ABSTRACT
Introduction:
The endothelial protein C receptor (EPCR) is a protein that regulates the protein C anticoagulant and anti-inflammatory pathways. A soluble form of EPCR (sEPCR) circulates in plasma and inhibits activated protein C (APC) activities. The clinical impact of sEPCR and its involvement in COVID-19 has not been explored. In this study, we investigated whether sEPCR levels were related to COVID-19 patients’ requirement for hospitalization.
Methods:
Plasma sEPCR levels were measured on hospital admission in 84 COVID-19 patients, and in 11 non-hospitalized SARS-CoV2-positive patients approximately 6 days after reported manifestation of their symptoms. Multiple logistic regression analysis was performed to identify potential risk factors for hospitalization and receiver operating characteristic (ROC) curves were generated to assess their value.
Results:
In our cohort, hospitalized patients had considerably higher sEPCR levels upon admission compared with outpatients [107.5 (76.7–156.3) vs. 44.6 (12.1–84.4) ng/mL; P < 0.0001)]. The ROC curve using hospitalization as the classification variable and sEPCR levels as the prognostic variable generated an area under the curve at 0.845 (95% CI = 0.710–0.981, P < 0.001). Additionally, we investigated the predictive value of sEPCR combined with BMI, age, or D-dimers.
Conclusions:
In our cohort, sEPCR levels in COVID-19 patients upon hospital admission appear considerably elevated compared with outpatients; this could lead to impaired APC activities and might contribute to the pro-coagulant phenotype reported in such patients. sEPCR measurement might be useful as a point-of-care test in SARS-CoV2-positive patients.
Keywords: APC, coagulation, COVID-19, sEPCR
INTRODUCTION
In the era of COVID-19, reports are being published discussing a possible role of activated protein C (APC) in patients suffering from COVID-19 (1, 2). Hypercoagulable states, microthrombosis, ischemic stroke, myocardial infarction, or digital ischemia are common in COVID-19 (2). The protein C (PC) anticoagulant system controls blood coagulation and inflammatory pathways (3). Protein S (PS) and the endothelial receptors thrombomodulin (TM) and endothelial protein C receptor (EPCR), are also involved in this system. Conversion of PC to APC is achieved by TM-bound thrombin, whereas the conversion is drastically augmented by the presence of EPCR (4). Besides its anticoagulant role in PC activation, there is evidence that the anti-inflammatory, anti-apoptotic, and cytoprotective functions of APC are exerted via an EPCR-dependent mechanism (5); more specifically, down-regulation of EPCR levels on injured endothelium impairs the ability to generate APC (6). A soluble form of EPCR (sEPCR), which exists under normal conditions in plasma, has been found elevated in inflammatory conditions (7). sEPCR binds with equal affinity both PC and APC; its binding to APC inhibits the anticoagulant activity of the latter, while its binding to PC precludes PC activation by thrombin/TM complexes (8–10). Indeed, increased sEPCR levels promote the procoagulant effects of APC due to sequestration, and are deemed a prognostic factor of deep vein thrombosis (11–13). Elevated sEPCR levels on intensive care unit (ICU) admission have also been associated with subsequent sepsis development (14), while carriers of the minor alleles of the EPCR gene were at reduced risk of developing septic shock in a cohort of critically ill patients (15).
While the role of membrane bound EPCR is clearly anti-thrombotic and anti-inflammatory, the physiological significance of circulating sEPCR has not been unraveled. Since in COVID-19 patients both inflammation and coagulation are disturbed, we hypothesized that sEPCR levels could stratify patients by disease severity.
PATIENTS AND METHODS
This observational, single-center study included 84 consecutive Caucasian, critically and non-critically ill COVID-19 patients, hospitalized from March 22, 2020 to October 28, 2020. Criteria for hospitalization included PaO2/FiO2 < 300 mm Hg, bilateral infiltrates in the chest X-ray, or existence of comorbidities. The decision for hospitalization relied exclusively on the attending physician at the Emergency Department. Patients receiving dexamethasone prior to blood sampling were excluded from the study. An additional group of SARS-CoV2-positive patients, with mild to moderate symptoms, not requiring hospitalization, were also included as a control group. SARS-CoV2 infection was diagnosed by real-time reverse transcription PCR (RT-PCR) in nasopharyngeal swabs. The study was approved by the Hospital's Research Ethics Committee (129/19–3-2020) and all procedures carried out on patients were in compliance with the Helsinki Declaration. Informed written consent was obtained from all patients or patients’ next-of-kin. Plasma soluble EPCR levels were measured on hospital admission (within 24-h), or for the non-hospitalized outpatients upon hospital visit [6 (4–7) days after reported manifestation of the first symptoms], by enzyme-linked immunosorbent assay (ELISA) (R&D Systems Inc, Minneapolis, Minn). sEPCR levels were also measured in 25 SARS-CoV-2-negative critically ill septic patients (16), matched for age, sex, and critical illness severity.
Statistical analysis
Data are presented as individual values, mean ± standard deviation (SD) for normally distributed variables, and median with interquartile range (IQR) for variables with skewed distribution. Two groups’ comparisons were performed by the t test or the non-parametric Mann–Whitney test. Kruskal–Wallis ANOVA followed by Dunn post hoc test was performed for more than two groups’ comparisons. Associations between qualitative variables were examined by the chi-square test. The patients’ age, sex, body mass index (BMI), sEPCR levels, presence of comorbidities, platelets, the international normalized ratio (INR), D-dimers, and the reported number of sickness days prior to sampling were recorded. Initially, multivariate logistic regression analysis was performed to identify potential risk factors for hospitalization using all variables (Backward method) and afterward, specific models were tested, including sEPCR and one or two more variables (Enter method). A receiver operating characteristic (ROC) curve was plotted using hospitalization as the classification variable and sEPCR levels on hospital admission/visit, and their linear combination with BMI, age, or D-dimers as prognostic variables. The optimal cut-off value for predicting hospitalization was calculated as the point with the greatest combined sensitivity and specificity. All P values were two-sided; P < 0.05 were considered significant.
RESULTS
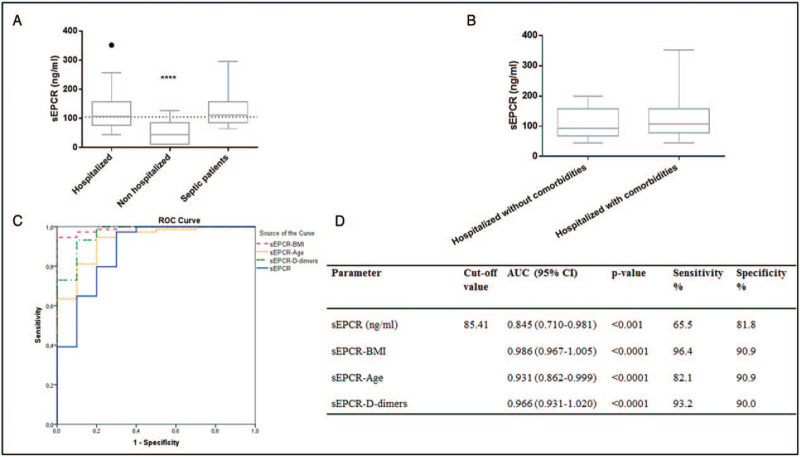
Demographics of the hospitalized and non-hospitalized patients are shown in Table 1. Hospitalized patients were older, had higher BMI and D-dimers, while 67% had bilateral infiltrates in their chest X-rays. In the hospitalized patients, 55 (65.5%) had comorbidities, while 15 patients (17.9%) eventually died (in-hospital mortality). None of the non-hospitalized patients had comorbidities, and no patient died. The reported sickness days prior to sampling did not differ between the two groups. Importantly, hospitalized patients had much higher sEPCR levels compared with non-hospitalized outpatients (107.5 ng/mL vs. 44.6 ng/mL; P < 0.0001; Fig. 1A). sEPCR correlated with age (rs = 0.29, P = 0.005), and BMI (rs = 0.22, P = 0.03), but did not correlate with sex, comorbidities, reported symptom days prior to sampling, platelets, nor D-dimers.
Table 1.
Demographics and coagulation indices of SARS-COV2-positive hospitalized and non-hospitalized patients
| Parameters | Hospitalized | Non-hospitalized | P value |
| Number of patients, N | 84 | 11 | |
| Age (years), (mean ± SD) | 60 ± 15 | 40 ± 9 | <0.0001 |
| Sex, N (%) | 0.2 | ||
| Male | 64 (76.2) | 6 (54.5) | |
| Female | 20 (23.8) | 5 (45.5) | |
| Reported sickness days prior to sampling, (median, IQR) | 6 (4–8) | 5 (3–6) | 0.1 |
| Body mass index (kg/m2), (median, IQR) | 26.0 (24.7–27.1) | 23.0 (22.0–24.0) | <0.0001 |
| D-dimers (μg/mL), (median, IQR) | 0.60 (0.36–1.17) | 0.24 (0.21–0.31) | <0.0001 |
| Platelets (per μL), (median, IQR) | 201,500 (155,750–269,000) | 175,000 (165,000–220,000) | 0.6 |
| INR, (median, IQR) | 1.05 (0.99–1.12) | 1.00 (0.99–1.02) | 0.09 |
| sEPCR (ng/mL), (median, IQR) | 107.5 (76.7–156.3) | 44.6 (12.1–84.4) | <0.0001 |
Patients were categorized according to whether they required hospitalization. Data are expressed either as number of patients (N) and percentages of total (%), mean ± SD, or median (IQR), as appropriate. Two-group comparisons were performed using the Student t test or the non-parametric Mann–Whitney test for skewed data. Associations between qualitative variables were examined by the chi-square test.
INR indicates international normalized ratio; sEPCR, soluble endothelial protein C receptor.
Fig. 1.
A, sEPCR levels in hospitalized and non-hospitalized COVID-19 patients, and in SARS-CoV2-negative critically ill septic patients. sEPCR levels were measured in 84 COVID-19 hospitalized patients on hospital admission (within 24-h), 11 SARS-CoV2-positive non-hospitalized patients, and in 25 SARS-CoV2-negative critically ill septic patients. Data are presented as box plots. Line in the box, median value; box edges, 25th to 75th centiles; whiskers, range of values; bullet points, outliers; horizontal line, median of the SARS-CoV2-positive group (N = 95). ∗∗∗∗P < 0.0001 from the COVID-19 hospitalized patients. B, COVID-19 hospitalized patients were divided into two subgroups; with or without comorbidities. sEPCR levels were thereafter compared in the two groups. C, Receiver operating characteristic (ROC) curve analysis. ROC curves were generated to determine the prognostic accuracy of either sEPCR (solid line), sEPCR combined with BMI (dashed line), sEPCR combined with age (dotted line), or sEPCR combined with D-dimers (dash-dotted line), to predict hospitalization. D, The corresponding areas under the curve (AUC) and 95% confidence intervals (CI), along with the respective sensitivities and specificities, are given. sEPCR levels, BMI, and D-dimers were estimated on hospital admission/visit, 4 to 7 days after reported manifestation of the first symptoms. BMI indicates body mass index; sEPCR, soluble endothelial protein C receptor.
sEPCR levels were additionally measured in 25 age, sex, and critical illness severity-matched ICU septic patients to investigate whether the increased sEPCR levels were significantly different in COVID-19 compared to sepsis. The mean patient age of the septic cohort was 61 ± 29, and 72% were males; five patients (20%) had comorbidities. sEPCR levels did not differ between COVID-19 and ICU septic patients (107.5 vs. 111.4 ng/mL, P = 0.4; Fig. 1A).
Since none of the non-hospitalized patients had comorbidities, we decided to compare their levels with the subset of hospitalized patients who did not have comorbidities (N = 29). The latter had higher sEPCR levels than the former (92.65 ng/mL, P = 0.001). Hospitalized patients with or without comorbidities did not differ with respect to sEPCR levels (P = 0.4; Fig. 1B).
Multivariate regression analysis (Backward method) revealed that sEPCR levels in the presence of BMI could be assumed as indicators for hospitalization [OR = 1.079 (CI = 1.018–1.144), P = 0.011 and 11.262 (1.993–63.650), P = 0.006, respectively)]. Although age and D-dimers could not be assumed as statistically significant predictors in the aforementioned model, nevertheless sEPCR levels in the presence of age [OR = 1.037, P = 0.004 and OR = 1.131, P = 0.006, respectively)], as well as sEPCR levels in the presence of D-dimers [OR = 1.063, P = 0.006 and OR = 670306, P = 0.026, respectively)], could also be assumed as indicators for hospitalization, following multivariate regression analysis applying the Enter method.
A ROC curve was generated to determine the prognostic accuracy of sEPCR in predicting hospitalization; the area under the curve (AUC) of sEPCR levels was 0.845 (95% CI = 0.710–0.981, P < 0.001; Fig. 1C). According to the ROC analysis, the optimal cut-off point was 85.41 ng/mL, with 65.5% (95% CI = 54.3–75.5) sensitivity and 81.8% (95% CI = 48.2–97.7) specificity. Additionally, we investigated the predictive value of sEPCR combined with BMI, age, or D-dimers. The AUCs and their respective sensitivities and specificities are given in Figure 1C, D.
In an effort to further approach a potential mechanism in the PC pathway, soluble (s)TM levels were also measured in a subset of patients. These data are presented in the Supplementary file. Our results showed no difference in sTM levels with respect to severity of COVID-19, as expressed by ICU/ward hospitalization and mortality (Fig. S1). Furthermore, no correlation was observed between sTM and sEPCR levels (P > 0.05). sTM levels were within normal values in all groups studied (< 45 ng/mL) (17).
DISCUSSION
Amounting evidence suggests that dysfunction of the endothelium, which is where EPCR is mainly located, plays a central role in COVID-19. Furthermore, EPCR binds to protein C modulating the generation of the anticoagulant and anti-inflammatory APC. Hence, we reasoned that levels of sEPCR in severe and critical COVID-19 patients would be high. Our findings suggest that sEPCR increases in response to COVID-19-related injury.
The factors and mechanisms controlling the release of soluble EPCR in vivo remain unclear. In vitro, sEPCR can be shed from the cell surface via metalloproteolytic activity, in a highly regulated process that is sensitive to both coagulation factors and inflammatory mediators (18). It is not surprising, therefore, that one report showed that sEPCR levels declined in response to treatment with anticoagulants, whose mechanism of action is known to decrease in vivo thrombin production (19). sEPCR can also be generated via alternative mRNA splicing in haplotype-H3-carrying cells (20).
When sEPCR binds to APC it causes a functional change in APC rendering it pro-coagulant, instead of allowing it to exert its normal anti-coagulant activity (8–10). In our cohort, sEPCR levels did not differ between COVID-19 and sepsis; it seems, therefore, that sEPCR acts as a general marker for systemic inflammatory acute illness, but this does not undermine its observed capability of differentiating COVID-19 hospitalized from non-hospitalized patients in our population. Hitherto, the clinical and functional impact of the EPCR soluble form remains to be established.
In our COVID-19 cohort, we did not find differences between hospital admission sEPCR and severity of COVID-19, as expressed by ICU/ward hospitalization, need for intubation, prolonged hospital stay, or mortality. However, we were able to demonstrate that sEPCR levels, measured in SARS-CoV2-positive patients 4 to 7 days after reported manifestation of the first symptoms, may act as an early indicator of hospitalization due to COVID-19 in the presence of BMI. Furthermore, ROC curve analysis demonstrated that the value of sEPCR in hospitalization prediction was greatly enhanced in our cohort when combined with the patients’ body mass index, age, or D-dimers; this could assist the emergency department (ED) clinician at triage, since age and BMI are readily available, whereas D-dimers are usually part of the laboratory measurements ordered by most EDs. These features point to the potential use of sEPCR assessment as a point-of-care test, and should be further investigated.
Our study has the following limitations: first, it was a single-center study with a modest sample size. It was a pilot study exploratory in nature and observational not allowing conclusions about causative mechanisms. We did not study the mechanisms controlling the release of sEPCR in COVID-19, nor did we genotype our patients; apparent discrepancies in sEPCR among studies have been partially explained by different frequencies of the H3-haplotype of the EPCR gene in various study populations (11, 21). In addition, it would be interesting to measure APC levels and seek potential correlations; however, this was not feasible.
To our knowledge this is the first report to study the role of sEPCR levels as possible predictors of hospitalization due to COVID-19. Our results indicated that sEPCR levels could distinguish patients requiring hospitalization, offering pathophysiological information on impaired APC activities; the latter might pinpoint to pro-coagulant phenotypes, although no overt thromboembolic disease was present in our patients at the time of sampling. sEPCR measurement might be useful as a point-of-care test in SARS-CoV2-positive patients for determining their need for hospitalization.
Supplementary Material
Footnotes
This work received no external funding.
The authors report no conflicts of interest.
Supplemental digital content is available for this article.
REFERENCES
- 1.Griffin JH, Lyden P. COVID-19 hypothesis: activated protein C for therapy of virus-induced pathologic thromboinflammation. Res Pract Thromb Haemost 4 (4):506–509, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzeffi M, Chow JH, Amoroso A, Tanaka K. Revisiting the protein C pathway: an opportunity for adjunctive intervention in COVID-19? Anesth Analg 131 (3):690–693, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maniatis NA, Letsiou E, Orfanos SE, Kardara M, Dimopoulou I, Nakos G, Lekka ME, Roussos C, Armaganidis A, Kotanidou A. Inhaled activated protein C protects mice from ventilator-induced lung injury. Crit Care 14 (2):R70, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci U S A 93 (19):10212–10216, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 296 (5574):1880–1882, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Zheng X, Li W, Song Y, Hu Y, Ferrell GL, Esmon NL, Esmon CT. Non-hematopoietic EPCR regulates the coagulation and inflammatory responses during endotoxemia. J Thromb Haemost 5 (7):1394–1400, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Kurosawa S, Stearns-Kurosawa DJ, Carson CW, D’Angelo A, Della Valle P, Esmon CT. Plasma levels of endothelial cell protein C receptor are elevated in patients with sepsis and systemic lupus erythematosus: lack of correlation with thrombomodulin suggests involvement of different pathological processes. Blood 91 (2):725–727, 1998. [PubMed] [Google Scholar]
- 8.Fukudome K, Kurosawa S, Stearns-Kurosawa DJ, He X, Rezaie AR, Esmon CT. The endothelial cell protein C receptor. Cell surface expression and direct ligand binding by the soluble receptor. J Biol Chem 271 (29):17491–17498, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Liaw PC, Neuenschwander PF, Smirnov MD, Esmon CT. Mechanisms by which soluble endothelial cell protein C receptor modulates protein C and activated protein C function. J Biol Chem 275 (8):5447–5452, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Regan LM, Stearns-Kurosawa DJ, Kurosawa S, Mollica J, Fukudome K, Esmon CT. The endothelial cell protein C receptor. Inhibition of activated protein C anticoagulant function without modulation of reaction with proteinase inhibitors. J Biol Chem 271 (29):17499–17503, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Saposnik B, Reny JL, Gaussem P, Emmerich J, Aiach M, Gandrille S. A haplotype of the EPCR gene is associated with increased plasma levels of sEPCR and is a candidate risk factor for thrombosis. Blood 103 (4):1311–1318, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Uitte de Willige S, Van Marion V, Rosendaal FR, Vos HL, de Visser MC, Bertina RM. Haplotypes of the EPCR gene, plasma sEPCR levels and the risk of deep venous thrombosis. J Thromb Haemost 2 (8):1305–1310, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Martos L, Oto J, Fernández-Pardo A, Plana E, Solmoirago MJ, Cana F, Hervás D, Bonanad S, Ferrando F, España F, et al. Increase of neutrophil activation markers in venous thrombosis-contribution of circulating activated protein C. Int J Mol Sci 21 (16):5651, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vassiliou AG, Kotanidou A, Mastora Z, Maniatis NA, Albani P, Jahaj E, Koutsoukou A, Armaganidis A, Orfanos SE. Elevated soluble endothelial protein C receptor levels at ICU admission are associated with sepsis development. Minerva Anestesiol 81 (2):125–134, 2015. [PubMed] [Google Scholar]
- 15.Vassiliou AG, Maniatis NA, Kotanidou A, Kallergi M, Karystinaki FS, Letsiou E, Glynos C, Kopterides P, Vassiliadi D, Nikitas N, et al. Endothelial protein C receptor polymorphisms and risk of severe sepsis in critically ill patients. Intensive Care Med 39 (10):1752–1759, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315 (8):801–810, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John S, Drobnik W, Lackner K, Schmieder RE. Soluble thrombomodulin and endothelial dysfunction in early atherosclerosis. Lancet 354 (9190):1647, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Qu D, Esmon NL, Esmon CT. Metalloproteolytic release of endothelial cell protein C receptor. J Biol Chem 275 (8):6038–6044, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Stearns-Kurosawa DJ, Swindle K, D’Angelo A, Della Valle P, Fattorini A, Caron N, Grimaux M, Woodhams B, Kurosawa S. Plasma levels of endothelial protein C receptor respond to anticoagulant treatment. Blood 99 (2):526–530, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Saposnik B, Lesteven E, Lokajczyk A, Esmon CT, Aiach M, Gandrille S. Alternative mRNA is favored by the A3 haplotype of the EPCR gene PROCR and generates a novel soluble form of EPCR in plasma. Blood 111 (7):3442–3451, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassiliou AG, Kotanidou A, Mastora Z, Tascini C, Cardinali G, Orfanos SE. The H3 haplotype of the EPCR gene determines high sEPCR levels in critically ill septic patients. Infect Dis Ther 7: (suppl 1): 3–14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.