Abstract
Objective
Permanent pacemaker implantation (PPI) after surgical aortic valve replacement (SAVR) remains a frequent complication. Predictors, however, have been mainly investigated in single‐center studies. Therefore, nationwide data were used to identify patients—and procedural risk factors for postoperative PPI.
Materials and Methods
Data were retrospectively collected from the Netherlands Heart Registration (NHR). Patients enrolled in the NHR undergoing isolated SAVR from 2013 to 2019 were analyzed. Primary endpoint was in‐hospital PPI during hospitalization after SAVR.
Results
From the NHR database, 5600 patients with symptomatic aortic valve stenosis were included in the study. Crude incidence of post‐SAVR PPI was 4.0%. Backward regression analysis identified previous cardiac surgery (odds ratio [OR]: 1.80; 95% confidence interval [CI]: 1.18–2.76), extra‐corporeal circulation time (OR: 1.01; 95% CI: 1.00–1.01), vasopressor use (OR: 2.66; 95% CI: 1.79–3.96) and in‐hospital cardiac conduction abnormalities (OR: 4.48; 95% CI: 3.36–5.98) as potential predictors for PPI. Across the time, PPI after SAVR significantly increased (OR: 1.11; 95% CI: 1.03–1.21).
Conclusions
From this nationwide analysis, PPI after SAVR remains a low but increasingly frequent complication. Several predictive factors for postoperative PPI after SAVR have been identified and might be useful for patient informed consent about potential adverse event rate.
Keywords: aortic stenosis, permanent pacemaker implantation, surgical aortic valve replacement
Abbreviations
- NHR
Netherland Heart Registration
- PPI
permanent pacemaker implantation
- SAVR
surgical aortic valve replacement
1. INTRODUCTION
Aortic stenosis is the most common valvular disease in adult patients, occurring in 2%–4% of subjects 1 , 2 and surgical aortic valve replacement (SAVR) is still the treatment of choice in younger adults. 3 Despite technical progresses and the increasing use of sutureless and rapid‐deployment protheses, permanent pacemaker implantation (PPI) remains a well‐known complication which occurs in 2%–8% of patients undergoing SAVR. 4 , 5 , 6 , 7 , 8
Post‐SAVR PPI increases intensive care unit and hospital length of stay although with variable duration between institutions. 5 , 6 , 7 , 8 Additionally, PPI after SAVR has been shown to independently reduce the long‐term survival. 7 Consequently, it would be a substantial clinical benefit to identify preoperatively those patients at high‐risk for atrio‐ventricular conduction abnormalities, to facilitate postoperative care and to deliver exhaustive informed consent preoperatively. 8 , 9 , 10 , 11
Several studies have suggested risk factors for PPI after SAVR. Perioperative mechanical injuries to the conduction heart system close to the aortic valve as annulus debridement or suture placement, are perceived as important contributing factors. 5 , 6 , 7 , 8 Other factors, like reoperations, longer cross‐clamping times, absence of preoperative sinus rhythm, preoperative concomitant aortic regurgitation and, pre‐existing conduction disorders, have also been found to be predictors of PPI post SAVR. 9 , 10 However, our ability to identify patients who are at high‐risk of requiring PPI pre‐operatively remains unfortunately sparse 12 and mainly linked to single‐centers studies. 9 , 10 , 12 , 13 , 14
Therefore, the purpose of this study was to use a large national database to determine the contemporary incidence of early postoperative PPI in patients undergoing isolated SAVR through a multicenter investigation and to identify patient's criteria and procedural risk factors for PPI after SAVR in a nationwide registry.
2. METHODS
2.1. The Netherlands Heart Registration (NHR)
The NHR is a nationwide registry which registers fundamental pre‐, operative‐ as well as postprocedural‐, (including follow‐up)‐, data related to cardiac interventions performed in 16 Dutch heart surgery centers. The aim of the NHR is to evaluate current practice in the treatment of heart disease, through all stages of the management process, from the preoperative diagnosis and work‐up, up to several years after the intervention. Data collection and registration are performed by the participating centers in a secured online environment. For this study, information related to patients undergoing SAVR was collected. This study complies with the Declaration of Helsinki and the use of the data for these purposes was approved by the Maastricht University Medical Centre Ethical Committee (METC 2020‐1528).
2.2. Study population
From January 1, 2013 to January 1, 2019, 19546 adult patients (age ≥18 years) were hospitalized in the Netherlands with a diagnosis of aortic stenosis. From this patient cohort, 9900 underwent an isolated SAVR. Patients with cardiac congenital pathology and/or concomitant cardiac surgical procedure were excluded from the study. Patients with bicuspid aortic valve were included. Patients with ascertainment of PPI in the first 30 days postoperatively were included in the final analysis (n = 5600). The variable PPI within 30 days postoperative is not a mandatory variable within the NHR database. Only 9 of the 16 heart surgery centers have collected this variable.
2.3. Statistical analysis
Continuous variables are described as mean ± SD or median (range), depending on normality. Categorical variables were presented as frequencies with percentages. The variables were largely complete with less than 5% missing per variable.
To allow for inclusion of all patients for the regression analysis, we used stochastic regression imputation with fully conditional specification to impute the dataset. Imputations were drawn using predictive mean matching.
First, a univariable logistic regression analysis was performed, with PPI status as dependent variable. The following variables were considered potential predictors of PPI: sex, age, weight, creatinine serum level, diabetes mellitus, left ventricular ejection fraction, systolic pulmonary pressure, history of lung disease, peripheral vascular disease, previous cardiac surgery, recent myocardial infarction, dialysis, Euroscore II, atrial fibrillation, previous coronary surgery, previous valve surgery, previous aortic valve surgery, previous mitral valve surgery, previous aortic surgery, prosthetic valve type, extra‐corporeal circulation time, ischemic time, inotropic use, vasopressor use, peri‐operative myocardial infraction, in‐hospital respiratory insufficiency, and in‐hospital cardiac conduction abnormalities. Subsequently, a multivariable logistic regression analysis was carried out, once with all potential predictor variables (fully adjusted model), and once with backward stepwise elimination on all potential predictors, to arrive at a model with only significant independent predictors. Due to the explorative nature of the study, every variable from the univariate analysis were put in the multivariate model. Multicollinearity across variables was assessed. In addition, the trend of PPI over time was also analyzed by univariable regression analysis. A p value of less than .05 was considered statistically significant. All analyses were performed using SPSS v26 (IBM Corp).
3. RESULTS
3.1. Baseline characteristics and procedural outcomes
Between 2013 and 2019, 5600 patients were identified in the database as receiving a SAVR with an ascertainment of the postoperative PPI status. The flowsheet of the study sample is described in Figure 1. The crude incidence of postoperative PPI was 4.0% (n = 222 patients). Baseline characteristics of the included patients are listed in Table 1. The total study group included 57.9% men and the median log Euroscore II was 1.4 (0.9–2.2). The procedural characteristics and outcomes are reported in Table 2. No patients included in the final analysis received an implantable cardioverter‐defibrillator (ICD) device. Between 2013 and 2019, a significative trend for increasing PPI across the time (odds ratio [OR]: 1.11; 95% confidence interval [CI]: 1.03–1.21; p < .05) was observed. The PPI rate remains stable from 2013 to 2015 before reaching a peak in 2017 with a PPI rate of 6.0% (Figure 2).
Figure 1.
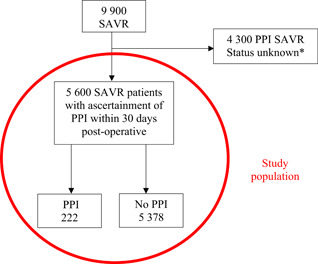
Flowchart of patients after surgical aortic valve replacement (SAVR) leading to permanent pacemaker implantation (PPI). *The variable PPI within 30 days postoperative is not a mandatory variable within the Netherlands Heart Registration database. Only 9 of the 16 heart surgery centers have collected this variable
Table 1.
Baseline characteristics of patients with or without a 30‐day permanent pacemaker implantation (PPI) after surgical aortic valve replacement (SAVR)
| Variables a | Total study group (n = 5 600) | PPI group (n = 222) | No PPI group (n = 5 378) |
|---|---|---|---|
| Male sex | 3 242 (57.9%) | 129 (58.1%) | 3 113 (57.9%) |
| Age, mean (SD), years | 68.2 (±10.2) | 68.6 (±10.5) | 68.1 (±10.2) |
| Weight, mean (SD), kg | 81.9 (±15.4) | 81.1 (±14.6) | 81.9 (±15.5) |
| Creatinine serum level, median (IQR), μmol/L | 82.0 (70.0–96.0) | 83.0 (71.0–97.0) | 82.0 (70.0–96.0) |
| History of lung disease | 699 (12.5%) | 24 (10.8%) | 675 (12.6%) |
| Peripheral vascular disease | 392 (7.0%) | 15 (6.8%) | 377 (7.0%) |
| Diabetes mellitus | 1 089 (19.5%) | 42 (19.6%) | 1 047 (19.7%) |
| Recent myocardial infarction | 69 (1.2%) | 4 (1.8%) | 65 (1.2%) |
| Dialysis | 20 (0.5%) | 0 (0%) | 20 (0.5%) |
| Log Euroscore II, median (IQR), % | 1.4 (0.9–2.2) | 1.8 (1.2–3.4) | 1.4 (0.9–2.1) |
| Atrial fibrillation | 413 (13.0%) | 18 (15.1%) | 395 (12.9%) |
| Left ventricular ejection fraction, median (IQR), % | 55.0 (55.0–60.0) | 55.0 (55.0–55.0) | 55.0 (55.0–60.0) |
| Systolic pulmonary pressure, median (IQR), mmHg | 25.0 (25.0–25.0) | 25.0 (25.0–25.0) | 25.0 (25.0–25.0) |
| Previous cardiac surgery | 489 (8.7%) | 40 (18.0%) | 449 (8.3%) |
| Previous coronary surgery | 180 (3.3%) | 12 (5.5%) | 168 (3.2%) |
| Previous valve surgery | 318 (5.8%) | 27 (12.3%) | 291 (5.5%) |
| Previous aortic valve surgery | 217 (5.1%) | 20 (14.2%) | 197 (4.8%) |
| Previous mitral valve surgery | 21 (0.6%) | 1 (0.9%) | 20 (0.6%) |
| Previous aortic surgery | 41 (0.9%) | 2 (1.0%) | 39 (0.8%) |
Note: Values are n (%).
Abbreviations: IQR, interquartile range; PPI, permanent pacemaker implantation.
Numbers are presented as valid percentage, excluding missing values.
Table 2.
Procedural characteristics of patients with or without a 30‐day permanent pacemaker implantation (PPI) after surgical aortic valve replacement (SAVR)
| Variables a | Total study group (n = 5 600) | PPI group (n = 222) | No PPI group (n = 5 378) |
|---|---|---|---|
| Procedural characteristics | |||
| Type of extra‐corporeal circulation (ECC) | |||
| Conventional ECC | 5 125 (91.5%) | 204 (91.9%) | 4 921 (91.5%) |
| Mini ECC | 153 (2.7%) | 7 (3.2%) | 146 (2.7%) |
| ECC type unknown | 284 (5.1%) | 11 (5.0%) | 273 (5.1%) |
| ECC time, mean (SD), min | 97.1 (±37.2) | 113.9 (±56.4) | 96.4 (±36.1) |
| Ischemic time, mean (SD), min | 68.9 (±24.4) | 77.9 (±36.7) | 68.5 (±23.8) |
| Circulation arrest | 7 (0.1%) | 0 (0%) | 7 (0.1%) |
| Circulation arrest time, mean (SD), min | 0.0 (± 0.03) | 0.0 (± 0.0) | 0.0 (± 0.03) |
| Prosthetic valve type | |||
| Bioprosthetic valves | 4604 (82.2%) | 180 (81.1%) | 4 424 (82.3%) |
| Mechanical valve | 995 (17.8%) | 42 (18.4%) | 953 (17.7%) |
| Bioprosthesis type | |||
| Stented valves | 4425 (96.1%) | 174 (96.7%) | 4251 (96.1%) |
| Stentless/sutureless valves | 159 (3.5%) | 2 (0.6%) | 157 (3.5%) |
| Inotropic use | 1 279 (44.4%) | 57 (50.4%) | 1 222 (44.1%) |
| Vasopressor use | 2 082 (72.2%) | 93 (82.3%) | 1 989 (71.8%) |
| Intraoperative PPI | 12 (0.2%) | 12 (5.4%) | 0 (0%) |
| Perioperative myocardial infarction | 285 (5.2%) | 14 (6.4%) | 271 (5.1%) |
| Intraoperative intra‐aortic balloon pump | 10 (0.3%) | 0 (0%) | 10 (0.3%) |
| Outcomes | |||
| In‐hospital respiratory insufficiency | 52 (1.1%) | 4 (2.1%) | 48 (1.0%) |
| In‐hospital cardiac conduction abnormalities | 1 723 (30.8%) | 143 (64.4%) | 1 580 (29.4%) |
| In‐hospital mortality | 60 (1.1%) | 2 (0.9%) | 58 (1.1%) |
Note: Values are n (%).
Abbreviations: CI, confidence interval; ECC, extra‐corporeal circulation; IQR, interquartile range; min, minutes; PPI, permanent pacemaker implantation.
Number value are presented as valid percentage, excluding missing values.
Figure 2.
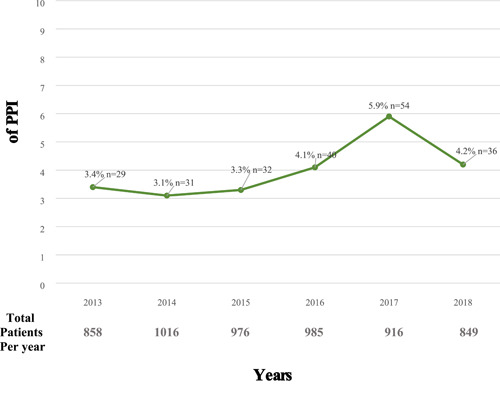
Trends of permanent pacemaker implantation (PPI) after surgical aortic valve replacement (SAVR) over time. Trends of PPI across time. The rate of PPI after SAVR significantly rises up over time (OR: 1.11; 95% CI: 1.03–1.21; p < .05). CI, confidence interval: OR, odds ratio
3.2. Univariable analysis
In the univariable analysis (Table 3), following variables showed a crude significant association with PPI postoperatively: previous cardiac surgery (OR: 2.41; 95% CI: 1.69–3.44, p < .001), higher log Euroscore II (OR: 1.03; 95% CI: 1.01–1.04, p < .01), previous valve surgery (OR: 2.35, 95% CI: 1.55–3.58, p < .001), previous aortic valve surgery (OR: 2.57; 95% CI: 1.62–4.07). Longer extra‐corporeal circulation time (OR: 1.01; 95% CI: 1.01–1.01; p < .001) and longer ischemic time (OR: 1.01, 95% CI: 1.01–1.02; p < .001) led to more risk of PPI. Also, use of vasopressors (OR: 2.34, 95% CI: 1.59–3.43; p < .001) and in‐hospital cardiac conduction abnormalities (OR: 4.35, 95% CI: 3.29–5.76; p < 0.001).001) significantly increased the risk of PPI.
Table 3.
Univariable analysis of 30‐day permanent pacemaker implantation (PPI) after surgical aortic valve replacement (SAVR)
| Variables | OR (95% CI) | p Value |
|---|---|---|
| Male sex | 0.99 (0.76–1.30) | .95 |
| Age, years | 1.00 (0.99 ‐ 1.02) | .54 |
| Weight, kg | 1.00 (0.99–1.01) | .40 |
| Creatinine serum level, μmol/L | 1.00 (1.00–1.00) | .68 |
| History of lung disease | 0.85 (0.55–1.30) | .44 |
| Peripheral vascular disease | 0.96 (0.56–1.64) | .89 |
| Diabetes mellitus | 1.00 (0.71–1.41) | .98 |
| Recent myocardial infarction | 1.50 (0.54–4.15) | .44 |
| Log Euroscore II,% | 1.03 (1.01–1.04) | <.01 |
| Atrial fibrillation | 1.30 (0.93–1.84) | .13 |
| Left ventricular ejection fraction, % | 0.99 (0.97–1.00) | .08 |
| Systolic pulmonary pressure, mmHg | 1.01 (0.99–1.03) | .55 |
| Previous cardiac surgery | 2.41 (1.69–3.44) | <.001 |
| Previous coronary surgery | 1.74 (0.95–3.18) | .07 |
| Previous valve surgery | 2.35 (1.55–3.58) | <.001 |
| Previous aortic valve surgery | 2.57 (1.62–4.07) | <.001 |
| Previous mitral valve surgery | 1.60 (0.77–3.31) | .21 |
| Previous aortic surgery | 0.77 (0.31–1.89) | .56 |
| Prosthetic valve characteristics | ||
| Mechanical valves bioprosthetic valves | Ref | |
| Stented valves | 0.92 (0.66–1.30) | .64 |
| Stentless valves | 0.28 (0.07–1.17) | .08 |
| ECC time, min | 1.01 (1.01–1.01) | <.001 |
| Ischemic time, min | 1.01 (1.01–1.02) | <.001 |
| Inotropic use | 1.23 (0.94–1.61) | .13 |
| Vasopressor use | 2.34 (1.59–3.43) | <.001 |
| Peri‐operative myocardial infarction | 1.35 (0.79–2.31) | .27 |
| In‐hospital respiratory insufficiency | 1.63 (0.59–4.52) | .35 |
| In‐hospital cardiac conduction abnormalities | 4.35 (3.29–5.76) | <.001 |
Abbreviations: CI, confidence interval; ECC, extra‐corporeal circulation; min, minutes; OR, odds ratio; PPI, permanent pacemaker implantation.
3.3. Multivariable analysis
By multicollinearity approach extra‐corporeal circulation time and ischemic time were correlated and therefore we decided to put only extra‐corporeal circulation time in the final multivariable model. In the multivariable analyses (Table 4), we identified seven variables that were independent risk factors of postoperative PPI. Previous cardiac surgery (OR: 3.23; 95% CI: 1.34–7.77; p = .01) and previous mitral valve surgery (OR: 3.25; 95% CI: 1.28–8.32; p < .01) increased the risk for PPI. Per‐operatively, the use of a stentless valve (OR: 0.17; 95% CI: 0.04–0.83; p = .03), longer extra‐corporeal circulation time (OR: 1.01; 95% CI: 1.00–1.01; p < .00) and the use of vasopressors (OR: 2.52; 95% CI: 1.68–3.79; p < .001) increased the risk of PPI. Postoperatively, in‐hospital cardiac conduction abnormalities (OR: 4.88; 95% CI: 3.61–6.60; p < .001) led to more risk for PPI.
Table 4.
Multivariable analysis of 30‐day permanent pacemaker implantation (PPI) after surgical aortic valve replacement (SAVR)
| Variables | OR (95% CI) | p Value |
|---|---|---|
| Male sex | 0.96 (0.69–1.34) | .82 |
| Age, years | 1.00 (0.98–1.02) | .80 |
| Weight, kg | 1.00 (0.99–0.99) | .31 |
| Creatinine serum level, μmol/L | 1.00 (0.99–1.00) | .34 |
| History of lung disease | 0.65 (0.40–1.04) | .07 |
| Peripheral vascular disease | 1.11 (0.62–1.97) | .73 |
| Diabetes mellitus | 0.88 (0.61–1.26) | .48 |
| Recent myocardial infarction | 1.48 (0.51–4.33) | .47 |
| Euroscore II,% | 0.99 (0.96–1.03) | .67 |
| Atrial fibrillation | 1.36 (0.93–1.99) | .12 |
| Left ventricular ejection fraction, % | 0.99 (0.97 ‐ 1.01) | .25 |
| Systolic pulmonary pressure, mmHg | 1.01 (0.99–1.03) | .54 |
| Previous cardiac surgery | 3.23 (1.34–7.77) | .01 |
| Previous coronary surgery | 0.43 (0.18–1.05) | .07 |
| Previous valve surgery | 0.40 (0.16–1.04) | .06 |
| Previous aortic valve surgery | 1.57 (0.74–3.34) | .24 |
| Previous mitral valve surgery | 3.25 (1.28–8.32) | .01 |
| Previous aortic surgery | 0.45 (0.15–1.34) | .15 |
| Prosthetic valve types | ||
| Mechanical valves bioprosthetic valves | Ref | |
| Stented valves | 0.96 (0.60–1.53) | .85 |
| Stentless valves | 0.17 (0.04–0.83) | .03 |
| ECC time, min | 1.01 (1.00–1.01) | <.001 |
| Inotropic use | 1.20 (0.89–1.62) | .24 |
| Vasopressor use | 2.52 (1.68–3.79) | <.001 |
| Perioperative myocardial infarction | 1.38 (0.78–2.45) | .27 |
| In‐hospital respiratory insufficiency | 1.40 (0.47–4.13) | .55 |
| In‐hospital cardiac conduction abnormalities | 4.88 (3.61–6.60) | <.001 |
Abbreviations: CI, confidence interval; ECC, extra‐corporeal circulation; min, minute.
By using a backward stepwise elimination (Table 5); previous cardiac surgery, extracorporeal circulation time, use of vasopressors and in‐hospital cardiac conduction abnormalities were confirmed to lead to a higher rate of postoperative PPI.
Table 5.
Backward stepwise elimination of 30‐day permanent pacemaker implantation (PPI) after surgical aortic valve replacement (SAVR)
| Variables | OR (95% CI) | p Value |
|---|---|---|
| Previous cardiac surgery | 1.80 (1.18–2.76) | <.01 |
| ECC time, min | 1.01 (1.00–1.01) | <.001 |
| Vasopressor use | 2.66 (1.79–3.96) | <.001 |
| In‐hospital cardiac conduction abnormalities | 4.48 (3.36–5.98) | <.001 |
Abbreviations: CI, confidence interval; ECC, extra‐corporeal circulation.
4. DISCUSSION
Our study provides a large‐sample analysis of potential patient's, procedural and electrophysiological‐related risk factors for PPI after SAVR. Additionally, we described the trend of the phenomenon across a 6‐year period. We identified four potential predictors for PPI, as followed: previous cardiac surgery, longer extra‐corporeal circulation time, use of vasopressor and in‐hospital cardiac conduction abnormalities. Between 2013 and 2019, the rate of PPI significantly increased over the time.
Association between conduction rhythm disorder and subsequent requirement of PPI with SAVR is well known. 5 In this study cohort, 3.9% of the patients received a PPI after isolated SAVR procedure. This rate is in accordance with previous published data from registries, as the GARY registry, reporting 3.9% to 5.1% 15 , 16 of PPI after isolated SAVR. Also, surgical cohorts of randomized control trials as the PARTNER studies 17 , 18 showed similar rates of PPI at 30 days in the SAVR study arms. Higher rates of postoperative PPI until 11.6% at 30 days have been described in study investigating sutureless valves. 19 Our work included different valve types, contributing maybe to the lower rate of PPI reported. Analysis in the valve subtype was unfortunately not available. Interestingly, rate of PPI may even be lower after transcatheter aortic valve implantation (TAVI) than SAVR in in high‐risk patients. 17 However, the extension of TAVI indications in low‐risk patients is still a matter of debate. Additional data with longer follow‐up in this specific population is currently needed, as the patients being less than 65 years of age still represent a grey area. 20
In our study, history of previous cardiac surgery was found to increase the risk of PPI postoperatively. Van Mieghem et al. 21 also demonstrated the negative effect of previous cardiac operation on PPI after SAVR. Multiple surgical valve procedures 9 and prior valve surgery 22 has also been described to be risk factors for postoperative PPI. This relationship highly suggests that direct trauma at the time of surgery or ischemic injury during previous surgery can hugely predispose patients to a PPI in the follow‐up. Suture injury, impingement of the implanted valve against conduction ways or localized pressure along the atrioventricular conduction axis due to residual calcic materials can provide the substrate for developing rhythm disturbances leading to PPI postoperatively. 23 , 24 , 25
Longer ischemic time peroperatively was found to increase the risk of PPI postoperatively significantly. These findings are in line with previous studies demonstrating the negative influence of longer cumulative cross‐clamp times. 9 , 26 , 27 , 28 Longer clamping time obviously increases the hemodynamic stress and is known to cause a higher degree of inflammation, that can lead to ischemic injury, and subsequently to further degradation of the conduction system. However, we reported a mean cardiac bypass time of 97.1 (±37.2) min, which is higher than reported in previous studies for SAVR. 9 , 10 , 29 Even if the PPI rate remains in the range of previous published materials, we do not have an explanation for such a finding.
In our patient cohort, presence of in‐hospital conductions abnormalities was a strong predictor of postoperatively PPI. Preoperative conduction system disease is well described as a cause of PPI postoperatively. 10 Also, the absence of preoperative sinus rhythm has been reported as predictors for PPI after valvular surgery. 9 Gonzales Barbeito et al. 30 in their series of 519 patients undergoing SAVR with a PERCEVAL S sutureless prosthesis identified disorders of intraventricular conduction and right bundle branch block as predictors for postoperative PPI. In their analysis, atrial fibrillation was not investigated as rhythm disturbances leading to PPI. A study reporting perioperative predictors of atrioventricular block after coronary artery bypass surgery identified atrial fibrillation as strong predictors for conductions disturbances postoperatively 31 confirming our findings.
The effect of using vasopressor during cardio‐surgical intervention is poorly investigated. 32 By increasing the automaticity of the sino‐atrial node and slowing down the atrio‐ventricular conduction, vasopressor may potentialize previous or concomitant lesions inducing a deceleration of the atrio‐ventricular conduction, leading to higher subsequent postoperative PPI. 22 , 33 Furthermore, the use of dobutamine has been related to the development of postoperative ventricular arrhythmias, emphasizing the potential impact of vasopressor on the heart conduction system. 34 However, as we had no details about the kind of vasopressor used, we were unable to conclude about a specific molecule causing post‐SAVR PPI.
Our findings indicate that PPI rate increased over the time, reaching a peak of 6% in 2017. This increasing trend has been also reported in the analysis of the GARY registry. 16 A possible explanation for this increase may be related to the use of rapid‐deployment and/or sutureless valves. As we were not able to provide information about the type of valves used, caution is the parent of safety but the use of sutureless and rapid deployment devices has been increased over the last 15 years, 35 for sure playing a role in the postoperative outcomes. A recent Italian experience 36 demonstrated the link between the surgeon's experience and the incidence of PPI postoperatively when using the Perceval aortic valve. The increasing trend of postoperative PPI observed in out cohort may reflect the extended use of sutureless and rapid deployment devices requiring an accomplished learning curve to reach comparable results in term of PPI when compared with classical stented valves.
5. LIMITATIONS
Our analysis has several limitations. The main limitation is inherent to the retrospective and observational nature of the study, with the related biases of this methodology. We obtain no data about the status of a preoperative PPI, pacemaker indication and type of pacemaker provided. Some other well‐known predictors for postoperative PPI (as bundle branch block, nature of in‐hospital conduction abnormalities, valve type and size) were not listed in the registry, making us unable to study it. Nevertheless, this national population‐based analysis provided a large series of patients, allowing us to conclude about trends and association, even if we cannot draw conclusions on possible cause and effects. We feel confident of our model being robust and statistically valid to identify important predictive factors for pacemaker implantation that may be relevant to decision making.
6. CONCLUSIONS
In this nationwide analysis, we identified several factors, like previous cardiac surgery, extra‐corporeal circulation time, use of vasopressors and in‐hospital conduction abnormalities, as predictive factors for postoperative PPI in adult patients undergoing isolated SAVR. The rate of PPI increased across the time, emphasizing the potential amplitude of this complication and the impact on current practice.
CONFLICT OF INTERESTS
Arnoud W Van't Hof: conflict of interests unrelated to this work (unrestricted grants form AZ, Medtronic, Boerhinger I, Abbott).
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We would like to acknowledge and thank all participating investigators for the data collection and their collaboration. We are also very grateful to the NHR for centralizing the data.
Ravaux JM, Van Kuijk SM, Di Mauro M, et al. Incidence and predictors of permanent pacemaker implantation after surgical aortic valve replacement: Data of the Netherlands Heart Registration (NHR). J Card Surg. 2021;36:3519‐3527. 10.1111/jocs.15803
REFERENCES
- 1. Nishimura RA, Otto CM, Bonow RO, et al. American College of Cardiology/American Heart Association, American Heart Association. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 148, 2014:e1‐e132. [DOI] [PubMed] [Google Scholar]
- 2. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005‐1011. [DOI] [PubMed] [Google Scholar]
- 3. Carrel TP, Dembitsky W, Dreyfus G, et al. Early treatment of aortic stenosis will prevent poor outcomes and save thousands of lives. J Thorac Cardiovasc Surg. 2013;146:4‐5. [DOI] [PubMed] [Google Scholar]
- 4. Erdogan H, Kayalar N, Ardal H, et al. Risk factors for requirement of permanent pacemaker implantation after aortic valve replacement. J Card Surg. 2006;21:211‐215. [DOI] [PubMed] [Google Scholar]
- 5. Schurr UP, Berli J, Berdajs D, et al. Incidence and risk factors for pacemaker implantation following aortic valve replacement. Interact Cardiovasc Thorac Surg. 2010;11(5):556‐560. [DOI] [PubMed] [Google Scholar]
- 6. Glikson M, Dearani Ja, Hyberger LK, Schaff HV, Hammill SC, Hayes DL. Indications, effectiveness, and long‐term dependency in permanent pacing after cardiac surgery. Am J Cardiol. 1997;80:1309‐1313. [DOI] [PubMed] [Google Scholar]
- 7. Mehaffey JH, Haywood NS, Hawkins RB, et al. Need for permanent pacemaker after surgical aortic valve replacement reduces long‐term survival. Ann Thorac Surg. 2018;106(2):460‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robich MP, Schiltz NK, Johnston DR, et al. Risk factors and outcome of patients requiring a permanent pacemaker after aortic valve replacement in the United States. J Card Surg. 2016;31:476‐485. [DOI] [PubMed] [Google Scholar]
- 9. Elahi MM, Lee D, Dhannapuneni RRV. Predictors of permanent pacemaker implantation during the early postoperative period after valve surgery. Tex Heart Inst J. 2006;33:455‐457. [PMC free article] [PubMed] [Google Scholar]
- 10. Dawkins S, Hobson AR, Kalra PR, Tang AT, Monro JL, Dawkins KD. Permanent Pacemaker implantation after isolated aortic valve replacement: incidence, indications, and predictors. Ann Thorac Surg. 2008;85:108‐112. [DOI] [PubMed] [Google Scholar]
- 11. Matthews IG, Fazal IA, Bates MGD, Turley AJ. In patients undergoing aortic valve replacement, what factors predict the requirement for permanent pacemaker implantation? Interact Cardiovasc Thorac Surg. 2011;12(3):475‐479. [DOI] [PubMed] [Google Scholar]
- 12. Limongelli G, Ducceschi V, D′Andrea A, et al. Risk factors for pacemaker implantation following aortic valve replacement: a single center experience. Heart. 2003;89(8):901‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Totaro P, Calamai G, Montesi G, Barzaghi C, Vaccari M. Continuous suture technique and impairment of the atrioventricular conduction after aortic valve replacement. J Card Surg Nov. 2000;15(6):418‐422. [DOI] [PubMed] [Google Scholar]
- 14. Brookes JDL, Mathew M, Brookes EM, Jaya JS, Almeida AA, Smith JA. Predictors of Pacemaker Insertion Post‐Sutureless (Perceval) Aortic Valve Implantation. Heart Lung Circ. 2020;S1443‐9506(20):31524‐31529. [DOI] [PubMed] [Google Scholar]
- 15. Mohr FW, Holzhey D, Mölmann H, et al, for the GARY Executive Board . The German Aortic Valve Registry: 1‐year results from 13 680 patients with aortic valve disease. Eur J Cardiothorac Surg. 2014;46(5):808‐816. [DOI] [PubMed] [Google Scholar]
- 16. Fujita B, Ensminger S, Bauer T, et al, GARY Executive Board . Trends in practice and outcomes from 2011 to 2015 for surgical aortic valve replacement: an update from the German Aortic Valve Registry on 42 776 patients. Eur J Cardiothoracic Surg. 2018;53(3):552‐559. [DOI] [PubMed] [Google Scholar]
- 17. Smith CR, Leon MB, Mack MJ, et al, PARTNER Trial Investigators . Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187‐2198. [DOI] [PubMed] [Google Scholar]
- 18. Leon MB, Smith CR, Mack MJ, et al, PARTNER 2 Investigators . Transcatheter or surgical aortic valve replacement in intermediate risk patients. N Engl J Med. 2016;374:1609‐1620. [DOI] [PubMed] [Google Scholar]
- 19. Laborde F, Fischlein T, Hakim‐Meibodi K, et al, Cavalier Trial Investigators . Clinical and haemodynamic outcomes in 658 patients receiving the Perceval sutureless aortic valve: early results from a prospective European Multicentre study (the Cavalier Trial). Eur J Cardiothorac Surg. 2016;49(3):978‐986. [DOI] [PubMed] [Google Scholar]
- 20. Mc Morrow R, Kriza C, Urban P, et al. Assessing the safety and efficacy of TAVR compared to SAVR in low‐to‐intermediate surgical risk patients with aortic valve stenosis: an overview of reviews. Int J Cardiol. 2020;314:43‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Mieghem NM, Head SJ, De Jong W, et al. Persistent annual permanent pacemaker implantation rate after surgical aortic valve replacement in patients with severe aortic stenosis. Ann Thorac Surg. 2019;94:1143‐1149. [DOI] [PubMed] [Google Scholar]
- 22. Hill TE, Kiehl EL, Shrestha NK, et al. Predictors of permanent pacemaker requirement after cardiac surgery for infective endocarditis. Eur Heart J Acute Cardiovasc Care. 2019;10(3):329–334. [DOI] [PubMed] [Google Scholar]
- 23. Peretto G, Durante A, Limite LR, Cianflone D. Postoperative arrhythmias after cardiac surgery: incidence, risk factors, and therapeutic management. Cardiol Res Pract. 2014;2014:615987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderson RH, Yanni J, Boyett MR, Chandler NJ, Dobrzynski H. The Anatomy of the Cardiac Conduction System. Clin Anat. 2009;22:99‐113. [DOI] [PubMed] [Google Scholar]
- 25. Coti I, Schukro C, Drevinja F, et al. Conduction disturbances following surgical aortic valve replacement with a rapid‐deployment bioprosthesis. J Thorac Cardiovasc Surg. 2020;Pii: S0022‐5223(20):30433‐30435. [DOI] [PubMed] [Google Scholar]
- 26. Klapkowski A, Pawlaczyk R, Kempa M, Jagielak D, Brzezinski M, Rogowski J. Complete atrioventricular block after isolated aortic valve replacement. Kardiol Pol. 2016;74(9):985‐993. [DOI] [PubMed] [Google Scholar]
- 27. Onalan O, Crystal A, Lashevsky I, et al. Determinants of pacemaker dependency after coronary and/or mitral or aortic valve surgery with long‐term follow‐up. Am J Cardiol. 2008;101(2):203‐208. [DOI] [PubMed] [Google Scholar]
- 28. Glikson M, Dearani JA, Hyberger LK, Schaff HV, Hammill SC, Hayes DL. Indications, effectiveness, and long‐term dependency in permanent pacing after cardiac surgery. Am J Cardiol. 1997;80(10):1309‐1313. [DOI] [PubMed] [Google Scholar]
- 29. Hwang YM, Kim J, Lee JH, et al. Conduction disturbance after isolated surgical aortic valve replacement in degenerative aortic stenosis. J Thorac Cardiovasc Surg. 2017;154(5):1556‐1565. [DOI] [PubMed] [Google Scholar]
- 30. Gonzales Barbeito M, Estévez‐Cid F, Pardo Martinez P, et al. Surgical technique modifies the postoperative atrioventricular bloc rate in sutureless prostheses. J Thorac Dis. 2019;11(7):2945‐2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Piantà RM, Di Leoni Ferrari A, Heck AA, et al. Atrioventricular block in coronary artery bypass surgery: perioperative predictors and impact on mortality. Braz J Cardiovasc Surg. 2015;30(2):164‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vail EA, Shieh M‐S, Pekow PS, et al. Use of vasoactive medications after cardiac surgery in the United States. Ann Am Thorac Soc. 2021;18(1):103‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tisdale JE, Patel R, Webb RC, Borzak S, Zarowitz BK. Electrophysiologic and proarrhythmic effects of intravenous inotropic agents. Prog Cardiovasc Dis. 1995;38(2):167‐180. [DOI] [PubMed] [Google Scholar]
- 34. Fellahi J‐L, Parienti J‐J, Hanouz J‐L, Plaud B, Riou B, Ouattara A. Perioperative use of dobutamine in cardiac surgery and adverse cardiac outcome: propensity‐adjusted analyses. Anesthesiology. 2008;108(6):979‐987. [DOI] [PubMed] [Google Scholar]
- 35. Glauber M, Miceli A, Di Bacco L. Sutureless and rapid deployment valves: implantation technique from A to Z – the Perceval valve. Ann Cardiothorac Surg. 2020;9(4):330‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mikus E, Calvi S, Tavazzi L, et al. Pacemaker need after sutureless aortic valve replacement: the role of the learning curve. J Cardiovasc Med (Hagerstown). 2020;22:133‐138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


