Abstract
Immunoglobulin A (IgA) is the dominant antibody isotype in the gut and has been shown to regulate microbiota. Mucosal IgA is also widely believed to prevent food allergens from penetrating the gut lining. Even though recent work has elucidated how bacteria-reactive IgA is induced, little is known about how IgA to food antigens is regulated. Although IgA is presumed to be induced in a healthy gut at steady state via dietary exposure, our data do not support this premise. We found that daily food exposure only induced low-level, cross-reactive IgA in a minority of mice. In contrast, induction of significant levels of peanut-specific IgA strictly required a mucosal adjuvant. Although induction of peanut-specific IgA required T cells and CD40L, it was T follicular helper (TFH) cell, germinal center, and T follicular regulatory (TFR) cell–independent. In contrast, IgG1 and IgE production to peanut required TFH cells. These data suggest an alternative paradigm in which the cellular mechanism of IgA production to food antigens is distinct from IgE and IgG1. We developed an equivalent assay to study this process in stool samples from healthy, nonallergic humans, which revealed substantial levels of peanut-specific IgA that were stable over time. Similar to mice, patients with loss of CD40L function had impaired titers of gut peanut-specific IgA. This work challenges two widely believed but untested paradigms about antibody production to dietary antigens: (i) the steady state/tolerogenic response to food antigens includes IgA production and (ii) TFH cells drive food-specific gut IgA.
INTRODUCTION
The gut faces a daily challenge of maintaining tolerance to commensals and dietary antigens while protecting the body from pathogens and toxins. Antigens delivered orally normally evoke an immunoregulatory response in the gut known as oral tolerance. This is characterized by active suppression of specific immune responses during subsequent intragastric or systemic exposures to the same antigens. The loss of oral tolerance can result in inflammatory type 2 immune responses to dietary antigens, in the form of food allergy. The cellular immune response that maintains tolerance to food antigens in the gut by regulatory T cells (Tregs) is well established (1–3). However, the contribution of humoral immunity to food tolerance remains underexplored (4, 5).
Immunoglobulin A (IgA) constitutes about 80% of all antibodies in the gut; almost all gut IgA is polymeric and transported into the gut lumen by the polymeric Ig receptor (6). Secretory IgA (sIgA) regulates the composition of gut commensal microbiota in both humans and mice while preventing pathogens and toxins from penetrating the mucosa (7–10). It is unclear whether gut IgA regulates immune responses to food antigens in the same way, although it has long been hypothesized that IgA mitigates allergic responses through immune exclusion of food antigens in the gut (11). Food-reactive IgA can be detected in the stool of humans and mice (12, 13), yet most studies of IgA to dietary antigens have focused on serum (14–16). In patients with food allergy, the value of serum IgA in predicting allergic status or therapeutic efficacy is controversial as both IgA1 and IgA2 against β-lactoglobulin (milk), ovomucoid, and ovalbumin (OVA; eggs) are variable (15–17). Therefore, the role of dietary antigen-specific IgA is still debated (4, 5).
To understand the role of dietary antigen-specific IgA, it is important to know when and how it is induced. Although it is not clear why certain foods are more allergenic than others, there is evidence of intrinsic adjuvant ability of some food antigens (18). One example is Ara h 1 protein in peanut, which can bind and activate dendritic cell–specific intercellular adhesion molecule-grabbing nonintegrin (DC-SIGN) on monocyte-derived DCs in vitro (19). This begets the question of whether a potent food allergen such as peanut induces IgA via intrinsic adjuvant activity or whether additional innate stimuli are required. One study using a milk allergy model argued that gut IgA is only induced under tolerogenic conditions [i.e., in the absence of a mucosal adjuvant such as cholera toxin (CT)] (13), whereas another found that OVA-specific IgA was only induced when CT was present (20). Studies evaluating IgA to model protein antigens such as keyhole limpit hemocyanin (KLH) found impaired IgA in the absence of CT (21–23) or in mice lacking CD19 (24), CD40 (25), or T cell costimulation (26). Most nonbacterial IgA studies focus on induction of IgA response to CT isolated from Vibrio cholerae, which causes severe diarrheal disease. These studies found that CT IgA is made in a DC-dependent (27) and T cell–dependent manner (28–30).
In contrast to dietary antigen-specific IgA, IgA induction to gut bacteria during steady state and pathogenic conditions has been defined (7, 31–33). Bacterial-reactive IgA can be induced through T cell–dependent and T cell–independent pathways in several gut-associated lymphoid tissues including Peyer’s patches (PPs), mesenteric lymph nodes (MLNs), and isolated lymphoid follicles (34, 35). A large fraction of IgA to commensal bacteria in mice and humans is polyreactive, transient, and produced in a T cell–independent manner (31, 36–40). However, IgA to pathobionts largely requires T cell help (32, 41), which drives high-affinity IgA to bacteria (42) and has been linked to high IgA coating of colitogenic bacteria (7). Both Tregs and TH17 cells have been demonstrated to adopt a T follicular helper (TFH) cell program to regulate PP IgA induction, and T follicular regulatory (TFR) cells were implicated in regulating high affinity IgA to enhance commensal diversity (28, 32, 43). Despite these clear T-dependent mechanisms, total gut IgA is intact in mice lacking CD4+ T cells, CD40, or T cell costimulation (25, 26, 29, 40).
Different targets of IgA could rely on distinct cellular mechanisms for its induction. Hence, with a dearth of knowledge of when and how IgA to dietary antigens are induced, it is difficult to define the normal immune response to food and, conversely, to understand whether the gut humoral immune response to dietary antigens differs between those with and without food allergy. Using peanut as a model food antigen, we show that there is only modest production of IgA to food antigens during daily exposure to food. However, in the presence of a mucosal adjuvant, a strong, highly specific, and long-lived IgA response is induced to peanut. Using mice with specific deletion of T cell subsets, we found that the induction of highly specific IgA to peanut requires CD4+ T cells, but unexpectedly, not TFH or TFR cells. Our data also revealed a dichotomy between peanut-specific IgA (PN IgA) as compared with IgG1 and IgE induction, whereby the latter two isotypes require TFH cells. We developed an assay to measure peanut-reactive IgA in human stool and found that IgA to peanut in healthy adults is highly specific and remains stable over time. Consistent with a T cell–dependent mechanism, peanut-specific stool IgA in both humans and mice requires CD40L. These findings define the fundamental immunological rules that govern the production of food-reactive IgA and enable the study of whether and how this process might go awry in those with food allergy.
RESULTS
Humans make detectable levels of gut PN IgA that are stable over time
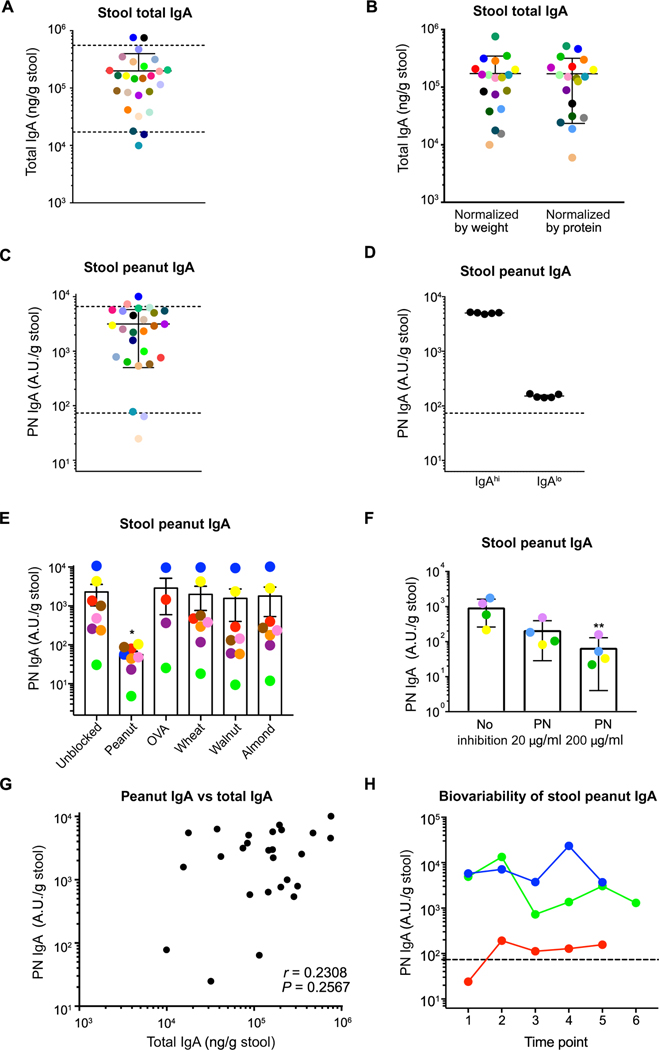
Because the gut is the major site where food antigens are encountered, we focused our study on examining food-reactive IgA in the gut. To study gut humoral responses to food antigens in healthy adults, we developed and validated an enzyme-linked immunosorbent assay (ELISA)–based stool PN IgA assay modified from a published salivary IgA assay (44). Because of the invasiveness of obtaining human gut samples, we used stool as a proxy for our study. Using filtered supernatants from weight-normalized stool suspensions, we measured total IgA using ELISA and established a reference range for stool total IgA in healthy adults (mean of 1.98 × 105 ng/g of stool and an interdecile range of 1.7 × 104 to 5.55 × 105 ng/g of stool; Fig. 1A). We then normalized stool samples to protein content, instead of weight, and found that the total stool IgA values derived from the two methods were comparable (Fig. 1B). We therefore used weight normalization for the rest of our assays. To ensure that these healthy adults do not have underlying peanut sensitization, we measured serum PN IgE and found them to be below 0.35 kUA/liter (fig. S1A).
Fig. 1. Humans make detectable levels of gut PN IgA that are stable over time.
(A) ELISA quantification of total IgA concentration in healthy human (n = 27) stool samples normalized by weight. Dotted lines denote the interdecile range. (B) ELISA quantification of stool total IgA normalized by weight and by total protein concentration. (C) ELISA quantification of PN IgA levels in stool samples of healthy individuals. Dotted lines denote the interdecile range. (D) ELISA quantification of PN IgA in five aliquots of a single stool sample from an IgAhi and an IgAlo individual to assess precision of stool PN IgA ELISA. Dotted line denotes the 10th percentile of stool PN IgA level. (E) Quantification of PN IgA with competitive ELISA in stool samples from eight individuals without or with prior incubation 200 μg/ml of peanut, OVA, wheat, walnut, or almond extract, and (F) with 0, 20, or 200 μg/ml peanut protein. (G) Correlation of PN IgA with total IgA in stool samples of healthy individuals (n = 27). (H) ELISA quantification of stool PN IgA in multiple stool samples collected from two IgAhi and one IgAlo individual at different time points over the course of 10 months to assess biological variability in stool PN IgA over time. Dotted line denotes the 10th percentile of stool PN IgA level. Samples are color-coded, whereby filled circles of the same color represent the same individual throughout each subfigure. Detection limit of 6.25 A.U. relative to a standard of pooled stools with high PN IgA is the intersection point of x and y axes. Median of each group was compared against control sample (no inhibition) using Kruskal-Wallis test with Dunn’s post hoc test, whereby (*) and (**) indicate significant differences with P < 0.05 and <0.01, respectively. Error bars indicate SD.
After exclusion of one individual with undetectable total IgA, we measured PN IgA by ELISA. We created a standard using pooled samples from five individuals to normalize values across all runs, reported in arbitrary units (A.U.). All individuals had detectable PN IgA, with a mean of 3151 A.U./g stool and an interdecile range of 73 to 6599 A.U./g stool (Fig. 1C). We also found detectable egg white IgA in healthy individuals (fig. S1B). To validate the precision of our assay, we assessed samples from an individual with high versus low PN IgA in technical replicates that yielded similar results (Fig. 1D). To test the specificity of PN IgA made in different individuals, we conducted a competitive ELISA in which various food antigens were preincubated with stool supernatant samples to compete for IgA binding to a peanut-coated ELISA plate. We found that peanut antigen incubated with the samples significantly inhibited PN IgA from binding to the peanut-coated plate, whereas none of the other antigens (OVA, walnut, almond, and wheat) did, indicating that PN IgA detected in human stool samples is specific to peanut (Fig. 1E). Further, we found that peanut antigen could block binding of stool PN IgA in a dose-dependent manner, which reinforces the specificity of the PN IgA detected (Fig. 1F). There was no correlation between stool PN IgA levels and total IgA levels (Fig. 1G), which suggested that individuals with more PN IgA did not result from greater gut IgA production in general. We examined the biovariability of PN IgA by assaying different samples from the same individuals over the course of 10 months and found that stool PN IgA values fluctuate within a limited range for each person (Fig. 1H). Last, we measured other antibody isotypes induced against PN and saw that PN IgA, IgG, and IgM, but not IgE, could be found in stool samples of healthy adults (fig. S1C). Similar to total gut antibodies, PN IgA was the most abundant isotype found in stool (fig. S1C) (45). Therefore, nonallergic adults produce a range of highly specific PN IgA that is stable over time.
Daily exposure to peanuts induces minimal peanut-reactive IgA, which is cross-reactive and produced in a T cell–independent manner
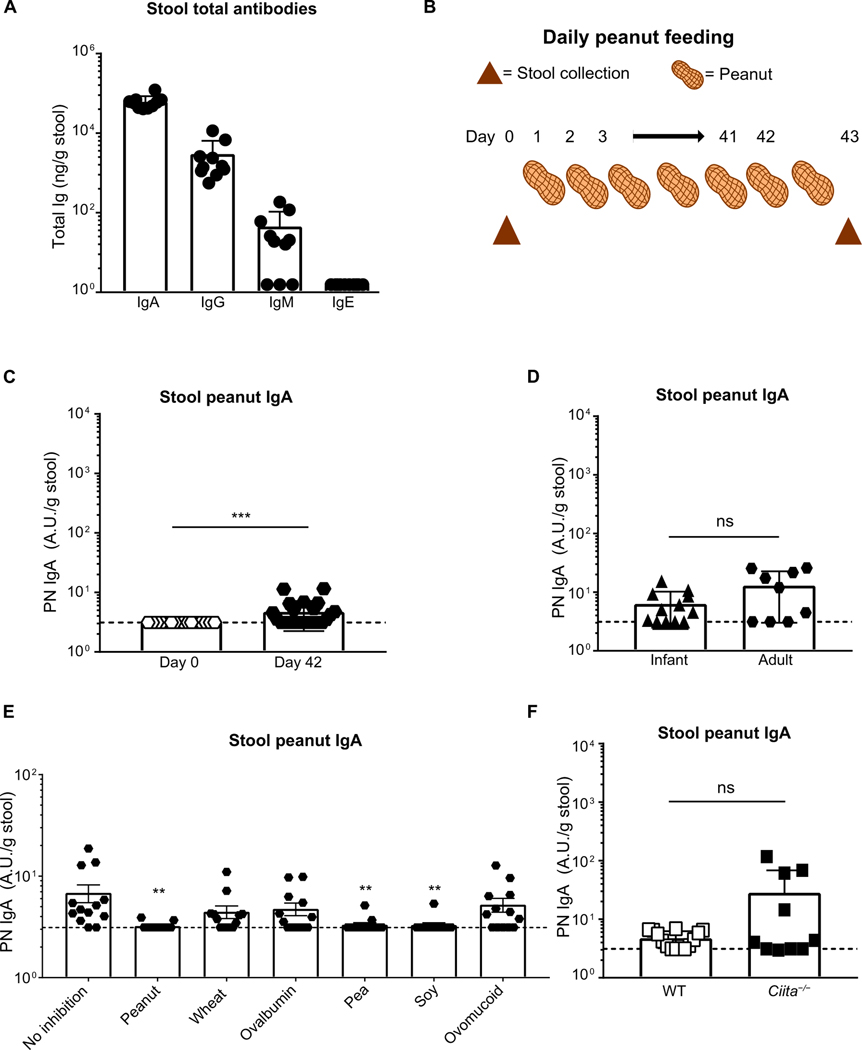
The high rate of peanut IgA–positive samples from our human cohort are consistent with the prevailing notion that gut IgA responses to food antigens are a tolerogenic response induced after any dietary exposure. This model predicts that a nonallergic individual should produce IgA to every encountered dietary antigen. However, these predictions have not been experimentally demonstrated. To understand how the gut immune response to peanut is initiated, we studied the IgA response to multiple food antigens in wild-type (WT) C57BL/6 mice and developed equivalent assays to measure total free IgA and food-reactive IgA from stool. Consistent with previous work (46), we found that free fecal total IgA is the most abundant antibody isotype in stool followed by IgG (Fig. 2A and fig. S2A). Free fecal IgM was minimally present, and IgE was below the detection limit of our assay. To test whether food-specific IgA is produced in mice and understand the cellular mechanisms of food-specific IgA production, we introduced peanut into the peanut-free mouse chow diet and collected stool samples before (day 0) and 1 day after 42 days of peanut exposure (day 42) (Fig. 2B). After 6 weeks of peanut feeding, a minority of the mice made IgA to peanut (PN IgA) and only at low levels, whereas the remaining mice did not produce detectable levels of PN IgA (Fig. 2C and fig. S2B). To address whether the lack of IgA production to peanut was mouse strain–specific, we used C3H/HeJ mice, which is a common mouse model for food allergy. Similar to C57BL/6 mice, C3H/HeJ mice make only a weak response to daily peanut feeding (fig. S2C) (47). Because wheat is a major component of mouse chow, and therefore a chronically exposed dietary antigen, we measured wheat-specific IgA and similarly found that only some mice made detectable levels of wheat-reactive IgA (fig. S2D).
Fig. 2. Daily exposure to peanut induces minimal peanut-reactive IgA that is cross-reactive and produced in a T cell–independent manner.
(A) ELISA quantification of total stool antibodies by isotype in WT mice. (B) Diagram of a 6-week peanut (PN) ad libitum feeding model. Time points for stool collection before (day 0) and after PN feeding (day 43) are indicated with triangles. (C) ELISA quantification of PN IgA in stool of 8-week-old adult mice exposed to PN for 6 weeks versus prepeanut exposure (day 0) or (D) 3-week-old infant mice exposed to PN for 6 weeks. (E) Quantification of PN IgA through competitive ELISA without or with prior incubation with 200 μg/ml PN, OVA, ovomucoid, wheat, soy, or pea extract. Median of each group was compared against median of control sample (no inhibition) using Kruskal-Wallis analysis and Dunn’s post hoc test. (F) ELISA quantification of PN IgA in stool of 8-week-old adult WT or MHCII transactivator knockout (Ciita−/−) mice exposed to PN for 6 weeks. Dotted line indicates the detection limit of assay at 3.12 A.U. relative to the standard. All data are pooled from three independent experiments with a minimum of three mice per group. Mice in each experiment were cohoused littermates. Medians were compared between groups with Mann-Whitney U test, unless otherwise indicated, whereby (**) and (***) indicate significant differences with P < 0.01 and <0.001, respectively, whereas ns denotes not significant. Error bars indicate SD.
The neonatal period is an important window for establishing gut tolerance to commensals and dietary antigens, and IgA plasma cells cannot be detected in mice before weaning (48, 49). Hence, we hypothesized that PN IgA induction might be different in infant and adult mice. Three-week-old mice (infants) were weaned onto regular chow supplemented with peanut for 6 weeks, and their stool PN IgA was compared with 8-week-old mice (adult) under the same regimen. No significant difference in PN IgA levels was observed, and the proportion of mice that made IgA to peanut was similar to our prior results (Fig. 2D).
To test the specificity of PN IgA induced during daily peanut exposure, we again performed competitive ELISA with peanut and other food antigens on samples with detectable PN IgA to see whether other food antigens could block its detection. The low level of PN IgA made during daily peanut exposure was blocked completely by peanut, pea, and soy in most samples; whereas wheat, OVA, and ovomucoid could block the signal in a subset of samples (Fig. 2E). In some patients with food allergy, serum IgE to peanut binds to other legumes such as soy and pea, which might have cross-reactive epitopes (50). However, cross-reactivity of PN IgE with OVA and ovomucoid is uncommon (50). Therefore, this weak peanut-reactive IgA we observed in the stool of mice after daily peanut feeding might contain both cross-reactive and polyreactive antibodies.
Because polyreactive IgA that binds to a variety of gut commensals (35) arises primarily from T-independent mechanisms (31, 36, 37), we tested whether cross-reactive PN IgA production requires T cells. Using class II major histocompatibility complex (MHCII) transactivator knockout (Ciita−/−) mice, which are deficient in MHCII and thereby deficient in CD4+ T cells, we found low-titer stool PN IgA in a small proportion of mice, similar to cohoused WT littermates after daily exposure to peanut (Fig. 2F). This demonstrates that low-titer cross-reactive IgA can be produced in a T-independent manner. Together, these results suggest that steady state daily food exposure in mice induces low-titer cross-reactive IgA that might be different from the highly specific, high-titer PN IgA observed in human stool.
Induction of specific IgA to food antigens requires adjuvant but only during the first exposure
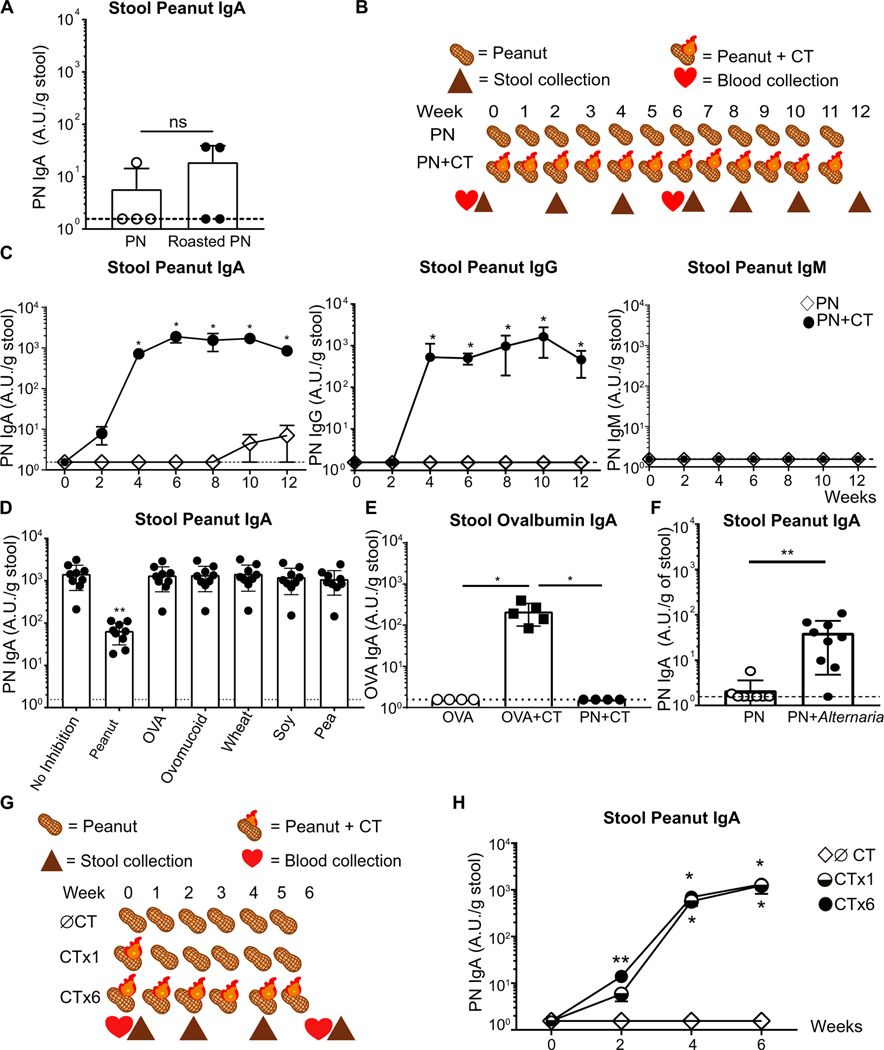
Because inclusion of peanut in the daily diet of mice induced only low levels of cross-reactive IgA, we hypothesized that an inflammatory stimulus, particularly of the innate immune system, is required for induction of food-specific IgA. It has been proposed that some food allergens, including peanut, contain unique immunostimulatory properties that directly activate innate immune pathways and thereby favor adaptive immune responses (18). Yet, peanut exposure alone induced little IgA (Fig. 2C). Food modifications such as roasting have been proposed to enhance the allergenicity of common targets such as peanut (51). We therefore exposed mice intragastrically to roasted or blanched peanut weekly for 6 weeks in the absence of adjuvant and observed poor induction of PN IgA (Fig. 3A).
Fig. 3. Induction of specific IgA to food antigens requires adjuvant but only during the first exposure.
(A) ELISA quantification of PN IgA in stool of WT mice exposed intragastrically weekly to blanched peanut alone (PN) or roasted peanut 1 week after the sixth immunization. (B) Diagram of a 12-week PN intragastric immunization model. Time points for stool and serum collection before and after immunization are indicated with symbols. (C) ELISA quantification of PN-specific antibodies by isotype in WT mice immunized with PN or peanut with cholera toxin (PN + VT) (D) Quantification of PN IgA through competitive ELISA without or with prior incubation with 200 μg/ml peanut, OVA, ovomucoid, wheat, soy, or pea extract. Median of each group was compared against median of control sample (no inhibition) using Kruskal-Wallis analysis and Dunn’s post hoc test. (E) ELISA quantification of OVA-specific IgA (OVA IgA) in stool of WT mice orally immunized with OVA, OVA with CT (OVA + CT), or PN + CT 1 week after the sixth immunization. (F) ELISA quantification of PN IgA in stool of WT mice orally immunized with PN or PN with Alternaria alternata extract (PN + Alternaria) 1 week after the sixth immunization. (G) Illustration of a 6-week PN immunization model using peanut alone (∅ CT), peanut with CT once followed by peanut alone for 5 weeks (CT ×1), or peanut with CT (CT ×6). Time points for stool and serum collection before and after immunization are indicated with symbols. (H) ELISA quantification of PN IgA production in stool of ∅ CT, CT ×1, and CT ×6 immunized mice over 6 weeks. Median of each group was compared using Kruskal-Wallis analysis and Dunn’s post hoc test. Dotted line indicates the detection limit of assay at 1.56 A.U. relative to the standard. Representative (A, C, E, and H) or pooled data (D and F) from three independent experiments with three to five mice per group. Mice in each group were cohoused littermates. Median of each group was compared with Mann-Whitney U test, unless otherwise stated, whereby (*) or (**) indicates significant differences with P < 0.05 and < 0.01, respectively. Error bars indicate SD.
The dominant murine food allergy models use the mucosal adjuvant CT to generate food-specific serum IgE after oral administration of ground peanut (18, 52). Further, CT has been shown to activate DCs in the gut and thereby enhance T cell activation (27) as well as induce T cell–dependent IgA responses (53). Using a weekly oral gavage peanut model with CT (PN + CT) or without (PN) this adjuvant for 12 weeks, we studied the kinetics of peanut-specific antibody production in the serum and stool of WT mice (Fig. 3B). CT induced robust production of stool PN IgA, which peaked at 6 weeks (Fig. 3C). Because the polymeric Ig receptor only transports IgA and IgM across the gut epithelium, we were surprised to discover a significant amount of PN IgG in the stool (Fig. 3C). A direct comparison of optical density of PN IgA and PN IgG in mouse and human stool samples confirmed that PN IgA was the dominant antibody isotype (figs. S1C and S3A), yet PN IgG was present in the stool in quantities higher than might be expected by transepithelial leakage. There is some evidence that neonatal Fc receptor (FcRn) might transport IgG into the lumen even in adult mice, and this IgG is important for combating mucosal bacterial infections (54, 55). Whether FcRn also transports food-specific IgG remains to be tested. Although secretory IgM is often produced and present at moderate levels in the gut, no PN IgM was detected in the stool of PN or PN + CT groups. An absence of free IgM in feces does not necessarily indicate an absence of gut IgM; instead, the majority of gut IgM may be commensal-bound, which would not be measured by this methodology (31).
Although significant PN IgE was detected in the serum of PN + CT mice, none was detectable in the stool, suggesting that either peanut IgE is not produced in the gut or cannot be transported across the gut lumen (fig. S3, B and C). PN IgA and IgG were also found at high levels in the serum of the PN + CT group (fig. S3C). Although a portion of IgA plasma cells in the bone marrow comes from gut priming (56, 57), the contribution of gut PN IgA to serum PN IgA and their relationship remains enigmatic (41, 57). In contrast to all other isotypes, PN IgM was detected in the serum of naïve mice, PN alone and PN + CT groups (fig. S3C), suggesting that a potentially cross-reactive IgM is present in the steady state.
To test whether CT-induced PN IgA is specific to peanut, we performed an ELISA-based blocking assay with six other dietary antigens to see whether any of them blocked detection of PN IgA. Unlike the weak and cross-reactive PN IgA produced during daily feeding of peanut, adjuvant-primed PN IgA is highly specific, as only peanut could significantly block its detection and did so in a dose-dependent manner (Fig. 3D and fig. S3D). Although legumes such as soy and pea have been shown to have cross-reactivity when tested with PN IgE clinically (50), neither antigens significantly blocked adjuvant-primed PN IgA, suggesting that either PN IgA made in this model is highly specific or that legume cross-reactive epitopes do not comprise most of the PN IgA induced during this form of dietary antigen exposure. Using another dietary protein, OVA, similar induction of OVA-specific IgA was observed, again only at significant levels when adjuvant was present (Fig. 3E). No OVA-specific IgA was detected in the PN + CT group (Fig. 3E). Consistent with previous studies, oral immunization with CT induces CT-specific IgA (CT IgA) in the stool, regardless of the coadministered food antigen (fig. S3, E and F) (22).
CT is a well-studied and potent mucosal adjuvant which can induce sIgA production (23, 58). Structurally, CT contains an A subunit with adenosine diphosphate–ribosyltransferase activity and five identical B subunits that bind to GM1 receptors, which is required for its adjuvanticity (58). To determine whether IgA responses to food are only induced in the presence of CT, we used a structurally dissimilar adjuvant, Alternaria alternata, a saprophyte of plants, including peanut, to see whether it promoted PN IgA (59). Albeit a weaker response as compared with PN + CT, we found that exposure of Alternaria together with peanut induced a gut sIgA response to peanut (Fig. 3F). However, unlike CT, coadministration of Alternaria with peanut did not lead to PN IgE in a majority of the mice (fig. S3G). These data suggest that stool PN IgA induction requires an adjuvant but that not all adjuvants lead to concurrent priming of both IgA and IgE to peanut.
From our data and the work of others, it is known that generation of high levels of antigen-specific IgA requires multiple oral exposures to antigen with adjuvant [Fig. 3B and (53)]. However, for innate instruction of adaptive immune responses, the role of an adjuvant is to activate the antigen-presenting cell, typically a DC. This initiates T cell priming. Subsequent antigen, but not adjuvant, is necessary to facilitate B cell–T cell interactions. Therefore, we hypothesized that CT should only be required during the first peanut exposure; this hypothesis presumes that the highly specific IgA antibody was made in a T-dependent manner. Using a model that involves one administration of peanut with CT and five subsequent administrations of peanut alone (CT ×1), we found that PN IgA was made at a similar magnitude and followed the same kinetics regardless of whether adjuvant was coadministered with every peanut exposure or only during the first exposure (Fig. 3, G and H). In line with the strong gut PN IgA response, comparably high levels of serum PN IgA and PN IgE were produced with both immunization regimens (fig. S3, H and I). These data demonstrate that production of highly specific gut IgA to peanut requires adjuvant when administered orally but only during the first exposure. This is likely to induce effective antigen presentation to T cells, with subsequent doses of antigen alone boosting the B cell response.
PN IgA is long-lived, and induction requires MHCII
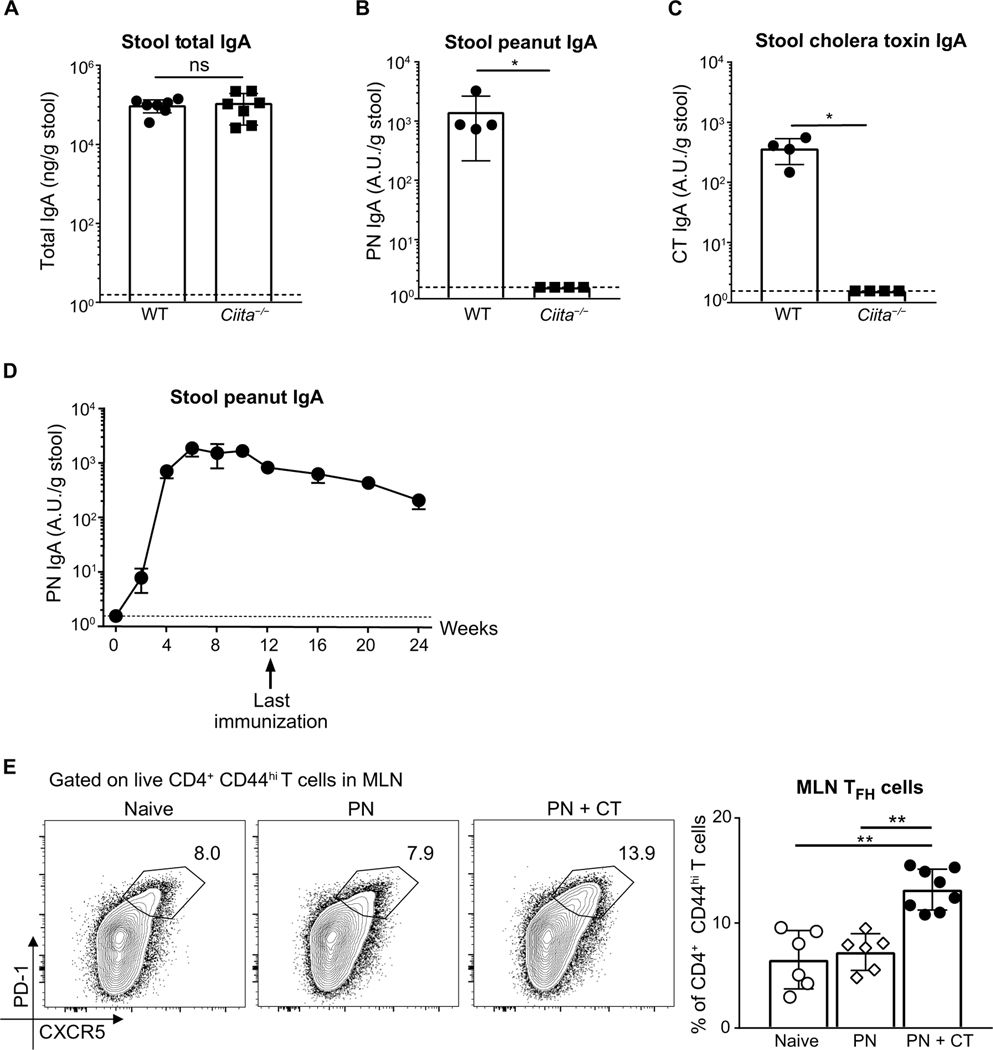
Adjuvant-initiated DC activation is required for T cell priming to a wide variety of target antigens. Given the specificity of PN IgA and the requirement for adjuvant, we hypothesized that PN IgA+ B cell induction requires CD4+ T cell help. We therefore immunized MHCII-deficient, thereby CD4+ T cell–deficient, (Ciita−/−) mice with peanut and CT and found that despite producing WT levels of total stool IgA (Fig. 4A), Ciita−/− mice are unable to make detectable stool PN IgA (Fig. 4B). In line with previous reports using mice lacking CD4+ T cells (29, 30), we found no stool CT IgA in Ciita−/− mice (Fig. 4C).
Fig. 4. PN IgA is long lived, and its induction requires CD4+ T cells.
ELISA quantification of (A) total IgA (B), PN IgA, and (C) CT IgA in stool of cohoused WT or MHCII transactivator knockout (Ciita−/−) mice 1 week after sixth immunization with PN + CT. (D) ELISA quantification of PN IgA in stool of WT mice 4, 8, and 12 weeks after 12 immunizations with PN + CT. Representative data from three independent experiments with four to seven mice per group. (E) Representative flow plot and frequency of TFH cells in MLNs from naïve, PN, or PN + CT–immunized WT mice 8 days after one PN + CT immunization. TFH cells (CXCR5hi PD-1hi) were gated on CD4+ CD44hi live activated T cells (TCRβ+). Pooled data from two independent experiments with three to four mice per group. Median of each group was compared using Kruskal-Wallis analysis and Dunn’s post hoc test. Dotted lines indicate detection limit of the PN-IgA assay at 1.56 A.U. relative to the standard. Median of each group was compared between groups with Mann-Whitney U test, unless otherwise stated, whereby (*) and (**) indicate significant differences with P < 0.05 and < 0.01, respectively. Error bars indicate SD.
A study using a reversible colonization system to study intestinal IgA responses to commensals suggested that gut IgA to commensals has little or no immunological memory and can adapt to changes in the microbes present in the gut (60). It is possible that this form of transient IgA is produced in a T cell–independent manner (31, 36, 40), whereas T cell–dependent CT IgA can still be detected up to 100 weeks after immunization (61). We therefore hypothesized that T cell–dependent PN IgA will generate a long-lived antibody response. To study the longevity of PN IgA, we collected stool samples from mice every 4 weeks after the last peanut exposure at week 12. Our data show that stool PN IgA is present at high levels even 3 months after ceasing immunization (Fig. 4D), supporting the hypothesis that T cell–dependent IgA to peanut is long-lived.
Production of peanut-specific IgG1 and IgE, but not gut IgA, requires TFH cells
We next wanted to define the subset of CD4+ T cells driving gut peanut IgA. Current models of specific, high-affinity antibody production center on CD40L-expressing TFH cells (62). Both TH17 and Treg cells have been shown to be precursors for TFH-like cells that promote IgA in the PP (28, 43). Mouse models resulting in enhanced TFH cell differentiation have been associated with increased gut IgA to commensals (42, 63). Using adoptive transfer of TCR transgenic cells, recent studies associated TFH cell differentiation with IgA production in MLNs and PPs (64, 65). We therefore tested whether PN + CT exposure induces TFH cells by analyzing CXCR5hi programmed cell death protein 1 (PD-1)hi activated T cells in the MLN and PPs. Using the gating strategy described in fig. S4A, we confirmed that this T cell population expressed BCL6 (fig. S4B). Upon intragastric exposure with PN + CT but not PN alone, we found an increase in TFH cell frequency 8 days after immunization (Fig. 4E) but not cell number (fig. S4C) in the MLN. This was not accompanied, however, by a change in TFH cell frequency or number in the PPs (fig. S4D). Because the small intestine has greater exposure to dietary antigens than the colon and given compartmentalization of different T cell fates in proximal versus distal gut-draining LNs (66), we separated colonic MLNs (cLN) from small intestinal-draining (siLN) MLNs and found similar TFH cell frequencies in siLN and cLN in both naïve and PN + CT–immunized mice (fig. S4E).
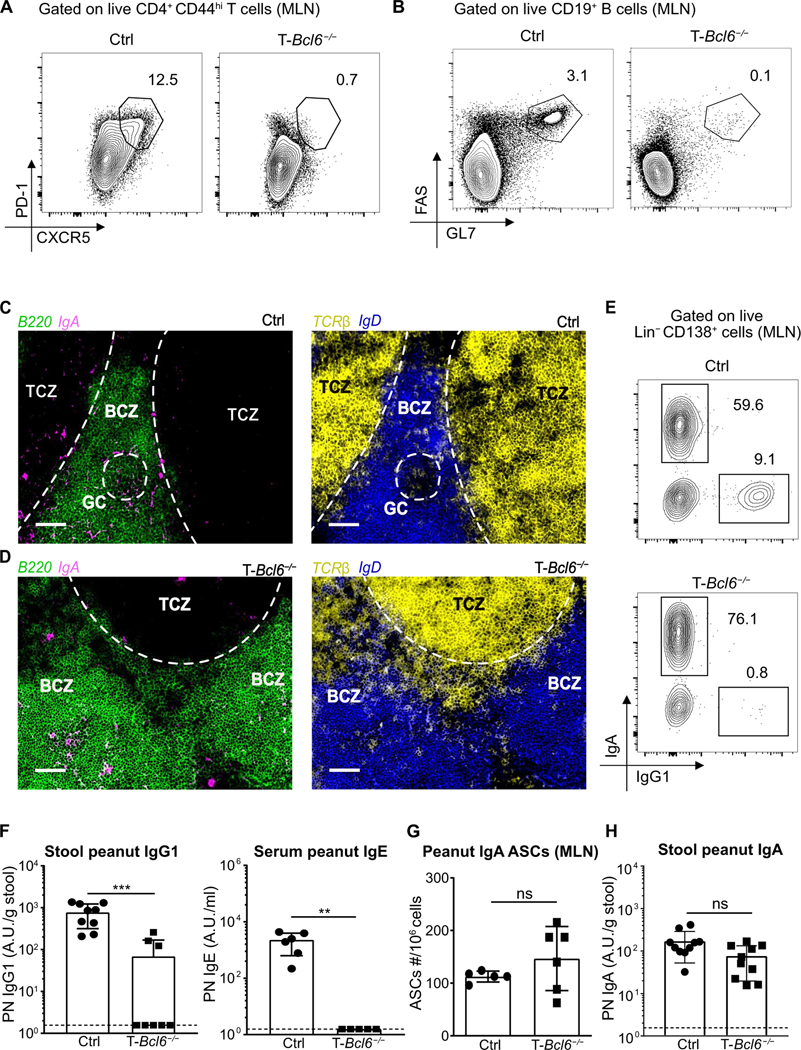
Although multiple studies have proposed that TFH-like cells induce production of gut IgA, using TFH cell-deficient mice, Bunker et al. (31) found that the majority of gut IgA to commensals is not reduced by TFH or even T cell deficiency. Whether TFH cells are actually required for IgA to nonbacterial antigens from any mucosal site has not been formally tested. To study whether TFH cells are necessary for the production of peanut IgA, we immunized T-Bcl6−/− mice (CD4creBcl6flox/flox) (67) with PN + CT and measured PN-specific antibody responses. As expected, T-Bcl6−/− mice exhibited a near total loss of TFH cells in the MLN and PP when compared with cohoused littermate controls (Fig. 5A and fig. S5, A and B). However, Treg and TH17 cell frequencies were not decreased in T-Bcl6−/− mice (fig. S5, C to F). In line with loss of TFH cells, germinal center (GC) B cells were almost completely absent in T-Bcl6−/− (Fig. 5B and fig. S6, A to C). Using immunofluorescent microscopy, we confirmed that GCs, defined as B220+ IgD− areas, were absent in MLNs of T-Bcl6−/− mice but present in control mice (Fig. 5, C and D). In WT mice, IgA+ B cells were found outside of GCs in the B cell zone (BCZ), some of which appear to colocalize with T cells (Fig. 5C). Similarly, using GL7 to define GCs, IgA+ B cells could be observed in the GC periphery and outside the GC in WT mice (fig. S7A). We confirmed that IgA staining outside of GCs was not due to nonspecific stromal cell binding using CD35 and CD45 staining along with IgA in WT mice (fig. S7, B and C). The specificity of IgA antibody was also validated using AID−/− mice, which showed the complete absence of IgA staining in the MLN (fig. S7D). Despite the lack of TFH cells and GCs in the MLN of T-Bcl6−/− mice, we observed IgA+ B cells in the BCZ as well as collections of B and T cells in close proximity (Fig. 5D). These data suggest that IgA+ B cells can be produced in the absence of TFH cells and might be induced distal to and independent of GCs.
Fig. 5. TFH cells are dispensable for antigen-specific gut IgA but are required for IgG and IgE production.
Representative flow plot of (A) TFH cells or (B) GC B cells in MLNs of control T-Bcl6+/+ (Bcl6flox/flox) (Ctrl) or T-Bcl6−/− (CD4creBcl6flox/flox) cohoused littermates 8 days after one peanut with CT (PN + CT) immunization. TFH cells (CXCR5hi PD-1hi) were gated on CD4+ CD44hi live activated T cells (TCRβ+). GC B cells (FAS+ GL7+) were gated on live B cells (CD19+). Immunofluorescence images of IgA+ B cells (B220+ IgA+) and colocalization of T cells (TCRβ+) and B cells (B220+) outside GC (B220+ IgD−) in BCZ of MLN of (C) control or (D) T-Bcl6−/− mice 8 days after one PN + CT immunization. T cell zones (TCZ) and GCs are demarcated with dotted lines. Scale bars, 100 μm. (E) Representative flow plot of IgA and IgG1 plasmablasts in MLN of control or T-Bcl6−/− cohoused littermates. Plasmablasts were gated on live CD138+ Lin− (TCRβ, NK1.1, CD14, CD4, and B220) cells. (F) ELISA quantification of stool PN IgG1 and serum PN IgE of cohoused control or T-Bcl6−/− littermates 1 week after sixth immunization with PN + CT. (G) Enumeration of PN IgA antibody-secreting cells (ASCs) in MLN of control or T-Bcl6−/− cohoused littermates by ELISPOT. All plasmablast and ASC analyses were performed 8 to 10 days after two weekly peanut with CT (PN + CT) immunizations. (H) ELISA quantification of stool PN IgA of cohoused control or T-Bcl6−/− littermates 1 week after sixth immunization with PN + CT. (A to E) Representative data from one mouse of each group from three to four independent experiments with three to five mice per group. (F to H) Pooled data from two to three independent experiments with a minimum of three mice per group. Median of each group was compared between groups with Mann-Whitney U test, whereby (**) or (***) indicates significant differences with P <0.01 or <0.001, respectively. Error bars indicate SD.
To test this, we used flow cytometry, ELISA, and the enzyme-linked immunosorbent spot (ELISPOT) assay to directly characterize the fate of gut B cells after peanut exposure. The presence of IgA plasmablasts in the MLNs and PPs was independent of TFH cells (Fig. 5E and fig. S8, A to C), and total IgA levels in the stool were unimpaired (fig. S8D). In contrast, IgG1 plasmablast were almost completely ablated in TFH-deficient mice (Fig. 5E and fig. S8, B and E). Consistent with work using other types of immunizations (68, 69), antigen-specific IgE and IgG1 in the serum and stool were significantly impaired in TFH-deficient mice (Fig. 5F and fig. S8F). However, PN IgA–secreting cells in MLNs and PPs and peanut-specific stool IgA were equivalent between T-Bcl6−/− mice and cohoused littermates (Fig. 5, G and H, and fig. S8G). Unlike gut IgA, PN IgA in the serum was partially impaired (fig. S8H), indicating potentially separate mechanisms regulating the production of gut versus systemic food-specific IgA. Last, we confirmed that the IgA response directed to a well-studied T cell–dependent gut antigen, CT, was also intact in the absence of TFH cells (fig. S8I). Together, these findings demonstrate that antigen-specific IgA production in the gut is CD4+ T cell–dependent but GC- and TFH cell–independent, whereas IgG1, IgE, and, at least in part, serum IgA require TFH cells.
Peanut-specific gut IgA production is independent of TFR cells
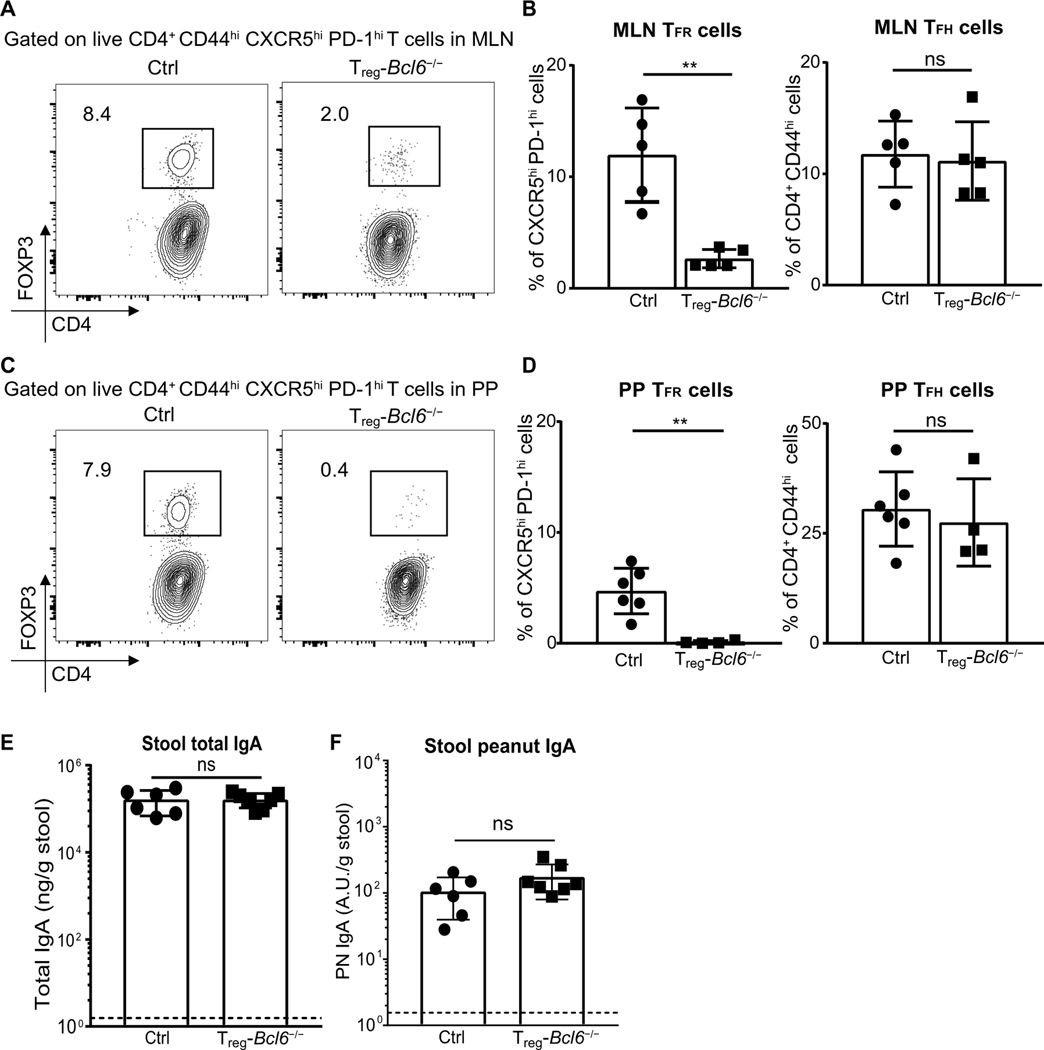
TFR cells are thymus-derived regulatory T cells that control GC B cell responses (70) and can be distinguished from TFH cells via FOXP3 expression (fig. S9A). TFR cells are important in modulating antibody responses (70). It was previously demonstrated that adoptively transferred Tregs adopt a TFH cell phenotype in PPs to modulate IgA production (43) and that TFR cells regulate production of high-affinity IgA to gut bacteria (32). Therefore, we tested for a possible role of TFR cells in shaping the gut IgA response to food antigens. Upon PN + CT immunization, TFR cell frequencies but not cell numbers were actually reduced in the MLN (fig. S9, B to D). There was also a modest decrease in TFR cell numbers, but not frequency, in the PPs of the PN + CT group (fig. S9, E and F). To assess the role of TFR cells in gut IgA production to peanut, we used Treg-Bcl6−/− mice (Foxp3creBcl6flox/flox), which have significant reductions in TFR cells but not TFH cells in both MLN (Fig. 6, A and B) and PP (Fig. 6, C and D). Despite deficiencies in TFR cell populations in these mice, we found comparable total IgA and PN IgA in the stool of Treg-Bcl6−/− as control mice (Fig. 6, E and F), suggesting that TFR cells are dispensable for the production of gut IgA to peanut.
Fig. 6. Production of PN IgA is independent of TFR cells.
(A and C) Representative flow plot and (B and D) frequencies of TFR and TFH cells in (A and B) MLN and (C and D) PP from control (Bcl6flox/flox) or Treg-Bcl6−/− (Foxp3creBcl6flox/flox) littermates 8 days after one PN + CT immunization. TFR cells (FOXP3+) were gated on TFH cells (CXCR5hi PD1hi). TFH cells were gated on CD4+ CD44hi live activated T cells (TCRβ+). (E and F) ELISA quantification of total IgA and PN IgA in stool of Treg-Bcl6+/+ or Treg-Bcl6−/− littermates 1 week after sixth immunization with PN + CT. Figures represent data from three independent experiments with three to six mice per group. Median of each group was compared with Mann-Whitney U test whereby (**) indicates significant differences with P <0.01. Error bars indicate SD
Production of peanut-specific gut IgA in mice and humans requires CD40L
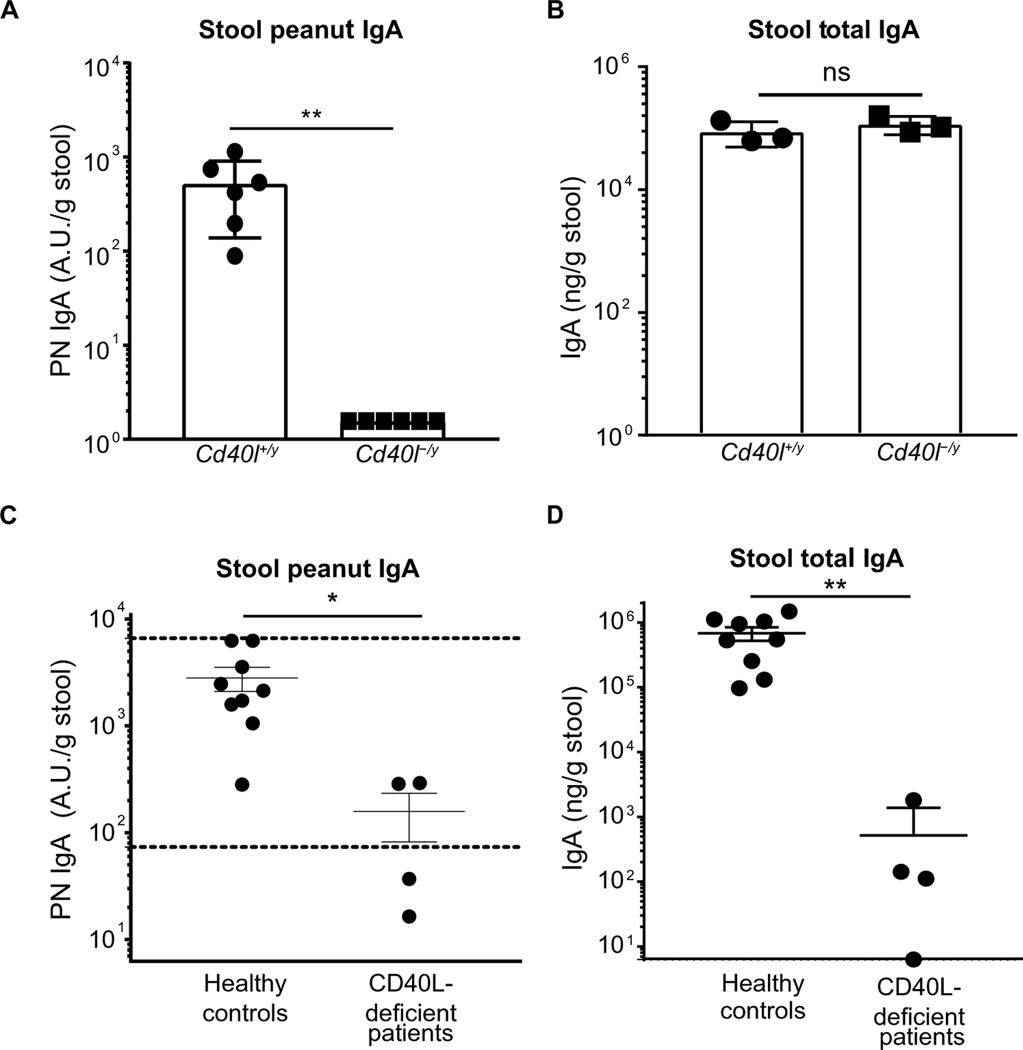
Previous work using Cd40−/− mice or Cd19−/− mice, which have defects in CD40 signaling, demonstrated abrogation of KLH- or CT-specific but not total gut IgA (24, 25). DCs can induce IgA class switching in the absence of CD40 (71). Given our results with TFH cell– and TFR cell–deficient mice, we tested whether PN IgA induction still depended on CD40-CD40L interaction. Our data show that mice deficient in CD40 ligand (Cd40l-/y) had no detectable PN IgA despite unaltered levels of total stool IgA (Fig. 7, A and B). Using our assay for human stool PN IgA, we found that the same pathway was important in human gut IgA responses to peanut. Patients with mutations in CD40L either had low levels of PN IgA in their stool compared with healthy adult controls or were below the reference range established in Fig. 1 (Fig. 7C). However, unlike in mice, CD40L expression is necessary for most of the gut IgA production in human stool (Fig. 7D). This demonstrates that although gut IgA production can occur in a CD40L–independent manner, both mice and humans require CD40L for specific IgA production to a food antigen.
Fig. 7. Induction of peanut-specific gut IgA requires CD40L in both mice and humans.
ELISA quantification of (A) PN IgA or (B) total IgA in stool samples from Cd40l+/y (WT) and Cd40l-/y (knockout) cohoused littermates 1 week after sixth immunization with PN + CT. Representative data from two independent experiments with three to six mice per group. ELISA quantification of (C) PN IgA or (D) Total IgA in stool samples from four CD40L-deficient patients and nine healthy individuals. Dotted lines represent interdecile range. Median of each group was compared between groups with Mann-Whitney U test, whereby (*) or (**) indicates significant differences with P < 0.05 or < 0.01, respectively. Error bars indicate SD.
DISCUSSION
The specificity of a large proportion of gut IgA remains unknown in both humans and mice (39, 72). In both species, polyreactive IgA that binds nucleic acids, insulin, and a broad range of microbiota has been demonstrated through cloning of IgA from B cells isolated from the intestine (36, 39). However, in humans, these antibodies are a minority of the IgA repertoire in the gut, whereas the target of much of the IgA is unknown (39). We propose that dietary antigens could be one of the major targets of this IgA, but the cause of their induction and the underlying cellular mechanisms differ from what has been assumed. In both humans and mice, food-reactive IgA has been observed in serum or stool by us and others (12, 13, 15, 16); this IgA is widely believed to be a constituent of the gut tolerogenic response, occurring through antigen feeding in the absence of adjuvants. We show that low levels of cross-reactive IgA to daily dietary antigens can be induced during steady state in a T-independent manner, similar to the pathway described for much of the commensal-reactive IgA (fig. S10); however, this occurs rarely. In the absence of substantial gut inflammation, dietary antigens are largely ignored, thus avoiding a metabolically costly endeavor of producing IgA to every food antigen ingested.
In contrast, high-titer IgA specific to dietary antigens is produced only during particular states of innate immune activation, induced in our model by mucosal adjuvants (fig. S10). It has been hypothesized that some food allergens, including peanut, have intrinsic adjuvant activity, particularly after modifications such as roasting (18, 51). Yet, similar to blanched peanut, roasted peanut alone induced negligible PN IgA in mice. Instead, coadministration with the strong mucosal adjuvant CT induced high titers of specific IgA to peanut. This induction of PN IgA is not unique to CT; we exposed mice to peanut with the fungus Alternaria and observed similar induction of IgA to peanut, albeit at a lower magnitude. The use of an adjuvant in murine models is often considered nonphysiological; however, on the basis of current understanding of naïve T cell priming, innate immune receptor activation of antigen-presenting cells is necessary for T cell priming and is accomplished either by antigen-intrinsic or antigen-extrinsic adjuvants (fig. S10). Such a model predicts that adjuvant activity would be required for a T cell–dependent antibody response, which concurs with our observations. Using a model of transient infection of the duodenum with a parasite, breach of oral tolerance to OVA led to increased OVA-specific IgG1 production (66). Whether this is true for IgA remains to be tested but is consistent with our model that an inflammatory stimulus in the gut can lead to production of antibodies against dietary antigens. Although we were able to show that nonallergic humans make IgA that is highly specific to peanut and is stable over time, we can only speculate on possible triggers of food-specific IgA until we gain a better mechanistic understanding of the relevant gut innate immune pathways. Using Alternaria, we found that particular adjuvants used in our mouse model induced IgA without concomitant IgE to peanut. Alternaria is a common saprophyte on plants, including peanut, and could be a contaminant in multiple food sources of plant origin (59). However, further work is necessary to define the cellular pathways common to adjuvants that can prime food-specific IgA and the relevance of these pathways to the human gut immune system.
Consistent with the need for adjuvant, we show that CD4+ T cells are required for PN IgA responses in mice. Further, CD40L is necessary in both humans and mice for this gut humoral response to peanut. Most T cell–dependent highly specific antibodies are induced by TFH cells, and multiple indirect lines of evidence suggested that the same was true for gut IgA (28, 32, 42, 43, 63, 64, 73); however, the requirement for TFH cells in IgA production to nonbacterial antigens has not been directly tested. Using T-Bcl6−/− mice lacking TFH cells, we unexpectedly found that TFH cells are neither required for the production of peanut-specific nor CT-specific IgA. In contrast, TFH cells were necessary for the production of peanut-specific IgG1 and IgE. Hence, the increase in TFH frequencies, which we observed in the MLN after peanut immunization, likely contributes to peanut-specific IgG1 and IgE but not to IgA production. We also did not observe a reduction in titer of PN IgA in the absence of TFR cells (Treg-Bcl6−/− mice), another T cell population associated with generation of gut IgA to bacteria (32). The finding that TFH and TFR cells are not necessary for T cell–dependent IgA switching is consistent with recent work demonstrating a failure to reduce IgA+ B cells in the PPs after eliminating interleukin-21–expressing T cells (74) and that transforming growth factor–β signaling in PP B cells is dispensable in the GC for IgA class switching (75). Multiple studies have also identified IgA B cells outside of GCs in PPs; instead, the subepithelial dome likely acts as an alternative site for T cell–dependent but TFH cell–independent specific IgA induction (76–78). Our data suggest that a dichotomy exists, whereby TFH cells and GCs are necessary for the induction of IgG1 and IgE responses to peanut; whereas highly specific IgA responses are induced outside of GCs and are promoted by a distinct T cell subset expressing CD40L. Further studies are required to identify the T cell that induces food-specific IgA and to assess whether this atypical model of B cell activation is a unique feature of gut-draining secondary lymphoid organs or widely applicable to IgA induction across the body.
Contrary to the belief that food-specific IgAs might prevent food sensitization, we did not observe an inverse relationship between the production of food-specific IgA and IgE. Instead, our data suggest a paradigm whereby, in the presence of an inflammatory gut insult, both a potentially harmful IgE response and a possibly mitigating IgA response could occur concomitantly to food antigens depending on the nature of the inflammatory stimulus. It is well accepted that IgA regulates intestinal commensal flora, neutralizes toxins, and opsonizes pathogenic microbes (7, 31, 32, 40, 75, 79, 80). Yet, how and even whether IgA produced in the gut regulates the immune response to dietary antigens is less clear (4, 81) and has not been explored experimentally. Mice without secretory IgM or IgA have disrupted commensal flora and epithelial integrity (82, 83); both are associated with increased sensitization to food antigens, making any interpretation difficult (84). To understand the role of dietary antigen-specific IgA, it is important to know when and how it is induced to develop models to selectively impair PN IgA without altering the IgA-regulated microbiome or the ability to generate IgE. Our work demonstrating distinct T cell requirements for IgA versus IgE induced in the gut will help enable the creation of such models.
MATERIALS AND METHODS
Study design
The study aimed to define when gut IgA is induced to food antigens and elucidate the cellular mechanisms underlying this process in mice. Experiments included 8 to 12 weeks old adult and 3 weeks old infant WT and knockout mouse strains (C57BL/6), which were either cohoused littermates or cohoused for at least 3 weeks. Peanut was used as a model food antigen and was given to mice ad libitum or by oral gavage. Analyses included T cell and B cell phenotyping by flow cytometry and antibody quantification by ELISA with food-specific antibodies as reference standards developed in the lab. With the use of an ELISA-based assay developed in the lab, stool IgA to food antigens were quantified in healthy human volunteers and CD40L-deficient patients. Healthy human volunteers and CD40L-deficient patients were recruited from separate studies, approved by local Institutional Review Boards [Human Investigation Committee (HIC) nos. 1607018104, 1008007275REG, and 2000021335] of the Yale School of Medicine. The number of experimental replicates are included in the figure legends. Samples were not double-blinded or randomized during experiments or analyses.
Mice
Age- and sex-matched 3- to 12-week-old mice were kept on peanut- and egg-free Teklad Global 18% Protein Rodent Diet (2018S, Harlan Laboratories). WT CD45.2 C57BL/6 mice were purchased from the National Cancer Institute. C3H/HeJ, Ciita−/− (B6.129s2-Ciitatm1Ccum/J) (85), and Cd40l-/y (B6.129S2-Cd40lgtm1Imx/J) (86) were purchased from the Jackson Laboratory (JAX). Cd4 Cre [Tg(Cd4-cre)1Cwi/BfluJ], Foxp3 Cre [B6.129(Cg)-Foxp3tm4(YFP/icre)Ayr/J], and Bcl6 flox [B6.129S(FVB)-Bcl6tm1.1Dent/J] (67) mice purchased from JAX were crossed to create conditional knockout of Bcl6 in CD4 (T-Bcl6−/− mice) and FOXP3 (Treg-Bcl6−/−)–expressing cells. AID−/− mice (87), backcrossed extensively onto the C57BL/6 background, were given by the Schatz lab (Yale University). Mice were kept in specific pathogen–free conditions. All protocols used in this study were approved by the Institutional Animal Care and Use Committee at the Yale University School of Medicine.
Immunization
Mice were immunized weekly for 1, 6, or 12 weeks by oral gavage with ground blanched peanut (Western Mixers Produce & Nuts), roasted and unsalted peanut (Whole Foods Market), or wheat flour (Bob’s Red Mill Company) or OVA (Sigma) at 5 mg food antigen, with or without 10 μg of CT (List Biologicals, lot nos. 10165A1 and 10167A2) or 100 μg of Alternaria alternata and Alternaria tenuis extract (Greer Laboratories, lot no. 322776) in 200 μl of 0.2 M sodium bicarbonate buffer per mouse based on a protocol modified from (52). For daily peanut exposure, one-half of a peanut was left in the cage for each mouse daily for a period of 6 weeks after weaning for infants and 6 weeks after for 8-week-old adult mice.
Mouse stool processing
Stool pellets were flash-frozen on dry ice upon collection. Frozen pellets were weighed and rehydrated with 1 ml of phosphate-buffered saline (PBS; Gibco) per 100 mg of stool for 15 min on ice. Rehydrated pellets were homogenized using sterilized wooden applicator (McKesson), vortexed, and centrifuged at 8000g for 10 min at 4°C. The supernatant was collected in sterile microfuge tubes that had been precoated with 1% bovine serum albumin (BSA) overnight. The supernatant was then stored at −80°C before analyses.
Human stool collection and processing
Human stool samples from healthy participants and CD40L-deficient patients were obtained from separate studies, approved by local Institutional Review Boards (HIC nos. 1607018104, 1008007275REG, and 2000021335) of the Yale School of Medicine. Both studies enrolled adult participants of >18 years of age, who were healthy by self-report. Informed consent was obtained from all participants and/or their legal guardians, and all methods were performed in accordance with relevant guidelines and regulations. All stool samples were collected at the participant’s convenience and then frozen at −18°C by the participant. The stool samples were then returned to the investigators either by prepaid express mailing envelopes or by personal delivery. Upon receipt, stool samples from human donors were divided into 1-g aliquots and kept at −80°C before analyses. Briefly, 1 g of stool was incubated with stool of protease inhibitor cocktail (4 ml/g; MP Biomedicals) overnight on a shaker at 4°C to homogenize the sample. Fecal sample mixtures were vortexed briefly for complete homogenization before centrifugation at 9000g for 10 min. The supernatant was filtered through a 70-μm cell strainer before centrifugation at 9000g for 10 min. The supernatant was collected into new microfuge tube and centrifuged again at 9000g for 10 min. The resulting supernatant was filtered through a 1.2-μm syringe filter (Whatman) and aliquoted into 1% BSA-coated microfuge tubes and stored at −80°C before further analyses.
Enzyme-linked immunosorbent assay
Stool and serum samples were analyzed by ELISA for measurement of total or peanut-specific antibodies. Briefly, 20 μg/ml crude peanut extract, wheat extract (Greer Laboratories), or OVA (Sigma) or 0.5 μg/ml, CT (List biologicals), anti-mouse IgA (MP Biomedicals), or anti-human IgA (BD Biosciences) capture antibodies in carbonate buffer were coated on 96-well Maxisorp plates (Thermo Fisher Scientific) overnight. Plates were blocked with 1% BSA in PBS at 37°C for 1 hour, followed by the addition of serially diluted serum or stool samples with a 2-hour incubation at 37°C. Competitive ELISAs to test antibody specificity were performed by preincubating stool samples with 20 or 200 μg/ml crude peanut extract (Greer Laboratories), wheat extract (Greer Laboratories), OVA (Sigma), ovomucoid (Sigma), pea (Anthony’s Premium Pea Protein), and soy flour (Sigma) before plating on peanut antigen-coated ELISA plates. Peanut-specific antibodies of each isotype were detected with horseradish peroxidase (HRP)–conjugated anti-human IgA, HRP-conjugated goat anti-mouse IgA (1040–05), HRP-conjugated goat anti-mouse IgE (1110–05), HRP-conjugated goat anti-mouse IgG1 (1073–05), HRP-conjugated goat anti-mouse IgG (1013–05) (SouthernBiotech), or HRP-conjugated rat anti-mouse IgM (#550588, BD Biosciences) antibodies at 37°C. For total mouse antibodies, purified mouse IgA, IgM, IgE (BD Biosciences), and IgG (Sigma) standards were used. Antigen-specific antibodies of each isotype were obtained from pooled serum or stool supernatant from hyperimmunized mice and used as standards for antigen-specific ELISAs. Purified human IgA standard (Bethyl Laboratories) or pooled stool supernatant from selected human donors was used for quantifying total human IgA, egg white–specific IgA, PN IgA, IgG, IgM, and IgE ELISA, respectively. Plates were developed with stabilized chromogen tetramethylbenzidine (Life Sciences) and stopped with 3N hydrochloric acid before reading at 450 nm on a microplate reader (Molecular Devices). PN IgE concentration in human serum samples was measured using the ImmunoCAP system (Phadia).
Statistical analysis
All statistical analysis was performed using GraphPad Prism software. Data were analyzed with Mann-Whitney U test (two experimental groups) or Kruskal-Wallis test (≥3 experimental groups) followed by Dunn’s test for post hoc analyses. Statistical significance is defined as *P < 0.05, **P < 0.01, and ***P < 0.001. Relationship between two variables were assessed using Spearman’s rank correlation coefficient. Nonparametric statistical analyses were used throughout the manuscript as normal distribution cannot be accurately assessed for small sample sizes (<30 per group).
Supplementary Material
Fig. S1. Healthy humans make stool IgA but not serum IgE against food antigens.
Fig. S2. Daily exposure to food induces minimal food antigen–reactive IgA.
Fig. S3. Specific IgA, IgG, IgM, and IgE to peanut are present in the serum of mice exposed to peanut and CT.
Fig. S4. Exposure of mice to peanut and CT does not increase TFH cell numbers in MLNs and PPs.
Fig. S5. TFH but not Treg or TH17 cells were decreased in MLNs and PPs of T-Bcl6−/− mice.
Fig. S6. GC B cells are almost absent in T-Bcl6−/− mice.
Fig. S7. IgA+ B cells can be found colocalizing with T cells outside GCs.
Fig. S8. IgG1+ but not IgA+ plasmablasts are reduced in T-Bcl6−/− mice.
Fig. S9. TFR cell numbers are reduced in PP but not MLN after peanut and CT exposure.
Fig. S10. Model of cross-reactive and specific gut IgA induction to peanut.
Table S1. Raw data file (Excel spreadsheet).
Acknowledgments:
We would like to thank M. Firla and J. Goldstein for technical assistance; M. Kulis for guidance on developing the human IgA assay; J. Chen, S. Olyha, S. Lewis, D. Liu, and X. Yin for helpful discussion; and C. Nagler and A. Williams for critical review of the manuscript.
Funding: This study was supported by Ira & Diana Riklis Family Research Award in Food Allergy, Food Allergy Research & Education (FARE), R01 AI108829 (to S.C.E.), CTSA UL1 TR001863 and R01 AI136942 (to S.C.E.), Sean N. Parker Center for Allergy and Asthma Research (to S.C.E.), and Agency for Science, Technology and Research, Singapore (to B.Z.).
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
REFERENCES AND NOTES
- 1.Esterházy D, Loschko J, London M, Jove V, Oliveira TY, Mucida D, Classical dendritic cells are required for dietary antigen–mediated induction of peripheral Treg cells and tolerance. Nat. Immunol. 17, 545–555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA, Oral tolerance in the absence of naturally occurring Tregs. J. Clin. Investig. 115, 1923–1933 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim KS, Hong S-W, Han D, Yi J, Jung J, Yang B-G, Lee JY, Lee M, Surh CD, Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 351, 858–863 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Berin MC, Mucosal antibodies in the regulation of tolerance and allergy to foods. Semin. Immunopathol. 34, 633–642 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tordesillas L, Berin MC, Mechanisms of oral tolerance. Clin. Rev. Allergy Immunol. 55, 107–117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandtzaeg P, Johansen F-E, Mucosal B cells: Phenotypic characteristics, transcriptional regulation, and homing properties. Immunol. Rev. 206, 32–63 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, Ruggiero E, Cho JH, Goodman AL, Flavell RA, Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000–1010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutherland DB, Suzuki K, Fagarasan S, Fostering of advanced mutualism with gut microbiota by immunoglobulin A. Immunol. Rev. 270, 20–31 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Fadlallah J, El Kafsi H, Sterlin D, Juste C, Parizot C, Dorgham K, Autaa G, Gouas D, Almeida M, Lepage P, Pons N, Le Chatelier E, Levenez F, Kennedy S, Galleron N, de Barros J-PP, Malphettes M, Galicier L, Boutboul D, Mathian A, Miyara M, Oksenhendler E, Amoura Z, Doré J, Fieschi C, Ehrlich SD, Larsen M, Gorochov G, Microbial ecology perturbation in human IgA deficiency. Sci. Transl. Med. 10, eaan1217 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Kubinak JL, Round JL, Do antibodies select a healthy microbiota? Nat. Rev. Immunol. 16, 767–774 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corthésy B, Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 4, 185 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamemura N, Takashima M, Morita H, Matsumoto K, Saito H, Kido H, Measurement of allergen-specific secretory IgA in stool of neonates, infants and toddlers by protection against degradation of immunoglobulins and allergens. J. Med. Invest. 62, 137–144 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Frossard CP, Hauser C, Eigenmann PA, Antigen-specific secretory IgA antibodies in the gut are decreased in a mouse model of food allergy. J. Allergy Clin. Immunol. 114, 377–382 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Husby S, Oxelius V-A, Teisner B, Jensenius JC, Svehag S-E, Humoral immunity to dietary antigens in healthy adults. Occurrence, isotype and IgG subclass distribution of serum antibodies to protein antigens. Int. Arch. Allergy Immunol. 77, 416–422 (1985). [DOI] [PubMed] [Google Scholar]
- 15.Konstantinou GN, Nowak-Węgrzyn A, Bencharitiwong R, Bardina L, Sicherer SH, Sampson HA, Egg-white-specific IgA and IgA2 antibodies in egg-allergic children: Is there a role in tolerance induction? Pediatr. Allergy Immunol. 25, 64–70 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konstantinou GN, Bencharitiwong R, Grishin A, Caubet J-C, Bardina L, Sicherer SH, Sampson HA, Nowak-Węgrzyn A, The role of casein-specific IgA and TGF-β in children with food protein-induced enterocolitis syndrome to milk. Pediatr. Allergy Immunol. 25, 651–656 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vazquez-Ortiz M, Pascal M, Juan M, Alsina L, Martín-Mateos MA, Plaza AM, Serum allergen-specific IgA is not associated with natural or induced tolerance to egg in children. Allergy 68, 1327–1332 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Berin MC, Shreffler WG, TH2 adjuvants: Implications for food allergy. J. Allergy Clin. Immunol. 121, 1311–1320 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, Burks AW, Sampson HA, The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J. Immunol. 177, 3677–3685 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Perrier C, Thierry A-C, Mercenier A, Corthésy B, Allergen-specific antibody and cytokine responses, mast cell reactivity and intestinal permeability upon oral challenge of sensitized and tolerized mice. Clin. Exp. Allergy 40, 153–162 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Mattsson J, Schön K, Ekman L, Fahlén-Yrlid L, Yrlid U, Lycke NY, Cholera toxin adjuvant promotes a balanced Th1/Th2/Th17 response independently of IL-12 and IL-17 by acting on Gsα in CD11b+ DCs. Mucosal Immunol. 8, 815–827 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Elson CO, Ealding W, Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J. Immunol. 132, 2736–2741 (1984). [PubMed] [Google Scholar]
- 23.Lycke N, Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology 59, 301–308 (1986). [PMC free article] [PubMed] [Google Scholar]
- 24.Gärdby E, Lycke NY, CD19-deficient mice exhibit poor responsiveness to oral immunization despite evidence of unaltered total IgA levels, germinal centers and IgA-isotype switching in Peyer’s patches. Eur. J. Immunol. 30, 1861–1871 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Bergqvist P, Gärdby E, Stensson A, Bemark M, Lycke NY, Gut IgA class switch recombination in the absence of CD40 Does not occur in the lamina propria and is independent of germinal centers. J. Immunol. 177, 7772–7783 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Gärdby E, Kagrdic D, Kjerrulf M, Bromander A, Vajdy M, Hörnquist E, Lycke N, The influence of costimulation and regulatory CD4+ T cells on intestinal IgA immune responses. Dev. Immunol. 6, 53–60 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fahlen-Yrlid L, Gustafsson T, Westlund J, Holmberg A, Strombeck A, Blomquist M, MacPherson GG, Holmgren J, Yrlid U, CD11chigh dendritic cells are essential for activation of CD4+ T cells and generation of specific antibodies following mucosal immunization. J. Immunol. 183, 5032–5041 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Hirota K, Turner J-E, Villa M, Duarte JH, Demengeot J, Steinmetz OM, Stockinger B, Plasticity of TH17 cells in Peyer’s patches is responsible for the induction of T cell–dependent IgA responses. Nat. Immunol. 14, 372–379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snider DP, Liang H, Switzer I, Underdown BJ, IgA production in MHC class II-deficient mice is primarily a function of B-1a cells. Int. Immunol. 11, 191–198 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Hörnquist CE, Ekman L, Grdic KD, Schön K, Lycke NY, Paradoxical IgA immunity in CD4-deficient mice. Lack of cholera toxin-specific protective immunity despite normal gut mucosal IgA differentiation. J. Immunol. 155, 2877–2887 (1995). [PubMed] [Google Scholar]
- 31.Bunker JJ, Flynn TM, Koval JC, Shaw DG, Meisel M, McDonald BD, Ishizuka IE, Dent AL, Wilson PC, Jabri B, Antonopoulos DA, Bendelac A, Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity 43, 541–553 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamoto S, Maruya M, Kato LM, Suda W, Atarashi K, Doi Y, Tsutsui Y, Qin H, Honda K, Okada T, Hattori M, Fagarasan S, Foxp3+ T cells regulate immunoglobulin A selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 41, 152–165 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Macpherson AJ, McCoy KD, Johansen F-E, Brandtzaeg P, The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Cerutti A, The regulation of IgA class switching. Nat. Rev. Immunol. 8, 421–434 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macpherson AJ, Köller Y, McCoy KD, The bilateral responsiveness between intestinal microbes and IgA. Trends Immunol. 36, 460–470 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Bunker JJ, Erickson SA, Flynn TM, Henry C, Koval JC, Meisel M, Jabri B, Antonopoulos DA, Wilson PC, Bendelac A, Natural polyreactive IgA antibodies coat the intestinal microbiota. Science 358, eaan6619 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fransen F, Zagato E, Mazzini E, Fosso B, Manzari C, El Aidy S, Chiavelli A, D’Erchia AM, Sethi MK, Pabst O, Marzano M, Moretti S, Romani L, Penna G, Pesole G, Rescigno M, BALB/c and C57BL/6 mice differ in polyreactive IgA abundance, which impacts the generation of antigen-specific IgA and microbiota diversity. Immunity 43, 527–540 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Pabst O, New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 12, 821–832 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Benckert J, Schmolka N, Kreschel C, Zoller MJ, Sturm A, Wiedenmann B, Wardemann H, The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J. Clin. Invest. 121, 1946–1955 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM, A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288, 2222–2226 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Wilmore JR, Gaudette BT, Gomez Atria D, Hashemi T, Jones DD, Gardner CA, Cole SD, Misic AM, Beiting DP, Allman D, Commensal microbes induce serum IgA responses that protect against polymicrobial sepsis. Cell Host Microbe. 23, 302–311.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proietti M, Cornacchione V, Rezzonico Jost T, Romagnani A, Faliti CE, Perruzza L, Rigoni R, Radaelli E, Caprioli F, Preziuso S, Brannetti B, Thelen M, McCoy KD, Slack E, Traggiai E, Grassi F, ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer’s patches to promote host-microbiota mutualism. Immunity 41, 789–801 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S, Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science 323, 1488–1492 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Kulis M, Saba K, Kim EH, Bird JA, Kamilaris N, Vickery BP, Staats H, Burks AW, Increased peanut-specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy. J. Allergy Clin. Immunol. 129, 1159–1162 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haneberg B, Aarskog D, Human faecal immunoglobulins in healthy infants and children, and in some with diseases affecting the intestinal tract or the immune system. Clin. Exp. Immunol. 22, 210–222 (1975). [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura K, Ikutani M, Yoshida T, Tanaka-Hayashi A, Yanagibashi T, Inoue R, Nagai Y, Adachi Y, Miyawaki T, Takatsu K, Mori H, Increased production of intestinal immunoglobulins in Syntenin-1-deficient mice. Immunobiology 220, 597–604 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Liu T, Navarro S, Lopata AL, Current advances of murine models for food allergy. Mol. Immunol. 70, 104–117 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Harris NL, Spoerri I, Schopfer JF, Nembrini C, Merky P, Massacand J, Urban JF Jr., A. Lamarre, K. Burki, B. Odermatt, R. M. Zinkernagel, A. J. Macpherson, Mechanisms of neonatal mucosal antibody protection. J. Immunol. 177, 6256–6262 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Turfkruyer M, Rekima A, Macchiaverni P, Le Bourhis L, Muncan V, van den Brink GR, Tulic MK, Verhasselt V, Oral tolerance is inefficient in neonatal mice due to a physiological vitamin A deficiency. Mucosal Immunol. 9, 479–491 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Sicherer SH, Clinical implications of cross-reactive food allergens. J. Allergy Clin. Immunol. 108, 881–890 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Moghaddam AE, Hillson WR, Noti M, Gartlan KH, Johnson S, Thomas B, Artis D, Sattentau QJ, Dry roasting enhances peanut-induced allergic sensitization across mucosal and cutaneous routes in mice. J. Allergy Clin. Immunol. 134, 1453–1456 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X-M, Serebrisky D, Lee S-Y, Huang C-K, Bardina L, Schofield BH, Stanley JS, Burks AW, Bannon GA, Sampson HA, A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J. Allergy Clin. Immunol. 106, 150–158 (2000). [DOI] [PubMed] [Google Scholar]
- 53.Hornqvist E, Goldschmidt TJ, Holmdahl R, Lycke N, Host defense against cholera toxin is strongly CD4+ T cell dependent. Infect. Immun. 59, 3630–3638 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, Wakatsuki Y, Roopenian DC, Mizoguchi A, Lencer WI, Blumberg RS, Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J. Clin. Investig. 116, 2142–2151 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamada N, Sakamoto K, Seo S-U, Zeng MY, Kim Y-G, Cascalho M, Vallance BA, Puente JL, Núñez G, Humoral immunity in the gut selectively targets phenotypically virulent attaching-and-effacing bacteria for intraluminal elimination. Cell Host Microbe 17, 617–627 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mei HE, Yoshida T, Sime W, Hiepe F, Thiele K, Manz RA, Radbruch A, Dörner T, Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood 113, 2461–2469 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Lemke A, Kraft M, Roth K, Riedel R, Lammerding D, Hauser AE, Long-lived plasma cells are generated in mucosal immune responses and contribute to the bone marrow plasma cell pool in mice. Mucosal Immunol. 9, 83–97 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Boyaka PN, Inducing mucosal IgA: A challenge for vaccine adjuvants and delivery systems. J. Immunol. 199, 9–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee HB, Patriarca A, Magan N, Alternaria in food: Ecophysiology, mycotoxin production and toxicology. Mycobiology 43, 93–106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hapfelmeier S, Lawson MAE, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, Geuking MB, Curtiss III R, McCoy KD, Macpherson AJ, Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328, 1705–1709 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bemark M, Hazanov H, Strömberg A, Komban R, Holmqvist J, Köster S, Mattsson J, Sikora P, Mehr R, Lycke NY, Limited clonal relatedness between gut IgA plasma cells and memory B cells after oral immunization. Nat. Commun. 7, 12698 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crotty S, T follicular helper cell biology: A decade of discovery and diseases. Immunity 50, 1132–1148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melo-Gonzalez F, Kammoun H, Evren E, Dutton EE, Papadopoulou M, Bradford BM, Tanes C, Fardus-Reid F, Swann JR, Bittinger K, Mabbott NA, Vallance BA, Willinger T, Withers DR, Hepworth MR, Antigen-presenting ILC3 regulate T cell–dependent IgA responses to colonic mucosal bacteria. J. Exp. Med. 216, 728–742 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gribonika I, Eliasson DG, Chandode RK, Schön K, Strömberg A, Bemark M, Lycke NY, Class-switch recombination to IgA in the Peyer’s patches requires natural thymus-derived Tregs and appears to be antigen independent. Mucosal Immunol. 12, 1268–1279 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Gustafsson T, Hua Y-J, Dahlgren MW, Livingston M, Johansson-Lindbom B, Yrlid U, Direct interaction between cholera toxin and dendritic cells is required for oral adjuvant activity. Eur. J. Immunol. 43, 1779–1788 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Esterházy D, Canesso MCC, Mesin L, Muller PA, de Castro TBR, Lockhart A, ElJalby M, Faria AMC, Mucida D, Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 569, 126–130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hollister K, Kusam S, Wu H, Clegg N, Mondal A, Sawant DV, Dent AL, Insights into the Role of Bcl6 in follicular Th cells using a new conditional mutant mouse model. J. Immunol. 191, 3705–3711 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meli AP, Fontés G, Leung Soo C, King IL, T follicular helper cell–derived IL-4 is required for IgE production during intestinal helminth infection. J. Immunol. 199, 244–252 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi T, Iijima K, Dent AL, Kita H, Follicular helper T cells mediate IgE antibody response to airborne allergens. J. Allergy Clin. Immunol. 139, 300–313.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KGC, Vinuesa CG, Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17, 975–982 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A, DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Macpherson AJ, Yilmaz B, Limenitakis JP, Ganal-Vonarburg SC, IgA function in relation to the intestinal microbiota. Annu. Rev. Immunol. 36, 359–381 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Kubinak JL, Petersen C, Stephens WZ, Soto R, Bake E, O’Connell RM, Round JL, MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe 17, 153–163 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones L, Ho WQ, Ying S, Ramakrishna L, Srinivasan KG, Yurieva M, Ng WP, Subramaniam S, Hamadee NH, Joseph S, Dolpady J, Atarashi K, Honda K, Zolezzi F, Poidinger M, Lafaille JJ, Curotto de Lafaille MA, A subpopulation of high IL-21-producing CD4+ T cells in Peyer’s patches is induced by the microbiota and regulates germinal centers. Sci. Rep. 6, 30784 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michetti P, Mahan MJ, Slauch JM, Mekalanos JJ, Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen salmonella typhimurium. Infect. Immun. 60, 1786–1792 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lycke NY, Bemark M, The regulation of gut mucosal IgA B-cell responses: Recent developments. Mucosal Immunol. 10, 1361–1374 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Biram A, Strömberg A, Winter E, Stoler-Barak L, Salomon R, Addadi Y, Dahan R, Yaari G, Bemark M, Shulman Z, BCR affinity differentially regulates colonization of the subepithelial dome and infiltration into germinal centers within Peyer’s patches. Nat. Immunol. 20, 482–492 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Reboldi A, Arnon TI, Rodda LB, Atakilit A, Sheppard D, Cyster JG, IgA production requires B cell interaction with subepithelial dendritic cells in Peyers patches. Science 352, aaf4822 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lycke N, Holmgren J, Long-term cholera antitoxin memory in the gut can be triggered to antibody formation associated with protection within hours of an oral challenge immunization. Scand. J. Immunol. 25, 407–412 (1987). [DOI] [PubMed] [Google Scholar]
- 80.Apter FM, Michetti P, Winner LS, Mack JA, Mekalanos JJ, Neutra MR, Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect. Immun. 61, 5279–5285 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mantis NJ, Rol N, Corthésy B, Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4, 603–611 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sait LC, Galic M, Price JD, Simpfendorfer KR, Diavatopoulos DA, Uren TK, Janssen PH, Wijburg OLC, Strugnell RA, Secretory antibodies reduce systemic antibody responses against the gastrointestinal commensal flora. Int. Immunol. 19, 257–265 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Johansen F-E, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, Betsholtz C, Brandtzaeg P, Absence of epithelial Immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component–deficient mice. J. Exp. Med. 190, 915–922 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wesemann DR, Nagler CR, The microbiome, timing, and barrier function in the context of allergic disease. Immunity 44, 728–738 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang C-H, Guerder S, Hong S-C, van Ewijk W, Flavell RA, Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity 4, 167–178 (1996). [DOI] [PubMed] [Google Scholar]
- 86.Renshaw BR, Humoral immune responses in CD40 ligand-deficient mice. J. Exp. Med. 180, 1889–1900 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T, Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Healthy humans make stool IgA but not serum IgE against food antigens.
Fig. S2. Daily exposure to food induces minimal food antigen–reactive IgA.
Fig. S3. Specific IgA, IgG, IgM, and IgE to peanut are present in the serum of mice exposed to peanut and CT.
Fig. S4. Exposure of mice to peanut and CT does not increase TFH cell numbers in MLNs and PPs.
Fig. S5. TFH but not Treg or TH17 cells were decreased in MLNs and PPs of T-Bcl6−/− mice.
Fig. S6. GC B cells are almost absent in T-Bcl6−/− mice.
Fig. S7. IgA+ B cells can be found colocalizing with T cells outside GCs.
Fig. S8. IgG1+ but not IgA+ plasmablasts are reduced in T-Bcl6−/− mice.
Fig. S9. TFR cell numbers are reduced in PP but not MLN after peanut and CT exposure.
Fig. S10. Model of cross-reactive and specific gut IgA induction to peanut.
Table S1. Raw data file (Excel spreadsheet).