Abstract
Objective
To report the temporal and spatial distribution of rainbow lorikeets presenting with lorikeet paralysis syndrome (LPS) and their clinicopathologic and pathologic findings, exposure to toxins, and response to treatment.
Methods
Records of lorikeets admitted in 2017 and 2018 to facilities in south‐east Queensland (QLD) were reviewed and LPS and non‐LPS cases were mapped and their distribution compared. Plasma biochemistries and complete blood counts were done on 20 representative lorikeets from south‐east QLD and Grafton, New South Wales (NSW). Tissues from 28 lorikeets were examined histologically. Samples were tested for pesticides (n = 19), toxic elements (n = 23), botulism (n = 15) and alcohol (n = 5).
Results
LPS occurred in warmer months. Affected lorikeets were found across south‐east QLD. Hotspots were identified in Brisbane and the Sunshine Coast. Lorikeets had a heterophilic leucocytosis, elevated muscle enzymes, uric acid and sodium and chloride. Specific lesions were not found. Exposure to cadmium was common in LPS and non‐LPS lorikeets. Treated lorikeets had a 60–93% See Table 2 depending on severity of signs.
Clinical significance
The primary differential diagnosis for lorikeets presenting with lower motor neuron signs during spring, summer and autumn in northern NSW and south‐east Queensland should be LPS. With supportive care, prognosis is fair to good.
Keywords: aetiology, clinical pathology, paralysis, prognosis, rainbow lorikeet, temporal distribution
Abbreviations
- Aka
also known as
- CNS
Central Nervous System
- GVC
Grafton Veterinary Clinic
- LPS
Lorikeet Paralysis Syndrome
- NS
not significant
- NSW
New South Wales
- QLD
Queensland
- RSPCA
Royal Society for the Prevention of Cruelty to Animals
- USA
United States of America
- VIC
Victoria
- WA
Western Australia
Lorikeet paralysis syndrome (LPS) is a paralytic disease of wild rainbow lorikeets (Trichoglossus haematodus) and less commonly other species of lorikeets in Australia. 1 , 2 Manifestations of this disease range from the inability to fly and hindlimb weakness and ataxia, to a flaccid paralysis of all limbs and the neck, inability to blink, paralysis of the tongue, inability to swallow and voice change. 1 , 2 The number of cases each year ranges from hundreds to thousands, making it one of the most important wildlife diseases and animal welfare concerns in Australia. 1 , 2
Despite its importance, there are no published data on the clinical pathological findings or aetiology of LPS and only one report on prognosis that suggests that a small proportion of lorikeets with LPS recover after several weeks of supportive care. 2 Lorikeets presenting with similar but not identical signs have been documented in Victoria (VIC), New South Wales (NSW) and Queensland (QLD). 1 , 2 , 3 , 4 , 5 , 6 Lorikeets described in these cases presented with flexed stifles, hocks and phalanges (aka Clench Claw Syndrome), signs not seen in birds with LPS. 6 In previous studies of lorikeets with central nervous system (CNS) signs and leg flexion, gross lesions were non‐specific and microscopic and ancillary diagnostic findings suggested multiple aetiologic agents may cause them. 1 , 2 , 3 , 4 , 5 , 6 One study found a high prevalence of non‐suppurative encephalitis, possibly of viral origin, in lorikeets with CNS signs and flexed legs. 3 More recently, a novel avulavirus (Avian avulavirus 5/Lorikeet/Australia/2016) was detected in four of five rainbow lorikeets presenting with CNS signs and flexed legs. 6 All five birds had a non‐suppurative encephalomyelitis and ganglioneuritis. In other studies, thiamine deficiency was postulated to cause CNS signs and leg flexion in VIC 5 and cadmium and lead exposure was found in lorikeets presenting with CNS signs and flexed legs in Sydney, NSW. 1
The objectives of this study were threefold. The first was to characterise the clinical and pathological changes present in lorikeets with LPS to better define this syndrome and help in the formulation of a treatment plan. The second was to determine the prognosis for lorikeets with LPS and to determine if the severity of the disease on presentation could be used to predict the chances of recovery. The third was to use epidemiologic, clinical pathologic, toxicologic and postmortem findings to narrow the list of possible aetiologies for LPS.
Materials and methods
Numbers, ages and temporal distribution of LPS cases presenting to the Royal Society for the Prevention of Cruelty of Animals (RSPCA)
Records used in the retrospective study included all rainbow lorikeets admitted to the RSPCA, Queensland (QLD) Wacol Animal Care Campus, Wacol, QLD and the RSPCA Eumundi Wildlife Centre, Eumundi, QLD from 1 January 2017 to 30 December 2018. Data were analysed using Microsoft Excel (Microsoft Corporation. 2018. Retrieved from https://office.microsoft.com/excel). Birds were classified as juveniles if the beak was uniformly black, subadults if the beak was a combination of black and orange, and adult if the beak was entirely orange.
Categorizing the severity of presenting signs
LPS cases were placed into categories based on severity of their signs. All LPS lorikeets were unable to fly and had no physical or radiographic evidence of trauma. Category 1 lorikeets were unable to stand, blink and swallow. Category 2 lorikeets were unable to blink but could swallow and stand and were ataxic when trying to walk. Category 3 lorikeets were able to blink, swallow, stand and walk, but were ataxic when hopping. Category 4 lorikeets were able to blink, swallow, walk and hop.
Spatial distribution of LPS cases and non‐LPS cases submitted to the RSPCA
LPS and non‐LPS cases were geocoded based on reported suburb and mapped (ArcGIS 10.5. ESRI, Redlands, CA) using a shape file of Australia (geographic coordinate system WGS 1984). The spatial clustering of LPS and non‐LPS cases was compared using a nearest neighbour statistic. 7 Clusters of LPS cases were identified using a time–space scan statistic based on a Bernoulli model (SaTScan 9.6. http://www.satscan.org/). 8 Data were scanned for clusters in size up to half of the study area and occurring up to half of the study period, and statistical significance was determined based on the likelihood statistic and Monte Carlo simulation (n = 999). Hotspots were interpreted based on the number of cases present compared with that expected and were mapped.
Distribution of body condition scores, treatment and survival rates
Distribution of body condition score
The distribution of the body condition scores for lorikeets presenting with LPS was determined by reviewing the body condition scores recorded (n = 304) for lorikeets that presented to the RSPCA in 2017. Lorikeets were given a body condition score ranging from one to five. Lorikeets with a score of one had a marked decrease in pectoral muscle mass (the pectoral muscles were concave in cross section) and no fat. Lorikeets with a body condition score of three were considered normal having limited body fat and a cross‐sectionally convex pectoral muscle mass. Lorikeets with a body condition score of 5 had convex muscle mass but excessive body fat.
Treatment in hospital
Treatment consisted of subcutaneous fluids (Hartmann's solution; Webster, Sydney, NSW, Australia) 100 mL/kg once on arrival for all and 50 mL/kg q24 hrs for 2 days for Category 1 lorikeets. Category 1 lorikeets were assist fed (5–8 mls, Neocare, Vetafarm, Wagga Wagga, NSW, Australia or Wombaroo Lorikeet Nectar, Adelaide, South Australia, Australia) q8–q12 hrs until they could self‐feed. Meloxicam (Boehringer Ingelheim, Vetmedica, Duluth, Georgia, USA) 1 mg/kg IM q12 hrs was administered for 3 days. Eye drops (Systane, Alcon Laboratories, Macquarie Park, NSW, Australia) were applied to both eyes q12 hrs for the lorikeets that were unable to blink. Once lorikeets were feeding on their own and were able to get some lift when flapping their wings, they were transferred into a 3 m high, 3 m wide and 6 m long flight cage. Lorikeets were released once they could fly for 5 min without developing open‐mouthed breathing.
Survival rates
Age (juvenile/subadult or adult), date of admission, number of days before transfer to an aviary, final outcome (died, euthanased, released, outcome unknown) and total number of days in care were recorded. A chi‐square test of independence was performed to determine if there were significant differences in survival between categories (Microsoft Corporation. 2018. Microsoft Excel). The normality of the distributions of the number of days in hospital and rehabilitation before release and the total number of days in care (days in hospital plus days in rehabilitation) for each category were tested by the Shapiro–Wilk test and the assessment of Q–Q plots and histograms using R version 3.6.0 for Windows. Given that the data were not consistently normally distributed, data sets were compared using Wilcoxon rank sum test in Excel. Differences were considered significant if the P value was ≤0.05.
Sample collection and timing of sample collection from lorikeets with LPS submitted to the Australia Zoo Wildlife Hospital (AZWH), RSPCA, and Grafton Veterinary Clinic (GVC)
Location and timing of specimen collection
Diagnostic specimens (Table 1) were collected from lorikeets with LPS presenting to the Australia Zoo Wildlife Hospital (AZWH), 1638 Steve Irwin Way, Beerwah, QLD, Australia, in November and December 2012 and February 2015; RSPCA, Wacol Animal Care Campus in January and February 2017, February 2018 and February 2020; and the GVC, 128 Bacon St., Grafton, NSW, Australia in February 2020. All specimens were collected upon arrival and none of the birds were treated prior to euthanasia, with the exception of two that had been in care for 20 and 27 days at the RSPCA. Histopathology, biochemistry and haematology were done on the 15 birds collected in January 2017 at the Wacol Campus of the RSPCA.
Table 1.
Assays, year, numbers, samples tested, origin of specimens and laboratories where the testing of lorikeets with suspect lorikeet paralysis syndrome (LPS) was undertaken
| Assay/laboratory | Date | N | Sample | Source a |
|---|---|---|---|---|
| Blood alcohol b , c | 2015 | 3 | Blood | AZWH b |
| 2 | Blood | GVC c | ||
| Avian influenza d | 2015 | 5 | Tracheal and cloacal swabs | AZWH |
| Cholinesterase activity d | 2015 | 3 | Brain | AZWH |
| Clostridium botulinum toxins C and D e | 2018 | 5 | Liver, digestive contents | RSPCA |
| Clostridium botulinum toxins A, B and E e | 2020 | 10 | Plasma | RSPCA |
| Newcastle disease d | 2015 | 5 | Tracheal and cloacal swabs | AZWH |
| Histopathology f | 2012 | 8 | Formalin‐fixed tissues | AZWH |
| 2017 | 15 | Formalin‐fixed tissues | RSPCA | |
| Haematology/biochemistry f | 2017 | 13 | Blood | RSPCA |
| 2020 | 5 | Blood | GVC | |
| Element profile g | 2017 |
12 LPS cases 1 non‐LPS |
Liver, kidney | RSPCA |
| Element profile h | 2020 |
5 i LPS cases 3 i non‐LPS cases |
Liver, kidney, digestive tract | RSPCA |
| 2020 |
6 j LPS cases |
Liver, digestive contents | GVC | |
| Pesticide profile h | 2020 |
5 i LPS cases 3 i non‐LPS cases |
Liver, kidney, digestive tract | RSPCA |
| 2020 |
6 j LPS cases |
Liver, digestive contents | GVC |
AZWH, Australia Zoo Wildlife Hospital; GVC, Grafton Veterinary Hospital; RSPCA, Royal Society for the Prevention of Cruelty to Animals.
Forensic and Scientific Services Forensic Toxicology; QLD Health, 39 Kessels Road, Coopers Plains, QLD 4108.
NSW Health Pathology North Grafton Base Hospital, Arthur Street Grafton, NSW 2460.
Biosecurity Sciences Laboratory, Department of Agriculture and Fisheries, Queensland Government, 39 Kessels Rd, Coopers Plains, QLD 4108.
Diagnostic Laboratory Services, Department of Primary Industries and Regional Development, 3 Baron‐Hay Court, South Perth, WA 6151.
University of Sydney, Camden, NSW 2570.
University of Sydney, Department of Chemical Pathology, Royal Prince Alfred Hospital, Camperdown, NSW 2050.
Environmental Forensics, Department of Planning, Industry and the Environment, NSW Government, 480 Weeroona Road, Lidcombe, NSW.
Liver samples were tested individually. Kidney samples from the non‐LPS lorikeets were pooled and tested as a composite sample. Kidney samples from the affected lorikeets were pooled and tested as a composite sample. Digestive tract samples (stomach and intestinal content) from affected lorikeets were pooled and tested as a composite sample. Digestive tract samples from non‐LPS lorikeets were not tested.
Digestive tract and liver samples were tested separately. Samples from lorikeets 1, 2 and 3 were pooled and tested as a composite sample and samples from lorikeets 4, 5 and 6 were also tested as a composite sample.
Blood collection
All lorikeets were anaesthetised with isoflurane admixed with oxygen and delivered via facemask (Baxter, Old Toongabbie, NSW, Australia). One millilitre of blood was collected from the right jugular vein from lorikeets from the RSPCA and GVC and blood smears were made. The remaining blood was placed in a 1.5 mL heparinized tube (Becton Dickenson, Macquarie Park, NSW, Australia) and mixed. Blood smears were stained with a Romanowsky stain (Diff‐Quik, RAL Diagnostics, Martillac, France).
Haematological and biochemical analysis
Estimated total white blood cell counts and differential counts were routinely done. 9 Thrombocyte numbers were considered normal if more than two thrombocytes were seen per field at 1000× magnification. Polychromasia was scored as 0 (no polychromatophilic cells) or 1+ (1–5% polychromatophilic cells). The heparinized blood was centrifuged and plasma was removed and frozen at −20°C. Plasma biochemical analytes were determined using a Konelab 20xti (Thermo Fisher Scientific, NSW, Australia).
Euthanasia and postmortem sample collection from lorikeets with LPS presenting to the RSPCA, AZWH and GVC
Anaesthetized lorikeets were euthanased with diluted intravenous pentobarbital sodium (Lethabarb, Virbac, NSW, Australia). Tissue samples collected at the AZWH included liver, kidneys, heart, lungs, proventriculus, ventriculus, intestinal tract, spleen, bursa of Fabricius (if present), gonads, adrenal glands, pancreas, brain, sections of the cervical, notarial and synsacral vertebral column, pectoral muscle, trachea, syrinx, skin and sciatic nerve. Additional samples collected from lorikeets from the RSPCA and GVC included thyroid, parathyroid, tongue, oral mucosa, tibiotarsus and surrounding muscle. The tibiotarsus was fractured to test bone strength. Tissues were formalin‐fixed. Sections of the vertebral column and tibiotarsus were demineralised in 14% ethylenediaminetetraacetic acid solution. All tissues were paraffin‐embedded, sectioned at 4 μm and stained with hematoxylin and eosin. Fresh liver, kidney and digestive tract contents were collected, frozen and stored at −20°C. Samples were collected under the Opportunistic Sample Collection Program of the Taronga Animal Ethics Committee (# R17B252).
Toxicological investigation of lorikeets with LPS presenting to the RSPCA and GVC and avian influenza (AI) and exotic Newcastle disease (END) exclusion (RSPCA)
Metal analysis
Metal analysis was done on liver and kidney tissues obtained from 13 rainbow lorikeets collected at the RSPCA (12 lorikeets affected by LPS and a non‐LPS case) as previously described (Table 1). 9 The results of testing are reported as mg/kg dry weight. The normality of the range of metal values was tested by the Shapiro–Wilk test and the assessment of Q–Q plots and histograms. Given that the data sets were predominately not normal, a Wilcoxon signed rank test was used for comparisons of metal concentrations in liver and kidney of lorikeets to those of grey‐headed (Pteropus poliocephalus) and black flying‐foxes (Pteropus alecto) 9 : species that feed on similar plant items. 10 , 11 Differences were considered significant if the P value was <0.05 after applying the Bonferroni correction for multiple comparisons. Median values and ranges of metals in the livers and kidneys of commercially raised chickens were also compared with the rainbow lorikeet tissues. 12 , 13 A statistical comparison to those of the chickens was not possible as the raw values for the chicken samples were not available. Additional testing for toxins and elements, including metals, was done on samples submitted to commercial laboratories (Table 1). The elements screened for by the Environmental Forensics Laboratory are reported as wet weight (Appendix A). They were converted to dry weight by multiplying values by four (assuming that they contained approximately 75% moisture) 14 and compared with the dry weight values determined for the 2017 lorikeets. Samples submitted for pesticide screening at the Environmental Forensics Laboratory were screened using gas chromatography with mass spectrometry and by liquid chromatography with mass spectrometry. The pesticides screened by gas chromatography are listed in Appendix B.
Avian influenza (AI) and Newcastle disease (ND)
To rule out the presence of AI and ND, oral swabs from five lorikeets were submitted to the Biosecurity Sciences Laboratory, Department of Agriculture and Fisheries, QLD, Australia (Table 1).
Results
Numbers, ages and temporal distribution of LPS cases presenting to the RSPCA
A total of 4035 rainbow lorikeets were admitted to the RSPCA during 2017 and 2018. Of these, 1119 (28%) were diagnosed with LPS. Five were juveniles and 1114 were subadults or adults. Most cases presented between October and June (Figure 1). Fewer presented in July 2017 (n = 2), September 2017 (n = 2) and July 2018 (n = 2). Total numbers of non‐LPS lorikeets and lorikeets with LPS presenting per month and the percentage of lorikeets per month presenting with LPS increased from October until they peaked in January and then declined again until June (Figure 1).
Figure 1.
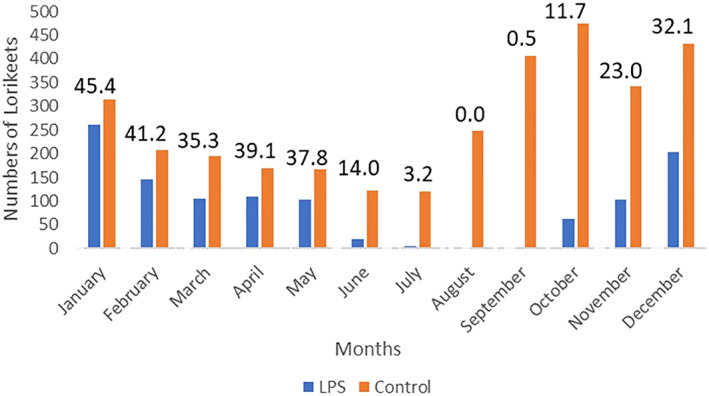
Temporal distribution of control and lorikeet paralysis syndrome affected lorikeets presenting to the RSPCA during 2017 and 2018. Values above the bars are the percentage of lorikeet paralysis cases as a function of the total number of lorikeets presented during that month.
Spatial distribution of LPS and non‐LPS cases submitted to the RSPCA
The spatial distribution of the sources of the LPS and non‐LPS cases is shown (Figure 2A,B). Significant (P < 0.0001) clustering of both LPS cases and non‐LPS cases (nearest neighbour indices 0.1372 and 0.0895, respectively) was found. LPS cases were most clustered during September 2017 to May 2018. Clusters of LPS cases were noted in both the interpolated map and the scan statistic at two locations south (primary) and south‐west (secondary) of Brisbane (Figure 2B). The primary cluster (radius 4.67 km; P < 0.0001) occurred between 10 December 2017 and 7 March 2018 (66 cases, 25 expected) and the secondary cluster (radius 4.57 km; P < 0.0001) occurred between 23 January 2018 and 14 March 2018 (33 cases, 9.3 expected) (Figure 2B). A cluster was also found in the Sunshine Coast (Figure 2A). This cluster (P < 0.0001), which occurred at a single location, lasted between 6 January and 5 June 2018 (33 cases, 11 expected). None of the locations where lorikeets were found included nocturnal roosting sites.
Figure 2.
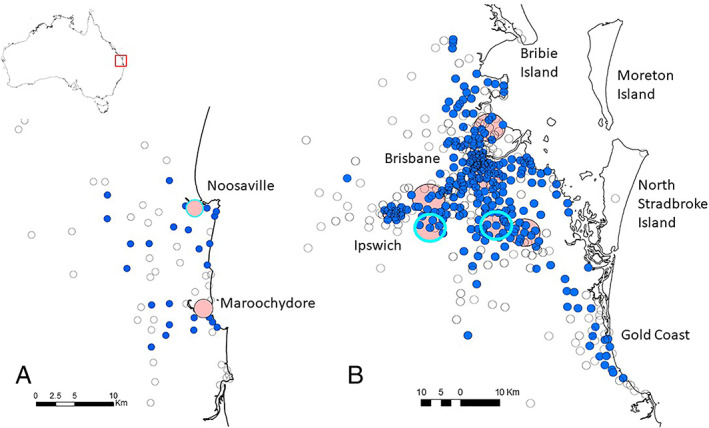
(A,B) Proportional symbol maps of cases of lorikeet paralysis syndrome (solid blue circles) and non‐lorikeet paralysis syndrome cases (empty circles) in rainbow lorikeets (Trichoglossus haematodus) in the Sunshine Coast area (A) and Brisbane, QLD area (B). Clusters are shown as pink‐filled circles and statistically significant clusters of cases (P < 0.0001) are indicated by cyan circles.
Body condition, severity of signs at presentation and outcome of treatment of lorikeets with LPS submitted to the RSPCA
The percentage of lorikeets presenting with LPS for which body condition scores were reported in 2017 (n = 304) were 17.8% (one), 24.7% (two), 46.7% (three), 6.6% (four) and 2.6% (five). The severity of signs exhibited by lorikeets with LPS is shown (Table 2). The largest percentage of lorikeets presented with advanced signs (Category 1) followed in descending order by Category 2, 3 and 4. Most of the Category 1 cases were euthanased given that they had a poor prognosis. However, of the 27 lorikeets in Category 1 that were treated, and the outcome known, 60% were released. The majority of lorikeets presenting in Categories 2, 3 and 4 were treated; treatment success significantly increased from 80% (Category 2) to 93% (Category 4) (Table 2). The average number of days in hospital and the average number of days in rehabilitation as a function of category on presentation are shown (Table 3). The average number of days in hospital decreased significantly as the severity of signs on presentation decreased. A similar trend was seen for the numbers of days in rehabilitation; however, the mean of Category 1 lorikeets was not significantly lower than the median of Category 2 and Category 3 lorikeets. Total time in care decreased significantly as the severity of signs decreased (Table 3).
Table 2.
Severity of signs, numbers treated and outcome of treatment
| Category | Total | Percent | Euthanased | Treated | Died after treatment | Treated lorikeets released | Outcome unknown |
|---|---|---|---|---|---|---|---|
| 1 | 571 | 50 | 543 | 28 | 10 | 17 (60%) | 1 |
| 2 | 264 | 24 | 65 | 199 | 19 | 161 (80%) a | 19 |
| 3 | 154 | 14 | 16 | 138 | 6 | 117 (85%) a | 15 |
| 4 | 130 | 12 | 3 | 127 | 3 | 118 (93%) a , b | 6 |
| Total | 1119 | 100 | 627 | 492 | 38 | 413 (84%) | 41 |
Percentage of lorikeets released is significantly higher (P ≤ 0.05) than in Category 1.
Percentage of lorikeets released is significantly higher (P < 0.05) than in Category 2.
Rainbow lorikeets with lorikeet paralysis syndrome, Royal Society for the Prevention of Cruelty to Animals 2017 and 2018.
Table 3.
Median and range of the numbers of days rainbow lorikeets with lorikeet paralysis syndrome spent in hospital and rehabilitation and combined hospital and rehabilitation days as a function of the severity of signs (category) on presentation
| Category on presentation | Median (range) of days in hospital | Median (range) of days in post‐hospitalisation rehabilitation | Median (range) of total days in care |
|---|---|---|---|
| 1 | 38 (22–73) | 68 (11–64) | 106 (63–117) |
| 2 | 24 (7–51) a | 51 (5‐204) | 75 (20‐247) a |
| 3 | 21 (7‐50) a , b | 49 (5‐180) b , c | 70 (18–222) a , b |
| 4 | 16 (4‐39) a , b , c | 37 (6‐85) a , b , c | 53 (13–96) a , b |
Days were significantly fewer (P < 0.05) than Category 1 lorikeets.
Days were significantly fewer (P < 0.05) than Category 2 lorikeets.
Days were significantly fewer (P ≤ 0.05) than Category 3 lorikeets.
Only lorikeets that lived to be released are included in these calculations.
Haematologic and biochemical findings from lorikeets presenting with lorikeet paralysis syndrome to the RSPCA and the GVC
Haematologic findings
Polychromasia was not observed in nine lorikeets (56%) and 1+ polychromasia was present in seven lorikeets (44%). All lorikeets had adequate numbers of thrombocytes. Twelve lorikeets (75.0%) had a moderate to marked leucocytosis, two (12.5%) had leucocyte counts within the expected range, and two (12.5%) had a leucopenia. Leucocytosis was the result of a moderate to marked heterophilia and mild to moderate monocytosis. Fourteen lorikeets had a lymphopenia. Both lorikeets hospitalised for 20 or more days had a leucocytosis, heterophilia and lymphopenia (Table 4).
Table 4.
Haematologic findings in rainbow lorikeets presenting with lorikeet paralysis syndrome
| Expected range a , b | Acute c RSPCA n = 11 mean (range) | Acute GVC n = 5 mean (range) | In care d RSPCA n = 2 actual values | |
|---|---|---|---|---|
| Total white blood cell count | 8.0–13.0 |
22.7 (4.9–36) Leucocytosis n = 8 Leucopenia n = 2 |
18.9 (10.2–35.2) Leucocytosis n = 4 |
19.2, 19.8 Leucocytosis n = 2 |
| Heterophil count | 3.2–7.8 |
20.44 (4.4–38.4) Heterophilia n = 9 |
17.8 (10.0–35.2) Heterophilia n = 5 |
11.7, 18.4 Heterophilia n = 2 |
| Lymphocyte count | 1.8–9.0 |
1.06 (0.2–3.6) Lymphopenia n = 9 |
0.73 (0–1.2) Lymphopenia n = 5 |
0.8, 1.2 Lymphopenia n = 2 |
| Monocyte count | 0.0–1.6 |
1.4 (0.3–2.6) Monocytosis n = 3 |
0.54 (0–1.2) | 0.6, 0.8 |
Cells × 103/μL.
Adapted from Hawkins et al. 15
Samples were collected on the day of presentation.
Samples were collected from lorikeets in hospital for 20 or more days.
GVC, Grafton Veterinary Clinic; RSPCA, Royal Society for the Prevention of Cruelty of Animals.
Plasma biochemistries
Biochemical analysis was undertaken on plasma of 16 LPS lorikeets (Table 5). Because there was insufficient plasma from some, only 15 values were available for some analytes and several analytes were not determined for lorikeets from the GVC. Striking findings were marked increases in the creatinine phosphokinase and similar but less severe increases in aspartate aminotransferase and moderate to marked increases in uric acid in most lorikeets. A mild to moderate increase in plasma amylase was seen in six of 16 lorikeets. Mild increases in plasma phosphorus concentrations and a mild to moderate hypernatremia and hyperchloremia were also seen.
Table 5.
Plasma biochemical findings in rainbow lorikeets with lorikeet paralysis syndrome, Royal Society for the Prevention of Cruelty to Animals (RSPCA) and Grafton veterinary clinic (GVC)
| Biochemical parameter | Expected range a | RSPCA mean (range) | GVC mean (range) |
|---|---|---|---|
| Creatine phosphokinase (U/L) | <600 |
10,492 (683–56,623) 11/11 elevated |
3700 (664–9940) 3/4 elevated |
| Aspartate amino transferase (U/L) | 120–360 |
1307 (241–4714) 8/11 elevated |
579 (222–1146) 3/4 elevated |
| Uric acid (umol/L) | 178–297 |
576 (122–892) 8/11 elevated |
972 (533–1516) 4/4 elevated |
| Calcium, uncorrected (mmol/L) | 2.1–2.7 |
2.0 (1.3–2.5) 7/11 low |
ND |
| Calcium, corrected (mmol/L) | 2.0–2.8 |
2.2 (1.5–2.2) 1/10 low |
ND |
| Phosphorus (mmol/L) | 1.0–1.6 |
1.85 (0.9–2.1) 9/11 elevated |
ND |
| Calcium:phosphorus ratio | 2:1 | 1.3 (0.8–2.5) | ND |
| Protein total (g/L) | 19–41 |
31.0 (20–44) 1/11 elevated |
29.0 (24–35) |
| Albumin (g/L) | 13–21 |
0.9 (7–14) 7/10 low |
12.75 (11–15) 1/4 low |
| Gamma glutamyl transferase (U/L) | <10 | 5.5 (3.0–6.0) | 7.8 (7–9) |
| Bile acid (umol/L) | < 70 | 4.0 (1.0–8.4) | 11.0 (2.9–17.7) |
| Triglycerides (mmol/L) | 0.4–1.2 | 0.8 (0.5–0.8) | 0.50 (0.49–0.94) |
| Cholesterol (mmol/L) | 3.6–10.6 |
7.3 (2.5–11.5) 1/11 low, 1/11 elevated |
7.6 (6.5–8.5) |
| Amylase (U/L) | 228–876 |
936 (238–1900) 3/11 elevated |
1069 (599–1813) 3/4 elevated |
| Glucose (mmol/L) | 11–25.5 |
17.4 (13–28.5) 1/11 elevated |
15.1 (12.9–18.1) |
| Potassium (mmol/L) | 2.5–3.5 |
3.8 (3.0–4.5) 6/11 mildly elevated |
ND |
| Sodium (mmol/L) | 145–149 |
157 (45–171) 10/11 elevated |
ND |
| Chloride (mmol/L) | 110–117 |
119 (109–127) 1/11 low, 7/11 slightly elevated |
ND |
Adapted from Hawkins et al. 15
ND, not done.
Necropsy findings of lorikeets with LPS presenting to the RSPCA, AZWH and GVC
Gross findings were available from 20 lorikeets, and tissues from 28 lorikeets were examined histologically. There were no consistent gross or microscopic lesions that would explain the signs observed in the lorikeets with LPS. Two lorikeets had traumatic injuries consistent with an attack by a predator. A single lorikeet had a ruptured liver and perihepatic haemorrhage suggesting blunt force trauma. Two lorikeets had dilation of their cloaca and one had dilation of the entire intestinal tract, suggesting a neurogenic ileus. Incidental gross findings included air sacullitis (n = 3), parathyroid hyperplasia (n = 9) and serpentine deviations of the keel consistent with metabolic bone disease when a nestling (n = 7). Tibiotarsal bone strength, reflecting recent calcium intake, was normal in all lorikeets.
Lesions of the CNS included mild focal haemorrhage of the cerebrum (n = 3), a thrombosed small vessel in the cerebrum (n = 1), extensive thrombosis of vessels in a section of the thoracic spinal cord in a lorikeet with aspergillus, and extensive haemorrhage in sections of the spinal cord and adjacent muscle (n = 1). The haemorrhagic brain lesions were thought to be caused by trauma and would not cause the signs observed in these lorikeets. Similarly, the thrombotic lesion seen in the brain would not have resulted in the observed signs. The thrombotic and haemorrhagic lesions seen in the spinal cord in two lorikeets were severe and could have accounted for the signs observed and as a result these birds were removed from the study. Metastatic mineralization of soft tissues, a lesion seen in cadmium poisoned animals 16 and other aberrations of calcium, phosphorus and vitamin D homeostasis, was present in four lorikeets. Muscle haemorrhage (n = 1) and mild focal to focally extensive myodegeneration (n = 3) were seen. Parathyroid hyperplasia was detected in six lorikeets. However, adjusted calcium levels were in the expected range 15 and hypocalcaemia was not considered as a differential.
Toxicological findings of lorikeets with LPS and AI and END exclusion
The results of the metal analysis from 13 rainbow lorikeets with LPS and one non‐LPS lorikeet submitted to the RSPCA in 2017 are shown (Table 6). One lorikeet had toxic concentrations of liver cadmium (145 mg/kg dry weight). 10 A second lorikeet had toxic concentrations of liver lead (81 mg/kg dry weight). 17 Since the renal concentrations of these elements were not elevated in either lorikeet, these findings are consistent with recent exposure. 18 Evidence for recent exposure to cadmium in other lorikeets is shown by the significant increase in cadmium liver concentrations compared with values from flying‐fox liver and the higher cadmium concentration in the liver compared with the kidney. The non‐LPS lorikeet also had evidence of recent cadmium exposure. All lorikeets had liver cadmium concentrations that exceeded the highest concentrations found in domestic chickens. With the exception of the one lorikeet with evidence for recent exposure to lead, liver and kidney concentrations of lead in the lorikeets were not significantly different than those in flying‐foxes.
Table 6.
Metal concentrations (mg/kg dry weight) of kidney and liver tissues in lorikeet paralysis syndrome (LPS) rainbow lorikeet cases and a non‐LPS case
| Element | Magnesium | Aluminium | Chromium | Manganese | Iron | Cobalt | Nickel | Copper | Zinc | |
|---|---|---|---|---|---|---|---|---|---|---|
| Liver | LPS lorikeet | |||||||||
| Median | 605 | 16.4 | 0.270 | 4.60 | 747 | 0.123 | 1.088 | 13.3 | 101.1 | |
| Range | 508–786 | 11.6–21.8 | 0.07–0.59 | 2.60–14.35 | 573–1039 | 0.04–0.28 | 0.77–1.58 | 5.3–28.4 | 56.7–196.7 | |
| Control lorikeet | 565 | 15.9 | 0.22 | 3.95 | 818 | 0.11 | 0.96 | 14.1 | 82.6 | |
| Chicken liver | ||||||||||
| Median | 608 | No value | 0.29 | 9.00 | 449 | 0.06 | 0.88 | 10.5 | 106 | |
| Range | 469–710 | 0.11–0.42 | 7.46–11.3 | 263–550 | 0.04–0.12 | 0.48–1.31 | 7.55–4.8 | (81.5–128) | ||
| Flying‐fox liver | ||||||||||
| Median | 393.3 | 0.24 | 3.32 | 390 | 0.1 | 0.74 | 8.91 | 58.1 | ||
| Range | 218–550 | 0.05–0.77 | 1.69–4.99 | 212–855 | 0.02–0.20 | 0.42–1.70 | 5.92–79.0 | 44.4–242 | ||
| Wilcox output |
W = 6 P < 0.001 |
Na | Ns | Ns |
W = 10 P < 0.001 |
Ns |
W = 2 P = 0.011 |
Ns |
W = 32 P = 0.037 |
|
| P value after Bonferroni correction | 0.001 | Ns | Ns | 0.004 | Ns | 0.180 | Ns | 0.636 |
| Element | Arsenic | Selenium | Cadmium a | Tin | Antimony | Mercury | Thallium | Lead b | Bismuth | |
|---|---|---|---|---|---|---|---|---|---|---|
| Liver | LPS lorikeet | |||||||||
| Median | 0.027 | 3.593 | 9.38 | 0.257 | 0.016 | 0.151 | 0.017 | 0.505 | 0.003 | |
| Range | 0.0–0.074 | 1.51–7.50 | 0.83–21.45 | 0.144–0.733 | 0.007–0.030 | 0.069–0.261 | 0.002–0.040 | 0.13–0.79 | 0.001–0.006 | |
| Control lorikeet | 0.034 | 3.712 | 25.85 | 0.132 | 0.013 | 0.110 | 0.046 | 0.465 | 0.004 | |
| Chicken liver | ||||||||||
| Median | 0.04 | 2.31 | 0.04 | 0.03 | 0.09 | 0.28 | 0.03 | 0.06 | <0.01 | |
| Range | 0.01–0.10 | 1.79–2.80 | 0.02–0.15 | <0.01–0.14 | 0.05–0.17 | 0.12–0.61 | 0.01–0.04 | 0.03–0.07 | <0.01–0.03 | |
| Flying‐fox liver | ||||||||||
| Median | 0.07 | 1.57 | 1.45 | 0.04 | 0.04 | 0.38 | <0.01 | 0.62 | <0.01 | |
| Range | 0.05–0.16 | 0.64–3.12 | 0.02–12.6 | 0.02–0.01 | <0.01–0.06 | <0.01–4.47 | <0.01–0.04 | 0.14–3.39 | <0.01–0.04 | |
| Wilcox output |
W = 125 P < 0.001 |
W = 11 P < 0.001 |
W = 16 P = 0.001 |
W = 7 P < 0.001 |
W = 11 P = 0.004 |
W = 110 P = 0.006 |
W = 32 P = 0.037 |
Ns |
W = 99 P = 0.044 |
|
| P value after Bonferroni correction | 0.001 | 0.005 | 0.022 | 0.001 | 0.076 | 0.096 | 0.636 | Ns | 0.746 |
| Element | Magnesium | Aluminium | Chromium | Manganese | Iron | Cobalt | Nickel | Copper | Zinc | |
|---|---|---|---|---|---|---|---|---|---|---|
| Kidney | LPS lorikeet | 111.731 | ||||||||
| Median | 598 | 12.0 | 0.305 | 9.50 | 1763 | 0.053 | 1.026 | 11.0 | ||
| Range | 452–831 | 6.2–26.8 | 0.89–0.69 | 6.53–18.53 | 655–4587 | 0.03–0.13 | 0.60–1.45 | 6.45–17.66 | 47.4–275.2 | |
| Control lorikeet | 762 | 16.1 | 0.189 | 12.86 | 4978 | 0.069 | 1.291 | 8.36 | 107.72 | |
| Chicken kidney | ||||||||||
| Median | 275.03 | 7.90 | 99.72 | 5.42 | 88.39 | 0.0450 | 0.023 | 3.92 | 28.67 | |
| ±standard deviation | ±8.17 | ±2.22 | ±6.24 | ±0.15 | ±14.65 | ±0.0082 | ±0.003 | ±0.20 | ±1.02 | |
| Flying‐fox kidney | ||||||||||
| Median | 547 | Na | 0.48 | 4.04 | 294 | 0.13 | 1.79 | 14.0 | 92.2 | |
| Range | 377–657 | 0.27–1.51 | 2.18–5.85 | 216–704 | 0.04–1.14 | 1.10–2.79 | 7.75–18.7 | 68.5–20 | ||
| Wilcox output | Ns | Na |
W = 97 P = 0.02 |
W = 0 P < 0.001 |
W = 1 P < 0.001 |
W = 100 P = 0.008 |
W = 116 P < 0.001 |
Ns | Ns | |
| P value after Bonferroni correction | Ns | Na | P = 0.27 | P < 0.001 | P < 0.001 | P = 0.142 | P < 0.001 | Ns | Ns |
| Element | Arsenic | Selenium | Cadmium | Tin | Antimony | Mercury | Thallium | Lead | Bismuth | |
|---|---|---|---|---|---|---|---|---|---|---|
| Kidney | LPS lorikeet | |||||||||
| Median | 0.015 | 1.890 | 1.771 | 0.222 | 0.011 | 0.35 | 0.01 | 0.349 | 0.003 | |
| Range | 0–0.05 | 1.14–3.27 | 0.16–6.05 | 0.04–1.05 | 0.003–0.019 | 0.11–0.74 | 0.00–0.015 | 0.09–0.89 | 0.001–0.006 | |
| Control | 0.038 | 2.141 | 1.882 | 0.346 | 0.080 | 0.314 | 0.035 | 0.853 | 0.003 | |
| Chicken kidney | ||||||||||
| Median | 0.008 | 0.534 | 0.38 | 0.042 | 0.003 | 0.004 | 0.003 | 0.153 | 0.295 | |
| ±standard deviation | ±1.77 | ±0.018 | ±0.10 | ±0.006 | ±0.0005 | ±0.001 | ±0.0002 | ±0.015 | ±0.065 | |
| Flying‐fox kidney (median and range) |
0.11 0.06–0.18 |
2.82 1.58–4.10 |
29.8 0.27–219 |
0.05 0.02–0.14 |
0.04 0.02–0.06 |
0.85 0.11–2.62 |
0.02 <0.01–0.04 |
0.59 0.19–1.86 |
0.01 0.01–0.02 |
|
| Wilcox output |
W = 121 P < 0.001 |
W = 99 P = 0.01 |
W = 108 P = 0.001 |
W = 23 P = 0.013 |
W = 121 P < 0.001 |
W = 99 P = 0.01 |
W = 101 P = 0.007 |
W = 95 P = 0.023 |
W = 121 P < 0.001 |
|
| P value after Bonferroni correction | 0.001 | 0.177 | 0.018 | 0.218 | < 0.001 | 0.177 | 0.113 | 0.396 | 0.001 |
One lorikeet had a toxic concentration of cadmium (145 mg/kg), 8 this value was removed from the analysis.
One lorikeet had a toxic concentration of lead in the liver (81 mg/kg), 14 this value was removed from the analysis.
Lorikeet values are compared with metal concentrations from chickens, 10 , 11 and grey‐headed and black flying‐foxes. 8
na, not applicable; ns, not significant.
Liver and kidney iron concentrations were significantly higher than flying‐fox values and kidney iron concentrations were elevated compared with liver iron concentrations. All values were higher than the upper value found in chicken liver and kidney. Liver and kidney tin concentrations were significantly higher than concentrations in the flying‐foxes and equal to or higher than those found in chickens, but below toxic concentrations. 19 Selenium concentrations were similar to those seen in the flying‐foxes and chicken controls and were considered adequate. Significantly but only moderately elevated concentrations of magnesium were found in the livers of lorikeets compared with flying‐foxes, and significantly but only mildly increased concentrations of manganese concentrations were found in the kidney of the lorikeets compared with the flying‐fox. Median values of liver magnesium and kidney manganese were also increased than those in chickens.
Liver samples from six lorikeets with LPS from the GVC and five LPS and three non‐LPS lorikeets from the RSPCA were tested for 29 elements (Appendix 1). Cadmium was not detected in the liver samples from the GVC lorikeets. Low‐level exposure to chromium was found in one. Toxic concentrations of other elements were not detected. Cadmium exposure was detected in both the non‐LPS and LPS lorikeets from the RSPCA. Median concentrations of cadmium were 1.6 mg/kg and 2.8 mg/kg dry matter in the liver of the LPS and non‐LPS lorikeets, respectively, and pooled kidney concentrations were 22.4 and 15 mg/kg dry weight in LPS and non‐LPs lorikeets, respectively. These values contrasted with those found in lorikeets tested in 2017, in that concentrations were higher in the kidney compared with the liver, suggesting a significant but not recent exposure, whereas the concentrations of cadmium were higher in the liver in the 2017 lorikeets suggesting recent exposure. Median iron concentrations in LPS (1944 mg/kg liver and 1000 kidney mg/kg dry weight) and non‐LPS (1548 mg/kg liver and 1700 kidney mg/kg dry weight) lorikeets from the RSPCA in 2020 were as high or higher than those found in rainbow lorikeets sampled from the RSPCA in 2017.
Pesticides, botulism toxins and alcohol were not detected. Cholinesterase levels were within the expected range ruling out exposure to organophosphate pesticides. Infection with avian influenza and Newcastle disease was excluded.
Discussion
Lorikeet paralysis syndrome is a common disease of rainbow lorikeets presenting to wildlife carers in south‐east QLD and north‐east NSW. 1 , 2 The importance of this disease is reflected in the findings of this study where 1119 cases of LPS, representing 26% of all lorikeet submissions, presented to the RSPCA in just 2 years.
Our findings show that LPS is a seasonal disease beginning in October with cases continuing until June, peaking in December, January and February. Therefore, LPS should be the primary differential diagnosis for lorikeets presenting with paresis and paralysis, voice change, inability to blink, and/or inability to swallow during the months of October through June in north‐east NSW and southern QLD. There is no sex predilection and most lorikeets will be subadults or adults. Lorikeets with LPS can present with normal or increased body condition scores (61%) or with varying degrees of pectoral muscle wasting (39%). Lorikeets presenting with normal or increased body condition likely reflect the acute onset of this disease, with most lorikeets being found soon after developing signs. In contrast, lorikeets with pectoral muscle atrophy are likely to have been down on the ground and unable to feed for a day or more. Changes in complete blood counts and plasma biochemistries are not specific for LPS but would support that diagnosis. Changes consistently seen in LPS affected lorikeets are heterophilia and lymphopenia, a marked elevation in muscle enzymes and uric acid, and elevations in sodium and chloride concentrations. The cause of the heterophilia and lymphopenia is not known but may represent a stress differential. 20
Given lorikeets with LPS have significant neurological deficits, it is expected that they may be prone to injury, including trauma and predation. One lorikeet submitted to the RSPCA was removed from the study because it had significant traumatic injuries to the spinal cord. Although trauma could have accounted for some of the signs observed in this lorikeet, it also exhibited signs consistent with LPS, therefore definitive diagnosis is uncertain. Four other lorikeets with LPS also had evidence of trauma. Therefore, it is possible that lorikeets in the early stages of LPS may have a compromised ability to navigate in flight and be more likely to run into objects or be hit by cars. In this case, lorikeets presenting with trauma, in some instances, may also have LPS. Given the lack of distinctive gross and histological lesions in birds with LPS, definitive postmortem diagnosis of the syndrome is not possible.
Lorikeets in Category 1 appear to have a fair (60%) chance of recovery; however, they will require intensive care, long hospital stays and long rehabilitation periods and therefore are likely to tax the resources of wildlife veterinarians and carers. In contrast, the prognosis for recovery improves and the time in care diminishes when lorikeets present with less severe signs, making these cases better candidates for treatment.
Our findings suggest that lorikeets with LPS would benefit with treatments that support kidney function, correct electrolyte abnormalities and relieve pain that might be associated with muscle injury. 21 The bulk of the lorikeets presenting with LPS in this study had increases in muscle enzymes and uric acid. The cause of the elevation of muscle enzymes is not known, as microscopic lesions of the muscle were not seen. Differentials for the elevated muscle enzymes are many but include trauma, exertional myopathy and a direct impact of a toxin on the muscle. Differentials for the elevated uric acid levels are also many but include a myoglobulin nephropathy, interference with uric acid secretion by a toxin, and increased metabolism of protein in a starved bird. 21 , 22 It is unlikely that the uric acid elevations were caused by dehydration, as uric acid levels are regulated by active secretion by the proximal tubule cells and not by glomerular filtration and uric acid does not elevate until there is a severe dehydration. 23 Additionally, we did not appreciate that these birds were severely dehydrated on presentation and urate sludging in the ureters and tubules, a characteristic of advanced dehydration, was not observed in the necropsy specimens. No matter what the cause of the elevation in uric acid and CPK, diuresis should be instituted, either by administration of parenteral or oral fluids, to support kidney function and correct electrolyte imbalances. 21
The aetiology of LPS is not known. Previous studies in VIC of lorikeets presenting with CNS signs found that most had a non‐suppurative encephalitis suggestive of a viral infection. 3 More recently, lorikeets submitted with CNS signs and leg flexion in the Greater Sydney area also had a non‐suppurative encephalitis, and avulavirus RNA was found in brain tissues. 6 That a virus was the cause of LPS cases in the current study is highly unlikely as no inflammatory lesions were found in the brains examined. Likewise, the lorikeets with non‐suppurative encephalitis presented throughout the year and many showed central nervous system signs not observed in the LPS cases described in this study.
The absence of any evidence of an infectious cause of LPS, plus the sudden onset of ascending flaccid paralysis that occurs in lorikeets with LPS, which is characteristic of disruption of the neuromuscular junction, suggests a toxin is the likely aetiology of LPS. However, the findings of this study argue that known toxins that can cause neurological signs in wild birds, including pesticides, botulinum toxins and alcohol, are not the cause of LPS and additional toxins should be considered.
In a previous study in the Greater Sydney area, elevated tissue concentrations of cadmium and elevated blood and tissue lead concentrations were found in lorikeets presenting with CNS signs and flexed legs. 1 Microscopically, a lesion associated with cadmium toxicity, mineralisation of soft tissues, was a common finding in these lorikeets. 1 In the current study, all but one of the lorikeets (n = 12) from RSPCA in 2017 were found to have evidence of recent exposure to cadmium. 16 But with the exception of a single lorikeet, the cadmium concentrations were not in the toxic range and would not have been expected to cause neuromuscular disease. Also, cadmium was not found to be significantly elevated in lorikeets from GVC and metastatic mineralisation was rare in all the lorikeets. Likewise, elevated kidney concentrations were found in lorikeets from the RSPCA in 2020, however concentrations were similar in both LPS and non‐LPS lorikeets. Therefore, it appears that cadmium exposure is an incidental finding.
Elevated tissue concentrations of cadmium in these lorikeets have implications beyond lorikeets themselves as it suggests exposure to an environmental source of cadmium, a source that might also pose a risk to humans and other animals. Cadmium exposure to animals on the East Coast of Australia is not limited to lorikeets. Pulscher et al. 10 found elevated concentrations of cadmium in liver and kidney samples obtained from flying‐foxes from the Greater Sydney area. Given that these flying‐fox species are nomadic and include south‐east QLD in their range, it is possible that they were exposed to the same source as the lorikeets. Alternatively, as cadmium exposure has also been documented in lorikeets from Sydney, 1 environmental cadmium contamination may be widespread or associated with urban areas. Potential environmental sources of cadmium include metal ore mining and processing facilities, power plants that burn fossil fuels, lubricating oils, fuel tanks, batteries and petrol. 26
One lorikeet had toxic liver lead concentrations (24.3 mg/kg wet weight) 17 and was likely to be experiencing signs of lead poisoning. However, unlike the lorikeets studied in the Sydney area, 1 lead concentrations in the other lorikeets were low and therefore did not contribute to the signs of LPS. Additional metal testing showed no evidence of arsenic, copper, mercury, selenium and zinc toxicity. 18 Neither was there evidence that these lorikeets were exhibiting deficiencies in copper, selenium or zinc. Liver concentrations of tin were elevated in lorikeets with LPS collected in 2017 compared with the chicken and flying‐fox samples. Although tin concentrations were on average nine times higher than those found in chicken livers 11 and six times higher than those found in flying‐fox livers, 8 these values were not considered toxic. 19
Median liver and kidney iron concentrations were mildly to moderately elevated in the LPS and control lorikeets from the RSPCA and GVC. However, liver concentrations were lower than median liver iron concentrations causing toxicity in other species of birds. 24 Little is known about kidney iron concentrations in lorikeets exposed to high iron concentrations and so these values could not be compared. The increased liver and kidney concentrations may be a natural phenomenon given that figs (Ficus spp.), a natural food source for lorikeets, are relatively high in iron. 10 Elevated kidney iron concentrations may represent an attempt to excrete iron into the urine as a means of eliminating excess iron. A similar means of iron excretion has also been proposed for flying‐foxes. 10
Given infectious diseases, clostridial toxins, alcohol and large number of anthropogenic toxins were not found to be associated with LPS, the remaining likely cause for LPS is a relatively fast‐acting plant toxin that works at the level of the neuromuscular junction. The seasonal occurrence of LPS suggests that the source of the toxin only blooms or has fruit during the warmer months and has a relatively limited range to that of northern NSW and southern QLD. We suggest that the toxin takes effect quickly but not immediately. None of the lorikeets presenting with LPS had food in their digestive system, so signs do not occur immediately upon ingestion. However, lorikeets with LPS were not found under the major roosts in south‐east QLD, so lorikeets must be impacted prior to returning to roost at night. Therefore, we suggest that the next step in seeking the cause of LPS is tracking blossoming and fruiting patterns of plants that lorikeets feed on and correlating them with the areas in which lorikeets with LPS are found or using DNA barcoding to identify plants consumed by lorikeets with LPS. 25
Conflicts of interest and sources of funding
The authors declare no conflicts of interest. Elements of this investigation were funded through Wildlife Rescue and Rehabilitation – an Australian Government initiative, the Sydney School of Veterinary Science, New South Wales Wildlife Information, Rescue and Education Service Inc. and National Significant Disease Investigation Program, Animal Health Australia.
Acknowledgments
We thank Robert McQuilty and the staff of the Department of Chemical Pathology, Royal Prince Alfred Hospital for their assistance with the element analysis. We also thank Anita Gordon and the staff of the Biosecurity Sciences Laboratory, Department of Agriculture and Fisheries, Queensland Government for testing samples for cholinesterase activity and exotic Newcastle disease and avian influenza, Christopher Doyle and the staff of the Environmental Forensics, Department of Planning, Industry and the Environment, New South Wales Government for the element and pesticide testing and Sam Hair of the Laboratory Animal Pathology DPIRD Diagnostics and Laboratory Services Sustainability and Biosecurity Department of Primary Industries and Regional Development Western Australia for testing for clostridial toxins.
A. Acid extractable concentrations of elements (mg/kg wet weight) from samples of liver (GVC and RSPCA), kidney and digestive system (RSPCA only) collected from lorikeets affected with lorikeet paralysis syndrome and control lorikeets as assessed by inductively coupled‐atomic emission spectroscopy
| Analyte | Liver Pool 1 affected three samples pooled. Grafton, NSW | Liver Pool 2 affected three samples pooled Grafton, NSW | Liver affected n = 5 mean range south‐east QLD | Liver control n = 3 mean range south‐east QLD | Kidney Pool affected five samples pooled. South‐east QLD | Kidney Pool control three samples pooled. South‐east QLD | GI Pool affected five samples pooled. South‐east QLD |
|---|---|---|---|---|---|---|---|
| Aluminium | 4 | 13 | ≤5 | <5 | <5 | <5 | 180 |
| Arsenic | <3 | <3 | <4 | <4 | <4 | <4 | <4 |
| Barium | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Beryllium | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Boron | <10 | <11 | <13 | <13 | <13 | <13 | <13 |
| Cadmium | <1 | <1 | 0.4 0.3–0.6 | 0.7 0.2–1.5 | 5.6 | 3.9 | 0.2 |
| Calcium | 66 | 94 | 116 78–210 | 156 68–230 | 250 | 400 | 680 |
| Chromium | <1 | 3 | <1 | <1 | <1 | <1 | <1 |
| Cobalt | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Copper | 6 | 7 | <4 | <4 | 5 | 4 | 5 |
| Iron | 670 | 600 | 486, 430–530 | 387, 230–590 | 250 | 430 | 230 |
| Lead | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Lithium | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Magnesium | 190 | 200 | 212, 180–230 | 223, 210–240 | 240 | 340 | 220 |
| Manganese | 3 | 3 | 3 | 3 | <2 | <2 | <2 |
| Mercury | ND | ND | <0.04 | <0.04 | <0.04 | <0.04 | ND |
| Molybdenum | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Nickel | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Phosphorus | 2700 | 2900 | 2960 2900–3100 | 2733 2700–2800 | 2800 | 2100 | 2500 |
| Potassium | 3100 | 3200 | 2960 2800–3100 | 2767 2600–2700 | 3000 | 2800 | 4000 |
| Selenium | <4 | <5 | <4 | <4 | <4 | <4 | <4 |
| Silver | <0.6 | <0.7 | <0.7 | <0.7 | <0.7 | <0.7 | <0.7 |
| Sodium | 1300 | 1400 | 1300 1100–1500 | 1400 1200–1500 | 1300 | 1200 | 1200 |
| Strontium | <3 | <3 | <3 | <3 | <3 | <3 | <3 |
| Sulphur | 2000 | 1900 | 2000 1900–2100 | 2000 1900–2100 | 1800 | 1900 | 2300 |
| Thallium | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Tin | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Titanium | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Vanadium | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Zinc | 27 | 38 | 27 | 27 | 27 | 24 | 20 |
B. Pesticide assayed for using gas chromatography with mass spectrometry
| Aldrin | DDD‐p,p′ | Fenithrotion | Oxyfluorfen |
| Allethrin | DDE‐p.p′ | Fenthion | Parathion |
| Alpha‐chlordane | DDT‐p,p′ | Fenvalerate | Phorate |
| Alpha‐HCH | Delta‐HCH | Gamma‐chlordane | Profenofos |
| Ametryn | Deltamethrin | Gamma‐HCh | Prometon |
| Atraton | Diazion | Heptachlor epoxide | Prometryn |
| Atrazine | Dichlorvos | Heptachlor | Propargite |
| Beta‐HCH | Dieldrin | Hexachlorobenzene | Propazine |
| Bifentrin | Dimethoate | Hexazione | Propetamphos |
| Carbophenothion | Endosulfan II | L‐Cyhalothrin | Simazine |
| Chlorpyrifos | Endosulfan 1 | Malathion | Simetryn |
| Cis‐pemethrin | Endosulfan sulfate | Methidathion | Tebuconazole |
| Crotoxyphos | Endrin Keton | Methozychlor,p,p′ | Terbuthylazine |
| Crotoxyphos | Endrin | Methyl Chlorpyrifos | Terbutryn |
| Cyfluthrin | Enthion | Methyl parathion | Tetrachlorvinphos |
| Cypermethrin | Fenamiphos | Mevinphos | Trans‐Permethrin |
Lacasse, C. , Rose, K. , Allen, M. , Ward, MP. , Pulscher, LA. , Giles, A. , Hall, J. and Phalen, DN. , Investigation into clinicopathological and pathological findings, prognosis, and aetiology of lorikeet paralysis syndrome in rainbow lorikeets (Trichoglossus haematodus). Aust Vet J. 2021;99:432–444. 10.1111/avj.13107
References
- 1. Rosenwax A, Phalen DN. Update on lorikeet paralysis . Proceeding Association of Avian Veterinarians Australasian Committee Annual Conference. Hobart, 2010:85–89.
- 2. Doneley B. Disorders of the nervous system. In: Avian medicine and surgery in practice. Manson Publishing/The Veterinary Press, London, 2011;203–211. [Google Scholar]
- 3. McOrist S, Perry RA. Encephalomyelitis in free‐living rainbow lorikeets (Trichoglossus haematodus). Avian Pathol 1986;15(4):783–789. [DOI] [PubMed] [Google Scholar]
- 4. Hartley WJ, Reece RL. Nervous diseases of Australian native and aviary lorikeets. Aust Vet Pract 1997;27:91–96. [Google Scholar]
- 5. Booth RJ, Hartley WJ, McKee JJ. Polioencephalomyelitis in rainbow lorikeets (Trichoglossus haematodus) . In: Martin A and Vogelnest L, editors. Veterinary Conservation Biology, Wildl Health Manag Australia. Proceedings of the International Joint Conference, Sydney 2001:157.
- 6. Chang W‐S, Eden J‐S, Hall J et al. Metatranscriptomic analysis of virus diversity in urban wild lorikeets with paretic disease. J Virol 2020;94:JVI.00606‐20. 10.1128/JVI.00606-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ward MP, Carpenter TE. Techniques for analysis of disease clustering in space and in time in veterinary epidemiology. Prev Vet Med 2000;45:257–284. [DOI] [PubMed] [Google Scholar]
- 8. Kulldorff M. Bernoulli, discrete Poisson and continuous Poisson models: A spatial scan statistic. Commun Stat Theory Methods 1997;26:1481–1496. [Google Scholar]
- 9. Fudge A. Laboratory medicine: Avian and exotic pets. Philadelphia, Saunders, 2000. [Google Scholar]
- 10. Pulscher LA, Gray R, McQuilty R et al. Investigation into the utility of flying foxes as bioindicators for environmental metal pollution reveals evidence of diminished lead but significant cadmium exposure. Chemosphere 2020;254:126839. [DOI] [PubMed] [Google Scholar]
- 11. Cannon CE. The diet of lorikeets Trichoglossus ssp. in the Queensland–New South Wales border region. Emu 1984;84:16–22. [Google Scholar]
- 12. Hu Y, Zhang W, Chen G et al. Public health risk of trace metals in fresh chicken meat products on the food markets of a major production region in southern China. Environ Pollut 2018;234:667–676. [DOI] [PubMed] [Google Scholar]
- 13. Zhang R, Wang Y, Wang C et al. Ameliorative effects of dietary selenium against cadmium. Toxicity is related to changes in trace elements in chicken kidneys. Biol Trace Elem Res 2017;176:391–400. [DOI] [PubMed] [Google Scholar]
- 14. Seong PN, Cho SH, Park KM et al. Characterization of chicken by‐products by mean of proximate and nutritional compositions. Korean J Food Sci An 2015;35(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hawkins MG, Barron HW, Speer BL et al. In: Carpenter JW, Marion CJ, editors. Exotic animal formulary. 4th edn. St. Louis, Missouri: Elsevier Health Sciences, 2012;184–438. [Google Scholar]
- 16. Brumbaugh WG, Mora MA, May TW et al. Metal exposure and effects in voles and small lorikeets near a mining haul road in Cape Krusenstern National Monument, Alaska. Environ Monit Assess 2020;170:73–86. [DOI] [PubMed] [Google Scholar]
- 17. Sriram A, Roe W, Booth M et al. Lead exposure in an urban, free‐ranging parrot: Investigating prevalence, effect and source attribution using stable isotope analysis. Sci Total Environ 2018;634:109–115. [DOI] [PubMed] [Google Scholar]
- 18. Keil DE, Berger‐Ritchie J, McMillin GA. Testing for toxic elements: a focus on arsenic, cadmium, lead, and mercury. Lab Med 2011;42:735–742. [Google Scholar]
- 19. Winship KA. Toxicity of tin and its compounds. Adverse Drug React Toxicol Rev 1988;7(1):19–38. [PubMed] [Google Scholar]
- 20. Gross WB, Siegel HS. Evaluation of lymphocyte/heterophils ratio as a measure of stress in chickens. Avian Dis 1983;27:972–979. [PubMed] [Google Scholar]
- 21. Clarkson PM. Exertional rhabdomyolysis and acute renal failure in marathon runners. Sports Med 2007;37:361–363. [DOI] [PubMed] [Google Scholar]
- 22. Handrich Y, Nicolas L, Maho Y. Winter starvation in captive common barn‐owls: physiological states and reversible limits. Auk 1993;110:458–469. [Google Scholar]
- 23. Lumeij J. Plasma urea, creatinine and uric acid concentrations in response to dehydration in racing pigeons (Columba livia domestica). Avian Pathol 1987;16:377–382. [DOI] [PubMed] [Google Scholar]
- 24. Olsen GP, Russell KE, Dierenfeld ED et al. A comparison of four regimens for treatment of iron storage disease using the European starling (Sturnus vulgaris) as a model. J Avian Med Surg 2006;20:74–79. [Google Scholar]
- 25. Dell'Agnello F, Natal C, Bertolino S et al. Assessment of seasonal variation of diet composition in rodents using DNA barcoding and real‐time PCR. Sci Rep 2019;9:14124–14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anonymous . Department of the Environment and Energy: Australian Government. Cadmium and compounds. Available at: http://www.npi.gov.au/resource/cadmium-and-compounds. Accessed 11 January 2020.


