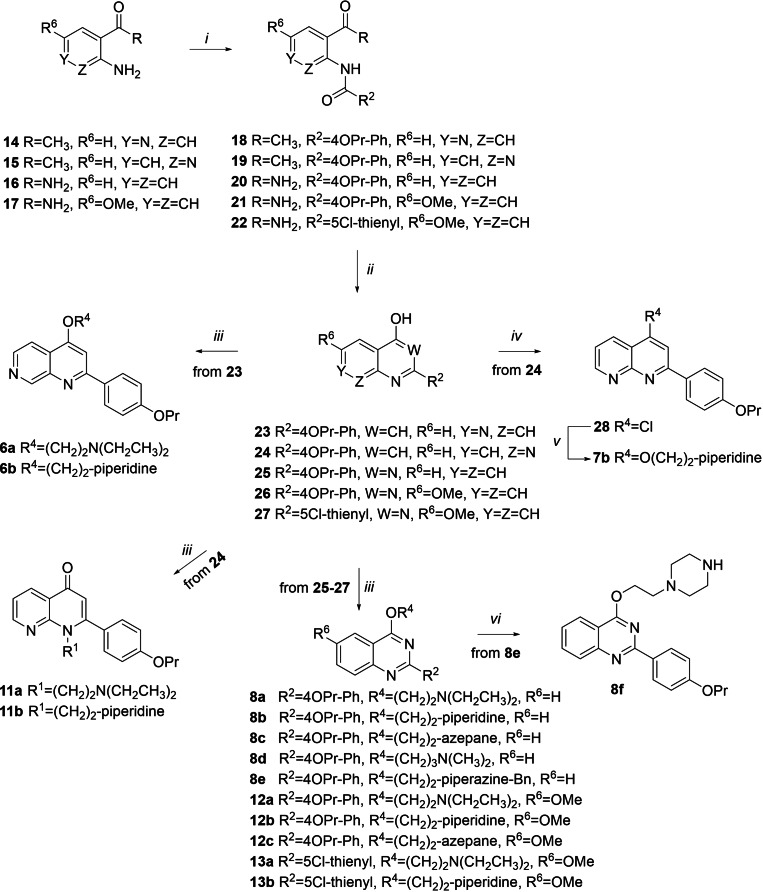
Scheme 1.
Reagents and condition: i) Et3N, dry THF, acyl chloride, rt – 60 °C, 3 h, 36–71 % or (for 18) DMAP, dry dioxane, 4‐propoxy benzoyl chloride, 100 °C, MW, 10 min, 43 %; ii) NaOH, dry dioxane, 110 °C, MW, 10 min, 40 % or tBuOK, tBuOH, rt – 90 °C, 90 min – 3 h, 75–90 %; iii) K2CO3, chloroalkylamines, dry DMF, 80–90 °C, 1–5 h, 11–84 % or (for 8 c and 8 d) 90–100 °C, MW, 10–15 min, 14–84 %; iv) POCl3, 100 °C, 3 h, 80 %; v) 60 % NaH, 1‐(2‐hydroxyethyl)piperidine, dry DMF, rt, 3 h, 41 %; vi) 10 % Pd/C, ammonium formate, MeOH, reflux, 2 h, 14 %.