Abstract
The First World Consensus Conference on Pancreas Transplantation provided 49 jury deliberations regarding the impact of pancreas transplantation on the treatment of diabetic patients, and 110 experts’ recommendations for the practice of pancreas transplantation. The main message from this consensus conference is that both simultaneous pancreas‐kidney transplantation (SPK) and pancreas transplantation alone can improve long‐term patient survival, and all types of pancreas transplantation dramatically improve the quality of life of recipients. Pancreas transplantation may also improve the course of chronic complications of diabetes, depending on their severity. Therefore, the advantages of pancreas transplantation appear to clearly surpass potential disadvantages. Pancreas after kidney transplantation increases the risk of mortality only in the early period after transplantation, but is associated with improved life expectancy thereafter. Additionally, preemptive SPK, when compared to SPK performed in patients undergoing dialysis, appears to be associated with improved outcomes. Time on dialysis has negative prognostic implications in SPK recipients. Increased long‐term survival, improvement in the course of diabetic complications, and amelioration of quality of life justify preferential allocation of kidney grafts to SPK recipients. Audience discussions and live voting are available online at the following URL address: http://mediaeventi.unipi.it/category/1st‐world‐consensus‐conference‐of‐pancreas‐transplantation/246.
Keywords: clinical research/practice, diabetes, pancreas/simultaneous pancreas‐kidney transplantation, survey
Short abstract
This article described how pancreas transplantation impacts on life expectancy, quality of life, and course of diabetic complications based on the jury deliberations and expert recommendations from the First World Consensus Conference on Pancreas Transplantation.
Abbreviations
- AGREE II
appraisal of guidelines for research and evaluation II
- BMI
body mass index
- CDC
complement‐dependent cytotoxicity
- CMV
cytomegalovirus
- CNI
calcineurin inhibitor
- DBD
donation after brainstem death
- DCD
donation after circulatory death
- DSA
donor‐specific antibody
- GRADE
grading of recommendations, assessment, development and evaluations
- HLA
human leukocyte antigens
- HTK
histidine‐tryptophan‐ketoglutarate
- IGL‐1
Institut Georges Lopez‐1
- IPTR
International Pancreas Transplant Registry
- m‐TOR
mechanistic‐target of rapamycin
- OPTN
Organ Procurement and Transplantation Network
- PAK
pancreas after kidney transplant
- PRA
panel reactive antibody
- PTA
pancreas transplant alone
- SIGN
Scottish Intercollegiate Guidelines Network
- SPK
simultaneous kidney and pancreas transplant
- UNOS
United Network for Organ Sharing
- UW
University of Wisconsin
1. INTRODUCTION
Guidelines are available for transplantation of all solid organs but the pancreas and the intestine. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 Unfortunately, pancreas transplantation is a relatively low volume but high complexity procedure that has never gained widespread acceptance. For instance, many of the medical protocols used in pancreas transplantation are borrowed from other types of transplantation, mostly from the kidney, and all immunosuppressive drugs are used off‐label in pancreas transplantation. 14 In addition, because most pancreas transplants are performed as either simultaneous pancreas‐kidney (SPK) or pancreas after kidney (PAK) transplants, the majority of recipients suffer from advanced diabetic nephropathy, a condition that has been associated with an increase in all‐cause mortality due to higher incidence of micro‐ and macrovascular complications of diabetes. 15 Few patients are referred for pancreas transplant alone (PTA) at a stage when extrarenal diabetic complications might be reversible. Although many uremic patients can still receive a pancreas transplant in conjunction with a kidney transplant, the high prevalence and severity of associated chronic complications of diabetes cause these recipients to be less likely to experience stabilization or reversal of progressive diabetic complications. 16 , 17
In recent years, there has been a decline in the number of pancreas transplants in the United States, Europe, and the United Kingdom. 18 , 19 , 20 Although the reasons for this decline are multifactorial, the lack of objective assessment of the impact of pancreas transplantation on the treatment of diabetic patients and absence of validated practice guidelines may be among the contributing factors. In selected patients, pancreas transplantation provides dramatic improvements in quality of life 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 and may prolong survival. 33 , 34 , 35 , 36 , 37 , 38 , 39 Additionally, some traditional deterrents have been minimized because pancreas transplantation currently requires the same immunosuppression as kidney transplantation 40 and surgical complications are observed at lower rates. 41
We report herein the expert recommendations for the practice of pancreas transplantation developed during the First World Consensus Conference on Pancreas Transplantation held in Pisa, Italy, on October 17–19, 2019. We also report several additional deliberations on the impact of the different types of pancreas transplantation on the course of diabetes that were crafted by an independent jury following an exhaustive review and presentation of data from the literature and audience discussions with experts.
2. SUMMARY OF METHODS
The methods used to achieve the consensus were presented in detail in a dedicated manuscript. 42
Briefly, the steering committee defined 144 questions (grouped in 12 topics). The 12 topics were categorized into two key domains. The first domain (three topics—35 questions) included “nontechnical” issues related to the impact of SPK transplant, PAK transplant, and PTA on the management of patients with diabetes. The second domain (nine topics—109 questions) dealt with technical issues related to the practice of pancreas transplantation. A systematic literature review was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions for each topic and was reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA). 43 , 44 Quality of evidence was assessed using the SIGN (Scottish Intercollegiate Guidelines Network) methodology. 45 Questions in the first domain were assessed using the Zurich‐Danish model 46 that charges an independent jury to draw the final deliberations. Questions in the second domain were assessed and approved by a panel of experts in pancreas transplantation and were validated by a distinct group of experts using the AGREE II instrument (Appraisal of Guidelines for Research and Evaluation II). 47 Jury deliberations and expert recommendations received a GRADE rating (Grading of Recommendations, Assessment, Development and Evaluations). 48 Consensus (agreement rate ≥85%) was reached by two online Delphi rounds and was finalized, after on‐site discussions and live voting (Pisa, Italy, October 18 and 19, 2019).
Audience discussions and live voting are available online at the following URL address: http://mediaeventi.unipi.it/category/1st‐world‐consensus‐conference‐of‐pancreas‐transplantation/246
3. DEFINITIONS
Sensitization (or sensitized patient) was defined as the presence of circulating antibodies directed against human leukocyte antigens (HLA). 49 High sensitization (or highly sensitized patients), was defined as a panel reactive antibody (PRA) >85%. 50
Obesity was defined according to World Health Organization (i.e., body mass index [BMI] ≥30 kg/m2). 51 Obesity classes (i.e., class I, class II, and class III) and ethnic variations that affect obesity definition were not considered due to lack of granular data in available literature.
Preemptive SPK transplantation was defined as the combined transplantation of a pancreas and a kidney in patients with stage 4/5 chronic kidney disease before they initiate dialysis.
4. RESULTS
4.1. Jury deliberations
The jury could not deliberate on two queries, due to lack of evidence, and released 49 deliberations. No deliberation was graded 1A. Twenty‐three of 49 deliberations could not be graded. The remaining 26 deliberations were rated GRADE 2B (n = 22) and GRADE 2C (n = 4) (Figure 1A).
FIGURE 1.
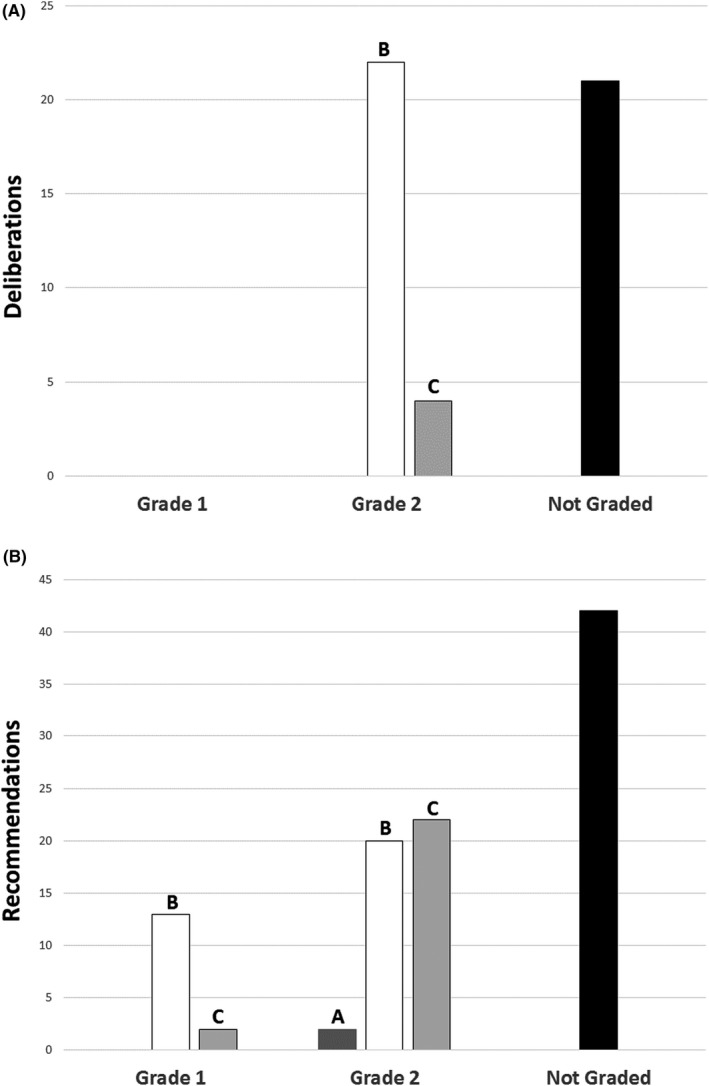
Level of evidence and strength of statements. (A) Jury deliberations; (B) expert recommendations.
Jury deliberations are reported in Tables 1, 2, 3.
TABLE 1.
Impact of simultaneous pancreas‐kidney (SPK) transplantation
| Query | Deliberation | Grade |
|---|---|---|
| A.1 – “In suitable recipients, does an SPK transplant increase life expectancy or improve quality of life?” | 1. SPK transplantation improves quality of life and long‐term survival compared to current medical treatment for people on the waitlist and compared to other transplant options | 2B |
| 2. The survival advantage with SPK transplantation is greater when a live donor kidney is not available or suitable | 2B | |
| 3. SPK transplantation improves quality of life and is not associated with an increased risk of premature loss of renal graft function | 2B | |
| A.2 – “In suitable SPK recipients with type 1 diabetes does an SPK transplant improve life‐expectancy or quality of life?” | 1. In type 1 diabetes, SPK transplantation improves quality of life and long‐term survival compared to current medical treatment for people on the waitlist and compared to other transplant options | 2B |
| 2. The survival advantage with SPK transplantation is greater when a live donor kidney is not available or suitable | 2B | |
| 3. SPK transplantation improves quality of life and is not associated with an increased risk of premature loss of renal graft function | 2B | |
| A.3 – “In suitable SPK recipients with type 2 diabetes, does an SPK transplant improve life‐expectancy or quality of life?” | 1. In suitable type 2 diabetes recipients, SPK transplantation improves quality of life and improves survival compared to patients remaining on dialysis | NG |
| 2. In type 2 diabetes, SPK transplantation improves survival compared to deceased donor kidney transplantation alone | 2B | |
| 3. In people with type 2 diabetes, there is insufficient evidence to determine whether survival is improved by SPK transplantation compared to living donor kidney transplant alone | NG | |
| A.4 – “In patients with type 1 diabetes and end stage‐renal disease on dialysis, does an SPK transplant increase longevity or improve quality of life?” | In patients with type 1 diabetes and end‐stage renal disease on dialysis, SPK transplantation both improves quality of life and increases longevity compared to current medical therapies | 2B |
| A.5 – “In patients with type 1 diabetes and end‐stage renal disease on dialysis, does an SPK transplant increase longevity or improve quality of life compared to live donor kidney transplantation?” | 1. Live donor kidney transplantation alone is an alternative to SPK transplantation in case of anticipated long wait times and in people who do not qualify for dual transplantation | 2C |
| 2. Live donor kidney transplantation alone achieves survival similar to SPK transplantation in the medium term, but SPK transplantation has improved long‐term survival | 2C | |
| A.6 – “In patients with type 1 diabetes and end‐stage renal disease on dialysis, does an SPK transplant increase longevity or improve quality of life compared to live donor kidney transplantation with islet cell transplantation?” | Because of lack of evidence, no conclusions can be drawn | ‐ |
| A.7 – “In patients with type 1 diabetes and end‐stage renal disease on dialysis, does an SPK transplant increase longevity or improve quality of life compared to deceased donor kidney transplantation?” | In selected patients, SPK transplantation improves long‐term survival, kidney graft function, and quality of life compared to patients who receive deceased donor kidney transplantation alone | 2C |
| A.8 – “In patients with type 1 diabetes and end‐stage renal disease on dialysis, does an SPK transplant increase longevity or improve quality of life compared to deceased donor kidney transplantation with islet cell transplantation?” | Because of lack of evidence, no conclusions can be drawn | ‐ |
|
A.9 – “In preemptive SPK recipients with type 1 diabetes does an SPK transplant improve longevity or quality of life?” |
There is indirect evidence that preemptive SPK transplantation improves longevity and quality of life in patients with type 1 diabetes | NG |
| A.10 – “In preemptive SPK recipients with type 1 diabetes does an SPK transplant improve longevity or quality of life compared to live donor kidney transplantation?” | Data are limited. Preemptive SPK transplantation and live donor kidney transplants both seem to provide excellent long‐term outcomes in patients with type 1 diabetes | NG |
| A.11 – “In preemptive SPK recipients with type 1 diabetes does an SPK transplant improve longevity or quality of life compared to live donor kidney transplantation with islet cell transplantation?” | Because of lack of evidence, no conclusions can be drawn | ‐ |
| A.12 – “In preemptive SPK recipients with type 1 diabetes does an SPK transplant improve longevity or quality of life compared to deceased donor kidney transplantation?” | Indirect evidence from deceased donor kidney transplant alone in patients with type 1 diabetes suggests that preemptive SPK transplantation is superior in terms of quality of life and longevity compared to deceased donor kidney transplantation alone | NG |
| A.13 – “In preemptive SPK recipients with type 1 diabetes does an SPK transplant improve longevity or quality of life compared to deceased donor kidney transplantation with islet cell transplantation?” | Because of lack of evidence, no conclusions can be drawn | |
| A.14 – “In patients with type 2 diabetes and end‐stage renal disease on dialysis, does an SPK transplant improve quality of life or increase longevity?” | Indirect evidence from kidney transplant recipients with type 2 diabetes suggests that, in selected patients, SPK transplantantation could be associated with improved quality of life and increased longevity compared to remaining on dialysis | NG |
| A.15 – “In patients with type 2 diabetes and end‐stage renal disease on dialysis, does an SPK transplant improve quality of life or increase longevity compared to live donor kidney transplantation?” | There is limited evidence. Indirect evidence suggests that in selected patients with type 2 diabetes on dialysis, the sustained normoglycemia after successful SPK transplantation offers additional advantages compared to live donor kidney transplantation alone | NG |
| A.16 – “In patients with type 2 diabetes and end‐stage renal disease on dialysis, does an SPK transplant improve quality of life or increase longevity compared to deceased donor kidney transplantation?” | There is limited evidence. Indirect evidence suggests that in selected patients with type 2 diabetes on dialysis, the sustained normoglycemia after successful SPK transplantation offers additional advantages compared to deceased kidney donor transplantation alone | NG |
| A.17 – “In preemptive recipients with type 2 diabetes does an SPK transplant improve quality of life or increase longevity compared to current medical therapy?” | There are limited data. Indirect evidence from type 1 diabetes suggests that in selected patients with type 2 diabetes, preemptive SPK transplant improve quality of life and increase longevity compared to current medical therapy | NG |
| A.18 – “In preemptive recipients with type 2 diabetes does an SPK transplant improve quality of life or increase longevity compared to live donor kidney transplantation?” | There are limited data. It is not known whether preemptive SPK transplantation improves quality of life or increases longevity compared to live donor kidney transplantation in type 2 diabetes | NG |
| A.19 – “In preemptive recipients with type 2 diabetes does an SPK transplant improve quality of life or increase longevity compared to deceased donor kidney transplantation?” | There are limited data. It is not known whether preemptive SPK transplantation improves quality of life or increases longevity compared to deceased kidney donor transplantation in type 2 diabetes | NG |
Abbreviations: NG, not graded; SPK, simultaneous pancreas kidney.
TABLE 2.
Impact of pancreas after kidney (PAK) transplantation
| Query | Deliberation | Grade |
|---|---|---|
| B.1 – “In suitable PAK recipients, is PAK transplant associated with additional risks? What is the risk of death compared to current medical therapies?” | 1. At 90 days, PAK transplantation is associated with an increased risk of mortality (compared to staying on the waitlist) which persists to 1 year | 2B |
| 2. After 1 year, PAK transplantation is associated with decreased mortality | 2B | |
| B.2 – “In suitable PAK recipients with type 1 diabetes, does PAK transplant prolong life or improve quality of life compared to current diabetes therapy?” | 1. Available evidence in patients with type 1 diabetes cannot determine whether PAK transplantation prolongs life expectancy | 2B |
| 2. PAK transplantation clearly improves quality of life due to superior renal graft survival and improved metabolic control | 2B | |
| B.3 – “In suitable PAK recipients with type 1 diabetes who received a live donor kidney, does PAK transplant increase life expectancy or improve quality of life?” | 1. Available evidence in patients with type 1 diabetes cannot determine whether PAK transplantation in live donor kidney recipients prolongs life expectancy | 2B |
| 2. PAK transplantation clearly improves quality of life due to superior renal graft survival and improves metabolic control compared to continued medical treatment of diabetes | 2B | |
| B.4 – “In suitable PAK recipients with type 1 diabetes who received a deceased kidney transplant, does PAK transplant increase life expectancy or improve quality of life?” | 1. Available evidence in patients with type 1 diabetes cannot determine whether PAK transplantation in deceased kidney transplant recipients prolongs life expectancy | NG |
| 2. PAK transplantation clearly improves quality of life due to superior renal graft survival and improves metabolic control compared to continued medical treatment of diabetes | NG | |
| B.5 – “In suitable PAK recipients with type 2 diabetes does PAK transplant increase life expectancy or improve quality of life?” | Based on available evidence, PAK transplant in people with type 2 diabetes is feasible, but further data are required before conclusions on the impact of PAK transplant on life expectancy or quality of life can be made | NG |
| B.6 – “In suitable PAK recipients with type 2 diabetes does PAK transplant after a live donor kidney transplant increase life expectancy or improve quality of life?” | Based on available evidence, PAK transplant after a live donor kidney transplant in people with type 2 diabetes is feasible. Further data are required before conclusions on the impact on life expectancy or quality of life can be made | NG |
| B.7 – “In suitable PAK recipients with type 2 diabetes does PAK transplant after deceased donor kidney transplant increase life expectancy or improve quality of life?” | Based on available evidence, PAK transplant after a deceased donor kidney transplant in people with type 2 diabetes is feasible. Further data are required before conclusions on the impact on life expectancy or quality of life can be made | NG |
Abbreviations: NG, not graded; PAK, pancreas after kidney.
TABLE 3.
Impact of pancreas transplantation alone (PTA)
| Query | Deliberation | Grade |
|---|---|---|
| C.1 – “In suitable recipients is PTA associated with an increased risk of death when compared to current medical therapies?” | 1. PTA is not associated with an increased long‐term risk of death compared with people remaining on the waiting list | 2B |
| 2. Indirect evidence suggests that PTA could be associated with a long‐term survival advantage compared to people who have diabetes and impaired hypoglycemia awareness | 2B | |
| C.2 – “In suitable PTA recipients, is PTA associated with an increased risk of earlier renal failure compared to current medical therapy?” | 1. Renal failure has occurred in people receiving PTA who had significant pretransplant renal impairment | 2B |
| 2. Renal failure post‐PTA is uncommon if pretransplant estimated glomerular filtration rate is ≥60 ml/min/1.73 m2 | 2B | |
| 3. In some people, there may be a decline in renal function after PTA with calcineurin inhibitor‐based immunosuppression | 2B | |
| 4. By improving glucose levels, PTA could have beneficial effects on underlying diabetic nephropathy in the long term | 2B | |
| C.3 – “In suitable PTA recipients, does PTA extend longevity or improve quality of life compared to current medical therapies?” | 1. Patients with diabetes and impaired hypoglycemia awareness or diabetes and autonomic neuropathy have a high mortality risk and indirect evidence suggests that this group has improved longevity after PTA | NG |
| 2. Overall PTA recipients have improved quality of life compared to patients remaining on the wait list | NG | |
| C.4 – “After the first post‐transplant year, is PTA superior to current medical therapies for metabolic control?” | Successful PTA provides normal or near normal glucose levels and therefore is superior to current medical therapies for hypoglycemia and hyperglycemia | 2B |
| C.5 – “Is PTA superior to current medical therapies in the course of chronic complications of diabetes?” | Indirect evidence suggests that successful PTA could improve the long‐term course of most chronic diabetes complications | NG |
| C.6 – “Is PTA superior to current medical therapies in the course of diabetic retinopathy?” | Depending on initial severity of diabetic retinopathy, successful PTA may contribute to stabilization or improvement of diabetic retinopathy | 2B |
| C.7 – “Is PTA superior to current medical therapies in the course of diabetic nephropathy?” | Depending on the severity of diabetic nephropathy, successful PTA may slow progression of diabetic nephropathy. These beneficial effects may be offset by calcineurin inhibitor‐related nephrotoxicity | NG |
| C.8 – “Is PTA superior to current medical therapies in the course of diabetic neuropathy?” | Depending on severity of diabetic neuropathy, evidence suggests that successful PTA slows the progression of diabetic neuropathy when compared to current medical therapies | 2C |
| C.9 – “Is PTA superior to current medical therapies in the course of cardiovascular disease?” | Insufficient evidence is available to determine whether PTA slows progression of cardiovascular disease | NG |
Abbreviations: NG, not graded; PTA, pancreas transplantation alone.
4.2. Experts’ recommendations
Experts released 110 recommendations. No recommendation was graded 1A. Fifty‐one recommendations could not be graded. The remaining 59 recommendations were rated GRADE 1B (n = 13), GRADE 1C (n = 2), GRADE 2A (n = 2), GRADE 2B (N = 20), and GRADE 2C (n = 22) (Figure 1B).
Experts’ recommendations are reported in Tables 4, 5, 6, 7, 8, 9, 10, 11, 12.
TABLE 4.
Expert panel recommendations on activity volume and innovation in pancreas transplantation
| Query | Recommendation | GRADE | Quality score | Agreement | Proposed action |
|---|---|---|---|---|---|
| 1.1 – “What is the minimally acceptable annual volume of pancreas transplants per center?” | The outcome of pancreas transplantation is multifactorial. Higher annual volume is expected to be among the factors contributing to better outcome, but available data do not allow for a clear definition of a minimum annual volume, as this could also be influenced by several geographical variables as well as donor and recipient selection. | NG | 83% | 97.3% | Investigate the impact of annual volume of pancreas transplants per center. Estimates should take into account the possible impact of concurrent volume of renal and hepatic transplantation. |
| 1.2 – “What is the minimally acceptable annual volume of pancreas transplants per surgeon?” | Pancreas transplantation should not be performed occasionally by the individual surgeon. Younger surgeons who are starting their practice are expected to have completed a formal training program in pancreas transplantation and/or act under the supervision of a proficient pancreas transplant surgeon. | NG | 96% | 97.3% | Investigate volume‐outcome relationship for individual surgeon. Investigate volume‐outcome relationship based on overall surgeon experience vs. current annual volume. |
| 1.3 – “Is there a role for segmental live donor pancreas transplantation in non‐immunized recipients?” | Live donor segmental pancreas transplantation could be an option even in nonimmunized patients in extremely well‐selected pairs provided that the center is able to ensure quality of the procedure and careful lifelong follow‐up of the donor. | NG | 68% | 88.4% | None. |
| 1.4 – “Is there a role for segmental live donor pancreas transplantation in immunized recipients?” | Live donor segmental pancreas transplantation is an option in immunized patients in extremely well‐selected pairs provided that the center is able to ensure quality of the procedure and careful lifelong follow‐up of the donor. | NG | 70% | 93.4% | None. |
| 1.5 – “What are the anticipated risks for the live donor?” | There is no enough specific evidence (i.e., direct evidence from live donors) to address this question, especially concerning the risks of simultaneous distal pancreatectomy and nephrectomy. There is a risk of early technical complications and a risk for delayed metabolic complications demanding for careful selection of donors and lifelong follow‐up. | NG | 83% | 88.4% | None. |
| 1.6 – “Is there evidence that minimally invasive pancreas transplantation increases the risk of the transplant procedure versus open pancreas transplantation?” | Robotic pancreas transplantation is feasible. Available data do not allow to draw a conclusion on safety, although there may be a potential benefit in obese recipients. | NG | 82% | 94.1% | Establish a training path for safe implementation and diffusion of robotic pancreas transplantation. Report on additional cases. |
| 1.7 – “Is there evidence that minimally invasive pancreas transplantation is associated with worse long‐term results versus open pancreas transplantation?” | Due to lack of data, this query cannot be answered at the present time. | NG | 97% | 100% | Establish a training path for safe implementation and diffusion of robotic pancreas transplantation. Report on additional cases. |
| 1.8 – “Is there evidence of benefits from minimally invasive pancreas transplantation?” | Due to lack of data, this query cannot be answered at the present time. | NG | 79% | 97.3% | Establish a training path for safe implementation and diffusion of robotic pancreas transplantation. Report on additional cases. |
| 1.9 – “Is there evidence that minimally invasive pancreas transplantation is more beneficial in obese versus lean pancreas transplant recipients” | Due to lack of data, this query cannot be answered at the present time. | NG | 93% | 97.3% | Establish a training path for safe implementation and diffusion of robotic pancreas transplantation. Report on additional cases. |
Abbreviation: NG, not graded.
TABLE 5.
Expert panel recommendations on pancreas donation
| Query | Recommendation | GRADE | Quality score | Agreement | Proposed action |
|---|---|---|---|---|---|
| 2.1 – “In the setting of DBD is age >40 years an absolute or relative contraindication to pancreas transplantation?” | In the setting of DBD, donor age >40 years should not be considered either an absolute or a relative contraindication to pancreas transplantation if the donor is otherwise suitable. Accumulation of risk factors and long ischemic times should be avoided. | 1B | 90% | 96.4% | Expand utilization of donors aged over 40 years and report on outcomes. |
| 2.2 – “In the setting of DBD is the use of pediatric donors an absolute or relative contraindication to pancreas transplantation?” | In the setting of DBD, pediatric pancreas donors should not be considered a contraindication to pancreas transplantation. Accumulation of risk factors and long ischemic times should be avoided. | 2B | 82% | 96.4% | Report outcomes of pancreas transplantation from donors of low body weight (<15 kg). |
| 2.3 – “In the setting of DBD is donor BMI >30 kg/m2 a contraindication to pancreas transplantation?” | Properly selected donors with a BMI > 30 kg/m2 can be used for pancreas transplantation. Accumulation of risk factors and long ischemic times should be avoided. | 2B | 80% | 93.2% |
Report on multicenter experience with pancreas transplantation from donors with a BMI > 30 kg/m2 compared to lower BMI donors. The ideal study should be prospective and should report on all pancreas offers with a focus on organ transplanted from donors with a BMI > 30 kg/m2. |
| 2.4 – “Is DCD an absolute or relative contraindication to pancreas transplantation?” | Controlled DCD is not a contraindication to pancreas transplantation. Accumulation of risk factors and long ischemic times should be avoided. | 2B | 85% | 100% | Report on further series of pancreas transplantation from DCD. |
| 2.5 – “Is University of Wisconsin solution superior to Celsior solution for pancreas preservation?” | There is no evidence that the use of University of Wisconsin vs. the use of Celsior solutions results in improved pancreas transplantation outcomes when pancreas allografts are preserved for relatively short periods of time. | 1B | 74% | 85.7% | None. |
| 2.6 – “Is University of Wisconsin solution superior HTK solution for pancreas preservation?” | University of Wisconsin solution appears to be superior to HTK solution for pancreas preservation. | 2B | 75% | 93.2% | None. |
| 2.7 – “Is University of Wisconsin solution superior to IGL‐1 solution for pancreas preservation?” | Due to lack of data, this query cannot be answered at the present time. | NG | 68% | 100% | Publish retrospective series before planning for prospective and randomized comparisons. |
| 2.8 – “Are quick en‐bloc techniques superior to conventional techniques for pancreas procurement?” | Due to lack of data, this query cannot be answered at the present time. | NG | 67% | 96.4% | Report on outcomes of pancreas transplantation following quick en‐bloc and conventional procurement techniques, after matching both donor and recipient population by propensity scores. Outcomes should include pancreas grafts discarded because of surgical injury. |
| 2.9 – “Is the outcome of local versus imported grafts superior in pancreas transplantation?” | There is no evidence that imported pancreatic grafts have inferior transplant outcomes compared to local grafts. A proficient team should perform the donor procedure, and strategies should be developed to reduce cold preservation time of imported grafts. | 2B | 84% | 96.4% | Report on outcomes of pancreas transplantation from local vs. imported grafts while matching donor and recipient populations for known prognostic factors predicting early graft failure. Outcomes should include pancreas grafts discarded because of surgical injury and the experience of the recovery surgeon/team. |
| 2.10 – “For how long can pancreas grafts be ideally preserved?” | While minimization of ischemia times (less than 12 h) are associated with superior outcomes, results remain acceptable up to 24 h of preservation time. Beyond this time limit, pancreas transplantation can still be performed if the individual graft is believed to be particularly suitable for a given recipient. | 1B | 78% | 85.7% | None. |
| 2.11 – “Is machine perfusion of pancreas allografts feasible and associated with improved pancreas transplant outcomes?” | Due to lack of data, this query cannot be answered at the present time. | NG | 74% | 96.4 | Conduct further studies in preclinical models. |
Abbreviations: DBD, donation after brainstem death; DCD, donation after circulatory death; HTK, Histidine‐tryptophan‐ketoglutarate; IGL‐1, Institut Georges Lopez‐1; NG, not graded.
TABLE 6.
Expert panel recommendations on pancreas graft allocation
| Query | Recommendation | GRADE | Quality score | Agreement | Proposed action |
|---|---|---|---|---|---|
| 3.1 – “In SPK transplants, are the results of AB0‐identical/‐compatible transplantation superior to those of AB0 incompatible transplantation?” | Anecdotal experience shows that AB0‐incompatible SPK transplantation can be performed safely. However, due to lack of supporting evidence, AB0‐incompatible SPK transplantation should only be considered in selected circumstances and according to national allocation rules. | NG | 61% | 94.2% | None. |
| 3.2 – “In solitary pancreas transplants are the results of AB0‐identical/‐compatible transplantation superior to those of AB0‐incompatible transplantation?” | In the absence of evidence, AB0‐incompatible solitary pancreas transplantation should not be performed. | NG | 60% | 88.2% | None. |
| 3.3 – “In SPK transplants are the results of crossmatch negative transplants superior to those of crossmatch positive transplants?” | The results of crossmatch‐negative SPK transplants are expected to be superior to those of crossmatch‐positive transplants in terms of risk of recipients developing donor‐specific antibody and higher rejection rates (including antibody‐mediated rejection). SPK transplantation should not be performed in the presence of positive crossmatch. | NG | 87% | 93.8 | Report on the outcome of SPK transplants performed in the setting of T or B cell‐positive crossmatch. |
| 3.4 – “In solitary pancreas transplants, are the results of crossmatch negative transplants superior to those of crossmatch positive transplants?” | The results of crossmatch‐negative solitary pancreas transplants are expected to be superior to those of crossmatch‐positive transplants in terms of risk of recipients developing donor‐specific antibody and higher rejection rates (including antibody‐mediated rejection). Solitary pancreas transplantation should not be performed in the presence of T cell and/or B cell CDC‐positive crossmatch. | NG | 77% | 87.5% | Report on the outcome of solitary transplants performed despite T or B cell‐positive crossmatch. |
| 3.5 – “In SPK transplants, in the setting of a negative crossmatch, are the results of transplantation affected by the presence of DSA with MFI levels <3000?” | In the setting of limited evidence, presence of pretransplant DSA with an <3000 MFI level in patients with a negative T cell and B cell flow cytometric crossmatch could be considered for SPK transplantation as per center‐specific policy. | NG | 65% | 87.5% | Retrospective and prospective studies are needed. |
| 3.6 – “In SPK transplants, in the setting of a negative crossmatch, are the results of transplantation affected by the presence of DSA with MFI levels <5000?” |
In the setting of a negative crossmatch, SPK transplantation could be considered, despite the presence of DSA with an MFI of <5000, as per center‐specific policy. |
NG | 60% | 95.9% | Retrospective and prospective studies are needed. |
| 3.7 – “In solitary pancreas transplants, in the setting of a negative crossmatch, are the results of transplantation affected by the presence of DSA with MFI levels <3000?” | In the setting of extremely limited evidence, presence of pretransplant DSA with a low MFI level (<3000) in patients with a negative crossmatch could be considered for solitary pancreas transplantation as per center policy. | NG | 60% | 90.6% | Retrospective and prospective studies are needed. |
| 3.8 – “In solitary pancreas transplants, in the setting of a negative crossmatch, are the results of transplantation affected by the presence of DSA with MFI levels <5000?” | Solitary pancreas transplantation could be considered in patients with a pretransplant DSA of intermediate (<5000) MFI level and a negative crossmatch, as per center‐specific policy. | NG | 57% | 93.8% | Retrospective and prospective studies are needed. |
| 3.9 – “In SPK transplants are the results of transplantation improved by reduced HLA mismatching?” | The overall results of SPK transplantation are not improved by reduced HLA mismatching. However, there is a correlation between number of HLA mismatches and rate of acute rejection. | 2C | 82% | 100% | Prospective studies should investigate the relationships between HLA‐matching, development of de novo DSA, and long‐term SPK transplant immunologic outcomes. |
| 3.10 – “In solitary pancreas transplants, are the results of transplantation improved by reduced HLA mismatching?” | In solitary pancreas transplantation, reduced HLA‐B and HLA‐DR mismatch are associated with lower acute rejection rates, but not with improved overall pancreas allograft survival. | 2C | 82% | 93.8% | Further prospective and retrospective studies are recommended. |
| 3.11 – “Should kidneys be preferentially allocated to SPK recipients, when compared to recipients of kidney alone transplants?” | Kidneys of donors suitable for pancreas donation should be preferentially allocated to SPK transplant recipients because of the higher survival advantage in this patient population, of improved results with simultaneous vs. sequential transplantation, and of practical reasons concerning the organization of multi‐organ procurement. | 1B | 86% | 100% | Provide additional analyses from registries to further evaluate the long‐term survival benefits of SPK transplantation. Because most of the currently available data are provided by US centers (due to mandatory reporting to the UNOS/OPTN and the IPTR), data from non‐US registry/collaborative studies should also be reported. |
| 3.12 – “Should kidneys be preferentially allocated to SPK recipients, when compared to recipients of kidney alone transplants with a PRA ≥ 80%?” | There is currently no evidence supporting priority for kidney allocation in the event of competition between HLA‐highly sensitized recipients of kidney alone transplants and SPK transplant recipients. Allocation should be done according to national allocation policy. | NG | 85% | 90.5% | Prospective and retrospective studies are strongly needed. |
| 3.13 – “Should kidneys be preferentially allocated to SPK recipients, when compared to recipients of other simultaneous transplants (i.e., liver‐kidney, heart kidney, lung‐kidney)?” | No evidence supports the priority for kidney allocation in the event of competition between recipients of SPK transplantation and recipients of other simultaneous transplants (i.e., liver‐kidney, heart‐kidney, and lung‐kidney). | NG | 89% | 90.5% | Prospective and retrospective studies are recommended. |
| 3.14 – “Are the results of SPK transplants in type 1 diabetic patients superior to the results of SPK transplants in type 2 diabetic patients so that a priority should be given to type 1 diabetics?” | There is no evidence to prioritize graft allocation for SPK transplantation to patients with type 1 vs. patients with type 2 diabetes. | 2B | 90% | 100% | Further prospective and retrospective studies are recommended. A clear definition of selection criteria for SPK transplantation in patients with type 2 diabetes is needed. |
| 3.15 – “Are the results of SPK transplants in patients aged ≤50 years superior to the results of SPK transplants in older patients so that a priority should be given to younger recipients?” | In selected patients, results of SPK transplantation are similar in younger and older recipients. There is no evidence to prioritize graft allocation based on recipient age. | 2B | 90% | 96.4% | Further prospective and retrospective (preferentially from large registries) studies are recommended to determine the benefit of SPK transplantation in older recipient categories. |
Abbreviations: CDC, complement‐dependent cytotoxicity; DSA, donor‐specific antibody; HLA, human leukocyte antigen; IPTR, International Pancreas and Transplant Registry; MFI, mean fluorescent intensity; NG, not graded; PRA, panel reactive antibody; SPK, simultaneous pancreas kidney; UNOS/OPTN, United Network for Organ Sharing/Organ Procurement and Transplantation Network; US, United States.
TABLE 7.
Expert panel recommendations on recipient selection for pancreas transplantation
| Query | Recommendation | GRADE | Quality score | Agreement | Proposed action |
|---|---|---|---|---|---|
| 4.1 – “Is there a higher risk of post‐transplant renal failure in potential PTA recipients with normal (eGFR ≥90 ml/min/1.73 m2) or mildly decreased (eGFR 60–89 ml/min/1.73 m2) renal function and nephrotic syndrome when compared to recipients without nephrotic syndrome?” | In patients referred for PTA with normal or mildly decreased (eGFR 60–89 ml/min/1.73 m2) renal function and nephrotic syndrome, the benefits of insulin independence should be balanced against the possible risk of accelerated renal failure. | NG | 70% | 88.2% | Retrospective and prospective studies on PTA in patients with normal or mildly decreased renal function and nephrotic syndrome are very much needed. |
| 4.2 – “Is there a higher risk of post‐transplant renal failure in potential PTA recipients with normal (eGFR ≥90 ml/min/1.73 m2) or mildly decreased (eGFR 60–89 ml/min/1.73 m2) renal function and proteinuria (without nephrotic syndrome) when compared to recipients without proteinuria?” | In PTA recipients, with normal or mildly decreased (eGFR 60–89 ml/min/1.73 m2) renal function and proteinuria (without nephrotic syndrome), the benefits of insulin independence should be balanced against the potential risk of worsening of nephropathy. | 2C | 74% | 90.6% | Specific registry analysis and prospective studies are both needed to further clarify the possible increase in the risk of renal failure in PTA recipients with normal or mildly decreased renal function with proteinuria, but without nephrotic syndrome. |
| 4.3 – “Does PTA improve the course of chronic diabetic complications as compared to state‐of‐the‐art medical therapies?” | Successful PTA is associated with an improved course of chronic complications of diabetes as compared to current therapies. | 2C | 83% | 90.6% | A prospective observational or randomized trial should probably be the next action to take. |
| 4.4 – “Are the results of PAK transplants performed in recipients with a creatinine clearance or eGFR ≤45 ml/min inferior to the results of PAK transplants performed in patients with higher creatinine clearance or eGFR levels?” | PAK transplantation in diabetic patients with a functioning kidney graft and a creatinine clearance or eGFR ≤ 45 ml/min could be performed after careful risk‐benefit analysis in the individual patient. Immunosuppression should be optimized to protect renal function. | NG | 82% | 90.6% | Ad hoc registry analysis as well as prospective studies are required to clarify if recipients with a creatinine clearance ≤45 ml/min are exposed to undue risk of renal graft failure when undergoing PAK transplant. |
| 4.5 – “Are the results of PAK transplants performed in recipients with a history of renal rejection inferior to the results of PAK transplants performed in patients without a history of renal rejection?” | Patients with history of renal allograft rejection should be selected very carefully for PAK transplantation. Optimal HLA matching and avoidance of donor‐specific antibodies are both expected to mitigate the risk of post‐PAK rejection. | NG | 84% | 90.6% | Further retrospective and prospective observational studies are both needed. |
| 4.6 – “Are the results of PAK transplants performed within 6 months from renal transplantation inferior to the results of PAK transplants performed after this time interval?” | PAK transplantation performed within 6 months of renal transplantation is associated with similar outcomes when compared to PAK transplantation performed after this time point. PAK transplantation provides better results when performed within 1 year after kidney transplantation. | 2C | 88% | 96.4% | Further retrospective and prospective observational studies are both needed. |
| 4.7 – “Are the results of preemptive SPK transplants superior to those of SPK transplants performed in patients undergoing dialysis?” | Preemptive SPK transplant is associated with improved outcomes when compared to SPK transplant performed in patients undergoing dialysis. | 2B | 98% | 100% | Further studies should define the level of renal function at which SPK transplantation becomes preferred as compared to PTA. |
| 4.8 – “Are the results of SPK transplants in obese patients inferior when compared to the results of SPK transplants in non‐obese patients?” | Obese patients undergoing SPK transplant may face a higher rate of early complications when compared to nonobese recipients. | 2B | 95% | 91.4% | The value of bariatric procedures and/or minimally invasive transplantation in obese SPK candidates should be explored to improve the outcome of SPK transplantation in obese recipients. |
| 4.9 – “Are the results of SPK transplants in patients with a lower limb amputation inferior to the results of SPK transplants in patients without history of lower limb amputation?” | Pre‐SPK transplant lower limb amputation, in the context of cardiovascular disease, may be a risk factor for inferior transplant results. | 2C | 85% | 94.3 | None. |
| 4.10 – “Are the results of SPK transplants in patients with an history of coronary heart disease inferior to the results of SPK transplants in patients without an history of coronary heart disease?” | History of treated coronary heart disease is associated with an increased risk of post‐SPK transplant cardiovascular events and inferior long‐term results. | 2C | 97% | 94.3% | Report outcomes of SPK transplantation based on severity of coronary heart disease. |
Abbreviations: eGFR, estimated glomerular filtration rate; HLA, human leukocyte antigen; NG, not graded; PAK, pancreas after kidney; PTA, pancreas transplantation alone; SPK, simultaneous pancreas kidney.
TABLE 8.
Expert panel recommendations on surgical techniques for pancreas transplantation
| Query | Recommendation | GRADE | Quality score | Agreement | Proposed action |
|---|---|---|---|---|---|
| 5.1 – “Is pancreas transplantation with bladder drainage associated with more frequent surgical complications when compared to pancreas transplantation with enteric drainage?” | Bladder drainage of whole pancreaticoduodenal grafts is not associated with higher rates of immediate surgical complications when compared to enteric drainage of whole pancreaticoduodenal grafts. Bladder drainage, however, is associated with a higher rate of late reintervention, mostly required for enteric conversion of exocrine drainage. | 2A | 96% | 96.8% | None. |
| 5.2 – “Is pancreas transplantation with bladder drainage associated with more frequent urologic and metabolic complications when compared to pancreas transplantation with enteric drainage?” | Bladder drainage of whole pancreaticoduodenal grafts is associated with higher rates of urological and metabolic complications when compared to enteric drainage of whole pancreaticoduodenal grafts. | 2C | 98% | 100% | None. |
| 5.3 – “Is SPK transplant with bladder drainage associated with superior immunologic outcomes when compared to SPK transplants with enteric drainage?” | Bladder drainage of pancreas allografts at the time of SPK transplantation is not associated with superior immunologic outcomes when compared to enteric drainage. | 2C | 100% | 96.8% | None. |
| 5.4 – “Is solitary pancreas transplant with bladder drainage associated with superior immunologic outcomes when compared to pancreas transplant with enteric drainage?” | Solitary pancreas transplantation with bladder drainage is not associated with superior immunologic outcomes when compared to pancreas transplantation with enteric drainage. | 2B | 95% | 94.2% | None. |
| 5.5 – “Is pancreas transplantation with portal venous drainage associated with higher rates of surgical complications when compared to pancreas transplantation with systemic venous drainage?” | Pancreas transplantation with portal venous drainage is not associated with higher rates of surgical complications when compared to pancreas transplantation with systemic venous drainage. | 1B | 93% | 96.8% | None. |
| 5.6 – “Is pancreas transplantation with portal venous drainage superior to pancreas transplantation with systemic venous drainage, with respect to immunologic outcomes?” | Pancreas transplantation with portal venous drainage does not appear to be superior to pancreas transplantation with systemic venous drainage, with respect to immunologic outcomes. | 2C | 99% | 94.2% | Report immunologic outcomes of PTA with portal and systemic drainage. |
| 5.7 – “Is pancreas transplantation with portal venous drainage superior to pancreas transplantation with systemic venous drainage with respect to metabolic parameters?” | Portal venous drainage of pancreatic allografts does not clearly improve metabolic parameters when compared to systemic venous drainage. | 1B | 95% | 94.2% | None. |
| 5.8 – “Is duodeno‐duodenal anastomosis associated with more frequent surgical complications when compared to duodeno‐jejunal anastomosis?” | There is no clear evidence that duodeno‐duodenostomy, when compared to duodeno‐jejunostomy, increases the overall rate of surgical complications after pancreas transplantation. Further data are required to clarify the early risk profile of duodeno‐duodenostomy vs. duodeno‐jejunonostomy. | 2C | 88% | 90.6% | Design and conduct of prospective and randomized studies comparing safety of duodeno‐duodenostomy vs. duodeno‐jejunostomy. |
| 5.9 – “Is duodeno‐duodenal anastomosis associated with improved immunologic outcomes when compared to duodeno‐jejunal anastomosis?” | Duodeno‐duodenostomy does not appear to be associated with an immunologic advantage when compared to duodeno‐jejunostomy. | NG | 93% | 96.8% | Define the impact of endoscopic protocol duodenal and pancreatic biopsy in patients with duodeno‐duodenal anastomosis on immunologic outcomes of pancreas transplantation. |
| 5.10 – “Is intraperitoneal pancreas placement associated with more frequent surgical complications when compared to retroperitoneal pancreas placement?” | In the setting of low‐quality data, there is no evidence that intraperitoneal graft placement is associated with increased rates of surgical complications when compared to retroperitoneal graft placement. | 2C | 73% | 94.2% | Conduct registry analysis and/or collaborative studies to compare the outcomes of pancreas transplantation based on site of graft placement (i.e., intraperitoneal vs. retroperitoneal). Initiation of a prospective and randomized study could also be considered. |
| 5.11 – “Is graft accessibility for percutaneous biopsy improved by retroperitoneal versus intraperitoneal pancreas graft placement?” | Percutaneous biopsy of pancreas grafts placed in the retroperitoneum appears feasible, but there is no proof that graft accessibility is improved when compared to grafts placed intraperitoneally due to a lack of comparative studies. | NG | 91% | 96.8% | Evaluate the rate of feasibility of percutaneous pancreas biopsy in pancreas allografts placed intra‐ and retroperitoneally. |
Abbreviations: NG, not graded; SPK, simultaneous pancreas kidney.
TABLE 9.
Expert panel recommendations on immunosuppression in pancreas transplantation
| Query | Recommendation | GRADE | Quality score | Agreement | Proposed action |
|---|---|---|---|---|---|
| 6.1 – “Is steroid usage versus steroid avoidance associated with improved immunologic outcomes?” | Available evidence does not demonstrate that steroid avoidance is associated with inferior immunologic outcomes when compared to a policy of steroid maintenance. | 1B | 87% | 96.7% | Retrospective and prospective studies to identify groups of patients who will better tolerate steroid avoidance. |
| 6.2 – “Is steroid usage versus early steroid withdrawal associated with improved immunologic outcomes?” | Available evidence does not demonstrate that early steroid withdrawal is associated with improved immunologic outcomes when compared to a policy of steroid maintenance. | 1B | 87% | 96.7% |
Retrospective and prospective studies to identify groups of patients who will better tolerate early steroid withdrawal. Prospective studies comparing early steroid withdrawal with steroid avoidance should be performed. |
| 6.3 – “Is steroid withdrawal versus steroid maintenance associated with improved metabolic parameters?” | Steroid withdrawal, when maintained long term, seems to be associated with improved metabolic parameters. | 1C | 81% | 86.7% | Design and conduct prospective studies adequately powered to define the impact of steroid avoidance on metabolic parameters after pancreas transplantation in the setting of a homogenous recipient population and concurrent immunosuppression. |
| 6.4 – “Is early steroid withdrawal versus steroid maintenance associated with improved metabolic parameters?” | Early steroid withdrawal seems to be associated with improved metabolic parameters. | 2C | 77% | 90.4% | Design and conduct prospective studies adequately powered to define the impact of early steroid withdrawal on metabolic parameters after pancreas transplantation in the setting of a homogenous recipient population and concurrent immunosuppression. |
| 6.5 – “Is induction versus no induction therapy associated with improved immunologic outcomes?” | The use of induction therapy is associated with improved immunologic outcomes when compared to a policy of no induction therapy. | 1B | 91% | 100% | Additional studies are required to identify optimal induction therapy. |
| 6.6 – “Is induction versus no induction therapy associated with more early complications?” | Induction with depleting antibodies, when compared to no induction, is associated with increased rates and severity of early posttransplant infections that do not result in inferior clinical outcomes. | 2B | 88% | 93.3% | Additional studies are required to identify optimal induction therapy. |
| 6.7 – “Is induction versus no induction therapy associated with more oncologic complications?” | There is no clear evidence that current induction agents increase oncologic complications. | 2B | 83% | 86.7% | Retrospective studies, including registry analysis, should report on induction therapy and long‐term oncologic complications in pancreas transplant recipients. |
| 6.8 – “Is induction therapy with depleting antibodies versus induction therapy with nondepleting antibodies associated with improved immunologic outcomes?” | In recipients at low immunologic risk (i.e., PRA <10%), there is no clear evidence that induction with depleting vs. nondepleting antibodies results in improved immunologic outcomes. | 2C | 82% | 86.7% | Design and conduct prospective and randomized trials, comparing policies of induction with depleting antibodies vs. policies of induction with nondepleting antibodies in the setting of “standardized” maintenance immunosuppression, after stratification of recipients based on immunologic risk according to current standards. |
| 6.9 – “Is induction therapy with depleting antibodies versus induction therapy with nondepleting antibodies associated with more early complications?” | Depleting antibodies are associated with increased rates of early complications that do not result in inferior patient and graft survival. | 2B | 86% | 90.4% | Further prospective randomized studies are required to identify optimal induction therapy and define the incidence and severity of early complications specifically caused by induction therapy. |
| 6.10 – “Is induction therapy with depleting antibodies versus induction therapy with nondepleting antibodies associated with more oncologic complications?” | There is no evidence that induction with depleting antibodies vs. induction with nondepleting antibodies is associated with more oncologic complications. | 2C | 83% | 93.3% | Retrospective studies, including registry analysis, should report on long‐term oncologic complications in pancreas transplant recipients. |
| 6.11 – “Is CNI‐free immunosuppression associated with inferior immunologic outcomes in pancreas transplantation when compared to CNI‐including immunosuppression?” | Current evidence suggests that CNI‐free immunosuppression is associated with inferior immunologic outcomes. | NG | 85% | 90.4% | Conduct multicenter and/or registry analyses to define recipient categories in which CNI‐free could be safely implemented. |
| 6.12 – “Is CNI‐free immunosuppression associated with reduced toxicity in pancreas transplantation when compared to CNI‐including immunosuppression?” | Due to lack of data, this query cannot be answered at the present time. | NG | 80% | 96.7% | Report on intention‐to‐treat studies describing the outcomes of long‐term use of CNI‐free protocols. |
| 6.13 – “Is tacrolimus superior to cyclosporine, with respect to immunologic outcomes, in SPK transplants?” | The use of tacrolimus is prevalent in pancreas transplantation as it achieves superior immunologic outcomes when compared to cyclosporine. | 1C | 82% | 90.5% | Tacrolimus has been established as the CNI of choice in pancreas transplantation. Future studies should focus on minimization strategies. |
| 6.14 – “Is tacrolimus superior to cyclosporine, with respect to immunologic outcomes, in solitary pancreas transplants?” | The use of tacrolimus is prevalent in solitary pancreas transplantation and is associated with excellent immunologic results. Despite lack of specific comparative studies, registry data and retrospective series show that tacrolimus achieves superior immunologic results. | 2C | 83% | 90.4% | Tacrolimus has been established as the CNI of choice in pancreas transplantation. Future studies should focus on minimization strategies, especially in patients with early renal dysfunction. |
| 6.15 – “Is once‐a‐day tacrolimus formulation superior to twice‐a‐day tacrolimus formulation in pancreas transplantation?” | Due to lack of data, this query cannot be answered at the present time. | NG | 79% | 96.7% |
Registry analysis and long‐term data should be reported to establish long‐term noninferiority of once‐a‐day vs. twice‐a‐day formulations of tacrolimus in SPK. Data on solitary pancreas transplants should also be provided. |
| 6.16 – “Is the use of mycophenolate formulations versus azathioprine associated with improved immunologic outcomes in pancreas transplantation?” | The use of mycophenolate formulations improves the immunologic outcomes of pancreas transplantation when compared to azathioprine. | 1B | 90% | 100% | None. |
| 6.17 – “Is the use of mycophenolate formulations versus azathioprine associated with more side effects in pancreas transplantation?” | Indirect evidence and retrospective data show that mycophenolate mofetil is associated with higher rates of gastrointestinal side effects than azathioprine. | NG | 68% | 90.4% | None. |
| 6.18 – “Is the use of m‐TOR inhibitors versus mycophenolate formulations associated with improved immunologic outcomes in pancreas transplantation?” | In the setting of conflicting data, there is no clear evidence that the use of m‐TOR inhibitors vs. mycophenolate formulations is associated with an immunologic advantage in pancreas transplantation. | NG | 71% | 96.7% |
The role of m‐TOR inhibitors in pancreas transplantation should be further explored. Future studies should be designed taking into consideration that m‐TOR inhibitors probably should not be used in the first few months after transplantation, because of the high incidence of surgical complications. |
| 6.19 – “Is the use of mycophenolate formulations versus m‐TOR inhibitors associated with more side effects in pancreas transplantation?” | The use of m‐TOR inhibitors vs. mycophenolate formulations as primary immunosuppressants in pancreas transplantation is associated with specific and less well‐tolerated side effects. | 1B | 82% | 90.4% | The role of m‐TOR inhibitors in pancreas transplantation should be further explored. Future studies should be designed taking into consideration that m‐TOR inhibitors probably should not be used in the first few months after transplantation, because of the high incidence of surgical complications. |
| 6.20 – “Is m‐TOR‐based immunosuppression versus CNI‐based immunosuppression associated with improved immunologic outcomes in pancreas transplantation?” | m‐TOR‐based immunosuppression is not associated with an immunologic advantage when compared to CNI‐based immunosuppression in pancreas transplantation. | 2C | 88% | 90.4% |
The role of m‐TOR inhibitors in pancreas transplantation should be further explored. Future studies should be designed taking into consideration that m‐TOR inhibitors probably should not be used in the first few months after transplantation, because of the high incidence of surgical complications. |
| 6.21 – “Is m‐TOR‐based immunosuppression versus CNI‐based immunosuppression associated with more side effects in pancreas transplantation?” | Due to lack of data, this query cannot be answered at the present time. | NG | 77% | 96.7% |
The role of m‐TOR inhibitors in pancreas transplantation should be further explored. Future studies should be designed taking into consideration that m‐TOR inhibitors probably should not be used in the first few months after transplantation, because of the high incidence of surgical complications. |
| 6.22 – “Is m‐TOR‐based immunosuppression versus CNI‐based immunosuppression associated with increased formation of donor specific antibodies in pancreas transplantation?” | Preliminary data suggest that the use of m‐TOR‐based immunosuppression vs. CNI‐based immunosuppression could be associated with increased formation of donor‐specific antibodies in pancreas transplantation. | 2B | 85% | 90.4% |
The role of m‐TOR inhibitors in pancreas transplantation should be further explored. Future studies should be designed taking into consideration that m‐TOR inhibitors probably should not be used in the first few months after transplantation, because of the high incidence of surgical complications. |
| 6.23 – “Is delayed introduction of m‐TOR inhibitor better tolerated than immediate m‐TOR inhibitor introduction in pancreas transplantation?” | Delayed introduction of m‐TOR inhibitors is better tolerated than immediate m‐TOR inhibitor introduction in pancreas transplantation. | NG | 84% | 96.7% |
The role of m‐TOR inhibitors in pancreas transplantation should be further explored. Future studies should be designed taking into consideration that m‐TOR inhibitors probably should not be used in the first few months after transplantation, because of the high incidence of surgical complications. |
Abbreviations: CNI, calcineurin inhibitor; m‐TOR, mammalian target of rapamycin; NG, not graded; PRA, panel reactive antibody; SPK, simultaneous pancreas kidney.
TABLE 10.
Expert panel recommendations on postoperative prophylaxis in pancreas transplantation
| Query | Recommendation | GRADE | Quality score | Agreement | Proposed action |
|---|---|---|---|---|---|
| 7.1 – “Does antithrombotic prophylaxis versus no prophylaxis reduce the rate of pancreas graft thrombosis in SPK transplants?” | Per protocol antithrombotic prophylaxis is suggested in SPK recipients as it may reduce the rate of pancreas graft loss due to vascular thrombosis | NG | 91% | 98.8% | Plan prospective randomized comparisons between different protocols of antithrombotic prophylaxis after stratification of patients in risk categories for vascular (graft and/or deep vein) thrombosis. |
| 7.2 – “Does antithrombotic prophylaxis versus no prophylaxis reduce the rate of pancreas graft thrombosis in solitary pancreas transplantation?” | Per protocol antithrombotic prophylaxis is recommended in recipients of solitary pancreas transplants as it may reduce the rate of pancreas graft loss due to vascular thrombosis. | 2C | 93% | 98.8% | Plan prospective randomized comparisons between different protocols of antithrombotic prophylaxis after stratification of patients in risk categories for vascular (graft and/or deep vein) thrombosis. |
| 7.3 – “Does antithrombotic prophylaxis versus no prophylaxis reduce the rate of deep venous thrombosis and pulmonary embolism in SPK transplants?” | There is no evidence to support the use of per protocol antithrombotic prophylaxis in SPK recipients for the prevention of deep venous thrombosis and pulmonary embolism. However, considering that SPK recipients are at higher risk for deep venous thrombosis and pulmonary embolism, as well as of vascular thrombosis of the pancreas allograft, standard antithrombotic prophylaxis, based on local protocols, is recommended. | NG | 90% | 100% | Report observational studies focusing on incidence and severity of deep venous thrombosis and pulmonary embolism in SPK and in solitary pancreas transplantation recipients. |
| 7.4 – “Does antithrombotic prophylaxis versus no prophylaxis reduce the rate of deep venous thrombosis and pulmonary embolism in solitary pancreas transplantation?” | Considering that recipients of solitary pancreas grafts recipients are at risk for deep venous thrombosis and pulmonary embolism, as well as of vascular thrombosis of the pancreas allograft, antithrombotic prophylaxis is recommended. Type and degree of antithrombotic prophylaxis can be trimmed based on local practice and recipient characteristics. | NG | 91% | 100% | Report observational studies focusing on incidence and severity of deep venous thrombosis and pulmonary embolism in SPK and in solitary pancreas transplantation recipients. |
| 7.5 – “Is anticoagulation superior to antiaggregation/antiplatelet therapy in antithrombotic prophylaxis to prevent pancreas graft thrombosis in pancreas transplant recipients?” | Either anticoagulation or antiaggregation/antiplatelet therapy, or a combination thereof, can be used in pancreas transplant recipients to reduce the risk of pancreas graft thrombosis. There is no evidence on which strategy is preferred. | NG | 90% | 96.4% | Report observational studies as well as comparative studies to study the benefits and risks of different therapies or combinations thereof. |
| 7.6 – “Does antiviral prophylaxis versus no prophylaxis reduce the incidence of CMV infection? in pancreas transplant recipients” | Antiviral prophylaxis is suggested in most pancreas transplant recipients. Type of drug as well as dose and duration of prophylaxis can be tailored based on donor/recipient matching for CMV serological status. | 2B | 98% | 98.8% | None. |
| 7.7 – “Is antiviral prophylaxis superior to preemptive therapy in reducing the rate of CMV infection in pancreas transplant recipients?” | 1. Anti‐CMV prophylaxis is recommended in seronegative recipients receiving grafts from CMV seropositive donors. | 2A | 94% | 100% | Retrospective and randomized studies in seropositive patients receiving grafts from either seronegative or seropositive donors comparing CMV prophylaxis with preemptive therapy. |
| 2. In other donor/recipient pairs, there is no clear evidence of which strategy should be preferred. Per center‐specific protocols may be applied according to specific guidelines. | NG | 94% | 100% | ||
| 7.8 – “Does antimycotic prophylaxis versus no prophylaxis reduce the rate of fungal infections in pancreas transplant recipients?” | Antimycotic prophylaxis should be used as per center protocol to mitigate the risk of invasive fungal infections. | NG | 90% | 100% | None. |
| 7.9 – “Does antimicrobial prophylaxis versus no prophylaxis reduce the rate of bacterial infections in pancreas transplant recipients?” | Antimicrobial prophylaxis, as per center protocol, is recommended in pancreas transplant recipients. | 1B | 98% | 100% | Observational and prospective studies focusing on specific antibiotic or combination of antibiotics. |
| 7.10 – “Does vaccination versus no vaccination reduce the rate of infections in pancreas transplant reciipients?” | Evidence derived from transplantation of other solid organs supports vaccination according to general consensus guidelines. | NG | 94% | 100% | Observational studies as well as comparative studies. |
Abbreviations: CMV, cytomegalovirus; NG, not graded; SPK, simultaneous pancreas kidney.
TABLE 11.
Expert panel recommendations on immunology in pancreas transplantation
| Query | Recommendation | GRADE | Quality score | Agreement | Proposed action |
|---|---|---|---|---|---|
| 8.1 – “Does surveillance evaluation of donor specific antibody levels improve the immunologic outcome of pancreas transplantation versus no protocol serology?” | De novo donor‐specific antibodies are associated with increased rates of pancreas allograft rejection, potentially affecting survival. DSA monitoring after pancreas transplantation is advised. | 2C | 94% | 100% | Conduct prospective studies on the consequences of development of de novo DSA in pancreas transplantation. |
| 8.2 – “Does surveillance pancreas biopsy improve the immunologic outcome of pancreas transplantation versus no protocol biopsy in SPK transplants?” | Protocol biopsy of the kidney or pancreas graft in SPK transplantation may help in surveillance. Use of surveillance biopsy remains center specific. | NG | 84% | 96.4% | Conduct prospective studies on protocol pancreas and kidney biopsy in SPK recipients. |
| 8.3 – “Does surveillance pancreas biopsy improve the immunologic outcome of pancreas transplantation versus no protocol biopsy in solitary pancreas transplants?” | Protocol biopsy in solitary pancreas transplants may help in graft surveillance. Use of surveillancebiopsy remains center specific. Combination with de novo donor‐specific antibodies detection is advisable. | NG | 88% | 98.8% | Conduct prospective studies on protocol pancreas biopsy in recipients of solitary pancreatic grafts. |
| 8.4 – “In SPK transplants, is a first rejection episode best treated with steroid pulses or T‐cell depleting antibodies?” | Steroids can be used for clinically diagnosed rejection episodes or biopsy‐proven grade 1 rejection. Higher biopsy grades require T‐cell depleting antibodies. Treatment can be individualized based on clinical history and immunologic data. | 2C | 81% | 98.8% | Conduct prospective and randomized comparisons between different treatment strategies in recipients of SPK, with stratification of rejection severity based on histology scores. |
| 8.5 – “In solitary pancreas transplant recipients, is a first rejection episode best treated with steroid pulses or T‐cell depleting antibodies?” | Steroids can be used for clinically diagnosed rejection episodes or biopsy‐proven grade 1 rejection. Higher biopsy grades require T‐cell depleting antibodies. Treatment can be individualized based on clinical history and immunologic data. | NG | 77% | 98.8% | Conduct prospective and randomized comparisons between different treatment strategies in recipients of SPK, with stratification of rejection severity based on histology scores. |
| 8.6 – “In SPK transplants, is a second rejection episode best treated with steroid pulses or T‐cell depleting antibodies?” | Treatment of second rejection episodes in SPK transplantation should be individualized based on clinical history, immunologic data, and/or biopsy results. In general, pancreas graft biopsy can add information. T‐cell depleting antibodies should be used in most patients. | NG | 85% | 100% | Conduct multicenter studies and/or registry analyses on treatment and outcome of second rejection episodes in SPK recipients. |
| 8.7 – “In solitary pancreas transplant recipients, is a second rejection episode best treated with steroid pulses or T‐cell depleting antibodies?” | Treatment of second rejection episodes in solitary pancreas transplantation should be individualized based on clinical history, immunologic data, and/or biopsy results. In general pancreas graft biopsy can add information. T‐cell depleting antibodies should be used in most patients. | NG | 86% | 100% | Conduct multicenter studies and/or registry analyses on treatment and outcome of second rejection episodes in recipients of solitary pancreas transplants. |
| 8.8 – “What is the ideal treatment of antibody‐mediated rejection in SPK transplants?” | Due to lack of specific data, treatment of antibody‐mediated rejection in SPK transplantation follows the protocols established in kidney transplantation. Treatment can be individualized based on clinical history and immunologic data. | NG | 86% | 100% | Conduct multicenter studies and/or registry analyses on treatment and outcome of antibody‐mediated rejection in recipients of solitary pancreas transplants. |
| 8.9 – “What is the ideal treatment of antibody‐mediated rejection in solitary pancreas transplantation?” | Due to lack of specific data, treatment of antibody‐mediated rejection in solitary pancreas transplantation follows the protocols established in kidney transplantation. Treatment can be individualized based on clinical history and immunologic data. | NG | 87% | 100% | Conduct multicenter studies and/or registry analyses on treatment and outcome of antibody‐mediated rejection in recipients of solitary pancreas transplants. |
| 8.10 – “Autoimmune recurrence. How patients should be surveilled?” | Autoantibodies related to autoimmune recurrence of type 1 diabetes can be assayed per protocol in patients with a functioning pancreas allografts. Pancreas allograft biopsy can be used to establish the diagnosis of autoimmune recurrence of diabetes in patients with rising antibodies and/or impaired pancreas allograft function (in the absence of other obvious reasons). The use of surveillance allograft biopsy in patients without laboratory and/or clinical suspicion of autoimmune recurrence can be performed per center‐specific protocols. | 2C | 83% | 100% | Systematically investigate autoimmune reactivity in pancreas transplant recipients and report on incidence, severity, and treatment of autoimmune recurrence. |
Abbreviations: DSA, donor‐specific antibody; NG, not graded; SPK, simultaneous pancreas kidney.
TABLE 12.
Expert panel recommendations on immunology in pancreas transplantation
| Query | Recommendation | GRADE | Quality score | Agreement | Proposed action |
|---|---|---|---|---|---|
| 9.1 – “What are the effects of SPK transplants on retinopathy?” | Successful SPK transplantation may contribute to stabilization/improvement in diabetic retinopathy depending on retinopathy stage. Patients must be monitored closely by an ophthalmologist for progression in advanced retinopathy stages. | 2B | 83% | 97.2% | Prospective studies comparing SPK transplantation with standard medical diabetes therapies are highly advisable. |
| 9.2 – “What are the effects of SPK transplants on the development/occurrence of diabetic nephropathy in the kidney graft?” | Successful SPK transplantation prevents development/occurrence of diabetic nephropathy in the kidney graft. | 2B | 82% | 91.7% | Further studies are highly advisable aiming to compare kidney graft survival rates in SPK and live donor renal transplantation. |
| 9.3 – “What are the effects of SPK transplants on neuropathy?” | SPK transplantation has beneficial effects on mild to moderate neuropathy. | 2B | 82 | 94.4% | Studies are needed on the impact of SPK transplantation on advanced neuropathy. |
| 9.4 – “What are the effects of SPK transplants on the cardiovascular system?” | SPK transplantation has beneficial effects on the cardiovascular system, including lower rate of cardiovascular death compared with either dialysis or kidney alone transplantation. | 2B | 85% | 94.4% | More prospective studies are advisable to confirm the positive impact of SPK transplantation at the cardiovascular level, in particular for peripheral arteries. |
| 9.5 – “What are the effects of SPK transplants on quality of life?” | Successful SPK transplantation is associated with improved quality of life. | 1B | 79% | 100% | None. |
| 9.6 – “What are the effects of PTA on retinopathy?” | Successful PTA contributes to stabilization/improvement in diabetic retinopathy. | 2B | 79% | 91.7% | Prospective studies comparing PTA with standard medical diabetes therapies highly advisable. |
| 9.7 – “What are the effects of PTA on nephropathy?” | Functioning PTA improves the evolution of diabetic nephropathy. These beneficial effects may be offset by CNI‐related nephropathy. | NG | 82% | 94.4% | More studies are needed to evaluate the role of associated albuminuria pre‐PTA and to explore whether genetic factors play a role in affecting the course of native kidney function in PTA recipients. |
| 9.8 – “What are the effects of PTA on neuropathy?” | Evidence suggests that successful PTA improves the course of diabetic neuropathy. | 2C | 81% | 97.2% | Studies are urgently needed on the impact of PTA on somatic and autonomic diabetic neuropathy. |
| 9.9 – “What are the effects of PTA on the cardiovascular system?” | There is insufficient evidence available on the effects of PTA on the cardiovascular system. | NG | 73% | 94.4% | Studies are urgently needed on the impact of PTA on the cardiovascular system. |
| 9.10 – “What are the effects of PTA on quality of life?” | Prospective studies are needed to assess the role of PTA on recipients’ quality of life in comparison vs. pre‐PTA. | NG | 78% | 97.2% | Prospective studies are needed to assess the role of PTA on recipients’ quality of life in comparison vs. pre‐PTA. |
Abbreviations: CNI, calcineurin inhibitor; NG, not graded; PTA, pancreas transplantation alone; SPK, simultaneous pancreas kidney.
5. DISCUSSION
This world consensus conference provides the first practice guidelines for pancreas transplantation. Islet cell transplantation, which is a further therapeutic option for beta‐cell replacement in selected diabetic patients, was intentionally not addressed. Some of the recommendations provided for pancreas transplantation might also apply to islet cell transplantation, but this was not the aim of this consensus conference and no commitment exists for their use in this setting.
This consensus conference provided 49 jury deliberations and 110 expert recommendations. It is interesting to note that no statement achieved GRADE 1A, as no meta‐analysis of prospective and randomized trials exists on discussed issues. Approximately 40% of approved statements could not be graded while an additional 10% resulted in extremely weak recommendations. This is probably the combined result of difficulties in designing and conducting clinical studies in the setting of a rarely performed procedure, lack of interest from stakeholders, paucity of investments from pharmaceutical companies in clinical trials, and the long period in which surgeons had to achieve clinical success rather than scientific evidence. On practical grounds, in pancreas transplantation, there are still many issues for which practice is not strongly supported by evidence, despite excellent clinical results. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39
5.1. Jury deliberations—impact of SPK
The jury deliberated that SPK transplantation improves both quality of life and long‐term survival of patients with insulin‐dependent diabetes in comparison to current medical treatments and other transplant options. 33 , 34 , 37 , 38 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 These deliberations were not based on a high level of evidence and applied more strictly to patients with type 1 diabetes. In patients with type 2 diabetes, it was not clear if SPK transplant conveyed a survival advantage over live donor renal transplantation alone, while it was deemed convenient over both dialysis and deceased donor kidney transplantation.
The association between SPK transplant and improved survival in type 1 diabetic recipients was reported several times. 33 , 34 , 37 , 38 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 The acknowledgment of this advantage by an independent jury prompts the transplant community to further pursue SPK transplantation, especially when a live kidney donor is not available.
The jury also provided deliberations regarding the value of SPK transplantation performed in preemptive recipients. 61 , 62 , 63 This is a key issue, considering donor shortage and the need to maintain a balance between equity and efficacy in graft allocation policy. 64 , 65 While preemptive SPK transplant seems to be an excellent option in the individual patient, sound evidence is still missing to demonstrate if and to which extent preemptive SPK transplantation could be convenient in the average SPK transplant recipient.
5.2. Jury deliberations—impact of PAK
PAK was criticized due to possibly increased risks compared to continued insulin therapy. Indeed, in addition to the general concerns that apply to all types of pancreas transplantation, PAK transplant was associated with increased risk of renal graft loss. 66 , 67
Jury deliberations indicate that PAK transplant increases the risk of mortality early after transplantation, but improves life expectancy thereafter. As already observed for the kidney, 68 higher early mortality is the consequence of the need for a major surgical procedure and administration of additional immunosuppression and should not discourage PAK transplantation. Indeed, after the early posttransplant period, the additional risk of mortality disappears while quality of life is greatly improved and renal graft function is better preserved. Considerations on quality of life and renal graft function apply well to patients with type 1 diabetes. In patient with type 2 diabetes, PAK transplant was deemed feasible but evidence on possible advantages was lacking.
5.3. Jury deliberations—impact of PTA
Deliberations on PTA were truly important because they underscored the high value of this type of transplantation. Indeed, contrary to a landmark study, 69 the jury deliberated that PTA does not increase the long‐term risk of death compared with people remaining on the waiting list. PTA might be actually associated with a long‐term survival advantage in diabetic patients who have impaired hypoglycemia awareness. Although these deliberations are not based on new data, 27 , 39 , 70 , 71 they are key since they are provided by an independent jury and unambiguously debunk the myth of PTA recipients exposed to undue risks.
A further concern with PTA is the risk of accelerated loss of renal function. 73 , 74 , 75 The jury deliberated that impaired pretransplant renal function is a risk factor for accelerated end‐stage renal failure after PTA, while an estimated glomerular filtration rate ≥60 ml/min/1.73 m2 is sufficient to protect most recipients against this risk. The use of calcineurin inhibitors (CNIs) may contribute to a decline in renal function after PTA, while normalization of glucose levels could have beneficial effects on underlying diabetic nephropathy in the long term. 76 , 77 These additional and important data underscore the key role of accurate recipient selection for safe PTA and appropriate management of immunosuppression. Probably, patients with hypoglycemia unawareness should be referred for PTA before development of diabetic nephropathy.
The jury also deliberated that PTA improves quality of life, may stabilize/improve diabetic retinopathy (depending on severity of initial retinal damage), and may slow the progression of diabetic neuropathy. 32 , 78 , 79 , 80 No conclusion could be drawn regarding the effects of PTA on progression of cardiovascular disease. The positive effect of PTA on the course of microvascular complications of diabetes is an important piece of information that sheds additional light on the role of PTA in the management of selected diabetic patients.
Overall, based on jury deliberations, PTA appears fully justified in patients with hypoglycemia unawareness and possibly in patients with other chronic complications of diabetes of mild/moderate severity. Regarding hypoglycemia unawareness, islet cell transplantation could be an alternative option, but this issue was not addressed in the consensus.
5.4. Expert panel recommendations—activity volume and innovation
5.4.1. Activity volume
For many surgical procedures, there is a clear relationship between volume of activity and outcomes. 81 In transplantation, volume‐outcome relationship has been shown for the kidney, 82 liver, 83 heart, 84 and lung. 85
In the United States, approximately 70% of transplant centers are low volume. Low volume programs (one to six pancreas transplants per year) may be associated with worse outcomes. 86 Volume‐outcome relationship was confirmed in Europe, 16 by the Scientific Registry of Transplant Recipients, 17 and in few studies. 18 , 19 Based on these data, low volume seems to be associated with a higher risk for pancreas failure, 86 but there is no study specifically addressing the issue of minimum annual volume of pancreas transplant per center. Therefore, and considering that outcomes after pancreas transplantation are multifactorial and not just determined by surgery and/or care in the immediate posttransplant period, experts could not define a minimum annual volume but suggested that higher annual volume could be among the factors contributing to good outcomes.
No specific study addressed the impact of surgeon volume on outcomes of pancreas transplantation. As a consequence, no annual volume threshold exists. Evidence from other high complexity and relatively low volume procedures, such as pancreatoduodenectomy, suggests that higher volume surgeons perform better as compared to lower volume surgeons. 87 Hospital volume can mitigate the impact of low volume surgeons on outcomes, 88 and experienced surgeons have results similar to those achieved by high volume surgeons. 89 Experts recommended that pancreas transplantation should not be performed occasionally by the individual surgeon and that younger surgeons should have received formal training and/or should operate under supervision.
5.4.2. Innovation
Regarding innovation, two issues were assessed: live donor segmental pancreas transplantation and robotic pancreas transplantation.
Live donor segmental pancreas transplantation has been performed only in a few centers, for a total of approximately 200 procedures worldwide. Most of these transplants were done at a single institution, the University of Minnesota. 90 , 91 In general, segmental live donor pancreas transplantation is an option in sensitized recipients who have a suitable donor with a negative crossmatch. Due to the limited experience, donor risks cannot be precisely defined. Experience with the so called “Warshaw procedure,” 92 corresponding to a live donor segmental pancreatectomy performed in patients with benign or low‐grade pancreatic tumors, 93 shows that this procedure is quite safe. 94 However, short‐ and long‐term risks do exist. The most frequent early complications include splenic infarction (potentially requiring splenectomy), postoperative pancreatic fistula, and postoperative hemorrhage. Delayed complications/sequelae include gastric varices, hypersplenism, and diabetes. Sinistral portal hypertension was reported to have no clinical consequence in a large series of Warshaw procedures with long‐term follow‐up, 94 but a live donor of a segmental pancreatic graft did present with an upper gastrointestinal hemorrhage 25 years after surgery. 95 Splenectomy is curative in these patients, but massive gastrointestinal bleeding can be life‐threatening. Therefore, experts recommended that live donor segmental pancreas transplantation could be carefully considered in sensitized recipients and in extremely well‐selected pairs. They also recommended that the center be responsible to ensure quality of the procedure and careful lifelong follow‐up of the donor.
The first robotic pancreas transplantation was performed in Pisa, Italy, on September 27, 2010 and the first three cases were reported in 2012. 96 Since then, only few additional cases (<20) were reported worldwide. 97 , 98 All procedures were successful, but the generalizability of these results remains to be established due to both selection biases and small sample size. The larger experience with robotic renal transplantation, 99 , 100 as well as with other complex intra‐abdominal procedures requiring vascular anastomoses, 101 , 102 shows that robotic assistance permits pancreas transplantation. Justification for the pursuit of further experience with robotic pancreas transplantation includes the possibility of minimizing the incidence and severity of local complications, such as perigraft fluid collections and surgical site infections, and potentially expediting postoperative recovery. Based on this background, experts could only conclude that robotic pancreas transplantation is feasible.
5.5. Expert panel recommendations—pancreas donation
5.5.1. Donor characteristics
In general, the use of donors not fulfilling ideal criteria was considered acceptable provided that the accumulation of additional risk factors and long ischemic times was avoided. In detail, in the setting of donation after brainstem death (DBD), experts did not recommend against the use of donors aged >40 years, 103 , 104 , 105 , 106 , 107 , 108 pediatric donors, 109 , 110 , 111 , 112 , 113 and donors with a BMI > 30 kg/m2. 114 , 115 , 116 In the discussion, experts underscored that the use of pediatric donors of low body weight (<15 kg) may increase the risk of technical failure, while a BMI < 35 kg/m2 reduces the impact of obesity. In the setting of donation after circulatory death (DCD), the use of young controlled DCD donors was not considered a contraindication to pancreas transplantation, as evidence showed that when donor age is <40 years, results are good irrespective of donor source (i.e., DCD or DBD). 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124
5.5.2. Preservation solutions
The comparative value of different preservation solutions was extensively debated due to concerns on outcomes with increasing preservation times. When grafts are preserved for <12 h, experts agreed that University of Wisconsin (UW) and Celsior solutions are equally safe and effective. This recommendation was mostly supported by two single center prospective and randomized studies. 125 , 126 On the contrary, UW was deemed to be superior to histidine‐tryptophan‐ketoglutarate (HTK) because of the description of higher rates of acute pancreatitis with HTK 127 and concerns on suitability of this preservation solution with increasing preservation times. However, in the discussion, experts acknowledged that HTK can also be employed if preservation time does not exceed 10 h and when using low perfusion volumes. 128 , 129 , 130 , 131 Finally, no conclusion could be drawn on Institut Georges Lopez‐1 (IGL‐1) solution, because of lack of a comparison group in available studies. 132 , 133 , 134
5.5.3. Procurement technique
Because of lack of comparative studies, experts could not decide about which procurement technique should be preferred (i.e., quick en‐bloc or conventional technique). Reported results suggest that both techniques can be used based on individual preference and experience, with a preference for quick en‐bloc techniques in hemodynamically unstable donors. 135 , 136 , 137
5.5.4. Local versus imported grafts
Imported grafts were not considered to be associated with inferior outcomes when compared to local grafts, provided that a proficient team performed the procurement and that cold preservation times were acceptably short. 138 , 139 The use of imported grafts increases costs and, despite efforts, is associated with longer preservation times that entail higher peak levels of pancreatic enzymes. Finally, results of available studies could have been influenced by several biases such as selective reporting (i.e., lack of intention‐to‐treat design), and use of different procurement techniques and preservation solutions.
5.5.5. Preservation time
Ideally, pancreatic grafts should be preserved for <12 h. 140 , 141 Preservation times up to 24 h can still be accepted. Beyond this time limit, acceptance of a pancreatic graft for transplantation is based on individual circumstances, such as specific recipient needs. As for other recommendations, accumulation of risk factors should be avoided.
5.5.6. Machine perfusion
No recommendation was drawn on the use of machine perfusion because of lack of clinical studies. 142 , 143 , 144
5.6. Expert panel recommendations—pancreas graft allocation
5.6.1. AB0‐incompatible pancreas transplantation
AB0‐incompatible pancreas transplantation was not considered an option for standard recipients of both SPK and solitary pancreas transplantations. Concerns about AB0‐incompatible SPK transplantation are justified by the extremely low number of reported cases 145 , 146 that include an episode of humoral rejection, eventually rescued with eculizumab, 145 and by the lack of comparisons with AB0‐compatible SPK transplants. Concerns about AB0‐incompatible solitary pancreas transplantations are strongly justified by the lack of reported cases. Therefore, AB0‐incompatible pancreas transplantation should be considered investigational and should be performed only under urgent conditions or in clinical trials.
5.6.2. Positive crossmatch
In general, a positive crossmatch contraindicates pancreas transplantation. Limited evidence shows that pretransplant B cell crossmatch positivity does not affect patient and pancreas graft survival, but is associated with higher rates of antibody‐mediated rejection. 147 , 148 Few solitary pancreas transplants were performed despite a positive crossmatch with good outcomes. 149 , 150
5.6.3. Donor‐specific antibodies
Detection of DSAs up to an MFI level <5000 may not be an absolute contraindication to pancreas transplantation if T and B cell crossmatch is negative. These recommendations are mostly supported by the lack of specific evidence showing the impact of pretransplant DSA on transplant outcomes. However, these recommendations may be subject to clinical and methodological limitations, as detection of de novo DSA was associated with worse outcomes, 150 , 151 , 152 , 153 , 154 and MFI values are method dependent and hence center specific.
5.6.4. HLA mismatching
Reduced HLA mismatching was not specifically recommended in either SPK or solitary pancreas transplantation. These recommendations are supported by evidence showing that in either transplant categories, reduced HLA mismatching decreases the incidence of acute rejection episodes and detection of de novo DSA, but does not improve overall results. 155 , 156 , 157 , 158 , 159 Additionally, matching for some HLA alleles, such as DR3, is associated with increased risk of autoimmune recurrence of diabetes. 160
5.6.5. Preferential allocations of renal grafts
Renal grafts should be preferentially allocated to SPK recipients because of improved results with simultaneous vs. sequential transplantation, practical implications in organization of multi‐organ procurement, and a more evident survival advantage of kidney transplantation in diabetic vs. nondiabetic patients. 33 , 34 , 37 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61
Preferential graft allocation to SPK could not be recommended in case of competition with highly sensitized recipients of a kidney alone transplantation with a negative crossmatch, because of lack of supporting evidence showing which transplant candidate could benefit most from that specific renal graft. 64
Similarly, there is no evidence supporting priority for kidney allocation in the event of competition between recipients of SPK transplantation and recipients of other simultaneous transplants (i.e., liver‐kidney, heart‐kidney, and lung‐kidney). 65 Finally, there is also no evidence to prioritize graft allocation for SPK transplantation based on the type of diabetes (i.e., type 1 vs. type 2) or recipient age (< vs. >50 years).
5.7. Expert panel recommendations—recipient selection
5.7.1. Native renal function in PTA recipients
Baseline renal function is considered key to reduce the risk of accelerated graft loss in PTA recipients function. 73 , 74 , 75 In patients with normal (eGFR ≥ 90 ml/min/1.73 m2) or mildly decreased (eGFR 60–89 ml/min/1.73 m2) renal function and proteinuria (without nephrotic syndrome), experts recommended that the benefits of insulin independence should be balanced against the potential risk of worsening of nephropathy. Despite few studies have addressed this issue, this recipient population does not seem to be exposed to an undue risk of renal failure after PTA. 161 , 162 , 163 , 164 The same recommendation was released for patients with the same level of renal function and nephrotic syndrome. However, this recommendation could not be graded as it was supported only by anecdotal cases. 165
5.7.2. Impact of PTA on the course of chronic complications
In general, PTA improves the course of chronic complications of diabetes as compared to current medical therapies, 76 , 77 , 79 , 80 , 164 , 166 , 167 so that patients with evolving chronic complications could be considered for PTA before severe renal damage has occurred.
5.7.3. Selection of PAK recipients
In potential PAK recipients, a creatinine clearance ≤45 ml/min was not considered an absolute contraindication to sequential pancreas transplantation. Few and conflicting data exist on the prognostic implication of pre‐PAK creatinine clearance using 45 ml/min as a cutoff. In a retrospective and multicenter study, a pre‐PAK eGFR ≤ 45 ml/min was associated with an increased probability of kidney graft failure. 66 On the other hand, in another retrospective study, eGFR significantly increased 3 months after grafting in patients with pretransplant eGFR ≤45 ml/min. 168
In a retrospective and multicenter study reporting on PAK transplant, history of renal rejection was associated with increased risk of posttransplant mortality, renal graft failure, and pancreas graft failure. 66 However, experts did not recommend against PAK transplant in patients with history of renal allograft rejection, provided that HLA matching is optimized and DSA are avoided, because of lack of clear evidence discouraging sequential pancreas transplantation in these recipients.
Regarding the timing of sequential pancreas transplantation, experts did not contraindicate early PAK transplant (i.e., <6 months from kidney transplant) and underscored that results are improved if PAK transplant is performed within 1 year after kidney transplantation. 66 , 169 , 170
5.7.4. Preemptive SPK
Experts acknowledged that preemptive SPK transplant is associated with improved outcomes when compared to SPK transplant performed in patients undergoing dialysis. Indeed, several retrospective studies, including registry analysis, show that preemptive SPK transplantation is associated with improved outcomes when compared to SPK transplantation performed in patients undergoing dialysis. Time on dialysis also has a negative prognostic impact in SPK recipients. 59 , 171 , 172 , 173 , 174
5.7.5. Other risk factors relevant to recipient selection
Obese patients may face a higher rate of early complications when compared to nonobese recipients 175 , 176 , 177 , 178 , 179 but obesity alone is not a contraindication to SPK transplant, considering that good results were reported. 116 Discussion highlighted also the importance of underweight (BMI < 18.5 kg/m2), as a risk factor of long‐term mortality. 116
History of amputation and coronary heart disease were both considered risk factors for inferior results, but neither was deemed an absolute contraindication to SPK transplantation. Advanced atherosclerotic peripheral arterial disease, including the need for limb amputation in diabetic patients, is associated with increased mortality. 180 The association of advanced atherosclerotic peripheral arterial disease with end‐stage renal failure increases the risk of mortality. 181 In general, pre‐SPK limb amputation predicts inferior transplant outcomes as it portends higher cardiovascular risk. 182 Similarly, pretransplant history of coronary artery disease increases the risk of major adverse cardiovascular events after transplantation. 183 , 184 However, coronary artery disease is not a major risk factor for mortality if medically treated and revascularized according to standard guidelines. 185 Discussion highlighted the importance of assessment of coronary artery disease in all patients undergoing pancreas transplantation.
5.8. Expert panel recommendations—surgical techniques
5.8.1. Exocrine drainage
Several studies, including three with a prospective design, have compared bladder and enteric drainage of exocrine secretions in pancreas transplantation. Bladder drainage, when compared to enteric drainage, does not increase immediate surgical complications but is associated with higher rates of late reintervention (mostly for enteric conversion). 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206
Only one study clearly showed a higher rate of surgical complications in bladder‐drained transplants (41% vs. 26%; p = .04). 186 Need for enteric conversion was not considered a surgical complication in these studies, and was reported to occur in up to 20% of recipients. 190 Two recent long‐term studies reported that >40% of patients with bladder drainage require enteric conversion at some point in time. 197 , 207 Additionally, bladder drainage increased the rate of metabolic and urologic complications, 188 , 198 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 and did not improve immunologic outcome of either SPK 187 , 190 , 191 , 192 , 196 , 197 , 198 , 205 , 206 , 208 , 214 , 217 , 218 or solitary pancreas transplantations. 70 , 171 , 219
Duodeno‐duodenostomy (vs. duodeno‐jejunostomy) was not considered to clearly increase the overall rate of surgical complications after pancreas transplantation, despite higher rates of bleeding. 220 , 221 , 222 , 223 , 224 , 225 Additionally, duodeno‐duodenostomy was not associated with improved immunologic outcomes, because of easier graft surveillance (endoscopic biopsy) with earlier diagnosis of rejection, 222 , 223 , 224 as reported in a study. 221 Indeed, duodenal biopsy alone may not be sufficient to rule out rejection, as suggested by both experimental 226 and clinical studies. 227 , 228
5.8.2. Venous drainage
No study demonstrated that portal venous drainage increases surgical risk 187 , 229 , 230 , 231 , 232 , 233 , 234 , 235 but, on the other hand, no study showed either an immunologic, 199 , 207 , 208 , 229 , 236 or a metabolic advantage. 233 , 234 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244
5.8.3. Graft placement
Regarding final graft position, intraperitoneal graft placement (vs. retroperitoneal graft placement) was not associated with higher incidence of surgical complications because of lack of comparative studies. 224 , 225 , 245 , 246 The hypothesis that retroperitoneal graft placement facilitates percutaneous graft biopsy remains to be proven.
5.9. Expert panel recommendations—immunosuppression
5.9.1. Steroids
The use of steroids remains prevalent in maintenance protocols after pancreas transplantation. 40 Despite heterogeneity in background immunosuppressive regimens complicating interpretation of data, steroid avoidance is feasible in a good proportion of pancreas transplant recipients and does not result in inferior results when compared to steroid maintenance. 247 , 248 , 249 , 250 , 251 , 252 Early steroid withdrawal is also feasible. 253 , 254 , 255 , 256 , 257 , 258 Steroids avoidance, if maintained long term, is associated with improved metabolic profile. 257 , 259 , 260 , 261 , 262
5.9.2. Induction therapy
The use of induction therapy, typically in the form of depleting antibodies, is prevalent across all pancreas transplant categories. 40 Two randomized controlled trials showed that induction therapy is associated with improved immunologic outcomes when compared to a policy of no induction therapy. 263 , 264 However, there is no clear evidence that induction with depleting vs. nondepleting antibodies results in improved immunologic outcomes in patients at low immunologic risk (i.e., PRA < 10%).
Regarding safety, induction with depleting antibodies is associated with cytokine release syndrome requiring premedication and with an increased incidence of early posttransplant infections, in particular CMV viremia, when compared with a policy of use of nondepleting antibodies or no induction therapy. 40 , 263 , 265 Despite experience in renal transplantation showing that induction therapy with depleting antibodies is associated with increased rates of oncologic complications, 266 there is no clear evidence that this applies to recipients of pancreas transplantation.
In comparison to a policy of no induction, experts agreed that induction is associated with improved immunologic outcomes, and that induction with depleting antibodies is associated with increased rates and severity of early posttransplant infections (that do not result in inferior patient and graft survival) without evidence of increased risk of oncologic complications.
In comparison to a policy of induction with nondepleting antibodies in recipients at low immunologic risk (i.e., PRA < 10%), experts agreed that induction with depleting antibodies vs. induction with nondepleting antibodies does not improve immunologic outcomes and is associated with increased rates and severity of early posttransplant infections (that do not result in inferior patient and graft survival). However, there is no clear evidence that induction with depleting antibodies increases the risk of oncologic complications.
5.9.3. CNI‐free regimen
The main rationale for CNI‐free immunosuppression is to avoid the side effects of CNI‐based immunosuppression. However, long‐term data on outcomes of patients maintained on CNI‐free regimens after pancreas transplantation are lacking. Short‐term data are sparse and suggest that this strategy is associated with inferior immunologic outcomes without a clear reduction in drug‐related toxicity. 252 , 262
Relatively more data are available for protocols of immunosuppression minimization and delayed withdrawal of CNI. In selected patients at low immunologic risk, these strategies may achieve immunologic results similar to CNI‐based immunosuppression. 256 , 275 , 276 Results of a prospective and randomized trial published after this Consensus Conference showed that CNI‐free immunosuppression based on sirolimus achieved good patient and graft survival rates, but at the price of high drop‐out rate (68%) and increased incidence of de novo DSA anti‐class II HLA antigens at 12 months (19% vs. 2%). Additionally, due to high surgical complication rates, introduction of sirolimus was delayed until posttransplant month 3. 277 A phase 2 multicenter open‐label randomized trial, that was also published after the Consensus Conference, compared the outcomes of SPK recipients treated with an immunosuppressive regimen including tacrolimus vs. a protocol using low‐dose CNI plus costimulation blockade (belatacept) with intended CNI withdrawal. In both arms, patients received induction therapy with rabbit thymoglobulin, while steroids were rapidly withdrawn, and maintenance therapy included also mycophenolate sodium or mycophenolate mofetil. CNI withdrawal was associated with increased rates of pancreas rejection, despite similar rates of kidney rejection. The study was terminated after randomization of 43 of 60 planned patients. The authors concluded that costimulation blockade with belatacept did not provide sufficient immunosuppression to reliably prevent rejection of the pancreas in SPK transplants undergoing CNI withdrawal. Low‐dose CNI used in conjunction with belatacept was sufficient to prevent rejection of both kidney and pancreas, while increasing the incidence of opportunistic infections. 278
In comparison to CNI‐based immunosuppression, experts agreed that CNI‐free immunosuppression is associated with inferior immunologic outcomes without evidence of reduced drug‐related toxicity.
5.9.4. CNI‐based regimen
The use of tacrolimus is prevalent in all categories of pancreas transplantation. 40 One multicenter, prospective, and randomized study showed that tacrolimus achieved superior immunologic results when compared to cyclosporine in SPK transplant recipients, although the high incidence of pancreas allograft thrombosis recorded in the cyclosporine arm may constitute a major bias of this study. 267 A single center, prospective, and randomized study did not confirm the superiority of tacrolimus over cyclosporine in SPK transplant recipients. 268 Basically, the introduction of tacrolimus corresponded to clinical success in solitary pancreas transplantation and comparison with historical series using cyclosporine showed improved results. 72 , 269
Reported experience with the use of once‐a‐day tacrolimus formulation in pancreas transplantation is limited. Data are available only for SPK transplantation and show that once‐a‐day tacrolimus formulation is associated with excellent patient and graft survival, and that patients can be safely converted from standard tacrolimus to long‐acting tacrolimus. 270 , 271 , 272 , 273
In comparison with cyclosporine, experts agreed that the use of tacrolimus is prevalent in all pancreas transplant categories and is associated with superior immunologic outcomes. No conclusion could be drawn on the comparative efficacy of once‐a‐day vs. twice‐a‐day tacrolimus formulations due to lack of supporting data.
5.9.5. Mycophenolate formulations
The use of mycophenolate formulations is clearly prevalent in pancreas transplantation. 40 A prospective, multicenter, randomized, open‐label study comparing mycophenolate mofetil to azathioprine, in the setting of OKT3 induction and steroid/cyclosporine maintenance, did not demonstrate the superiority of mycophenolate mofetil in SPK transplantation. 279 An additional prospective and randomized study conducted at a single center showed that mycophenolate mofetil significantly decreased the incidence of biopsy‐proven acute rejection in SPK transplantation. 280 A review showed that the use of mycophenolate mofetil in combination with a CNI and steroids, after induction treatment, was associated with a 40% reduction in the incidence of acute rejection at 1 year after pancreas transplantation. 281 Retrospective studies have shown that mycophenolate mofetil compared to azathioprine improves immunologic outcome of pancreas transplantation when used in combination with either tacrolimus or cyclosporine, but at the price of more gastrointestinal side effects that frequently require dose reduction. 269 , 282 , 283
In comparison to azathioprine, experts agreed that mycophenolate formulations improve immunologic outcomes but are associated with more gastrointestinal side effects.
5.9.6. m‐TOR inhibitors
An analysis of all pancreas transplants included in the UNOS database from 1987 to 2016 showed that the use of m‐TOR inhibitors when compared to immunosuppressive protocols without m‐TOR inhibitors was associated with improved allograft survival and patient survival up to 10 years after transplantation. 284 However, there is no evidence that the use of m‐TOR inhibitors improves immunologic outcomes of pancreas transplantation when compared to mycophenolate formulations. The results of a multicenter, prospective, and randomized study comparing sirolimus and mycophenolate mofetil in SPK recipients were never published. Preliminary data from this trial showed that sirolimus was potentially associated with improved immunologic outcomes 285 but at the price of a higher incidence of surgical complications (i.e., delayed wound healing, lymphocele, and incisional hernia) and hyperlipidemia. 286 Two retrospective studies showed that the results of sirolimus and mycophenolate mofetil were similar when used in combination with tacrolimus. 254 , 287 A single center, randomized, and prospective study with 10‐year follow‐up showed significantly better rates of rejection with sirolimus, 288 although allograft and patient survival rates were similar.
There are only few data on comparative efficacy of m‐TOR‐based immunosuppression vs. CNI‐based immunosuppression in pancreas transplantation when these drugs are used as primary immunosuppressants. In general, CNI‐free immunosuppression in pancreas transplantation is associated with inferior immunologic outcomes. 251 , 266 In selected patients at low immunologic risk, m‐TOR inhibitors may allow CNI minimization, while maintaining satisfactory immunologic results. 252 , 275 , 276 Data from a recently published prospective and randomized study showed that immediate use of sirolimus after SPK transplantation, in the context of CNI‐free immunosuppression, is associated with an increased rate of surgical complications. 277 Additionally, the use of m‐TOR inhibitors in the setting of CNI‐free immunosuppression could increase the formation of DSA. 289 This issue is not fully addressed in the literature. Reported outcomes range from no effect, 290 to increased development on nondonor‐specific HLA antibodies, with immediate evidence of worse graft outcome, 291 and to an increased incidence of de novo DSA anti‐class II HLA agents at 1 year after transplantation. 277
In comparison with mycophenolate formulations, and in the context of limited evidence, experts acknowledged that the use of m‐TOR inhibitors is not clearly associated with an immunologic advantage. Additionally, when both drugs are used as primary immunosuppressants, experts agreed that the use of m‐TOR inhibitors vs. mycophenolate formulations is associated with specific and less well‐tolerated side effects.
In comparison with CNI‐based immunosuppression, experts agreed that the use of m‐TOR inhibitors is not associated with an immunologic advantage. Lack of specific evidence did not allow experts to define if m‐TOR‐based immunosuppression is associated with more side effects.
5.9.7. Summary of immunosuppression
State of the art immunosuppressive regimen for all categories of pancreas transplantation consists in induction with depleting antibody and maintenance with tacrolimus, mycophenolate, and steroids. Early steroid withdrawal is feasible and may result in improved metabolic parameters in the long‐term period.
The avoidance of CNI is associated with inferior immunologic outcomes without clear evidence of reduced toxicity. Concerns about early outcomes of CNI‐free immunosuppression are an additional and major clinical issue.
Mycophenolate formulations improve immunologic outcomes when compared to azathioprine but are associated with high rates of gastrointestinal side effects.
m‐TOR‐based immunosuppression is not associated with an immunologic advantage when compared to CNI‐based immunosuppression. The use of m‐TOR inhibitors vs. mycophenolate formulations could be associated with improved immunologic outcomes but carry more side effects, especially if used as primary immunosuppressants. Immediate posttransplant use of m‐TOR inhibitors is associated with high rates of surgical complications, making delayed introduction preferable. In the context of CNI‐free regimens, m‐TOR‐based immunosuppression may increase the development of DSA.
5.10. Expert panel recommendations—postoperative prophylaxis
5.10.1. Antithrombotic prophylaxis
Vascular thrombosis is the leading cause of early graft loss in pancreas transplantation. 292 The high incidence of vascular thrombosis in pancreas grafts is explained by multiple factors such as the hypercoagulable state of diabetic patients, 292 , 293 increased donor age, 294 donor obesity, 292 cerebrovascular cause of donor death, 294 low microcirculatory blood flow of the pancreas allograft, 295 need for back table vascular reconstructions, 294 preservation injury, 292 long preservation times, 296 occurrence of graft pancreatitis, 292 , 294 endothelial damage promoted by high CNI levels, 293 and the disproportion in size between the large vascular pedicles and the small pancreatic branches following splenectomy and enterectomy. 292 Finally, vascular allograft thrombosis may also be caused, or promoted, by missed rejection. 297
Experts recommended that per protocol antithrombotic prophylaxis should be given to all pancreas transplant recipients, although there is not enough evidence to define which prophylaxis protocol should be used. 293 , 298 , 299 , 300 , 301 , 302 , 303 , 304 , 305
Regarding deep venous thrombosis, recipients of both SPK and solitary pancreas transplantation are at increased risk for deep venous thrombosis and pulmonary embolism. However, no study is available to compare a policy of no antithrombotic prophylaxis vs. a policy of per protocol antithrombotic prophylaxis in pancreas transplant recipients for the prevention of deep venous thrombosis and pulmonary embolism. There are also no studies comparing different anticoagulation prophylaxis protocols. 293 , 298 , 299 , 300 , 301 , 302 , 303 , 304 , 305 Experts did not recommend antithrombotic prophylaxis for the prevention of deep venous thrombosis in SPK recipients, due to lack of supporting evidence, while recommended antithrombotic prophylaxis in recipients of solitary pancreas transplants, taking into consideration also the higher risk of graft thrombosis in this recipient categories. Due to lack of evidence, decision on type and degree of antithrombotic prophylaxis could not be specified.
A further question was about the use of anticoagulation vs. anti‐aggregation antiplatelet therapy. Many pancreas transplant recipients are already under chronic anti‐aggregant therapy at the time of transplantation due to underlying cardiovascular disease or cardiovascular risk factors. Therefore, postoperative anticoagulant prophylaxis typically occurs in the setting of preexisting anti‐aggregation. Because of lack of comparative studies, experts could not indicate a preference for a specific strategy.
5.10.2. Antiviral prophylaxis
Recipients of pancreas transplantation are at high risk for virus activation or infection due to the frequent use of induction therapy with T‐cell depleting antibodies, in particular when steroids are also used. 40 Most of the available literature focuses on cytomegalovirus infection as infection with other viruses occurs less frequently. Published studies 251 , 263 , 306 , 307 , 308 , 309 , 310 , 311 , 312 and a Consensus Conference 313 on the management of cytomegalovirus in solid organ transplantation show that antiviral prophylaxis should be provided to pancreas transplant recipients. The type of antiviral drug, as well as duration of prophylaxis, can be tailored based on donor/recipient matching for cytomegalovirus serological status. When anti‐cytomegalovirus medications are not administered, prophylaxis against herpes simplex virus and varicella‐zoster virus should be considered.
Based on this background, experts recommended implementation of antiviral prophylaxis in most pancreas transplant recipients.
Regarding the use of prophylaxis or preemptive cytomegalovirus therapy, experts recommended prophylaxis in seronegative recipients receiving grafts from CMV‐seropositive donors. 306 , 313 In other donor/recipient pairs, either strategies were considered acceptable.
5.10.3. Antimycotic prophylaxis
Pancreas transplantation is associated with a risk of fungal infection. Fungal infections are associated with reduced patient and graft survival. Available literature does not provide clear evidence that fungal prophylaxis should be used in all pancreas transplant recipients. A selective policy of antifungal prophylaxis in patients at higher risk for invasive fungal infection is justified. Most centers use a protocolized short duration, systemic antifungal prophylaxis strategy. 312 , 314 , 315 , 316 , 317 , 318 , 319 , 320 , 321 Experts recommended the use of antimycotic prophylaxis, as per center protocol, to mitigate the risk of invasive fungal infections.
5.10.4. Antimicrobial prophylaxis
Pancreas transplantation is associated with a high risk of bacterial infection. Antibacterial prophylaxis is largely prescribed following pancreas transplantation and is associated with a reduced incidence and severity of posttransplant bacterial infections. Debate remains concerning the ideal combination of antibiotics to use for prophylaxis as well as duration of prophylaxis. 322 , 323 , 324 , 325 , 326 , 327 , 328 Experts recommended the use of antimicrobial prophylaxis, as per center protocol.
5.10.5. Vaccination
This consensus was held before the SARS‐CoV‐2 pandemic. Therefore, any recommendations on vaccination against SARS‐CoV‐2 have not been included.
While vaccination strategies have not been studied specifically in the setting of pancreas transplantation, evidence derived from experience in transplantation of other solid organs 329 supports a role for multiple vaccinations, based on individual needs, to reduce the incidence of late post‐transplant infections. Therefore, experts recommended vaccinations in pancreas transplant recipients, based on general consensus guidelines.
5.11. Expert panel recommendations—immunology
5.11.1. DSA monitoring
The role of DSA is emerging as an important factor in immunological graft failure. Regarding a policy of per protocol evaluation of DSA, there is no specific study that has compared the immunologic outcome of pancreas transplant recipients with vs. without DSA monitoring. However, despite conflicting data, 154 , 330 several studies showed an association between de novo DSA and increased rate of rejection episodes/poorer graft survival in pancreas transplantation. 150 , 151 , 152 , 153 Experts recommended DSA monitoring after pancreas transplantation.
5.11.2. Per protocol pancreas graft biopsy
There is no specific evidence supporting protocol biopsies in SPK transplant recipients, but in solitary pancreas grafts protocol biopsy improved immunologic outcomes. 331 , 332 Considering also that concordance between renal and pancreatic biopsy is not complete, 333 , 334 , 335 experts concluded that per protocol biopsy in SPK transplant recipients is center specific and may help in immunologic surveillance.
In solitary pancreas transplantation, few studies showed that protocol pancreas biopsy may improve immunologic outcome. 331 , 332 Pancreatic biopsy should be preferred over duodenal biopsy, when feasible, because concordance between pancreatic and duodenal biopsies is limited. 228 Experts concluded that use of protocol biopsy in solitary pancreas transplants is center specific. It may help in graft surveillance, especially if combined with DSA monitoring.
5.11.3. Treatment of first rejection episodes
No prospective and randomized study has compared steroids vs. T‐cell depleting antibody as a treatment of first rejection episodes in pancreas transplantation. Most authors treat first, or mild, rejection episodes with steroid pulses. Treatment with T cell depleting antibodies is typically reserved to patients with recurrent, or moderate/severe, rejection episodes. 333 , 336 , 337 , 338 , 339 A recent study found that outcome of first rejection episodes is not improved by administration of T‐cell depleting antibodies when mild, but is improved when moderate or severe. 340 Experts recommended the use of steroids for treatment of clinically diagnosed rejection episodes or biopsy‐proven grade 1 rejection. T‐cell depleting antibodies can be used for higher rejection grades or based on clinical history and immunologic data.
5.11.4. Treatment of second rejection episodes
There is basically no evidence in the literature supporting how a second rejection episode should be treated in recipients of solitary pancreas transplants. Experts recommended that treatment of second rejection episodes should be individualized. T‐cell depleting antibodies should be used in most patients.
5.11.5. Treatment of antibody‐mediated rejection
The importance of antibody‐mediated rejection in pancreas transplantation is becoming increasingly evident. However, the definition of antibody‐mediated rejection has changed over time. Earlier studies defined antibody‐mediated rejection as the combined presence of DSAs, graft dysfunction, and C4d positivity on histology slides. 341 , 342 These criteria were incorporated in the Banff schema for grading of pancreas allograft rejection published in 2008. 343 However, DSAs can be detected in the absence of rejection, graft dysfunction can occur without rejection and, as shown in kidney transplantation, C4d positivity may not be sufficient to establish a diagnosis of antibody‐mediated rejection. 344 Updated Banff grading schema replaced graft dysfunction with histologic evidence of acute tissue injury. 344 , 345
Currently available treatment strategies are basically derived from renal transplantation, and there are no comparative studies that specifically address the efficacy of these protocols in pancreas transplantation. Treatment options include the use of plasma exchange and intravenous immunoglobulins either alone 342 , 346 or in combination with rituximab. 341 , 347 , 349 A management algorithm was proposed by Redfield et al in 2015. 348
Because of lack of specific data, experts could not draw a specific recommendation, and suggested that treatment of antibody‐mediated rejection in pancreas transplantation can be individualized based on clinical history and immunologic data.
5.11.6. Surveillance for autoimmune recurrence of diabetes
After the first description by Sutherland, Goetz, and Sibley in 1989, 350 autoimmune recurrence of diabetes is increasingly recognized as an important cause of graft loss. While the presence of autoantibodies before pancreas transplantation has no impact on graft outcome, major autoantibody changes (serum conversion, spreading from one to multiple autoantibodies, or titer increase) are predictive of subsequent loss of graft function. 160 , 351 , 352 , 354 More recently, the recurrence of autoreactive CD4 T cells has been described in both recipients’ blood and pancreas grafts. 354 Monitoring of autoreactive CD4 T cells, in combination with autoantibodies and biopsies, was described in three SPK recipients with autoimmune recurrence. 355 Current status of autoimmune monitoring in pancreas transplantation is described in several reviews. 356 , 357
Experts recommended per protocol assay of autoantibodies related to autoimmune recurrence of type 1 diabetes. In patients with rising antibodies and/or impaired pancreas allograft function (in the absence of other obvious reasons of graft injury), experts recommended also the use of pancreas allograft biopsy to establish the diagnosis of autoimmune recurrence of diabetes.
5.12. Expert panel recommendations—follow‐up
5.12.1. Effects of pancreas transplantation on diabetic retinopathy
The impact of SPK transplantation on diabetic retinopathy is controversial. 78 , 358 , 359 , 360 , 361 , 362 , 363 In the most recent studies, diabetic retinopathy is stabilized/improved after successful SPK transplantation. 78 , 358 , 359 It should be noted that diabetic retinopathy is often severe in these patients, which makes reversal of the retinal damage unlikely. Results are better if accurate retinal examination is performed pre‐SPK transplantation and appropriate ocular treatment ensured. Experts acknowledged that successful SPK transplantation may contribute to stabilization/improvement of diabetic retinopathy depending on retinopathy stage, and recommended that patients are monitored closely by an ophthalmologist for progression in advanced retinopathy stages.
Few studies have addressed the effects of PTA on retinopathy (including one in comparison with insulin therapy and one in comparison with failed PTA). Generally, successful PTA is associated with improved stabilization of advanced retinopathy and increased lesion reversal in nonproliferative retinopathy. One study reports the deceleration of retinal damage early after PTA, with potential stabilization over time. 26 , 78 , 80 Experts acknowledged that successful PTA contributes to stabilization/improvement of diabetic retinopathy.
5.12.2. Effects of pancreas transplantation on diabetic nephropathy
Several studies have compared the effects of SPK transplantation on the survival of the transplanted kidney in comparison with the survival of renal alone grafts from deceased or living donors. The superiority of SPK vs. deceased donor renal transplantation is well established, whereas that vs. live donor renal transplantation is still uncertain. A few studies suggest that the function of the grafted kidney is better in SPK transplant than in recipients of live donor renal transplantation. 364 , 365 , 366 Experts acknowledged that successful SPK transplantation prevents development/occurrence of diabetic nephropathy in the kidney graft.
Several studies have evaluated the effects of PTA on the native kidneys, which can be damaged by immunosuppressive drug nephrotoxicity. Over the years, due to better titration of immunosuppression and selection of recipients, the rate of chronic kidney disease in PTA recipients has progressively diminished. Currently, the 10‐year cumulative incidence of post‐PTA chronic kidney disease ranges from 10 to 30% when the pre‐PTA eGFR is >60 ml/min/1.73 m2, with some authors suggesting a threshold of eGFR pre‐PTA of 70 ml/min/1.73 m2. Less information is available on the role of associated albuminuria. Some data show that in patients with a functioning PTA and not evolving toward chronic kidney disease, the decrease in eGFR over time is similar to that observed in the general T1D population. 26 , 27 , 76 Experts acknowledged that functioning PTA improves the evolution of diabetic nephropathy, but underscored as these beneficial effects may be sometimes offset by CNI‐related nephropathy.
5.12.3. Effects of pancreas transplantation on diabetic neuropathy
Several studies, including prospective analyses, have evaluated the effects of SPK transplantation on somatic and autonomic neuropathy, also in comparison with kidney transplant alone and standard insulin therapy. Overall, evidence suggests that SPK transplantation improves symptoms of somatic neuropathy, parameters of peripheral nerve function, and autonomic nervous system cardiorespiratory tests, possibly also due to rescue from uremia. Insufficient data are available on the impact of SPK transplantation on advanced autonomic nervous system alterations, such as gastroparesis and neurogenic bladder. 79 , 367 , 368 , 369 , 370 , 371 , 372 Experts acknowledged that SPK transplantation has beneficial effects on mild to moderate neuropathy.
Scant information is available on the effects of PTA on diabetic neuropathy. Published data suggest some improvements in nerve conduction velocity, autonomic function, and epinephrine response. 28 , 166 Experts acknowledged that successful PTA may improve the course of diabetic neuropathy.
5.12.4. Effects of pancreas transplantation on cardiovascular system
A few studies evaluated the effects of SPK transplantation on the cardiovascular system, also in comparison with kidney transplant alone. SPK transplantation has been reported to be associated with lower rate of cardiovascular death and reduced progression of carotid and lower limb arterial damage. 52 , 182 , 373 , 374 , 375 , 376 , 377 Experts acknowledged that SPK transplantation has beneficial effects on the cardiovascular system, including lower rate of cardiovascular death compared with either dialysis or kidney alone transplantation.
Limited data are available on the effects of PTA on the cardiovascular system, and mainly from a single group. PTA can lead to early and persistent reduction of a few cardiovascular risk factors (total and LDL cholesterol, blood pressure) and improved cardiac morphology and function (including diastolic parameters) as assessed by ultrasound evaluation. 29 , 167 , 375 , 376 , 377 Experts concluded that evidence available on the effects of PTA on the cardiovascular system is not sufficient to draw a final conclusion.
5.12.5. Effects of pancreas transplantation on quality of life
Several studies have evaluated the effects of SPK transplantation on recipients’ quality of life, mostly in comparison with kidney graft alone recipients or diabetic patients on dialysis. Consistently, successful grafting is associated with improved scores in multiple domains. 21 , 22 , 23 , 24 , 25 Experts acknowledged that successful SPK transplantation is associated with improved quality of life.
Little information is available on the effects of PTA on recipients’ quality of life. Available data suggest enhanced quality of life after PTA. 30 , 31 , 32 Experts acknowledged that PTA improves recipient quality of life compared to patients on waiting list.
5.13. Research agenda
Opportunities for research are presented as proposed actions for each recommendation in Tables 4, 5, 6, 7, 8, 9, 10, 11, 12. In general, the level of evidence was quite low demonstrating that well‐designed studies as well as meta‐analyses are greatly needed for many topics.
Additional studies are more urgently needed for volume‐outcome relationship, pancreas allocation strategies, efficacy of IGL‐1 solution (vs. UW solution), clinical role of machine perfusion, induction with depleting antibodies vs. induction without depleting antibodies in patients at low immunologic risk, pancreas transplantation in patients with type 2 diabetes, long‐term results of preemptive SPK (vs. SPK in patients in dialysis), comparison of different anticoagulation prophylaxis regimens (including comparison between anticoagulation and anti‐aggregation protocols), strategies for immunologic surveillance, treatment of rejection episodes (in particular, treatment of second rejection episodes and treatment of antibody‐mediated rejections), effects of PTA on cardiovascular system, and effects of PTA on recipients’ quality of life.
Multicenter studies are particularly needed.
5.14. Limitations
As already reported while describing the methods of this consensus conference, 42 the main limitation of our collaborative effort was the need to review 50+ years of literature and consequently to extract data from several hundreds of articles. This extraordinary effort has intrinsic limitations and carries the risk of unintentional selection bias. Despite the creation of several dedicated teams for literature review, sharing and presentation of results of literature search, and online and in‐person discussion of each statement, we acknowledge that some articles could have been missed. Additionally, Ovid/Medline was not included in the systematic reviews, and only data from full peer‐reviewed manuscripts were considered. Consequently, we might have been missed additional information from these data sources.
Some of the data examined and discussed to reach the consensus may have been influenced by local practice as well as geographical and institutional variations. As most studies were provided by the United States and Europe, the applicability of these guidelines in other countries may require adaptations to local practice, legislative framework, organizational needs, epidemiology of organ donation, and other geographical/cultural variations.
Despite our effort to include all major transplant centers, and to specifically involve all physicians with known competence in pancreas transplantation, some prominent centers and influential colleagues may have not been invited or could not participate. However, having reached consensus among a large group of internationally recognized experts ensures balanced and competent assessment of available evidence.
5.15. Conclusions
In conclusion, we have reported on 49 jury deliberations and 110 experts’ recommendations, that we believe can be used to support and improve practice of pancreas transplantation worldwide. The main message from this consensus conference is that both SPK and PTA have the potential to improve patient survival in the long‐term period, while all types of pancreas transplantation dramatically improve the quality of life of recipients. These advantages clearly appear to outweigh potential disadvantages, thus encouraging further implementation of pancreas transplantation.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
ACKNOWLEDGMENTS
This Consensus Conference is dedicated in the loved memory of Mr. Fabrizio Iacopini, who made most of the local arrangements for the live sessions and unexpectedly died due to COVID‐19 before these proceeding could be published. The first World Consensus Conference had no funding from commercial companies. The conference received a main unrestricted grant from Fondazione Pisa. The following Institutions also provided additional financial support: Regione Toscana, Università di Pisa, and Azienda Ospedaliero Universitaria Pisana.
Boggi U, Vistoli F, Andres A, et al. First World Consensus Conference on pancreas transplantation: Part II – recommendations. Am J Transplant. 2021;21(Suppl. 3):17–59. 10.1111/ajt.16750
Piero Marchetti, Raja Kandaswamy, and Thierry Berney are senior authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lentine KL, Kasiske BL, Levey AS, et al. KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation. 2017;101(8S Suppl 1):S1‐S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levey AS, Eckardt K‐U, Dorman NM, et al. Nomenclature for kidney function and disease: report of a Kidney Disease: improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97(6):1117‐1129. [DOI] [PubMed] [Google Scholar]
- 3. Chadban SJ, Ahn C, Axelrod DA, et al. KDIGO Clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation. 2020;104(4S1 Suppl 1):S11‐S103. [DOI] [PubMed] [Google Scholar]
- 4. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group . KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1‐155. [DOI] [PubMed] [Google Scholar]
- 5. Berenguer M, Burra P, Ghobrial M, et al. Posttransplant management of recipients undergoing liver transplantation for hepatocellular carcinoma. Working group report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104(6):1143‐1149. [DOI] [PubMed] [Google Scholar]
- 6. Sapisochin G, Javle M, Lerut J, et al. Liver transplantation for cholangiocarcinoma and mixed hepatocellular cholangiocarcinoma: working group report from the ILTS transplant tncology consensus conference. Transplantation. 2020;104(6):1125‐1130. [DOI] [PubMed] [Google Scholar]
- 7. Tsochatzis E, Coilly A, Nadalin S, et al. International Liver Transplantation Consensus Statement on end‐stage liver disease due to nonalcoholic steatohepatitis and liver transplantation. Transplantation. 2019;103(1):45‐56. [DOI] [PubMed] [Google Scholar]
- 8. Charlton M, Levitsky J, Aqel B, et al. International liver transplantation society consensus statement on immunosuppression in liver transplant recipients. Transplantation. 2018;102(5):727‐743. [DOI] [PubMed] [Google Scholar]
- 9. European Association for the Study of the Liver . EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64(2):433‐485. [DOI] [PubMed] [Google Scholar]
- 10. de Jonge N, Kirkels JH, Klöpping C, et al. Guidelines for heart transplantation. Neth Heart J. 2008;16(3):79‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Banner NR, Bonser RS, Clark AL, et al. UK guidelines for referral and assessment of adults for heart transplantation. Heart. 2011;97(18):1520‐1527. [DOI] [PubMed] [Google Scholar]
- 12. Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update–a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25(7):745‐755. [DOI] [PubMed] [Google Scholar]
- 13. Maurer JR, Frost AE, Estenne M, et al. International guidelines for the selection of lung transplant candidates. The International Society for Heart and Lung Transplantation, the American Thoracic Society, the American Society of Transplant Physicians, the European Respiratory Society. Transplantation. 1998;66(7):951‐956. [DOI] [PubMed] [Google Scholar]
- 14. Potter LM, Maldonado AQ, Lentine KL, et al. Transplant recipients are vulnerable to coverage denial under Medicare Part D. Am J Transplant. 2018;18(6):1502‐1509. [DOI] [PubMed] [Google Scholar]
- 15. Allen KV, Walker JD. Microalbuminuria and mortality in long‐duration type 1 diabetes. Diabetes Care. 2003;26(8):2389‐2391. [DOI] [PubMed] [Google Scholar]
- 16. Kakio Y, Uchida HA, Takeuchi H, et al. Diabetic nephropathy is associated with frailty in patients with chronic hemodialysis. Geriatr Gerontol Int. 2018;18(12):1597‐1602. [DOI] [PubMed] [Google Scholar]
- 17. Adame Perez SI, Senior PA, Field CJ, et al. Frailty, health‐related quality of life, cognition, depression, vitamin D and health‐care utilization in an ambulatory adult population with type 1 or type 2 diabetes mellitus and chronic kidney disease: a cross‐sectional analysis. Can J Diabetes. 2019;43(2):90‐97. [DOI] [PubMed] [Google Scholar]
- 18. Stratta RJ, Fridell JA, Gruessner AC, et al. Pancreas transplantation: a decade of decline. Curr Opin Organ Transplant. 2016;21(4):386‐392. [DOI] [PubMed] [Google Scholar]
- 19. Benjamens S, Leemkuil M, Margreiter C, et al. A steady decline in pancreas transplantation rates. Pancreatology. 2019;19(1):31‐38. [DOI] [PubMed] [Google Scholar]
- 20. Stratta RJ, Gruessner AC, Odorico JS, et al. Pancreas transplantation: an alarming crisis in confidence. Am J Transplant. 2016;16(9):2556‐2562. [DOI] [PubMed] [Google Scholar]
- 21. Smith GC, Trauer T, Kerr PG, et al. Prospective quality‐of‐life monitoring of simultaneous pancreas and kidney transplant recipients using the 36‐item short form health survey. Am J Kidney Dis. 2010;55(4):698‐707. [DOI] [PubMed] [Google Scholar]
- 22. Pera PI, Vasallo JM, Rabasa AT, et al. Quality of life in simultaneous pancreas–kidney transplant recipients. Clin Transplant. 2009;23(5):600‐605. [DOI] [PubMed] [Google Scholar]
- 23. Sureshkumar KK, Patel BM, Markatos A, et al. Quality of life after organ transplantation in type 1 diabetics with end‐stage renal disease. Clin Transplant. 2006;20(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 24. Martins LS, Outerelo C, Malheiro J, et al. Health‐related quality of life may improve after transplantation in pancreas‐kidney recipients. Clin Transplant. 2015;29(3):242‐251. [DOI] [PubMed] [Google Scholar]
- 25. Rajkumar T, Mazid S, Vucak‐Dzumhur M, et al. Health‐related quality of life following kidney and simultaneous pancreas kidney transplantation. Nephrology. 2019;24(9):975‐982. [DOI] [PubMed] [Google Scholar]
- 26. Kim SJ, Smail N, Paraskevas S, et al. Kidney function before pancreas transplant alone predicts subsequent risk of end‐stage renal disease. Transplantation. 2014;97(6):675‐680. [DOI] [PubMed] [Google Scholar]
- 27. Gruessner RWG, Gruessner AC. Pancreas transplant alone. A procedure coming of age. Diabetes Care. 2013;36(8):2440‐2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kendall DM, Rooney DP, Smets YFC, et al. Pancreas transplantation restores epinephrine response and symptom recognition during hypoglycemia in patients with long‐standing type I diabetes and autonomic neuropathy. Diabetes. 1997;46(2):249‐257. [DOI] [PubMed] [Google Scholar]
- 29. Coppelli A, Giannarelli R, Mariotti R, et al. Pancreas transplant alone determines early improvement of cardiovascular risk factors and cardiac function in type 1 diabetic patients. Transplantation. 2000;76(6):974‐976. [DOI] [PubMed] [Google Scholar]
- 30. Sutherland DER. Present status of pancreas transplantation alone in non‐uremic diabetic patients. Transplant Proc. 1994;26:379‐383. [PubMed] [Google Scholar]
- 31. Stratta RJ, Weide LG, Sindhi R, et al. Solitary pancreas transplantation. Experience with 62 consecutive cases. Diabetes Care. 1997;20(3):362‐368. [DOI] [PubMed] [Google Scholar]
- 32. Scalea JR, Pettinato L, Fiscella B, et al. Successful pancreas transplantation alone is associated with excellent self‐identified health score and glucose control: a retrospective study from a high‐volume center in the United States. Clin Transplant. 2018;32(2):e13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tibell A, Solders G, Larsson M, et al. Superior survival after simultaneous pancreas and kidney transplantation compared with transplantation of a kidney alone in diabetic recipients followed for 8 years. Transplant Proc. 1997;29(1–2):668. [DOI] [PubMed] [Google Scholar]
- 34. Lee CM, Scandling JD, Krieger NR, et al. Outcomes in diabetic patients after simultaneous pancreas‐kidney versus kidney alone transplantation. Transplantation. 1997;64(9):1288‐1294. [DOI] [PubMed] [Google Scholar]
- 35. Rayhill SC, D’Alessandro AM, Odorico JS, et al. Simultaneous pancreas‐kidney transplantation and living related donor renal transplantationin patients with diabetes: is there a difference in survival? Ann Surg. 2000;231(3):417‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reddy KS, Stablein D, Taranto S, et al. Long‐term survival following simultaneous kidney‐pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Transplant Proc. 2001;33(1–2):1659‐1660. [DOI] [PubMed] [Google Scholar]
- 37. Mohan P, Safi K, Little DM, et al. Improved patient survival in recipients of simultaneous pancreas‐kidney transplant compared with kidney transplant alone in patients with type 1 diabetes mellitus and end‐stage renal disease. Br J Surg. 2003;90(9):1137‐1141. [DOI] [PubMed] [Google Scholar]
- 38. Sung RS, Zhang M, Schaubel DE, et al. A reassessment of the survival advantage of simultaneous kidney‐pancreas versus kidney‐alone transplantation. Transplantation. 2015;99(9):1900‐1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant. 2004;4(12):2018‐2026. [DOI] [PubMed] [Google Scholar]
- 40. Amorese G, Lombardo C, Tudisco A, et al. Induction and immunosuppressive management of pancreas transplant recipients. Curr Pharm Des. 2020;26(28):3425‐3439. [DOI] [PubMed] [Google Scholar]
- 41. Humar A, Kandaswamy R, Granger D, et al. Decreased surgical risks of pancreas transplantation in the modern era. Ann Surg. 2000;231(2):269‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boggi U, Vistoli F, Marchetti P, Kandaswamy R, Berney T; on behalf of the World Consensus Group on Pancreas Transplantation . First world consensus conference on pancreas transplantation: part I – Methods and results of literature search. Am J Transplant. (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8(5):336‐341. [DOI] [PubMed] [Google Scholar]
- 44. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;21(339):b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. SIGN 50: a guideline developer’s handbook. https://www.sign.ac.uk/what‐we‐do/methodology/sign‐50‐a‐guideline‐developers‐handbook/. last accessed 14 July 2021
- 46. Lesurtel M, Perrier A, Bossuyt PMM, et al. An independent jury‐based consensus conference model for the development of recommendations in medico‐surgical practice. Surgery. 2014;155(3):390‐397. [DOI] [PubMed] [Google Scholar]
- 47. Brouwers MC, Kho ME, Browman GP, et al.; AGREE Next Steps Consortium . The Global Rating Scale complements the AGREE II in advancing the quality of practice guidelines. J Clin Epidemiol. 2012;65(5):526‐534. [DOI] [PubMed] [Google Scholar]
- 48. Grading Tutorial. https://www.uptodate.com/home/grading‐tutorial. last accessed 14 July 2021
- 49. Loupy A, Lefaucheur C. Antibody‐mediated rejection of solid‐organ allografts. N Engl J Med. 2018;379(12):1150‐1160. [DOI] [PubMed] [Google Scholar]
- 50. May FNJ, Rees MT, Griffin S, Fildes JE. Understanding immunological response to desensitisation strategies in highly sensitised potential kidney transplant patients. Transplant Rev. 2021;35(2):100596. [DOI] [PubMed] [Google Scholar]
- 51. World Health Organization . Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series 854. Geneva: World Health Organization. 1995. https://apps.who.int/iris/handle/10665/37003. last accessed 14 July 2021 [PubMed] [Google Scholar]
- 52. Lindahl JP, Hartmann A, Aakhus S, et al. Long‐term cardiovascular outcomes in type 1 diabetic patients after simultaneous pancreas and kidney transplantation compared with living donor kidney transplantation. Diabetologia. 2016;59(4):844‐852. [DOI] [PubMed] [Google Scholar]
- 53. Tufveson G, Brynger H, Dimeny E, et al. Renal transplantation in diabetic patients with or without simultaneous pancreatic transplantation 1986: data from the EDTA Registry. Nephrol Dial Transplant. 1991;6(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 54. Douzdjian V, Rice JC, Gugliuzza KK, et al. Renal allograft and patient outcome after transplantation: pancreas‐kidney versus kidney‐alone transplants in type 1 diabetic patients versus kidney‐alone transplants in nondiabetic patients. Am J Kidney Dis. 1996;27(1):106‐116. [DOI] [PubMed] [Google Scholar]
- 55. Rayhill SC, D’Alessandro AM, Odorico JS, et al. Simultaneous pancreas‐kidney transplantation and living related donor renal transplantation in patients with diabetes: is there a difference in survival? Ann Surg. 2000;231(3):417‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reddy KS, Stablein D, Taranto S, et al. Long‐term survival following simultaneous kidney‐pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am J Kidney Dis. 2003;41(2):464‐470. [DOI] [PubMed] [Google Scholar]
- 57. Navarro MD, Pérez R, Castillo D, et al. Simultaneous pancreas‐kidney transplant compared with kidney transplant in type I diabetic patients with end‐stage renal disease. Transplant Proc. 2002;34(1):204‐205. [DOI] [PubMed] [Google Scholar]
- 58. Bunnapradist S, Cho YW, Cecka JM, et al. Kidney allograft and patient survival in type I diabetic recipients of cadaveric kidney alone versus simultaneous pancreas/kidney transplants: a multivariate analysis of the UNOS database. J Am Soc Nephrol. 2003;14(1):208‐213. [DOI] [PubMed] [Google Scholar]
- 59. Knoll GA, Nichol G. Dialysis, kidney transplantation, or pancreas transplantation for patients with diabetes mellitus and renal failure: a decision analysis of treatment options. J Am Soc Nephrol. 2003;14(2):500‐515. [DOI] [PubMed] [Google Scholar]
- 60. Myint TM, O’Shaughnessy DV, Marshall S, et al. Health‐related quality of life of patients awaiting kidney and simultaneous pancreas‐kidney transplants. Nephrology. 2013;18(12):827‐832. [DOI] [PubMed] [Google Scholar]
- 61. Grochowiecki T, Szmidt J, Gałazka Z, et al. The comparison of treatment results of type 1 diabetes mellitus complicated by end‐stage diabetic nephropathy in patients undergoing simultaneous pancreas and pre‐emptive kidney transplantation (SPPkTx) and patients enrolled into the dialysis program–a c. Ann Transplant. 2005;10(3):31‐35. [PubMed] [Google Scholar]
- 62. Huang E, Wiseman A, Okumura S, et al. Outcomes of preemptive kidney with or without subsequent pancreas transplant compared with preemptive simultaneous pancreas/kidney transplantation. Transplantation. 2011;92(10):1115‐1122. [DOI] [PubMed] [Google Scholar]
- 63. Wiseman AC, Huang E, Kamgar M, et al. The impact of pre‐transplant dialysis on simultaneous pancreas‐kidney versus living donor kidney transplant outcomes. Nephrol Dial Transplant. 2013;28(4):1047‐1058. [DOI] [PubMed] [Google Scholar]
- 64. Westphal SG, Langewisch ED, Robinson AM, et al. The impact of multi‐organ transplant allocation priority on wait‐listed kidney transplant candidates. Am J Transplant. 2021;21(6):2161‐2174. Epub ahead of print. PMID: 33140571. [DOI] [PubMed] [Google Scholar]
- 65. Asch WS, Bia MJ. New organ allocation system for combined liver‐kidney transplants and the availability of kidneys for transplant to patients with stage 4–5 CKD. Clin J Am Soc Nephrol. 2017;12(5):848‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pavlakis M, Khwaja K, Mandelbrot D, et al. Renal allograft failure predictors after PAK transplantation: results from the New England collaborative association of pancreas programs. Transplantation. 2010;89(11):1347‐1353. [DOI] [PubMed] [Google Scholar]
- 67. Browne S, Gill J, Dong J, et al. The impact of pancreas transplantation on kidney allograft survival. Am J Transplant. 2011;11(9):1951‐1958. [DOI] [PubMed] [Google Scholar]
- 68. Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725‐1730. [DOI] [PubMed] [Google Scholar]
- 69. Venstrom JM, McBride MA, Rother KI, et al. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. J Am Med Assoc. 2003;290(21):2817‐2823. [DOI] [PubMed] [Google Scholar]
- 70. Gruessner RWG, Sutherland DER, Kandaswamy R, et al. Over 500 solitary pancreas transplants in nonuremic patients with brittle diabetes mellitus. Transplantation. 2008;85(1):42‐47. [DOI] [PubMed] [Google Scholar]
- 71. Kiberd BA, Larson T. Estimating the benefits of solitary pancreas transplantation in nonuremic patients with type 1 diabetes mellitus: a theoretical analysis. Transplantation. 2000;70(7):1121‐1127. [DOI] [PubMed] [Google Scholar]
- 72. Boggi U, Vistoli F, Amorese G, et al. Long‐term (5 years) efficacy and safety of pancreas transplantation alone in type 1 diabetic patients. Transplantation. 2012;93(8):842‐846. [DOI] [PubMed] [Google Scholar]
- 73. Singh SK, Kim SJ, Smail N, et al. Outcomes of recipients with pancreas transplant alone who develop end‐stage renal disease. Am J Transplant. 2016;16(2):535‐540. [DOI] [PubMed] [Google Scholar]
- 74. Le Dinh H, DeRoover A, Coimbra C, et al. Evolution of native kidney function after pancreas transplantation alone. Transplant Proc. 2012;44(9):2829‐2833. [DOI] [PubMed] [Google Scholar]
- 75. Mazur MJ, Rea DJ, Griffin MD, et al. Decline in native renal function early after bladder‐drained pancreas transplantation alone. Transplantation. 2004;77(6):844‐849. [DOI] [PubMed] [Google Scholar]
- 76. Fioretto P, Steffes MW, Sutherland DER, et al. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339(2):69‐75. [DOI] [PubMed] [Google Scholar]
- 77. Fioretto P, Mauer SM, Goetz F, et al. Effects of pancreas transplantation on glomerular structure in insulin‐dependent diabetic patients with their own kidneys. Lancet. 1993;342(8881):1193‐1196. [DOI] [PubMed] [Google Scholar]
- 78. Kim YJ, Shin S, Han DJ, et al. Long‐term effects of pancreas transplantation on diabetic retinopathy and incidence and predictive risk factors for early worsening. Transplantation. 2018;102(1):e30‐e38. [DOI] [PubMed] [Google Scholar]
- 79. Navarro X, Sutherland DER, Kennedy WR. Long‐term effects of pancreatic transplantation on diabetic neuropathy. Ann Neurol. 1997;42(5):727‐736. [DOI] [PubMed] [Google Scholar]
- 80. Giannarelli R, Coppelli A, Sartini MS, et al. Pancreas transplant alone has beneficial effects on retinopathy in type 1 diabetic patients. Diabetologia. 2006;49(12):2977‐2982. [DOI] [PubMed] [Google Scholar]
- 81. Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high‐risk surgery. N Engl J Med. 2011;364:2128‐2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Barbas AS, Dib MJ, Rege AS, et al. The volume‐outcome relationship in deceased donor kidney transplantation and implications for regionalization. Ann Surg. 2018;267(6):1169‐1172. [DOI] [PubMed] [Google Scholar]
- 83. Macomber CW, Shaw JJ, Santry H, et al. Centre volume and resource consumption in liver transplantation. HPB. 2012;14(8):554‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shuhaiber JH, Moore J, Dyke DB. The effect of transplant center volume on survival after heart transplantation: a multicenter study. J Thorac Cardiovasc Surg. 2010;139(4):1064‐1069. [DOI] [PubMed] [Google Scholar]
- 85. Weiss ES, Allen JG, Meguid RA, et al. The impact of center volume on survival in lung transplantation: an analysis of more than 10,000 cases. Ann Thorac Surg. 2009;88(4):1062‐1070. [DOI] [PubMed] [Google Scholar]
- 86. Alhamad T, Malone AF, Brennan DC, et al. Transplant center volume and the risk of pancreas allograft failure. Transplantation. 2017;101(11):2757‐2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Macedo FIB, Jayanthi P, Mowzoon M, et al. The impact of surgeon volume on outcomes after pancreaticoduodenectomy: a meta‐analysis. J Gastrointest Surg. 2017;21(10):1723‐1731. [DOI] [PubMed] [Google Scholar]
- 88. Wood TW, Ross SB, Bowman TA, et al. High‐volume hospitals with high‐volume and low‐volume surgeons: is there a "field effect" for pancreaticoduodenectomy? Am Surg. 2016;82(5):407‐411. [PubMed] [Google Scholar]
- 89. Schmidt CM, Turrini O, Parikh P, et al. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single‐institution experience. Arch Surg. 2010;145(7):634‐640. [DOI] [PubMed] [Google Scholar]
- 90. Sutherland DER, Radosevich D, Gruessner R, et al. Pushing the envelope: living donor pancreas transplantation. Curr Opin Organ Transplant. 2012;17(1):106‐115. [DOI] [PubMed] [Google Scholar]
- 91. Boggi U, Amorese G, Marchetti P, et al. Segmental live donor pancreas transplantation: review and critique of rationale, outcomes, and current recommendations. Clin Transplant. 2011;25(1):4‐12. [DOI] [PubMed] [Google Scholar]
- 92. Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg. 1988;123:550‐553. [DOI] [PubMed] [Google Scholar]
- 93. Sutherland DE, Najarian JS. Conservation of the spleen with distal pancreatectomy. Arch Surg. 1988;123(12):1525. [DOI] [PubMed] [Google Scholar]
- 94. Ferrone CR, Konstantinidis IT, Sahani DV, et al. Twenty‐three years of the Warshaw operation for distal pancreatectomy with preservation of the spleen. Ann Surg. 2011;253(6):1136‐1139. [DOI] [PubMed] [Google Scholar]
- 95. Serrano OK, Cunha RD, Mettler T, et al. Sinistral portal hypertension after live segmental pancreas donation: a long‐term sequelae presenting with life‐threatening upper gastrointestinal hemorrhage. Transplant Proc. 2017;49(1):221‐224. [DOI] [PubMed] [Google Scholar]
- 96. Boggi U, Signori S, Vistoli F, et al. Laparoscopic robot‐assisted pancreas transplantation: first world experience. Transplantation. 2012;93(2):201‐206. [DOI] [PubMed] [Google Scholar]
- 97. Yeh CC, Spaggiari M, Tzvetanov I, et al. Robotic pancreas transplantation in a type 1 diabetic patient with morbid obesity: a case report. Medicine. 2017;96(6):e5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Spaggiari M, Tulla KA, Okoye O, et al. The utility of robotic assisted pancreas transplants ‐ a single center retrospective study. Transpl Int. 2019;32(11):1173‐1181. [DOI] [PubMed] [Google Scholar]
- 99. Tzvetanov IG, Spaggiari M, Tulla KA, et al. Robotic kidney transplantation in the obese patient: 10‐year experience from a single center. Am J Transplant. 2020;20(2):430‐440. [DOI] [PubMed] [Google Scholar]
- 100. Ahlawat R, Sood A, Jeong W, et al. Robotic kidney transplantation with regional hypothermia versus open kidney transplantation for patients with end‐stage renal disease: an ideal stage 2B study. J Urol. 2021;205(2):595‐602. [DOI] [PubMed] [Google Scholar]
- 101. Kauffmann EF, Napoli N, Cacace C, et al. Resection or repair of large peripancreatic arteries during robotic pancreatectomy. Updates Surg. 2020;72(1):145‐153. [DOI] [PubMed] [Google Scholar]
- 102. Kauffmann EF, Napoli N, Menonna F, et al. Robotic pancreatoduodenectomy with vascular resection. Langenbecks Arch Surg. 2016;401(8):1111‐1122. [DOI] [PubMed] [Google Scholar]
- 103. Boggi U, Del Chiaro M, Signori S, et al. Pancreas transplants from donors aged 45 years or older. Transplant Proc. 2005;37(2):1265‐1267. [DOI] [PubMed] [Google Scholar]
- 104. Boggi U, Del Chiaro M, Vistoli F, et al. Pancreas transplantation from marginal donors. Transplant Proc. 2004;36(3):566‐568. [DOI] [PubMed] [Google Scholar]
- 105. Schenker P, Wunsch A, Ertas N, et al. Long‐term results after simultaneous pancreas‐kidney transplantation using donors aged 45 years or older. Transplant Proc. 2008;40(4):923‐926. [DOI] [PubMed] [Google Scholar]
- 106. Hilling DE, Baranski AG, Haasnoot A, et al. Contribution of donor and recipient characteristics to short‐ and long‐term pancreas graft survival. Ann Transplant. 2012;17(4):28‐38. [DOI] [PubMed] [Google Scholar]
- 107. Fellmer PT, Pascher A, Kahl A, et al. Influence of donor‐ and recipient‐specific factors on the postoperative course after combined pancreas‐kidney transplantation. Langenbecks Arch Surg. 2010;395(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 108. Arenas‐Bonilla AJ, Campos‐Hernandez JP, Carrasco‐Valiente J, et al. Influence of donor and recipient ages in survival of simultaneous pancreas‐kidney transplantation. Transplant Proc. 2016;48:3033‐3036. [DOI] [PubMed] [Google Scholar]
- 109. Fernandez LA, Turgeon NA, Odorico JS, et al. Superior long‐term results of simultaneous pancreas‐kidney transplantation from pediatric donors. Am J Transplant. 2004;4(12):2093‐2101. [DOI] [PubMed] [Google Scholar]
- 110. Spaggiari M, Bissing M, Campara M, et al. Pancreas transplantation from pediatric donors: a united network for organ sharing registry analysis. Transplantation. 2017;101(10):2484‐2491. [DOI] [PubMed] [Google Scholar]
- 111. Spaggiari M, Di Bella C, Di Cocco P, et al. Pancreas transplantation from pediatric donors: a single‐center experience. Transplantation. 2018;102(10):1732‐1739. [DOI] [PubMed] [Google Scholar]
- 112. Chiari D, Bissolati M, Gazzetta PG, et al. Pancreas transplantation from very small pediatric donor using the "cephalic placement" technique: a case report. Transplant Proc. 2016;48(2):435‐437. [DOI] [PubMed] [Google Scholar]
- 113. Socci C, Orsenigo E, Santagostino I, et al. Pancreata from pediatric donors restore insulin independence in adult insulin‐dependent diabetes mellitus recipients. Transplant Proc. 2010;42(6):2068‐2070. [DOI] [PubMed] [Google Scholar]
- 114. Alhamad T, Malone AF, Lentine KL, et al. Selected mildly obese donors can be used safely in simultaneous pancreas and kidney transplantation. Transplantation. 2017;101(6):1159‐1166. [DOI] [PubMed] [Google Scholar]
- 115. Ito T, Gotoh M. Report from the Japan Registry of Pancreas Transplantation (2000–2012): outcomes of pancreas transplantation from marginal donors. Clin Transpl. 2013:53‐61. [PubMed] [Google Scholar]
- 116. Fridell JA, Mangus RS, Taber TE, et al. Growth of a nation part I: impact of organ donor obesity on whole‐organ pancreas transplantation. Clin Transplant. 2011;25(3):E225‐E232. [DOI] [PubMed] [Google Scholar]
- 117. Barlow AD, Hosgood SA, Nicholson ML. Current state of pancreas preservation and implications for DCD pancreas transplantation. Transplantation. 2013;95(12):1419‐1424. [DOI] [PubMed] [Google Scholar]
- 118. Shahrestani S, Webster AC, Lam VWT, et al. Outcomes from pancreatic transplantation in donation after cardiac death: a systematic review and meta‐analysis. Transplantation. 2017;101(1):122‐130. [DOI] [PubMed] [Google Scholar]
- 119. Fridell JA, Mangus RS, Thomas CM, et al. Donation after circulatory arrest in pancreas transplantation: a report of 10 cases. Transplant Proc. 2017;49(10):2310‐2314. [DOI] [PubMed] [Google Scholar]
- 120. Anderson PT, Aquil S, McLean K, et al. First Canadian experience with donation after cardiac death simultaneous pancreas and kidney transplants. Can J Surg. 2017;60(5):323‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Siskind E, Akerman M, Maloney C, et al. Pancreas transplantation from donors after cardiac death: an update of the UNOS database. Pancreas. 2014;43(4):544‐547. [DOI] [PubMed] [Google Scholar]
- 122. Leemkuil M, Leuvenink HGD, Pol RA. Pancreas transplantation from donors after circulatory death: an irrational reluctance? Curr Diab Rep. 2019;19(11):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kopp WH, Lam HD, Schaapherder AFM, et al. Pancreas transplantation with grafts from donors deceased after circulatory death: 5 years single‐center experience. Transplantation. 2018;102(2):333‐339. [DOI] [PubMed] [Google Scholar]
- 124. Romano A, Alsabeah K, Wilczek H, et al. Simultaneous pancreas‐kidney transplant from donors after brain death vs donors after circulatory death: a single‐center follow‐up study over 3 decades. Transplant Proc. 2019;51(3):845‐851. [DOI] [PubMed] [Google Scholar]
- 125. Boggi U, Vistoli F, Chiaro MD, et al. Pancreas preservation with University of Wisconsin and Celsior solutions: a single‐center, prospective, randomized pilot study. Transplantation. 2004;77(8):1186‐1190. [DOI] [PubMed] [Google Scholar]
- 126. Nicoluzzi J, Macri M, Fukushima J, et al. Celsior versus Wisconsin solution in pancreas transplantation. Transplant Proc. 2008;40(10):3305‐3307. [DOI] [PubMed] [Google Scholar]
- 127. Alonso D, Dunn TB, Rigley T, et al. Increased pancreatitis in allografts flushed with histidine‐tryptophan‐ketoglutarate solution: a cautionary tale. Am J Transplant. 2008;8(9):1942‐1945. [DOI] [PubMed] [Google Scholar]
- 128. Schneeberger S, Biebl M, Steurer W, et al. A prospective randomized multicenter trial comparing histidine‐tryptophane‐ketoglutarate versus University of Wisconsin perfusion solution in clinical pancreas transplantation. Transpl Int. 2009;22(2):217‐224. [DOI] [PubMed] [Google Scholar]
- 129. Fridell JA, Mangus RS, Powelson JA. Histidine‐tryptophan‐ketoglutarate for pancreas allograft preservation: the Indiana University experience. Am J Transplant. 2010;10(5):1284‐1289. [DOI] [PubMed] [Google Scholar]
- 130. Englesbe MJ, Moyer A, Kim DY, et al. Early pancreas transplant outcomes with histidine‐tryptophan‐ketoglutarate preservation: a multicenter study. Transplantation. 2006;82(1):136‐139. [DOI] [PubMed] [Google Scholar]
- 131. Becker T, Ringe B, Nyibata M, et al. Pancreas transplantation with histidine‐tryptophan‐ketoglutarate (HTK) solution and University of Wisconsin (UW) solution: is there a difference? JOP. 2007;8(3):304‐311. [PubMed] [Google Scholar]
- 132. Igreja MR, Wiederkehr JC, Wiederkehr BA, et al. Use of Georges Lopez Institute preservation solution IGL‐1 in pancreas transplantation: a series of 47 cases. Transplant Proc. 2018;50(3):702‐704. [DOI] [PubMed] [Google Scholar]
- 133. Niclauss N, Wojtusciszyn A, Morel P, et al. Comparative impact on islet isolation and transplant outcome of the preservation solutions Institut Georges Lopez‐1, University of Wisconsin, and Celsior. Transplantation. 2012;93(7):703‐708. [DOI] [PubMed] [Google Scholar]
- 134. Garca‐Gil FA, Fuentes‐Broto L, Albendea CD, et al. Evaluation of Institut Georges Lopez‐1 preservation solution in pig pancreas transplantation: a pilot study. Transplantation. 2014;97(9):901‐907. [DOI] [PubMed] [Google Scholar]
- 135. Squifflet J‐P. A quick technique for en bloc liver and pancreas procurement. Transpl Int. 1996;9(5):520‐521. [DOI] [PubMed] [Google Scholar]
- 136. Boggi U, Vistoli F, Del CM, et al. A simplified technique for the en bloc procurement of abdominal organs that is suitable for pancreas and small‐bowel transplantation. Surgery. 2004;135(6):629‐641. [DOI] [PubMed] [Google Scholar]
- 137. Hakim NS, Papalois VE. Successful procurement of 50 pancreatic grafts using a simple and fast technique. Int Surg. 1998;83(4):327‐329. [PubMed] [Google Scholar]
- 138. Fridell JA, Mangus RS, Hollinger EF, et al. No difference in transplant outcomes for local and import pancreas allografts. Transplantation. 2009;88(5):723‐728. [DOI] [PubMed] [Google Scholar]
- 139. Finger EB, Radosevich DM, Bland BJ, et al. Comparison of recipient outcomes following transplant from local versus imported pancreas donors. Am J Transplant. 2012;12(2):447‐457. [DOI] [PubMed] [Google Scholar]
- 140. Rudolph EN, Dunn TB, Sutherland DER, et al. Optimizing outcomes in pancreas transplantation: impact of organ preservation time. Clin Transplant. 2017;31(9):e13035. [DOI] [PubMed] [Google Scholar]
- 141. Humar A, Kandaswamy R, Drangstveit MB, et al. Prolonged preservation increases surgical complications after pancreas transplants. Surgery. 2000;127(5):545‐551. [DOI] [PubMed] [Google Scholar]
- 142. Leemkuil M, Lier G, Engelse MA, et al. Hypothermic oxygenated machine perfusion of the human donor pancreas. Transplant Direct. 2018;4(10):e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Barlow AD, Hamed MO, Mallon DH, et al. Use of ex vivo normothermic perfusion for quality assessment of discarded human donor pancreases. Am J Transplant. 2015;15(9):2475‐2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kuan KG, Wee MN, Chung WY, et al. A study of normothermic hemoperfusion of the porcine pancreas and kidney. Artif Organs. 2017;41(5):490‐495. [DOI] [PubMed] [Google Scholar]
- 145. Biglarnia A‐R, Nilsson BO, Nilsson T, et al. Prompt reversal of a severe complement activation by eculizumab in a patient undergoing intentional ABO‐incompatible pancreas and kidney transplantation. Transpl Int. 2011;24(8):e61‐66. [DOI] [PubMed] [Google Scholar]
- 146. Kenmochi T, Asano T, Maruyama M, et al. Living donor pancreas transplantation in Japan. J Hepatobiliary Pancreat Sci. 2010;17(2):101‐107. [DOI] [PubMed] [Google Scholar]
- 147. Heilman RL, Chakkera H, Mazur M, et al. Outcomes of simultaneous kidney‐pancreas transplantation with positive cross‐match. Transplant Proc. 2009;41(1):303‐306. [DOI] [PubMed] [Google Scholar]
- 148. Khwaja K, Wijkstrom M, Gruessner A, et al. Pancreas transplantation in crossmatch‐positive recipients. Clin Transplant. 2003;17(3):242‐248. [DOI] [PubMed] [Google Scholar]
- 149. Peltenburg HG, Tlebosch A, Van Den Berg‐Loonen PM, et al. A positive T cell crossmatch and accelerated acute rejection of a pancreas‐spleen allograft. Transplantation. 1992;53(1):226‐228. [PubMed] [Google Scholar]
- 150. Parajuli S, Alagusundaramoorthy S, Aziz F, et al. Outcomes of pancreas transplant recipients with de novo donor‐specific antibodies. Transplantation. 2019;103(2):435‐440. [DOI] [PubMed] [Google Scholar]
- 151. Cantarovich D, De Amicis S, Akl A, et al. Posttransplant donor‐specific anti‐HLA antibodies negatively impact pancreas transplantation outcome. Am J Transplant. 2011;11(12):2737‐2746. [DOI] [PubMed] [Google Scholar]
- 152. Mittal S, Page SL, Friend PJ, Sharples EJ, Fuggle SV. De novo donor‐specific HLA antibodies: biomarkers of pancreas transplant failure. Am J Transplant. 2014;14(7):1664‐1671. [DOI] [PubMed] [Google Scholar]
- 153. Malheiro J, Martins LS, Tafulo S, et al. Impact of de novo donor‐specific anti‐HLA antibodies on grafts outcomes in simultaneous pancreas‐kidney transplantation. Transpl Int. 2016;29(2):173‐183. [DOI] [PubMed] [Google Scholar]
- 154. Mujtaba MA, Fridell JA, Higgins N, et al. Early findings of prospective anti‐HLA donor specific antibodies monitoring study in pancreas transplantation: Indiana University Health Experience. Clin Transplant. 2012;26(5):e492‐e499. [DOI] [PubMed] [Google Scholar]
- 155. Berney T, Malaise J, Morel P, et al. Impact of HLA matching on the outcome of simultaneous pancreas‐kidney transplantation. Nephrol Dial Transplant. 2005;20(Suppl. 2):ii48‐53, ii62. [DOI] [PubMed] [Google Scholar]
- 156. Rudolph EN, Dunn TB, Mauer D, et al. HLA‐A, ‐B, ‐C, ‐DR, and ‐DQ matching in pancreas transplantation: effect on graft rejection and survival. Am J Transplant. 2016;16(8):2401‐2412. [DOI] [PubMed] [Google Scholar]
- 157. Lo A, Stratta RJ, Alloway RR, et al. A multicenter analysis of the significance of HLA matching on outcomes after kidney‐pancreas transplantation. Transplant Proc. 2005;37(2):1289‐1290. [DOI] [PubMed] [Google Scholar]
- 158. Gruessner AC, Sutherland DER, Gruessner RWG. Matching in pancreas transplantation‐a registry analysis. Transplant Proc. 2001;33(1–2):1665‐1666. [DOI] [PubMed] [Google Scholar]
- 159. Mancini MJ, Connors JF, Wang XQ, et al. HLA matching for simultaneous pancreas kidney transplantation in the United States: a multivariable analysis of the UNOS data. Clin Nephrol. 2002;57(1):27‐37. [DOI] [PubMed] [Google Scholar]
- 160. Vendrame F, Hopfner YY, Diamantopoulos S, et al. Risk factors for type 1 diabetes recurrence in immunosuppressed recipients of simultaneous pancreas‐kidney transplants. Am J Transplant. 2016;16(1):235‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Boggi U, Vistoli F, Amorese G, et al. Results of pancreas transplantation alone with special attention to native kidney function and proteinuria in type 1 diabetes patients. Rev Diabet Stud. 2011;8(2):259‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Chatzizacharias NA, Vaidya A, Sinha S, et al. Risk analysis for deterioration of renal function after pancreas alone transplant. Clin Transplant. 2012;26(3):387‐392. [DOI] [PubMed] [Google Scholar]
- 163. Shin S, Jung CH, Choi JY, et al. Long‐term effects of pancreas transplant alone on nephropathy in type 1 diabetic patients with optimal renal function. PLoS One. 2018;13(1):e0191421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Coppelli A, Giannarelli R, Vistoli F, et al. The beneficial effects of pancreas transplant alone on diabetic nephropathy. Diabetes Care. 2005;28(6):1366‐1370. [DOI] [PubMed] [Google Scholar]
- 165. Coppelli A, Giannarelli R, Boggi U, et al. Disappearance of nephrotic syndrome in type 1 diabetic patients following pancreas transplant alone. Transplantation. 2006;81(7):1067‐1068. [DOI] [PubMed] [Google Scholar]
- 166. Kennedy WR, Navarro X, Goetz FC, et al. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med. 1990;322(15):1031‐1037. [DOI] [PubMed] [Google Scholar]
- 167. Occhipinti M, Rondinini L, Mariotti R, et al. Amelioration of cardiac morphology and function in type 1 diabetic patients with sustained success of pancreas transplant alone. Diabetes Care. 2014;37(8):171‐172. [DOI] [PubMed] [Google Scholar]
- 168. Chatzizacharias NA, Vaidya A, Sinha S, et al. Renal function in type 1 diabetics one year after successful pancreas transplantation. Clin Transplant. 2011;25(5):e509‐e515. [DOI] [PubMed] [Google Scholar]
- 169. Gruessner AC, Sutherland DER, Dunn DL, et al. Pancreas after kidney transplants in posturemic patients with type I diabetes mellitus. J Am Soc Nephrol. 2001;12(11):2490‐2499. [DOI] [PubMed] [Google Scholar]
- 170. Luan FL, Kommareddi M, Cibrik DM, et al. The time interval between kidney and pancreas transplantation and the clinical outcomes of pancreas after kidney transplantation. Clin Transplant. 2012;26(3):403‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Gruessner AC, Gruessner RWG. Pancreas transplantation of US and non‐US cases from 2005 to 2014 as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud. 2016;13(1):35‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Becker BN, Rush SH, Dykstra DM, et al. Preemptive transplantation for patients with diabetes‐related kidney disease. Arch Intern Med. 2006;166(1):44‐48. [DOI] [PubMed] [Google Scholar]
- 173. Pruijm MT, de Fijter HJW, Doxiadis II, et al. Preemptive versus nonpreemptive simultaneous pancreas‐kidney transplantation: a single‐center, long‐term, follow‐up study. Transplantation. 2006;81(8):1119‐1124. [DOI] [PubMed] [Google Scholar]
- 174. Grochowiecki T, Szmidt J, Gałązka Z, et al. Comparison of 1‐year patient and graft survival rates between preemptive and dialysed simultaneous pancreas and kidney transplant recipients. Transplant Proc. 2006;38(1):261‐262. [DOI] [PubMed] [Google Scholar]
- 175. Bumgardner GL, Henry ML, Elkhammas E, et al. Obesity as a risk factor after combined pancreas/kidney transplantation. Transplantation. 1995;60(12):1426‐1430. [DOI] [PubMed] [Google Scholar]
- 176. Rogers J, Chavin KD, Baliga PK, et al. Influence of mild obesity on outcome of simultaneous pancreas and kidney transplantation. J Gastrointest Surg. 2003;7(8):1096‐1101. [DOI] [PubMed] [Google Scholar]
- 177. Sampaio MS, Reddy PN, Kuo HT, et al. Obesity was associated with inferior outcomes in simultaneous pancreas kidney transplant. Transplantation. 2010;89(9):1117‐1125. [DOI] [PubMed] [Google Scholar]
- 178. Bédat B, Niclauss N, Jannot AS, et al. Impact of recipient body mass index on short‐term and long‐term survival of pancreatic grafts. Transplantation. 2015;99(1):94‐99. [DOI] [PubMed] [Google Scholar]
- 179. Hanish SI, Petersen RP, Collins BH, et al. Obesity predicts increased overall complications following pancreas transplantation. Transplant Proc. 2005;37(8):3564‐3566. [DOI] [PubMed] [Google Scholar]
- 180. Beach KW, Strandness DE Jr. Arteriosclerosis obliterans and associated risk factors in insulin‐dependent and non‐insulin‐dependent diabetes. Diabetes. 1980;29(11):882‐888. [DOI] [PubMed] [Google Scholar]
- 181. Nakamura S, Sasaki O, Nakahama H, et al. Clinical characteristics and survival in end‐stage renal disease patients with arteriosclerosis obliterans. Am J Nephrol. 2002;22(5–6):422‐428. [DOI] [PubMed] [Google Scholar]
- 182. Sucher R, Rademacher S, Jahn N, et al. Effects of simultaneous pancreas‐kidney transplantation and kidney transplantation alone on the outcome of peripheral vascular diseases. BMC Nephrol. 2019;20(1):453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Yiannoullou P, Summers A, Goh SC, et al. Major adverse cardiovascular events following simultaneous pancreas and kidney transplantation in the United Kingdom. Diabetes Care. 2019;42(4):665‐673. [DOI] [PubMed] [Google Scholar]
- 184. Mangus RS, Powelson J, Kinsella SB, et al. Pretransplant coronary artery disease associated with worse clinical outcomes in pancreas transplantation. Clin Transplant. 2013;27(4):E442‐E447. [DOI] [PubMed] [Google Scholar]
- 185. Witczak BJ, Jenssen T, Endresen K, et al. Risk factors for mortality in diabetic nephropathy patients accepted for transplantation. Transplantation. 2007;84(3):356‐361. [DOI] [PubMed] [Google Scholar]
- 186. Corry RJ, Chakrabarti P, Shapiro R, et al. Comparison of enteric versus bladder drainage in pancreas transplantation. Transplant Proc. 2001;33(1–2):1647‐1651. [DOI] [PubMed] [Google Scholar]
- 187. Cattral MS, Bigam DL, Hemming AW, et al. Portal venous and enteric exocrine drainage versus systemic venous and bladder exocrine drainage of pancreas grafts: clinical outcome of 40 consecutive transplant recipients. Ann Surg. 2000;232(5):688‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Stratta RJ, Gaber AO, Shokouh‐Amiri MH, et al. A prospective comparison of systemic bladder versus portal‐enteric drainage in vascularized pancreas transplantation. Surgery. 2000;127:217‐226. [DOI] [PubMed] [Google Scholar]
- 189. Adamec M, Janouŝek L, Lipár K, et al. A prospective comparison of bladder versus enteric drainage in vascularized pancreas transplantation. Transplant Proc. 2004;36(5):1524‐1525. [DOI] [PubMed] [Google Scholar]
- 190. Arjona‐Sánchez A, Muñoz‐Casares FC, Ruiz‐Rabelo J, et al. Consolidation of enteric drainage for exocrine secretions in simultaneous pancreas‐kidney transplant. Transplant Proc. 2010;42(5):1815‐1818. [DOI] [PubMed] [Google Scholar]
- 191. Newell KA, Bruce DS, Cronin DC, et al. Comparison of pancreas transplantation with portal venous and enteric exocrine drainage to the standard technique utilizing bladder drainage of exocrine secretions. Transplantation. 1996;62:1353‐1356. [DOI] [PubMed] [Google Scholar]
- 192. Bloom RD, Olivares M, Rehman L, et al. Long‐term pancreas allograft outcome in simultaneous pancreaskidney transplantation: a comparison of enteric and bladder drainage. Transplantation. 1997;64:1689‐1695. [DOI] [PubMed] [Google Scholar]
- 193. Douzdjian V, Baliga PK, Rajagopalan PR. Primary enteric drainage of the pancreas revisited: a viable alternative to bladder drainage in simultaneous pancreas‐kidney transplants. Transplant Proc. 1998;30(2):440. [DOI] [PubMed] [Google Scholar]
- 194. Sansalone CV, Aseni P, Follini ML, et al. Enteric versus bladder drainage in pancreas transplantation: initial experience at Niguarda Hospital, Milan. Transplant Proc. 1998;30(2):251‐252. [DOI] [PubMed] [Google Scholar]
- 195. Pirsch JD, Odorico JS, D’Alessandro AM, et al. Posttransplant infection in enteric versus bladder‐drained simultaneous pancreas‐kidney transplant recipients. Transplantation. 1998;66:1746‐1750. [DOI] [PubMed] [Google Scholar]
- 196. Sugitani A, Gritsch HA, Shapiro R, et al. Surgical complications in 123 consecutive pancreas transplant recipients: comparison of bladder and enteric drainage. Transplant Proc. 1998;30(2):293‐294. [DOI] [PubMed] [Google Scholar]
- 197. Lo A, Stratta RJ, Hathaway DK, et al. Long‐term outcomes in simultaneous kidney‐pancreas transplant recipients with portal‐enteric versus systemic‐bladder drainage. Am J Kidney Dis. 2001;38(1):132‐143. [DOI] [PubMed] [Google Scholar]
- 198. Kaufman DB, Leventhal JR, Gallon LG, et al. Technical and immunologic progress in simultaneous pancreas‐kidney transplantation. Surgery. 2002;132(4):545‐553. [DOI] [PubMed] [Google Scholar]
- 199. Friedrich J, Charpentier K, Marsh CL, et al. Outcomes with the selective use of enteric exocrine drainage in pancreas transplantation. Transplant Proc. 2004;36:3101‐3104. [DOI] [PubMed] [Google Scholar]
- 200. Orsenigo E, Florina P, Cristallo M, et al. Outcome of simultaneous kidney pancreas transplantation: a single center analysis. Transplant Proc. 2004;36(5):1519‐1523. [DOI] [PubMed] [Google Scholar]
- 201. Stratta RJ, Alloway RR, Lo A, et al. Does surgical technique influence outcomes after simultaneous kidney‐pancreas transplantation? Transplant Proc. 2004;36:1076‐1077. [DOI] [PubMed] [Google Scholar]
- 202. Sansalone CV, Maione G, Aseni P, et al. Surgical complications are the main cause of pancreatic allograft loss in pancreas‐kidney transplant recipients. Transplant Proc. 2005;37(6):2651‐2653. [DOI] [PubMed] [Google Scholar]
- 203. Monroy‐Cuadros M, Salazar A, Yilmaz S, et al. Bladder vs enteric drainage in simultaneous pancreas‐kidney transplantation. Nephrol Dial Transplant. 2006;21(2):483‐487. [DOI] [PubMed] [Google Scholar]
- 204. Kave B, Yii M, Bell R, et al. Initial Australasian experience with portal‐enteric drainage in simultaneous pancreas‐kidney transplantation. ANZ J Surg. 2010;80(10):722‐727. [DOI] [PubMed] [Google Scholar]
- 205. Jiménez‐Romero C, Manrique A, Morales JM, et al. Conversion from bladder to enteric drainage for complications after pancreas transplantation. Transplant Proc. 2009;41(6):2469‐2471. [DOI] [PubMed] [Google Scholar]
- 206. Adler JT, Zaborek N, Redfield RR 3rd, Kaufman DB, Odorico JS, Sollinger HW. Enteric conversion after bladder‐drained pancreas transplantation is not associated with worse allograft survival. Am J Transplant. 2019;19(9):2543‐2549. [DOI] [PubMed] [Google Scholar]
- 207. Riad SM, Keys DO, Jackson S, et al. Enteric conversion of bladder‐drained pancreas as a predictor of outcomes in almost 600 recipients at a single center. Transplant Direct. 2020;6(5):e550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Kuo PC, Johnson LB, Schweitzer EJ, et al. Simultaneous pancreas/kidney transplantation–a comparison of enteric and bladder drainage of exocrine pancreatic secretions. Transplantation. 1997;63(2):238‐243. [DOI] [PubMed] [Google Scholar]
- 209. Pearson TC, Santamaria PJ, Routenberg KL, et al. Drainage of the exocrine pancreas in clinical transplantation: comparison of bladder versus enteric drainage in a consecutive series. Clin Transplant. 1997;11:201‐205. [PubMed] [Google Scholar]
- 210. Sollinger HW, Odorico JS, Knechtle SJ, et al. Experience with 500 simultaneous pancreas‐kidney transplants. Ann Surg. 1998;228:284‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Feitosa Tajra LC, Dawhara M, Benchaib M, et al. Effect of the surgical technique on long‐term outcome of pancreas transplantation. Transpl Int. 1998;11(4):295‐300. [DOI] [PubMed] [Google Scholar]
- 212. Orsenigo E, Cristallo M, Socci C, et al. Urological complications after simultaneous renal and pancreatic transplantation. Eur J Surg. 2002;168(11):609‐613. [DOI] [PubMed] [Google Scholar]
- 213. Medina Polo J, Morales JM, Blanco M, et al. Urological complications after simultaneous pancreas‐kidney transplantation. Transplant Proc. 2009;41:2457‐2459. [DOI] [PubMed] [Google Scholar]
- 214. Wai PY, Sollinger HW. Long‐term outcomes after simultaneous pancreas‐kidney transplant. Curr Opin Organ Transplant. 2011;16(1):128‐134. [DOI] [PubMed] [Google Scholar]
- 215. Herrero‐Martínez JM, Lumbreras C, Manrique A, et al. Epidemiology, risk factors and impact on long‐term pancreatic function of infection following pancreas‐kidney transplantation. Clin Microbiol Infect. 2013;19(12):1132‐1139. [DOI] [PubMed] [Google Scholar]
- 216. Byrne M, Singh A, Mowbray CA, et al. Bladder‐drained pancreas transplantation: urothelial innate defenses and urinary tract infection susceptibility. J Surg Res. 2019;235:288‐297. [DOI] [PubMed] [Google Scholar]
- 217. Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233(4):463‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Sollinger HW, Stratta RJ, D'Alessandro AM, et al. Experience with simultaneous pancreas‐kidney transplantation. Ann Surg. 1988;208(4):475‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Vrakas G, Arantes RM, Gerlach U, et al. Solitary pancreas transplantation: a review of the UK experience over a period of 10 yr. Clin Transplant. 2015;29(12):1195‐1202. [DOI] [PubMed] [Google Scholar]
- 220. Hummel R, Langer M, Wolters HH, et al. Exocrine drainage into the duodenum: a novel technique for pancreas transplantation. Transpl Int. 2008;21(2):178‐181. [DOI] [PubMed] [Google Scholar]
- 221. Horneland R, Paulsen V, Lindahl JP, et al. Pancreas transplantation with enteroanastomosis to native duodenum poses technical challenges– but offers improved endoscopic access for scheduled biopsies and therapeutic interventions. Am J Transplant. 2015;15:242‐250. [DOI] [PubMed] [Google Scholar]
- 222. Khubutia M, Pinchuk A, Dmitriev I, et al. Simultaneous pancreas‐kidney transplantation with duodeno‐duodenal anastomosis. Transplant Proc. 2014;46:1905‐1909. [DOI] [PubMed] [Google Scholar]
- 223. Lindahl JP, Horneland R, Nordheim E, et al. Outcomes in pancreas transplantation with exocrine drainage through a duodenoduodenostomy versus duodenojejunostomy. Am J Transplant. 2018;18(1):154‐162. [DOI] [PubMed] [Google Scholar]
- 224. Walter M, Jazra M, Kykalos S, et al. 125 Cases of duodenoduodenostomy in pancreas transplantation: a single‐centre experience of an alternative enteric drainage. Transpl Int. 2014;27:805‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Gunasekaran G, Wee A, Rabets J, et al. Duodenoduodenostomy in pancreas transplantation. Clin Transplant. 2012;26:550‐557. [DOI] [PubMed] [Google Scholar]
- 226. Nakhleh RE, Sutherland DE, Tzardis P, et al. Correlation of rejection of the duodenum with rejection of the pancreas in a pig model of pancreaticoduodenal transplantation. Transplantation. 1993;56(6):1353‐1356. [DOI] [PubMed] [Google Scholar]
- 227. Nakhleh RE, Benedetti E, Gruessner A, et al. Cystoscopic biopsies in pancreaticoduodenal transplantation. Are duodenal biopsies indicative of pancreas dysfunction? Transplantation. 1995;60(6):541‐546. [DOI] [PubMed] [Google Scholar]
- 228. Nordheim E, Horneland R, Aandahl EM, et al. Pancreas transplant rejection episodes are not revealed by biopsies of the donor duodenum in a prospective study with paired biopsies. Am J Transplant. 2018;18(5):1256‐1261. [DOI] [PubMed] [Google Scholar]
- 229. Ollinger R, Margreiter C, Bösmüller C, et al. Evolution of pancreas transplantation: long‐term results and perspectives from a high‐volume center. Ann Surg. 2012;256:780‐786. [DOI] [PubMed] [Google Scholar]
- 230. Dawahra M, Petruzzo P, Lefrançois N, et al. Portal drainage of pancreas allograft: surgical complications and graft survival. Transplant Proc. 2002;34(3):817‐818. [DOI] [PubMed] [Google Scholar]
- 231. Bazerbachi F, Selzner M, Marquez MA, et al. Portal venous versus systemic venous drainage of pancreas grafts: impact on long‐term results. Am J Transplant. 2012;12(1):226‐232. [DOI] [PubMed] [Google Scholar]
- 232. Quintela J, Aguirrezabalaga J, Alonso A, et al. Portal and systemic venous drainage in pancreas and kidney‐pancreas transplantation: early surgical complications and outcomes. Transplant Proc. 2009;41:2460‐2462. [DOI] [PubMed] [Google Scholar]
- 233. Petruzzo P, Lefrancois N, Berthillot C, et al. Impact of pancreatic venous drainage site on long‐term patient and graft outcome in simultaneous pancreas‐kidney transplantation. Clin Transplant. 2008;22(1):107‐112. [DOI] [PubMed] [Google Scholar]
- 234. Alonso A, Fernández C, Cillero S, et al. Effects of portal versus systemic venous drainage in pancreas and kidney‐pancreas transplantation. Transplant Proc. 2007;39(7):2335‐2337. [DOI] [PubMed] [Google Scholar]
- 235. Stratta RJ, Shokouh‐Amiri MH, Egidi MF, et al. A prospective comparison of simultaneous kidney‐pancreas transplantation with systemic‐enteric versus portal‐enteric drainage. Ann Surg. 2001;233(6):740‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Troppmann C, Gjertson DW, Cecka JM, et al. Impact of portal venous pancreas graft drainage on kidney graft outcome in simultaneous pancreas‐kidney recipients reported to UNOS. Am J Transplant. 2004;4(4):544‐553. [DOI] [PubMed] [Google Scholar]
- 237. Föger B, Königsrainer A, Palos G, et al. Effect of pancreas transplantation on lipoprotein lipase, postprandial lipemia, and HDL cholesterol. Transplantation. 1994;58(8):899‐904. [DOI] [PubMed] [Google Scholar]
- 238. Petruzzo P, Laville M, Badet L, et al. Effect of venous drainage site on insulin action after simultaneous pancreas‐kidney transplantation. Transplantation. 2004;77(12):1875‐1879. [DOI] [PubMed] [Google Scholar]
- 239. Petruzzo P, Badet L, Lefrançois N, et al. Metabolic consequences of pancreatic systemic or portal venous drainage in simultaneous pancreas‐kidney transplant recipients. Diabet Med. 2006;23(6):654‐659. [DOI] [PubMed] [Google Scholar]
- 240. Bak MI, Grochowiecki T, Gałazka Z, et al. Proinsulinemia in simultaneous pancreas and kidney transplant recipients. Transplant Proc. 2006;38(1):280‐281. [DOI] [PubMed] [Google Scholar]
- 241. Havrdova T, Boucek P, Jedinakova T, et al. Portal versus systemic venous drainage of the pancreatic graft: the effect on glucose metabolism in pancreas and kidney transplant recipients. Transplant Proc. 2014;46(6):1910‐1912. [DOI] [PubMed] [Google Scholar]
- 242. Frystyk J, Ritzel RA, Maubach J, et al. Comparison of pancreas‐transplanted type 1 diabetic patients with portal‐venous versus systemic‐venous graft drainage: impact on glucose regulatory hormones and the growth hormone/insulin‐like growth factor‐I axis. J Clin Endocrinol Metab. 2008;93(5):1758‐1766. [DOI] [PubMed] [Google Scholar]
- 243. Carpentier A, Patterson BW, Uffelman KD, et al. The effect of systemic versus portal insulin delivery in pancreas transplantation on insulin action and VLDL metabolism. Diabetes. 2001;50(6):1402‐1413. [DOI] [PubMed] [Google Scholar]
- 244. Bagdade JD, Ritter MC, Kitabchi AE, et al. Differing effects of pancreas‐kidney transplantation with systemic versus portal venous drainage on cholesteryl ester transfer in IDDM subjects. Diabetes Care. 1996;19(10):1108‐1112. [DOI] [PubMed] [Google Scholar]
- 245. Boggi U, Vistoli F, Signori S, et al. A technique for retroperitoneal pancreas transplantation with portal‐enteric drainage. Transplantation. 2005;79(9):1137‐1142. [DOI] [PubMed] [Google Scholar]
- 246. Ferrer J, Molina V, Rull R, et al. Pancreas transplantation: advantages of a retroperitoneal graft position. Cir Esp. 2017;95(9):513‐520. [DOI] [PubMed] [Google Scholar]
- 247. Fridell JA, Mangus RS, Chen JM, et al. Steroid‐free three‐drug maintenance regimen for pancreas transplant alone: comparison of induction with rabbit antithymocyte globulin +/− rituximab. Am J Transplant. 2018;18(12):3000‐3006. [DOI] [PubMed] [Google Scholar]
- 248. Muthusamy ASR, Vaidya AC, Sinha S, et al. Alemtuzumab induction and steroid‐free maintenance immunosuppression in pancreas transplantation. Am J Transplant. 2008;8(10):2126‐2131. [DOI] [PubMed] [Google Scholar]
- 249. Uemura T, Ramprasad V, Matsushima K, et al. Single dose of alemtuzumab induction with steroid‐free maintenance immunosuppression in pancreas transplantation. Transplantation. 2011;92(6):678‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Rajab A, Pelletier RP, Ferguson RM, et al. Steroid‐free maintenance immunosuppression with rapamune and low‐dose neoral in pancreas transplant recipients. Transplantation. 2007;84(9):1131‐1137. [DOI] [PubMed] [Google Scholar]
- 251. Axelrod D, Leventhal JR, Gallon LG, et al. Reduction of CMV disease with steroid‐free immunosuppresssion in simultaneous pancreas‐kidney transplant recipients. Am J Transplant. 2005;5(6):1423‐1429. [DOI] [PubMed] [Google Scholar]
- 252. Gruessner RWG, Kandaswamy R, Humar A, et al. Calcineurin inhibitor‐ and steroid‐free immunosuppression in pancreas‐kidney and solitary pancreas transplantation. Transplantation. 2005;79(9):1184‐1189. [DOI] [PubMed] [Google Scholar]
- 253. Thomusch O, Wiesener M, Opgenoorth M, et al. Rabbit‐ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (Harmony): an open‐label, multicentre, randomised controlled trial. Lancet. 2016;388(10063):3006‐3016. [DOI] [PubMed] [Google Scholar]
- 254. Kaufman DB, Leventhal JR, Koffron AJ, et al. A prospective study of rapid corticosteroid elimination in simultaneous pancreas‐kidney transplantation: comparison of two maintenance immunosuppression protocols: Tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus. Transplantation. 2002;73(2):169‐177. [DOI] [PubMed] [Google Scholar]
- 255. Reddy KS, Devarapalli Y, Mazur M, et al. Alemtuzumab with rapid steroid taper in simultaneous kidney and pancreas transplantation: comparison to induction with antithymocyte globulin. Transplant Proc. 2010;42(6):2006‐2008. [DOI] [PubMed] [Google Scholar]
- 256. Knight RJ, Podder H, Kerman RH, et al. Comparing an early corticosteroid/late calcineurin‐free immunosuppression protocol to a sirolimus‐, cyclosporine A‐, and prednisone‐based regimen for pancreas‐kidney transplantation. Transplantation. 2010;89(6):727‐732. [DOI] [PubMed] [Google Scholar]
- 257. Aoun M, Eschewege P, Hamoudi Y, et al. Very early steroid withdrawal in simultaneous pancreas‐kidney transplants. Nephrol Dial Transplant. 2007;22(3):899‐905. [DOI] [PubMed] [Google Scholar]
- 258. Montero N, Webster AC, Royuela A, et al. Steroid avoidance or withdrawal for pancreas and pancreas with kidney transplant recipients. Cochrane Database Syst Rev. 2014;2014(9):CD007669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Luzi L, Picena Sereni L, Battezzati A, et al. Metabolic effects of a corticosteroid‐free immunosuppressive regimen in recipients of pancreatic transplant. Transplantation. 2003;75(12):2018‐2023. [DOI] [PubMed] [Google Scholar]
- 260. Cantarovich D, Karam G, Hourmant M, et al. Steroid avoidance versus steroid withdrawal after simultaneous pancreas‐kidney transplantation. Am J Transplant. 2005;5(6):1332‐1338. [DOI] [PubMed] [Google Scholar]
- 261. Jordan ML, Chakrabarti P, Luke P, et al. Results of pancreas transplantation after steroid withdrawal under tacrolimus immunosuppression. Transplantation. 2000;69(2):265‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Gruessner R, Sutherland D, Parr E, et al. A prospective, randomized, open‐label study of steroid withdrawal in pancreas transplantation‐a preliminary report with 6‐month follow‐up. Transplant Proc. 2001;33(1–2):1663‐1664. [DOI] [PubMed] [Google Scholar]
- 263. Kaufman DB, Iii GWB, Bruce DS, et al. Prospective, randomized, multi‐center trial of antibody induction therapy in simultaneous pancreas‐kidney transplantation. Am J Transplant. 2003;3(7):855‐864. [DOI] [PubMed] [Google Scholar]
- 264. Stratta RJ, Alloway RR, Lo A, et al. Two‐dose daclizumab regimen in simultaneous kidney‐pancreas transplant recipients: primary endpoint analysis of a multicenter, randomized study. Transplantation. 2003;75(8):1260‐1266. [DOI] [PubMed] [Google Scholar]
- 265. Thai NL, Khan A, Tom K, et al. Alemtuzumab induction and tacrolimus monotherapy in pancreas transplantation: one‐ and two‐year outcomes. Transplantation. 2006;82(12):1621‐1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Thiyagarajan UM, Ponnuswamy A, Bagul A. Thymoglobulin and its use in renal transplantation: a review. Am J Nephrol. 2013;37(6):586‐601. [DOI] [PubMed] [Google Scholar]
- 267. Bechstein WO, Malaise J, Saudek F, et al. Efficacy and safety of tacrolimus compared with cyclosporine microemulsion in primary simultaneous pancreas‐kidney transplantation: 1‐year results of a large multicenter trial. Transplantation. 2004;77(8):1221‐1228. [DOI] [PubMed] [Google Scholar]
- 268. Boggi U, Vistoli F, Del Chiaro M, et al. Neoral versus prograf in simultaneous pancreas‐kidney transplantation with portal venous drainage: three‐year results of a single‐center, open‐label, prospective, randomized pilot study. Transplant Proc. 2005;37(6):2641‐2643. [DOI] [PubMed] [Google Scholar]
- 269. Prieto M, Sutherland DE, Fernandez‐Cruz L, et al. Experimental and clinical experience with urine amylase monitoring for early diagnosis of rejection in pancreas transplantation. Transplantation. 1987;43(1):73‐79. [DOI] [PubMed] [Google Scholar]
- 270. Cattral M, Luke S, Knauer MJ, et al. Randomized open‐label crossover assessment of Prograf vs Advagraf on immunosuppressant pharmacokinetics and pharmacodynamics in simultaneous pancreas‐kidney patients. Clin Transplant. 2018;32(2):e13180. [DOI] [PubMed] [Google Scholar]
- 271. Falconer SJ, Jansen C, Oniscu GC. Conversion from twice‐daily to once‐daily tacrolimus in simultaneous pancreas‐kidney transplant patients. Transplant Proc. 2014;46(5):1458‐1462. [DOI] [PubMed] [Google Scholar]
- 272. Torabi J, Campbell A, Ajaimy M, et al. Utilization of LCP‐tacrolimus (Envarsus XR) in simultaneous pancreas and kidney transplant recipients. Ochsner J. 2018;18(3):190‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Kerstenetzky L, Descourouez JL, Jorgenson MR, et al. A single‐center experience with tacrolimus LCP (Envarsus XR) in pancreas transplant recipients. Ann Pharmacother. 2018;52(4):392‐396. [DOI] [PubMed] [Google Scholar]
- 274. Vincenti F, Stock P. De novo use of sirolimus in immunosuppression regimens in kidney and kidney‐pancreas transplantation at the University of California, San Francisco. Transplant Proc. 2003;35(3 Suppl):183S‐186S. [DOI] [PubMed] [Google Scholar]
- 275. Knight RJ, Kerman RH, Mckissick E, et al. Selective corticosteroid and calcineurin‐inhibitor withdrawal after pancreas‐kidney transplantation utilizing thymoglobulin induction and sirolimus maintenance therapy. Clin Transplant. 2008;22(5):645‐650. [DOI] [PubMed] [Google Scholar]
- 276. Knight RJ, Kerman RH, Zela S, et al. Thymoglobulin, sirolimus, and reduced‐dose cyclosporine provides excellent rejection prophylaxis for pancreas transplantation. Transplantation. 2003;75(8):1301‐1306. [DOI] [PubMed] [Google Scholar]
- 277. Cantarovich D, Kervella D, Karam G, et al. Tacrolimus‐ versus sirolimus‐based immunosuppression after simultaneous pancreas and kidney transplantation: 5‐year results of a randomized trial. Am J Transplant. 2020;20(6):1679‐1690. [DOI] [PubMed] [Google Scholar]
- 278. Stock PG, Mannon RB, Armstrong B, et al. Challenges of calcineurin inhibitor withdrawal following combined pancreas and kidney transplantation: results of a prospective, randomized clinical trial. Am J Transplant. 2020;20(6):1668‐1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Merion RM, Henry ML, Melzer JS, et al. Randomized, prospective trial of mycophenolate mofetil versus azathioprine for prevention of acute renal allograft rejection after simultaneous kidney‐pancreas transplantation. Transplantation. 2000;70(1):105‐111. [PubMed] [Google Scholar]
- 280. Stegall MD, Simon M, Wachs ME, et al. Mycophenolate mofetil decreases rejection in simultaneous pancreas‐ kidney transplantation when combined with tacrolimus or cyclosporine. Transplantation. 1997;64(12):1695‐1700. [DOI] [PubMed] [Google Scholar]
- 281. Demartines N, Schiesser M, Clavien PA. An evidence‐based analysis of simultaneous pancreas‐kidney and pancreas transplantation alone. Am J Transplant. 2005;5(11):2688‐2697. [DOI] [PubMed] [Google Scholar]
- 282. Odorico JS, Pirsch JD, Knechtle SJ, et al. A study comparing mycophenolate mofetil to azathioprine in simultaneous pancreas‐kidney transplantation. Transplantation. 1998;66(12):1751‐1759. [DOI] [PubMed] [Google Scholar]
- 283. Oh JM, Wiland AM, Klassen DK, et al. Comparison of azathioprine and mycophenolate mofetil for the prevention of acute rejection in recipients of pancreas transplantation. J Clin Pharmacol. 2001;41(8):861‐869. [DOI] [PubMed] [Google Scholar]
- 284. Siskind EJ, Liu C, Collins DT, et al. Use of mammalian target of rapamycin inhibitors for pancreas transplant immunosuppression is associated with improved allograft survival and improved early patient survival. Pancreas. 2019;48(5):644‐651. [DOI] [PubMed] [Google Scholar]
- 285. Girman P, Lipár K, Kočík M, et al. Sirolimus vs mycophenolate mofetil (MMF) in primary combined pancreas and kidney transplantation. Results of a long‐term prospective randomized study. Am J Transplant. 2020;20(3):779‐787. [DOI] [PubMed] [Google Scholar]
- 286. Malaise J, De Roover A, Squifflet JP, et al. Immunosuppression in pancreas transplantation: the Euro SPK trials and beyond. Acta Chir Belg. 2008;108(6):673‐678. [DOI] [PubMed] [Google Scholar]
- 287. Garcia VD, Keitel E, Santos AF, et al. Immunosuppression in pancreas transplantation: mycophenolate mofetil versus sirolimus. Transplant Proc. 2004;36(4):975‐977. [DOI] [PubMed] [Google Scholar]
- 288. Ciancio G, Sageshima J, Sageshima L, et al. Advantage of rapamycin over mycophenolate mofetil when used with tacrolimus for simultaneous pancreas kidney transplants: randomized, single center trial at 10 years. Am J Transplant. 2012;12(12):3363‐3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Pelletier RP, Rajab AA, Diez A, et al. Early immunosuppression treatment correlates with later de novo donor‐specific antibody development after kidney and pancreas transplantation. Clin Transplant. 2015;29(12):1119‐1127. [DOI] [PubMed] [Google Scholar]
- 290. Mithani Z, Gralla J, Adebiyi O, et al. De novo donor‐specific antibody formation in tacrolimus‐based, mycophenolate versus mammalian target of rapamycin immunosuppressive regimens. Exp Clin Transplant. 2018;16(1):23‐30. [DOI] [PubMed] [Google Scholar]
- 291. Knight RJ, Kerman RH, Zela S, et al. Pancreas transplantation utilizing thymoglobulin, sirolimus, and cyclosporine. Transplantation. 2006;81(8):1101‐1105. [DOI] [PubMed] [Google Scholar]
- 292. Muthusamy AS, Giangrande PL, Friend PJ. Pancreas allograft thrombosis. Transplantation. 2010;90(7):705‐707. [DOI] [PubMed] [Google Scholar]
- 293. Burke GW, Ciancio G, Figueiro J, et al. Hypercoagulable state associated with kidney‐pancreas transplantation. Thromboelastogram‐directed anti‐coagulation and implications for future therapy. Clin Transplant. 2004;18(4):423‐428. [DOI] [PubMed] [Google Scholar]
- 294. Troppmann C, Gruessner AC, Benedetti E, et al. Vascular graft thrombosis after pancreatic transplantation: univariate and multivariate operative and nonoperative risk factor analysis. J Am Coll Surg 1996;182(4):285‐316. [PubMed] [Google Scholar]
- 295. Stratta RJ, Taylor RJ, Gill IS. Pancreas transplantation: a managed cure approach to diabetes. Curr Probl Surg. 1996;33:709‐808. [DOI] [PubMed] [Google Scholar]
- 296. Humar A, Ramcharan T, Kandaswamy R, et al. Technical failures after pancreas transplants: why grafts fail and the risk factors ‐ a multivariate analysis. Transplantation. 2004;78(8):1188‐1192. [DOI] [PubMed] [Google Scholar]
- 297. Wallace DF, Bunnett J, Fryer E, Drage M, Horsfield C, Callaghan CJ. Early allograft pancreatectomy‐technical failure or acute pancreatic rejection? Clin Transplant. 2019;33(10):e13702. [DOI] [PubMed] [Google Scholar]
- 298. Fertmann JM, Wimmer CD, Arbogast HP, et al. Single‐shot antithrombin in human pancreas‐kidney transplantation: reduction of reperfusion pancreatitis and prevention of graft thrombosis. Transpl Int. 2006;19(6):458‐465. [DOI] [PubMed] [Google Scholar]
- 299. Aboalsamh G, Anderson P, Al‐Abbassi A, et al. Heparin infusion in simultaneous pancreas and kidney transplantation reduces graft thrombosis and improves graft survival. Clin Transplant. 2016;30(9):1002‐1009. [DOI] [PubMed] [Google Scholar]
- 300. Vaidya A, Muthusamy AS, Hadjianastassiou VG, et al. Simultaneous pancreas‐kidney transplantation: to anticoagulate or not? Is that a question? Clin Transplant. 2007;21(4):554‐557. [DOI] [PubMed] [Google Scholar]
- 301. Schenker P, Vonend O, Ertas N, et al. Incidence of pancreas graft thrombosis using low‐molecular‐weight heparin. Clin Transplant. 2009;23(3):407‐414. [DOI] [PubMed] [Google Scholar]
- 302. Humar A, Johnson EM, Gillingham KJ, et al. Venous thromboembolic complications after kidney and kidney‐pancreas transplantation: a multivariate analysis. Transplantation. 1998;65(2):229‐234. [DOI] [PubMed] [Google Scholar]
- 303. Abualhassan N, Aljiffry M, Thalib L, et al. Post‐transplant venous thromboembolic events and their effect on graft survival. Saudi J Kidney Dis Transpl. 2015;26(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 304. Lam NN, Garg AX, Knoll GA, et al. Venous thromboembolism and the risk of death and graft loss in kidney transplant recipients. Am J Nephrol. 2017;46(4):343‐354. [DOI] [PubMed] [Google Scholar]
- 305. Farney AC, Rogers J, Stratta RJ. Pancreas graft thrombosis: causes, prevention, diagnosis, and intervention. Curr Opi Organ Transplant. 2012;17(1):87‐92. [DOI] [PubMed] [Google Scholar]
- 306. Luan FL, Stuckey LJ, Park JM, et al. Six‐month prophylaxis is cost effective in transplant patients at high risk for cytomegalovirus infection. J Am Soc Nephrol. 2009;20(11):2449‐2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Humar A, Lebranchu Y, Vincenti F, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high‐risk kidney transplant recipients. Am J Transplant. 2010;10(5):1228‐1237. [DOI] [PubMed] [Google Scholar]
- 308. Keven K, Basu A, Tan HP, et al. Cytomegalovirus prophylaxis using oral ganciclovir or valganciclovir in kidney and pancreas‐kidney transplantation under antibody preconditioning. Transplant Proc. 2004;36(10):3107‐3112. [DOI] [PubMed] [Google Scholar]
- 309. Rayes N, Seehofer D, Kahl A, et al. Long‐term outcome of cytomegalovirus infection in simultaneous pancreas‐kidney transplant recipients without ganciclovir prophylaxis. Transpl Int. 2007;20(11):974‐981. [DOI] [PubMed] [Google Scholar]
- 310. Fallatah SM, Marquez MA, Bazerbachi F, et al. Cytomegalovirus infection post‐pancreas‐kidney transplantation ‐ results of antiviral prophylaxis in high‐risk patients. Clin Transplant. 2013;27(4):503‐509. [DOI] [PubMed] [Google Scholar]
- 311. Shah AP, Chen JM, Fridell JA. Incidence and outcomes of cytomegalovirus in pancreas transplantation with steroid‐free immunosuppression. Clin Transplant. 2015;29(12):1221‐1229. [DOI] [PubMed] [Google Scholar]
- 312. Stratta RJ. Ganciclovir/acyclovir and fluconazole prophylaxis after simultaneous kidney‐pancreas transplantation. Transplant Proc. 1998;30(2):262. [DOI] [PubMed] [Google Scholar]
- 313. Kotton CN, Kumar D, Caliendo AM, et al. The third international consensus guidelines on the management of cytomegalovirus in solid‐organ transplantation. Transplantation. 2018;102(6):900‐931. [DOI] [PubMed] [Google Scholar]
- 314. Benedetti E, Gruessner AC, Troppmann C, et al. Intra‐abdominal fungal infections after pancreatic transplantation: incidence, treatment, and outcome. J Am Coll Surg. 1996;183(4):307‐316. [PubMed] [Google Scholar]
- 315. Nath DS, Kandaswamy R, Gruessner R, Sutherland DE, Dunn DL, Humar A. Fungal infections in transplant recipients receiving alemtuzumab. Transplant Proc. 2005;37(2):934‐936. [DOI] [PubMed] [Google Scholar]
- 316. Shaikh SA, Zimmerman A, Nolan A, et al. The incidence of fungal infections in pancreas transplant recipients in the absence of systemic antifungal prophylaxis. Clin Transplant. 2019;33(10):e13691. [DOI] [PubMed] [Google Scholar]
- 317. Garg N, Jorgenson M, Descourouez J, et al. Pneumocystis jiroveci pneumonia in kidney and simultaneous pancreas kidney transplant recipients in the present era of routine post‐transplant prophylaxis: risk factors and outcomes. BMC Nephrol. 2018;19(1):332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. van Hal SJ, Marriott DJ, Chen SC, et al. Candidemia following solid organ transplantation in the era of antifungal prophylaxis: the Australian experience. Transpl Infect Dis. 2009;11(2):122‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Singh N. Antifungal prophylaxis for solid organ transplant recipients: seeking clarity amidst controversy. Clin Infect Dis. 2000;31(2):545‐553. [DOI] [PubMed] [Google Scholar]
- 320. Fishman JA. Prevention of infection caused by Pneumocystis carinii in transplant recipients. Clin Infect Dis. 2001;33(8):1397‐1405. [DOI] [PubMed] [Google Scholar]
- 321. Silveira FP, Kusne S; AST Infectious Diseases Community of Practice . Candida infections in solid organ transplantation. Am J Transplant. 2013;13(4):220‐227. [DOI] [PubMed] [Google Scholar]
- 322. Pfundstein J, Roghmann MC, Schwalbe RS, et al. A randomized trial of surgical antimicrobial prophylaxis with and without vancomycin in organ transplant patients. Clin Transplant. 1999;13(3):245‐252. [DOI] [PubMed] [Google Scholar]
- 323. Everett JE, Wahoff DC, Statz C, et al. Characterization and impact of wound infection after pancreas transplantation. Arch Surg. 1994;129(12):1310‐1317. [DOI] [PubMed] [Google Scholar]
- 324. Barone GW, Hudec WA, Sailors DM, et al. Prophylactic wound antibiotics for combined kidney and pancreas transplants. Clin Transplant. 1996;10(4):386‐388. [PubMed] [Google Scholar]
- 325. Smets YFC, Van Der Piji JW, Van Dissel JT, et al. Infectious disease complications of simultaneous pancreas kidney transplantation. Nephrol Dial Transplant. 1997;12(4):764‐771. [DOI] [PubMed] [Google Scholar]
- 326. Bonatti H, Berger N, Kafka R, et al. Experience with ATG short course high dose induction therapy in a series of 112 enteric drained pancreatic transplants. Ann Transplant. 2002;7(3):22‐27. [PubMed] [Google Scholar]
- 327. Freise CE, Stock PG, Roberts JP, et al. Low postoperative wound infection rates are possible following simultaneous pancreas‐kidney transplantation. Transplant Proc. 1995;27(6):3069‐3070. [PubMed] [Google Scholar]
- 328. Anesi JA, Blumberg EA, Abbo LM. Perioperative antibiotic prophylaxis to prevent surgical site infections in solid organ transplantation. Transplantation. 2018;102(1):21‐34. [DOI] [PubMed] [Google Scholar]
- 329. Danziger‐Isakov L, Kumar D; AST ID Community of Practice . Vaccination of solid organ transplant candidates and recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9):e13563. [DOI] [PubMed] [Google Scholar]
- 330. Chaigne B, Geneugelijk K, Bédat B, et al. Immunogenicity of anti‐HLA antibodies in pancreas and islet transplantation. Cell Transplant. 2016;25(11):2041‐2050. [DOI] [PubMed] [Google Scholar]
- 331. Stratta RJ, Taylor RJ, Grune MT, et al. Experience with protocol biopsies after solitary pancreas transplantation. Transplantation. 1995;60(12):1431‐1437. [DOI] [PubMed] [Google Scholar]
- 332. Casey ET, Smyrk TC, Burgart LJ, et al. Outcome of untreated grade II rejection on solitary pancreas allograft biopsy specimens. Transplantation. 2005;79(12):1717‐1722. [DOI] [PubMed] [Google Scholar]
- 333. Troxell ML, Koslin DB, Norman D, et al. Pancreas allograft rejection: analysis of concurrent renal allograft biopsies and posttherapy follow‐up biopsies. Transplantation. 2010;90(1):75‐84. [DOI] [PubMed] [Google Scholar]
- 334. Parajuli S, Arpali E, Astor BC, et al. Concurrent biopsies of both grafts in recipients of simultaneous pancreas and kidney demonstrate high rates of discordance for rejection as well as discordance in type of rejection – a retrospective study. Transpl Int. 2018;31(1):32‐37. [DOI] [PubMed] [Google Scholar]
- 335. Uva PD, Papadimitriou JC, Drachenberg CB, et al. Graft dysfunction in simultaneous pancreas kidney transplantation (SPK): results of concurrent kidney and pancreas allograft biopsies. Am J Transplant. 2019;19(2):466‐474. [DOI] [PubMed] [Google Scholar]
- 336. Papadimitriou JC, Drachenberg CB, Wiland A, et al. Histologic grading of acute allograft rejection in pancreas needle biopsy: correlation to serum enzymes, glycemia, and response to immunosuppressive treatment. Transplantation. 1998;66(12):1741‐1745. [DOI] [PubMed] [Google Scholar]
- 337. Trofe J, Stratta RJ, Egidi MF, et al. Thymoglobulin for induction or rejection therapy in pancreas allograft recipients: a single centre experience. Clin Transplant. 2002;16(Suppl 7):34‐44. [DOI] [PubMed] [Google Scholar]
- 338. Dong M, Parsaik AK, Kremers W, et al. Acute pancreas allograft rejection is associated with increased risk of graft failure in pancreas transplantation. Am J Transplant. 2013;13(4):1019‐1025. [DOI] [PubMed] [Google Scholar]
- 339. Moinuddin I, Yaqub MS, Taber T, et al. Isolated pancreas rejections do not have an adverse impact on kidney graft survival whereas kidney rejections are associated with adverse pancreas graft survival in simultaneous pancreas kidney transplantation. J Nephrol. 2018;31(2):307‐315. [DOI] [PubMed] [Google Scholar]
- 340. Aziz F, Parajuli S, Uddin S, et al. How should pancreas transplant rejection be treated? Transplantation. 2019;103(9):1928‐1934. [DOI] [PubMed] [Google Scholar]
- 341. Torrealba JR, Samaniego M, Pascual J, et al. C4d‐positive interacinar capillaries correlates with donor‐specific antibody‐mediated rejection in pancreas allografts. Transplantation. 2008;86(12):1849‐1856. [DOI] [PubMed] [Google Scholar]
- 342. De Kort H, Munivenkatappa RB, Berger SP, et al. Pancreas allograft biopsies with positive c4d staining and anti‐donor antibodies related to worse outcome for patients. Am J Transplant. 2010;10(7):1660‐1667. [DOI] [PubMed] [Google Scholar]
- 343. Drachenberg CB, Odorico J, Demetris AJ, et al. Banff schema for grading pancreas allograft rejection: working proposal by a multi‐disciplinary international consensus panel. Am J Transplant. 2008;8(6):1237‐1249. [DOI] [PubMed] [Google Scholar]
- 344. Loupy A, Haas M, Solez K, et al. The Banff 2015 kidney meeting report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017;17(1):28‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Drachenberg CB, Torrealba JR, Nankivell BJ, et al. Guidelines for the diagnosis of antibody‐mediated rejection in pancreas allografts‐updated Banff grading schema. Am J Transplant. 2011;11(9):1792‐1802. [DOI] [PubMed] [Google Scholar]
- 346. Rangel ÉB, Malheiros DMAC, De Castro MCR, et al. Antibody‐mediated rejection (AMR) after pancreas and pancreas‐kidney transplantation. Transpl Int. 2010;23(6):602‐610. [DOI] [PubMed] [Google Scholar]
- 347. Pascual J, Pirsch JD, Odorico JS, et al. Alemtuzumab induction and antibody‐mediated kidney rejection after simultaneous pancreas‐kidney transplantation. Transplantation. 2009;87(1):125‐132. [DOI] [PubMed] [Google Scholar]
- 348. Niederhaus SV, Leverson GE, Lorentzen DF, et al. Acute cellular and antibody‐mediated rejection of the pancreas allograft: incidence, risk factors and outcomes. Am J Transplant. 2013;13(11):2945‐2955. [DOI] [PubMed] [Google Scholar]
- 349. Redfield RR, Kaufman DB, Odorico JS. Diagnosis and treatment of pancreas rejection. Curr Transplant Rep. 2015;2(2):169‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Sutherland DER, Goetz FC, Sibley RK. Recurrence of disease in pancreas transplants. Diabetes. 1989;38(Suppl 1):85‐87. [DOI] [PubMed] [Google Scholar]
- 351. Bosi E, Bottazzo GF, Secchi A, et al. Islet cell autoimmunity in type I diabetic patients after HLA‐mismatched pancreas transplantation. Diabetes. 1989;38(Suppl 1):82‐84. [DOI] [PubMed] [Google Scholar]
- 352. Occhipinti M, Lampasona V, Vistoli F, et al. Zinc transporter 8 autoantibodies increase the predictive value of islet autoantibodies for function loss of technically successful solitary pancreas transplant. Transplantation. 2011;92(6):674. [DOI] [PubMed] [Google Scholar]
- 353. Vendrame F, Pileggi A, Laughlin E, et al. Recurrence of type 1 diabetes after simultaneous pancreas‐kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T‐cells. Diabetes. 2010;59(4):947‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Laughlin E, Burke G, Pugliese A, et al. Recurrence of autoreactive antigen‐specific CD4+ T cells in autoimmune diabetes after pancreas transplantation. Clin Immunol. 2008;128(1):23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Abreu JRF, Roep BO. Immune monitoring of islet and pancreas transplant recipients. Curr Diab Rep. 2013;13(5):704‐712. [DOI] [PubMed] [Google Scholar]
- 356. Burke GW, Vendrame F, Virdi SK, et al. Lessons from pancreas transplantation in type 1 diabetes: recurrence of islet autoimmunity. Curr Diab Rep. 2015;15(12):1‐9. [DOI] [PubMed] [Google Scholar]
- 357. Monti P, Vignali D, Piemonti L. Monitoring inflammation, humoral and cell‐mediated immunity in pancreas and islet transplants. Curr Diabetes Rev. 2015;11(3):135‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. KožNarová R, Saudek F, Sosna T, et al. Beneficial effect of pancreas and kidney transplantation on advanced diabetic retinopathy. Cell Transplant. 2000;9:903‐908. [DOI] [PubMed] [Google Scholar]
- 359. Giannarelli R, Coppelli A, Sartini M, et al. Effects of pancreas–kidney transplantation on diabetic retinopathy. Transpl Int. 2005;18(5):619‐622. [DOI] [PubMed] [Google Scholar]
- 360. Ramsay RC, Goetz FC, Sutherland DE, et al. Progression of diabetic retinopathy after pancreas transplantation for insulin‐dependent diabetes mellitus. N Engl J Med. 1998;318(4):208‐214. [DOI] [PubMed] [Google Scholar]
- 361. Landgraf R. Impact of pancreas transplantation on diabetic secondary complications and quality of life. Diabetologia. 1996;39(12):1415‐1424. [DOI] [PubMed] [Google Scholar]
- 362. Königsrainer A, Miller K, Steurer W, et al. Does pancreas transplantation influence the course of diabetic retinopathy? Diabetologia. 1991;34(Suppl 1):S86‐S88. [DOI] [PubMed] [Google Scholar]
- 363. Bandello F, Vigano C, Secchi A, et al. Effect of pancreas transplantation on diabetic retinopathy: a 20‐case report. Diabetologia. 1991;34(Suppl 1):S92‐S94. [DOI] [PubMed] [Google Scholar]
- 364. Lindahl JP, Trond J, Hartmann A. Long‐term outcomes after organ transplantation in diabetic end‐stage renal disease. Diabetes Res Clin Pract. 2014;105(1):14‐21. [DOI] [PubMed] [Google Scholar]
- 365. Wilczek HE, Jaremko G, Tyden G, et al. Evolution of diabetic nephropathy in kidney grafts. Evidence that a simultaneously transplanted pancreas exerts a protective effect. Transplantation. 1995;59:51‐57. [DOI] [PubMed] [Google Scholar]
- 366. Lindahl J, Reinholt FP, Eideet IA, et al. In patients with type 1 diabetes simultaneous pancreas and kidney transplantation preserves long‐term kidney graft ultrastructure and function better than transplantation of kidney alone. Diabetologia. 2014;57:2357‐2365. [DOI] [PubMed] [Google Scholar]
- 367. Müller‐Felber W, Landgraf R, Wagner S, et al. Follow‐up study of sensory‐motor polyneuropathy in Type 1 (insulin‐dependent) diabetic subjects after simultaneous pancreas and kidney transplantation and after graft rejection. Diabetologia. 1991;34(1):113‐117. [DOI] [PubMed] [Google Scholar]
- 368. Solders G, Tyden G, Persson A, et al. Improvement of nerve conduction in diabetic neuropathy. A follow‐up study 4 yr after combined pancreatic and renal transplantation. Diabetes. 1992;41(8):946‐951. [DOI] [PubMed] [Google Scholar]
- 369. Hathaway DK, Abell T, Cardoso S, et al. Improvement in autonomic and gastric function following pancreas‐kidney versus kidney‐alone transplantation and the correlation with quality of life. Transplantation. 1994;57(6):816‐822. [DOI] [PubMed] [Google Scholar]
- 370. Müller‐Felber W, Landgraf R, Scheue R, et al. Diabetic neuropathy 3 years after successful pancreas and kidney transplantation. Diabetes. 1993;42(10):1482‐1486. [DOI] [PubMed] [Google Scholar]
- 371. Martinenghi S, Comi G, Galardi G, et al. Amelioration of nerve conduction velocity following simultaneous kidney/pancreas transplantation is due to the glycaemic control provided by the pancreas. Diabetologia. 1997;40:1110‐1112. [DOI] [PubMed] [Google Scholar]
- 372. Navarro X, Kennedy WR, Sutherland DE. Autonomic neuropathy and survival in diabetes mellitus: effects of pancreas transplantation. Diabetologia. 1991;34(Suppl 1):S108‐S112. [DOI] [PubMed] [Google Scholar]
- 373. La Rocca E, Fiorina P, Di Carlo V, et al. Cardiovascular outcomes after kidney–pancreas and kidney–alone transplantation. Kidney Int. 2001;60:1964‐1971. [DOI] [PubMed] [Google Scholar]
- 374. Jukema JW, Smets YFC, van der Pijl JW. Impact of simultaneous pancreas and kidney transplantation on progression of coronary atherosclerosis in patients with end‐stage renal failure due to type 1 diabetes. Diabetes Care. 2002;25(5):906‐911. [DOI] [PubMed] [Google Scholar]
- 375. Larsen JL, Christopher W, Colling CW, et al. Pancreas transplantation improves vascular disease in patients with type 1 diabetes. Diabetes Care. 2004;27(7):1706‐1711. [DOI] [PubMed] [Google Scholar]
- 376. La Rocca E, Minicucci F, Secchi A, et al. Evolution of carotid vascular lesions in kidney‐pancreas and kidney‐alone transplanted insulin‐dependent diabetic patients. Transplant Proc. 1995;27(6):3072. [PubMed] [Google Scholar]
- 377. Biesenbach G, Konigsrainer A, Gross C, Margreiter R. Progression of macrovascular diseases is reduced in type 1 diabetic patients after more than 5 years successful combined pancreas‐kidney transplantation in comparison to kidney transplantation alone. Transpl Int. 2005;18(9):1054‐1060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


