Abstract
Assessing the scope and severity of threats is necessary for evaluating impacts on populations to inform conservation planning. Quantitative threat assessment often requires monitoring programs that provide reliable data over relevant spatial and temporal scales, yet such programs can be difficult to justify until there is an apparent stressor. Leveraging efforts of wildlife management agencies to record winter counts of hibernating bats, we collated data for 5 species from over 200 sites across 27 U.S. states and 2 Canadian provinces from 1995 to 2018 to determine the impact of white‐nose syndrome (WNS), a deadly disease of hibernating bats. We estimated declines of winter counts of bat colonies at sites where the invasive fungus that causes WNS (Pseudogymnoascus destructans) had been detected to assess the threat impact of WNS. Three species undergoing species status assessment by the U.S. Fish and Wildlife Service (Myotis septentrionalis, Myotis lucifugus, and Perimyotis subflavus) declined by more than 90%, which warrants classifying the severity of the WNS threat as extreme based on criteria used by NatureServe. The scope of the WNS threat as defined by NatureServe criteria was large (36% of Myotis lucifugus range) to pervasive (79% of Myotis septentrionalis range) for these species. Declines for 2 other species (Myotis sodalis and Eptesicus fuscus) were less severe but still qualified as moderate to serious based on NatureServe criteria. Data‐sharing across jurisdictions provided a comprehensive evaluation of scope and severity of the threat of WNS and indicated regional differences that can inform response efforts at international, national, and state or provincial jurisdictions. We assessed the threat impact of an emerging infectious disease by uniting monitoring efforts across jurisdictional boundaries and demonstrated the importance of coordinated monitoring programs, such as the North American Bat Monitoring Program (NABat), for data‐driven conservation assessments and planning.
Keywords: conservation, data sharing, disease, Endangered Species Act, monitoring, North American Bat Monitoring Program, NatureServe, conservación, enfermedad, intercambio de datos, Ley de Especies en Peligro de Extinción, monitoreo, NatureServe, Programa de Monitoreo de Murciélagos Norteamericanos
Short abstract
Article impact statement: Data sharing and coordinated monitoring are needed to assess species’ response to threats to inform conservation planning at relevant scales.
Abstract
Alcance y Severidad del Síndrome de Nariz Blanca en los Murciélagos Hibernando en América del Norte
Resumen
La evaluación del alcance y la severidad de las amenazas es necesaria para los análisis de impacto sobre las poblaciones que se usan para orientar a la planeación de la conservación. La evaluación cuantitativa de amenazas con frecuencia requiere de programas de monitoreo que proporcionen datos confiables en escalas espaciales y temporales, aunque dichos programas pueden ser difíciles de justificar hasta que exista un estresante aparente. Gracias a una movilización de esfuerzos de las agencias de manejo de fauna para registrar los conteos invernales de murciélagos hibernadores, recopilamos datos para cinco especies en más de 200 sitios a lo largos de 27 estados de EUA y dos provincias canadienses entre 1995 y 2018 para determinar el impacto del síndrome de nariz blanca (SNB), una enfermedad mortal de los murciélagos hibernadores. Estimamos declinaciones en los conteos invernales de las colonias de murciélagos en sitios en donde el hongo invasivo que ocasiona el SNB (Pseudogymnoascus destructans) había sido detectado para evaluar el impacto de amenaza del SNB. Tres especies que se encuentran bajo valoración por parte del Servicio de Pesca y Vida Silvestre de los EUA (Myotis septentrionalis, Myotis lucifugus y Perimyotis subflavus) tuvieron una declinación de más del 90%, lo que justifica la clasificación de la severidad de la amenaza del SNB como extrema con base en el criterio usado por NatureServe. El alcance de la amenaza del SNB definido por el criterio de NatureServe fue desde amplio (36% de la distribución de Myotis lucifugus) hasta dominante (79% de la distribución de Myotis septentrionalis) para estas especies. Las declinaciones de otras dos especies (Myotis sodalis y Eptesicus fuscus) fueron menos severas, pero de igual manera quedaron clasificadas desde moderada hasta seria con base en los criterios de NatureServe. El intercambio de datos entre las jurisdicciones proporcionó una evaluación completa del alcance y la severidad de la amenaza del SNB e indicó las diferencias regionales que pueden guiar a los esfuerzos de respuesta realizados en las jurisdicciones internacionales, nacionales, estatales o provinciales. Evaluamos el impacto de amenaza de una enfermedad infecciosa emergente mediante la combinación de los esfuerzos de monitoreo que sobrepasan fronteras jurisdiccionales y demostramos la importancia que tienen para la planeación y la evaluación basadas en datos de la conservación los programas de monitoreo coordinados, como el Programa de Monitoreo de los Murciélagos Norteamericanos (NABat).
INTRODUCTION
A paradox of conservation planning is that data to inform critical decisions are often lacking for species most in need of conservation (Frick et al., 2019; Stanton et al., 2019; Sutherland et al., 2004). Conservation planning hinges on reasonable assessment of population status and trends to assess vulnerability and determine appropriate management actions (CMP, 2020; Thogmartin et al., 2012; Voyles et al., 2015). When new threats arise, the urgency to inform management decisions and attempts toward mitigative action can outpace availability of empirical data to inform adaptive management strategies. Monitoring programs that provide reliable, comparable data over broad spatial scales and through time are essential for informing and prioritizing management decisions but can be difficult to justify until there is an immediate or obvious stressor (Langwig et al., 2015; Voyles et al., 2015).
The emergence of the disease, white‐nose syndrome (WNS), in the northeastern United States in 2006 caused mass mortality of hibernating bats and sparked immediate attention to and concern over rapid population declines and potential regional extirpation of once‐common species (Blehert et al., 2009; Frick et al., 2010; Turner et al., 2011; Langwig et al., 2012; Thogmartin et al., 2012). The disease is caused by an invasive fungal pathogen, Pseudogymnoascus destructans (Pd) (Blehert et al., 2009; Gargas et al., 2009), likely originating from Eurasia (Leopardi et al., 2015; Drees et al., 2017). The fungus has spread from its presumed point of introduction in upstate New York to at least 38 U.S. states and 7 Canadian provinces, and new detections of Pd and WNS are reported each year (White‐nose Syndrome Response Team, 2020). Currently, 12 bat species have been confirmed with WNS based on established case definitions, and another 6 bat species have been documented with Pd but without signs of disease.
When WNS first emerged, dramatic local die‐offs of multiple bat species, including the Indiana bat (Myotis sodalis), a species protected under the U.S. Endangered Species Act, catalyzed a collaborative response framed by national plans in the United States and Canada outlining jurisdictional responsibilities among federal, state, and tribal partners to identify management and research priorities (USFWS, 2011; Canadian Wildlife Health Cooperative, 2015). In the United States, existing state‐led monitoring programs and coordination of data sharing for federally protected species by the U.S. Fish and Wildlife Service (USFWS) provided a valuable precedent for a collaborative coordinated response (USFWS, 2019). Similarly, the Canadian Wildlife Health Cooperative coordinated a broad collaborative response effort via a Canadian interagency WNS response team.
Resulting from this WNS response effort, the North American Bat Monitoring Program (NABat) was initiated in 2015 as the first broad‐scale coordinated effort to monitor bat species across North America (Loeb et al., 2015). Although continental monitoring programs are established for birds (North American Breeding Bird Survey, Christmas Bird Count) and amphibians (Amphibian Research and Monitoring Initiative), the difficulty of observing bats limited development of a comparable program (O'Shea et al., 2003). Globally, data to inform population trends of bats are scarcer than for other mammals or birds (Frick et al., 2019).
Direct counts of bats in winter roosts (hibernacula) are among the 4 primary sources (others include stationary acoustic, mobile acoustic, and external roost counts) of data for NABat (Loeb et al., 2015). The NABat effort builds on existing monitoring efforts of state wildlife and natural heritage programs. Across the eastern half of North America, where many bat species aggregate in subterranean roosts during hibernation, counts of bats during hibernation have provided the best available data for estimating changes in abundance related to the invasion and progression of WNS (Frick et al., 2010, 2015; Turner et al., 2011; Langwig et al., 2012; Thogmartin et al., 2012). Collating counts of hibernating bats, especially across jurisdictions, can be used to assess species’ status and trends and can be applied to standardized criteria for threat impact, such as NatureServe criteria (Master et al., 2012), to inform conservation priorities.
We estimated changes to winter colonies in 5 species of hibernating bats and classified these estimates based on NatureServe criteria to assess the impact level of the threat of WNS to bat populations. We used NatureServe criteria because they provide standard definitions for threat assessment, including the scope (geographic extent to which a threat affects a species) and severity of a threat (amount of population reduction) to classify threat impact (Fig. 1) (Master et al., 2012). Furthermore, many state resource managers are familiar with NatureServe criteria and use them in statewide assessments to identify species in conservation need and prioritize monitoring of species.
FIGURE 1.
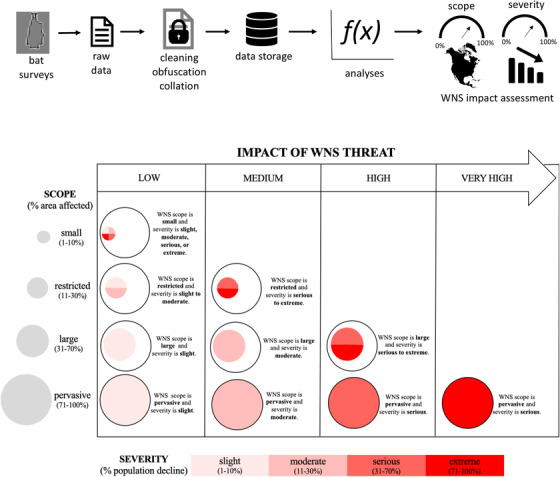
Framework from data collection to threat assessment, and NatureServe criteria used to assess the impact of the threat of white‐nose syndrome (WNS)
We collated the most comprehensive data set on counts of hibernating bat species to date by collaborating with management agencies and researchers across 27 U.S. states and 2 Canadian provinces. Data were collected over 23 years to assess status and trends of bat species across the expanding range of WNS in North America. To inform management response at different spatial scales, we compared estimates of changes in winter counts of bats at multiple jurisdictional scales. We also examined changes in species incidence and size of persisting winter colonies relative to the progression of WNS to inform specific types of management actions, such as site‐level conservation. We sought to provide a critical foundation for making data‐driven conservation decisions.
METHODS
Data collation and curation
Counts of bats were collected and contributed by state, federal, and provincial biologists and their partners (Appendix S1). In most cases, surveys were conducted using visual or photographic methods to identify and count bats inside hibernacula, typically from January to March when winter colonies are most stable, and to minimize disturbance (Loeb et al., 2015; Appendix S1). We limited analyses to sites where Pd had been detected and had at least one winter count before and after detection. We restricted analyses to 5 species (Myotis septentrionalis, Perimyotis subflavus, Myotis lucifugus, M. sodalis, and Eptesicus fuscus) that were sampled (∼50 winter counts) to achieve 80% power to detect a 50% decline in colony size (Appendix S1). We used counts of these 5 species compiled from over 200 sites total (M. sodalis, 204; P. subflavus, 228; M. lucifugus, 208; M. septentrionalis, 62; E. fuscus, 54) across 27 U.S. states and 2 Canadian provinces from 1995 to 2018 (Appendix S2).
The year of first detection of Pd was determined either by past research efforts (e.g., Frick et al., 2017) or designation by state or provincial biologists. Evidence of disease included histological confirmation (Meteyer et al., 2009), molecular evidence of the pathogen (Muller et al., 2013), physical and behavioral field signs of disease (Janicki et al., 2015), or some combination per established case definitions for WNS (Appendix S1). We categorized the pathogen invasion process at each site into 4 stages as described by Langwig et al. (2015): prearrival, years prior to the first detection of Pd; invasion, year of first detection of Pd at a site and year following; epidemic, years 2–4 following detection of Pd when disease was manifest; established, years 5–7 following detection of Pd when the pathogen is established. Surveys completed over 7 years after detection were removed due to limited sample size so as to avoid spatial bias.
We made the following assumptions: each count represents an accurate estimate of number of bats present at a site at the time of survey; site surveys are comparable among years and differences in counts among years are due to changes in number of bats present at a site rather than differences in survey methods or effort; species were identified correctly (Appendix S1); and changes in counts following Pd arrival to a site are due to stochastic yearly variation or the effects of WNS.
We included sites only if a count for a given species had been conducted at a site at least once before and once after the prearrival stage. We retained true 0s in the data set and defined a true 0 as the absence of a given species in a survey event in which the species was intentionally surveyed. We resolved multiple surveys within a winter year to a single count by averaging multiple counts within a day or month and taking the latest count within a winter year. We filtered prearrival count data to 10 years prior to Pd arrival at a site. For analyses examining changes in counts over time, we removed sites where average count for a species prior to Pd arrival was <10 individuals because of variability in probability of detection of bats at small colony sizes (Appendix S1).
Calculating scope of WNS threat
We defined scope of threat (Master et al., 2012) from WNS as the geographic extent of WNS relative to a species range. We estimated the geographic extent of WNS with a convex hull polygon (R package sf; Pebesma, 2018) around WNS‐confirmed counties (www.whitenosyndrome.org), excluding spatially disjunct cases in Washington state. Scope was the proportion of the species’ range (using IUCN, 2019 range maps) where WNS had been confirmed (Fig. 2). We assigned scope of WNS threat as small (1–10%), restricted (11–30%), large (31–70%), or pervasive (71–100%), following NatureServe (Master et al., 2012) (Fig. 1).
FIGURE 2.
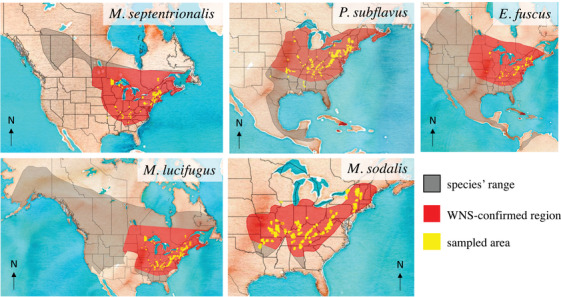
Overlap between species range and the range of white‐nose syndrome (WNS) (based on WNS case definitions [White‐nose Syndrome Response Team, 2019]) to define scope of the threat of WNS as determined by convex hull polygon around WNS‐confirmed counties (yellow, counties contributing monitoring data). Species range map source: International Union for the Conservation of Nature (2019)
Estimating severity of WNS threat
We used R 3.6.2 (R Core Team, 2019) for all analyses. We used a Bayesian hierarchical model with a lognormal distribution to estimate changes in counts of hibernating bats (Ci ). We modeled mean count and standard deviation () by disease stage (i) and treated site as a random intercept (ε γ) (Eq. 1). We applied this model to each of the 5 species (R package rstanarm [Goodrich et al., 2018]). For each model, we ran 3 chains for 20,000 iterations, discarding the first 10,000 iterations as burn‐in, and thinned by 3 to reduce serial correlation. To ensure convergence of model parameters, we examined the Gelman–Rubin diagnostic and confirmed that Rhat was <1.1. We performed visual inspections to ensure chains were properly mixed. We report mean values from the posterior with 95% credible interval (CRI).
| (1) |
We calculated change in winter counts as the percent change in the model estimate for μi (back‐transformed from log scale) in i relative to the prearrival count estimate (μ prearrival):
| (2) |
We report mean estimates percent change from the posterior with 95% CRI. We considered colonies to exhibit significant declines when percent change was negative and CRI did not overlap 0. If a species exhibited significant decline in winter counts, we used the absolute value of the decline estimate in the WNS‐established stage to assign levels of severity of WNS threat following NatureServe criteria: slight (1–10%), moderate (11–30%), serious (31–70%), or extreme (71–100%) (Master et al., 2012) (Fig. 1).
We also estimated changes in counts of hibernating bats by using Eqs. 1 and 2 at 2 jurisdictional spatial scales: legacy USFWS regions (northeast, Region 5 [includes Canadian provinces]; southeast, Region 4 [includes Oklahoma and Texas from Region 2]; and Midwest, Region 3) and U.S. state or Canadian province for jurisdictions with >1 site/species. We created data subsets at the regional or state or province level and estimated counts (Eq. 1) and severity of decline (Eq. 2) separately for regions and states or provinces.
We used a linear mixed‐effects model (package lme4 [Bates et al., 2015]) to estimate log‐transformed counts by disease stage. We allowed site to vary by intercept and by disease stage to calculate site‐ and stage‐specific estimates of decline. We compared model estimate consistency with Eq. 1 so as to resolve observed discrepancies in model results for M. sodalis, a species with highly skewed colony sizes (Appendix S1):
| (3) |
Estimating changes to species incidence at sites
We defined species incidence as the probability of a nonzero count for a species at a site in a survey and included sites where incidence of the species had been documented at least once among all surveys at that site. We modeled species incidence ( as a binomial process in which probability of observation () varied by disease stage (i) and randomly by site (). We used the same priors as described in Eq. 1 (rstanarm in R [Goodrich et al., 2018]):
| (4) |
We report species incidence as the mean estimates of the posterior draws with 95% CRI. A decline in species incidence by disease establishment was considered significant if CRIs did not overlap with CRIs from the prearrival stage.
Describing size classes of observed winter colonies
We categorized colonies based on observed counts into 6 size classes (0, 1–9, 10–99, 100–999, 1,000–9,999, >10,000 bats) and calculated the proportion of colonies in each size class by disease stage. We included all counts (including counts with fewer than 10 bats) to capture changes in small colonies prior to and following Pd arrival at a site. In addition, we calculated the proportion of counts by colony size class at occupied sites to document the distribution of colony sizes where the species was present.
RESULTS
Scope of WNS threat
The estimated geographic extent of where WNS has been confirmed varied over each species’ range, existing over a large part of the range of M. sodalis (93%), M. septentrionalis (79%), and P. subflavus (59%) to about one‐third of the range for the more widespread species, M. lucifugus (36%) and E. fuscus (32%) (Fig. 2). The scope of WNS threat was pervasive for M. sodalis and M. septentrionalis, large for P. subflavus and M. lucifugus, and restricted for E. fuscus (Fig. 2; Table 1).
TABLE 1.
Scope, severity, and impact of the threat of white‐nose syndrome (WNS) on 5 hibernating bat species based on NatureServe criteria
| Species | Scope of WNS threat (%) | Percent severity of WNS threat (95% credible interval) | Scope level | Severity level | Impact of WNS threat |
|---|---|---|---|---|---|
| Myotis septentrionalis | 79 | 100 (97, 100) | pervasive | extreme | very high |
| Myotis lucifugus | 36 | 98 (96, 100) | large | extreme | high |
| Perimyotis subflavus | 59 | 93 (90, 100) | large | extreme | high |
| Myotis sodalis | 93 | 28 | pervasive | moderate | medium |
| Eptesicus fuscus | 32 | 35 (13, 54) | restricted | serious | medium |
Percent overlap of species and WNS occurrence ranges weighted by proportion of sites with observed declines.
Estimate of percent mean declines at hibernacula with WNS establishment; 95% credible interval from Eq. 1.
Due to extreme skew in colony sizes and variation in declines of M. sodalis, estimates of severity of WNS threat ranged from 84% (95% credible interval 78–100%) based on Eq. 1 to 28% based on mean site‐level declines derived from Eq 3. Severity and impact is 28% based on our best understanding of the model fit and data (Appendix S2).
Severity of WNS threat
We found that winter counts of 3 species (M. septentrionalis, P. subflavus, and M. lucifugus) had declined to such low levels (>90%) within 7 years following the detection of WNS to warrant classifying the severity of WNS as extreme (Table 1 & Fig. 3). Overall declines for M. sodalis were estimated at 84% (CRI: 78–100%), indicating extreme severity. Yet strong skew in colony sizes resulted in poor model fit for this species, and the mean of site‐level estimates from Eq. 3 indicated declines were closer to moderate severity (28%) (Table 1 & Appendix S2). At 35% estimated declines, E. fuscus qualified as a serious level of severity (CRI: 13–54%) (Table 1; Fig. 3).
FIGURE 3.
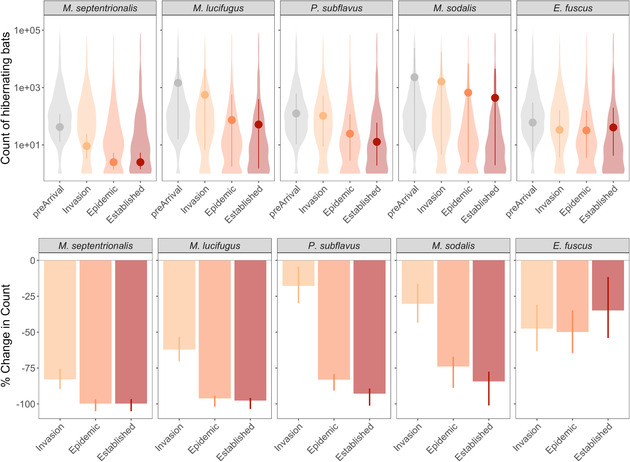
Counts of hibernating bats and proportional change in winter counts during progression of white‐nose syndrome: (top) distribution of counts of hibernating bats (violin shapes) used to estimate mean colony size (points) and (bottom) percent change in mean estimates of counts of hibernating bats relative to prearrival (vertical lines, 95% credible intervals)
Changes in winter counts were highly variable during disease invasion but coalesced by the epidemic and established stages for all species except E. fuscus (Fig. 3 & Appendix S2). Thus, although M. septentrionalis initially had higher levels of decline, at 83% (CRI: 75–90%), compared with P. subflavus (35% CRI: 24–44%) and M. lucifugus (62% CRI: 54–70%) during the disease invasion stage, declines for all 3 species reached similar extreme levels by the disease epidemic (86–100%) and establishment stages (93–100%) (Table 1, Fig. 3, & Appendix S2). For E. fuscus, declines in winter colony counts were highly variable within and across disease stages: 47% (CRI: 31−63%) declines during disease invasion, 50% (CRI: 35−65%) during disease epidemic stage, and 35% (CRI: 13−54%) by disease establishment (Table 1, Fig. 3, & Appendix S2). The proportion of sites increasing or decreasing in count relative to the prearrival count reflected overall changes in winter colonies; over 97% of sites had decreased counts by the established stage for M. septentrionalis, P. subflavus, and M. lucifugus, roughly 80% of sites had decreased counts for M. sodalis, and 73% of sites had decreased counts for E. fuscus (Appendix S3).
Declines in winter colonies were most variable among regions and states or provinces during the invasion stage (Fig. 4 & Appendix S2). Generally, estimates at state and provincial jurisdictions had high levels of uncertainty due to low sample size (fewer than ∼5 sites surveyed) or lacked enough sites (at least >1 site surveyed) to estimate declines independently (Fig. 4; Appendices S2 & S3). Regional estimates of declines coalesced toward study‐wide averages during the epidemic and established disease stages for most species, but declines were generally least variable and most severe in the northeast and least severe in the southeast (Fig. 4 & Appendix S2).
FIGURE 4.
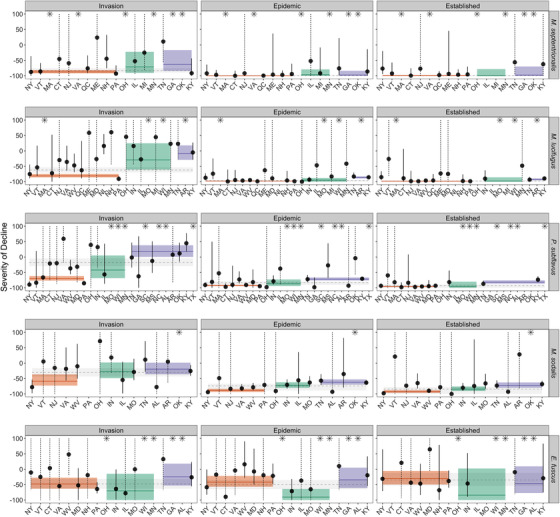
Regional and state or province estimates of percent change (points), relative to prearrival of white‐nose syndrome (WNS), in winter counts for 5 bat species in hibernacula (vertical lines, 95% credible intervals; *, states or provinces for which sample size was insufficient [1 site] to estimate severity; dotted lines, credible interval exceeds 100% growth and 100% mortality; colored lines, regional estimates; colored bands, 95% credible intervals; orange, northeast; green, Midwest; purple, southeast; dashed gray lines, study‐wide estimates of proportional change in winter counts; gray bands, 95% credible intervals). For purposes of representing results by region, Quebec and Newfoundland are included in the northeast and Oklahoma and Texas are included in the southeast
Changes in species incidence at sites
The proportion of sites where a species occurred in our count record decreased significantly from prearrival to disease establishment for M. septentrionalis, P. subflavus, M. lucifugus, and M. sodalis but not E. fuscus (Appendices S2 & S3). Incidence of M. septentrionalis decreased the most dramatically from 98% (CRI: 96−100%) in the prearrival stage to 21% (CRI: 9–36%) by disease establishment (Appendix S2). Declines in incidence were less dramatic for M. lucifugus, P. subflavus, and M. sodalis, whose incidence at sites decreased from near 100% in prearrival to 92% (CRI: 86−96%), 93% (CRI: 87−97%), and 93% (CRI: 87−97%) by disease establishment, respectively (Appendix S2). Incidence of E. fuscus at sites changed from 98% (CRI: 95−100%) in prearrival to 93% (CRI: 82−98%) by disease establishment, but this decrease was not significant (Appendix S2).
Changes to size classes of observed winter colonies
We found that 90% of the few sites where M. septentrionalis remained by disease establishment had fewer than 10 bats (Fig. 5 & Appendix S2). Where P. subflavus persisted in disease establishment, 63% of sites had fewer than 10 bats and no large colonies (>1000 bats) remained (Fig. 5 & Appendix S2). Distribution of remaining M. lucifugus colony sizes in disease establishment became strongly skewed toward sites with fewer than 10 bats (44%) or fewer than a hundred bats (33%), and only a single very large colony (>10,000 bats) persisted (Fig. 5 & Appendix S2). For M. sodalis, sites with fewer than 10 bats also increased (from 20% prior to Pd arrival to 27% in disease establishment), but the proportion of large colony sizes remained largely unchanged through disease progression (Fig. 5 & Appendix S2). Similarly, for E. fuscus, the proportions of colony size classes did not change by disease establishment (Fig. 5 & Appendix S2).
FIGURE 5.
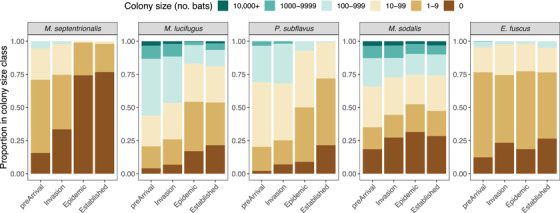
Changes in the proportions of colony sizes as white‐nose syndrome progresses for 5 species of hibernating bats
DISCUSSION
The scope of the WNS threat is pervasive and large, and the severity of the WNS threat is extreme for 3 of the 5 most commonly monitored hibernating bat species in North America, indicating a high to very high level of WNS impact for M. septentrionalis, M. lucifugus, and P. subflavus (Table 1). These 3 species are currently undergoing a species status assessment by the USFWS to inform determination of the need for regulatory protection under the Endangered Species Act (Smith et al., 2018). Myotis septentrionalis was listed as threatened under the U.S. Endangered Species Act in 2015 but was recently court‐ordered for reevaluation of its endangered status (U.S. Fish & Wildlife Service, 2015). All 3 of these species were listed in 2014 as endangered in Canada under the Species at Risk Act. Our comprehensive assessment showed that counts of bats have declined by more than 90% at monitored hibernacula within the decade since WNS emerged for these species. Furthermore, the geographic extent of the WNS threat now overlaps 36–79% of the ranges of these species and pathogen and disease detection continue to expand each year (White‐nose Syndrome Response Team, 2020).
The severity of declines caused by WNS was more variable and complex for M. sodalis, a species federally listed as endangered in the United States since the 1970s (USFWS, 2019). Our results indicate a mean decline in wintering colonies of 84%; however, a small number of sites with very large colonies (tens of thousands of bats) exhibited less severe declines than the majority of smaller sites, suggesting that overall the severity of WNS is more moderate. Our results concur with a recent report that shows a disproportionate number of M. sodalis occur at just a few sites that have not experienced severe declines to date (U.S. Fish & Wildlife Service, 2019). Finally, our results indicate that E. fuscus may be more affected by WNS than previously realized, but the declines were highly variable and remained much lower than for the other species.
Pooling data across jurisdictions improved precision of estimated declines and allowed for a comprehensive assessment of the threat impact from WNS. Declines from WNS were highly variable when estimated at the scale of states and provinces due to constraints at smaller spatial scales, including low number of sites surveyed or available to survey within a jurisdiction, geographic variation in winter behavior of bats, sampling errors, as well as natural variability in initial declines. These sources of variability may be difficult, or impossible, to control or account for within smaller jurisdictions, which reinforces the value of data sharing and coordinated monitoring efforts. Incorporating standardized survey protocols that account for sampling effort and imperfect detection would further improve ability to interpret variability in counts. Estimates of declines from data pooled at the regional scale were also more variable than the comprehensive estimates, but they indicated there may be regional differences in the severity of WNS. Declines were generally more severe in the northeast than in midwestern and southeastern regions for all species.
Our results showing changes to species incidence at sites and colony sizes can inform strategies for monitoring and management in areas where WNS is established. For M. septentrionalis and P. subflavus, a majority of the sites that are still occupied have fewer than 10 bats, which can challenge efforts for reliable monitoring and for managing these species (e.g., applying any developed treatments or vaccines) (Hoyt et al., 2019; Rocke et al., 2019; Fletcher et al., 2020). For P. subflavus and M. lucifugus, colonies with fewer than 10 bats were not commonly monitored prior to WNS, and understanding the stability and status of these colonies is a high conservation priority (Frick et al., 2015). For M. lucifugus and M. sodalis, only a few very large colonies of >10,000 bats remain. These aggregations represent a high proportion of the total number of bats observed for each species and are important foci for conservation and research (U.S. Fish & Wildlife Service, 2019). Understanding why bats are persisting at these sites could provide important insights for conservation strategies.
Across most of the current range of disease spread of WNS, winter counts represent the best available data for assessing the impact of WNS on hibernating bats. However, in western and southwestern United States and western Canada, most hibernating bat species do not typically roost in large aggregations in subterranean environments (Weller et al., 2018), and thus other survey methods are required to monitor species’ response and status over time (Loeb et al., 2015). Our assessment of scope of the WNS threat is conservative and may be underestimated because we made a simplifying assumption that populations are uniformly distributed across the species range polygon, which does not account for variation in density or seasonal variation between winter and summer occurrences. For example, although the range map of M. septentrionalis extends into western Canada, the vast majority of known winter occurrences are within the range of WNS. Our scope of inference on severity of declines is limited to sites where monitoring was conducted because these sites were not chosen at random. In some regions, site selection was originally based on presence of threatened or endangered species.
Although our data come from surveys spanning a variety of hibernacula types, including mines, tunnels, and natural caves, there are several hibernacula used by bats that are not regularly or easily monitored (e.g., in buildings, rock crevices, talus slopes, and tree boles) (Whitaker & Gummer, 2000; Lemen et al., 2016). A better understanding of the extent to which hibernating bats may use other habitats would help evaluate our implicit assumption that winter surveys at hibernacula provide a representative sample. Two observations could lead to new insights about the ecology of hibernating bats. First, there is some evidence that M. septentrionalis are active during the winter in coastal habitats, which may serve as a potential refugia for these species (Grider et al., 2016). Second, E. fuscus appears to roost in human structures (e.g., buildings) relatively often during hibernation (Whitaker & Gummer, 2000). The count of E. fuscus at typical hibernacula (e.g., mines, caves) may represent a small proportion of the wintering population, and alternate methods of monitoring (e.g., acoustic surveys, etc.) may be warranted. Research is needed to better characterize wintering ecology and determine the extent of disease prevalence, severity, and mortality for bats roosting in different types of winter habitats, especially for M. septentrionalis, given the severity of its decline due to WNS at monitored winter hibernacula.
Our results inform several potential conservation strategies. First, protect sites. Despite widespread declines in wintering colonies from WNS, a handful of sites remain where bats persist. For M. sodalis and M. lucifugus, a small number of sites contain a disproportionate amount of their remaining known winter populations. These sites are priorities for protection and provide the opportunity to maximize conservation value per unit effort. Efforts focused on characterizing overwintering roosts for M. septentrionalis, such as human‐made habitats, could also help provide areas of focus for conservation and highlight the importance of public awareness and education. Second, continue to monitor and collaborate. Individual monitoring efforts gain additional value when combined to provide the opportunity for species’ status assessments conducted at broad spatial scales. Continued monitoring and data contributions are especially critical to inform periodic assessments and updates on WNS impacts as the pathogen spreads and disease progresses. Third, conduct Pd surveillance. Continued monitoring for Pd and disease status could be conducted regularly where the disease has established and where the pathogen continues to invade to improve understanding of the scope and severity of the threat of WNS, not only to these species, but also to other bat species at the frontier of disease spread. Fourth, conduct other forms of seasonal population monitoring (e.g., acoustic monitoring, mist netting, summer maternity counts). Alternate forms of monitoring can provide additional information on species status and can be combined with winter monitoring to provide full‐annual‐cycle assessment of species status and trends.
Collaborative monitoring and data sharing are essential for producing comprehensive assessments of species status, particularly for wide‐ranging species. We provide a framework for assessing the threat of an emerging infectious disease by uniting monitoring efforts across jurisdictional boundaries and through use of a quantitative approach to inform threat impact assessment. This work represents an ongoing effort to collaboratively assess WNS impacts through quantitative estimates of the scope and severity of the WNS threat to hibernating bats in North America. We demonstrated the process and tools the North American Bat Monitoring Program uses to compile, store, and visualize information from a large collaborative community for reproducible and comparable estimates that can incorporate additional data in support of species management. The success of these monitoring efforts highlights the value and importance of collaborative data sourcing to inform conservation efforts.
Supporting information
Appendix S1. Supporting information on acknowledgements, terminology, and methods
Table S2‐1. Number of contributed winter surveys (and sites)
Table S2‐2. Comparison of estimated severity of decline (with 95% credible intervals) from equations 1 and 3
Table S2‐3. Comparison of estimated severity of decline calculated from mean and median site counts and model estimates (Eq. 3) with established and pre‐arrival counts in parentheses (countestablished/countprearrival)
Table S2‐4. Proportion of site incidence (mean estimates with 95% credible intervals) by species and disease stage
Table S2‐5. Number of occupied sites in each colony size class by species and disease stage.
Table S2‐6. Sample size of count surveys (and number of sites) by species, region, and state or province.
Table S2‐7. Severity of decline (lower and upper credible intervals) at regional and state/provincial levels
Figure S3‐1. Proportion of increasing or decreasing sites at each stage in comparison to pre‐arrival counts for that species.
Figure S3‐2. Relationship between sample size and power to detect a decline of 30%, 50%, and 80% population change following WNS and given 4 levels of variance in colony counts.
Figure S3‐3. Density of site‐specific count estimates in the pre‐arrival and established disease stages from the complex model allowing site to vary with disease stage (Eq. 3)
Figure S3‐4. Relationship between precision of state and province‐level severity estimates of WNS decline and number of sites surveyed/included in analyses.
ACKNOWLEDGMENTS
We extend our appreciation to those who diligently collect and, importantly, share information on hibernating bats, without whose effort this study would not be possible (Appendix S1). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Fish and Wildlife Service. Funding for this analysis was provided by U.S. Fish and Wildlife Service White‐nose Syndrome Research Grants F17AP00585 and F18AP00576.
Cheng Tina L., et al. (2021). The scope and severity of white‐nose syndrome on hibernating bats in North America Conservation Biology. 35:1586–1586. 10.1111/cobi.13739.
Article impact statement: Data sharing and coordinated monitoring are needed to assess species’ response to threats to inform conservation planning at relevant scales.
Contributor Information
Tina L. Cheng, Email: tcheng@batcon.org.
Winifred F. Frick, Email: wfrick@batcon.org.
REFERENCES
- Bates, D. , Maechler, M. , Bolker, B. , & Walker, S . (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Blehert, D. S. , et al. (2009). Bat white‐nose syndrome: an emerging fungal pathogen? Science, 323, 227. [DOI] [PubMed] [Google Scholar]
- Canadian Wildlife Health Cooperative . (2015). A national plan to manage white nose syndrome in bats in Canada. Canadian Wildlife Health Cooperative, Halifax, Canada. Available from http://www.cwhc‐rcsf.ca/ (accessed February 2021). [Google Scholar]
- CMP (Conservation Measures Partnership) . (2020). Open standards for the practice of conservation. Version 4. Available from https://conservationstandards.org/ (accessed August 2020).
- Drees, K. P. , et al. (2017). Phylogenetics of a fungal invasion: origins and widespread dispersal of white‐nose syndrome. Molecular Biology, 8, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, Q. E. , Webber, Q. M. R. , & Willis, C. K. R . (2020). Modelling the potential efficacy of treatments for white‐nose syndrome in bats. Journal of Applied Ecology, 57, 1283–1291. [Google Scholar]
- Frick, W. F. , Pollack, J. F. , Hicks, A. C. , Langwig, K. E. , Reynolds, S. D. , Turner, G. G. , Butchkoski, C. M. , & Kunz, T. H . (2010). An emerging disease causes regional population collapse of a common North American bat species. Science, 329, 678–682. [DOI] [PubMed] [Google Scholar]
- Frick, W. F. , et al. (2015). Disease alters macroecological patterns of North American bats. Global Ecology and Biogeography, 24, 741–749. [Google Scholar]
- Frick, W. F. , Cheng, T. L. , Langwig, K. E. , Hoyt, J. R. , Janicki, A. F. , Parise, K. L. , Foster, J. T. , & Kilpatrick, A. M . (2017). Pathogen dynamics during invasion and establishment of white‐nose syndrome explain mechanisms of host persistence. Ecology, 98, 624–631. [DOI] [PubMed] [Google Scholar]
- Frick, W. F. , Kingston, T. , & Flanders, J . (2019). A review of the major threats and challenges to global bat conservation. Annals of the New York Academy of Sciences, 1469, 5–25. [DOI] [PubMed] [Google Scholar]
- Gargas, A. , Trest, M. T. , Christensen, M. , Volk, T. J. , & Blehert, D. S . (2009). Geomyces destructans sp. nov. associated with bat white‐nose syndrome. Mycotaxon, 108, 147–154. [Google Scholar]
- Grider, J. F. , Larsen, A. L. , Homyack, J. A. , & Kalcounis‐Rueppell, M. C . (2016). Winter activity of coastal plain populations of bat species affected by white‐nose syndrome and wind energy facilities. PLOS ONE, 11(e0166512) 10.1371/journal.pone.0166512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich, B. , Gabry, J. , Ali, I. , & Brilleman, S . (2018). rstanarm: Bayesian applied regression modeling via Stan. R package version 2.17.4. Available from http://mc‐stan.org/. Accessed July 25, 2020.
- Hoyt, J. R. , Langwig, K. E. , White, J. P. , Kaarakka, H. M. , Redell, J. A. , Parise, K. L. , Frick, W. F. , Foster, J. T. , & Kilpatrick, A. M . (2019). Field trial of a probiotic bacteria to protect bats from white‐nose syndrome. Scientific Reports, 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUCN (International Union for the Conservation of Nature) . (2019). The IUCN red list of threatened species. Version 2018‐2. IUCN, Gland, Switzerland. Available from https://www.iucnredlist.org (accessed December 2019). [Google Scholar]
- Janicki, A. F. , Frick, W. F. , Kilpatrick, A. M. , Parise, K. L. , Foster, J. T. , & McCracken, G. F . (2015). Efficacy of visual surveys for white‐nose syndrome at bat hibernacula. PLOS ONE, 10(e0133390) 10.1371/journal.pone.0133390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langwig, K. E. , Frick, W. F. , Bried, J. T. , Hicks, A. C. , Kunz, T. H. , & Marm Kilpatrick, A . (2012). Sociality, density‐dependence and microclimates determine the persistence of populations suffering from a novel fungal disease, white‐nose syndrome. Ecology Letters, 15, 1050–1057. [DOI] [PubMed] [Google Scholar]
- Langwig, K. E. , et al. (2015). Context‐dependent conservation responses to emerging wildlife diseases. Frontiers in Ecology and the Environment, 13, 195–202. [Google Scholar]
- Lemen, C. A. , Freeman, P. W. , & White, J. A . (2016). Acoustic evidence of bats using rock crevices in winter: A call for more research on winter roosts in North America. Transactions of the Nebraska Academy of Sciences, 36, 9–13. [Google Scholar]
- Leopardi, S. , Blake, D. , & Puechmaille, S. J . (2015). White‐nose syndrome fungus introduced from Europe to North America. Current Biology, 25, R217–R219. [DOI] [PubMed] [Google Scholar]
- Loeb, S. C. et al. (2015). A plan for the North American Bat Monitoring Program (NABat). General Technical Report SRS‐208. U.S. Forest Service, Southern Research Station, Asheville, North Carolina. [Google Scholar]
- Master, L. , Faber‐Langendoen, D. , Bittman, R. , Hammerson, G. A. , Heidel, B. , Ramsay, L. , Snow, K. , Teucher, A. , & Tomaino, A . (2012). NatureServe conservation status assessments: Factors for evaluating species and ecosystem risk. NatureServe, Arlington, Virginia. [Google Scholar]
- Meteyer, C. U. , Buckles, E. L. , Blehert, D. S. , Hicks, A. C. , Green, D. E. , Shearn‐Bochsler, V. , Thomas, N. J. , Gargas, A. , & Behr, M. J . (2009). Histopathologic criteria to confirm white‐nose syndrome in bats. Journal of Veterinary Diagnostic Investigation, 21, 411–414. [DOI] [PubMed] [Google Scholar]
- Muller, L. K. , Lorch, J. M. , Lindner, D. L. , O'connor, M. , Gargas, A. , & Blehert, D. S . (2013). Bat white‐nose syndrome: a real‐time TaqMan polymerase chain reaction test targeting the intergenic spacer region of Geomyces destructans . Mycologia, 105, 253–259. [DOI] [PubMed] [Google Scholar]
- O'Shea, T. J. , Ellison, L. E. , & Bogan, M. A . (2003). Monitoring trends in bat populations of the United States and territories: Status of the science and recommendations for the future. Wildlife Society Bulletin, 31, 16–29. [Google Scholar]
- Pebesma, E . (2018). Simple features for R: standardized support for spatial vector data. The R Journal, 10, 439–446. [Google Scholar]
- R Core Team . (2019). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from https://www.R‐project.org/. Accessed July 25, 2020. [Google Scholar]
- Rocke, T. E. , et al. (2019). Virally‐vectored vaccine candidates against white‐nose syndrome induce anti‐fungal immune response in little brown bats (Myotis lucifugus). Scientific Reports, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D. R. , Allan, N. L. , McGowan, C. P. , Szymanski, J. A. , Oetker, S. R. , & Bell, H. M . (2018). Development of a species status assessment process for decisions under the U.S. Endangered Species Act. Journal of Fish and Wildlife Management, 9, 302–320. [Google Scholar]
- Stanton, J. C. , Marek, J. , Hall, L. S. , Kus, B. , Alvarado, A. , Orr, B. , Morrissette, E. , Riege, L. , & Thogmartin, W. E . (2019). Collaborative model development for recovery planning: creation of a decision support tool for an endangered songbird. Ecology and Society, 24, 11. [Google Scholar]
- Sutherland, W. J. , Pullin, A. S. , Dolman, P. M. , & Knight, T. M . (2004). The need for evidence‐based conservation. Trends in Ecology & Evolution, 19, 305–308. [DOI] [PubMed] [Google Scholar]
- Thogmartin, W. E. , King, R. A. , McKann, P. C. , Szymanski, J. A. , & Pruitt, L . (2012). Population‐level impact of white‐nose syndrome on the endangered Indiana bat. Journal of Mammalogy, 93, 1086–1098. [Google Scholar]
- Turner, G. G. , Reeder, D. , & Coleman, J. T. H . (2011). A five‐year assessment of mortality and geographic spread of white‐nose syndrome in North American bats, with a look to the future. Update of white‐nose syndrome in bats. Bat Research News, 52, 13–27. [Google Scholar]
- USFWS (U.S. Fish and Wildlife Service) . (2011). A national plan for assisting states, federal agencies, and tribes in managing white‐nose syndrome in bats. USFWS, Northeast Region, Hadley, Massachusetts. Available from https://www.whitenosesyndrome.org (accessed August 2020). [Google Scholar]
- USFWS (U.S. Fish and Wildlife Service) . (2015). Endangered and threatened wildlife and plants; threatened species status for the northern long‐eared bat with 4(d) rule. Federal Registry, 80, 17973–18033. [Google Scholar]
- USFWS (U.S. Fish and Wildlife Service) . (2019). Indiana bat (Myotis sodalis) 5‐year review: summary and evaluation. USFWS, Midwest Region, Bloomington, Indiana. [Google Scholar]
- Voyles, J. , et al. (2015). Moving beyond too little, too late: Managing emerging infectious diseases in wild populations requires international policy and partnerships. Ecohealth, 12, 404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller, T. J. , Rodhouse, T. J. , Neubaum, D. J. , Ormsbee, P. C. , Dixon, R. D. , Popp, D. L. , Williams, J. A. , Osborn, S. D. , Rogers, B. W. , Beard, L. O. , & McIntire, A. M . (2018). A review of bat hibernacula across the western United States: implications for white‐nose syndrome surveillance and management. PLOS ONE, 13(e0205647) 10.1371/journal.pone.0205647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker, J. O. , & Gummer, S. L . (2000). Population structure and dynamics of big brown bats (Eptesicus fuscus) hibernating in buildings in Indiana. The American Midland Naturalist, 143, 389–396. [Google Scholar]
- White‐nose Syndrome Response Team . (2019). White‐nose syndrome case definitions. U.S. Fish and Wildlife Service, Northeast Region, Hadley, Massachusetts. Available from https://s3.us-west-2.amazonaws.com/prod-is-cms-assets/wns/prod/de91e7d0-9c0e-11e9-ad22-19882a049409-WNS-Case-Definitions_v5162019_FINAL-clean-logo.pdf (accessed January 2020). [Google Scholar]
- White‐nose Syndrome Response Team . (2020). WNS spread map. U.S. Fish and Wildlife Service, Northeast Region, Hadley, Massachusetts. Available from www.whitenosesyndrome.org (accessed August 2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information on acknowledgements, terminology, and methods
Table S2‐1. Number of contributed winter surveys (and sites)
Table S2‐2. Comparison of estimated severity of decline (with 95% credible intervals) from equations 1 and 3
Table S2‐3. Comparison of estimated severity of decline calculated from mean and median site counts and model estimates (Eq. 3) with established and pre‐arrival counts in parentheses (countestablished/countprearrival)
Table S2‐4. Proportion of site incidence (mean estimates with 95% credible intervals) by species and disease stage
Table S2‐5. Number of occupied sites in each colony size class by species and disease stage.
Table S2‐6. Sample size of count surveys (and number of sites) by species, region, and state or province.
Table S2‐7. Severity of decline (lower and upper credible intervals) at regional and state/provincial levels
Figure S3‐1. Proportion of increasing or decreasing sites at each stage in comparison to pre‐arrival counts for that species.
Figure S3‐2. Relationship between sample size and power to detect a decline of 30%, 50%, and 80% population change following WNS and given 4 levels of variance in colony counts.
Figure S3‐3. Density of site‐specific count estimates in the pre‐arrival and established disease stages from the complex model allowing site to vary with disease stage (Eq. 3)
Figure S3‐4. Relationship between precision of state and province‐level severity estimates of WNS decline and number of sites surveyed/included in analyses.


