Abstract
Background:
We assessed the impact of the coronavirus disease 2019 (COVID-19) pandemic on HIV suppression rates in people living with HIV (PLWH) attending a large Italian HIV clinic.
Setting:
The HIV outpatient clinic of the Infectious Diseases Department of Luigi Sacco Hospital, Milan, Italy, which serves more than 5000 PLWH per year.
Methods:
A before and after quasi-experimental study design was used to make a retrospective assessment of the monthly trend of HIV-RNA determinations of ≥50 among the PLWH attending our clinic, with “before” being the period from January 1, 2016 to February 20, 2020, and “after” being the period from February 21, 2020 to December 31, 2020 (the COVID-19 period). Interrupted time series analysis was used to evaluate any changes in the trend.
Results:
During the study period, 70,349 HIV-RNA viral load determinations were made, and the percentage of HIV-RNA viral load determinations of <50 copies/mL increased from 88.4% in 2016 to 93.2% in 2020 (P < 0.0001). There was a significant monthly trend toward a decrease in the number of HIV-RNA determinations of ≥50 copies/mL before the pandemic (β −0.084; standard error 0.015; P < 0.001), and this did not significantly change after it started (β −0.039, standard error 0.161; P = 0.811).
Conclusions:
A high prevalence of viral suppression was maintained among the PLWH referring to our clinic, despite the structural barriers raised by the COVID-19 pandemic. The use of simplified methods of delivering care (such as teleconsultations and multiple antiretroviral treatment prescriptions) may have contributed to preserving this continuum.
Key Words: PLWH, viral suppression, HIV-RNA, continuum of care, COVID-19 epidemic
INTRODUCTION
The start of coronavirus disease 2019 (COVID-19) pandemic was an unprecedented health emergency.1 Many hospitals experienced intense pressure to increase their capacity to treat patients with COVID-19 which, in many cases, reduced the access to health care of patients with other diseases.2,3 HIV infection requires chronic treatment to guarantee the suppression of viral replication,4,5 and this raised serious concerns about the impact of COVID-19 on access to care and uninterrupted antiretroviral treatment (ART) for people living with HIV (PLWH).6,7 The provision of HIV services was particularly challenging in countries such as Italy, where the delivery of HIV care and ART is almost exclusively ensured by specialists working in hospital-based infectious disease clinics, many of whom had to be transferred to newly created, dedicated COVID-19 units.8 Italy was the first European country to be hard hit by COVID-19, and the first to face the consequent severe hospital crisis.9–11
The aim of this study was to evaluate the impact of COVID-19 on HIV suppression and the continuum of care among the PLWH attending a large HIV clinic in Milan, Italy.
MATERIALS AND METHODS
Setting
The study was conducted at the Infectious Diseases Department of Luigi Sacco Hospital, one of the largest infectious disease centers in Milan, Italy. The Department runs an HIV outpatient clinic that serves more than 5000 patients a year.
Normally, clinical follow-up of patients receiving stable ART is performed every 3–6 months in accordance with national12 and international guidelines13 and enough antiretroviral medication to cover a maximum of 3 months is dispensed by the hospital pharmacy.
With the massive waves of COVID-19 in Italy in March–May and October–December of 2020, most of the health care professionals running our HIV clinic were redeployed to newly created COVID-19 inpatient units, thus leaving only a few to carry out the service. In-person appointments were guaranteed for patients with urgent clinical needs, but patients in stable clinical condition had to maintain contact by telephone, email, or WhatsApp messages.
Multiple ART prescriptions (covering up to 6 months of treatment) were given to stable patients to reduce the need for nonurgent appointments; an ART home delivery service was also established for regional residents who could not visit the hospital during lockdown.
Study Design
This retrospective study had a before and after quasi-experimental design to be able to assess virological suppression (HIV-RNA levels of <50 copies/mL). “Before” was from January 1, 2016 to February 20, 2020 (the date on which the first patient with COVID-19 was admitted to our hospital), and “after” was from February 21, 2020 to December 31, 2020 (the COVID-19 period).
Participants
All the PLWH attending our HIV outpatient service during the study period.
Variables
All the HIV-RNA determinations made during the study period were included in the analysis, which considered all the individual unique codes used to identify PLWH for purposes of laboratory analyses.
Accesses to our antiretroviral drug dispensary were retrieved from the hospital pharmacy's drug accountability records using the individual unique codes used to identify PLWH for purposes of drug dispensing monitoring.
Data Sources and Measurements
The data concerning HIV-RNA determinations and ART dispensing were automatically collected as case-based data.
HIV-RNA determinations were considered positive if the result was ≥50 copies/mL. HIV-RNA determinations of ≥50 copies/mL were considered to be related to determination-naive subjects if no corresponding patient code had been recorded earlier in the study period.
Every access to the drug dispensary was extracted as the number of months of antiretroviral coverage collected.
Objectives
The primary objective of the study was to assess the monthly trend of HIV-RNA determinations of ≥50 copies/mL before and during the pandemic.
The secondary objectives were to assess the percentage of patients lost to follow-up (ie, those who interrupted their virological follow-up and ART collections) in 2020 and compare this with previous years.
Statistical Analysis
All the HIV-RNA determinations were included in the analysis. The percentage of determinations of ≥50 copies/mL was calculated per month and per year, as was the mean number (±SD) of determinations per month (2016–2019 vs 2020). Interrupted time series analysis was used to assess changes in the percentage and monthly trends before and during the pandemic. The Durbin–Watson statistic showed the presence of a first-order positive autocorrelation, and so the error structure was fitted using an autoregressive model of order one; the final model included the adjusted estimates for the intercept, slopes, and the first-order autocorrelation parameter. A sensitivity analysis was made of the patients with an available HIV-RNA determination in 2016, who were considered a cohort starting from 2017.
The Cochran–Armitage trend test was used to assess the trend of determination-naive PLWH attending the clinic overtime.
The percentage of patients interrupting their virological follow-up in a given year was calculated, with the numerator indicating the number of patients who did not undergo a viremia determination in that year and the denominator the number of patients who had undergone at least one determination in the previous year. The percentages of PLWH interrupting their virological follow-up in 2018, 2019, and 2020 were compared using the χ2 test. Similarly, the percentages of patients not collecting medication in 2018, 2019, and 2020 were calculated and compared using the χ2 test.
The data were analyzed using SAS software, version 9.4, and a P-value of <0.05 was considered statistically significant.
The study did not require ethical approval because only aggregate data without any sensitive information were used for the analysis.
RESULTS
HIV-RNA Determinations and Annual Rates of Viral Suppression
A total of 70,349 HIV-RNA determinations were made during the study period. There was a progressive reduction in the annual number of determinations from 15,939 in 2016 to 15,217 in 2017, 14,424 in 2018, 13,723 in 2019, and 11,046 in 2020. The 2020 decrease in the number of determinations was particularly marked during the months corresponding to the peaks of the COVID-19 epidemic, when the mean number was more than 2 SDs lower than that of the pre-epidemic period: 668 vs 1420 (SD = 112) in March, 539 vs 1162 (SD = 65) in April, 856 vs 1417 (SD = 115) in May, and 848 vs 1215 (SD = 68) in November.
Virological suppression progressively increased from 88.4% in 2016 to 93.2% in 2020 (P < 0.0001), and there was a similar increase when the analysis was restricted to the patients followed up from 2017 (from 92.6% in 2017 to 95.5% in 2020, P < 0.0001).
There was a progressive decline in the number of determination-naive PLWH: 235 (4.2%) in 2017, 159 (2.8%) in 2018, 157 (2.8%) in 2019, and 97 (1.8%) in 2020 (P < 0.0001).
Interrupted Time Series Analysis of HIV-RNA Determinations of ≥50 Copies/mL Before and During the Pandemic
There was a significant monthly trend toward a decrease in the percentage of HIV-RNA determinations of ≥50 copies/mL before the pandemic [β −0.084, standard error (SE) 0.015; P < 0.001], but no significant change in the monthly trend after the start of the pandemic (β −0.039, SE = 0.161; P = 0.811) (Fig. 1A). The numbers were similar after restricting the analysis to the patients followed up from 2017: β −0.053, SE = 0.013; P < 0.001 before the pandemic and β 0.075, SE = 0.099; P = 0.453 during the pandemic (Fig. 1B).
FIGURE 1.
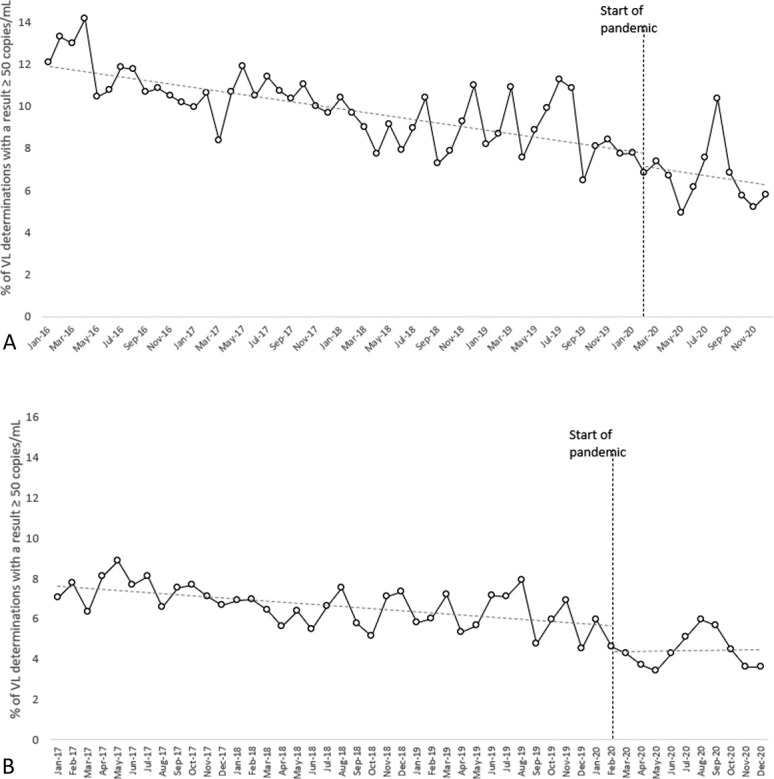
A, Graphical representation of the trend of HIV-RNA VL load determinations of ≥50 copies/mL between 2016 and 2020 (interrupted time series analysis). A significant monthly trend toward a small proportion of determinations of ≥50 copies/mL was observed before the pandemic (P < 0.001), and there was no significant change after the start of the pandemic (P = 0.811). B, Graphical representation of the monthly trend of HIV-RNA VL load determinations of ≥50 copies/mL between 2017 and 2020, restricted to subjects beginning treatment in 2017 and considered as a cohort observed over time (interrupted time series analysis). There was a significant monthly trend toward a small proportion of HIV-RNA determinations of ≥50 copies/mL before the pandemic (P < 0.001), with no significant change after its start (P = 0.453). VL, viral load.
Interruption of Virological Follow-Up and Medication Collection
The number of patients who collected antiretroviral medication at least once in 2020 and the preceding years (2018–2019) is shown in Figure 2A. There was no significant difference in the percentage of patients who stopped collecting their medications regularly in 2018 (478/6029, 7.9%), 2019 (542/5950, 9.1%), and 2020 (535/6040, 8.9%) (P = 0.052).
FIGURE 2.
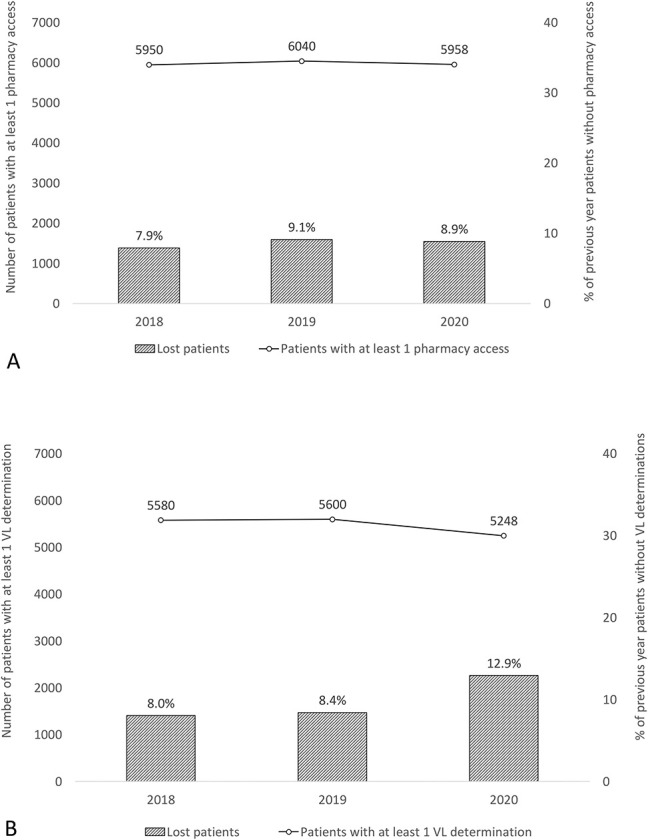
A, Graphical representation of the number of patients with access to antiretroviral drug dispensing over time (2018–2020) and the related percentage of patients lost to follow-up (ie, patients accessing drug dispensing in one year but not in the following year). There was no significant difference in the lost to follow-up rates of 2018, 2019, and 2020 (P = 0.052). B, Graphical representation of the number of patients with at least one HIV-RNA VL determination (2018–2020) and the percentage of patients lost to follow-up (ie, patients with an HIV-RNA VL determination in 1 year but not in the following year). There was no difference in the lost to follow-up rates between 2018 and 2019 (P = 0.490), but there were significant differences between 2018 and 2020 (P < 0.0001) and between 2019 and 2020 (P < 0.0001). VL, viral load.
The number of patients who underwent at least one HIV-RNA determination in 2018, 2019, and 2020 is shown in Figure 2B. The percentage of patients who interrupted their virological follow-up in 2020 (724/5600, 12.9%) was significantly higher than that in 2018 (448/5568, 8.0%) and 2019 (469/5580, 8.4%) (P < 0.0001 for both).
Among the 724 patients who interrupted their virological follow-up in 2020, 352 (48.6%) continued to access the drug dispensary to renew their supply.
DISCUSSION
The delivery of routine care at our HIV clinic in Milan has been severely challenged by the COVID-19 pandemic: long periods when patients struggled to collect their ART because of lockdowns and movement restrictions, a dramatic reduction in available professional resources, a need to reduce access to our clinic to avoid the health care transmission of SARS-CoV-2, and a consequent reduction in routine in-person appointments. To overcome these barriers, telephone calls and/or digital messaging were widely used for delivering consultations and patients in stable conditions were given multiple ART prescriptions covering up to 6 months of treatment to avoid nonurgent visits. Moreover, ART home delivery was offered to patients in lockdown (a strategy previously used in Shenzhen).14
Our data showing a consolidated decreasing trend of virological determinations ≥50 copies/mL during the pandemic support the efficacy of our efforts to ensure continuity of HIV care. This is different from the experience of Spinelli et al,15 who introduced telemedicine because of shelter‐in‐place mandates in San Francisco and found that it led to a considerable decrease in suppression rates, with alarmingly higher odds of viral nonsuppression in special populations such as ethnic minorities and the homeless. Socioeconomic factors are among the most frequent reasons preventing PLWH from accessing quality care,16 and the innovative strategies of delivering HIV care introduced during the state of emergency are likely to fail unless complementary action is simultaneously taken to overcome social and structural barriers to health care.17–19 This is more relevant now than ever given the estimated global increase in poverty and health inequalities due to the COVID-19 pandemic.20
Despite the progressive increase of virological suppression rate among our PLWH from 2016 to 2020, the percentage of patients interrupting their virological follow-up in 2020 (12.9%) was higher than in previous years. Almost half of these also interrupted their collections of ART from our drug dispensary, but unfortunately, we could not ascertain whether this was due to the interruption of care or a move to another HIV center. Moreover, the virological outcomes of the patients who collected their ART but have not attended follow-up visits for a long time remains to be established.
Finally, in line with the findings of another Italian study, we observed a decline in the number of determination-naive PLWH; however, this trend existed before the pandemic, which may therefore not have been a causal factor.8 On the other hand, a large study investigating the impact of lockdown on HIV care in South Africa found that there was a 47.6% reduction in HIV testing in April 2020, and a significant reduction in the number of patients starting ART although, like us, the authors did not find any significant change in the number of patients picking up their medications.21 Given the greater consideration that needs to be given to strategies aimed at facilitating HIV testing and promptly starting ART during the pandemic, it is interesting to note that an American study has highlighted the importance of implementing HIV self-testing.22
STUDY LIMITATIONS AND STRENGTHS
The main limitations are potential biases because some patients could not travel to our hospital because of movement restrictions. Moreover, as this was an observational study, no definite causative pathway can be inferred between our observations and the pandemic.
The main strength of the study is its use of data concerning more than 5000 PLWH actively followed up in a hospital that is also a reference center for COVID-19, thus making it a real stress test for the provision of HIV care during the pandemic.
CONCLUSIONS
Despite the structural barriers raised by the COVID-19 pandemic, a high prevalence of viral suppression was maintained among the PLWH attending our clinic. The provision of care was assured by simplifying the methods of delivering care (such as teleconsultations and multiple ART prescriptions), which might even prove to be useful once the pandemic is over.
ACKNOWLEDGMENTS
The authors thank all of the medical and paramedical staff involved in the care of PLWH during the current COVID-19 crisis.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Contributor Information
Cecilia Bonazzetti, Email: cecilia.bonazzetti@unimi.it.
Federico Conti, Email: federico.conti@unimi.it.
Laura Pezzati, Email: laura.pezzati@unimi.it.
Letizia Oreni, Email: letizia.oreni@alice.it.
Valeria Micheli, Email: valeria.micheli@asst-fbf-sacco.it.
Alessandro Mancon, Email: alessandro.mancon@live.com.
Stefania Vimercati, Email: stefania.vimercati@asst-fbf-sacco.it.
Maria Albrecht, Email: maria.albrecht@asst-fbf-sacco.it.
Matteo Passerini, Email: matteopasserini1@gmail.com.
Maria Vittoria Cossu, Email: maria.cossu@asst-fbf-sacco.it.
Amedeo Ferdinando Capetti, Email: amedeo.capetti@asst-fbf-sacco.it.
Paola Meraviglia, Email: paola.meraviglia@asst-fbf-sacco.it.
Spinello Antinori, Email: spinello.antinori@unimi.it.
Giuliano Rizzardini, Email: giuliano.rizzardini@asst-fbf-sacco.it.
Massimo Galli, Email: massimo.galli@unimi.it.
Anna Lisa Ridolfo, Email: annalisa.ridolfo@gmail.com.
REFERENCES
- 1.Roosa K, Lee Y, Luo R, et al. Real-time forecasts of the COVID-19 epidemic in China from February 5th to February 24th, 2020. Infect Dis Model. 2020;5:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study. Lancet. 2018;392:1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modesti PA, Wang J, Damasceno A, et al. Indirect implications of COVID-19 prevention strategies on non-communicable diseases. BMC Med. 2020;18:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Valk M, Reiss P. Noncommunicable diseases in people living with HIV: time for integrated care. J Infect Dis. 2017;216:1481–1483. [DOI] [PubMed] [Google Scholar]
- 5.Brault MA, Spiegelman D, Hargreaves J, et al. Treatment as prevention: concepts and challenges for reducing HIV incidence. J Acquir Immune Defic Syndr. 2019;82:S104–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS Board concludes with key decisions taken related to the colliding epidemics of HIV and COVID-19. Available at: https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2020/february/20200218_china_covid19. Accessed April 17, 2021.
- 7.Guo W, Weng HL, Bai H, et al. Quick community survey on the impact of COVID-19 outbreak for the healthcare of people living with HIV [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:662–666. [DOI] [PubMed] [Google Scholar]
- 8.Quiros-Roldan E, Magro P, Carriero C, et al. Consequences of the COVID-19 pandemic on the continuum of care in a cohort of people living with HIV followed in a single center of Northern Italy. AIDS Res Ther. 2020;17:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. [DOI] [PubMed] [Google Scholar]
- 10.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giacomelli A, Ridolfo AL, Milazzo L, et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158:104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linee Guida Italiane sull'utilizzo dei farmaci antiretrovirali e sulla gestione diagnostico-clinica delle persone con infezione da HIV-1. 2017. Società Italiana di Malattie Infettive. http://www.salute.gov.it/imgs/C_17_pubblicazioni_2696_allegato.pdf. Accessed April 17, 2021. [Google Scholar]
- 13.DHHS HIV Clinical Guidelines. Available at: https://clinicalinfo.hiv.gov/en/guidelines. Accessed April 17, 2021.
- 14.Wang H. HIV care during the coronavirus disease-2019 pandemic in Shenzhen, China. Curr Opin HIV AIDS. 2020;15:341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spinelli MA, Hickey MD, Glidden DV, et al. Viral suppression rates in a safety-net HIV clinic in San Francisco destabilized during COVID-19. AIDS. 2020;34:2328–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burch LS, Smith CJ, Phillips AN, et al. Socioeconomic status and response to antiretroviral therapy in high-income countries: a literature review. AIDS. 2016;30:1147–1162. [DOI] [PubMed] [Google Scholar]
- 17.Nosyk B, Zang X, Krebs E, et al. Ending the HIV epidemic in the USA: an economic modelling study in six cities. Lancet HIV. 2020;7:e491–e503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickey MD, Imbert E, Glidden DV, et al. Viral suppression during COVID-19 among people with HIV experiencing homelessness in a low-barrier clinic-based program. AIDS. 2021;35:517–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong WS, Agwu AL, Barrette EP, et al. Innovations in human immunodeficiency virus (HIV) care delivery during the coronavirus disease 2019 (COVID-19) pandemic: policies to strengthen the ending the epidemic initiative—a policy paper of the infectious diseases Society of America and the HIV medicine association. Clin Infect Dis. 2021;72:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitehead M, Taylor-Robinson D, Barr B. Poverty, health, and COVID-19. BMJ. 2021;372:n376. [DOI] [PubMed] [Google Scholar]
- 21.Dorward J, Khubone T, Gate K, et al. The impact of the COVID-19 lockdown on HIV care in 65 South African primary care clinics: an interrupted time series analysis. Lancet HIV. 2021;8:e158–e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menza TW, Garai J, Ferrer J, et al. Rapid uptake of home-based HIV self-testing during social distancing for SARS-CoV2 infection in Oregon. AIDS Behav. 2021;25:167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]


