Graphical abstract
Keywords: Intrusive fungus, Immunosuppressed, Sepsis, Corticosteroids, COVID-19
Abstract
Being considered minor vexations, fungal infections hinder the life of about 15% of the world population superficially, with rare threats to life in case of invasive sepsis. A significant rise in the intrusive mycoses due to machiavellian fungal species is observed over the years due to increased pathology and fatality in people battling life-threatening diseases. Individuals undergoing therapy with immune suppressive drugs plus recovering from viral infections have shown to develop fungal sepsis as secondary infections while recovering or after. Currently, the whole world is fighting against the fright of Coronavirus disease (COVID-19), and corticosteroids being the primitive therapeutic to combat the COVID-19 inflammation, leads to an immune-compromised state, thereby allowing the not so harmful fungi to violate the immune barrier and flourish in the host. A wide range of fungal co-infection is observed in the survivors and patients of COVID-19. Fungal species of Candida, Aspergillus and Mucorales, are burdening the lives of COVID-19 patients/survivors in the form of Yellow/Green, White and Black fungus. This is the first article of its kind to assemble note on fungal infections seen in the current human health scenario till date and provides a strong message to the clinicians, researchers and physicians around the world “non-pathological fungus should not be dismissed as contaminants, they can quell immunocompromised hosts”.
Introduction
The high degree of similarity between the host and the eukaryotic pathogen (fungus) often sets back the development of compounds against it. In addition, the fungus is still least considered an important pathogen by health officials and the public [1]. Booming counts of immunodeficient individuals have diverted attention to the mortal fungal afflictions as they spike the global health burden [2]. Currently, the whole world is tackling the holocaust of severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) and adding to the health hazard burden, a wide range of harmless fungal species are breaching the immune barriers and overwhelming the hospitals with a range of fungal sepsis in SARS CoV-2 infected or survivors. By far, >500 fungal species are known to infect humans with mild superficial sepsis to lethal invasive sepsis, mainly in immune deficit persons [3,4]. As of now, 233, 186, 5631 cases (according to worldometer.info) are battling with Coronavirus disease (COVID-19) around the globe and are administered with corticosteroids [Dexamethasone (DEX)] as therapeutic measures to downregulate the SARS CoV-2 viral replication and infectivity in the infected patients by modulating cytokines, majorly the interleukins (IL-1,4,6,8,12) and tumor necrosis factor (TNF)α [5]. Studies in 2008 by Zhang et al. [6] on the SARS animal model showed a decrease in the cytokines production of both innate (Interferons α and β) and Th1 cytokines (IL-1,4,6,8 & TNFα) in DEX treated animal model initially. However, long term use at a higher dose or prolonged usage exacerbates lung lesions with a significant increase in IL-4. In addition, hemato-lymphohistiocytosis syndrome along with COVID-19 are at higher risk to infect with invasive fungal infection due to immune modulation caused by both the SARS-CoV-2 and the corticosteroids used for treatment [7]. Similarly, studies by de León-Borrás and his colleagues pointed out the use of corticosteroids as treatment increases the risk of invasive fungal sepsis by 3.33 times than the patients receiving non-steroid treatments [8] (Fig. 1 ). We want to bring to notice the trauma observed in few COVID-19 survivors post SARS CoV-2 infection, specifically the fungal invasion through this narrative review.
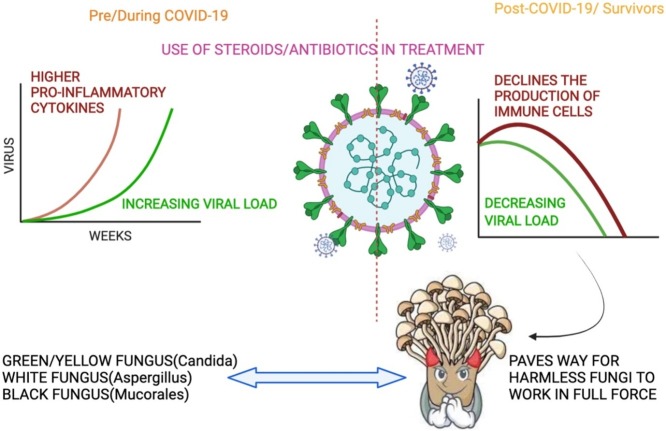
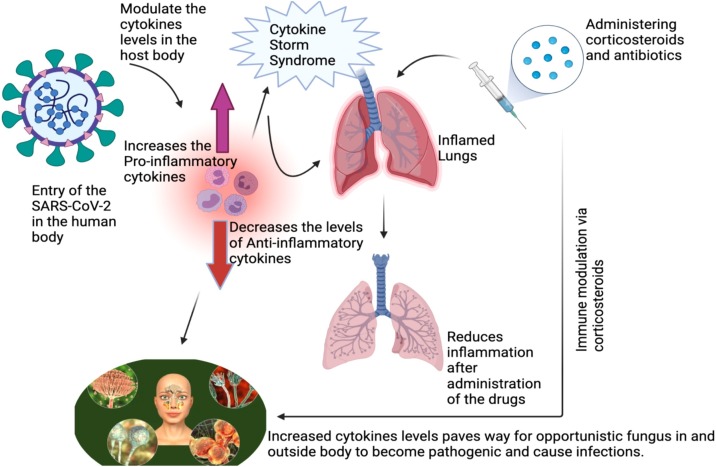
Fig. 1.
Elucidates the effects induced by SARS-CoV-2 in the human host after its entry. The cytokine levels are zapped to cause Cytokine Storm Syndrome (CSS), namely the levels of pro-inflammatory cytokines are increased and anti-inflammatory cytokines are reduced leading to inflammation in the lungs and administering corticosteroids reduces the inflammations in the lungs initially. But the CSS in the body along with the corticosteroids actions makes the host more susceptible to get infected by the opportunistic fungi present inside and outside of the body to flourish in full swing. Which culminated into secondary invasive fungal infections like mucormycosis, yellow/green fungus or white fungus formation.
First-hand sources
Talento and Hoenigl [9] through their editorial highlighted the occurrence of secondary fungal infections of candida and Aspergillus species as COVID-19-associated pulmonary aspergillosis (CAPA) and candida infections in blood due to tampered immune reactions like hyperimmune state and non-functional T-lymphocytes. In addition, classical risk factors such as longer duration in intensive care, mechanical ventilation support or vascular devices and corticosteroids and antibiotic administration [10]. Likewise, another opportunistic filamentous fungus named Mucorales are observed in post COVID-19 patients as Mucormycosis, with higher susceptibility in immune compromise peoples or diabetes [11] lays foundation for the current study.
Usual repressive role of corticosteroids
Corticosteroids are the front liners in the therapeutically world for dealing with inflammatory diseases and immune-suppressive procedures. Corticosteroids virtually affect every immune system's cells, and the result depends on the cells differentiation and activation mode [12]. Act as an antagonist for differentiation of macrophages and cytokine production like IL-1,6, TNFα along with declining production of pro-inflammatory factors like prostaglandins and leukotrienes. They are also capable of conquering the activated macrophage's microbe and tumor execution property, decline the adhesion of neutrophils with endothelial cells and breaks down the release of enzymes from lysosomes plus chemotaxis in the inflamed region [13].
In addition, corticosteroids, especially glucocorticoids, suppress the lymphocytes population, leading to lymphopenia conditions in the host by declining IL’s (2,3,4,6) production to stop T-cells specially released The effects of glucocorticoids is also observed on dendritic cells maturation and activity [14].
COVID-19 pathogenesis and mode of corticosteroid action
Majority of COVID-19 patients battles with mild to moderate infection. Only a few cases, about 20–40% hospitalized with COVID-19 and 67–85% intensive care unit (ICU) COVID-19 patients suffers from acute respiratory distress syndrome (ARDS) due to hemophagocytic lymphohistiocytosis [5]. Basically, the SARS-CoV-2 pathogenesis occurs as early infection with viral incubation followed by viral replication leads to restricted inflammation in the pulmonary region and ultimately produces inflammatory response causing pneumonia [15]. Though the virion load declines in the later stages, the inflammatory response keeps progressing to cause uncontrolled immune responses called cytokine release syndrome (CRS) [16]. IL-6 in the serum of the patients is mainly associated with the CRS causing ARDS and collapsed respiratory functions [17]. In CRS and ARDS, increased permeability of epithelial and endothelial cells in the lungs damages the alveoli. It accumulates fluids full of proteins in the interstitium of the lungs, provoking the production of pro-inflammatory cytokines (IL-1,6,8, M1 macrophage, and TNF), causing neutrophil activation and release of toxic mediators such as reactive oxygen species as well as injuries in endothelial cells of capillaries and epithelial cells of alveoli. This situation leads to fibroblast multiplication producing fibrosis in the interstitial and intra-alveolar region followed by a need for ventilation via machines (MV) [18], as shown in Fig. 2 .
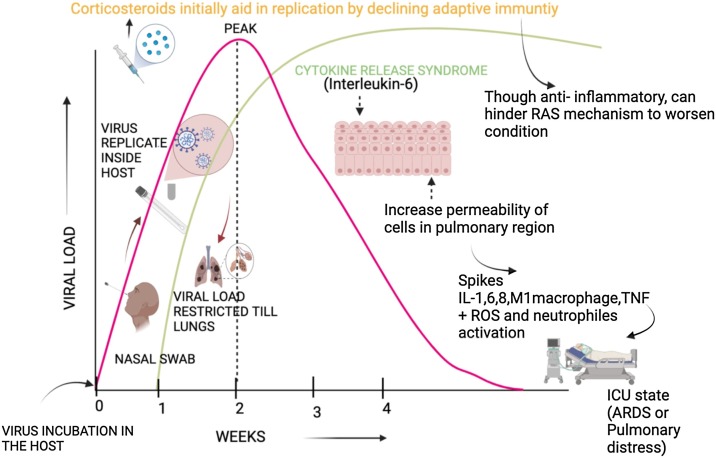
Fig. 2.
The timeline of SARS-CoV-2 in the human host and the mechanism of interaction with corticosteroids to battle the COVID-19 infection. Blocking the immune cells helps the virion replicate and further disorganize the RAAS metabolism to worsen the conditions of succumbing to mechanical ventilation.
Abbreviation: ARDS-Acute respiratory distress syndrome, ICU-Intensive care unit, IL-Interleukin, TNF-Tumor necrosis factor, RAAS-Renin-angiotensin-aldosterone system, ROS-Reactive oxygen species.
Being parallel in pathology like ARDS, COVID-19 is also treated with corticosteroids. Administration of corticosteroids mainly depends on the timing, as early treatment elevates viral reproduction and postpones adaptive immunity. According to some studies, corticosteroids must be given at the pulmonary phase in COVID-19 pathology to obtain its benefit by declining the state of hyper inflammation [15,17,19]. Generally, the glucocorticoids and mineralocorticoid receptors are triggered by the corticosteroids to induce anti-inflammatory and hyperglycemia sequels along with hindrance in the renin-angiotensin-aldosterone system to retain sodium. DEX might be effective again inflammation but can lead to withholding of sodium and fluid to further worsen the injury in the lungs. Due to a longer half-life, DEX is used as a slow therapy taper than other corticosteroids [19].
Corticosteroids endorsement for COVID-19
Apart from concomitant manifestations like ARDS, corticosteroids were not administered to COVID-19 cases. However, methylprednisolone has shown few positive observations in a Chinese study [20]. With randomized trials, the recommendations for corticosteroids were altered. Another recovery trial study carried out in the United Kingdom showed the utilization of DEX (6 mg/day) for 10 days or till discharge showed a reduction in mortality along with MV [21]. Use of systemic corticosteroids as amalgamation with antivirals and immune alternators like tocilizumab (Interluckin-6 inhibitor) and baricitinib (Kinase inhibitors are shown to be effective in COVID-19 patients. Sometimes variation in duration and dosage of formulated systemic corticosteroids have shown benefits in patients from smaller randomized trails. Other Glucocorticoids like Hydrocortisone (160 mg), Prednisone (40 mg) and Methylprednisolone (32 mg) is recommended in the absence of DEX [22]. Additionally, corticosteroids with inhalation property like Budesonide is shown to impair virion replication plus downregulation of receptors of cellular entry [23]. According to the REACT group (World Health Organization’s rapid evidence appraisal for COVID-19 therapies), the mortality rate is reduced after the inclusion of corticosteroids rather than placebo and usual care in COVID-19 critical cases [24]. Hence, corticosteroids were commended to patients with pneumonia and the need for oxygen supplementation or ventilators. Not mandatory for cases without breathing difficulty.
Fungal blooms in severe COVID-19 cases
Using the approved corticosteroid level for a longer duration leads to fluid retentions in the body with swelling and weight gain plus diabetes-like conditions as plausible side effects in the patients [25]. In addition to mimicking natural products to reduce inflammation, corticosteroids are highly potent to reduce the normal functioning of the immune system in the body (Fig. 3 ). Likewise to attenuate the CRS anti- interleukin-6 (IL-6) therapy is given in COVID-19 cases, which can lead to adverse conditions as seen in mice models with lower or nil expression of IL-6. Since the mice loses it immunity against microbes specially like bacteria and fungus [26]. This situation allows opportunistic pathogens like fungi to bloom in full swing and the invasion of fungal sepsis is dependent on the host’s immune potency and the species of the fungi. In some cases, the probability of retriggering the dormant fungal spore is high under immune-compromised states, increasing the chances of invasive fungal infections in person. ICU COVID-19 patients, patients requiring MV and those who had longer stay in hospital were seen to be infected with fungus due to suppression of immunity in the patients due to extensive steroids and antibiotics administration of broad-spectrum [27]. To date, the ubiquitous fungal species like Mucorales, Aspergillus and Candida are seen to invade the immunity jeopardized COVID-19 survivors and cause infections as mucormycosis (black fungus), green/yellow fungus and white fungus by taking advantage of their immune deficiency due to extensive treatment along with predisposing characters of diabetes ketoacidosis [28,29], and obesity as enlarged adipose tissues are apoptotic with immune cells attraction ability, causes inflammation by disorganizing the metabolic pathway of fatty acid [30].
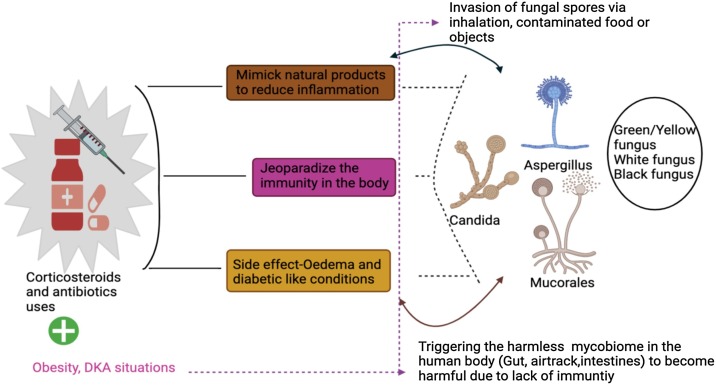
Fig. 3.
The above figure depicts the limitations of the extensive use of corticosteroids and antibiotics to impede the current catastrophe COVID-19. The presence of other clinical manifestations like obesity and diabetes ketoacidosis are seen to spike post COVID infections.
Abbreviations: DKA-Diabetes ketoacidosis.
Invasion of the Mucorales
Abraded scars on the skin, inhalation and taking in contaminated food are the entry point of Mucorales in a host body to infect the cutaneous, pulmonary, gastrointestinal and rhino-orbital or cerebrum region by rapidly invaginating in the blood vessels to cause thrombosis and dissemination, leading to necrosis of the tissue [19]. The endothelial and epithelial cells separated from stroma through the basement membrane constitute laminin and collagen IV extracellular proteins. The lining of epithelial cells is damaged usually during diabetes and extensive medications or ventilations process. Thus leading to the exposure of the proteins to directly interact with the surrounding fungal spores, and one of the most common mucorales genus Rhizopus binds with the collagen and laminin proteins using the endothelial glucose-regulator protein 78 (GRP 78) receptor Rhizopus endocytose itself inside the host and forms its hype [29]. The GRP78 binding ligand belongs to the spore coating proteins family, universally seen only in Mucorales [31]. Collectively, the spore coating protein of the Mucorales and GRP78 of the host endothelium leads to invasive fungal sepsis.
In addition, the presence of diabetes and related ketoacidosis like conditions can further enhance the pathogenesis of the Rhizopus genus as the lower pH of blood, higher levels of glucose, iron and the β-hydroxybutyrate impairs chelation of iron from transferrin and acidotic condition necessary for the growth of the fungus [29]. Huge number of cases were registered under mucormycosis associated with SARS-CoV-2 infection in COVID-19 patients in ICU’S or with mechanical ventilators in middle or later stage of the infections, with increased mortality and longer hospital stay with ventilators [32]. Recent review highlighted the prevalence rate of about 81.2% (82/101 cases) in India (80 times higher prevalence compared to whole world) with mortality of 30.7% [33]. Cases of mucormycosis was also registered without known risk factors suggesting the virion and the drugs administered could induce immune suppression, there by expose the patients to opportunistic fungus [34].
Candida albicans pathogenesis
Candida showcases dichotomous morphological form depending on the immune reactions of the host, the surroundings and its ability to transition from single-celled yeast to hyphae. Yeast is the commensal form causing infection circulation or disperse, and hyphae as the pathogenic infectious form can invaginate deeper in the tissues and destroy them [3]. The hyphae wall protein and agglutinin like sequence interact with the adhering host cell by dissolving the membranes through aspartic protease secretion [35]. These fungi evade immune cells by declining or blocking the pathogen-associated molecular patterns (PAMPs), lowering the fusion of phagosomes with lysosomes, declining opsonization, breaking down the complement factors or by releasing catalases and superoxide dismutases, reduce the concentrations of antimicrobial peptides, disturbing the balance between Th1 and 2 cells [3]. In addition, they also produce a toxin called Candidalysin capable of changing the membrane integrity in macrophages [36]. They can also trigger M1 macrophagae differentiation into M2 to protect themselves and increase their livelihood [37]. Cohort studies on COVID-19 secondary infections has reported the presence of Candida infection in bloodstream in about 0.03–10% of COVID-19 patients admitted in the hospital with the invasion of the Fungai after about 7 days of admission and show cased >%50 mortality [38]
Aspergillus species mode of action
These species are rarely observed in immune-compromised persons, but they are quite morbid and mortal by increasing the susceptibility for mortality from 40 to 90% in immune deficit patients [3]. Through asexual reproduction, they produce conidiospores inhaled and engulfed in the macrophages of the alveolar region to trigger the pro-inflammation reaction to recruit neutrophils at the site of infectivity in the immunity compromised persons. The spores trick macrophages and germinate by invaginating the vessels lumen through the cells in the epithelial and endothelial region [39]. Like Candida species, Aspergillus evade immune cells by blocking important PAMPs, the interaction between lysosome-phagosome, increasing the production of catalases capable of antioxidants, mannitol and SOD. Aspergillus produce secondary metabolic products like fumagillin, cytochalasin E, gliotoxin and actibind to express a wide range of immunocompromised reactions. Another quirky Aspergillus feature includes producing melanin pigments to protect its spores from environmental adversities outside the host. It helps in scavenging intermediates of reactive oxygen in the human cells and masking β-glucans and trafficking conidiospores intracellularly to cause infectivity [3]. Till date few hundreds of CAPA related mortality have been registered and studies showed the prevalence of 23.3%–34.4% in COVID-19 patients from asymptomatic to superinfection [40]
Communion of fungus and host
In general, the infection rate depends on the level of interaction between the pathogen and host; in this case, the PAMPs of the pathogen and the pathogen recognition receptors (PRRs) on the host initiate the immune response in the host. The diverse immune cells like polymorphic nuclear leukocytes, macrophages, dendritic cells and non-immune cells like fibroblast and epithelial cells of the host express the PRRs on their surfaces to tackle the pathogens. The usual PAMPs are glucans (α and β), mannans (N and o linked), peptidoglycan proteins, liposaccharides and phospholipomannan. These constitute the cell wall of fungus and are acknowledged by the highly conserved host PRRs like toll-like receptors (TLRs), C-type lectin receptors (CLRs) and oligomerization domains of nucleotide-binding like receptors (NLRs) (Table 1 ). Anti-fungal activity is actively carried out by TLRs (2,4,9) and CLR’s [3]. The complementary system of the host recognizes the mannoproteins of the fungal cell wall to cause phagocytosis or opsonization, followed by elevated production of antimicrobial peptides, cytokines and chemokines in the host. TLRs(2 and 4) recognize derivatives of mannan and lipomannan to induce Th1 and Treg cells [41]. Similarly, immune cells express mincle to ligate with the mannose of fungi and trigger the release of TNF α and IL-10. Being commensal fungi interact with a host by creating immune response tolerance; when the balance is distorted in an immune-deficient person, the infection spreads to various major organs [3].
Table 1.
Tabulates the ligands released by the cell walls of the pathogen (PAMPs) and the cells recognizing the ligands through PRRs using a wide range of receptors to facilitate its entry inside the host.
| Pathogen (Fungus) | Host cells (Humans) | Receptors of the host |
|---|---|---|
| Produce pathogen-associated molecular patterns (PAMPs) | Recognized by pathogen recognition receptors (PRRs) | The interaction between PAMPs and PRRs occurs |
| Examples | ||
| α-Glucans and β-Glucans | Leukocytes | Toll like receptor (TLRs) |
| N -Mannans and O-Mannans | Macrophages | C-type lectinreceptor (CLRs) |
| Peptidoglycans proteins | Dendritic cells | Oligomerization domain of nucleotide-binding like receptor (NLRs) |
| Liposaccharides | Fibroblast | |
| Phospholipomannan | Epithelial cells | |
Other persuasive entry routes of fungal spores in COVID patients or survivors
Considerably, fungal infection is observed in immunocompromised and people with comorbid predisposition like diabetic ketoacidosis with a 100% fatality rate in a severe infectious state. Primarily the ubiquitous spores enter through various routes like ingestion, inoculation in the injured region, inhalation. Apart from these, the fungal contamination in medical supplies like bandages, ostomy adhesive bags, linens [42] can also contribute to hospital outbreaks of invasive fungal growth in COVID patients undergoing treatment in hospitals. There is another possibility of inhalation spores during the hospitalization period; due to the immunosuppressive effects of the drugs administered to mitigate COVID, the individual can become a susceptible host after discharge from the hospital or while under surveillance due to higher iron levels in patient’s serum under COVID treatment or survivor. As already known, the spores’ viability depends on the suitable conditions, here iron supply makes them stay alive for a longer duration [29].
Reformed mycobiome in SARS CoV-2 infection
Apart from bacteria, the gastrointestinal track nurses some fungal species know as mycobiome to provide immunity alone with the other microbiota [43]. Studies by Zuo et al. [44] showed maladjustment in the alimentary track mycobiome of COVID-19 patients compared with healthy even on the last day of infection. They observed a heterogenous group of microbiome groups growing in the gut due to reduced T cells production in the host body due to SARS CoV-2 infection [45]. Reports show the presence of Candida and Aspergillus species in the faeces of COVID-19 patients, and there is a possibility of these fungal species at the post-recovery phase to trigger secondary infections like black and white, yellow or green fungus in the COVID-19 survivors. In addition to the intestinal mycobiome, alteration in pulmonary mycobiome is also observed in pneumonia infections [44].
The above paragraphs highlight the mode of interaction, entry and the effects of the lesser considerate pathogen- fungus in COVID-19 patients and survivors. Immune cells malfunction triggers the catastrophic events in their pre and post COVID-19 phase due to the use of corticosteroids and anti-inflammatory drugs during the hospitalization period [11]. In addition, presence of comorbidities like diabetes, hypertension further adds up to the malaise. Revising the drug administration and checking the fungal markers in respiratory samples, body fluid can allow us to tackle it at its earliest [46]
Conclusion
Harmless fungus conquers the human body in an exclusively immune-suppressed state, i.e. when the defense mode is impeded. Hence making immune-suppressing drugs act as a two-sided sword used to treat the severe inflammatory condition with a sign of spiking the peril of exposing the host body to various infections. The rife COVID-19 mainly increases pro-inflammatory compounds' levels to cause cytokine storms, and immune suppressive drugs play a significant role in combating the pandemic. In addition, pneumonia and acute respiratory distress syndrome cause fungal concomitance by altering the gut and pulmonary mycobiome. Immune compromised condition is one of the prime predisposing features to harbor fungal sepsis of medical significance. The utilization of aggressive treatment methods, corticosteroids, antibiotics steeps down the immunity of the host against the invading fungus to comprehend its pathology leading to a morbid situation. The limitations of the current study includes the exclusion of other less frequent opportunistic fungus like Histoplasma sp., Cryptococcus sp., and Pneumocystis jirovecii in COVID-19 secondary infections and patients with pre-existing immune compromised states due to organ transplantations, hematological malignancies etc. Due to the scarcity of mycoses animal models, the anti-fungal effect of the host is lesser-known when compared with bacteria and viruses, as fungal sepsis is majorly seen in immune response jeopardized peoples. Proper guidelines are required to control the administration of drugs related to declining immunity in the COVID-19 cases, survivors and COVID-19 people with additional malaises like diabetes, inflammatory disorders and those undergoing chemotherapies or other treatments to identify the mycoses in the initial stages to increase their livelihood. More rigorous research must be done to refine the drug administration and management guidelines in patients/survivors of COVID-19, along with patients with other comorbidity plus COVID-19.
Author contributions
Conceptualization, G.D.R., A.M., and M.S.; Writing-original draft preparation, K.P., H.K., A.M., and B.B.; Literature search, selected bibliographic sources, B.B., V.T., A.A.R., M.P., K.P., A.M.A., R.M.A., and V.A.; Figures, H.K., and K.P. B.B and A.M were coordinated the working group; Supervision, Validation, Writing-review & editing, M.S., A.A.R., A.M.A., R.M.A., G.D.R., A.M., and H.K. All authors have read and agreed to the published version of the manuscript.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
References
- 1.Rodrigues M.L., Nosanchuk J.D. Fungal diseases as neglected pathogens: a wake-up call to public health officials. PLoS Negl Trop Dis. 2020;14(2):e0007964. doi: 10.1371/journal.pntd.0007964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira L.V.N., Wang R., Specht C.A., Levitz S.M. Vaccines for human fungal diseases: close but still a long way to go. npj Vaccines. 2021;6(1):1–8. doi: 10.1038/s41541-021-00294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pathakumari B., Liang G., Liu W. 2020. Immune defence to invasive fungal infections: a comprehensive review.https://pubmed.ncbi.nlm.nih.gov/32739740/ [DOI] [PubMed] [Google Scholar]
- 4.Kuchi Bhotla H., Balasubramanian B., Arumugam V.A., Pushparaj K., Easwaran M., Baskaran R., et al. Insinuating cocktailed components in biocompatible-nanoparticles could act as an impressive neo-adjuvant strategy to combat COVID-19. Nat Resour Human Health. 2021;1(1):3–7. [Google Scholar]
- 5.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X., Alekseev K., Jung K., Vlasova A., Hadya N., Saif L.J. Cytokine responses in porcine respiratory coronavirus-infected pigs treated with corticosteroids as a model for severe acute respiratory syndrome. J Virol. 2008;82(9):4420–4428. doi: 10.1128/JVI.02190-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segrelles-Calvo G., De Saraújo G.R., Frases S. 2020. Systemic mycoses: a potential alert for complications in COVID-19 patients.https://pubmed.ncbi.nlm.nih.gov/33085538/ [DOI] [PubMed] [Google Scholar]
- 8.de León-Borrás R., DelPilar-Morales E., Rivera-Pérez N., Pallens-Feliciano M., Tirado-Gómez M., González-Sepúlveda L., et al. Factors associated to invasive fungal infection in Hispanic patients with hematological malignancies. Bol Asoc Med P R. 2017;109(1):43–48. [PMC free article] [PubMed] [Google Scholar]
- 9.Talento A.F., Hoenigl M. Fungal infections complicating COVID-19: with the rain comes the spores. J Fungi (Basel) 2020;6(4):1–2. doi: 10.3390/jof6040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szarpak L., Chirico F., Pruc M., Szarpak L., Dzieciatkowski T., Rafique Z. Mucormycosis-a serious threat in the COVID-19 pandemic? J Infect. 2021;83(2):237–279. doi: 10.1016/j.jinf.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pushparaj K., Kuchi Bhotla H., Arumugam V.A., Pappusamy M., Easwaran M., Liu W.-C., et al. 2021. Mucormycosis (black fungus) ensuing COVID-19 and comorbidity meets-Magnifying global pandemic grieve and catastrophe begins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEwen B.S., Biron C.A., Brunson K.W., Bulloch K., Chambers W.H., Dhabhar F.S., et al. 1997. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions.https://pubmed.ncbi.nlm.nih.gov/9063588/ [DOI] [PubMed] [Google Scholar]
- 13.Youssef J., Novosad S.A., Winthrop K.L. 2016. Infection risk and safety of corticosteroid use.https://pubmed.ncbi.nlm.nih.gov/26611557/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coutinho A.E., Chapman K.E. 2011. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights.https://pubmed.ncbi.nlm.nih.gov/20398732/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhotla H.K., Kaul T., Balasubramanian B., Easwaran M., Arumugam V.A., Pappusamy M., et al. Platelets to surrogate lung inflammation in COVID-19 patients. Med Hypotheses. 2020;143:110098. doi: 10.1016/j.mehy.2020.110098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 19.Williams D.M. Clinical pharmacology of corticosteroids. Respir Care. 2018;63(6):655–670. doi: 10.4187/respcare.06314. [DOI] [PubMed] [Google Scholar]
- 20.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani W., et al. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J Virol. 2020;95(1) doi: 10.1128/JVI.01648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner E.J., Ungaro R.C., Gearry R.B., Kaplan G.G., Kissous-Hunt M., Lewis J.D., et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(2):481–491.e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimmig L.M., Wu D., Gold M., Pettit N.N., Pitrak D., Mueller J., et al. IL-6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. Front Med. 2020;7:583897. doi: 10.3389/fmed.2020.583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song G., Liang G., Liu W. 2020. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China.https://pubmed.ncbi.nlm.nih.gov/32737747/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puebla L.E.J. Fungal infections in immunosuppressed patients. Immunodeficiency. 2012 [Google Scholar]
- 29.Baldin C., Ibrahim A.S. Molecular mechanisms of mucormycosis—the bitter and the sweet. PLoS Pathog. 2017;13(8) doi: 10.1371/journal.ppat.1006408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammad S., Aziz R., Al Mahri S., Malik S.S., Haji E., Khan A.H., et al. 2021. Obesity and COVID-19: what makes obese host so vulnerable?https://pubmed.ncbi.nlm.nih.gov/33390183/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gebremariam T., Liu M., Luo G., Bruno V., Phan Q.T., Waring A.J., et al. CotH3 mediates fungal invasion of host cells during mucormycosis. J Clin Invest. 2014;124(1):237–250. doi: 10.1172/JCI71349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song G., Liang G., Liu W. 2020. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China.https://pubmed.ncbi.nlm.nih.gov/32737747/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh A.K., Singh R., Joshi S.R., Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr Clin Res Rev. 2021;15(4):102146. doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pemán J., Ruiz-Gaitán A., García-Vidal C., Salavert M., Ramírez P., Puchades F., et al. 2020. Fungal co-infection in COVID-19 patients: should we be concerned?https://pubmed.ncbi.nlm.nih.gov/33041191/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naglik J.R., Moyes D.L., Wächtler B., Hube B. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect. 2011;13(12–13):963. doi: 10.1016/j.micinf.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasper L., Franke A., Mogavero S., Hoefs S., Martin R., Wilson D.W., et al. Role of the fungal peptide toxin Candidalysin in macrophage damage and inflammatory response. Mycoses. 2016;59:1. [Google Scholar]
- 37.Reales-Calderón J.A., Aguilera-Montilla N., Corbí Á.L., Molero G., Gil C. Proteomic characterization of human proinflammatory M1 and anti-inflammatory M2 macrophages and their response to Candida albicans. Proteomics. 2014;14(12):1503–1518. doi: 10.1002/pmic.201300508. [DOI] [PubMed] [Google Scholar]
- 38.Chiurlo M., Mastrangelo A., Ripa M., Scarpellini P. Invasive fungal infections in patients with COVID-19: a review on pathogenesis, epidemiology, clinical features, treatment, and outcomes. New Microbiol. 2021;44(2):71–83. [PubMed] [Google Scholar]
- 39.Amitani R., Murayama T., Nawada R., Lee W.J., Niimi A., Suzuki K., et al. Aspergillus culture filtrates and sputum sols from patients with pulmonary aspergillosis cause damage to human respiratory ciliated epithelium in vitro. Eur Respir J. 1995;8(10):1681–1687. doi: 10.1183/09031936.95.08101681. [DOI] [PubMed] [Google Scholar]
- 40.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brouwer N., Dolman K.M., van Houdt M., Sta M., Roos D., Kuijpers T.W. Mannose-binding lectin (MBL) facilitates opsonophagocytosis of yeasts but not of bacteria despite MBL binding. J Immunol. 2008;180(6):4124–4132. doi: 10.4049/jimmunol.180.6.4124. [DOI] [PubMed] [Google Scholar]
- 42.Duffy J., Harris J., Gade L., Sehulster L., Newhouse E., O’Connell H., et al. Mucormycosis outbreak associated with hospital linens. Pediatr Infect Dis J. 2014;33(5):472–476. doi: 10.1097/INF.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 43.van Tilburg Bernardes E., Pettersen V.K., Gutierrez M.W., Laforest-Lapointe I., Jendzjowsky N.G., Cavin J.-B., et al. Intestinal fungi are causally implicated in microbiome assembly and immune development in mice. Nat Commun. 2020;11(1):1–16. doi: 10.1038/s41467-020-16431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuo T., Zhan H., Zhang F., Liu Q., Tso E.Y.K., Lui G.C.Y., et al. Alterations in fecal fungal microbiome of patients with COVID-19 during time of hospitalization until discharge. Gastroenterology. 2020;159(4):1302. doi: 10.1053/j.gastro.2020.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;0:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasero D., Sanna S., Liperi C., Piredda D., Pietro Branca G., Casadio L., et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2020;49(5) doi: 10.1007/s15010-020-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]