Abstract
Objective
Wearable seizure detection devices could provide more reliable seizure documentation outside the hospital compared to seizure self‐reporting by patients, which is the current standard. Previously, during the SeizeIT1 project, we studied seizure detection based on behind‐the‐ear electroencephalography (EEG). However, the obtained sensitivities were too low for practical use, because not all seizures are associated with typical ictal EEG patterns. Therefore, in this paper, we aim to develop a multimodal automated seizure detection algorithm integrating behind‐the‐ear EEG and electrocardiography (ECG) for detecting focal seizures. In this framework, we quantified the added value of ECG to behind‐the‐ear EEG.
Methods
This study analyzed three multicenter databases consisting of 135 patients having focal epilepsy and a total of 896 seizures. A patient‐specific multimodal automated seizure detection algorithm was developed using behind‐the‐ear/temporal EEG and single‐lead ECG. The EEG and ECG data were processed separately using machine learning methods. A late integration approach was applied for fusing those predictions.
Results
The multimodal algorithm outperformed the EEG‐based algorithm in two of three databases, with an increase of 11% and 8% in sensitivity for the same false alarm rate.
Significance
ECG can be of added value to an EEG‐based seizure detection algorithm using only behind‐the‐ear/temporal lobe electrodes for patients with focal epilepsy.
Keywords: behind‐the‐ear EEG, ECG, epilepsy, multimodal algorithms, reduced electrode montage, seizure detection, wearable sensors
Key Points.
ECG can be of added value to an EEG‐based seizure detection algorithm using only behind‐the‐ear or temporal lobe electrodes for patients with focal epilepsy
1. INTRODUCTION
Epilepsy is among the most common neurological disorders, affecting almost 1% of the population worldwide. 1 Antiepileptic drugs provide adequate treatment for about 70% of patients. 2 The remaining 30% continue to have seizures, which drastically affect their quality of life. For optimizing therapeutic interventions for these patients, objective measures of seizure documentation and counting are needed during daily life. 3 However, seizure self‐reporting by patients, which is the current standard, is unreliable. 4 , 5 , 6 , 7 , 8 Alternatively, wearable seizure detection devices could provide more reliable seizure documentation. Previously, during the SeizeIT1 project, we investigated whether behind‐the‐ear electroencephalography (EEG) could be useful to capture seizure patterns as part of a wearable device. Consequently, we developed algorithms using only behind‐the‐ear EEG channels for patients with focal epilepsy. 9 , 10 However, we concluded that the obtained sensitivities were too low for practical use. Low sensitivities are explained by the fact that not all seizures are associated with typical ictal EEG patterns. Independent research studies reported that the sensitivity of full‐scalp EEG for localizing focal seizures is around 21% in focal aware 11 and 50%–70% in focal impaired awareness seizures. 12 , 13 , 14 Furthermore, from the seizures with EEG correlation on full scalp EEG, 62% could be annotated as a seizure by a neurologist using only the behind‐the‐ear EEG channels in our study. 10 To improve overall sensitivity, a complementary monitoring modality is necessary.
Ictal heart rate (HR) changes as measured with electrocardiography (ECG) was shown to be useful for seizure detection in patients with focal epilepsy, 15 , 16 , 17 , 18 especially temporal lobe epilepsy. Ictal tachycardia was observed in 71%–84% of the seizures. 19 , 20 , 21 To illustrate this, Figure 1 shows the behind‐the‐ear EEG and ECG signals during a selected seizure.
FIGURE 1.
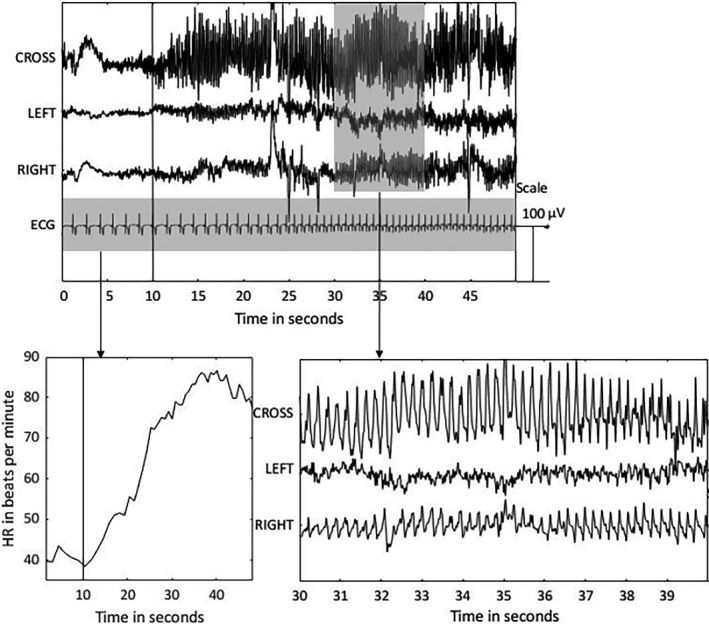
The behind‐the‐ear electroencephalogram (EEG) and electrocardiogram (ECG) are shown during a temporal focal impaired awareness seizure from the right hemisphere. The top panel contains four channels: crosshead (CROSS), left channel, right channel, and ECG. The amplitude of the ECG signal is decreased by a factor 10. The seizure onset is depicted with a vertical black line at 10 s. The bottom left panel shows the extracted heart rate (HR) in beats per minute during the seizure. A heart rate increase from 40 to 80 beats per minute is observed. The bottom right panel shows a close‐up of the behind‐the‐ear EEG channels during 20–30 s after the seizure onset
Multimodal algorithms have been developed combining ECG with full/reduced EEG montages. 22 , 23 , 24 Fürbass et al. 22 showed the added value of ECG to a 7/8 electrode EEG montage in terms of sensitivity. However, the question of whether adding ECG can increase sensitivity while maintaining the same false alarm rate (FAR) remains unanswered. Furthermore, the added value of ECG compared to behind‐the‐ear EEG remains uninvestigated.
In this paper, we develop a patient‐specific multimodal seizure detection algorithm using three behind‐the‐ear/temporal EEG channels and single‐lead ECG, with the focus on obtaining high sensitivity. The EEG and ECG data are processed separately using machine learning methods, which give rise to the unimodal EEG and unimodal ECG models, respectively. A late integration approach is applied to fuse the predictions from the unimodal models. We analyze data from three independent centers, with a total of 135 patients with focal epilepsy and 896 seizures.
The novelties of the paper are twofold. First, we propose a novel ECG‐based seizure detection algorithm. Second, we propose a multimodal algorithm that combines this ECG‐based algorithm and our existing behind‐the‐ear/temporal EEG‐based algorithm. 10 We investigate the added value of ECG in comparison with seizure detection using only behind‐the‐ear/temporal EEG. To this end, we construct graphs that demonstrate sensitivity in relation to FAR, allowing a comparison of sensitivities for different modalities at the same FAR. Furthermore, we investigate the dependence of detection performance on seizure type and localization.
This is a Phase 1 study according to the proposed standards for testing seizure detection devices, 25 because real seizure data from patients were included and gold standard annotations were used for validation. However, the data were not acquired with wearable devices. [Correction added on August 10, 2021, after first online publication: In the preceding sentence, “Class 1” has been changed to “Phase 1”.]
2. MATERIALS AND METHODS
The methods section starts with an overview of the datasets used. This is followed with a description of the seizure detection algorithm.
2.1. Datasets
The data used in this study consist of three different databases: SeizeIT1, Epilepsiae‐Freiburg, and Epilepsiae‐Paris. A detailed description is given in the following two paragraphs.
2.1.1. SeizeIT1 dataset
The SeizeIT1 dataset contained recordings from 82 patients with refractory focal epilepsy, who underwent presurgical evaluation at UZ Leuven, Belgium. Only those patients having at least two focal epileptic seizures were used in this study. This inclusion criterion was set for training and testing of a personalized algorithm. The dataset consisted of 42 patients having in total 221 seizures. Most seizures were focal impaired awareness (FIA) seizures, originating from the temporal lobe with a length between 10 and 30 s. A total of 176 (80%) seizures had ictal EEG changes on full scalp EEG. The overview of seizure types, localization, lateralization, and duration is given in Figure 2. Patients were recorded using the 10–20 EEG system and one bipolar ECG. Additionally, four behind‐the‐ear EEG electrodes (two at each side) were recorded and used in this study. Of the 176 seizures with ictal EEG changes, 109 (62%) could be blindly recognized by a neurologist using only the behind‐the‐ear EEG channels. More information about the SeizeIT1 dataset is described in detail in Vandecasteele et al. 10
FIGURE 2.
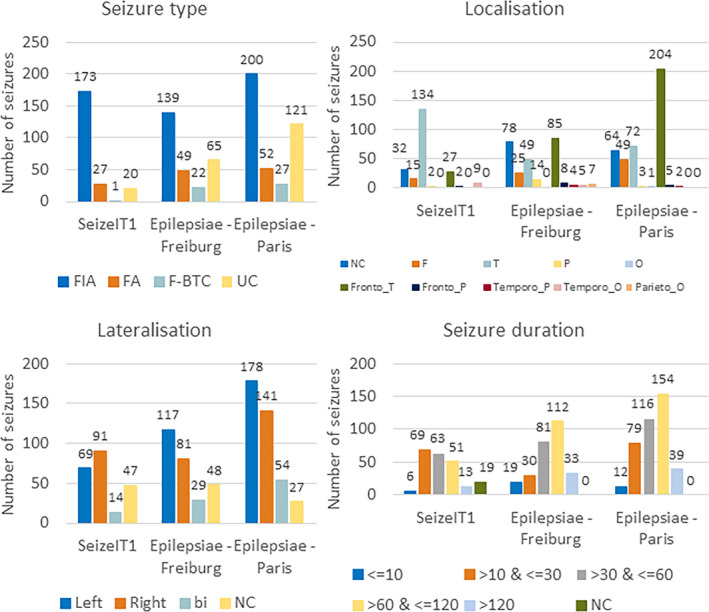
An overview of the seizure type (A), localization (B), lateralization (C), and duration (D) for the three different datasets. bi, bilateral; F, frontal; FA, focal aware; F‐BTC, focal to bilateral tonic–clonic; FIA, focal impaired awareness; NC, not clear; O, occipital; P, parietal; T, temporal; UC, unclassified
2.1.2. Epilepsiae‐Freiburg and Epilepsiae‐Paris datasets
The Epilepsiae‐Freiburg and Epilepsiae‐Paris datasets are subsets of the surface EEG Epilepsiae Database, 26 , 27 recorded in Freiburg, Germany and Paris, France. The Freiburg dataset consisted of 30 patients having 275 seizures in total, whereas the Paris dataset contained 63 patients and 400 seizures in total. Information about seizure localization/lateralization was provided for these datasets by the electrodes involved during seizure onset, listed for each seizure. Most of the seizures were FIA seizures originating in the (fronto‐)temporal lobe with a duration between 60 and 120 s for both datasets. The overview of seizure types, localization, lateralization, and duration is provided in Figure 2. In the Freiburg dataset, 243 (88%) seizures had ictal EEG changes, whereas all the seizures from the Paris dataset were associated with ictal EEG changes. From the Freiburg/Paris datasets, 196 (71%)/278 (70%) seizures had temporal lobe involvement.
The patients were recorded with 10–20 scalp EEG with one bipolar ECG. No behind‐the‐ear channels were recorded as in the SeizeIT1 database. In our study, we have used the closest electrodes of the behind‐the‐ear placements as surrogates: namely, the T7, T8, P7, and P8 electrodes. 28
2.2. Automated seizure detection algorithms using EEG and ECG
A schematic overview of the different steps of the automated seizure detection algorithm is shown in Figure 3. First, the steps of the unimodal EEG‐based seizure detection are described. Second, the unimodal ECG‐based seizure detection is explained. Third, the multimodal algorithm, combining the outcomes of the unimodal models, is clarified. Last, the performance metrics are depicted.
FIGURE 3.
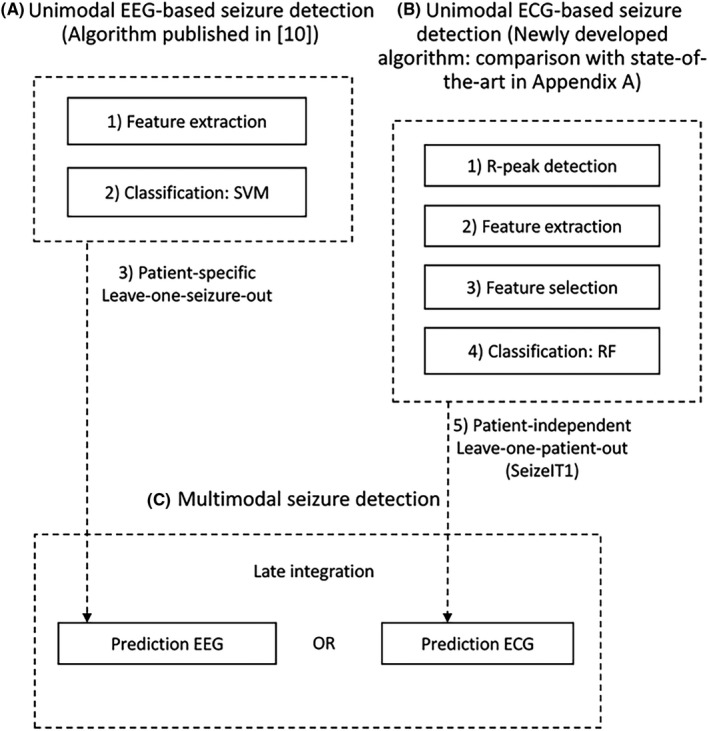
A schematic overview of the different steps in the automated seizure detection algorithm. ECG, electrocardiography; EEG, electroencephalography; RF, random forest; SVM, support vector machine
2.2.1. Unimodal EEG‐based seizure detection algorithm using behind‐the‐ear/temporal EEG electrodes
A patient‐specific algorithm for offline seizure detection 10 was used as a unimodal EEG‐based seizure detection algorithm. For SeizeIT1, only the behind‐the‐ear EEG channels were used. 10 For the Epilepsiae databases, those specific EEG channels were not recorded and the midposterotemporal electrodes T7, T8, P7, and P8 were used instead.
Feature extraction: The data were segmented into 2‐s windows with 1‐s overlap. For each window, time domain, frequency domain, entropy derived, and asymmetry features were extracted. 10
Classification: For classifying those 2‐s windows, a support vector machine (SVM) with a radial basis function kernel was applied. For training the SVM model for SeizeIT1, original seizure annotations (onset and end of seizure) from the neurologist were adapted by an engineer for excluding artifacts and nontypical EEG patterns. 10 For the Epilepsia datasets, the provided annotations were used for training the models. To obtain “sensitivity in relation to FAR” graphs, the continuous output of the SVM classifier was rescaled to a probability between 0 (very low probability) and 1 (very high probability to be a seizure segment). By changing the cutoff value for this probability, receiver operating characteristic (ROC) graphs were obtained with the sensitivity in relation to FAR.
Cross‐validation: A patient‐specific seizure detection algorithm was applied in this work, meaning that we used data from the test patient for training the models. We have shown before that patient‐specific algorithms achieve better performances than patient‐independent ones. 10 The cross‐validation scheme was leave‐one‐seizure‐out.
2.2.2. Unimodal ECG‐based seizure detection algorithm
A new patient‐independent ECG algorithm was developed using the SeizeIT1 database. The performance of the algorithm is compared to two state‐of‐the‐art algorithms in Appendix S1. The different steps of the algorithm are summarized.
R‐peak detection: The method consists of an ensemble of three different R‐peak detection algorithms. The first one is based on a wavelet decomposition, 29 the second one extracts the R‐peaks from the derivative signal with an adaptive threshold, 16 and the last one uses an adapted version of the Pan–Tompkins algorithm. Upper and lower envelopes were extracted for obtaining a clean signal. 30 The different R‐peak detection algorithms generate three different R‐peak series. These R‐peak locations were combined with an ensemble method. 31
Feature extraction: The data were segmented in windows of 60 s with 10‐s overlap. Compared to EEG (windows of 2 s), larger windows are needed for extracting reliable ECG features. For each window, 133 features were extracted (the detailed description of all the features is provided in Appendix S2). The feature set consisted of different features from both the time domain HR variability (HRV) and frequency domain HRV and other features also applied in various (non‐)epilepsy publications. 16 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40
Feature selection: For selecting discriminatory features, a random forest‐based feature selection scheme 41 resulted in a subset of the six most discriminant features, namely the modified cardiac sympathetic index based on Lorenz plot (CSI100), 32 circadian rhythm‐based feature, duration of the HR increase, HR after tachycardia/HR before tachycardia, very low frequency power and low frequency power.
Classification: For classifying each 60‐s window, a random forest classifier was used. Following the rationale used in the EEG algorithm, the continuous output of the random forest was converted to a probability, and an ROC “sensitivity in relation to FAR” graph was computed.
Cross‐validation: A patient‐independent seizure detection algorithm was developed. In an ideal case, the same patient‐specific cross‐validation scheme would be applied for ECG as for the EEG model. However, due to the lack of multiple available data points per patient, this was not possible without overfitting the model. In the case of EEG, multiple 2‐s windows are available per seizure, whereas for ECG, only one HR increase is present per seizure. The cross‐validation scheme was leave‐one‐patient‐out for SeizeIT1. The Epilepsiae databases were tested with a model trained on SeizeIT1.
2.2.3. Multimodal seizure detection algorithm
Different strategies for fusing multiple modalities exist; the main categories of such fusing strategies are the early and late fusion. In this work, we opted for a late integration strategy due to the difference in the cross‐validation schemes used and the different alignment of the events in both modalities. The typical patterns of EEG and ECG do not occur exactly at the same time. The HR increase/decrease can follow or precede the EEG onset. 20 Hence, a late integration, “OR” strategy, has been applied; a seizure should be detected by at least one of the modalities (EEG or ECG) for generating a seizure detection alarm, similarly as in Fürbass et al. 22 This choice was made because not all seizures have ictal ECG changes or EEG correlation and we aimed for high sensitivity.
For this multimodal algorithm, there are two varying thresholds (one for EEG and one for ECG). Visualizing sensitivity and FAR for all possible combinations of the two threshold values would give rise to a three‐dimensional plot that is difficult to read. Therefore, for simplicity, a threshold was selected for each modality leading to 0.2 false positives (FP)/h and those two thresholds were used for producing the multimodal results. This procedure was also done for 0.5 and 1 FP/h.
2.2.4. Performance evaluation
The following measures were applied to determine the performance of the seizure detection algorithm:
Detection sensitivity: TPs / (TPs + FNs), where TP is true positive and FN is false negative. A seizure was detected correctly (TP) if a detection occurred between the EEG onset and offset of the seizure.
FAR per hour: FPs/recording length. FPs within 10 s of each other were counted as one FP.
The “sensitivity in relation to FAR” graphs were constructed by changing the cutoff values of the probabilities for both the ECG‐ and EEG‐based algorithms. For each patient, a graph was constructed, and the graphs of all patients were averaged.
3. RESULTS
3.1. Performance of the unimodal models: EEG and ECG
In Figure 4, the “sensitivity in relation to FAR” graphs are shown for the unimodal algorithms: EEG and ECG. Table 1A shows the average sensitivities corresponding to an FAR of one FP/h. For the EEG‐based algorithm, Epilepsiae‐Freiburg had the lowest sensitivity (77%), SeizeIT1 had a sensitivity of 79%, whereas Epilepsiae‐Paris had the highest sensitivity (82%). For the ECG‐based algorithm, Epilepsiae‐Freiburg had the highest sensitivity (74%), SeizeIT1 had a sensitivity of 65%, whereas Epilepsiae‐Paris had the lowest sensitivity (52%). The unimodal algorithms, developed originally using the SeizeIT1 dataset, had similar performance on completely independent datasets, recorded at other centers (Freiburg and Paris). The performance of the unimodal ECG algorithm is compared a against state‐of‐the‐art algorithm in Figure 5. Details on this state‐of‐the‐art comparison can be found in Appendix S1.
FIGURE 4.

Sensitivity in relation to false alarm rate (FAR) for the different datasets. The blue graph depicts the unimodal results of electroencephalography (EEG), the orange one the results of electrocardiography (ECG). On those graphs, the sensitivities at an FAR at 0.2 false positives (FP)/h, 0.5 FP/h, and 1 FP/h are depicted with circles. The black symbols indicate the results of the multimodal algorithm at three discrete thresholds (squares, 0.2 FP/h; diamonds, 0.5 FP/h; stars, 1 FP/h). For comparison, the results on the EEG graph with the same FAR are indicated with blue marks. std, standard deviation
TABLE 1.
(A) FAR and Sens for the unimodal EEG and ECG at an FAR of 1 FP/h for the different databases; (B) FAR and Sens of the multimodal (at a threshold generating 1FP/h for the unimodal modalities) and EEG‐based detectors at the same FAR for the different databases.
| A | FAR, FP/h | Sens, unimodal EEG, % | Sens, unimodal ECG, % |
|---|---|---|---|
| SeizeIT1 | 1,00 | 79 (70–89)/100 (0 100) | 65 (55–76)/67 (0 100) |
| Epilepsiae‐Freiburg | 1,00 | 77 (68–87)/85 (0 100) | 74 (66–83)/79 (12 100) |
| Epilepsiae‐Paris | 1,00 | 82 (74–89)/100 (0 100) | 52 (44–61)/50 (0 100) |
| B | FAR, FP/h | Sens, multimodal, % | Sens, unimodal EEG, % |
|---|---|---|---|
| SeizeIT1 | 1.85 | 92 (87–96)/100 (50 100) | 81 (72–91)/100 (0 100) |
| Epilepsiae‐Freiburg | 1.94 | 90 (85–95)/100 (50 100) | 82 (73–91)/88 (6 100) |
| Epilepsiae‐Paris | 1.90 | 84 (77–91)/100 (0 100) | 84 (78–91)/100 (0 100) |
Data are shown as mean (95% confidence interval) / median (minimum maximum).
Abbreviations: ECG, electrocardiography; EEG, electroencephalography; FAR, false alarm rate; FP, false positives; Sens, sensitivity.
FIGURE 5.
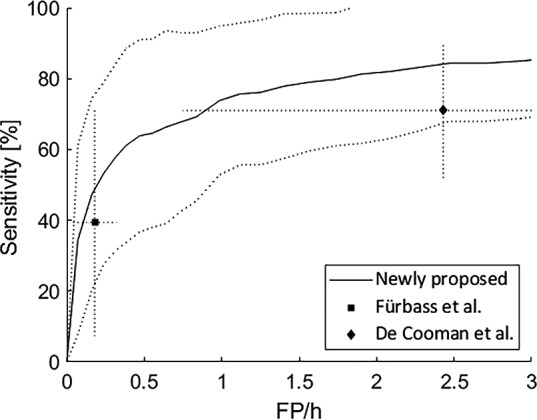
Performance comparison of our proposed electrocardiography‐based seizure detection algorithm. The sensitivity versus false alarm rate (FAR) is plotted together with the standard deviation on the sensitivity (dotted lines). The sensitivity and FAR are shown for two state‐of‐the‐art solutions: Fürbass et al. 22 and De Cooman et al. 16 The dotted lines indicate the standard deviation in two directions: the FAR and sensitivity. FP, false positives
For all the databases, the EEG‐based seizure detection algorithm had the highest average and median sensitivities. By analyzing individual patients, 88 patients (65%) exhibited higher sensitivity with EEG, 29 patients (21%) had equal sensitivity with EEG and ECG, and 18 patients (13%) exhibited higher sensitivity with ECG. In total, 463 seizures (52%) were detected with both EEG and ECG, 244 seizures (27%) were detected only with EEG, and 131 seizures (15%) were not detected either with EEG or with ECG. It should be noted that 58 seizures (6%), from 38 patients, were detected only with ECG. Appendix S3 describes the percentage of seizures detected in relation to seizure type and localization for the different modalities, which is shown as well in Figure 6.
FIGURE 6.
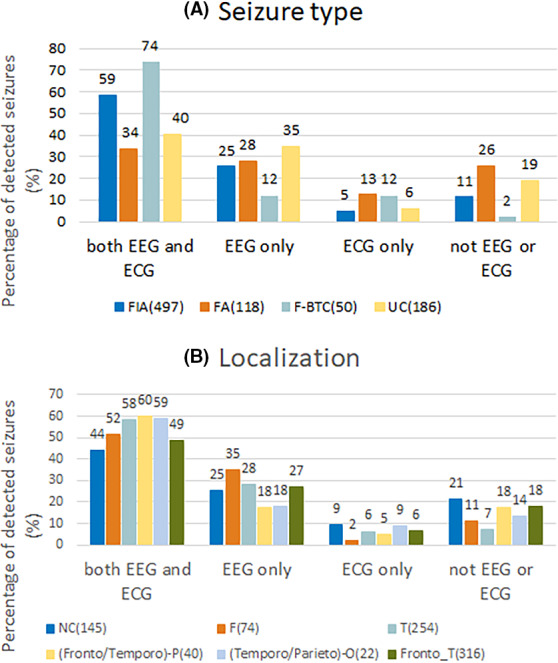
Percentage of detected seizures at a false alarm rate (FAR) of 1/h is displayed for the four groups: seizures detected with both electroencephalography (EEG) and electrocardiography (ECG), only with EEG, only with ECG, or not detected either with EEG or with ECG, in relation to seizure type (A) and in relation to localization (B). The number of seizures in each group is indicated. F, frontal; FA, focal aware; F‐BTC, focal to bilateral tonic–clonic; FIA, focal impaired awareness; NC, not clear; T, temporal; UC, unclassified
3.2. Performance of the multimodal model
In Figure 4, we present the results of the multimodal algorithm. The outcome of the two unimodal algorithms were combined with a late integration approach at three discrete thresholds: the thresholds leading to 0.2, 0.5, and 1 FP/h for both the EEG and ECG algorithms. Those three (sensitivity, FP/h) pairs were shown together with the corresponding sensitivities leading to the same FAR on the EEG graph. By analyzing those three pairs, the lowest increase in sensitivity was observed with the 0.2 FP/h threshold and the highest with the 1 FP/h threshold. In Table 1B, the sensitivity of the multimodal algorithm (threshold leading to 1 FP/h) is presented along with the outcome of the EEG‐based detectors with the same FP/h. For SeizeIT1, an increase was observed from 81% (EEG unimodal) to 92% (multimodal). A similar increase was seen for Epilepsiae‐Freiburg (from 82% to 90%). No increase was observed for Epilepsiae‐Paris (from 84% to 84%). In total, 31 (23%) patients had increased sensitivity (with the multimodal algorithm compared to the EEG unimodal), 88 (65%) patients had the same sensitivity, and 16 (11%) patients had decreased sensitivity.
4. DISCUSSION
Mobile health and digital biomarkers involve the application of wearable sensors to obtain data pertinent to wellness and disease diagnosis, prevention, and management. 42 Current noninvasive wearable devices in epilepsy are accelerometer‐ and surface electromyography (sEMG)‐based devices for online automated detection of tonic–clonic seizures, the easiest group of seizures to detect. Accelerometry can be recorded unobtrusively with wristbands, which are commonly used in daily life nowadays. sEMG can be recorded with a wearable device placed on the upper arm. These devices have a sensitivity of around 90% and a positive predictive value of around 55%. The F1‐score of these devices is approximately 0.68. 43 , 44 Focal nonmotor seizures are typically not accompanied by such strong and typical movement and/or muscle activity. For those focal seizures, the authors are aware of only one automated seizure detection device, made by UNEEG medical. 45 This device is a minimally invasive, subscalp, implantable EEG system allowing ultra‐long‐term seizure monitoring.
No noninvasive wearable devices are currently available for the detection of focal seizures. An important methodological problem is the diversity of focal epileptic seizures; there are three main focal seizure types, namely focal aware, focal impaired awareness, and focal to bilateral tonic–clonic seizures, which can be generated from any lobe, namely temporal, frontal, occipital, or parietal lobe. 46 We observe that a single biosignal is not able to detect all types of focal seizures. Therefore, including multiple biosignals is recommended for achieving higher sensitivities. As variability in performance across datasets is high, we included multiple datasets in this study, allowing us to derive general claims. More specifically, we used a multicenter dataset of 135 patients with 896 seizures recorded during presurgical evaluation. The multimodal algorithm using behind‐the‐ear/temporal EEG and ECG was able to capture 85% of the seizures with 1.90 FP/h. Fifty‐two percent of seizures were captured with both EEG and ECG, 27% with only EEG, and 6% with only ECG. For those 6%, probable reasons why those seizures could not be picked up with EEG are listed in Appendix S4. Approximately 21% of the seizures remained difficult to detect with behind‐the‐ear EEG, which was due to unclear ictal EEG patterns, short seizures, or artifacts on the restricted set of temporal lobe or behind‐the‐ear electrodes. These observations suggest that wearable devices to detect focal seizures should be multimodal, that is, able to record different biosignals. In an ideal setting, a neurologist supervises the use of the wearable device. Neurologists should be aware of ictal EEG and ECG changes of an individual patient beforehand and select which biosignals to capture with the multimodal wearable device. Moreover, neurologists could provide labels for a portion of seizure and nonseizure data to create a personalized algorithm, as done in our study.
Our "sensitivity in relation to FAR" graphs showed that high sensitivity can be obtained at the cost of a higher number of FP detections. In our view, the FAR is too high to use this algorithm for online/immediate seizure detection in daily practice. It would be very useful, however, to use the algorithm for reducing the amount of data to be reviewed. Neurologists have only to review the automatically flagged regions and decide whether they represent seizures or FP detections. Our multimodal algorithm had a high average sensitivity of 90% at 2 FP/h. This means that two data points should be reviewed per hour, which would reduce the review time probably more than 90%. A follow‐up study will be conducted for measuring the performance against visual analysis by a neurologist/EEG expert, and the review time will be quantified. Furthermore, this study will investigate whether ECG can improve visual behind‐the‐ear EEG seizure recognition by a neurologist/EEG expert.
With our current algorithm, 15% of all seizures were not captured with EEG or ECG. The majority of those seizures had an unclear or frontotemporal onset zone. Seizures with an unclear origin often have motion and noise artifacts on the EEG, which hampers localization. Seizures with frontotemporal origin could have an onset zone in the frontal lobe. Those seizures are typically associated with motor and muscle components and could be picked up with EMG. Adding EMG/motion to the analysis could potentially increase the sensitivity even more.
For integrating the EEG and ECG, a late integration approach with an “OR” strategy was applied; the ECG or the EEG should detect a seizure for generating a seizure detection alarm. Assigning the same contribution to the different modalities might be suboptimal. Different weights attributed to the different modalities can be trained in an additional second classification level. Alternatively, those weights can be assigned based on the level of noise of the raw signals or the certainties of detections (seizure/no seizure). Also, different weights could be assigned to the output seizure probabilities of the two modalities. For quantifying uncertainties, trust scores could be a good methodology. 48
In this work, the EEG model is patient‐specific, trained on data from a specific patient. We have shown before that patient‐specific algorithms are more sensitive than patient‐independent ones. 10 However, limited seizure data from patients are available due to low seizure frequencies, possibly leading to overfitted models. Improvements can be made by starting with a patient‐independent model and adapting this model to a particular patient with limited patient‐specific data. Deep transfer learning approaches have been shown to be successful for reduced EEG montages in other fields, such as automatic sleep staging. 49 The ECG model in this work is patient‐independent, using data from other patients for training the model. Patient‐independent models achieved better performance than fully patient‐specific ones. 47 This is due to limited patient‐specific seizure data, as only one HR increase per seizure is available. Transfer learning approaches could be helpful for the ECG modality as well. 47
When developing a wearable device, good quality of signals will be vital for obtaining reliable results. In the current study, a lower sensitivity for the ECG‐based algorithm was observed for Epilepsiae‐Paris. By inspecting the raw ECG data, an increased number of artifacts was observed. Those artifacts looked like motion artifacts with usually high‐frequency muscle activity on top.
The present work was part of the SeizeIT1 project, in which we used EEG and ECG data from standard hospital equipment. We are continuing this work in the SeizeIT2 project (https://eithealth.eu/project/seizeit2/; ClinicalTrials.gov: NCT04284072) with a multimodal wearable device based on Byteflies's Sensor‐Dot (https://www.byteflies.com/), measuring two channels EEG behind the ear, ECG, accelerometry, and EMG of the left deltoid muscle. We are planning to determine F1 scores for typical absences, focal seizures, and tonic–clonic seizures in a hospital‐based and home‐based international multicenter study. We are further developing new and improved electrode patches for measuring EEG, ECG, EMG, respiration, oxygen saturation, and skin temperature in the study of epileptic seizures (ClinicalTrials.gov: NCT04642105). We will study the prevalence of epileptic seizures and sleep–wake disturbances in Alzheimer disease using this wearable device (ClinicalTrials.gov: NCT03617497) and tonic, atonic, and myoclonic seizures in childhood epilepsy (ClinicalTrials.gov: NCT04584385). Development of improved seizure detection algorithms based on artificial intelligence and machine learning will be an essential step for integrating this wearable device into the everyday clinical practice of the neurologist–epileptologist to improve management of patients with refractory focal epilepsy.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We wish to thank our partners in the SeizeIT1 consortium: Hans Danneels, Hans De Clercq (Byteflies), Gergely Vértes, Kasper Claes (UCB Pharma), and Brecht Bonte (Pilipili). We are grateful for the use of the EPILEPSIAE database, created and supported by the European Union (grant 211713). We offer special thanks to the technicians and clinical teams of the epilepsy centers of the Centro Hospitalar, University of Coimbra, Coimbra, Portugal, Hôpital de la Pitié‐Salpêtrière, Paris, France, and University Medical Center Freiburg, Freiburg, Germany. This study was supported by Bijzonder Onderzoeksfonds KU Leuven (BOF: Prevalentie van epilepsie en slaapstoornissen in de ziekte van Alzheimer; C24/18/097), Fonds voor Wetenschappelijk Onderzoek‐Vlaanderen (PhD/postdoctoral grants), EIT (19263–SeizeIT2: Discreet Personalized Epileptic Seizure Detection Device), and the Flemish Government (AI Research Program). S.V.H., M.D.V., and K.V. are affiliated with KU Leuven Institute for AI, Leuven, Belgium.
Vandecasteele K, De Cooman T, Chatzichristos C, Cleeren E, Swinnen L, Macea Ortiz J, et al. The power of ECG in multimodal patient‐specific seizure monitoring: Added value to an EEG‐based detector using limited channels. Epilepsia. 2021;62:2333–2343. 10.1111/epi.16990
Wim Van Paesschen and Borbála Hunyadi share last authorship.
REFERENCES
- 1. Forsgren L, Beghi E, Oun A, Sillanpää M. The epidemiology of epilepsy in Europe—a systematic review. Eur J Neurol. 2005;12:245–53. [DOI] [PubMed] [Google Scholar]
- 2. French JA. Refractory epilepsy: clinical overview. Epilepsia. 2007;48:3–7. [DOI] [PubMed] [Google Scholar]
- 3. Elger CE, Mormann F. Seizure prediction and documentation—two important problems. Lancet Neurol. 2013;12:531–2. [DOI] [PubMed] [Google Scholar]
- 4. Blum DE, Eskola J, Bortz JJ, Fisher RS. Patient awareness of seizures. Neurology. 1996;47:260–4. [DOI] [PubMed] [Google Scholar]
- 5. Fisher RS, Blum DE, DiVentura B, Vannest J, Hixson JD, Moss R, et al. Seizure diaries for clinical research and practice: limitations and future prospects. Epilepsy Behav. 2012;24:304–10. [DOI] [PubMed] [Google Scholar]
- 6. Hoppe C, Poepel A, Elger CE. Epilepsy: accuracy of patient seizure counts. Arch Neurol. 2007;64:1595–9. [DOI] [PubMed] [Google Scholar]
- 7. Poochikian‐Sarkissian S, Tai P, del Campo M , Andrade DM, Carlen PL, Valiante T, et al. Patient awareness of seizures as documented in the epilepsy monitoring unit. Can J Neurosci Nurs. 2009;31:22–3. [PubMed] [Google Scholar]
- 8. Tatum WO, Winters L, Gieron M, Passaro EA, Benbadis S, Ferreira J, et al. Outpatient seizure identification: results of 502 patients using computer‐assisted ambulatory EEG. J Clin Neurophysiol. 2001;18:14–9. [DOI] [PubMed] [Google Scholar]
- 9. Gu Y, Cleeren E, Dan J, Claes K, Van Paesschen W, Van Huffel S, et al. Comparison between scalp EEG and behind‐the‐ear EEG for development of a wearable seizure detection system for patients with focal epilepsy. Sensors. 2017;18:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vandecasteele K, De Cooman T, Dan J, Cleeren E, Van Huffel S, Hunyadi B, et al. Visual seizure annotation and automated seizure detection using behind‐the‐ear electroencephalographic channels. Epilepsia. 2020;61:766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devinsky O, Kelley K, Porter RJ, Theodore WH. Clinical and electroencephalographic features of simple partial seizures. Neurology. 1988;38:1347–52. [DOI] [PubMed] [Google Scholar]
- 12. Foldvary N, Klem G, Hammel J, Bingaman W, Najm I, Lüders H. The localizing value of ictal EEG in focal epilepsy. Neurology. 2001;57:2022–8. [DOI] [PubMed] [Google Scholar]
- 13. Walczak TS, Radtke RA, Lewis DV. Accuracy and interobserver reliability of scalp ictal EEG. Neurology. 1992;42:2279–85. [DOI] [PubMed] [Google Scholar]
- 14. Spencer SS, Williamson PD, Bridgers SL, Mattson RH, Cicchetti DV, Spencer DD. Reliability and accuracy of localization by scalp ictal EEG. Neurology. 1985;35:1567–75. [DOI] [PubMed] [Google Scholar]
- 15. Pavei J, Heinzen RG, Novakova B, Walz R, Serra AJ, Reuber M, et al. Early seizure detection based on cardiac autonomic regulation dynamics. Front Physiol. 2017;8:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Cooman T, Varon C, Hunyadi B, Van Paesschen W, Lagae L, Van Huffel S. Online automated seizure detection in temporal lobe epilepsy patients using single‐lead ECG. Int J Neural Syst. 2017;27:1750022. [DOI] [PubMed] [Google Scholar]
- 17. Jeppesen J, Fuglsang‐Frederiksen A, Johansen P, Christensen J, Wüstenhagen S, Tankisi H, et al. O‐45 automated seizure detection for epilepsy patients using wearable ECG‐device. Neurophysiol Clin. 2019;130:36. [Google Scholar]
- 18. Vandecasteele K, De Cooman T, Gu Y, Cleeren E, Claes K, Van Paesschen W, et al. Automated epileptic seizure detection based on wearable ECG and PPG in a hospital environment. Sensors. 2017;17:2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eggleston KS, Olin BD, Fisher RS. Ictal tachycardia: the head‐heart connection. Seizure. 2014;23:496–505. [DOI] [PubMed] [Google Scholar]
- 20. Zijlmans M, Flanagan D, Gotman J. Heart rate changes and ECG abnormalities during epileptic seizures: prevalence and definition of an objective clinical sign. Epilepsia. 2002;43:847–54. [DOI] [PubMed] [Google Scholar]
- 21. Chen W, Guo CL, Zhang PS, Liu C, Qiao H, Zhang JG, et al. Heart rate changes in partial seizures: analysis of influencing factors among refractory patients. BMC Neurol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fürbass F, Kampusch S, Kaniusas E, Koren J, Pirker S, Hopfengärtner R, et al. Automatic multimodal detection for long‐term seizure documentation in epilepsy. Clin Neurophysiol. 2017;128:1466–72. [DOI] [PubMed] [Google Scholar]
- 23. Qaraqe M, Ismail M, Serpedin E, Zulfi H. Epileptic seizure onset detection based on EEG and ECG data fusion. Epilepsy Behav. 2016;58:48–60. [DOI] [PubMed] [Google Scholar]
- 24. Cogan D, Birjandtalab J, Nourani M, Harvey J, Nagaraddi V. Multi‐biosignal analysis for epileptic seizure monitoring. Int J Neural Syst. 2017;27:1650031. [DOI] [PubMed] [Google Scholar]
- 25. Beniczky S, Ryvlin P. Standards for testing and clinical validation of seizure detection devices. Epilepsia. 2018;59:9–13. [DOI] [PubMed] [Google Scholar]
- 26. Klatt J, Feldwisch‐Drentrup H, Ihle M, Navarro V, Neufang M, Teixeira C, et al. The EPILEPSIAE database: an extensive electroencephalography database of epilepsy patients. Epilepsia. 2012;53:1669–76. [DOI] [PubMed] [Google Scholar]
- 27. Ihle M, Feldwisch‐Drentrup H, Teixeira CA, Witon A, Schelter B, Timmer J, et al. EPILEPSIAE—a European epilepsy database. Comput Methods Programs Biomed. 2012;106:127–38. [DOI] [PubMed] [Google Scholar]
- 28. Seeck M, Koessler L, Bast T, Leijten F, Michel C, Baumgartner C, et al. The standardized EEG electrode array of the IFCN. Clin Neurophysiol. 2017;128:2070–7. [DOI] [PubMed] [Google Scholar]
- 29. Li C, Zheng C, Tai C. Detection of ECG characteristic points using wavelet transforms. IEEE Trans Biomed Eng. 1995;42:21–8. [DOI] [PubMed] [Google Scholar]
- 30. Varon C, Caicedo A, Testelmans D, Buyse B, Van Huffel S. A novel algorithm for the automatic detection of sleep apnea from single‐lead ECG. IEEE Trans Biomed Eng. 2015;62:2269–78. [DOI] [PubMed] [Google Scholar]
- 31. De Cooman T, Goovaerts G, Varon C, Widjaja D, Willemen T, Van Huffel S. Heart beat detection in multimodal data using automatic relevant signal detection. Physiol Meas. 2015;36:1691–704. [DOI] [PubMed] [Google Scholar]
- 32. Jeppesen J, Beniczky S, Johansen P, Sidenius P, Fuglsang‐Frederiksen A. Detection of epileptic seizures with a modified heart rate variability algorithm based on Lorenz plot. Seizure. 2015;24:1–7. [DOI] [PubMed] [Google Scholar]
- 33. Doyle OM, Temko A, Marnane W, Lightbody G, Boylan GB. Heart rate based automatic seizure detection in the newborn. Med Eng Phys. 2010;32:829–39. [DOI] [PubMed] [Google Scholar]
- 34. Orphanidou C, Bonnici T, Charlton P, Clifton D, Vallance D, Tarassenko L. Signal‐quality indices for the electrocardiogram and photoplethysmogram: derivation and applications to wireless monitoring. IEEE J Biomed Health Inform. 2015;19:832–8. [DOI] [PubMed] [Google Scholar]
- 35. Johnson AE, Behar J, Andreotti F, Clifford GD, Oster J. Multimodal heart beat detection using signal quality indices. Physiol Meas. 2015;36:1665–77. [DOI] [PubMed] [Google Scholar]
- 36. Ganeshapillai G, Liu JF, Guttag J. Reconstruction of ECG signals in presence of corruption. Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:3764–7. [DOI] [PubMed] [Google Scholar]
- 37. Citi L, Klerman E, Brown E, Barbieri R. Point process heart rate variability assessment during sleep deprivation. Comput Cardiol. 2010;37:721–4. [PMC free article] [PubMed] [Google Scholar]
- 38. Osorio I. Automated seizure detection using EKG. Int J Neural Syst. 2014;24:1450001. [DOI] [PubMed] [Google Scholar]
- 39. Osorio I, Manly BF. Is seizure detection based on EKG clinically relevant? Clin Neurophysiol. 2014;125:1946–51. [DOI] [PubMed] [Google Scholar]
- 40. Ungureanu C, Bui V, Roosmalen W, Aarts RM, Arends JB, Verhoeven R, et al. A wearable monitoring system for nocturnal epileptic seizures. ISMICT. Proceedings of the 8th International Symposium on Medical Information and Communication Technology (ISMICT'14), April 2‐4, 2014, Firenze, Italy. Piscataway, NJ: Institute of Electrical and Electronics Engineers; 2014. p. 1–5. [Google Scholar]
- 41. Deviaene M, Testelmans D, Borzee P, Buyse B, Van Huffel S, Varon C. Feature selection algorithm based on random forest applied to sleep apnea detection. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:2580–3. [DOI] [PubMed] [Google Scholar]
- 42. Sim I. Mobile devices and health. N Engl J Med. 2019;381:956–68. [DOI] [PubMed] [Google Scholar]
- 43. Kurada AV, Srinivasan T, Hammond S, Ulate‐Campos A, Bidwell J. Seizure detection devices for use in antiseizure medication clinical trials: a systematic review. Seizure. 2019;66:6169. [DOI] [PubMed] [Google Scholar]
- 44. Verdru J, Van Paesschen W. Wearable seizure detection devices in refractory epilepsy. Acta Neurol Belg. 2020;120:1271–81. [DOI] [PubMed] [Google Scholar]
- 45. Duun‐Henriksen J, Baud M, Richardson MP, Cook M, Kouvas G, Heasman JM, et al. A new era in electroencephalographic monitoring? Subscalp devices for ultra–long‐term recordings. Epilepsia. 2020;61:1805–17. [DOI] [PubMed] [Google Scholar]
- 46. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. De Cooman T, Vandecasteele K, Varon C, Hunyadi B, Cleeren E, Van Paesschen W, et al. Personalizing heart rate‐based seizure detection using supervised SVM transfer learning. Front Neurol. 2020;11:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Becker T, Vandecasteele K, Chatzichristos C, Van Paesschen W, Valkenborg D, Van Huffel S, et al. Classification with a deferral option and low‐trust filtering for automated seizure detection. Sensors. 2021;21:1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Phan H, Chén OY, Koch P, Lu Z, McLoughlin I, Mertins A, et al. Towards more accurate automatic sleep staging via deep transfer learning. IEEE Trans Biomed Eng. 2021;68:1787–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


