascular triad: Dysregulation, plasticity and restoration in low vision and blindness‐a review. Restor Neurol Neurosci 2018; 36: 767–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bennett CR, Bex PJ, Bauer CM, Merabet LB. The assessment of visual function and functional vision. Semin Pediatr Neurol 2019; 31: 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luna B, Velanova K, Geier CF. Development of eye‐movement control. Brain Cogn 2008; 68: 293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martín MBC, Santos‐Lozano A, Martín‐Hernández J, et al. Cerebral versus ocular visual impairment: the impact on developmental neuroplasticity. Front Psychol 2016; 7: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dutton G, Bax M. Visual impairment in children due to damage to the brain. London: John Wiley, 2010. [Google Scholar]
- 25. Bauer CM, Heidary G, Koo B‐B, Killiany RJ, Bex P, Merabet LB. Abnormal white matter tractography of visual pathways detected by high‐angular‐resolution diffusion imaging (HARDI) corresponds to visual dysfunction in cortical/cerebral visual impairment. J AAPOS 2014; 18: 398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yin W, Chen MH, Hung SC, Baluyot KR, Li T, Lin W. Brain functional development separates into three distinct time periods in the first two years of life. NeuroImage 2019; 189: 715–26. [DOI] [PubMed] [Google Scholar]
- 27. Sale A, Berardi N, Maffei L. Enrich the environment to empower the brain. Trends Neurosci 2009; 32: 233–9. [DOI] [PubMed] [Google Scholar]
- 28. Ismail FY, Fatemi A, Johnston MV. Cerebral plasticity: windows of opportunity in the developing brain. Eur J Paediatr Neurol 2017; 21: 23–48. [DOI] [PubMed] [Google Scholar]
- 29. Elsman EBM, Al Baaj M, van Rens GHMB, et al. Interventions to improve functioning, participation, and quality of life in children with visual impairment: a systematic review. Surv Ophthalmol 2019; 64: 512–57. [DOI] [PubMed] [Google Scholar]
- 30. Barraga NC. Effects of experimental teaching on the visual behavior of children with low vision. Optom Vis Sci 1965; 42: 557–61. [DOI] [PubMed] [Google Scholar]
- 31. Moore MW. Visual efficiency training with low vision children. Am Orthopt J 1972; 22: 68–70. [PubMed] [Google Scholar]
- 32. Vervloed MPJ, Janssen N, Knoors H. Visual rehabilitation of children with visual impairments. J Dev Behav Pediatr 2006; 27: 493–506. [DOI] [PubMed] [Google Scholar]
- 33. Williams C, Northstone K, Borwick C, et al. How to help children with neurodevelopmental and visual problems: a scoping review. Br J Ophthalmol 2014; 98: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chorna OD, Guzzetta A, Maitre NL. Vision assessments and interventions for infants 0–2 years at high risk for cerebral palsy: a systematic review. Pediatr Neurol 2017; 76: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lanners J, Piccioni A, Fea F, Goergen E. Early intervention for children with cerebral visual impairment: preliminary results. J Intellect Disabil Res 1999; 43: 1–12. [DOI] [PubMed] [Google Scholar]
- 36. Sonksen PM, Petrie A, Drew KJ. Promotion of visual development of severely visually impaired babies: evaluation of a developmentally based programme. Dev Med Child Neurol 1991; 33: 320–35. [DOI] [PubMed] [Google Scholar]
- 37. Fazzi E, Signorini SG, La Piana R, et al. Neuro‐ophthalmological disorders in cerebral palsy: Ophthalmological, oculomotor, and visual aspects. Dev Med Child Neurol 2012; 54: 730–6. [DOI] [PubMed] [Google Scholar]
- 38. Griffith R. Griffiths mental developmental scale‐revised: birth to 2 years (GMDS‐R). Florence: Hogrefe, 1996. [Google Scholar]
- 39. Iodice A, Galli J, Molinaro A, et al. Neurovisual assessment in children with ataxia telangiectasia. Neuropediatrics 2018; 49: 26–34. [DOI] [PubMed] [Google Scholar]
- 40. Amiel‐Tison C. Neurological development from birth to six years: guide for examination and evaluation. Baltimore MD: Johns Hopkins University Press, 2001. [Google Scholar]
- 41. Teller DY, McDonald MA, Preston K, Sebris SL, Dobson V. Assessment of visual acuity in infants and children; the acuity card procedure. Dev Med Child Neurol 1986; 28: 779–89. [DOI] [PubMed] [Google Scholar]
- 42. Hyvarinen L. Contrast sensitivity testing in clinical practice. British Journal of Ophthalmology. 1995;79: 9:867–868. 10.1136/bjo.79.9.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferreira V, Albuquerque CP. Adaptation of a developmental test to accommodate young children with low vision. J Vis Impair Blind 2017; 111: 97–111. [Google Scholar]
- 44. Ivanov IV, Kuester S, MacKeben M, et al. Effects of visual search training in children with hemianopia. PLoS ONE 2018; 13: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lewis TL, Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Dev Psychobiol 2005; 46: 163–83. [DOI] [PubMed] [Google Scholar]
- 46. Sgandurra G, Lorentzen J, Inguaggiato E, et al. A randomized clinical trial in preterm infants on the effects of a home‐based early intervention with the “CareToy System”. PLoS ONE 2017; 12: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Polat U, Ma‐Naim T, Spierer A. Treatment of children with amblyopia by perceptual learning. Vision Res 2009; 49: 2599–603. [DOI] [PubMed] [Google Scholar]
- 48. Alimović S, Mejaški‐Bošnjak V. Stimulation of functional vision in children with perinatal brain damage. Coll Antropol 2011; 35: 3–9. [PubMed] [Google Scholar]
- 49. Tsai LT, Hsu JL, Wu CT, Chen CC, Su YC. A new visual stimulation program for improving visual acuity in children with visual impairment: a pilot study. Front Hum Neurosci 2016; 10: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramenghi LA, Ricci D, Mercuri E, et al. Visual performance and brain structures in the developing brain of pre‐term infants. Early Hum Dev 2010; 86: 73–5. [DOI] [PubMed] [Google Scholar]
- 51. Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Res 1978; 153: 563–76. [DOI] [PubMed] [Google Scholar]
- 52. Berardi N, Sale A, Maffei L. Brain structural and functional development: Genetics and experience. Dev Med Child Neurol 2015; 57: 4–9. [DOI] [PubMed] [Google Scholar]
- 53. Pieh C, Proudlock F, Gottlob I. Smooth pursuit in infants: maturation and the influence of stimulation. Br J Ophthalmol 2012; 96: 73–7. [DOI] [PubMed] [Google Scholar]
- 54. Alahyane N, Lemoine‐Lardennois C, Tailhefer C, Collins T, Fagard J, Doré‐Mazars K. Development and learning of saccadic eye movements in 7‐ to 42‐month‐old children. J Vis 2016; 16: 1–12. [DOI] [PubMed] [Google Scholar]
- 55. Lemoine‐Lardennois C, Alahyane N, Tailhefer C, Collins T, Fagard J, Doré‐Mazars K. Saccadic adaptation in 10–41 month‐old children. Front Hum Neurosci 2016; 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rösblad B. Roles of visual information for control of reaching movements in children. J Mot Behav 1997; 29: 174–82. [DOI] [PubMed] [Google Scholar]
- 57. Hosang L, Yusifov R, Löwel S. Long‐term visual training increases visual acuity and long‐term monocular deprivation promotes ocular dominance plasticity in adult standard cage‐raised mice. eNeuro 2018; 5: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spittle Orton J, Anderson P, Boyd R, Doyle L. Early developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database Syst Rev 2009; 1: 1–56. [DOI] [PubMed] [Google Scholar]
- 59. Bigelow AE. The development of joint attention in blind infants. Dev Psychopathol 2003; 15: 259–75. [DOI] [PubMed] [Google Scholar]
- 60. Lueck AH. Developmental guidelines for infants with visual impairments: a guidebook for early intervention. Louisville, KY: American Printing House for the Blind Incorporated Press, 2008. [Google Scholar]
- 61. Rogers SJ. Characteristics of social interactions between mothers and their disabled infants: a review. Child Care Health Dev 1988; 14: 301–17. [DOI] [PubMed] [Google Scholar]
- 62. Cioni G, Inguaggiato E, Sgandurra G. Early intervention in neurodevelopmental disorders: underlying neural mechanisms. Dev Med Child Neurol 2016; 58: 61–6. [DOI] [PubMed] [Google Scholar]
- 63. Dale NJ, Sakkalou E, O’Reilly MA, et al. Home‐based early intervention in infants and young children with visual impairment using the Developmental Journal: longitudinal cohort study. Dev Med Child Neurol 2019; 61: 697–709. [DOI] [PubMed] [Google Scholar]
- 64. Guzzetta A, Fiori S, Scelfo D, Conti E, Bancale A. Reorganization of visual fields after periventricular haemorrhagic infarction: potentials and limitations. Dev Med Child Neurol 2013; 55: 23–6. [DOI] [PubMed] [Google Scholar]
- 65. Merabet LB, Mayer DL, Bauer CM, Wright D, Kran BS. Disentangling how the brain is “wired” in cortical (cerebral) visual impairment. Semin Pediatr Neurol 2017; 24: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nyström M, Andersson R, Holmqvist K, van de Weijer J. The influence of calibration method and eye physiology on eyetracking data quality. Behav Res Methods 2013; 45: 272–88. [DOI] [PubMed] [Google Scholar]
- 67. Kooiker MJG, van der Linden Y, van Dijk J, et al. Early intervention for children at risk of visual processing dysfunctions from 1 year of age: a randomized controlled trial protocol. Trials 2020; 21: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Flow chart of the pilot study protocol.
Appendix S1: Training procedures.
Table S1: Neurovisual profile of the study sample.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


