Abstract
We show the synthesis of an in vivo stable mercury compound with functionality suitable for radiopharmaceuticals. The designed cyclic bisarylmercury was based on the water tolerance of organomercurials, higher bond dissociation energy of Hg−Ph to Hg−S, and the experimental evidence that acyclic structures suffer significant cleavage of one of the Hg−R bonds. The bispidine motif was chosen for its in vivo stability, chemical accessibility, and functionalization properties. Radionuclide production results in 197(m)HgCl2(aq), so the desired mercury compound was formed via a water‐tolerant organotin transmetallation. The Hg‐bispidine compound showed high chemical stability in tests with an excess of sulfur‐containing competitors and high in vivo stability, without any observable protein interaction by human serum assay, and good organ clearance demonstrated by biodistribution and SPECT studies in rats. In particular, no retention in the kidneys was observed, typical of unstable mercury compounds. The natHg analogue allowed full characterization by NMR and HRMS.
Keywords: Bispidine, Mercury, Organomercury, Radiopharmaceuticals, Theranostics
Stable and versatile: The cyclic bisarylmercury bispidine structure shows exceptional stability against sulfur compounds known to usually react very easily with mercury. Aside from its novelty in mercury chemistry, the in vivo stability paired with the functionalizability of this motif is an important step forward in the development of useful radiomercury pharmaceuticals.

Conventional radiopharmaceutical drugs used in oncology fulfil one of two roles: diagnostic (tumour imaging) or therapeutic (cancer treatment). Due to the biochemical heterogeneity between the diagnostic drug and the therapeutic drug, the in vivo interactions observed for the diagnostic drug are not directly interchangeable with the actual behaviour of the therapeutic drug. Theranostics, the designed combination of therapeutic and diagnostic radiopharmaceuticals with biochemical homogeneity, aims to bridge this information gap, thus allowing continual treatment evaluation. [1] A current example of a theranostic matched pair is 68Ga and 177Lu labelled DOTA‐TATE for the treatment of neuroendocrine tumours. [2] Although no single radionuclide is suitable for every type of treatment (due to tumour size, receptor expression, etc.), ideally the radiopharmaceutical would use the same element for both imaging and therapy as the in vivo interactions would then be identical. [1]
Therefore, our interest in radiomercury was motivated by the theranostically suitable decay emissions of 197Hg and its metastable nuclear isomer 197mHg (collectively referred to as 197(m)Hg). A broader selection of radionuclides in developed pharmaceuticals would allow for more personalized care, [3] and compared with currently used radionuclides the mercury isotope possesses many apt qualities. The emitted conversion electrons have energies closely corresponding with the beta emissions of 177Lu used for therapy, [4] whilst a gamma emission is suitably near the energy of the 99mTc gamma emission diagnostically used in single‐photon emission computed tomography (SPECT) medical imaging. [5] The high number of Auger electron (AE) emissions per decay (197mHg: 19, 197Hg: 23) also have great therapeutic potential, with similar energies to 125I, which has already shown that even extracellular AE radiotherapy can effectively cause cell death.[ 6 , 7 , 8 ] Combined with the practical half‐lives of 23.82 h and 64.81 h (for the metastable and ground state nuclear isomers respectively) [9] and the stable 197Au decay product, the innately theranostic nuclide 197(m)Hg is a promising candidate for radiopharmacy.[ 10 , 11 ]
There is historical precedence for the medicinal use of radiomercury, as during the 1960s and early 1970s reactor produced 197Hg with low specific activity did see clinical use in diagnostics, for kidney and brain imaging, in the radiolabelled chlormerodrin, [12] a mercurial diuretic commercially traded from 1952 [13] till 1974. [14] 197Hg‐chlormerodrin, whilst once popular amongst some physicians, [15] was discontinued by the FDA in 1989. [16] The lack of viable mercury compounds, coupled with the rapid progress of the 99mTc alternative, led to radiomercury's discontinuation and current obscure status in the long list of radionuclides in modern radiopharmaceutical research.[ 17 , 18 , 19 , 20 ] Modern cyclotron production, using natAu as the target material, yields 197(m)Hg with such high specific activity that the medically relevant amount falls well below the lower limits of concern for mercury's chemical toxicity. [10]
In contrast to historical popularity, mercury has attracted far less interest in modern chemistry, mainly due to concerns over toxicity. [21] Today, a lot of mercury research is focused on its detection and removal from the environment and, although being phased out, organomercury's present‐day main use is still in disinfectants (e. g. thiomersal). None of these uses require long term in vivo stability.[ 22 , 23 ] Chelation therapy for mercury poisoning often uses thiols, such as dimercaptosuccinic acid (DMSA), due to low side‐effects and cost, but as the priority is fast clearance, long term stability of the mercury compound formed is not essential. [24] Beyond radiopharmaceuticals, the formation of a mercury compound that is highly stable in nanomolar concentrations could also transfer to trace mercury analysis in environmental science and toxicology.
As with other radiometals, the bifunctional chelator (BFC) model was deemed the most appropriate route for securing the radiomercury and providing a link to a tumour‐targeting vector. [19] Organomercurials inherently have the necessary high water tolerance and the Hg−Ph bond is preferred due to having the highest bond dissociation energy for any Hg bond. [25] Research has shown that acyclic organomercury compounds suffer significant cleavage[ 26 , 27 ] therefore a cyclic structure was chosen for increased stability. Functionalization of the bridge would allow for improved solubility and attachment of a tumour specific carrier. Therefore a bispidine backbone was chosen as a suitable candidate due to its known in vivo stability, ease of synthesis, and functionalization capabilities, along with its already thoroughly investigated potential for radiopharmaceuticals.[ 28 , 29 , 30 , 31 , 32 ] Protonation of the tertiary amine moieties under physiological conditions would also improve the compound's water solubility due to the known bispidine ‘proton sponge’ effect. [33] In order to form the desired highly stable bispidine carbon‐metal bond, a different route from previous studies, which focussed on catalysis chemistry, was necessary.[ 34 , 35 , 36 , 37 , 38 , 39 ] Trimethylstannyl was chosen as a viable leaving group for the transmetallation with mercury due to its tolerance for the unavoidable aqueous conditions used for the radiometal's production and handling. [40]
The objective of this work was the synthesis of a mercury compound, stable in vivo, which has solubility and functionality such that it could be attached to a cancer‐targeting carrier for drug development in radiopharmacy.
The natHg‐bispidine reference compound 3 a was synthesized under nonaqueous conditions in three main steps (Scheme 1): a 4‐fold Mannich reaction, a lithiation followed by stannylation and finally a mercury transmetallation. Formation of 1 in high purity was possible with simple recrystallisation in a yield of 75 % but the formation of 2 a proved more troublesome due to many difficult to separate side‐products, affording the stannylated compound with a ∼90 % purity and 9 % yield. However, the mercuration step proved high yielding with simple purification.
Scheme 1.
Cyclic bisarylmercury bispidine synthesis.
The reduced bispidine analogue was also prepared as the bispidol 1H in high yield of 96 %. Because its less sterically hindered or more reactive OH group it is better suited as the starting material for further conjugation and then afterward stannylation reactions. But the stannylated compound 2 b itself, before functionalization was carried out on the hydroxyl group, was lower yielding, with lower purity and much more prone to degradation than the butylated analogue 2 a. In apolar solvents the OH⋅⋅⋅N hydrogen bond causes the chair‐boat conformer to be visible in NMR, overlapping with the chair‐chair conformer useful in the mercuration reaction. [41] The shorter reaction path along with the butyl moiety serving as a pseudo‐conjugate, with regards to sterics, meant the focus of this study centred on 3 a for the proof of principle. The product 3 a was characterized by HPLC, MS (Figures 1 and 2), and NMR (see SI). Radiolabelling experiments of the stannylated precursor 2 a with 197(m)HgCl2(aq) were carried out in 1 : 1 (v/v) water/EtOH with a buffer to ensure a constant pH 6. After initial radio‐TLC analyses of the reaction mixture showed a single peak not corresponding to Hg salts, the reaction was checked against a test developed by our lab in which the TLC plate is presoaked with a trithiol – tris(2‐ mercaptoethyl)ammonium oxalate [42] – which readily binds to reactive Hg species (i. e. Hg2+ or RHg+), fixing them to the baseline.
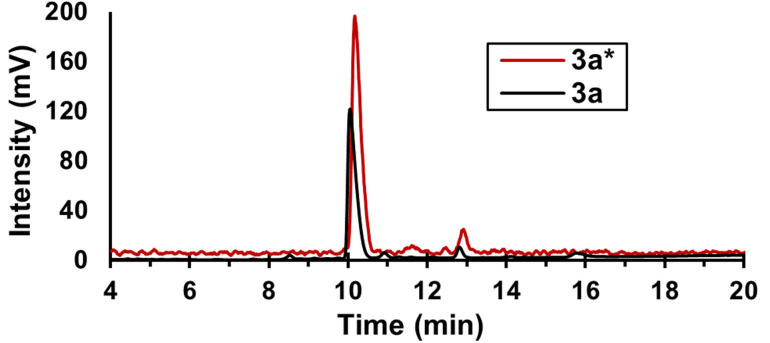
Figure 1.
Overlay of radiochromatogram (γ, red) and UV absorption chromatogram (220 nm, black), for co‐injection of 3 a* (retention time 10.2 min) and 3 a (retention time 10.1 min).
Figure 2.

Relative abundance of the calculated and measured [M+H]+ isotopic distribution pattern of substance 3 a as analysed by mass spectrometry.
The results (Figure 3) showed the reaction product to be stable to this test, indicating complete binding of the Hg. The reaction solution was then measured by HPLC, with the gamma ray detector showing a single main peak. Confirmation for the formation of the 197(m)Hg‐ radiolabelled bispidine 3 a* was done by co‐injection with the non‐radioactive analogue 3 a, which showed the same retention time for the UV trace as the gamma trace of the HPLC (Figure 1). Chemical stability tests were performed on three aliquots of 3 a*, each left for two days in a vial with an excess of sulfur‐containing competitor and then analysed by radio‐TLC, as shown in Figure 4.
Figure 3.
Species analysis of 197(m)Hg. Overlay of four radio‐TLCs: RP−C18 (dotted lines) and trithiol impregnated RP−C18 (solid lines) of 197(m)HgCl2 reference and 3 a*. NS3=tris(2‐mercaptoethyl)ammonium oxalate.
Figure 4.
Degradation analysis of 197(m)Hg‐species. Overlay of five radio‐TLCs (iTLC‐SG) of 197(m)HgCl2 reference, 3 a* reference, and 3 a* with competitor trials after 2 d at room temperature. aIntensity normalized with respect to 3 a*. GSH=l‐glutathione. NS3=tris(2‐mercaptoethyl)ammonium oxalate.
The results show high chemical stability, as only the sulfide caused any measurable degradation (∼4 %), as seen at the baseline. The lipophilicity of 3 a* was determined, indicating how the compound would interact in vivo, [43] by measuring the distribution coefficient under the physiological pH of blood (pH 7.4) using the “shake flask” method. [44] The value logD7.4=2.27 shows an acceptable level of lipophilicity for in vivo testing, being below the recommended upper limit of 3, over which the poor solubility can cause adverse effects, but above the lower limit of 1, below which would cause too fast a renal clearance. [45] For the low amounts of 3 a* needed for in vivo testing, the water solubility is high enough for the aqueous conditions.
As a preliminary assessment for in vivo stability, 3 a* was incubated with human serum as per the procedure of Zarschler et al. [46] The results (Figure 5) show that, unlike with 197(m)Hg‐radiolabelled EDTA used as reference, neither 197(m)Hg release (demetallation) nor binding to serum proteins is detectable for 3 a*. The only radioactive species detected matches with the mass of 3 a* highlighting its remarkable stability under these conditions. In contrast, a range of protein bands of different sizes are visible when 197(m)Hg‐radiolabelled EDTA was incubated with human serum due to substantial decomplexation and transchelation. For example, a prominent protein band in the size range of approximately 65 kDa is detectable in the autoradiographic scans, which corresponds most likely to radioalabelled serum albumin.
Figure 5.
Analysis of 197(m)Hg‐incorporation into human serum proteins by SDS‐PAGE. 20 % SDS‐polyacrylamide gel Coomassie blue staining (A), showing bands of human serum proteins, and autoradiograph (B), showing 197(m)Hg‐labelled bands. Lane 1: 197(m)Hg‐radiolabelled bispidine 3 a*. Lane 2: 197(m)Hg‐radiolabelled EDTA. M: molecular‐weight size marker.
To test the actual in vivo stability of 3 a*, a biodistribution was performed in healthy rats. The results (Figure 6) show rapid renal clearance by normal micturition, during a 24 h period, and no indication of demetallation\retention in the kidneys as observed with mercury salts.
Figure 6.
Biodistribution of 3 a* in healthy male Wistar rats (197(m)HgCl2: n=4, 3 a*: n=8). ∼300 kBq per animal. All other organs measured well below 1 %ID/g.
These results were confirmed by the SPECT/CT study (Figure 7). No substantial retention or accumulation of activity in the kidneys was observed 24 hours after injection. In contrast to unbound mercury, however, there is fractional uptake by the liver with subsequent hepatobiliary clearance. 3 a also showed high bench stability, being left in solution for over eight months without degradation (measured by HPLC).
Figure 7.

Standardized Uptake Value coloured SPECT/CT images of rats 24 h after injection of 197(m)HgCl2 (left, retention in kidneys) and 3 a* (right, rapid renal excretion with minor liver uptake followed by hepatobiliary clearance); maximum intensity projections (top) and coronal slice images (bottom); intestine (int), kidneys (ki), liver (li), rectum (re), stomach (st).
Limitations: Stannylation of the bispidine precursors 1 in order to form precursor 2 a, under milder conditions with Pd‐based catalysts were unsuccessful after many attempts. Instead, the harsher conditions for the lithiation/stannylation low‐yield route were required. The stannylated compounds 2 a/b are unstable under acidic conditions so purification with silica gel was not possible (even with wetting of a basic solvent system), therefore basic alumina was required. They are also prone to hydrolysis and as such have a poor shelf‐life.
The radiolabelling conditions are pH‐sensitive as a very low pH would risk hydrolysing the stannyl moieties, whilst too high a pH would form a mixture of different Hg species affecting the uniformity of the reaction conditions.
Future work: The next steps for this work would be testing of carrier attachment at the bispidine backbone, using methods already well established, e. g. amide, carbamate, thiourea, thiol and ‘click’ coupling.[ 47 , 48 , 49 ] Followed by optimization of reaction conditions (with continued searching for suitable alternative leaving groups to remove the current bottle‐neck in the synthesis), testing of structural derivatives in search of improved characteristics (e. g. solubility) and investigations into the potential for mercury analytics.
Overall, these results show good promise for the development of a new class of thermodynamically stable organomercury compounds suitable for theranostic applications in radiopharmacy.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
The authors gratefully acknowledge the excellent technical assistance of Ulrike Gesche for radiolabelling, and that of Regina Herrlich for the animal studies. The authors also thank Karin Landrock for the elemental analyses and the lipophilicity determination, Markus Laube for the HRMS assistance, and Constantin Mamat for the NMR assistance. The irradiations for 197(m)Hg production were carried out at the CANAM infrastructure of the NPI CAS Řež supported through MEYS project no. LM2011019. Open access funding enabled and organized by Projekt DEAL.
I. M. F. Gilpin, M. Ullrich, T. Wünsche, K. Zarschler, O. Lebeda, J. Pietzsch, H.-J. Pietzsch, M. Walther, ChemMedChem 2021, 16, 2645.
Contributor Information
Ian Moore F. Gilpin, Email: m.walther@hzdr.de.
Dr. Martin Walther, Email: iammooregilpin@gmail.com.
References
- 1. Srivastava S. C., Semin. Nucl. Med. 2012, 42, 151–163. [DOI] [PubMed] [Google Scholar]
- 2. Hennrich U., Kopka K., Pharmaceuticals 2019, 12, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Srivastava S. C., J. Postgrad. Med. 2013, 47, 31–46. [Google Scholar]
- 4. Pillai A. M. R., Knapp F. F. (R)., Curr. Radiopharm. 2015, 8, 78–85. [DOI] [PubMed] [Google Scholar]
- 5. Boros E., Marquez B. V., Ikotun O. F., Lapi S. E., Ferreira C. L., in Ligand Des. Med. Inorg. Chem. (Ed.: Storr T.), John Wiley & Sons, Ltd., 2014, pp. 47–79. [Google Scholar]
- 6. Ku A., Facca V. J., Cai Z., Reilly R. M., EJNMMI Radiopharm. Chem. 2019, 4, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Randhawa P., Olson A. P., Chen S., Gower-Fry K. L., Hoehr C., Engle J. W., Ramogida C. F., Radchenko V., Curr. Radiopharm. 2021, 14, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai Z., Al-saden N., Georgiou C. J., Reilly R. M., Int. J. Radiat. Biol. 2020, 1–11. [DOI] [PubMed] [Google Scholar]
- 9. Lebeda O., Kondev F. G., Červenák J., Nucl. Inst. Methods Phys. Res. A 2020, 959, 163481. [Google Scholar]
- 10. Walther M., Preusche S., Bartel S., Wunderlich G., Freudenberg R., Steinbach J., Pietzsch H.-J., Appl. Radiat. Isot. 2015, 97, 177–181. [DOI] [PubMed] [Google Scholar]
- 11. Freudenberg R., Apolle R., Walther M., Hartmann H., Kotzerke J., EJNMMI Phys. 2018, 5, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sodee D. B., Di Stefano B., Ohio State Med. J. 1965, 61, 819–822. [PubMed] [Google Scholar]
- 13. Sittig M., Pharmaceutical Manufacturing Encyclopedia, Noyes Publications, Westwood, 1988. [Google Scholar]
- 14. Crout J. R., Fed. Regist. 1974, 39, 42018–42019. [Google Scholar]
- 15. Sodee D. B., J. Nucl. Med. 1968, 9, 645. [PubMed] [Google Scholar]
- 16.FDA, Approved Drug Products with Therapeutic Equivalence Evaluations, US Department Of Health And Human Service, 1989.
- 17. Daly M. J., Jones W., Rudd T. G., Tremann J., J. Nucl. Med. 1979, 20, 63–66. [PubMed] [Google Scholar]
- 18. Staum M. M., J. Nucl. Med. Technol. 1992, 20, 209–213. [Google Scholar]
- 19. Kostelnik T. I., Orvig C., Chem. Rev. 2019, 119, 902–956. [DOI] [PubMed] [Google Scholar]
- 20. Anthony E. J., Bolitho E. M., Bridgewater H. E., Carter O. W. L., Donnelly J. M., Imberti C., Lant E. C., Lermyte F., Needham R. J., Palau M., Sadler P. J., Shi H., Wang F.-X., Zhang W.-Y., Zhang Z., Chem. Sci. 2020, DOI 10.1039/d0sc04082 g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gad S. C., Pham T., in Encycl. Toxicol. (Ed.: Wexler P.), Academic Press, 2014, pp. 207–210. [Google Scholar]
- 22. Horvat M., in Encycl. Anal. Sci. (Eds.: Townshend A., Poole C. F., Worsfold P. J.), Academic Press, 2005, pp. 545–557. [Google Scholar]
- 23. Wang L., Hou D., Cao Y., Ok Y. S., Tack F. M. G., Rinklebe J., O'Connor D., Environ. Int. 2020, 134, 105281. [DOI] [PubMed] [Google Scholar]
- 24. Wax P. M., J. Med. Toxicol. 2013, 9, 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dean J. A., Lange's Handbook of Chemistry, McGraw-Hill, Inc., 1999. [Google Scholar]
- 26. Wilhelm M., Saak W., Strasdeit H., Zeitschrift fur Naturforsch. - Sect. B J. Chem. Sci. 2000, 55 b, 35–38. [Google Scholar]
- 27. Bytautas L., Croat. Chem. Acta 2013, 86, 453–462. [Google Scholar]
- 28. Comba P., Kerscher M., Rück K., Starke M., Dalton Trans. 2018, 47, 9202–9220. [DOI] [PubMed] [Google Scholar]
- 29. Comba P., Haaf C., Wadepohl H., Inorg. Chem. 2009, 48, 6604–6614. [DOI] [PubMed] [Google Scholar]
- 30. Cui H., Goddard R., Pörschke K.-R., Hamacher A., Kassack M. U., Inorg. Chem. 2014, 53, 3371–3384. [DOI] [PubMed] [Google Scholar]
- 31. Nonat A. M., Roux A., Sy M., Charbonnière L. J., Dalton Trans. 2019, 48, 16476–16492. [DOI] [PubMed] [Google Scholar]
- 32. Bruchertseifer F., Comba P., Martin B., Morgenstern A., Notni J., Starke M., Wadepohl H., ChemMedChem 2020, 15, 1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miyahara Y., Goto K., Inazu T., Tetrahedron Lett. 2001, 42, 3097–3099. [Google Scholar]
- 34. Cui H., Goddard R., Pörschke K.-R., Organometallics 2011, 30, 6241–6252. [Google Scholar]
- 35. Gogoll A., Johansson C., Axén A., Grennberg H., Chem. Eur. J. 2001, 7, 396–403. [DOI] [PubMed] [Google Scholar]
- 36. Spieler J., Huttenloch O., Waldmann H., Eur. J. Org. Chem. 2000, 391–399. [Google Scholar]
- 37. Bulygina L. A., Khrushcheva N. S., Peregudov A. S., Sokolov V. I., Russ. Chem. Bull. 2016, 65, 2479–2484. [Google Scholar]
- 38. Bulygina L. A., Kagramanov N. D., Khrushcheva N. S., Lyssenko K. A., Peregudov A. S., Sokolov V. I., J. Organomet. Chem. 2017, 846, 169–175. [Google Scholar]
- 39. Bulygina L. A., Khrushcheva N. S., Lyssenko K. A., Peregudov A. S., J. Organomet. Chem. 2019, 887, 64–70. [Google Scholar]
- 40. Walther M., Lebeda O., Preusche S., Pietzsch H.-J., Steinbach J., in AIP Conf. Proc., American Institute of Physics, 2017, p. 020023. [Google Scholar]
- 41. Comba P., Kerscher M., Schiek W., in Prog. Inorg. Chem., (Ed.: Karlin K.D.), John Wiley & Sons, Inc., 2007, 55, 613–704. [Google Scholar]
- 42. Spies H., Glaser M., Pietzsch H.-J., Hahn F. E., Lügger T., Inorg. Chim. Acta 1995, 240, 465–478. [Google Scholar]
- 43. Johnson T. W., Dress K. R., Edwards M., Bioorg. Med. Chem. Lett. 2009, 19, 5560–5564. [DOI] [PubMed] [Google Scholar]
- 44. Leo A. J., Methods Enzymol. 1991, 202, 544–591. [DOI] [PubMed] [Google Scholar]
- 45. Lindsley C. W., in Encycl. Psychopharmacol. (Eds.: Stolerman I. P., Price L. H.), Springer, Berlin, Heidelberg, 2014. [Google Scholar]
- 46. Zarschler K., Kubeil M., Stephan H., RSC Adv. 2014, 4, 10157–10164. [Google Scholar]
- 47. Stephan H., Walther M., Fähnemann S., Ceroni P., Molloy J. K., Bergamini G., Heisig F., Müller C. E., Kraus W., Comba P., Chem. Eur. J. 2014, 20, 17011–17018. [DOI] [PubMed] [Google Scholar]
- 48. Singh G., Zarschler K., Hunoldt S., Martínez I. I. S., Ruehl C. L., Matterna M., Bergmann R., Máthé D., Hegedüs N., Bachmann M., Comba P., Stephan H., Chem. A Eur. J. 2020, 26, 1989–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Price E. W., Orvig C., Chem. Soc. Rev. 2014, 43, 260–290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information