Abstract
Introduction
We conducted six cross‐sectional nationwide questionnaire studies among all patients with hemophilia in the Netherlands from 1972 until 2019 to assess how health outcomes have changed, with a special focus on patients >50 years of age.
Methods
Data were collected on patient characteristics, treatment, (joint) bleeding, joint impairment, hospitalizations, human immunodeficiency virus and hepatitis C infections, and general health status (RAND‐36).
Results
In 2019, 1009 patients participated, of whom 48% had mild, 15% moderate, and 37% severe hemophilia. From 1972 to 2019, the use of prophylaxis among patients with severe hemophilia increased from 30% to 89%. Their median annual bleeding rate decreased from 25 to 2 bleeds. Patients with severe hemophilia aged <16 years reported joint impairment less often over time, but in those aged >40 years joint status did not improve. In 2019, 5% of all 1009 patients were positive for the human immunodeficiency virus. The proportion of patients with an active hepatitis C infection drastically decreased from 45% in 2001 to 2% in 2019 due to new anti‐hepatitis C treatment options. Twenty‐five percent had significant liver fibrosis even after successful therapy. Compared to the general male population, patients aged >50 years reported much lower scores on the RAND‐36, especially on physical functioning.
Discussion/Conclusion
Our study shows that increased use of prophylactic treatment and effective hepatitis C treatment have improved joint health and nearly eradicated hepatitis C infection in patients with hemophilia in the Netherlands. However, patients still suffer from hemophilia‐related complications, especially patients aged >50 years.
Keywords: bleeding, clinical outcomes, hemophilia, joint damage, quality of life
Essentials.
We conducted six consecutive studies among patients with hemophilia in the Netherlands.
The annual bleeding rate and the level of joint impairment have decreased dramatically.
Furthermore, almost all previously hepatitis C virus‐‐infected patients have been successfully treated.
However, there is still significant morbidity, especially among patients over 50 years of age.
1. INTRODUCTION
Hemophilia is a hereditary X‐linked bleeding disorder, characterized by a lack of functional coagulation factor VIII (FVIII; hemophilia A) or IX (FIX; hemophilia B). Patients with severe hemophilia suffer from spontaneous bleeds in joints/muscles, leading to disability. Patients with moderate/mild hemophilia mainly develop bleeds after trauma or surgery. 1
Effective treatment was lacking before the 1970s, and most patients with severe hemophilia lived with serious physical disabilities, and only survived until childhood or early adulthood due to bleeding in vital organs (with intracranial bleeds being especially common). 2 , 3 The introduction of cryoprecipitate and subsequently coagulation factor concentrates greatly improved survival.
Transmission of human immunodeficiency virus (HIV) and hepatitis C virus (HCV) through contaminated coagulation factor products during the 1980s led to many deaths. 4 New viral inactivation techniques were introduced from 1985 onward that eliminated the contamination risk after 1990. During this time, the first treatment for HIV and HCV became widely available. 3 Also, the first national consensus‐based treatment guidelines were established. 5
Around the 2000s, hemophilia treatment in the Netherlands was gradually centralized. From 2013 onward, a standard set of quality criteria was introduced for comprehensive hemophilia treatment centers. 6 Additionally, the national consensus‐based treatment guidelines from 1987 were revised in 2009 to harmonize treatment practices. 7 Last, treatment with direct‐acting antivirals became available for all HCV‐infected patients in 2014. 8
Along with these developments, the life expectancy of patients with hemophilia is increasing. 9 Elderly patients are now increasingly experiencing age‐related diseases that require a more tailored approach. Additionally, as elderly patients age, the effect of bleeding‐induced arthropathy on daily life may worsen despite adequate treatment.
From 1972 until 2019, six nation‐wide surveys have been performed to assess the health status of the Dutch hemophilia population. 10 , 11 , 12 In this study we evaluated health outcomes of patients during the past five decades of hemophilia treatment, with a special focus on the health status of aging patients with hemophilia >50 years of age.
2. METHODS
2.1. Study design
In 2019, a cross‐sectional study was performed among all patients with congenital hemophilia in the Netherlands. The current study was preceded by five surveys in 1972, 1978, 1985, 1992, and 2001. 10 , 11 , 12 All patients registered at one of the six national hemophilia treatment centers were invited to participate. The first five surveys consisted of a questionnaire. The current study consisted of a questionnaire, as well as clinical data collection from medical records and sampling of blood and urine. For the current analysis, only data derived from the questionnaires and medical records were used. From June 2018 until July 2019, questionnaires were sent to patients by e‐mail or regular mail, followed by two reminders. The study was approved in 2018 by the Medical Ethics committee at Leiden University Medical Center. Informed consent was obtained from all patients.
2.2. Measurements
Of the 2019 study participants, information on age, severity of hemophilia, HIV status, HCV status, and inhibitor status was obtained from electronic health records. When electronic health record data were missing, answers from the questionnaire were used if available. In case of discrepancies between the electronic health records and questionnaire, data from the electronic health records were used. All other 2019 data were obtained from the questionnaire only.
The following patient characteristics were collected: age, type and severity of hemophilia, and family history of hemophilia. Hemophilia severity was categorized as severe (<0.01 IU/ml), moderate (0.01–0.05 IU/ml), or mild (>0.05–0.40 IU/ml). The following treatment characteristics were collected: treatment modality (prophylactic treatment or on‐demand treatment), the annual coagulation factor consumption, and the type of coagulation factor product.
The questionnaires contained the following self‐reported outcomes: annual (joint) bleeding rate, level of joint impairment, orthopedic interventions, hospital admission rate and duration of stay, HIV status, HCV status, age‐related co‐morbidities and general health status.
2.3. Definition of outcome variables
Prophylaxis was defined as periodic infusion of coagulation factor products to prevent bleeding. Annual coagulation factor consumption was defined as the total number of units of coagulation factor used divided by bodyweight per year (IU/kg/year). The annual (joint) bleeding rate was defined as the number of self‐reported (joint) bleeds in the preceding 12 months. In children, the annual (joint) bleeding rate was based on the results of the last 3 months, which was then multiplied by four.
Joint impairment was calculated using a point system; no joint impairment (0 points), mild impairment (no daily problems, 1 point), moderate impairment (daily problems, 2 points), or severe impairment (no movement in joint, 3 points). This information was reported for the knee, elbow, ankle, and wrist joints. Hospital admission was defined as having been admitted to the hospital in the preceding 12 months for at least 1 day (day admissions were included). Hospital duration was calculated as the number of nights spent in the hospital (day admissions were excluded). Age‐related co‐morbidities were defined as being treated by a medical specialist or a general practitioner for a set of age‐related conditions (see Table S1 in supporting information for the full list).
Inhibitor status was based on the Bethesda unit (BU) assay, using each center’s own cut‐off level, which varied from >0.6 BU to >1.0 BU. A current inhibitor was defined as being currently inhibitor‐positive. A past inhibitor was defined as having been inhibitor‐positive in the past but currently inhibitor‐negative. HIV status was reported for patients treated with coagulation factor before 1985 and was defined as positive if the patient had a confirmed clinical diagnosis of HIV. HCV status was reported for patients treated with coagulation factor before 1992. Patients were classified as having a “past infection” when they had a confirmed clinical diagnosis of HCV infection in the past and “current infection” if they were currently HCV‐RNA positive.
General health status was assessed in adults using the RAND 36‐Item Health Survey (RAND‐36). 13 The RAND‐36 is a 36‐item questionnaire that measures perceived health status across eight different domains: physical functioning, social functioning, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well‐being, energy/fatigue, bodily pain, and general health perceptions. Domain scores were calculated when a patient had completed at least half of the items of a domain according to RAND‐36 scoring instructions. 14 Domain scores were converted to a 100‐point scale. Based on a review of the literature, a difference of 4 points on any RAND‐36 domain between groups was regarded as clinically significant. 15 Scores were compared to RAND‐36 scores of the Dutch general male population. 16
2.4. Statistical analysis
Descriptive statistics were reported as mean/standard deviation, median/interquartile range (IQR), or as proportions. Treatment characteristics and health outcomes were summarized and compared across all six surveys stratified by age or severity of hemophilia. Patients with missing data for a given analysis were excluded.
To measure the response rate, the total number of unique patients registered at each hemophilia treatment center was retrieved. This was done by anonymizing and then merging patient data of all registered patients. A trusted third party (ZorgTTP) was responsible for the process of anonymization and merging of data.
3. RESULTS
3.1. Response and patient characteristics
From 1972–2019 the number of participants in the questionnaire varied from 447 to 1009 patients (Table 1) In the latest study 2192 patients were invited to participate, of whom 33% had severe hemophilia, 13% had moderate hemophilia, and 54% had mild hemophilia (Table 2). Of these, 1312 patients participated in at least one part of the study (by filling in the questionnaire, consenting to the use of their clinical data, or both); 1009 patients completed the questionnaire (a response rate of 46%). Of these 1009, 729 patients also consented to the use of their clinical data. Response rates of the previous questionnaires were 84% in 1972, 70% in 1978, 81% in 1985, 78% in 1992, 68% in 2001. 10 , 11 , 12
TABLE 1.
Characteristics of participants in the Hemophilia in the Netherlands studies obtained from questionnaire data
|
1972 (N = 447) |
1978 (N = 560) |
1985 (N = 935) |
1992 (N = 980) |
2001 (N = 1066) |
2019 (N = 1009) |
|
|---|---|---|---|---|---|---|
| Mean age in years (range) a | 21 (0–47) | 23 (0–70) | 27 (0–85) | 30 (0–84) | 35 (0–90) | 40 (0–88) |
| Severity of hemophilia (%) | ||||||
| Severe | 159 (36) | 245 (44) | 384 (41) | 387 (39) | 420 (39) | 378 (37) |
| Moderate | 83 (19) | 106 (19) | 175 (19) | 173 (18) | 176 (17) | 149 (15) |
| Mild | 172 (38) | 138 (25) | 376 (40) | 420 (43) | 470 (44) | 482 (48) |
| Type of hemophilia (%) b | ||||||
| Hemophilia A | 377 (84) | 481 (86) | 801 (86) | 853 (87) | 925 (87) | 867 (87) |
| Hemophilia B | 70 (16) | 79 (14) | 134 (14) | 127 (13) | 141 (13) | 129 (13) |
| Family history of hemophilia (%) c | ||||||
| Negative | 112 (25) | 128 (23) | 237 (25) | 195 (20) | 246 (23) | 168 (18) |
| Positive | 335 (75) | 432 (77) | 698 (75) | 785 (80) | 820 (77) | 753 (82) |
| HIV infection (%) d | ||||||
| Current infection | — | — | 36 (4) | 55 (8) | 29 (5) | 22/412 (5) e |
| Hepatitis C infection (%) f | ||||||
| Current infection | — | — | — | — | 344 (45) | 8/412 (2) g |
| Past infection | — | — | — | — | 97 (13) | 226/412 (55) g |
| Inhibitory antibodies (%) h | ||||||
| Ever inhibitors | — | — | 31/384 (8) | 51/388 (13) | 52/420 (13) | 66/361 (19) i |
| Current inhibitors | — | — | 19 (5) | 29 (7) | 15 (4) | 6/361 (2) i |
| Past inhibitors | — | — | 12 (3) | 22 (6) | 37 (9) | 60/361 (17) i |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Age was unknown for 8 patients.
Type of hemophilia was unknown for 13 patients.
Family history of hemophilia was unknown for 88 patients.
Reported for patients treated with coagulation factor before 1985.
HIV status was unknown for 4 patients.
Reported for patients treated with coagulation factor before 1992.
HCV status was unknown for 84 patients.
Reported for patients with severe hemophilia.
Inhibitor status was unknown for 17 patients.
TABLE 2.
Comparison of age distribution and severity hemophilia of the 2019 HiN‐6 study with the Dutch hemophilia population
|
Dutch hemophilia population a (N = 2192) |
2019 HiN−6 study (N = 1009) |
|
|---|---|---|
| Age category (%) | ||
| 0–17 years | 446 (21) | 196 (20) |
| 18–25 years | 254 (12) | 108 (11) |
| 26 years or older | 1436 (67) | 697 (70) |
| Missing | 56 b | 8 |
| Severity of hemophilia (%) | ||
| Severe | 704 (33) | 378 (37) |
| Moderate | 282 (13) | 149 (15) |
| Mild | 1148 (54) | 482 (48) |
| Missing | 58 b | 0 |
Abbreviation: HiN‐6, Haemophilia in the Netherlands‐6 study.
All patients who were registered at a hemophilia treatment center in the Netherlands.
56 patients from one treatment center had missing data for age and severity of hemophilia. Furthermore, two patients from another treatment center had evaluable data for age but not for severity of hemophilia.
Table 1 shows the patient characteristics of each survey. Of the 1009 patients, 378 (37%) had severe hemophilia, 149 (15%) moderate hemophilia, and 482 (48%) mild hemophilia. The mean age of participants increased from 21 years in 1972 to 40 years in 2019. During this period the mean age of the Dutch male population increased from 32 years to a similar mean age of 41 years. 17
3.2. Treatment characteristics
From 1972–2019, the proportion of patients with severe hemophilia receiving prophylactic treatment increased from 30% to 89%. In 2019, almost all (98%) patients aged 0 to 16 years were on prophylaxis (Table 3, Figure 1A). Also, 25% of patients aged 0 to 16 years with moderate hemophilia were on prophylactic treatment. As expected only 3% of patients aged 0 to 16 years with mild hemophilia were treated with prophylaxis. The median age at initiation of prophylaxis in patients with severe hemophilia decreased from 8 years (range: 0–15) in 1978 to 3 years (range: 0–79) in 2019 (Table 3). Median annual coagulation factor consumption (in IU/kg) for patients with severe hemophilia on prophylaxis increased from 886 IU/kg (IQR: 632–1259) in the 1970s 18 to 2535 IU/kg (IQR: 1885–3614) in 2019.
TABLE 3.
Prophylaxis usage, annual bleeding rates, and hospital admission
|
1972 (N = 447) |
1978 (N = 560) |
1985 (N = 935) |
1992 (N = 980) |
2001 (N = 1066) |
2019 (N = 1009) |
|
|---|---|---|---|---|---|---|
| Severe hemophilia | N = 159 | N = 245 | N = 384 | N = 387 | N = 420 | N = 378 |
| Patients on prophylaxis (%) | ||||||
| Children, 0–16 y | 22/65 (34) | 41/91 (45) | 69/111 (62) | 64/92 (70) | 112/130 (86) | 93/95 (98) |
| Young adults, 17–25 y | 12/39 (31) | 27/54 (50) | 43/72 (60) | NR | 38/42 (90) | 42/46 (91) |
| Adults, older than 25 y | 8/57 (14) | 28/99 (28) | 71/201 (35) | 119/232 (51) | 134/248 (54) | 193/228 (85) |
| Median age at first prophylaxis, years (range) | NR | 8 (0–15) | 5 (1–15) | NR | 2 (0 −11) | 3 (0–79) |
| Median ABR a by age (range, IQR) | ||||||
| Children, 0–16 y | 20 (0–98) | 20 (0–70) | 10 (0–65) | 10 (0–98) | 5 (0–51) | 4 (0–228, 0–12) |
| Young adults, 17–25 y | 20 (0–98) | 17 (0–100) | 10 (0–90) | 10 (0–98) | 6 (0–75) | 1 (0–12, 0–2) |
| Adults, older than 25 y | 14 (0–97) | 15 (0–100) | 10 (0–90) | 10 (0–82) | 7 (0–75) | 2 (0–100, 0–6) |
| Hospital admissions | ||||||
| Hemophilia patients (%) | 51 | 38 | 25 | 22 | 22 | 73/330 (22) |
| Median duration, (range) | 28 (2–252) | 20 (1–180) | 11 (1–100) | 5 (1–330) | 7 (1–89) | 7 (1–125) |
| Moderate hemophilia | N = 23 | N = 106 | N = 175 | N = 173 | N = 176 | N = 149 |
| Patients on prophylaxis (%) | ||||||
| Children, 0–16 y | 6/41 (15) | 9/41 (22) | 7/59 (12) | 7/41 (17) | 7/46 (15) | 6/24 (25) |
| Young adults, 17–25 y | 4/14 (29) | 7/26 (27) | 1/19 (5) | NR | 4/23 (17) | 4/19 (21) |
| Adults, older than 25y | 1/27 (4) | 4/39 (10) | 10/97 (10) | 11/98 (11) | 10/107 (9) | 14/104 (13) |
| Median ABR a by age (range, IQR) | ||||||
| Children, 0–16 y | 4 (0–40) | 10 (0–104) | 3 (0–66) | 7 (0–33) | 2 (0–57) | 4 (0–32, 0–8) |
| Adults, older than 17 y | 4 (0–50) | 5 (0–100) | 2 (0–40) | 3 (0–52) | 1 (0–71) | 1 (0–100, 0–2) |
| Hospital admissions | ||||||
| Hemophilia patients (%) | 51 | 27 | 23 | 22 | 15 | 21/136 (15) |
| Median duration (range) | 17 (2–180) | 10 (1–50) | 7 (1–50) | 5 (1–72) | 6 (1–31) | 6 (1–120) |
| Mild hemophilia | N = NR | N = NR | N = NR | N = NR | N = NR | N = 482 |
| Patients on prophylaxis (%) | ||||||
| Children, 0–16 y | NR | NR | NR | NR | NR | 2/68 (3) |
| Young adults, 17‐25 y | NR | NR | NR | NR | NR | 1/43 (2) |
| Adults, older than 25 y | NR | NR | NR | NR | NR | 7/346 (2) |
| Median ABR a by age (range, IQR) | ||||||
| Children, 0–16 y | NR | NR | NR | NR | NR | 4 (0–100, 0–14) |
| Young adults, 17–25 y | NR | NR | NR | NR | NR | 0 (0–88, 0–1) |
| Adults, older than 25 y | NR | NR | NR | NR | NR | 0 (0–40, 0–0.5) |
| Hospital admissions | ||||||
| Hemophilia patients (%) | NR | NR | NR | NR | NR | 103/415 (25) |
| Median duration (range) | NR | NR | NR | NR | NR | 5 (1–175) |
Abbreviations: ABR, annual bleeding rate; IQR, interquartile range; NR, not reported.
Annual bleeding rate.
FIGURE 1.
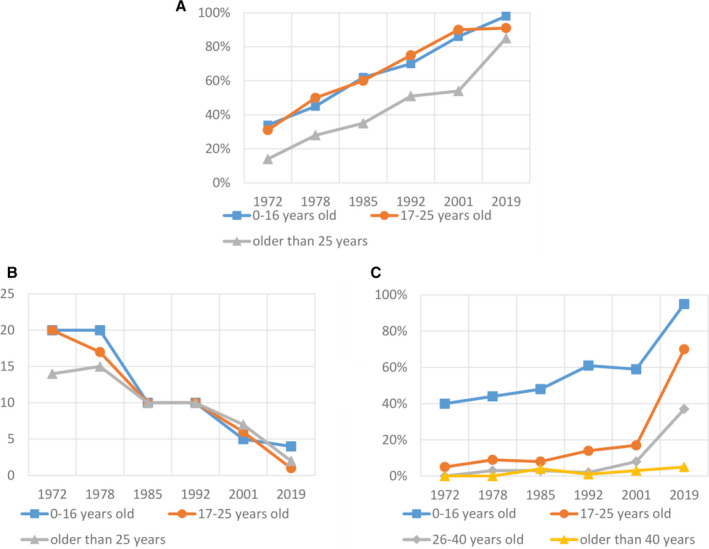
Health and treatment outcomes over time. A, Proportion of patients with severe hemophilia on prophylactic treatment, from 1972 to 2019, stratified by age. B, Median annual bleeding rate of patients with severe hemophilia, from 1972 to 2019, stratified by age. C, Self‐reported absence of joint impairment in ankles, knees, and elbows in patients with severe hemophilia, from 1972 to 2019, stratified by age
In 2019, only 5 out of 827 patients (1%) were treated with a plasma‐derived coagulation factor product. In patients with hemophilia A, 48 out of 724 (7%) were treated with extended half‐life FVIII products. Among patients with hemophilia B, 30 out of 103 (29%) used extended half‐life FIX products. Six out of 724 patients with hemophilia A (1%) were treated with emicizumab, three of which were patients with an active inhibitor.
3.3. Treatment outcomes, 1972–2019
3.3.1. Annual bleeding rates
Since 1972, the median annual bleeding rate (ABR) of patients with severe hemophilia decreased from 25 to 2 bleeds (Figure 1B). In 2019, the highest ABR (4 bleeds) was reported by patients in the youngest age group, aged 0 to 16 years (Table 3 and Figure 1B). The same ABR was reported in 0 to 16‐year‐olds with moderate and mild hemophilia. The vast majority were nosebleeds (55%). For comparison, only 6% of bleeds were classified as nosebleeds in patients >25 years.
In patients with severe hemophilia on prophylaxis, 125 out of 285 patients (44%, 95% confidence interval [CI]: 38–50%) had at least one joint bleed in the past year (Table 4). The median annual joint bleeding rate (AJBR) in 2019 for patients with severe hemophilia <25 years was 0 (n 118, IQR 0–0), in both patients treated on‐demand (n 4) or on prophylactic treatment (n 113; Table 4). In patients with mild hemophilia (n 417) and moderate hemophilia (n 128), the AJBR in 2019 was 0 (IQR 0–0) for all age groups (Table 4). In patients with severe hemophilia with an active inhibitor the AJBR was 6 (n 5, IQR 0–12) versus 0 (n 52, IQR 0–3) in patients with a previously cleared inhibitor and 0 (n 259, IQR 0–3) in non‐inhibitor patients (Table 4). The median AJBR was the same (zero) for both patients with severe hemophilia A and patients with severe hemophilia B (Table S6 in supporting information).
TABLE 4.
Self‐reported annualized joint bleeding rates in 2019
| N of patients | Median annual joint bleeding rate (range, IQR) | |
|---|---|---|
| Severe hemophilia 378 a | ||
| Overall | 322 | |
| Children, 0–16 y | 78 | 0 (0–12, 0–0) |
| Young adults, 17–25 y | 40 | 0 (0–6, 0–1) |
| Adults, older than 25 y | 204 | 2 (0–70, 0–4) |
| Patients on prophylactic treatment | ||
| Children, 0–16 y | 77 | 0 (0–12, 0–0) |
| Young adults, 17–25 y | 36 | 0 (0–6, 0–1) |
| Adults, older than 25 y | 172 | 2 (0–70, 0–4) |
| Patients on prophylactic treatment with at last one joint bleed | ||
| 0 bleeds | 160 | NA |
| ≥ 1 bleeds | 125 | NA |
| Patients treated on‐demand | ||
| Children, 0–16 y | 1 | 0 (0–0, 0–0) |
| Young adults, 17–25 y | 3 | 0 (0–0, 0–0) |
| Adults, older than 25 y | 32 | 1.5 (0–50, 0–6) |
| Inhibitory antibodies | ||
| Never inhibitor‐positive | 259 | 0 (0–50, 0–3) |
| Currently inhibitor‐positive | 5 | 6 (0–15, 0–12) |
| Previously inhibitor‐positive | 52 | 0 (0–70, 0–3) |
| Moderate hemophilia | 149 b | |
| Children, 0–16 y | 23 | 0 (0–4, 0–0) |
| Adults, older than 17 y | 105 | 0 (0–20, 0–1) |
| Mild hemophilia | 482 c | |
| Children, 0–16 y | 59 | 0 (0–0, 0–0) |
| Young adults, 17–25 y | 40 | 0 (0–4, 0–0) |
| Adults, older than 25 y | 313 | 0 (0–25, 0–0) |
Abbreviations: IQR, interquartile range; NA, not applicable.
Annualized joint bleeding rate was unknown for 56 patients with severe hemophilia.
Annualized joint bleeding rate was unknown for 21 patients with moderate hemophilia.
Annualized joint bleeding rate was unknown for 65 patients with mild hemophilia.
3.3.2. Joint impairment
Between 1972–2019, there was an increase in patients with severe hemophilia with no joint impairment in the ankles, elbows, and knees (Figure 1C, Table S2 in supporting information). The proportion of patients reporting no joint impairment changed between 1972and 2019 from 40% to 95% in patients aged 0 to 16 years, from 5% to 70% in patients aged 17 to 25 years, and from 3% to 37% in patients 25 to 40 years old. In patients >40 years, there were none without joint impairment in 1972, and this percentage did not improve much, only 5% in 2019. In patients with moderate hemophilia, a similar, but less pronounced, trend was seen over time (Table S2). In 2019, the proportion of patients with mild hemophilia with an absence of joint impairment ranged from 98% among the 0 to 16‐year‐olds to 87% in the 40+ group (Table S2). Patients with severe hemophilia B had similar joint impairment and instances of joint replacement surgery as patients with severe hemophilia A (Table S6).
3.3.3. Hospital admissions
The proportion of patients with severe hemophilia requiring hospitalization in the previous year decreased from 51% in 1972 to 22% in 2019 (Table 3). The hospital admission rate in patients with mild hemophilia (25%) and severe hemophilia (22%) was similar (Table 3) However, hospitalization for a non‐hemophilia‐related problem was more common in patients with mild hemophilia (29%) than in patients with severe hemophilia (17%).
3.3.4. Inhibitor development, HIV status, and HCV status
The percentage of patients with severe hemophilia A or B with a past or current inhibitor increased from 8% in 1985 to 19% in 2019 (Table 1). In 2019, 21% and 7% of patients with severe and mild hemophilia A, respectively, reported having a past or current inhibitor.
HIV was first reported in 1985 when 4% of patients were HIV infected. Among still‐living patients treated with coagulation factor before 1985, the prevalence of HIV increased to 8% in 1992 and afterward decreased to 5% in 2019. Currently, out of 412 patients who were treated with coagulation factor before 1985, 22 are HIV‐positive (Table 1). HCV infections among patients treated with coagulation factor before 1992 were common in the year 2001 with 45% of patients reporting to have an active HCV infection. In 2019, 8 (2%) patients had an active HCV infection (Table 1).
3.3.5. Self‐reported general health status
There were no clinically relevant differences in reported general health status measured using the RAND‐36 between the 2001 cohort and the 2019 cohort (Table 5). Compared to the Dutch general population, the 2019 cohort scored lower on all domains, except for emotional well‐being (2019 cohort score: 77.1, general population score: 77.9) and role limitations due to personal or emotional problems (2019 cohort score: 85.0, general population score: 85.8; Table 5). Patients under 50 years of age had scores similar to the general population, except for the domain of energy/fatigue (2019 cohort <50 score: 65.6, general population score: 71.6).
TABLE 5.
General health status of patients in HiN‐5 cohort, overall HiN‐6 cohort, HiN‐6 cohort >50 years, and Dutch general male population
| Domains of the RAND 36‐Item Health Survey | HiN−5 (2001) |
HiN−6 (2019) |
HiN−6 (≤50) | HiN−6 (>50) |
HiN−6 (>50) severe hemophilia |
HiN−6 (>50) non‐severe hemophilia |
General Male Population (all ages) 16 |
|---|---|---|---|---|---|---|---|
| N | 623 | 706–757 a | 368–398 a | 339–358 a | 101–108 a | 236–250 a | ‐ |
| Physical functioning, mean (SD) | 75.8 (29) | 77.9 (27.5) | 87.5 (20.1) | 67.1 (30.5) | 43.0 (27.3) | 77.5 (25.6) | 88.3 (21) |
| Social functioning, mean (SD) | 82.0 (24) | 83.3 (20.8) | 86.1 (20.2) | 80.3 (21.2) | 77.0 (20.8) | 81.7 (21.2) | 87.5 (20) |
| Role limitations (physical health problems), mean (SD) | 73.3 (40) | 76.4 (37.5) | 83.6 (32.6) | 68.4 (40.9) | 52.9 (43.7) | 75.1 (37.9) | 83.3 (32) |
| Role limitations (personal/emotional problems), mean (SD) | 83.4 (34) | 85.0 (31.4) | 87.7 (28.3) | 82.1 (34.3) | 77.3 (36.5) | 84.1 (33.2) | 85.8 (30) |
| Emotional well‐being, mean (SD) | 76.9 (18) | 77.1 (15.6) | 77.7 (14.5) | 76.4 (16.7) | 75.5 (17.7) | 76.8 (16.3) | 77.9 (17) |
| Energy/fatigue, mean (SD) | 67.1 (20) | 64.7 (17.7) | 65.6 (17.0) | 63.7 (18.3) | 60.0 (18.3) | 65.3 (18.2) | 71.6 (18) |
| Bodily pain, mean (SD) | 78.8 (24) | 77.4 (22.6) | 82.0 (21.0) | 72.3 (23.3) | 64.9 (21.8) | 75.5 (23.2) | 83.5 (23) |
| General health perception, mean (SD) | 67.0 (23) | 64.5 (22.3) | 69.1 (21.8) | 59.6 (21.7) | 54.7 (21.5) | 61.7 (21.4) | 72.9 (20) |
Abbreviations: HiN, Haemophilia in the Netherlands study; SD, standard deviation.
Scores for each domain were calculated if a participant had completed at least half of the items of that domain. Therefore, the total of number of participants for which a score was calculated differs per domain.
3.4. Current health status of patients older than 50 years
3.4.1. Bleeding rate and joint impairment
Only 4% of older patients with severe hemophilia had no joint impairment in the ankles, elbows, and/or knees versus 75% of patients with non‐severe hemophilia (Table 6). In addition, 75% of older patients with severe hemophilia had undergone orthopedic surgery and the mean number of lifetime orthopedic interventions was 1.9 (Table 6). Twenty percent of older patients had joint impairment in their wrists; this number increased to 48% in patients with some knee impairment.
TABLE 6.
Health outcomes in patients with hemophilia over 50 years old
| <50 (N = 613) | 50+, overall (N = 388) | 50+, severe hemophilia (N = 115) | 50+, non‐severe hemophilia (N = 273) | |
|---|---|---|---|---|
| Median annual bleeding rate | 613 | 388 | 115 | 273 |
| Rate (IQR) | 1 (0–228) | 0 (0–100) | 3 (0–100) | 0 (0–100) |
| Missing | 109 | 45 | 14 | 31 |
| Median annual joint bleeding rate | 613 | 388 | 115 | 273 |
| Rate (IQR) | 0 (0–70) | 0 (0–70) | 2 (0–70) | 0 (0–25) |
| Missing | 95 | 44 | 14 | 30 |
| Hospital admissions (%) | 613 | 388 | 115 | 273 |
| No | 419 (82) | 261 (72) | 86 (77) | 175 (69) |
| Yes | 93 (18) | 103 (28) | 26 (23) | 77 (31) |
| Missing | 101 | 24 | 3 | 21 |
| d Duration of hospital stay in days | 66 | 83 | 25 | 58 |
| Median (range) | 5 (1–80) | 6 (1–175) | 7 (1–125) | 5 (1–175) |
| Missing | 5 | 2 | 0 | 2 |
| Joint impairment (%) | 613 | 388 | 115 | 273 |
| Some impairment | 123 (25) | 153 (47) | 96 (96) | 57 (25) |
| No impairment | 376 (75) | 175 (53) | 4 (4) | 171 (75) |
| Missing | 114 | 60 | 15 | 45 |
| Orthopedic surgery in the past, any type (%) | 613 | 388 | 115 | 273 |
| No | 280 (82) | 219 (60) | 28 (25) | 191 (76) |
| Yes | 63 (18) | 145 (40) | 84 (75) | 61 (24) |
| Missing | 270 | 24 | 3 | 21 |
| Orthopedic surgery in the past, joint replacement surgery (%) | 343 | 364 | 112 | 252 |
| No | 325 (95) | 274 (75) | 46 (41) | 228 (90) |
| Yes | 18 (5) | 90 (25) | 66 (59) | 24 (10) |
| Orthopedic surgery in the past, arthrodesis (%) | 343 | 364 | 112 | 252 |
| No | 327 (95) | 311 (85) | 72 (64) | 239 (95) |
| Yes | 16 (5) | 53 (15) | 40 (36) | 13 (5) |
| Orthopedic surgery in the past, synovectomy (%) | 343 | 364 | 112 | 252 |
| No | 334 (97) | 346 (95) | 100 (89) | 246 (98) |
| Yes | 9 (3) | 18 (5) | 12 (11) | 6 (2) |
| Number of orthopedic interventions | 343 | 364 | 112 | 252 |
| Mean (SD) | 0.4 (0.9) | 0.9 (1.4) | 1.9 (1.5) | 0.5 (1.1) |
| Missing | 2 | 4 | 1 | 3 |
| c HIV status (%) | 136 | 280 | 108 | 172 |
| Negative | 126 (93%) | 264 (95%) | 95 (88%) | 169 (100%) |
| Positive | 9 (7%) | 13 (5%) | 13 (12%) | 0 (0%) |
| Missing | 1 | 3 | 0 | 3 |
| a HCV status (%) | 198 | 298 | 108 | 190 |
| Always HCV‐negative | 85 (51) | 93 (38) | 3 (3) | 90 (62) |
| Past infection | 80 (48) | 146 (60) | 92 (93) | 54 (37) |
| Current infection | 2 (1) | 6 (2) | 4 (4) | 2 (1) |
| Missing | 31 | 53 | 9 | 44 |
| HCV treatment among HCV‐positive patients (%) | 82 | 152 | 96 | 56 |
| No | 12 (15) | 23 (15) | 11 (12) | 12 (22) |
| Yes | 67 (85) | 126 (85) | 83 (88) | 43 (78) |
| Missing | 3 | 3 | 2 | 1 |
| Last treatment (%) | 167 | 126 | 83 | 43 |
| DAA | 15 (28) | 28 (25) | 19 (26) | 9 (24) |
| DAA +RBV | 2 (4) | 24 (21) | 13 (18) | 11 (29) |
| DAA +RBV + PEG‐IFN | 3 (6) | 5 (4) | 4 (5) | 1 (3) |
| PEG‐IFN +RBV | 19 (35) | 24 (21) | 16 (22) | 8 (21) |
| IFN +RBV | 11 (20) | 21 (19) | 13 (18) | 8 (21) |
| IFN | 4 (7) | 10 (9) | 9 (12) | 1 (3) |
| Missing | 13 | 14 | 19 | 5 |
| b Liver fibrosis/cirrhosis (%) | 82 | 152 | 96 | 56 |
| No significant fibrosis (<9.5 kPa) | 32 (91) | 56 (75) | 37 (76) | 19 (73) |
| Significant fibrosis (9.5 −12.4 kPa) | 1 (3) | 7 (9) | 4 (8) | 3 (12) |
| Cirrhosis (>12.4 kPa) | 2 (6) | 12 (16) | 8 (16) | 4 (15) |
| Missing | 47 | 77 | 47 | 30 |
Abbreviations: DAA, direct acting antivirals; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IFN, interferon; PEG‐IFN, pegylated‐interferon; RBV, ribavirin; SD, standard deviation.
Reported for 298 patients >50 years treated with coagulation factor before 1992.
Based on FibroScan measurements.
Reported for 280 patients >50 years treated with coagulation factor before 1985.
Reported for patients that stayed at least one night in the hospital (day admissions were excluded).
3.4.2. HCV status
Among older patients who were treated with coagulation factor products before 1992, 62% were currently or previously infected with HCV. Among patients with severe hemophilia, 97% were currently or previously infected (Table 6). Overall, only 2% of older patients were currently HCV‐positive (Table 6).
Eighty‐five percent of older patients had received antiviral treatment in the past. Half of these were treated with older treatment methods (interferon, peg‐interferon, and/or ribavirin), while the other half were treated with direct‐acting antiviral drugs (Table 6). Among patients who were or had been HCV‐positive, 25% had clinically significant liver fibrosis or cirrhosis (Table 6).
3.4.3. Self‐reported general health status
Patients >50 years scored substantially lower on the RAND‐36 than the Dutch general population and younger patients with hemophilia (Table 5). Patients with severe hemophilia reported even more pronounced limitations, especially on the domains of physical functioning and role limitations due to physical health problems (Table 5). Emotional well‐being scores of older patients were similar to those of the general population (Table 5).
3.4.4. Age‐related co‐morbidities
Among 367 patients >50 years, the most common age‐related co‐morbidities were hypertension (37%), hypercholesterolemia (17%), malignancies (13%), and type 2 diabetes (10%; see Table S1). The prevalence of hypertension was even higher in patients with severe hemophilia (47%).
4. DISCUSSION
We evaluated clinical outcomes in patients with hemophilia in the Netherlands from 1972–2019 using a series of six national questionnaires. The same outcome definitions were used for all questionnaires, enabling direct comparison of different cohorts over time. Bleeding rate and joint impairment decreased dramatically. Furthermore, HCV has almost been eradicated.
The prevalence of hemophilia in the Netherlands was 25.5 cases per 100,000 males, which is higher than reported previously, 6 , 19 but similar to a recent estimate of the birth prevalence (29.6 cases per 100,000 live male births). 20 The higher prevalence is most likely due to the high level of care increasing survival, 9 as well as improved diagnosis and registration of patients with previously undetected mild hemophilia over time (Table 1). Although our reported prevalence is high, it is still lower than the reported birth prevalence, 20 indicating the presence of unregistered patients with mild hemophilia and/or excess mortality due to hemophilia.
4.1. Change in health outcomes, 1972–2019
The annual bleeding rate has decreased due to more prophylaxis usage and higher dosing schemes, enabling children with hemophilia to participate safely in sports (which improves muscle function and quality of life 21 ). Over time, factor consumption in patients on prophylaxis has increased, from 886 IU/kg/year in the 1970s, 18 1514 IU/kg/year in the 1980s, 18 1880 IU/kg/year in the 1990s, 18 and finally 2534 IU/kg in the 2010s. Despite coagulation factor accounting for >90% of total treatment costs, 22 , 23 direct comparisons of prophylactic dosing schemes are scarce. A previous study showed that a high‐dose protocol (4000 IU/kg per year) only marginally improved outcomes compared to an intermediatedose protocol (2100 IU/kg per year), while being 66% more expensive. 24 Our results seem to confirm that intermediate‐dose prophylaxis can lead to good joint outcomes.
The median ABR was highest in the 0–16 group (4 bleeds). However, joint bleeds were rare and most bleeds were nosebleeds, which were far less common in adults. Among non‐hemophilic males, the prevalence of epistaxis is also highest in children, 25 and is commonly caused by irritation due to digital trauma. 26
The median AJBR for patients with severe hemophilia on prophylaxis was zero. Still, 44% of patients (95%CI: 38%–50%) had at least one joint bleed, leaving room for improvement. This is similar to a report from the UK (another high‐income country), which found that in 2018, between 32.5% and 59.9% of patients on prophylaxis still reported at least one joint bleed per year. 27 Details on the cause/severity of joint bleeds were not available.
The hospital admission rate in patients with severe hemophilia after 1985 was 22%, which is higher than for Dutch men (9.8% in 1986 to 8.6% in 2017). 28 The hospital admission rate in patients with mild hemophilia was similarly high (25%). Interestingly, the proportion of hospitalizations for non‐hemophilic problems was higher in mild hemophilia (29%) than in severe hemophilia (17%). The reason for hospitalization was not included in the questionnaire and similar studies to compare our results with were not available.
Unlike patients <40 years, patients >40 years did not improve in joint function over time. This is due to accrued irreversible joint damage (in a period of time when there was no treatment or when it was still suboptimal). There is some evidence that hemophilia A and B differ in their clinical phenotype. 29 In the 2019 cohort, patients with severe hemophilia and A and B reported roughly similar bleeding and joint outcomes. However, given the small sample size, no conclusion can be drawn from these results.
The proportion of patients with severe hemophilia (A or B) with a past or current inhibitor increased from 8% in 1985 to 19% in 2019. Among patients with severe hemophilia A the percentage is 20%, which is low compared to most clinical trials. 1 In contrast, a US study reported that between 1998 and 2011, 11.5% to 17.0% of patients with severe hemophilia (A or B) had a past or current inhibitor. 30 The increasing prevalence over time may be due to more low‐titer inhibitors being detected. 31 Also, due to lower sensitivity tests and less testing in the past, some low‐titer inhibitors in patients in the 1980s/1990s would have been missed (this probably also explains the similarly low inhibitor prevalence in the US study).
General health status did not change meaningfully from 2001 to 2019. The probability of not detecting a meaningful improvement is unlikely as the RAND‐36 questionnaire is reported to be sensitive to changes in health over time. 32 Similar results have been reported by several European studies. 33 , 34 , 35 A possible explanation for this may be response shift, which is defined as a change in the meaning of one’s self‐evaluation of quality of life as a result of changes in internal standards, values, and the conceptualization of quality of life. 36 Persons with hemophilia may have changed their internal standards over time: while their health has deteriorated (e.g., as a result of recurrent bleeding), their previous idea of a bad health status may have been lower than what they currently experience.
4.2. Current health status of older patients
The prevalence of joint replacement surgery among patients with severe hemophilia of all ages was high (30%), which is in line with an earlier Dutch study (31%). 37 For comparison, a UK study reported a prevalence of 5% for joint replacement surgery among males >60 years. 38 Eighty‐four percent of patients with knee impairment also reported having wrist problems. This most likely due to these patients putting more weight on their hands when standing up, in order to alleviate their knees.
Mental health status among patients age 50+ patients appeared to be similar to that of the Dutch general male population, both in the 2001 survey, 39 and in the 2019 survey, which is in agreement with several other studies. 40 , 41 , 42 The high level of mental health might be due to adequate hemophilia treatment in a multidisciplinary care setting.
Although HCV has almost been eradicated, 25% of cured patients still have moderate‐to‐severe liver fibrosis. Follow‐up of these patients is warranted as they remain at increased risk for complications. 43
4.3. Limitations
Reported study response rates have decreased over time (from 84% in 1972, to 46% in 2019). The burden of participating in multiple studies (which is becoming more common), as well as the requirement of a hospital visit may have dissuaded some. However, participation rates in previous studies may have been overestimated, as evidenced by the high prevalence of hemophilia in 2019 (25.5 cases per 100,000 males). Despite lower participation, the 2019 cohort was similar to the Dutch hemophilia population with regard to age distribution and severity of hemophilia (Table 2). Therefore, the results are likely to be highly generalizable. Nevertheless, non‐response bias cannot be ruled out. Patients who participated in the questionnaire might have been more adherent to treatment, which would have skewed results in a more positive direction.
Differentiating between joint bleeds and flare‐ups of chronic arthropathy is difficult. 44 Therefore, the bleeding rate in patients with significant hemophilic arthropathy is probably slightly overestimated. The annual bleeding rate in children was based on the results of the last 3 months, multiplied by four. This may have artificially increased bleeding rates due to recall bias.
The RAND‐36 reference values were obtained from a validation study from 1992 to 1996 16 and may not be representative of the current Dutch population. Yet, RAND‐36 domain scores were shown to remain relatively stable over a time period of almost 20 years. 45 In addition, age‐specific domain scores were not available, so domain scores of the overall population (mean age: 43.1) were used for comparisons with the hemophilia cohort.
Last, patients tend to underreport co‐morbidities. 46 This might explain the higher prevalence of hypertension reported by other studies. 47 , 48
5. CONCLUSION
Even though the increase in prophylactic treatment, coagulation factor dosage, and centralization of care has improved outcomes, many patients with severe hemophilia still experience joint bleeds and report decreased physical health. Many older patients with severe hemophilia suffer from severe painful joint impairment, which greatly decreases quality of life. This emphasizes the need for personalized treatment focusing on bleed control, adequate pain management, and timely reference to an orthopedic surgeon or physiatrist. 49 With the increased use of novel treatment options and expected further health gains, regular measurements of patient‐relevant outcomes may identify areas for improvement and directions for further research.
In conclusion, our study shows that bleeding rates, joint health, and HCV cure rates have strongly improved over the past five decades. However, there are still opportunities for improvement.
CONFLICTS OF INTEREST
S. Hassan, E.C. van Balen, C. Smit, E.P. Mauser, E.A.M. Beckers, L. Hooimeijer, P.F. Ypma, L. Nieuwenhuizen, S.E.M. Schols, M.H. Driessens, and F.R. Rosendaal have no conflicts of interest to disclose. L.F.D. van Vulpen received a research grant form CSL Behring, and is a consultant for Sobi and Tremeau. All fees go to the institution. J. Eikenboom received research support from CSL Behring and he has been a teacher on educational activities of Roche. M. Coppens has received financial support for research from Bayer, CSL Behring, Daiichi Sankyo, Portola/Alexion, Roche, Sanquin Blood Supply, and UniQure and consultancy or lecturing fees from Bayer, CSL Behring, Medcon International, MEDtalks, NovoNordisk, Pfizer, and Sobi. F.W.G. Leebeek received unrestricted research grants from CSL Behring, Takeda, uniQure, and Sobi; is consultant for uniQure, Novo Nordisk, Biomarin, and Takeda, of which the fees go to the institution; and has received a travel grant from Sobi. He is also a DSMB member for a study by Roche. J.G. van der Bom has been a teacher on the educational activities of Bayer. S.C. Gouw has received unrestricted research grants from Sobi.
AUTHOR CONTRIBUTIONS
S. Hassan, E.C. van Balen, and S.C. Gouw developed the protocol, collected the data, and analyzed the data. E.P. Mauser, L.F.D. van Vulpen, J. Eikenboom, E.A.M. Beckers, L. Hooimeijer, P.F. Ypma, L. Nieuwenhuizen, M. Coppens, S.E.M. Schols, and F.W.G. Leebeek were involved in the development of the protocol, provided data from participating hemophilia centers, and reviewed the manuscript. M.H. Driessens was involved the development of the protocol and provided feedback on the manuscript. C. Smit, J.G. van der Bom, F.R. Rosendaal, and S.C. Gouw initiated and coordinated the research project, supervised data collection, and the final interpretation of the data. All authors reviewed and approved the final version.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank all patients who participated in this study, data managers K.M. van Beurden and E.M. Taal, trial coordinator V. Schmidt, and all health‐care professionals at the hemophilia treatment centers who were involved in this study.
Hassan S, van Balen EC, Smit C, et al. Health and treatment outcomes of patients with hemophilia in the Netherlands, 1972–2019. J Thromb Haemost. 2021;19:2394–2406. 10.1111/jth.15424
Manuscript handled by: Jill Johnsen
Final decision: Jill Johnsen, 07 June 2021
Funding information
This study was funded by an unrestricted grant from the Dutch Ministry of Health, Welfare and Sport (Dutch: Ministerie van Volksgezondheid, Welzijn en Sport; VWS).
DATA AVAILABILITY STATEMENT
For original data, please contact S.C. Gouw@lumc.nl.
REFERENCES
- 1. Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388:187‐197. [DOI] [PubMed] [Google Scholar]
- 2. Larsson SA. Life expectancy of Swedish haemophiliacs, 1831–1980. Br J Haematol Br J Haematol. 1985;59:593‐602. [DOI] [PubMed] [Google Scholar]
- 3. Franchini M, Mannucci PM. The history of hemophilia. Semin Thromb Hemost. 2014;40:571‐576. [DOI] [PubMed] [Google Scholar]
- 4. Triemstra M, Rosendaal FR, Smit C, Van Der Ploeg HM, Briët E. Mortality in Patients with Hemophilia: Changes in a Dutch Population from 1986 to 1992 and 1973 to 1986. Ann Intern Med. 1995;123(11):823. [DOI] [PubMed] [Google Scholar]
- 5. Nederlandse vereniging van hemofiliebehandelaren i.o. CBO. Consensus bijeenkomst. Behandeling van Hemofilie. 1987.
- 6. Leebeek FWG, Fischer K. Quality of haemophilia care in the Netherlands: New standards for optimal care. Blood Transfus SIMTI Servizi Sri. 2014;12(suppl 3):s501‐s504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leebeek FWG, Mauser‐Bunschoten EP. Nederlandse‐Vereniging‐voor‐Hemofiliebehandelaars‐(NVHB); Richtlijn Diagnostiek en behandeling van hemofilie en aanverwante hemostasestoornissen. Van Zuiden Commun BV 2009.
- 8. Berden FA, Kievit W, Baak LC, et al. Dutch guidance for the treatment of chronic hepatitis C virus infection in a new therapeutic era. Neth J Med. 2014;72:388‐400. [PubMed] [Google Scholar]
- 9. Hassan S, Monahan RC, Mauser‐Bunschoten EP, et al. Mortality, life expectancy, and causes of death of persons with hemophilia in the Netherlands 2001–2018. J Thromb Haemost Wiley. 2021;19(3):645‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Plug I, Van Der Bom JG, Peters M, et al. Thirty years of hemophilia treatment in the Netherlands, 1972–2001. Blood Blood. 2004;104:3494‐3500. [DOI] [PubMed] [Google Scholar]
- 11. Triemstra AHM, Smit C, Ploeg HM, Briët E, Rosendaal FR. Two decades of haemophilia treatment in the Netherlands, 1972–92. Haemophilia. 1995;1(3):165‐171. [DOI] [PubMed] [Google Scholar]
- 12. Smit C, Rosendaal FR, Varekamp I, et al. Physical condition, longevity, and social performance of Dutch haemophiliacs, 1972–85. BMJ. 1989;298:235‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hays RD, Sherbourne CD, Mazel RM. The RAND 36‐Item Health Survey 1.0. Heal Econ. 1993;2:217‐227. [DOI] [PubMed] [Google Scholar]
- 14. van der Zee KI , Sanderman R. Het meten van de algemene gezondheidstoestand met de RAND‐36 : een handleiding. 2e druk. Groningen: Noordelijk Centrum voor Gezondheidsvraagstukken, NCG; 2012.
- 15. Hays RD, Morales LS. The RAND‐36 measure of health‐related quality of life. Ann Med Royal Soc Med Press Ltd. 2001;33(5):350‐357. [DOI] [PubMed] [Google Scholar]
- 16. Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF‐36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51:1055‐1068. [DOI] [PubMed] [Google Scholar]
- 17. Centraal Bureau voor de Statistiek. Bevolking; Kerncijfers [Dataset].
- 18. Fischer K, van der Bom JG , Mauser‐Bunschoten EP, et al. Changes in treatment strategies for severe haemophilia over the last 3 decades: effects on clotting factor consumption and arthropathy. Haemophilia. 2001;7:446‐452. [DOI] [PubMed] [Google Scholar]
- 19. Stonebraker JS, Bolton‐Maggs PHB, Michael Soucie J, Walker I, Brooker M. A study of variations in the reported haemophilia A prevalence around the world. Haemophilia Haemophilia. 2010;16:20‐32. [DOI] [PubMed] [Google Scholar]
- 20. Iorio A, Stonebraker JS, Chambost H, et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a meta‐analytic approach using national registries. Ann Intern Med. 2019;171:540‐546. [DOI] [PubMed] [Google Scholar]
- 21. Zetterberg E, Ljungkvist M, Salim M. Impact of Exercise on Hemophilia. Semin Thromb Hemost. 2018;44:787‐795. [DOI] [PubMed] [Google Scholar]
- 22. Neufeld EJ, Sidonio RF, O’Day K, Runken MC, Meyer K, Spears J. Cost analysis of plasma‐derived factor VIII/von Willebrand factor versus recombinant factor VIII for treatment of previously untreated patients with severe hemophilia A in the United States. J Med Econ Taylor and Francis Ltd. 2018;21:762‐769. [DOI] [PubMed] [Google Scholar]
- 23. O’Hara J, Hughes D, Camp C, Burke T, Carroll L, Diego DAG. The cost of severe haemophilia in Europe: the CHESS study. Orphanet J Rare Dis BioMed Central Ltd. 2017;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fischer K, Carlsson KS, Petrini P, et al. Intermediate‐dose versus high‐dose prophylaxis for severe hemophilia: Comparing outcome and costs since the 1970s. Blood The American Society of Hematology. 2013;122:1129‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tunkel DE, Anne S, Payne SC, et al. Clinical Practice Guideline: Nosebleed (Epistaxis) Executive Summary. Otolaryngol Head Neck Surg. 2020;162(1):8‐25. [DOI] [PubMed] [Google Scholar]
- 26. Fatakia A, Winters R, Amedee RG. Epistaxis: A common problem. Ochsner Journal. Ochsner Clinic, L.L.C. and Alton Ochsner Medical Foundation; 2010. p. 176‐8. [PMC free article] [PubMed]
- 27. Wilkins R, Stephensen D, Siddle H, Scott M, Palmer B, Chapman G, Richards M, Walwyn R, Redmond A. Prevalence of Haemarthrosis and Clinical Impact on The Musculoskeletal System in People With Haemophilia in The United Kingdom; Evaluation of UK National Haemophilia Database and Haemtrack Patient Reported Data. Hua Xiang UKHCDO National Haemophilia Database, Manchester Elizabeth Horn Leeds haemophilia Comprehensive Care Centre, Leeds Teaching Hospitals NHS trust, Leeds Teaching Hospitals NHS trust, Leeds. 2020.
- 28. Centraal Bureau voor de Statistiek. Ziekenhuisopnamen en ‐patiënten; diagnose‐indeling VTV .
- 29. Castaman G, Matino D. Hemophilia A and B: molecular and clinical similarities and differences. Haematologica. 2019;104(9):1702‐1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mazepa MA, Monahan PE, Baker JR, Riske BK, Soucie JM. Men with severe hemophilia in the United States: Birth cohort analysis of a large national database. Blood American Society of Hematology. 2016;127:3073‐3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van den Berg HM , Hashemi SM, Fischer K, et al. Increased inhibitor incidence in severe haemophilia A since 1990 attributable to more low titre inhibitors. Thromb Haemost Schattauer GmbH. 2016;115:729‐737. [DOI] [PubMed] [Google Scholar]
- 32. Stafford M, Stansfeld S, Shipley M, Marmot M, Hemingway H. Is the SF‐36 a valid measure of change in population health? Results from the Whitehall II study. BMJ British Medical Journal Publishing Group. 1997;315:1273‐1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gringeri A, Mantovani LG, Scalone L, Mannucci PM. Cost of care and quality of life for patients with hemophilia complicated by inhibitors: The COCIS study group. Blood Blood. 2003;102:2358‐2363. [DOI] [PubMed] [Google Scholar]
- 34. Solovieva S. Clinical severity of disease, functional disability and health‐related quality of life. Three‐year follow‐up study of 150 Finnish patients with coagulation disorders. Haemophilia Haemophilia. 2001;7:53‐63. [DOI] [PubMed] [Google Scholar]
- 35. Lindvall K, Von Mackensen S, Berntorp E. Quality of life in adult patients with haemophilia ‐ a single centre experience from Sweden. Haemophilia Haemophilia. 2012;18:527‐531. [DOI] [PubMed] [Google Scholar]
- 36. Sprangers MAG, Schwartz CE. Integrating response shift into health‐related quality of life research: a theoretical model. Soc Sci Med. 1999;48(11):1507‐1515. [DOI] [PubMed] [Google Scholar]
- 37. Den Uijl IEM, Roosendaal G, Fischer K. Insufficient evidence to suggest less stringent therapy in hemophilia B? Blood. 2009;114(23):4907. [DOI] [PubMed] [Google Scholar]
- 38. Steel N, Melzer D, Gardener E, McWilliams B. Need for and receipt of hip and knee replacement ‐ A national population survey. Rheumatology (Oxford). 2006;45:1437‐1441. [DOI] [PubMed] [Google Scholar]
- 39. Plug I, Peters M, Mauser‐Bunschoten EP, et al. Social participation of patients with hemophilia in the Netherlands. Blood. 2008;111:1811‐1815. [DOI] [PubMed] [Google Scholar]
- 40. Negri L, Buzzi A, Aru AB, et al. Perceived well‐being and mental health in haemophilia. Psychol Heal Med. 2020;25(9):1062‐1072. [DOI] [PubMed] [Google Scholar]
- 41. Ingvorsen EB, Schnohr C, Andersen T, et al. “Development in well‐being and social function among Danish hemophilia patients with HIV: a three‐wave panel study spanning 24 years”. BMC Public Health. 2019;19(1):1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Curtis R, Baker J, Riske B, et al. Young adults with hemophilia in the U.S.: demographics, comorbidities, and health status. Am J Hematol. 2015;90(suppl 2):S11‐S16. [DOI] [PubMed] [Google Scholar]
- 43. Ioannou GN, Beste LA, Green PK, et al. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients With Baseline Cirrhosis or High FIB‐4 Scores. Gastroenterology W.B. Saunders. 2019;157:1264‐1278.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Timmer MA, Pisters MF, de Kleijn P , de Bie RA , Fischer K, Schutgens RE. Differentiating between signs of intra‐articular joint bleeding and chronic arthropathy in haemophilia: a narrative review of the literature. Haemophilia. 2015;21:289‐296. [DOI] [PubMed] [Google Scholar]
- 45. Jacobsen EL, Bye A, Aass N, et al. Norwegian reference values for the Short‐Form Health Survey 36: development over time. Qual Life Res. 2018;27:1201‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lucke T, Herrera R, Wacker M, et al. Systematic Analysis of Self‐Reported Comorbidities in Large Cohort Studies ‐ A Novel Stepwise Approach by Evaluation of Medication. PLoS One. 2016;11:e0163408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Holme PA, Combescure C, Tait RC, Berntorp E, Rauchensteiner S, de Moerloose P . Hypertension, haematuria and renal functioning in haemophilia ‐ a cross‐sectional study in Europe. Haemophilia Blackwell Publishing Ltd. 2016;22:248‐255. [DOI] [PubMed] [Google Scholar]
- 48. Sood SL, Cheng D, Ragni M, et al. A cross‐sectional analysis of cardiovascular disease in the hemophilia population. Blood Adv American Society of Hematology. 2018;2:1325‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shapiro S, Makris M. Haemophilia and ageing. Br J Haematol. 2019;184:712‐720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
For original data, please contact S.C. Gouw@lumc.nl.


