Abstract
Background
Two populations of Dermacentor reticulatus ticks (Western and Eastern) in Poland are among the most dynamic tick populations in Central Europe. Expansion and settlement of ticks in new localizations depend on the presence of suitable hosts, for both adult and juvenile ticks.
Methods
The current study was planned to complement our previous studies on questing adult ticks and was focused on a collection of juvenile D. reticulatus ticks from rodents from three regions in Poland, defined by the presence/absence of adult ticks (regions of the Western and Eastern tick population and the gap area between them) to confirm the existence of stable populations. Rodent trapping was conducted in open habitats (fallow lands, wasteland and submerged meadows) in 2016–2018 in June, July and/or August to encompass seasonal peaks of larvae and nymph activity.
Results
Altogether, three tick species were collected, 2866 D. reticulatus, 2141 Ixodes ricinus and 427 Haemaphysalis concinna. Dermacentor reticulatus was the most common (72.3%) and abundant (mean 17.94 ± 2.62 ticks/rodent) tick species on rodents from the Eastern region; in the Western region infestation of rodents was only 6.8%. Ixodes ricinus was found in all three regions and was the only tick species collected from rodents from the gap area. Haemaphysalis concinna was noted only in the Western region. The highest infestation of juvenile D. reticulatus was recorded on voles (Myodes and Microtus spp.), infestation of I. ricinus was the highest on Apodemus mice, and the majority of H. concinna ticks were collected from root voles Alexandromys oeconomus.
Conclusions
Our study confirmed a stable population of D. reticulatus in Eastern and Central Poland and a lower prevalence and mean abundance of this tick species among rodents from the Western region. A lack of juvenile D. reticulatus on rodents in Niewiadów confirmed the existence of the gap area, free of D. reticulatus ticks.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-021-05039-z.
Keywords: Dermacentor reticulatus, Haemaphysalis concinna, Ixodes ricinus, Larvae, Nymphs, Poland, Rodents
Background
Occurrence of vector-borne diseases depends on the availability of appropriate vectors. In recent years a growing number of papers have reported on the spread of numerous vectors, including a cluster of mosquitoes and tick species in Southern and Central Europe [1–3].
Two populations of Dermacentor reticulatus ticks (Western and Eastern) in Poland are among the most dynamic tick populations in Central Europe [4–6]. We have recently documented seasonal and annual changes in the geographical range of both these populations, resulting in a measurable decrease in the size of the area historically free of this tick species (gap) and a continuous spread of canine babesiosis in the area of Poland, particularly in the eastern expansion zone [6]. This study was based on the collection of adult questing D. reticulatus ticks from vegetation.
The present study was planned to complement our previous studies on adult ticks, exploring the occurrence of juvenile D. reticulatus ticks on rodents in two endemic regions with their respective expansion zones and the gap area. Rodents are an important host for juvenile ticks, including Ixodes ricinus, Haemaphysalis concinna and D. reticulatus [7–9]. Thus, availability of these hosts for larvae and nymphs is necessary for the survival of newly settled ticks in new locations [1, 10, 11]. Larvae and nymphs of D. reticulatus are nidiculous and feed mainly on voles (Microtus spp. and Alexandromys oeconomus) [7, 8, 12, 13]. Without an appropriate host species for the juvenile stages of D. reticulatus, completion of a tick life cycle and subsequent progressive colonization of new areas are impossible. Therefore, recording of juvenile D. reticulatus ticks on rodents is likely the most convincing evidence for the successful establishment of a stable population at a particular site.
The main aim of this present study was to determine the occurrence of D. reticulatus instars on rodents from open habitats in two regions (Eastern and Western) and in the gap area, presumably free of D. reticulatus. We expected to witness similar trends as for adult ticks [6]: high rodent infestation with D. reticulatus juvenile stages in Eastern Poland (hyperendemic for D. reticulatus occurrence with high tick abundance) and lower on rodents in the western part of the country. Since in Central Poland adult D. reticulatus ticks still have not been detected, we also expected that rodents from the gap region should be free from D. reticulatus instar infestation.
Methods
The study was performed each summer in the period 2016–2018. Rodent trapping was conducted at each site in June to encompass the seasonal peak of larval D. reticulatus activity and then in July and/or August to encompass the seasonal peak of nymph activity [14].
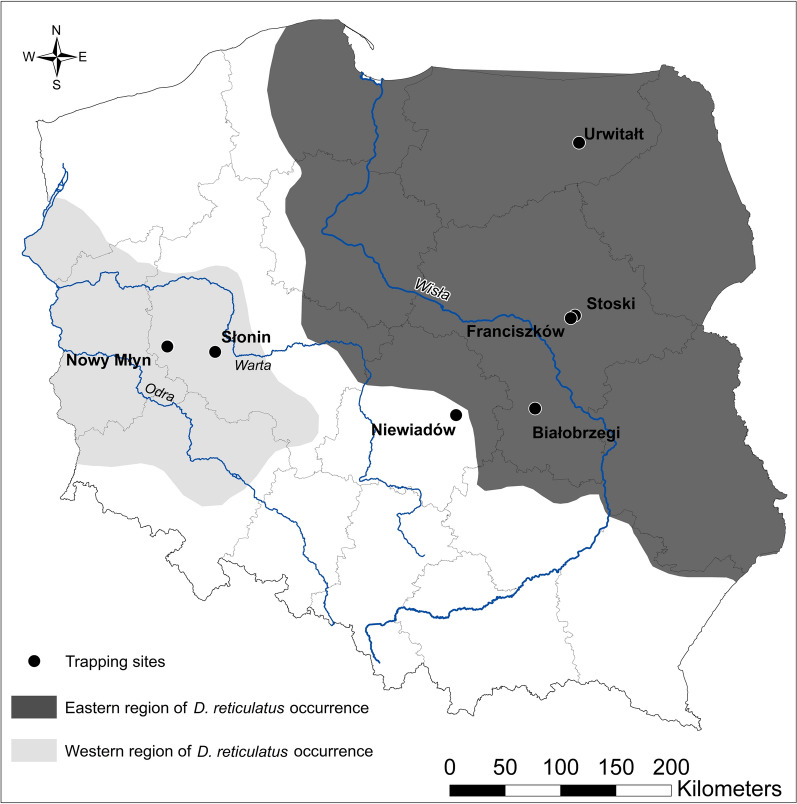
Altogether, three sites in the area endemic for the eastern population of D. reticulatus were monitored in the Mazovia voivodeship—Białobrzegi (51.66445N, 20.94700E); Stoski (52.409226N, 21.509125E); Franciszków (52.388847N, 21.452349E)—and one site in the Warmia-Mazuria voivodeship: Urwitałt (53.809318N, 21.647636E) (Fig. 1).
Fig. 1.
Rodent trapping sites
Two sites were selected in the western region of D. reticulatus occurrence: Słonin (52.119201N, 16.747274E) and Nowy Młyn (52.148505N, 16.114098E) in the Greater Poland voivodeship and finally one site in the gap (non-endemic region for D. reticulatus), Niewiadów (51.6237N, 19.9150E), in the Łódź voivodeship (Fig. 1). In 2016, two sites from the eastern endemic region were examined (Franciszków and Stoski); in 2017 two sites from the eastern endemic region and one in the gap region (Białobrzegi, Urwitałt, Niewiadów), and finally in 2018, three sites in the eastern region, one in the gap region and two in the western region were examined (Białobrzegi, Franciszków, Stoski, Nowy Młyn, Słonin, Niewiadów).
Trapping sites were located in habitats preferable for D. reticulatus, such as meadows, fallow lands and wetlands. The main target constituted voles from the genera Microtus and Alexandromys, the preferable hosts for larvae and nymphs of D. reticulatus [7, 8, 13–15]. All methods for rodent trapping and tick collection were described in detail in our previous papers [7, 8, 16]. In short, traps were set along 2–3 transects about 100 to 300 m long, depending on the topography of the examined site. Transects were from 10 to 20 m distant. One live trap was set at one point in 10-m intervals. Traps were set in the evening and inspected next day in the morning (6–9 a.m.). We used 50 traps during 3 nights at each trapping site. In each site two trapping sessions were conducted, in June or the first days of July and then in the last week of July or the first days of August. The bait used was a mixture of grains (for mice Apodemus spp.) and chopped fresh fruits and vegetables (apples, cucumbers, carrots) (for voles Microtus and Alexandromys). Rodents were live-trapped and live-processed, under light isoflurane anesthesia, and then released near their trapping point. Selected measurements of the rodent body were performed: head length, head width, nose-anus length, tail length and foot length, and weight. Species, age, sex and sex activity were determined as previously described [16, 17]. During the handling, rodents were carefully examined for the presence of ticks. All collected ticks were preserved in 70% ethanol.
Identification of collected ticks to species and stages was made according to a morphological key [18] using a stereoscopic microscope Zeiss Stemi 508 in the Department of Parasitology, Faculty of Biology, UW.
Statistical analysis
All statistical analyses were conducted in the IBM SPSS Statistics v. 21 software package (IBM Corp.). For the analysis of prevalence (% of infected rodents), we applied maximum likelihood techniques based on log linear analysis. REGION of tick origin (three levels: eastern, western and gap regions; see Fig. 1), RODENT SPECIES (7 levels: Apodemus agrarius, A. flavicollis, A. sylvaticus, Microtus arvalis, M. agrestis, A. oeconomus, Myodes glareolus), SEX (males or females) and AGE (two levels: juvenile or adult, based on breeding status) were used as the factors in the models, with the presence or absence of ticks considered as a binary factor (0, 1) and referred to as presence/absence of TICK. For each level of analysis in turn, beginning with the most complex model, involving all possible main effects and interactions, those combinations which did not contribute significantly to explaining variation in the data were eliminated in a stepwise fashion beginning with the highest level interaction (backward selection procedure). A minimum sufficient model was then obtained, for which the likelihood ratio of chi-square was not significant, indicating that the model was sufficient to explain the data [19, 20].
General linear models (GLMs in SPSS v.21) were used for analysis of the mean tick abundance, using models with normal errors, incorporating the region of rodent origin, species, sex and age of rodents as fixed factors. Means are presented with the standard error of the mean (SE).
Results
In total, 345 rodents were involved in the study: 132 Apodemus spp.: 77 striped field mice A. agrarius, 39 yellow-necked mice A. flavicollis, 16 wood mice A. sylvaticus; 158 root voles A. oeconomus; 47 Microtus spp.: 25 common voles M. arvalis, 22 field voles M. agrestis and 8 bank voles M. glareolus. Seven rodent species were trapped in the Eastern region, five in the Western region and four in the gap area (Additional file 1: Table S1).
Altogether, 5434 instars of three tick species were collected: 2866 D. reticulatus (2397 L and 469 N); 2141 I. ricinus (1957 L and 184 N) and 427 H. concinna (405 L and 22 N). In the region of the Eastern population, D. reticulatus was the dominant tick species collected from rodents (Additional file 1: Table S1, 2853 of juvenile ticks, 72.3%). In the region of the Western D. reticulatus population, I. ricinus was the most abundant tick species, followed by H. concinna and D. reticulatus (only 13 specimens collected). In the gap area, only I. ricinus instars were found on rodents (Additional file 1: Table S1).
Juvenile D. reticulatus were found in every site in the Eastern endemic region of D. reticulatus occurrence: in Białobrzegi (on 79.7% of 74 rodents), Stoski (76.2% of 21 rodents), Franciszków (71.4% of 14 rodents) and Urwitałt (60% of 50 rodents) and in one site in the Western region of D. reticulatus occurrence: Nowy Młyn (14% of 50 examined rodents). According to our expectations, in Niewiadów site (located in the gap area) no D. reticulatus instars were recorded during all trapping sessions. Ixodes ricinus was found in all trapping locations, and H. concinna was recorded in one site in the Western region of D. reticulatus occurrence (Nowy Młyn).
The overall prevalence of tick infestation was very high (88.4%). Among all examined hosts from all regions, the dominant species was I. ricinus (74.8% of infestation), followed by D. reticulatus (35.4%) and H. concinna (6.2%). Overall mean tick abundance differed among the three tick species (D. reticulatus: 8.32 ± 1.84 ticks/individual; I. ricinus: 6.21 ± 0.66 ticks/individual; H. concinna: 1.24 ± 0.51 ticks/individual); 60.3% of rodents harbored one tick species, and 28.1% were infested by two tick species.
Prevalence of tick infestation by region
Total prevalence of ticks was slightly different among the three examined regions and was the highest in the Western region (three tick species present, 91.3%) and the lowest in the gap area (only I. ricinus noted, 80.7%); in the Eastern region it reached 90.6% (REGION × TICK presence/absence: χ2 = 5.74, df = 2, P = 0.06).
Juvenile I. ricinus were collected in all sites in all examined regions; the highest infestation was recorded in the Western and the lowest in the Eastern region of the D. reticulatus populations (REGION × I. ricinus presence/absence: χ2 = 21.53, df = 2, P < 0.001; Fig. 2a).
Fig. 2.
a Prevalence of tick species by region. b Mean abundance ± SE of tick species by region
Juvenile D. reticulatus were collected from rodents trapped in the areas of Eastern and Western D. reticulatus populations (infestation more than ten times higher in the Eastern region; Fig. 2a), but not from rodents trapped in the gap area (REGION × D. reticulatus presence/absence: χ2 = 209.54, df = 2, P < 0.001).
Haemaphysalis concinna (21.4%) were collected only from rodents trapped in the Western region of D. reticulatus occurrence (REGION × H. concinna presence/absence: χ2 = 56.83, df = 2, P < 0.001; Fig. 2a).
Mean abundance of tick infestation by region
Overall mean abundance of ticks was high (15.75 ± 2.04 ticks/individual). The highest mean abundance was noted in rodents trapped in the Eastern region of D. reticulatus occurrence (> 20 ticks/individual; Additional file 1: Table S1) compared to rodents from the Western region (about 14 ticks/individual; Additional file 1: Table S1). Since only one tick species was present in the gap area (I. ricinus), the lowest mean abundance was recorded for rodents from that area, only 4.06 ± 1.32 (main effect of REGION on tick abundance: F(2, 344) = 7.21, P < 0.001; Additional file 1: Table S1).
Mean abundance of juvenile D. reticulatus was > 100 × higher in the Eastern than in the Western region (main effect of REGION on D. reticulatus abundance: F(2, 344) = 12.56, P < 0.001; Fig. 2b). In contrast, juvenile I. ricinus ticks were the most abundant in the Western region of D. reticulatus occurrence (Fig. 2b) with similar abundance in rodents from the Eastern region and the gap area (main effect of REGION on I. ricinus abundance: F(2, 344) = 6.51, P = 0.01). Juvenile H. concinna was the second most abundant tick species in the Western region (main effect of REGION on H. concinna: F(2, 344) = 7.20, P < 0.001; Fig. 2b).
Co-infestation with different tick species by region
Since I. ricinus was the only tick species detected on rodents from the gap area, co-infestation could be observed only in the Eastern and Western regions. An identical percentage of examined rodents (45.3%) was infested with one (I. ricinus or D. reticulatus) or two tick species (I. ricinus + D. reticulatus) in the Eastern region. In the Western region, 67% of rodents were infested with one tick species, and 24.3% were infested with two tick species (I. ricinus + D. reticulatus or I. ricinus + H. concinna) (REGION × TICK CO-INFESTATION: χ2 = 78.71, df = 4, P < 0.001). Rodent species had no significant effect on occurrence of tick co-infestations (not significant, NS).
Monthly activity of juvenile Dermacentor reticulatus
Instars of D. reticulatus were collected in each month of the study. The highest infestation with larvae was observed in June (56.4% of rodents), with a similar low prevalence in July and August (9.5% and 9.7%, respectively) (D. reticulatus LARVAE presence/absence × MONTH: χ2 = 47.16, df = 2, P < 0.001). The highest infestation with D. reticulatus nymphs was observed in July (38.1%), followed by August (29.0%) and June (25.6%) (D. reticulatus NYMPH presence/absence × MONTH: χ2 = 35.80, df = 2, P < 0.001).
Effect of host species on tick infestation
All bank voles M. glareolus were infested with ticks (Additional file 1: Table S1). High prevalence of infestation (> 90%) was found on three species of Apodemus mice (Additional file 1: Table S1). Similarly, almost 90% of root voles A. oeconomus were tick-infested. Tick infestation of both M. agrestis and M. arvalis was slightly lower and similar (about 80%) (RODENT SPECIES × TICK presence/absence: χ2 = 14.90, df = 6, P = 0.02; Additional file 1: Table S1). Neither host age nor host sex had any significant effect on tick infestation (NS).
Juvenile D. reticulatus ticks were collected from all rodent species (7 species; Additional file 1: Table S1). Because of definitely higher prevalence in the endemic (Eastern) region of D. reticulatus compared to the Western region (only 13 specimens of D. reticulatus collected from rodents), we compared prevalence of D. reticulatus instars only for rodent species from the Eastern region. The highest D. reticulatus infestation (97.4%) was recorded in A. oeconomus, followed by M. agrestis (86.7%), A. agrarius (77.8%) and M. glareolus (71.4%). Lower prevalence of D. reticulatus infestation was detected in M. arvalis (68.4%), A. sylvaticus and A. flavicollis (66.7 and 65%, respectively) (RODENT SPECIES × D. reticulatus presence/absence: χ2 = 16.07, df = 6, P = 0.01; Additional file 1: Table S1).
Larvae and nymphs of I. ricinus were also collected from all seven host species, but the infestation was particularly high for Apodemus mice (Additional file 1: Table S1), the highest being for A. flavicollis (89.7%), followed by A. sylvaticus (87.5%) and A. agrarius (84.4%), and much lower for voles (Microtus + Alexandromys): A. oeconomus 72.8%, M. arvalis 56.0% and M. agrestis (45.5%), for M. glareolus 62.5% (RODENT SPECIES × I. ricinus presence/absence: χ2 = 24.98, df = 6, P < 0.001) in all regions (Additional file 1: Table S1).
Only three rodent species from the Western region of D. reticulatus occurrence harbored H. concinna ticks. The highest tick infestation was observed in A. oeconomus, followed by M. agrestis and A. agrarius (RODENT SPECIES × H. concinna presence/absence: χ2 = 23.50, df = 6, P = 0.01; Additional file 1: Table S1).
Tick location on the host body
Some differences were observed in the location of ticks on the host body; however, this was based on general observations, not measured in exact numbers. About 75% of I. ricinus instars fed on the ear surface, between the toes and on the rodent’s head region. About 80% of D. reticulatus larvae and nymphs were found on the surface of the ears (larvae) and deep in the ear canals (nymphs), while about 90% of juvenile H. concinna were found on the ventral site of the rodent body.
Discussion
In the current study we supported the results of our previous study on questing ticks: both larvae and nymphs of D. reticulatus were found on rodents from the regions of the Western and Eastern tick populations, and no juvenile ticks were recorded at the site located in the gap area. This finding confirms the existence of two stable populations of ornate dog ticks, including the areas recently invaded by this tick species (expansion zones; [5, 6]).
Furthermore, very high mean infestation of rodents trapped in the Eastern region of D. reticulatus occurrence by juvenile stages of D. reticulatus was in agreement with extremely high mean abundance of adult ticks recorded previously in Białobrzegi (91 ticks/100 m2), Urwitałt (44 ticks/100 m2) and Stoski (50.5 ticks/100 m2 [6]).
Interestingly, the share of D. reticulatus in the tick community of rodents was reversed in two regions, as D. reticulatus instars constituted the majority of ticks collected from rodents in the region of the Eastern population and were the least common/abundant among the tick community in Western Poland, where H. concinna was more common/abundant than D. reticulatus [7]. In one of the sites in the Western region (Słonin) we did not detect larvae and nymphs of D. reticulatus, although it was located about 40 km from the outer border of the D. reticulatus Western population and only 8–10 km from the nearest site positive for adult D. reticulatus [5, 6]. In Nowy Młyn site adult D. reticulatus were collected in spring 2018 with a density of 9.50 ticks/100 m2 [6], but then we collected only 10 D. reticulatus nymphs from 39 rodents in the summer months of instar activity [7]. To the best of our knowledge, this present study is also the first to report the occurrence of juvenile D. reticulatus ticks in Western Poland; to date only adult questing D. reticulatus ticks have been sampled in that region [21, 22].
Again, this region-dependent structure of the rodent tick community reflects ideally the dominance of adult D. reticulatus among ticks collected from hosts in the region of the Eastern tick metapopulation (in both Poland [23] and Ukraine [24]). The low share of this tick species in the tick community of different rodent species was also observed in the Western metapopulation: in Germany [25–28] and The Netherlands [9], which is an interesting repeatable observation of unknown reason. In the current study, the lack of D. reticulatus in the gap area and high prevalence/abundance in the region of the Eastern population resulted in 100 × higher total abundance of ticks on rodents from this region. Thus, the gap area, historically free of D. reticulatus, was also characterized by the very low total tick abundance on rodents. However, in view of dynamic expansion of D. reticulatus populations in the area of Poland [6], we may expect that the gap area will be colonized by these ticks within a 10-year period.
It is worth underlining that our trapping sites were located in open habitats to focus trapping efforts on Microtus/Alexandromys voles, predicted as the main hosts for juvenile D. reticulatus [7, 8, 12, 13]. However, the selected sites were also rich in vegetation (plant cover up to 1.5 m) and humid even in the hot summer period (submerged meadows, surface water borders). As a result, we sampled a rich rodent community comprising both species typical for open habitats (Microtus/Alexandromys voles, A. agrarius) and ecotone/woodland species (A. sylvaticus, M. glareolus, A. flavicollis). We think that this species-rich rodent community together with suitable humidity conditions of the habitats resulted in the occurrence of three tick species, including species with high humidity requirements (I. ricinus, H. concinna) [2, 29, 30]. In our previous study in forest sites in NE Poland, I. ricinus instars were clearly dominant in the tick community of woodland rodents and D. reticulatus was dominant only among ticks collected from the common vole, M. arvalis, which prefers less humid habitats than the root vole [13, 31]. Our study revealed also that a wide range of open habitats or woodland rodents may constitute suitable hosts for juvenile D. reticulatus (n = 7, present study). As all these rodent species are common and widespread in the whole area of Poland/Europe, they can easily support the settlement of new D. reticulatus populations following the transportation/introduction of engorged females by wildlife (i.e. cervids, wild boar [32] or dogs traveling with their owners [33] and contribute to fast expansion of this tick species.
As both D. reticulatus and I. ricinus instars were observed on rodent ears (current study), this co-occurrence may have resulted in co-feeding [34] and predisposed to the exchange of pathogens between these two species. This phenomenon may have important consequences as both tick species are competent vectors of tick-borne encephalitis virus (TBEV) [4, 35, 36]. The majority of these rodent species are known as a TBEV reservoir hosts [37], and co-feeding has been found to enable virus transmission in an experimental study [38]. For these reasons, co-feeding may increase the density of TBEV-infected I. ricinus as well as D. reticulatus in such habitats, bringing enhanced health risks.
Influence of co-feeding of D. reticulatus and I. ricinus on Borrelia burgdorferi-infected rodents cannot be simply predicted, as ticks from genus Dermacentor were not experimentally confirmed as vectors of any B. burgdorferi (s.l.) spirochaetes [39, 40]. It would be interesting to investigate whether the prevalence of Borrelia burgdorferi (s.l.) is lower in adult I. ricinus ticks in such habitats, where D. reticulatus' contribution to the tick community in rodents is very high and may have resulted in a type of ‘dilution effect’ [41].
Our results about seasonality of larvae and nymphs of D. reticulatus are similar to other studies regarding the activity of D. reticulatus instars [14, 15]. Most of the larvae were collected in June, and the peak of activity of nymphs was noted in July. However, due to the emerging climate changes we can expect changes in seasonal tick activity [42].
During current and previous studies on ticks collected from the environment we did not collect juvenile D. reticulatus from vegetation on any occasion. Additionally, juvenile ticks of this species were not collected from medium-sized mammals, i.e. domestic dogs and red foxes, hosts of adult ticks [20, 23, 43]. Interestingly, recently few D. reticulatus nymphs were collected by the flagging method from vegetation during the highest peak activity of instars in Germany [44]. However, this may be more of an exceptional situation than a common phenomenon and needs to be verified.
Conclusions
We demonstrated that the presence of juvenile D. reticulatus on rodents from open habitats reflects the distribution of adult ticks and can be used as an indicator of the existence of a stable tick population. We have also demonstrated the wide range of rodents as important hosts for juvenile ticks, including D. reticulatus, and the great contribution of this tick species to the tick community in endemic regions, especially in the area of the Eastern tick population/metapopulation. Finally, co-infestation of I. ricinus and D. reticulatus can result in co-feeding and may affect the circulation of pathogens of medical significance in such regions.
Supplementary Information
Additional file 1: Table S1. Tick prevalence and mean abundance by host species by region; nt: number of ticks collected.
Acknowledgements
We sincerely thank Ms Caroline Rust, UK, for the linguistic proofreading of this article.
Abbreviations
- L
Larvae
- N
Nymphs
- NS
Not significant
- SE
Standard error
- TBEV
Tick-borne encephalitis virus
Authors’ contributions
DDS: data collection and analysis, laboratory and field studies, statistical and geospacial analyses, drafiting the manuscript; EJM: data collection and field studies; AB: conceptualization, data collection, project funding, supervision. All authors read and approved the final manuscript.
Funding
The study was funded by the National Science Centre (NCN) Sonata Bis grant no. 2014/14/E/NZ7/00153 (AB).
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The study was performed with the approval of the First Warsaw Local Ethics Committee for Animal Experimentation in Poland (ethical license number: 706/2015).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dorota Dwużnik-Szarek, Email: dorota.dwuznik@biol.uw.edu.pl.
Ewa Julia Mierzejewska, Email: ewajuliamierzejewska@gmail.com.
Anna Bajer, Email: anabena@biol.uw.edu.pl.
References
- 1.Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George JC, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubel F, Brugger K, Walter M, Vogelgesang JR, Didyk YM, Fu S, et al. Geographical distribution, climate adaptation and vector competence of the Eurasian hard tick Haemaphysalis concinna. Ticks Tick Borne Dis. 2018;9:1080–1089. doi: 10.1016/j.ttbdis.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Uiterwijk M, Ibáñez-Justicia A, Vossenberg BVD, Jacobs F, Overgaauw P, Nijsse R, et al. Imported Hyalomma ticks in The Netherlands 2018–2020. Parasit Vectors. 2021;14:244. doi: 10.1186/s13071-021-04738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mierzejewska EJ, Pawełczyk A, Radkowski M, Welc-Falęciak R, Bajer A. Pathogens vectored by the tick, Dermacentor reticulatus, in endemic regions and zones of expansion in Poland. Parasit Vectors. 2015;8:490. doi: 10.1186/s13071-015-1099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mierzejewska EJ, Estrada-Peña A, Alasarraf M, Kowalec M, Bajer A. Mapping of Dermacentor reticulatus expansion in Poland in 2012–2014. Ticks Tick Borne Dis. 2016;7:94–106. doi: 10.1016/j.ttbdis.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Dwużnik-Szarek D, Mierzejewska EJ, Rodo A, Goździk K, Behnke-Borowczyk J, Kiewra D, et al. Monitoring of expansion of Dermacentor reticulatus and occurrence of canine babesiosis in Poland in 2016–2018. Parasit Vectors. 2021;14:267. doi: 10.1186/s13071-021-04758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwużnik D, Mierzejewska EJ, Alsarraf M, Bajer A. A new focus of the tick Haemaphysalis concinna in Western Poland. Exp Appl Acarol. 2019;78:93–112. doi: 10.1007/s10493-019-00371-7. [DOI] [PubMed] [Google Scholar]
- 8.Dwużnik D, Mierzejewska EJ, Drabik P, Kloch A, Alsarraf M, Behnke JM, et al. The role of juvenile Dermacentor reticulatus ticks as vectors of microorganisms and the problem of 'meal contamination'. Exp Appl Acarol. 2019;78:181–202. doi: 10.1007/s10493-019-00380-6. [DOI] [PubMed] [Google Scholar]
- 9.Krawczyk AI, van Duijvendijk GLA, Swart A, Heylen D, Jaarsma RI, Jacobs FHH, et al. Effect of rodent density on tick and tick-borne pathogen populations: consequences for infectious disease risk. Parasit Vectors. 2020;13:34. doi: 10.1186/s13071-020-3902-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCoy KD, Léger E, Dietrich M. Host specialization in ticks and transmission of tick-borne diseases: a review. Front Cell Infect Microbiol. 2013;3:1–12. doi: 10.3389/fcimb.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadolny RM, Gaff HD. Modelling the effects of habitat and hosts on tick invasions. Lett Biomath. 2018;5:2–29. doi: 10.30707/LiB5.1Nadolny. [DOI] [Google Scholar]
- 12.Grzeszczuk A, Karbowiak G, Ziarko S, Kovalchuk O. The root vole Microtus oeconomus (Pallas, 1776): a new potential reservoir of Anaplasma phagocytophilum. Vector Borne Zoonotic Dis. 2006;6:240–243. doi: 10.1089/vbz.2006.6.240. [DOI] [PubMed] [Google Scholar]
- 13.Paziewska A, Zwolińska L, Harris PD, Bajer A, Siński E. Utilisation of rodent species by larvae and nymphs of hard ticks (Ixodidae) in two habitats in NE Poland. Exp Appl Acarol. 2010;50:79–91. doi: 10.1007/s10493-009-9269-8. [DOI] [PubMed] [Google Scholar]
- 14.Földvári G, Široký P, Szekeres S, Majoros G, Sprong H. Dermacentor reticulatus: a vector on the rise. Parasit Vectors. 2016;9:314. doi: 10.1186/s13071-016-1599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karbowiak G. Kleszcz łąkowy Dermacentor reticulatus- występowanie, biologa i rola jako wektora chorób odkleszczowych. Habilitation thesis. Ed. Instytut Parazytologii im. Witolda Stefańskiego PAN, Warsaw 2009.
- 16.Tołkacz K, Bednarska M, Alsarraf M, Dwużnik D, Grzybek M, Welc-Faleciak R, et al. Prevalence, genetic identity and vertical transmission of Babesia microti in three naturally infected species of vole, Microtus spp. (Cricetidae) Parasit Vectors. 2017;10:66. doi: 10.1186/s13071-017-2007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behnke JM, Barnard CJ, Bajer A, Bray D, Dinmore J, Frake K, Osmond J, et al. Variation in the helminth community structure in bank voles (Clethrionomys glareolus) from three comparable localities in the Mazury Lake District region of Poland. Parasitology. 2001;123:401–414. doi: 10.1017/S0031182001008605. [DOI] [PubMed] [Google Scholar]
- 18.Estrada-Peña A, Mihalca AD, Petney TN. Ticks of Europe and North Africa. A guide to species identification. Springer, Cham. 2017;ISBN 978-3-319-63760-0.
- 19.Bajer A, Behnke JM, Pawełczyk A, Kuliś K, Sereda MJ, Siński E. Medium-term temporal stability of the helminth component community structure in bank voles (Clethrionomys glareolus) from the Mazury Lake District region of Poland. Parasitology. 2005;130:213–228. doi: 10.1017/S0031182004006389. [DOI] [PubMed] [Google Scholar]
- 20.Dwużnik D, Mierzejewska EJ, Kowalec M, Alsarraf M, Stańczak Ł, Opalińska P, et al. Ectoparasites of red foxes (Vulpes vulpes) with a particular focus on ticks in subcutaneous tissues. Parasitology. 2020;147:1359–1368. doi: 10.1017/S003118202000116X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiewra D, Czułowska A. Evidence for an increased distribution range of Dermacentor reticulatus in south-west Poland. Exp Appl Acarol. 2013;59:501–506. doi: 10.1007/s10493-012-9612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiewra D, Czułowska A, Lonc E. Winter activity of Dermacentor reticulatus (Fabricius, 1794) in the newly emerging population of Lower Silesia, south-west Poland. Ticks Tick Borne Dis. 2016;7:1124–1127. doi: 10.1016/j.ttbdis.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Mierzejewska EJ, Welc-Faleciak R, Karbowiak G, Kowalec M, Behnke JM, Bajer A. Dominance of Dermacentor reticulatus over Ixodes ricinus (Ixodidae) on livestock, companion animals and wild ruminants in central and eastern Poland. Exp Appl Acarol. 2015;66:83–101. doi: 10.1007/s10493-015-9889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levytska VA, Mushinsky AB, Zubrikova D, Blanarova L, Długosz E, Vichova B, et al. Detection of pathogens in ixodid ticks collected from animals and vegetation in five regions of Ukraine. Ticks Tick Borne Dis. 2021;12:101586. doi: 10.1016/j.ttbdis.2020.101586. [DOI] [PubMed] [Google Scholar]
- 25.Obiegala A, Pfeffer M, Pfister K, Karnath C, Silaghi C. Molecular examinations of Babesia microti in rodents and rodent-attached ticks from urban and sylvatic habitats in Germany. Ticks Tick Borne Dis. 2015;6:445–449. doi: 10.1016/j.ttbdis.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Pfäffle M, Littwin N, Petney T. Host preferences of immature Dermacentor reticulatus (Acari: Ixodidae) in a forest habitat in Germany. Ticks Tick Borne Dis. 2015;6(508):15. doi: 10.1016/j.ttbdis.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Silaghi C, Woll D, Mahling M, Pfister K, Pfeffer M. CandidatusNeoehrlichia mikurensis in rodents in an area with sympatric existence of the hard ticks Ixodes ricinus and Dermacentor reticulatus, Germany. Parasit Vectors. 2012;5:285. doi: 10.1186/1756-3305-5-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silaghi C, Woll D, Hamel D, Pfister K, Mahling M, Pfeffer M. Babesia spp. and Anaplasma phagocytophilum in questing ticks, ticks parasitizing rodents and the parasitized rodents—Analyzing the host-pathogen-vector interface in a metropolitan area. Parasit Vectors. 2012;5:191. doi: 10.1186/1756-3305-5-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubel F, Brugger K, Pfeffer M, Chitimia-Dobler L, Didyk YM, Leverenz S, et al. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016;7:224–233. doi: 10.1016/j.ttbdis.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Hauser G, Rais O, Morán Cadenas F, Gonseth Y, Bouzelboudjen M, Gern L. Influence of climatic factors on Ixodes ricinus nymph abundance and phenology over a long-term monthly observation in Switzerland (2000–2014) Parasit Vectors. 2018;11:289. doi: 10.1186/s13071-018-2876-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welc-Falęciak R, Bajer A, Behnke JM, Siński E. Effects of host diversity and the community composition of hard ticks (Ixodidae) on Babesia microti infection. Int J Med Microbiol. 2008;298:235–242. doi: 10.1016/j.ijmm.2007.12.002. [DOI] [Google Scholar]
- 32.Ciebiera O, Łopińska A, Gabryś G. Ticks on game animals in the fragmented agricultural landscape of western Poland. Parasitol Res. 2021;120:1781–1788. doi: 10.1007/s00436-021-07132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright I. Babesiosis in Essex, UK: monitoring and learning lessons from a novel disease outbreak. Parasit Vectors. 2018;11:132. doi: 10.1186/s13071-018-2718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartosik K, Buczek A, Borzęcki A, Kulina D. Study of the non-parasitic stage in Ixodes ricinus after co-feeding with Dermacentor reticulatus in three infestations. Ann Agric Environ Med. 2017;24:90–95. doi: 10.5604/12321966.1234005. [DOI] [PubMed] [Google Scholar]
- 35.Bajer A, Rodo A, Bednarska M, Mierzejewska E, Welc-Faleciak R. Babesia canis and tick-borne encephalitis virus (TBEV) co-infection in a sled dog. Ann Agric Environ Med. 2011;20:426–430. [PubMed] [Google Scholar]
- 36.Liebig K, Boelke M, Grund D, Schicht S, Springer A, Strube C, et al. Tick populations from endemic and non-endemic areas in Germany show differential susceptibility to TBEV. Sci Rep. 2020;10:15478. doi: 10.1038/s41598-020-71920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grzybek M, Alsarraf M, Tołkacz K, Behnke-Borowczyk J, Biernat B, Stańczak J, et al. Seroprevalence of TBEV in bank voles from Poland-a long-term approach. Emerg Microbes Infect. 2018;7:145. doi: 10.1038/s41426-018-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Randolph SE. Transmission of tick-borne pathogens between co-feeding ticks: Milan Labuda's enduring paradigm. Ticks Tick Borne Dis. 2011;2:179–182. doi: 10.1016/j.ttbdis.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Eisen L. Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: a review. Ticks Tick Borne Dis. 2020;11:101359. doi: 10.1016/j.ttbdis.2019.101359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pal U, Kitsou C, Drecktrah D, Yaş OB, Fikrig E. Interactions between ticks and Lyme disease spirochetes. Curr Issues Mol Biol. 2021;42:113–114. doi: 10.21775/cimb.042.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kowalec M, Szewczyk T, Welc-Falęciak R, Siński E, Karbowiak G, Bajer A. Ticks and the city - are there any differences between city parks and natural forests in terms of tick abundance and prevalence of spirochaetes? Parasit Vectors. 2017;10:573. doi: 10.1186/s13071-017-2391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dantas-Torres F. Climate change, biodiversity, ticks and tick-borne diseases: the butterfly effect. Int J Parasitol Parasites Wildl. 2015;4:452–461. doi: 10.1016/j.ijppaw.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Król N, Kiewra D, Lonc E, Janaczyk B, Chodorowska-Skubiszewska A, Dzieciol M. Dermacentor reticulatus (Fabricius, 1794) and Babesia canis (Piana et Galli-Valerio, 1895) as the parasites of companion animals (dogs and cats) in the Wroclaw area, south-western Poland. Ann Parasitol. 2016;62:125–130. doi: 10.17420/ap6202.44. [DOI] [PubMed] [Google Scholar]
- 44.Schmuck HM, Chitimia-Dobler L, Król N, Kacza J, Pfeffer M. Collection of immature Dermacentor reticulatus (Fabricius, 1794) ticks from vegetation and detection of Rickettsia raoultii in them. Ticks Tick Borne Dis. 2020;11:101543. doi: 10.1016/j.ttbdis.2020.101543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Tick prevalence and mean abundance by host species by region; nt: number of ticks collected.
Data Availability Statement
All data generated or analysed during this study are included in this published article.