Abstract
Tin is the frontrunner for substituting toxic lead in perovskite solar cells. However, tin suffers the detrimental oxidation of SnII to SnIV. Most of reported strategies employ SnF2 in the perovskite precursor solution to prevent SnIV formation. Nevertheless, the working mechanism of this additive remains debated. To further elucidate it, we investigate the fluoride chemistry in tin halide perovskites by complementary analytical tools. NMR analysis of the precursor solution discloses a strong preferential affinity of fluoride anions for SnIV over SnII, selectively complexing it as SnF4. Hard X‐ray photoelectron spectroscopy on films shows the lower tendency of SnF4 than SnI4 to get included in the perovskite structure, hence preventing the inclusion of SnIV in the film. Finally, small‐angle X‐ray scattering reveals the strong influence of fluoride on the colloidal chemistry of precursor dispersions, directly affecting perovskite crystallization.
Keywords: lead-free systems, perovskite solar cells, tin fluoride, tin halide perovskites, tin oxidation
Fluoride chemistry in tin halide perovskites improves the crystallization process. Fluoride anions selectively coordinate and remove SnIV and affect the colloidal properties in solution. This study describes the working mechanism of SnF2 and highlights the importance of solution chemistry for controlling crystallization and SnII oxidation in tin halide perovskites.
Introduction
Metal halide perovskite materials have shown enormous potential for the processing of efficient and stable photovoltaics. [1] However, the dominant type of perovskite solar cells (PSCs) are based on lead, a metal whose toxicity and environmental hazard can hinder its commercial application in numerous fields. [2] The lead threat pushed the scientific community to develop lead‐free perovskite materials to maintain excellent photovoltaic performance while avoiding environmental risks. In this sense, tin halide perovskites are the best candidate to replace the dominant lead‐based counterparts.[ 3 , 4 ] Nevertheless, these materials face some difficulties related to their inherent physicochemical characteristics. [5] The most important one is the ease with which SnII oxidizes into SnIV species, leading to the substantial decline in the performance through the undesirable formation of electron traps and p‐doping of the material. [6] Previous studies have reported many origins of this oxidation, such as the solvent,[ 7 , 8 ] the processing conditions [9] or even spontaneously through disproportionation in tin‐poor environments. [10] Stopping this oxidation is one of the requirements to achieve efficient and stable tin halide PSCs. For this reason, several trials have been made tackling the oxidation of SnII. These include the use of new solvent systems to avoid the oxidation by dimethyl sulfoxide (DMSO), [11] employing reducing agents to eliminate the content of SnIV, such as metallic Sn powder [12] or hypophosphorous acid [13] or introducing additives for alleviating the formation of SnIV, like the ever‐present SnF2.[ 6 , 14 ]
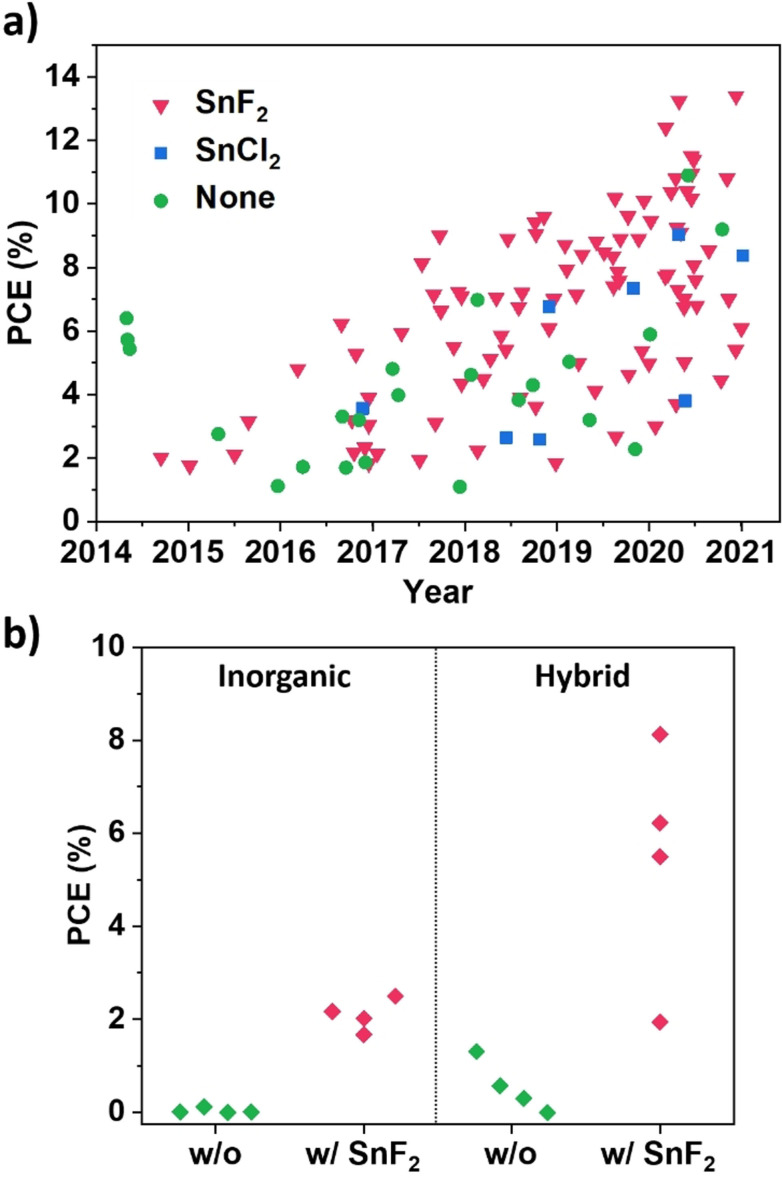
SnF2 has achieved remarkable success as an additive in the tin halide perovskite field. Since its first use in PSCs by Kumar and co‐workers, [15] it has been proven over time as an imperative to achieve good results (Figure 1 a). There is barely any good cell performance report without SnF2; exceptional cases use SnCl2,[ 16 , 17 ] which may behave similarly to SnF2, or 2D materials, which are another popular strategy for processing tin‐based perovskites.[ 18 , 19 , 20 ] The appeal of SnF2 in the community is such that the number of studies not using it (nor SnCl2) quickly stagnated over the years, being in 2020 below 10 % of the total publications on tin halide perovskites in that year (Figure S1). Its success lies mainly in the impossibility to obtain photovoltaic behaviour in the solar cells fabricated without it. In Figure 1 b, we collected data from studies in which solar cells were made with and without SnF2.[ 15 , 21 , 22 , 23 , 24 , 25 , 26 , 27 ] The improvement in efficiency for both inorganic and hybrid tin halide perovskites is enormous, with negligible efficiency for the SnF2‐free cases. One of the most reported improvements is the better substrate coverage and film morphology obtained with SnF2,[ 22 , 25 , 28 ] which implies that SnF2 affects the film crystallization, a factor that remains unexplored. Nevertheless, its addition needs to be controlled, as a too‐high content of SnF2 is reported to induce phase separation.[ 22 , 25 , 28 ] The other most explored effect is the ability of SnF2 to reduce the formation of SnIV and its related defects, with the reported benefits usually being reduced recombination [24] and a blue shift of the absorption onset.[ 24 , 29 , 30 ] Besides, Savill and co‐workers found out that even low amounts of SnF2 are sufficient to positively impact mitigating SnIV formation in tin/lead perovskites. [29] This effect on oxidation suppression could originate from introducing a Sn‐rich environment, reducing Sn vacancies. [10] This interpretation has been proposed already in the first use of this additive by Kumar et al. [15] However, the question of what fluoride is doing and why we do not provide the Sn‐rich environment simply with a higher SnI2 ratio in respect to FAI remains unanswered. Related to this, other SnII species that could provide the same beneficial effect were already discussed by Yokoyama et al. [31] Using SnI2 excess also led to good results in one of the first studies on inorganic tin halide perovskites. [32]
Figure 1.
a) Highest PCE reported in tin halide perovskites literature containing solar cell data using SnF2, SnCl2, or none of them, ordered by date until Jan. 2021. b) Comparison of solar cell performance improvement in inorganic and hybrid tin halide perovskites before and after the addition of SnF2, extracted from the studies in literature in which the comparison is made.[ 15 , 21 , 22 , 23 , 24 , 25 , 26 , 27 ]
Overall, reports in literature consistently lead to the same results: SnF2 has a critical positive influence on the formation of high‐quality ASnX3 (where A=methylammonium (MA+), formamidinium (FA+) and Cs+, and X=Cl−, Br− and I−) films and holds a particular role in the stability of these materials against their oxidation to SnIV. These two possibly related aspects are the key to SnF2 being the predominant, most robust additive in the tin‐based perovskite field. While there has been extensive exploration of the impact and functioning of SnF2 in these thin films, the chemical mechanism and influence in the processing are entirely unknown. Introducing an exact Scheme of its working procedure remains a must in the field. This move would open the door to optimized application and help identify new additives in the future.
In this work, we explain the origin of the beneficial effects of SnF2 in the processing and stability to oxidation of tin halide perovskites by studying the chemistry of fluoride in these solutions. Using a combination of complementary solution and film characterization techniques, we propose that the role of SnF2 is not limited to the resulting thin film but also affects the precursor solution properties critically and hence their processing. The study of the solution chemistry of fluoride in formamidinium (FA)‐based FASnI3 precursor solutions by 119Sn‐ and 1H‐NMR revealed a strongly predominant affinity of the fluoride anion for SnIV over SnII. With the help of hard X‐ray photoelectron spectroscopy (HAXPES) analysis, we show how SnF2 increases the SnII content in perovskite samples, an indication that SnIV is partially prevented from being incorporated in the perovskite film. Meanwhile, small‐angle X‐ray scattering (SAXS) enables an understanding of how fluoride anion modifies perovskite subunits’ interaction in solution, generating improved homogeneous crystal growth conditions. Furthermore, experiments with other fluoride species and SnCl2 prove that these effects are not exclusive to SnF2. Thus, the chemistry of a hard Lewis base like fluoride, combined with the Sn‐rich environment, make SnF2 a highly suitable additive for processing tin halide perovskites.
Results and Discussion
Previous works concluded that SnF2 could reduce the SnIV content in solutions and films.[ 14 , 30 ] However, the redox activity cannot explain the multiple effects of SnF2 in ASnX3 perovskites entirely. Therefore, we postulated that SnF2 must be involved in a different type of chemical reaction. In the early stages of tin halide perovskites development, it was thought that a yellow color for the solution implied the elimination of SnIV through its reduction by SnF2. [28] Using NMR, we uncover that SnIV and SnF2 do not undergo a redox reaction, but a simple ligand exchange reaction, producing colorless SnF4 in solution. In this regard, we prepared FASnI3 precursors solutions with and without SnF2 and SnIV to investigate their signature chemistry. 119Sn‐NMR is sensitive to Sn nuclei in different electronic environments, allowing to identify of the Sn species existing in the solution, including other oxidation states of the same nucleus.
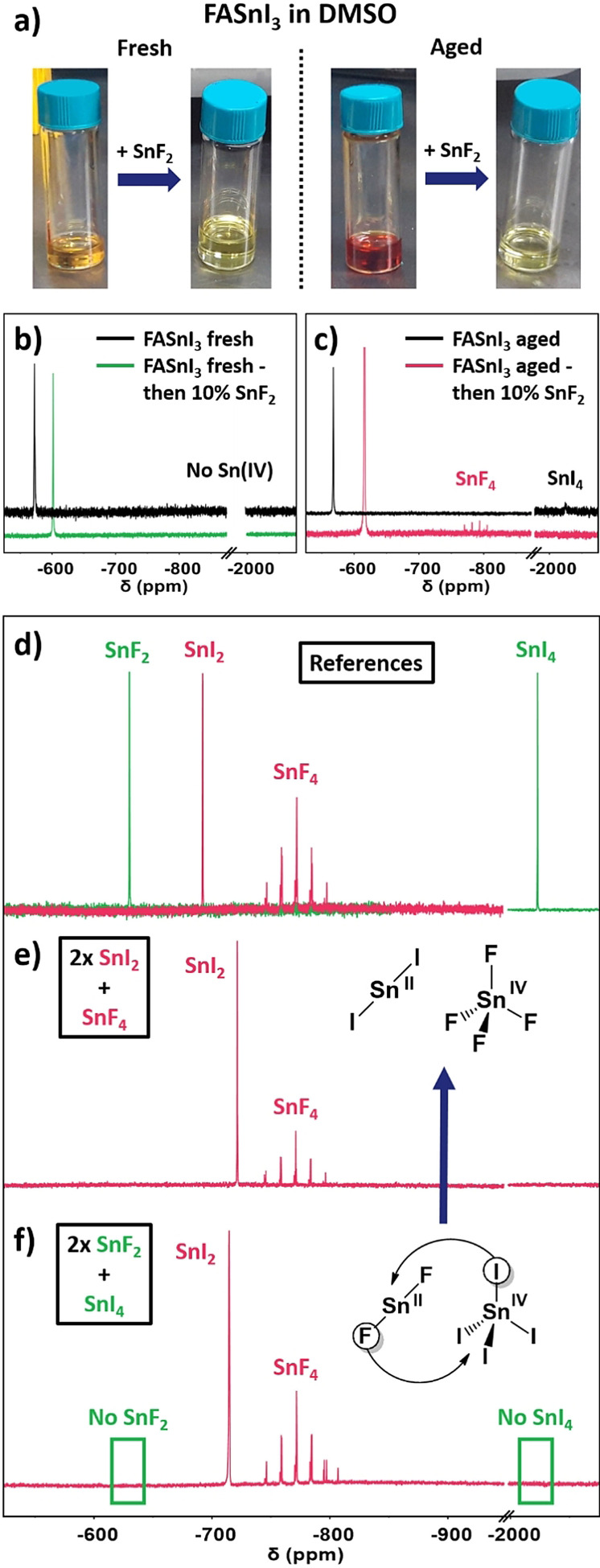
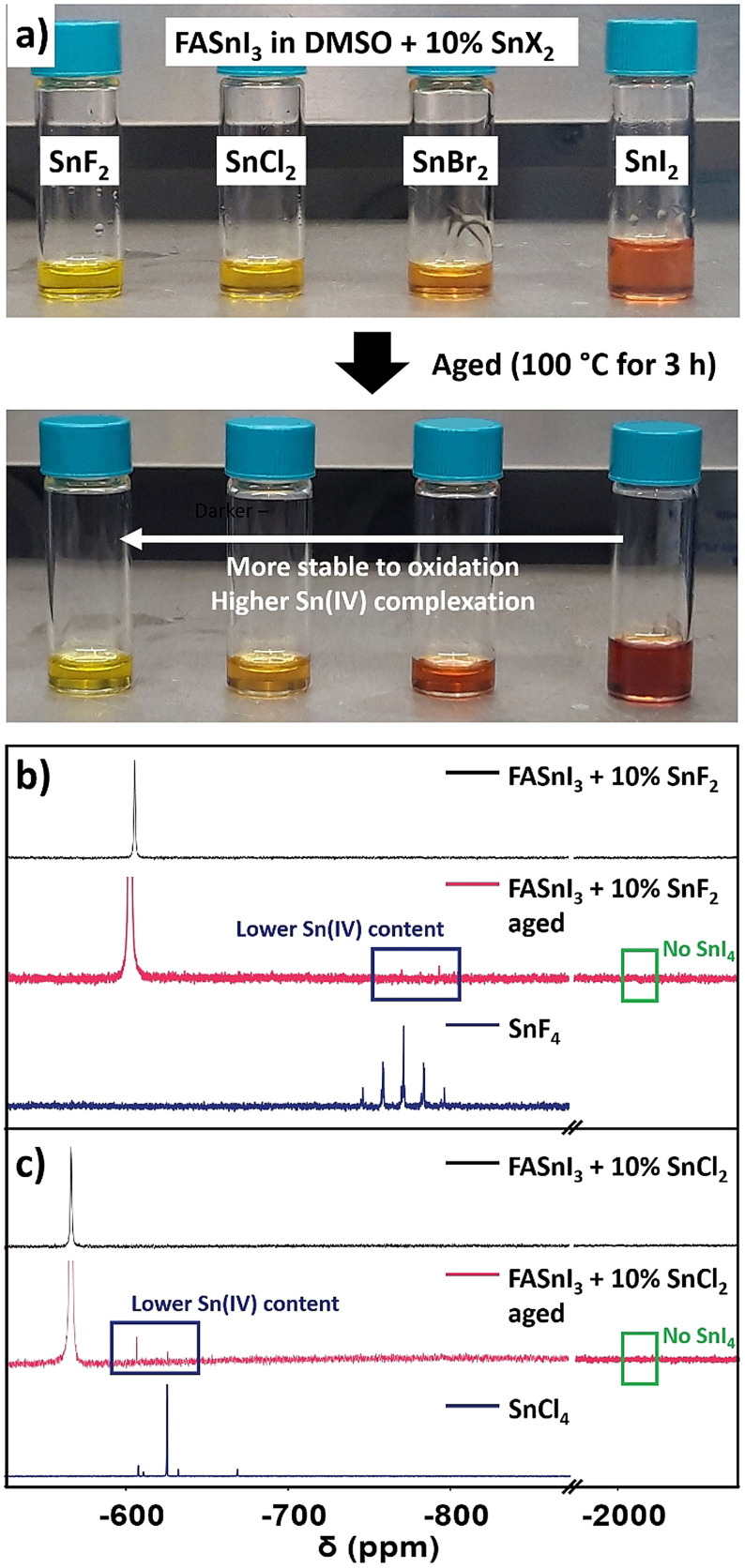
Figure 2 a depicts the change in color from orange to the pale yellow of a 1 M FASnI3 solution in DMSO after the addition of SnF2. Following the same method, we added SnF2 to a SnIV‐containing solution after being aged by heating at 100 °C for 3 h. Saidaminov et al. and we recently described that this thermal treatment promotes SnII oxidation in DMSO solutions.[ 7 , 8 ] The color of the aged solution goes from an intense red to a pale yellow to that of the fresh sample. Unlike the fresh solution, we know that certain content of SnIV is present for the aged one. Still, the color after SnF2 for the aged solution is the same as for the fresh solution, suggesting that the characteristics and species in the solution are changing, no matter the SnIV content.
Figure 2.
a) Pictures of fresh and aged FASnI3 solutions in DMSO before and after the addition of SnF2 in 10 mol %; the respective 119Sn‐NMR spectra of b) fresh and c) aged solutions; d) 119Sn‐NMR spectra of SnF2, SnI2, SnF4 and SnI4 in DMSO. Further information about solution preparation in Figure S3. 119Sn‐NMR spectra and solution pictures of the mixing of e) 2× SnI2 and SnF4 and f) 2× SnF2 and SnI4. The signal magnification was adapted accordingly for illustrative purposes.
119Sn‐NMR of the fresh solution indicates neither elimination nor formation of SnIV, even though its color changes to pale yellow (Figure 2 b). However, we observe the SnII shielding resulting in a chemical shift change from −574 ppm to −604 ppm. Regarding the SnII species (SnI2 and SnF2), they cannot be differentiated in solution, as they show up in a single signal belonging to the average electronic environment of SnII in solution. In this sense, the addition of increasing amounts of SnF2 shifts the SnII peak to lower chemical shift values in a fairly linear manner, as we show in Figure S2. In contrast to the fresh solution, the aged FASnI3 solution showed the expected SnI4 signal at −2025 ppm. After the addition of SnF2, this peak disappeared, and a new quintuplet rose at −770 ppm (Figure 2 c). To identify the newly formed species, we measured solutions in DMSO of SnI2, SnI4, SnF2 and SnF4 by 119Sn‐NMR (Figures 2 d), indicating that the species corresponds to SnF4. This result, therefore, implies that SnF2 cannot reduce SnIV from an oxidized sample. Instead, it coordinates SnIV via a ligand exchange reaction between the fluorides from SnIIF2 and the iodides from oxidized SnIVI4 [Eq. 1]:
| (1) |
As shown in Figure 2 e, a colorless solution comprising SnI2 and SnF4 presented both mixed species’ signals in NMR. However, mixing SnF2 and SnI4, the resulting NMR species observed were SnI2 and SnF4. Consequently, the complexation selectivity of fluoride ions towards SnIV is absolute (Figure 2 f). This can be easily explained by the “hard and soft (Lewis) acids and bases” (i.e. HSAB theory) nature of the different solution species. Fluoride is a small, non‐polarizable, very electronegative anion that shows a stronger affinity for a cation of a similar nature, that is, SnIV, which is smaller and more electronegative than its reduced analogue SnII. This hard Lewis base character of fluoride anions was already applied in previous works on lead halide perovskites, owing to its ability to passivate vacancies due to their strong bonds with PbII. [33] In the present case, we find that fluoride's role is connected to SnIV complexation. Simultaneously, the passivation of undercoordinated SnII in the thin film should not be excluded.
The only appearance so far of this species can be found in Nakamura and co‐workers’ work. The authors use an SnF2‐selective reducing agent to effectively generate Sn0 nanoparticles to scavenge SnIV from the solution. [34] Even though there is no particular discussion on the formation of SnF4 and it does not affect their mechanism, the 119Sn‐NMR spectra provided in their work show the signal, also a multiplet, corresponding to SnF4 at approximately −750 ppm when adding SnF2 to a SnIV‐containing solution. This different multiplicity that the SnF4 signal presents in 119Sn‐NMR compared to the rest of the Sn species can be explained by coupling between the Sn and halide nuclei. SnF4 has four chemically equivalent 19F‐spins (each with spin 1/2) to couple with, which results in a perfect quintuplet as observed. In this sense, we would expect to observe a triplet for SnF2. However, the four coordination sites are not saturated in SnF2, and, thus, there is an exchange with other impurity‐related compounds, such as water. Birchall and Dénès found the same behaviour. They claimed that the missing coupling between 19F and 119Sn is due to the exchange between different hydrated species of SnF2. [35] Our samples contain water since non‐anhydrous [D6]DMSO was used as a solvent for the NMR experiments, therefore agreeing with the previously reported experiences. For other species, no splitting occurs since chloride, bromide, and iodide do not have NMR‐active nuclei in significant amounts or with detectable line widths.
This affinity of fluorides towards SnIV can have several important implications that may reduce SnIV content in the final film. For instance, the strong preference for SnIV means that fluorides could complex it as soon as it is generated, whether from O2 in the environment or DMSO‐driven oxidation.[ 7 , 8 , 11 ] In fact, one should think if SnF2 would be as valuable for other solvents as in DMSO, as fluorides could be critical in sequestrating SnIV as soon as it is oxidized by this solvent, making it less harmful. The conversion of SnI4 into SnF4 will also prevent the SnI4‐driven degradation pathways recently described by Lanzetta et al. [36] Furthermore, the selective complexation of SnIV as SnF4 may hinder its ability to form any perovskite‐like complex in solution. It has been widely reported for SnF2 that this material's excess tends to undergo phase separation.[ 22 , 25 , 28 ] Conclusively, if SnIV is retained as SnF4, it would be challenging to incorporate this form into the perovskite lattice. Instead, it would be displaced to grain boundaries or even removed from the film. As a result, the point defects resulting from incorporated SnIV in the perovskite lattice can be significantly reduced. To prove this, we compared the different SnIV species’ ability to coordinate with FAI by analyzing the 1H‐NMR of these solutions. Figure S4 shows how all SnI2 (FASnI3), SnF4 and SnI4 cause the splitting of the FAI aminic protons, pointing out a certain degree of interaction between the species. However, the signals for all N‐ and C‐attached protons are slightly shielded in the FASnI3 solution (where the formation of perovskite adducts in solution occurs), whilst for the case of SnF4, there is no shielding, suggesting that the interaction of SnF4 species might have a lower affinity towards perovskite precursors. Moreover, the shift is very pronounced for SnI4, which might imply strong coordination with FAI and an increased ability to get incorporated in the perovskite, resulting in adverse consequences for the photovoltaic properties of the films.
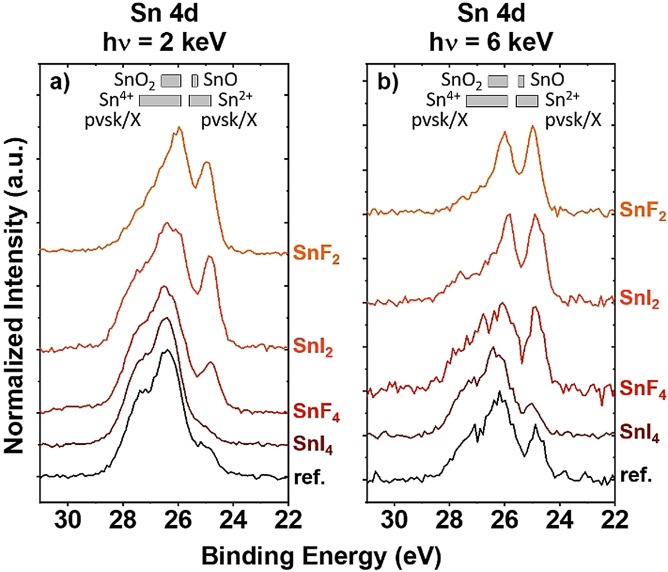
The impact of SnIV complexation by fluoride in the preparation of FASnI3 on the Sn chemical environment in the resulting films was investigated via HAXPES. For that, 10 mol % of SnF2, SnI2, SnF4 and SnI4 was deliberately added to FASnI3 perovskite precursor solutions. Figure 3 presents HAXPES spectra of the Sn 4d energy region of FASnI3 samples prepared with and without various additives, measured with excitation energies of 2 keV and 6 keV, respectively. It is possible to vary the probing depths of the HAXPES measurements using different excitation energies, that is, the 2 keV data is more surface‐sensitive than the 6 keV data (see methods section). The spectra shown in Figure 3 do not exhibit a line shape that resembles a single Sn 4d5/2‐4d3/2 doublet peak (i.e., with a 3:2=4d5/2:4d3/2 area ratio and a 4d5/2‐4d3/2 spin‐orbit separation of ≈1.1 eV);[ 30 , 40 ] this is a clear indication that spectral contributions from more than one Sn chemical species are detected. Overall, the Sn 4d spectra suggest substantial contributions in the binding energy (BE) regions (24.9±0.1) eV and (26.2±0.2) eV, corresponding to values reported in the literature for Sn 4d5/2 of Sn‐based perovskite/halide and oxide reference compounds with Sn being in Sn2+ and Sn4+ environments, respectively.[ 30 , 37 , 38 , 39 ] Because the Sn 4d BE values of Sn2+ (SnO) and Sn4+ (SnO2) oxide compounds are energetically overlapping with the respective Sn environments expected to be present in the sample set (i.e., FASnI3 and the different Sn‐based additives) and O‐related lines were detected in the HAXPES measurements, the Sn 4d spectra in Figure 3 likely contain Sn oxide derived spectral features and thus consist of more than two doublet peaks. Comparing the spectra in Figure 3 a with the spectra in Figure 3 b demonstrates that the high BE Sn4+‐related features are more prominent in the 2 keV measurements than in the 6 keV, which indicates an increased prevalence of the Sn4+ related species (in the form of SnO2 or SnX4) near the surface of samples than deeper within their bulk. However, significant differences in the line shape of the spectra for a given excitation set reveal pronounced changes in the Sn chemical environment of the investigated samples concerning the presence/absence and kind of additives during processing. The spectra of samples with additives containing Sn2+ or F− display a significant increase in the low BE Sn2+‐related signal. This finding seems to be in line with the NMR results described above, that capturing SnIV in the form of fluorinated species prevents its incorporation in the films. However, the observed variability of properties at the surface of the samples associated with handling conditions of the Sn‐based perovskite samples (as has been already reported, [30] and further discussed in Supporting Information, see Figure S5) prevents the present interpretation of the HAXPES results from reaching further conclusions on the impact of individual additives with statistical certainty.
Figure 3.
HAXPES spectra of Sn 4d core levels of FASnI3 films prepared without (“ref.”) and with 10 mol % of SnF2, SnI2 (excess), SnF4 and SnI4, measured using a) 2 keV and b) 6 keV excitation and normalized to maximum intensity (after background subtraction). The used additives are labelled next to the corresponding spectra. The grey‐filled boxes denote the binding energy of Sn 4d5/2 of Sn‐based reference compounds reported in the literature.[ 30 , 37 , 38 , 39 ] “Sn2+ pvsk/X” stands for perovskite (tin halide salt) compounds with various ASnX3 (SnX2) compositions. “Sn4+ pvsk/X” stands for perovskite (organotin halide) compounds with various ASnX6 (Ph3SnX) compositions.
As depicted previously in Figure 2 a, we attribute the color change of FASnI3 in DMSO to the change in solution properties and not to the SnIV content. Nevertheless, it is crucial to understand the underlying reasons that led to this change, as we expect it to exhibit a decisive influence on the crystallization dynamics.
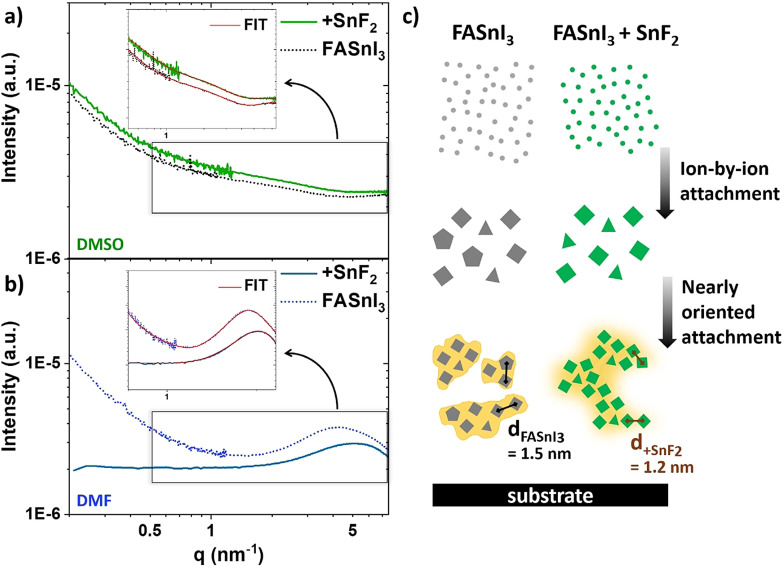
Here, we perform transmission small‐angle X‐ray scattering (SAXS) to reveal the effect of SnF2 on the perovskite precursors in solution. Thereby, a proposed nucleation mechanism indicates that the use of SnF2 promotes homogeneously distributed growth, yielding improvements in the overall crystal quality. Using the SAXS instrument at BESSY II at X‐ray energies of 8 keV and 10 keV (ΔE/E=2×10−4), we cover a q‐range from 0.05 to 8.5 nm−1 (size range: 209.4–0.74 nm). Figure 4 a compares the SAXS scattering curve of a plain FASnI3 solution in DMSO with FASnI3 containing SnF2. At first sight, the comparison does not show significant variations.
Figure 4.
SAXS performed on different FASnI3 precursor solutions. SAXS curves of FASnI3 (dotted line) compared to FASnI3 with SnF2 addition (solid line) in a) DMSO and b) DMF, as well as the corresponding fit given in red in the magnified representations. c) Proposed nucleation and growth mechanism in FASnI3 precursor solution affected by SnF2 addition, where the different forms (triangle, quadrangle, pentagon) should schematically describe the potential variety of subunits.
Nevertheless, by applying a model fit using the software SASfit©, [41] which offers several different form and structure factors describing various shapes of particles and their interaction, small changes regarding the particle interplay in the high q‐region can be observed. Here, however, interpretation requires particular precaution since we are already in the proximity of interatomic distances. The general behaviour of the initial perovskite precursor stage is highly dependent on the specific solvent environment. Literature shows that solvents with a lower donating number interact weakly, whereas stronger donating solvents interact strongly with the metal of a perovskite precursor solution. [42] Therefore, stronger donating solvents tend to hinder the iodide coordination of the metal. Since DMSO is known to be strongly donating and hence decelerates the perovskite crystallization process, we here include N,N‐dimethylformamide (DMF) with lower donating effect to investigate further the possible influence of SnF2 on the early stages of crystallization. Figure S6 shows the same effect on the color of SnF2 in DMF as in DMSO, as proof that the same visual transformation occurred.
The evolution of a maximum in the SAXS scattering curve of FASnI3 in DMF given in Figure 4 b shows a clear difference compared to the scattering curve of pure FASnI3 in DMSO. The maximum emerges based on a dominant structure factor, which evolves due to particle interaction. The mean spacing d between the mass centers of the individual interacting particles can be calculated as discussed by Raghuwanshi et al. using the magnitude of q at the peak maximum. [43] In the plain FASnI3 solution in DMF, this results in a mean spacing d of approximately 1.5 nm. Adding SnF2 to the solution leads to a shift of this peak maximum to higher q and, consequently, lower mean d spacing of 1.2 nm. Besides the shift of the maximum, also the slope at lower q‐values disappears. The shallow negative slope for both DMF and DMSO solutions gives rise to the presence of larger structures with a broad size distribution (>100 nm) in the solution. We propose that the larger sizes represent aggregates consisting of small interacting subunits formed by ion‐to‐ion attachment. We assign these subunits to particles or clusters in an average dimension of 0.4 nm observed in all scattering curves. In the DMF case, we assume that these aggregates form by nearly oriented attachment, as described in the non‐classical nucleation theory being pre‐ordered arrangements (Figure 4 c).[ 44 , 45 , 46 ] The well‐pronounced structure factor peak can evidence this, showing the recurring distance d between subunits, representing the average distance between the mass centers of the units and could thus be considered the tin‐to‐tin distance due to the high electron density of tin. A specific recurring distance d can also be noticed in the case of SnF2 addition. However, there is no negative slope at low q‐values assigned to larger higher‐level structures. Therefore, we conclude that the total size distribution generally appears to be more homogeneous; the nearly oriented attachment with the recurring distance d of 1.2 nm might be considerably more extensive than in the plain FASnI3 solution or even of infinite size. Additionally, we performed several runs for every sample to prove no damage caused by the beam (Figure S7).
Pre‐ordered arrangements of subunits set the starting point for the further crystallization of a thin film on a substrate. The broad size distribution of comparable smaller aggregates might result in films including unordered pores or pinholes because solvent evaporation leaves holes between the pre‐ordered totals. Instead, the more uniform size distribution due to a larger oriented attachment of the subunits supports homogeneously distributed crystal growth, a suitable substrate coverage and improved film morphology, precisely what is observed by SnF2 addition in literature.[ 22 , 25 , 28 ] With the premise of the already advanced aggregation of elements in the DMF solvent, a similar mechanism during the late stages of enhanced crystallization may be expected for the case of DMSO. By applying pressure via spin coating and solvent evaporation, the concentration of FASnI3 in the solution increases. Following the evolution of SAXS scattering curves for FASnI3 concentration series in DMF and DMSO, it suggests that a structure factor maximum is formed at higher concentrations in the case of DMSO comparable to the DMF solution (Figure S8). In this sense, the observed behaviour for DMF can be extrapolated to a more advanced stage of precursor formation for the DMSO precursor solution. Therefore, SnF2 as an additive leads to an in total more homogeneous crystallization of the tin halide perovskite thin film, and thus to a better morphology.
Regarding the change in solution color caused by SnF2 addition, we speculate that fluoride modifies the coordination level of tin centers by iodide ions, hindering the formation of colorful, highly coordinated [SnI x ]2−x units. The fact that better morphology films are obtained through the pale yellow, SnF2‐containing solution points out that, with solution color as an indication, the properties of the existing formations in solution critically influence the crystallization dynamics of tin halide perovskites. This feature is currently underexplored for these materials and proves to be much more complex and sensitive than for their lead analogues due to its quite restricted processing conditions.
Other SnX2 (X=Cl, Br, I) and their influence on perovskite properties are also frequently discussed in the literature.[ 16 , 17 , 47 ] We further performed SAXS on FASnI3 precursor solutions according to SnX2 addition, given in Figure S9, to compare their respective functionality to the SnF2 addition. Similar to the scattering curves shown in Figure 4 a, no significant influence or difference between different X can be noted. However, it should not be ruled out that they could have the same behavior difference as SnF2 in other solvents like DMSO and DMF. This confirms the need to investigate the strong dependence of additives and compositions used for tin halide perovskites. Finally, a scattering curve for the presence of SnIV is given in the inset window in Figure S9, for which we measured an aged sample of FASnI3. The effect of temperature‐induced degradation of DMSO solutions on its properties seems relevant, confirming that we did not influence unexpected SnIV content in FASnI3 with or without SnX2.
Although SAXS detected no difference for the different SnX2 additives in DMSO solutions, they still caused a color change in a clear trend (Figure 5 a). Both SnF2 and SnCl2 led to a similar yellow coloration of FASnI3 solution, while SnBr2 affected it mildly more. This trend could mean that the colorful, highly coordinated [SnI x ]2−x iodostannates were hindered more strongly as the halide X is a harder Lewis base. The fresh solutions were analyzed by 119Sn‐NMR (Figure S10), although no notable difference was found except for the shielding effect from SnF2, already discussed in Figure 2. In this sense, SnF2 is the only SnX2 additive shifting the FASnI3 signal upfield, while SnCl2 and SnBr2 go slightly in the opposite direction. This effect correlates well with the chemical shifts in the different SnX2‐pure solutions in DMSO (Figure S11), except for the SnI2 case. Hence, the final SnII signal position might be an average value of all SnII species. After heating the solutions, we observed that the solution's darkening was negligible for chloride‐ and fluoride‐containing solutions, which could be due to both lower SnII oxidation and more efficient SnIV complexation by these anions, as explained in Figure 2. The heated solutions were measured by 119Sn‐NMR (Figure 5 b), showing that the content of SnIV, all in the form of SnF4, had been significantly reduced in comparison to SnF2‐free heated solution (Figure 2 c). We hypothesize that fluoride could affect DMSO and SnII environments, maybe through the modulation of [SnI x ]2−x adducts, making these two species less eager to undergo a redox reaction. Also, we observed that SnCl2 addition had the same effect as SnF2, leading to both reduction of the oxidation and the selective complexation of SnIV through the formation of SnCl4 (Figure 5 c). These results prove that hard Lewis bases like chloride and fluoride can block the formation of SnIV in the solution and their introduction into the perovskite film through two different mechanisms: complexation of SnIV and antioxidative character. Our findings agree with previous reports on reducing SnIV by the addition of SnF2[ 23 , 28 , 30 ] or SnCl2[ 16 , 17 ] and suggest that many of the other additives employed in literature for tin halide perovskites may work in the same fashion.
Figure 5.
a) Fresh and aged solutions of FASnI3 in DMSO with 10 % SnF2, SnCl2, SnBr2 and SnI2. An arrow points out the difference in darkening as an effect of the ageing treatment. 119Sn‐NMR spectra of b) 10 % SnF2‐ and c) 10 % SnCl2‐containing FASnI3 solution before and after being aged. SnF4 and SnCl4 solutions spectra are added as indicative of the species formed under the ageing process.
To confirm that fluoride was responsible for these changes in solution, we prepared FASnI3 solutions containing other fluoride‐based compounds. Unfortunately, other common species (i.e. CsF and NaF) had limited solubility in common solvents. Therefore we had to saturate the FASnI3 solution below a 5 % molar ratio (Figure S12). Even though the concentration was lower than for SnF2, we observed the exact change in color from orange to pale yellow, potentially affecting perovskite subunits in solution in the same fashion. Similarly, these solutions experienced no darkening of solutions aged at 100 °C for 3 h in different conditions, proving the complexation of SnIV in the form of SnF4. Even though these particular additives may not be directly implementable due to the strong influence that Cs+ and Na+ cations can have in the perovskite solar cells processing and performance, these results confirm the universality of the working principle for fluoride‐based compounds. Furthermore, they suggest that SnF2 additive may be eventually replaceable by other fluoride‐based species if applied in the right conditions.
To complete the study, we wanted to investigate how these changes in perovskite solution properties affect the thin film formation and the corresponding solar cells performance. We fabricated pristine FASnI3 films and with 10 % of the excess of FAI, SnI2, SnBr2, SnCl2 and SnF2. Excess FAI was tried to study both stoichiometry sides of FASnI3. While there was no notable change in the X‐ray diffraction patterns (Figure S13), the scanning electron microscopy (SEM) results offered some differences among samples (Figure S14). The sample with 10 % SnI2 excess was the only one showing a high density of extensive pinholes. In contrast, the film with 10 % SnF2 was the most homogeneous one, free of pinholes and other minor irregularities that are present in the rest of the films, agreeing with previous papers that used SnF2 on its beneficial effect on morphology.[ 22 , 25 , 28 ]
There is an evident change in the resulting grain size with the changing halide element (Figure S15). Fluoride led to the smallest average grain size (568 nm) compared to chloride (629 nm) and bromide (623 nm). These results are orthogonal to those reported previously, where fluoride [25] and chloride [17] were said to increase the perovskite crystals’ grain size. However, tin halide perovskites’ sensitive nature implies that significant changes can be expected from minor modifications in the perovskite composition or processing. Therefore, the effect of halides introduction can vary from study to study. Also, stoichiometry in pure FASnI3 perovskite strongly affects the pinhole density and the average grain size. The largest size was found for equimolar FASnI3 (695 nm), which went down when increasing or decreasing the SnI2 ratio (647 and 568 nm, respectively). Moreover, SnF2 addition shows an impact on the size distribution itself, which is significantly narrowed to plain FASnI3 thin film. These observations agree with the results by SAXS, assuming that a uniformly nearly oriented attachment in solution leads to a more homogeneous distribution of the grain sizes in the film.
We then used these films for solar cells fabrication (more details in the Supporting Information) to investigate any possible trend between additives and performance. In this sense, adding a small portion of tin halides to FASnI3 solutions seems beneficial for the device performance, showing some positive trend when moving to lower size halides (Figure S16). Though it appears that smaller halides—harder Lewis bases—work better by having a more decisive influence in the processing, chloride was the exception. Even though NMR and SAXS found SnCl2 to have very similar behavior to SnF2, the resulting devices yielded no efficiency, suggesting that chloride brings other factors into play. Previous works point out how chloride can be incorporated in the lattice and its tendency to form massive aggregates,[ 16 , 17 ] making its application not as trivial as SnF2 and requiring a more careful optimization. We suspect SnCl2 could mimic SnF2 to some extent in these solutions if the processing conditions are adjusted accordingly. It is also worth noting the slight improvement in efficiency produced just by using a 1,1:1 SnI2:FAI stoichiometry (i.e. 10 % SnI2 excess), despite the content of irregularly sized pores (Figure S14). This matches well with the results in previous works,[ 11 , 32 ] proving the importance of providing a Sn‐rich environment in the film.
Conclusion
SnF2 is a widely used additive for tin and lead/tin halide perovskites, systematically showing the same beneficial effects in all reported studies: perovskite films with lower SnIV content and improved morphology. We uncovered the different roles of fluoride in SnF2 on SnIV complexation and colloidal arrangement in the precursor solution. By studying the fluoride chemistry in perovskite solutions and films with different complementary techniques, we demonstrated that the fluoride in SnF2 has a critical role in reducing SnIV content in the precursor solution and the final perovskite film. We showed by NMR the selective complexation of SnIV in the form of SnF4, which HAXPES revealed to have a lower tendency to get introduced in the film than SnI4. Moreover, we showed how the introduction of SnF2 in perovskite solutions increases their stability against the oxidation caused by DMSO. This antioxidative character was also found for SnCl2, meaning that many other reported additives for tin halide perovskites may also block SnII oxidation by simple tuning of solution properties. Apart from reducing SnIV content in the thin film, SAXS measurements on the related precursor solutions evidenced that fluoride alters the essential formation of pre‐organized perovskite clusters. We identified an advanced colloidal arrangement in DMF compared to the DMSO solutions that are notably influenced by the addition of SnF2. We assigned this arrangement to an advanced nucleation process in DMF compared to DMSO. Finally, based on our findings, we proposed a nucleation mechanism that occurs in solution and is affected by the SnF2 addition resulting in improved overall crystal quality. In this sense, the effect of SnF2 on the film processing will be strongly determined by the environment in which it is applied (i.e. solvent, perovskite composition). Consequently, there is an immediate need to fundamentally understand and optimize solution properties, their processing and studying the effect of additives. As we are doing in this study with the example of SnF2 as pioneering work and impulse for further research. Overall, we presented a complete comprehensive picture of the working mechanism of SnF2 in tin halide perovskites processing and provided the community with the guidelines for finding new additives with specific chemical properties to selectively complex SnIV species and regulate the crystallization.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
The authors thank HZB for the allocation of synchrotron radiation beamtime for HAXPES experiments. We like to thank the PTB for the ability to use their facilities at BESSY II to carry out SAXS measurements. Further, we acknowledge Uwe Keiderling for providing a suitable software for data treatment. M.F. acknowledges the PhD program of University of Potsdam. G.L. would like to acknowledge China Scholarship Council (CSC) for financial support (Grant No. 201906150131). The authors acknowledge the support of the joint Research School HyPerCells of Helmholtz‐Zentrum Berlin and University of Potsdam. Open access funding enabled and organized by Projekt DEAL.
J. Pascual, M. Flatken, R. Félix, G. Li, S.-H. Turren-Cruz, M. H. Aldamasy, C. Hartmann, M. Li, D. Di Girolamo, G. Nasti, E. Hüsam, R. G. Wilks, A. Dallmann, M. Bär, A. Hoell, A. Abate, Angew. Chem. Int. Ed. 2021, 60, 21583.
Contributor Information
Dr. Jorge Pascual, Email: jorge.mielgo@helmholtz-berlin.de.
Prof. Antonio Abate, Email: antonio.abate@helmholtz-berlin.de.
References
- 1.Best Research-Cell Efficiencies. NREL, accessed May 8, 2021.
- 2. Li J., Cao H.-L., Jiao W.-B., Wang Q., Wei M., Cantone I., Lü J., Abate A., Nat. Commun. 2020, 11, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abate A., Joule 2017, 1, 659–664. [Google Scholar]
- 4. Jiang X., Zang Z., Zhou Y., Li H., Wei Q., Ning Z., Acc. Mater. Res. 2021, 2, 210–219. [Google Scholar]
- 5. Nasti G., Abate A., Adv. Energy Mater. 2020, 10, 1902467. [Google Scholar]
- 6. Konstantakou M., Stergiopoulos T., J. Mater. Chem. A 2017, 5, 11518–11549. [Google Scholar]
- 7. Saidaminov M. I., Spanopoulos I., Abed J., Ke W., Wicks J., Kanatzidis M. G., Sargent E. H., ACS Energy Lett. 2020, 5, 1153–1155. [Google Scholar]
- 8. Pascual J., Nasti G., Aldamasy M. H., Smith J. A., Flatken M., Phung N., Di Girolamo D., Turren-Cruz S.-H., Li M., Dallmann A., Avolio R., Abate A., Mater. Adv. 2020, 1, 1066–1070. [Google Scholar]
- 9. He X., Wu T., Liu S., Wang Y., Meng X., Wu J., Noda T., Yang X., Moritomo Y., Segawa H., Han L., J. Mater. Chem. A 2020, 8, 2760–2768. [Google Scholar]
- 10. Ricciarelli D., Meggiolaro D., Ambrosio F., De Angelis F., ACS Energy Lett. 2020, 5, 2787–2795. [Google Scholar]
- 11. Di Girolamo D., Pascual J., Aldamasy M. H., Iqbal Z., Li G., Radicchi E., Li M., Turren-Cruz S.-H., Nasti G., Dallmann A., De Angelis F., Abate A., ACS Energy Lett. 2021, 6, 959–968. [Google Scholar]
- 12. Gu F., Ye S., Zhao Z., Rao H., Liu Z., Bian Z., Huang C., Sol. RRL 2018, 2, 1800136. [Google Scholar]
- 13. Li W., Li J., Li J., Fan J., Mai Y., Wang L., J. Mater. Chem. A 2016, 4, 17104–17110. [Google Scholar]
- 14. Gupta S., Cahen D., Hodes G., J. Phys. Chem. C 2018, 122, 13926–13936. [Google Scholar]
- 15. Kumar M. H., Dharani S., Leong W. L., Boix P. P., Prabhakar R. R., Baikie T., Shi C., Ding H., Ramesh R., Asta M., Graetzel M., Mhaisalkar S. G., Mathews N., Adv. Mater. 2014, 26, 7122–7127. [DOI] [PubMed] [Google Scholar]
- 16. Marshall K. P., Walker M., Walton R. I., Hatton R. A., Nat. Energy 2016, 1, 16178. [Google Scholar]
- 17. Tai Q., Guo X., Tang G., You P., Ng T.-W., Shen D., Cao J., Liu C.-K., Wang N., Zhu Y., Lee C.-S., Yan F., Angew. Chem. Int. Ed. 2019, 58, 806–810; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 816–820. [Google Scholar]
- 18. Liao Y., Liu H., Zhou W., Yang D., Shang Y., Shi Z., Li B., Jiang X., Zhang L., Quan L. N., Quintero-Bermudez R., Sutherland B. R., Mi Q., Sargent E. H., Ning Z., J. Am. Chem. Soc. 2017, 139, 6693–6699. [DOI] [PubMed] [Google Scholar]
- 19. Shao S., Liu J., Portale G., Fang H.-H., Blake G. R., ten Brink G. H., Koster L. J. A., Loi M. A., Adv. Energy Mater. 2018, 8, 1702019. [Google Scholar]
- 20. Li M., Zuo W.-W., Yang Y.-G., Aldamasy M. H., Wang Q., Turren-Cruz S.-H., Feng S.-L., Saliba M., Wang Z.-K., Abate A., ACS Energy Lett. 2020, 5, 1923–1929. [Google Scholar]
- 21. Sabba D., Mulmudi H. K., Prabhakar R. R., Krishnamoorthy T., Baikie T., Boix P. P., Mhaisalkar S., Mathews N., J. Phys. Chem. C 2015, 119, 1763–1767. [Google Scholar]
- 22. Liao W., Zhao D., Yu Y., Grice C. R., Wang C., Cimaroli A. J., Schulz P., Meng W., Zhu K., Xiong R.-G., Yan Y., Adv. Mater. 2016, 28, 9333–9340. [DOI] [PubMed] [Google Scholar]
- 23. Gupta S., Bendikov T., Hodes G., Cahen D., ACS Energy Lett. 2016, 1, 1028–1033. [Google Scholar]
- 24. Handa T., Yamada T., Kubota H., Ise S., Miyamoto Y., Kanemitsu Y., J. Phys. Chem. C 2017, 121, 16158–16165. [Google Scholar]
- 25. Zhao Z., Gu F., Li Y., Sun W., Ye S., Rao H., Liu Z., Bian Z., Huang C., Adv. Sci. 2017, 4, 1700204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee S. J., Shin S. S., Im J., Ahn T. K., Noh J. H., Jeon N. J., Seok S. I., Seo J., ACS Energy Lett. 2018, 3, 46–53. [Google Scholar]
- 27. Gupta S., Hodes G., SN Appl. Sci. 2019, 1, 1066. [Google Scholar]
- 28. Koh T. M., Krishnamoorthy T., Yantara N., Shi C., Leong W. L., Boix P. P., Grimsdale A. C., Mhaisalkar S. G., Mathews N., J. Mater. Chem. A 2015, 3, 14996. [Google Scholar]
- 29. Milot R. L., Klug M. T., Davies C. L., Wang Z., Kraus H., Snaith H. J., Johnston M. B., Herz L. M., Adv. Mater. 2018, 30, 1804506. [DOI] [PubMed] [Google Scholar]
- 30. Hartmann C., Gupta S., Bendikov T., Kozina X., Kunze T., Félix R., Hodes G., Wilks R. G., Cahen D., Bär M., ACS Appl. Mater. Interfaces 2020, 12, 12353–12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yokoyama T., Song T. B., Cao D. H., Stoumpos C. C., Aramaki S., Kanatzidis M. G., ACS Energy Lett. 2017, 2, 22–28. [Google Scholar]
- 32. Marshall K. P., Walton R. I., Hatton R. A., J. Mater. Chem. A 2015, 3, 11631–11640. [Google Scholar]
- 33. Li N., Tao S., Chen Y., Niu X., Onwudinanti C. K., Hu C., Qiu Z., Xu Z., Zheng G., Wang L., Zhang Y., Li L., Liu H., Lun Y., Hong J., Wang X., Liu Y., Xie H., Gao Y., Bai Y., Yang S., Brocks G., Chen Q., Zhou H., Nat. Energy 2019, 4, 408–415. [Google Scholar]
- 34. Nakamura T., Yakumaru S., Truong M. A., Kim K., Liu J., Hu S., Otsuka K., Hashimoto R., Murdey R., Sasamori T., Kim H. D., Ohkita H., Handa T., Kanemitsu Y., Wakamiya A., Nat. Commun. 2020, 11, 3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Birchall T., Dénès G., Can. J. Chem. 1984, 62, 591–595. [Google Scholar]
- 36. Lanzetta L., Webb T., Zibouche N., Liang X., Ding D., Min G., Westbrook R. J. E., Gaggio B., Macdonald T. J., Islam M. S., Haque S. A., Nat. Commun. 2021, 12, 2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stranick M. A., Moskwa A., Surf. Sci. Spectra 1993, 2, 45. [Google Scholar]
- 38. Hoste S., Willeman H., Van De Vondel D., Van Der Kelen G. P., J. Electron Spectrosc. Relat. Phenom. 1974, 5, 227. [Google Scholar]
- 39. Karim M. M. S., Ganose A. M., Pieters L., Leung W. W. W., Wade J., Zhang L., Scanlon D. O., Palgrave R. G., Chem. Mater. 2019, 31, 9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Padova P., Fanfoni M., Larciprete R., Mangiantini M., Priori S., Perfetti P., Surf. Sci. 1994, 313, 379. [Google Scholar]
- 41. Breßler I., Kohlbrecher J., Thünemann A. F., J. Appl. Crystallogr. 2015, 48, 1587–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hamill J. C., Schwartz J., Loo Y. L., ACS Energy Lett. 2018, 3, 92–97. [Google Scholar]
- 43. Raghuwanshi V. S., Ochmann M., Hoell A., Polzer F., Rademann K., Langmuir 2014, 30, 6038. [DOI] [PubMed] [Google Scholar]
- 44. Niederberger M., Cölfen H., Phys. Chem. Chem. Phys. 2006, 8, 3271–3287. [DOI] [PubMed] [Google Scholar]
- 45. Liu Y., Geng H., Qin X., Yang Y., Zeng Z., Chen S., Lin Y., Xin H., Song C., Zhu X., Li D., Zhang J., Song L., Dai Z., Kawazoe Y., Matter 2019, 1, 690–704. [Google Scholar]
- 46. De Yoreo J. J., Gilbert P. U. P. A., Sommerdijk N. A. J. M., Penn R. L., Whitelam S., Joester D., Zhang H., Rimer J. D., Navrotsky A., Banfield J. F., Wallace A. F., Michel F. M., Meldrum F. C., Cölfen H., Dove P. M., Science 2015, 349, aaa6760. [DOI] [PubMed] [Google Scholar]
- 47. Heo J. H., Kim J., Kim H., Moon S. H., Im S. H., Hong K. H., J. Phys. Chem. Lett. 2018, 9, 6024–6031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information