Abstract
Background and Aims
Patients with cirrhosis on the liver transplant (LT) waiting list may die or be removed because of complications of portal hypertension (PH) or infections. von Willebrand factor antigen (vWF‐Ag) and C‐reactive protein (CRP) are simple, broadly available markers of these processes.
Approach and Results
We determined whether addition of vWF‐Ag and CRP to the Model for End‐Stage Liver Disease‐Sodium (MELD‐Na) score improves risk stratification of patients awaiting LT. CRP and vWF‐Ag at LT listing were assessed in two independent cohorts (Medical University of Vienna [exploration cohort] and Mayo Clinic Rochester [validation cohort]). Clinical characteristics, MELD‐Na, and mortality on the waiting list were recorded. Prediction of 3‐month waiting list mortality was assessed by receiver operating characteristics curve (ROC‐AUC). In order to explore potential mechanisms underlying the prognostic utility of vWF‐Ag and CRP in this setting, we evaluated their association with PH, bacterial translocation, systemic inflammation, and circulatory dysfunction. In the exploration cohort (n = 269) vWF‐Ag and CRP both improved the predictive value of MELD‐Na for 3‐month waitlist mortality and showed the highest predictive value when combined (AUC: MELD‐Na, 0.764; MELD‐Na + CRP, 0.790; MELD‐Na + vWF, 0.803; MELD‐Na + CRP + vWF‐Ag, 0.824). Results were confirmed in an independent validation cohort (n = 129; AUC: MELD‐Na, 0.677; MELD‐Na + CRP + vWF‐Ag, 0.882). vWF‐Ag was independently associated with PH and inflammatory biomarkers, whereas CRP closely, and MELD independently, correlated with biomarkers of bacterial translocation/inflammation.
Conclusions
The addition of vWF‐Ag and CRP—reflecting central pathophysiological mechanisms of PH, bacterial translocation, and inflammation, that are all drivers of mortality on the waiting list for LT—to the MELD‐Na score improves prediction of waitlist mortality. Using the vWFAg‐CRP‐MELD‐Na model for prioritizing organ allocation may improve prediction of waitlist mortality and decrease waitlist mortality.
Abbreviations
- ACLF
acute‐on‐chronic liver failure
- CRP
C‐reactive protein
- ESLD
end‐stage liver disease
- HVPG
hepatic venous pressure gradient
- LBP
lipopolysaccharide‐binding protein
- LT
liver transplantation
- MAP
mean arterial pressure
- MELD
Model for End‐Stage Liver Disease
- MELD‐Na
MELD‐Sodium
- PH
portal hypertension
- PRC
plasma renin concentration
- ROC
receiver operating characteristics
- SBP
spontaneous bacterial peritonitis
- vWF‐Ag
von Willebrand factor antigen
- WBCs
white blood cells
Mortality rates on the liver transplantation (LT) waitlist remain high,( 1 , 2 , 3 ) illustrating the need for improved risk stratification in this patient cohort. Model for End‐Stage Liver Disease (MELD) and later MELD‐Sodium (MELD‐Na) scores were introduced to standardize assessment of the risk of death on the waitlist. MELD‐Na score is now applied to prioritize organ allocation throughout the USA and Europe.( 4 , 5 , 6 ) Limitations of the use of the MELD and MELD‐Na score include the underestimation of complications of portal hypertension (PH) and the risk of infections that may result in acute‐on‐chronic liver failure (ACLF) and death.( 7 , 8 , 9 , 10 ) Further optimization of the predictive ability of the MELD equation may reduce mortality before LT by granting higher priority to those most at risk. Patients with cirrhosis and PH have an elevated level of von Willebrand factor antigen (vWF‐Ag).( 11 , 12 ) In this context, we recently demonstrated that the addition of vWF‐Ag, a key indicator of endothelial cell dysfunction and associated PH,( 11 ) significantly improved the predictive performance of MELD‐Na for 3‐month waitlist mortality.( 13 ) Besides vWF‐Ag, accumulating evidence suggests that an elevated C‐reactive protein (CRP) may also exhibit a predictive potential in patients with end‐stage liver disease (ESLD).( 14 , 15 , 16 ) CRP presumably reflects ongoing systemic inflammation that leads to worsening of vasodilatation and hyperdynamic circulation( 17 , 18 ) and associated complications, as well as development of ACLF.( 19 )
Accordingly, we aimed to assess the predictive capacity of the addition of vWF‐Ag and CRP to MELD‐Na in patients listed for LT. Using an exploration cohort for model generation, we further validated the predictive ability of our combined score in an independent validation set. Finally, we elaborate the pathophysiological processes underlying increased CRP and vWF‐Ag levels in a cohort of thoroughly characterized patients with an indication for LT, by assessing hepatic venous pressure gradient (HVPG) and a detailed profile of circulating markers of pathophysiological processes involved in chronic liver disease.
Materials and Methods
Study Population and Definition of Outcome
For the exploration cohort, all patients listed for LT between 2003 and 2016 at the Medical University of Vienna (Vienna, Austria) were included in this study. The Reviewer approved waiver of the requirement to obtain informed consent in accordance with 45 CFR 46,116. as justified by the Investigator, and waiver of HIPAA authorization in accordance with applicable HIPAA regulations. This particular set of patients was recently assessed for the relevance of vWF‐Ag in 3‐month waitlist mortality prediction and was now further evaluated.( 13 ) Routine blood samples at time of listing included baseline coagulation parameters, liver and kidney function tests, as well as CRP and vWF‐Ag levels.
For the validation cohort, patients who were listed for LT at the Mayo Clinic (Rochester, MN) between 2009 and 2019 and had CRP level measured were included.
During the study period, patients in Vienna were listed according to their MELD score. Patients were further able to accumulate points for each month on the waiting list. For the Mayo cohort, patients were listed exclusively according to their MELD‐Na score except for only 15 cases that were listed before 2016 (during the MELD‐only era). To account for differences in allocation systems between institutions and time point of listing, only calculated MELD‐Na was used for the analyses. MELD‐Na score was calculated as described elsewhere.( 20 ) Patients undergoing transplantation for acute liver failure or undergoing retransplantation were excluded from this study. Whereas any patient with HCC would be granted a total of 15 points at listing in Vienna, within this analysis, they are included according to their actual MELD‐Na score. Thereby, we aimed to reflect the individual patient risk for early waitlist mortality based on the MELD‐Na system without an inherent bias.
Postlisting 3‐month mortality was defined as death within 3 months of listing. Patients who were transplanted within 3 months were censored at the time of transplant and classified as alive (subgroup analyses are included in the supplement [Supporting Fig. S1A,B] illustrating results if these patients were classified as deaths or were excluded from the analyses). Patients who were removed because they became too sick for transplant were counted as deaths at the time of delisting, and patients who recovered and were removed because they no longer needed transplant were censored at the time of delisting. Three‐month waitlist mortality therefore includes both patients who died on the list or who were removed from the waitlist because they became too sick to transplant.
For our analysis on the pathophysiological mechanisms underlying the prognostic ability of vWF/CRP, we included prospectively characterized patients undergoing HVPG measurement at the Vienna Hepatic Hemodynamic Laboratory of the Medical University of Vienna (Vienna, Austria) from January 2017 until July 2020. Only patients with an indication for LT,( 21 ) that is, decompensated cirrhosis with either Child‐Turcotte stage B/C or MELD ≥15, were included. Patients suffering from an HCC outside of the Milan criteria were excluded from the analyses.
Informed consent in writing was obtained for each patients prior to blood sampling and the study protocols, conformed to the ethical guidelines of the 1975 Declaration of Helsinki, were approved by the institutional ethics committee/review board.
Assessment of vWF‐Ag in the Validation Cohort
Whereas vWF‐Ag was assessed using routine laboratory tests in the exploration cohort, in the validation cohort, EDTA plasma samples were obtained from a previously established biobank. EDTA blood was drawn at listing and immediately processed to plasma and stored at −80°C. Concentration of vWF‐Ag was assessed using a commercially available ELISA (Diagnostica Stago, Inc., Asnières‐sur‐Seine, France).
Assessment of HVPG and Markers of Pathophysiological Processes
To further explore pathophysiological processes involved in the association of vWF‐Ag, CRP, and sodium with waitlist mortality, we assessed their MELD‐independent associations with the degree of portal hypertension (reflected by: HVPG), bacterial translocation (reflected by: lipopolysaccharide‐binding protein [LBP]), and other inflammation markers (IL‐6), as well as markers of systemic hemodynamic impairment (reflected by: mean arterial pressure [MAP], copeptin [as a marker for the activity of the vasopressin system], and plasma renin concentration [PRC]). HVPG was measured in the fasting condition and in the absence of vasoactive treatments following the standardized operating procedure of the Vienna Hepatic Hemodynamic Laboratory.( 22 ) Biomarkers were assessed based on central venous blood obtained at the day of the procedure using standard laboratory methods.
Statistical Analyses
Statistical analyses were performed using R software (version 3.6.2; R Foundation for Statistical Computing, Vienna, Austria). Logistic regression and ROC analysis were applied to assess the discriminatory potential of CRP and vWF‐Ag for predicting waitlist mortality. In addition, this statistical approach was used to identify high‐ and low‐risk groups for 3‐month mortality by stratifying according to Youden’s J statistic. A Hosmer‐Lemeshow goodness‐of‐fit test was performed in order to assess calibration of the logistic regression model. Bootstrap analyses were used to provide CI of the AUC and test for differences between AUCs according to prediction using different scores. Generalized additive models with restricted smoothing cubic splines were used to determine the (nonlinear) continuous risk (log odds) for 3‐month waitlist mortality for measured parameters CRP and vWF‐Ag. Kaplan‐Meier curves were plotted to visualize survival according to defined risk groups, and log‐rank tests were used to assess statistical differences. The MELD‐independent associations of vWF‐Ag, CRP, and sodium with PH (HVPG), bacterial translocation (LBP), and other inflammation markers (IL‐6), as well as resulting systemic hemodynamic impairment (MAP, copeptin, and PRC), were investigated by multiple linear regression models adjusted for MELD. P values < 0.05 were considered statistically significant.
Results
Patient Selection and Demographics
We recently presented our results of the addition of vWF‐Ag to the MELD‐Na score in this exploration cohort.( 13 ) Briefly, a total of 1,237 patients were listed for LT at the General Hospital in Vienna (Medical University of Vienna) between 2003 and 2016 and included in our prospective database. Of these 1,237 patients, 38 who were listed for retransplantation, 14 who were listed for fulminant hepatic failure, and an additional 369 patients who were removed from the waitlist for other reasons than death or being too sick for transplant were excluded, as described.( 13 ) In total, 830 patients listed for LT were eligible. Detailed information on vWF‐Ag at the time point of listing was available in 269 patients. As previously indicated, these patients did not significantly differ from patients who did not have vWF‐Ag and CRP measurements at listing, except of being slightly older (Supporting Table S1). Fifty patients (21.6%) were transplanted within 3 months after listing. In our validation cohort, 127 patients had plasma samples collected and a CRP level measured at the time of listing between 2009 and 2019 and were therefore included in this analysis. Twelve patients (9.3%) were transplanted within 3 months of listing. Patient characteristics of both cohorts, including underlying liver pathology, are displayed in Table 1.
TABLE 1.
Cohort Characteristics
| Vienna (Exploration) Cohort (N = 269) | Mayo (Validation) Cohort (N = 129) | |
|---|---|---|
| Sex | ||
| Male | 203 (75.5%) | 80 (62.0%) |
| Female | 66 (24.5%) | 49 (38.0%) |
| Age at listing (years) | 56 [50‐61] | 60 [48‐66] |
| Laboratory values at listing | ||
| MELD‐Na | 19 [15‐23] | 16 [10‐21] |
| vWF‐Ag (%) | 419 [314‐420] | 397 [223‐723] |
| CRP (mg/dL) | 0.80 [0.31‐1.71] | 0.94 [0.30‐2.06] |
| Bilirubin (mg/dL) | 2.95 [1.68‐6.07] | 2.20 [1.10‐4.60] |
| Creatinine (mg/dL) | 0.90 [0.75‐1.16] | 1.00 [0.80‐1.34] |
| Na (mmol/L) | 136 [132‐139] | 139 [135‐141] |
| Indication for transplant | ||
| Alcohol‐associated cirrhosis | 89 (33.1%) | 16 (12.4%) |
| Tumor | 62 (23.0%) | 54 (41.9%) |
| Viral hepatitis | 42 (15.6%) | 3 (2.3%) |
| Biliary disorders | 23 (8.6%) | 11 (8.5%) |
| AI hepatitis | 14 (5.2%) | 1 (0.8%) |
| Cryptogenic cirrhosis | 13 (4.8%) | 2 (1.6%) |
| Other indications | 26 (9.7%) | 42 (32.6%) |
| Clinical characteristics | ||
| Varices | ||
| None | 127 (47.2%) | 69 (49.6%) |
| Small | 71 (26.4%) | 39 (30.2%) |
| Large | 71 (26.4%) | 26 (20.2%) |
| Ascites | ||
| None | 109 (40.5%) | 61 (47.3%) |
| Mild/medically controlled | 83 (30.9%) | 42 (32.6%) |
| Severe/refractory | 77 (28.6%) | 26 (20.2%) |
| HE | ||
| None | 168 (62.5%) | 79 (61.2%) |
| Mild/medically controlled | 59 (21.9%) | 37 (28.7%) |
| Severe | 42 (15.6%) | 13 (10.1%) |
| Previous variceal bleeding | 54 (20.1%) | 26 (20.2%) |
Continuous features are given as medians [IQRs], categorical features are given as counts (%).
Abbreviations: AI, autoimmune; IQRs, interquartile ranges.
To assess potential mechanisms contributing to the association of vWF‐Ag and CRP with 3‐month survival on the waiting list for LT, we included a third cohort of 168 patients with a detailed assessment of pathophysiologically oriented biomarkers, involved in ESLD, meeting the above‐mentioned inclusion and exclusion criteria.
Association Between CRP and vWF‐Ag and 3‐Month Survival on the Waitlist For LT
Generalized additive models for baseline (i.e., at time of listing) CRP and vWF‐Ag were implemented to assess the independent predictive value of these parameters on 3‐month waitlist mortality. Indeed, after adjustment for MELD‐Na, both parameters showed an association with the risk of 3‐month waitlist mortality (Fig. 1). Whereas an elevated CRP seemed to primarily increase the risk proportionally to its increase, vWF‐Ag seemed to exert a bidirectional risk modification with a cutoff at 399.7%. In particular, vWF‐Ag levels <400% were associated with a risk reduction, whereas values above this cutoff were associated with an increased risk.
FIG. 1.
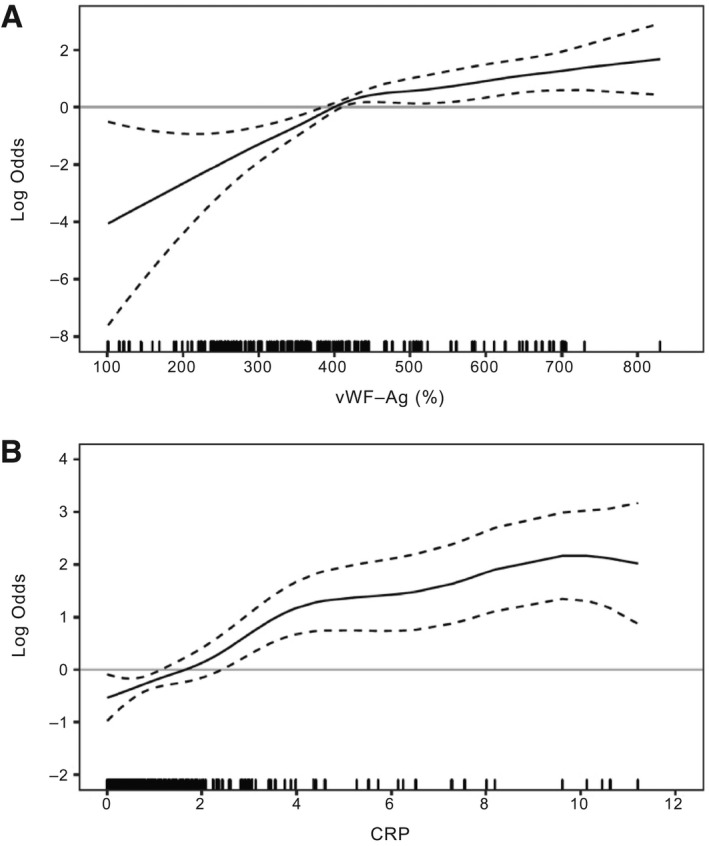
CRP and vWF‐Ag are associated with waitlist mortality for LT. Illustration of continuous risk (log odds) for 3‐months waitlist mortality removal because of clinical deterioration for vWF‐Ag (A) and CRP (B) using generalized additive models. Restricted cubic splines for CRP and vWF‐Ag, as well as covariate adjustment for MELD‐Na were applied. One vWF‐Ag value of 1,106.7 was regarded as an outlier and therefore excluded.
Addition of vWF‐Ag and CRP to MELD‐Na to Predict 3‐Month Waitlist Mortality
Logistic regression models were used to investigate the predictive performance of MELD‐Na if patients who were transplanted within 3 months after listing were included as alive (Fig. 2A), as deaths (Supporting Fig. S1A) or were fully excluded (Supporting Fig. S1B). Although including transplanted patients as deaths did yield the lowest predictive potential for 3‐month waitlist mortality (AUC, 0.650), inclusion of these patients as alive did show a comparable AUC as if these patients were excluded (including as alive, AUC 0.764; exclusion, AUC 0.782). Accordingly, we proceeded with our analyses based on including patients transplanted within 3 months after listing as alive. Furthermore, logistic regression models used to assess the predictive performance of MELD‐Na with the addition of CRP (Fig. 2B), vWF‐Ag (Fig. 2C), or both (Fig. 2D). MELD‐Na showed an AUC of 0.764 (95% CI, 0.660‐0.850). The addition of vWF‐Ag increased the predictive ability of MELD‐Na (AUC, vWF‐Ag = 0.803; 95% CI, 0.721‐0.882). Similarly, the addition of CRP also increased the predictive potential of MELD‐Na with an AUC of 0.790 (95% CI, 0.700‐0.874). However, the highest predictive performance was achieved when both CRP and vWF‐Ag were added to MELD‐Na (AUC, 0.824; 95% CI, 0.739‐0.898), which was statistically significantly superior to MELD‐Na (likelihood ratio test, P = 0.006). Similar results were obtained when patients transplanted within 3 months were excluded from the analyses (Supporting Fig. S2; combined score: AUC, 0.841).
FIG. 2.
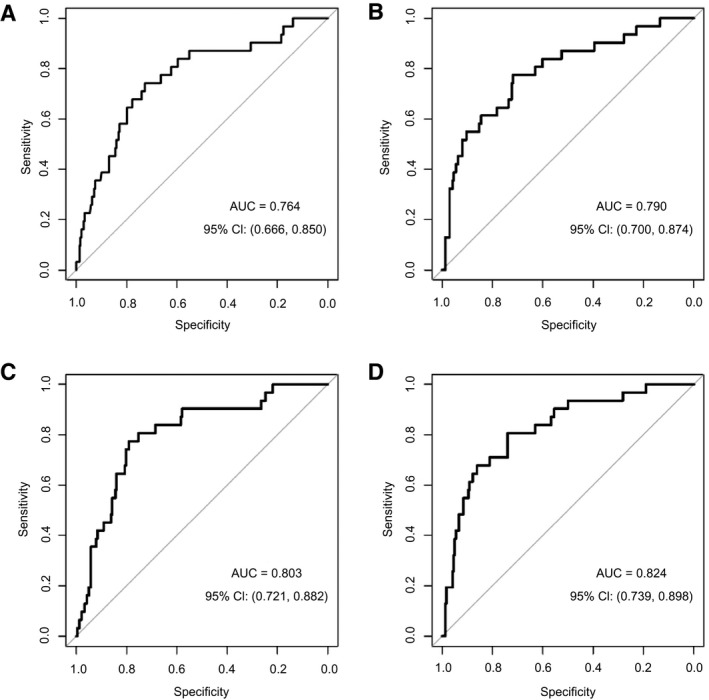
Incorporation of CRP and vWF‐Ag into the MELD‐Na score does improve the predictive potential for 3‐month waitlist mortality. ROC curves for the logistic regression models are illustrated for MELD‐Na (A), MELD‐Nae + CRP (B), MELD‐Na + vWF‐Ag (C), and MELD‐Na + CRP + vWF‐Ag (D).
Following from the logistic model considering MELD‐Na, CRP, and vWF‐Ag as separate predictors, a combined MELD‐Na + CRP + vWF‐Ag was constructed by weighting each parameter by its model effect size.
To facilitate easy clinical translation, a constant scalar of 4.6 was included to maintain the same scale as MELD‐Na. Accordingly, the resulting equation for the MELD‐NA + CRP + vWF‐Ag score was:
Thereby, a bidirectional reclassification was achieved, allowing patients to either gain or lose MELD‐Na points in accordance with the combination of vWF‐Ag and CRP. Indeed, the model could lead to redistribution of 36.4% of patients as illustrated in Fig. 3.
FIG. 3.
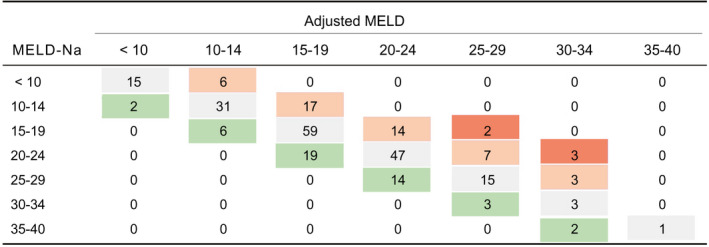
Redistribution of patients according to the adjusted MELD‐Na score. Gray cells illustrate patients who remained in the same category after MELD‐Na adjustment with vWF‐Ag and CRP. Green cells indicate a reduction in the score, whereas red illustrates an increase. Dark red suggests a very intense change in score of ≥6 points.
A logistic regression model was then fit using MELD‐Na + CRP + vWF‐Ag to predict 3‐month waitlist mortality. This MELD‐Na + CRP + vWF‐Ag model achieved an AUC of 0.824, which was significantly better than the current MELD‐Na model (bootstrap test, P = 0.023). Additionally, a Hosmer‐Lemeshow test of the goodness of fit for the MELD‐Na + CRP + vWF‐Ag score found no evidence of poor fit (P = 0.49), indicating that our model is well calibrated to the observed incidence of 3‐month waitlist mortality.
Because we included calculated MELD‐Na, exception points for HCC patients were eliminated. However, to exclude the possibility that 3‐month waitlist mortality prediction was limited to patients with a low MELD‐Na score or affected by the diagnosis of HCC, we further performed a subgroup analyses of patients with MELD‐Na >15, MELD‐Na >20, and excluding HCC patients, revealing similar results (Supporting Fig. S3).
Prediction of 3‐Month Waitlist Mortality According to the Combined MELD‐Na + CRP + vWF‐Ag Score
Using Youden’s J statistics, we classified patients into high‐ and low‐risk according to their combined MELD‐Na + CRP + vWF‐Ag score. The optimal MELD‐Na + CRP + vWF‐Ag cutoff was identified at 21 points, which achieved a sensitivity of 81.6% and a specificity of 73.2% to predict 3‐month waitlist mortality. Accordingly, 6 (3.3%) of the 180 low‐risk patients (<21) died on the waitlist within 3 months, compared to 25 (28%) of the 89 high‐risk patients (≥21). A log‐rank test found that the high‐risk MELD‐Na + CRP + vWF‐Ag patients were correctly assigned at a higher risk for waitlist mortality compared to low‐risk patients (P < 0.0001). In total, 51 patients (19.0%) were correctly reclassified as being high risk when using the MELD‐Na + CRP + vWF‐Ag system compared to the standard MELD‐Na cutoff of 20 points, whereas 9 patients (3.4%) were reclassified inappropriately.
Validation of Increased Predictive Potential of Waitlist Mortality of the Combined Score in an Independent Cohort of Patients
We further attempted to validate our results in an independent validation cohort. Accordingly, we found that in the validation cohort, MELD‐Na predicted 3‐month waitlist survival with an AUC of 0.677. However, when we used the full model combining MELD‐Na, CRP, and vWF, we observed a strikingly increased predictive potential with an AUC of 0.882. Corresponding ROC curves are illustrated in Fig. 4.
FIG. 4.
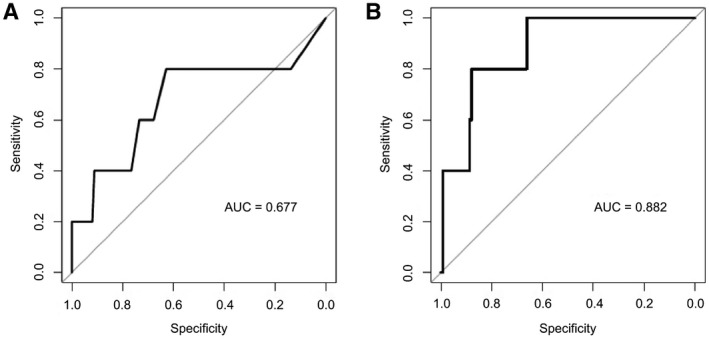
Validation of increased predictive potential of the incorporation of CRP and vWF‐Ag into the MELD‐Na score to predictive 3‐month LT waitlist mortality LT in an independent validation cohort. ROC curves for the logistic regression models as established in the exploration cohort are illustrated for MELD‐Na (A) and the combined model of MELD‐Na + CRP + vWF‐Ag (B).
Although all patients in the exploration cohort were listed with the same criteria, our validation included 15 patients who were listed before 2016, when organ allocation was performed according to MELD only. Accordingly, we performed a subgroup analyses of patients listed after 2016 in the MELD‐Na era, revealing similar results as the entire cohort (AUC, 0.864; Supporting Fig. S4).
Association Between CRP and vWF‐Ag and Other Complications of Cirrhosis
To identify whether CRP and vWF‐Ag reflect different pathophysiological processes, we assessed their correlation in our exploration cohort. CRP and vWF‐Ag were found to be significantly correlated (P = 0.016); however, this correlation was very weak (r = 0.15), suggesting that these markers reflect partly overlapping, but also independent, pathophysiological processes.
To further explore pathophysiological processes involved in the association of vWF‐Ag, CRP, and Na with waitlist mortality, we assessed their MELD‐independent associations with degree of portal hypertension (HVPG), bacterial translocation (LBP), and other inflammation markers (IL‐6), as well as markers of systemic hemodynamic impairment (MAP, copeptin, and PRC; Table 2). Interestingly, vWF‐Ag was the only marker that showed a MELD‐independent positive association with HVPG (change in vWF‐Ag per mm Hg; B = 5.859; P = 0.005), while also being linked to inflammation (IL‐6: change in vWF‐Ag per pg/mL; B = 0.662; P = 0.007). As expected, CRP—as a simple laboratory test for inflammation—remained closely related to other, more sophisticated markers of bacterial translocation (LBP: change in CRP per µg/mL; B = 0.259; P < 0.001) and inflammation (IL‐6; change CRP per pg/mL; B = 0.016; P < 0.001). Interestingly, CRP was also linked to systemic hemodynamic impairment, as evidenced by a negative association with MAP (change in CRP per mm Hg; B = −0.022; P = 0.007) and a positive association with copeptin (change in CRP per pmol/L; B = 0.014; P = 0.006). Finally, Na showed associations with some of the previously mentioned parameters/mechanisms except for HVPG/PH, but was additionally positively linked to PRC as an indicator of renal hypoperfusion/circulatory dysfunction.
TABLE 2.
Cohort Characteristics: Pathophysiology Cohort
| Vienna (Pathophysiology) Cohort (N = 168) | |
|---|---|
| Sex | |
| Male | 112 (67%) |
| Female | 56 (33%) |
| Age, years | 56.4 ± 0.9 |
| Etiology | |
| Alcohol‐associated cirrhosis | 103 (61%) |
| Viral hepatitis | 14 (9%) |
| Alcohol‐associated and viral hepatitis | 12 (7%) |
| NASH | 5 (3%) |
| Biliary disorders | 4 (2%) |
| Other etiologies | 30 (18%) |
| Clinical characteristics | |
| Varices | |
| Large | 76 (45%) |
| Small | 51 (31%) |
| None | 39 (23%) |
| Unknown | 2 (1%) |
| Ascites | |
| None | 7 (4%) |
| Mild/medically controlled | 127 (76%) |
| Severe/refractory | 34 (20%) |
| HE | |
| None | 97 (58%) |
| Mild/medically controlled | 70 (41%) |
| Severe | 1 (1%) |
| Previous variceal bleeding | 21 (13%) |
| Laboratory values | |
| MELD‐Na | 17 (13‐20) |
| CTP score | 8 (7‐10) |
| HVPG (mm Hg) | 20 (17‐24) |
| MAP (mm Hg) | 97 (87‐105) |
| INR | 1.5 (1.3‐1.7) |
| Bilirubin (mg/dL) | 1.72 (1.02‐3.10) |
| Albumin (g/L) | 31.7 ± 0.4 |
| Creatinine (mg/dL) | 0.77 (0.60‐1.05) |
| Na (mmol/L) | 136 (134‐139) |
| vWF‐Ag (%) | 350 (266‐420) |
| WBC (G/L) | 4.72 (3.49‐6.37) |
| CRP (mg/dL) | 0.61 (0.25‐1.51) |
| LBP (µg/mL) | 7.03 (6.18‐9.83) |
| IL‐6 (pg/mL) | 17.80 (8.57‐32.40) |
| Copeptin (pmol/L) | 14.20 (7.45‐26.50) |
| PRC (µIU/mL) | 100 (35‐297) |
Abbreviations: CTP, Child‐Turcotte‐Pugh score; NA, sodium; INR, international normalized ratio.
Discussion
Within this study, we found that the integration of CRP and vWF‐Ag into the MELD‐Na score was able to significantly increase the predictive potential for 3‐month waitlist mortality. Importantly, we were able to confirm this association in an independent validation cohort. We further provide exploratory evidence that vWF‐Ag and CRP may reflect very distinct pathophysiological processes that are not captured by MELD and only incompletely captured by MELD‐Na, presumably accounting for their strong predictive potential when combined. If the results on the prognostic performance can be prospectively validated in a larger cohort, incorporation of these two easily measurable laboratory parameters in clinical decision making might significantly impact survival prediction on the waitlist for LT.
The drawbacks of MELD are associated with an incorrect estimation of kidney function by measurement of serum creatinine. This might lead to a significant underestimation of certain subpopulations awaiting LT.( 23 , 24 , 25 ) Indeed, creatinine was found to be reduced in patients with cirrhosis, particularly patients with ascites who commonly have low muscle mass, and might ultimately often not accurately reflect extent of liver disease and concomitant pathophysiological changes.( 26 , 27 ) Furthermore, creatinine levels have been shown to be lower in women as compared to men given their lower muscle mass, highlighting the critical issue of systematic misclassification.( 28 ) Furthermore, studies from Europe have suggested that certain patients on the waitlist may be disadvantaged by the current MELD‐based allocation system.( 29 ) Hence, refining the MELD score is critical to further improvement of allocation policy and to reduce waitlist mortality. Although the addition of Na to MELD significantly improves risk stratification for patients on the waitlist for LT( 4 ) (as also very recently confirmed in the Europtransplant database( 5 )), significant shortcomings remain. In particular, the identification of high‐risk patients with low MELD‐Na score at listing as well as further stratification of patients with a very high MELD‐Na score pose difficult challenges.( 7 , 8 , 9 , 10 ) We previously documented that vWF‐Ag significantly improved prediction of 3‐month waitlist mortality.( 13 ) However, a previous report from our group that was based on a different patient population indicated that the combination of CRP and vWF‐Ag is particularly powerful to predict outcome in ESLD patients.( 12 ) In line with these results, we were able to document that integration of CRP or vWF‐Ag in the MELD‐Na score did improve prediction of 3‐month LT waitlist mortality, but their combination was found to exhibit the highest predictive potential. This could be explained by a divergent predictive potential of these two parameters. Indeed, as indicated by the generalized additive model curves, the predictive phenotype of CRP and vWF‐Ag did differ significantly. Although CRP primarily identified patients at increased risk for 3‐month waitlist mortality, vWF‐Ag did also exhibit the potential to identify patients who actually had a lower risk for 3‐month waitlist mortality than expected based on MELD‐Na.
vWF‐Ag has been shown to be a highly versatile biomarker in patients with underlying chronic liver disease, given that it provides clinically meaningful information in several different clinical contexts/stages of liver disease.( 11 , 12 , 13 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 ) VWF‐Ag is stored in endothelial cells’ Weibel‐Palade‐bodies and alpha‐granules of platelets. Shear stress and hemodynamic alternations were suggested to represent the main activators of vWF‐Ag secretion.( 38 , 39 ) Accordingly, elevated vWF‐Ag levels has been suggested as a noninvasive marker for portal hypertension,( 11 ) as also indicated by its correlation with HVPG in our analyses. However, vWF‐Ag might also reflect other pathophysiological processes, given that it confers HVPG‐independent prognostic information.( 12 ) Indeed, an association of vWF‐Ag with markers of bacterial translocation and the risk of spontaneous bacterial peritonitis (SBP) was observed,( 12 ) which may be explained by the binding of endotoxin to Toll‐like receptor 4, leading to endothelial cell activation.( 40 ) In line with these results, we observed an association of vWF‐Ag with IL‐6 and CRP within our analyses. Interestingly, despite the potential association of vWF‐Ag with systemic inflammation in ESLD patients, we found that CRP did offer incremental predictive potential for 3‐month waitlist mortality, which was also observed in a previous study by our group, focusing on another patient population.( 12 ) However, it should be noted that limited knowledge exists as to how interventions such as TIPS, nonselective beta blockers (NSBBs), or other vasoactive drugs might affect circulating levels of vWF‐Ag. Given that recent evidence suggests that vWF levels are higher in the portal circulation,( 41 ) TIPS might affect systemic levels of this biomarker. On the one hand, direct shunting of the portal venous blood could lead to higher systemic levels. On the other hand, vWF‐Ag is an indicator of endothelial dysfunction and used as a noninvasive test for PH and correlates with HVPG, and, accordingly, decreases in portal pressure gradient by TIPS placement may even lower vWF release. However, at the least, the latter scenario may not be of concern, given that in this case, TIPS likely improves both vWF‐Ag and prognosis. Similarly, NSBB and vasoactive drugs might affect portal venous pressure and, concomitantly, vWF‐Ag levels. Although changes in vWF‐Ag associated with these treatments might ultimately also allow to monitor clinical improvement and concomitant prognosis of these patients, our current analyses is unable to answer the effects of these potential modulators of vWF‐Ag levels, which should be addressed in future analyses.
The prognostic implications of CRP in patients with ESLD are well established.( 14 , 15 , 16 ) Of note, given that CRP is predominantly synthesized in the liver, a lack in increase of CRP after liver surgery has been associated with decreased liver function.( 42 ) Indeed, patients with higher CRP actually had slightly lower albumin levels in our analyses (Table 3). However, we found a positive correlation with 3‐month waitlist mortality, suggesting that the association with inflammatory processes might be the predominant source of its predictive value in these patients and might overcome potential effects of reduced synthetic function. Furthermore, we could demonstrate that CRP and vWF‐Ag show only a very weak correlation (Pearson’s r = 0.15). In particular, when looking at the distribution of CRP and vWF‐Ag in association with incidences of 3‐month LT waitlist mortality, we found that only 33.3% had high CRP and high vWF‐Ag levels, whereas 62.6% had either high vWF‐Ag or high CRP levels (as shown in Supporting Fig. S5). Indeed, pathophysiological parameters of inflammation (IL‐6) and bacterial translocation (LBP) seemed to be more closely/consistently associated with CRP than with vWF‐Ag. Accordingly, CRP—as a readily available laboratory test—may serve as a surrogate for more sophisticated inflammation markers that are not routinely available. Indeed, besides PH‐driven variceal bleeding, SBP, and other infections are predominant causes of mortality in patients on the waitlist for LT, given that they are important triggers of ACLF.( 43 , 44 , 45 ) Furthermore, an increase in CRP was also associated with low MAP,( 46 ) high copeptin.( 47 ) and systemic hemodynamic impairment, a critical determinant of outcome that is only captured by MELD if kidney function is substantially impaired. Accordingly, the synergistic predictive improvement of the integration of vWF‐Ag and CRP into the MELD‐Na score for 3‐month LT waitlist mortality might indeed be caused by their optimized combined quantification of PH, bacterial translocation, and inflammation, as well as the resulting systemic hemodynamic impairment, which was also supported by their significant association with clinical variables such as previous bleeding, HE, or severe ascites.
TABLE 3.
MELD‐Independent Associations of Circulating Pathophysiological Markers With vWF‐Ag, CRP, and Na in the Pathophysiology Cohort
| vWF‐Ag | CRP | Na | ||||
|---|---|---|---|---|---|---|
| B | P Value | B | P Value | B | P Value | |
| vWF‐Ag (%)* | — | — | 0.002 | 0.003 | −0.005 | 0.015 |
| CRP (mg/dL)* | 20.134 | 0.004 | — | — | −0.789 | <0.001 |
| Na (mmol/L)* | −8.096 | 0.001 | −0.100 | <0.001 | — | — |
| HVPG (mm Hg)* | 5.859 | 0.005 | 0.007 | 0.730 | −0.096 | 0.076 |
| WBC (G/L)* | 9.406 | <0.001 | 0.115 | <0.001 | −0.190 | 0.017 |
| LBP (µg/mL)* | 3.968 | 0.106 | 0.259 | <0.001 | −0.207 | 0.006 |
| IL‐6 (pg/mL)* | 0.662 | 0.007 | 0.016 | <0.001 | −0.012 | 0.115 |
| Albumin (g/L)* | −6.085 | 0.008 | −0.065 | 0.010 | −0.012 | 0.870 |
| MAP (mm Hg)* | −1.097 | 0.143 | −0.022 | 0.007 | 0.063 | 0.005 |
| Copeptin (pmol/L)* | 0.590 | 0.216 | 0.014 | 0.006 | −0.014 | 0.333 |
| PRC (µIU/mL)* | 0.020 | 0.081 | 0.001 | 0.342 | −0.018 | <0.001 |
| Varices (yes)* | 26.512 | 0.314 | −0.025 | 0.931 | −1.743 | 0.028 |
| Previous variceal bleeding (yes)* | 73.978 | 0.022 | −0.026 | 0.942 | 0.067 | 0.947 |
| Ascites (any)* | 74.603 | 0.196 | 0.895 | 0.158 | −1.920 | 0.279 |
| Ascites (severe/refractory)* | −3.900 | 0.887 | 1.057 | <0.001 | −1.693 | 0.039 |
| HE (any)* | 61.183 | 0.005 | −0.422 | 0.075 | 0.417 | 0.533 |
Adjusted for MELD.
Abbreviation: NA, sodium.
Significant P values are bolded.
Of note, given that previous studies have suggested the superiority of white blood cells (WBCs) over CRP,( 48 ) we also assessed the predictive potential of combining WBCs with MELD‐Na (Supporting Fig. S6). Indeed, we observed that combining WBC counts with MELD‐Na did result in a comparable predictive improvement of 3‐month waitlist mortality prediction as CRP (AUC: MELD‐Na + WBCs, 0.771; MELD‐Na + CRP, 0.791). Although we are limited in power to quantify these potential small differences, it should be mentioned that these parameters could be interchangeable, which is also supported by their highly significant correlation. However, WBCs might be confounded by different degrees of PH‐induced hypersplenism, which does not apply for CRP values. Future evaluations will have to determine the superiority of each parameter over another.
Na significantly overlapped in pathophysiological associations with CRP and vWF‐Ag. Serum Na+ decreases in patients with ESLD as a result of systemic vasodilatation and subsequent fluid retention. In this line, activation of the renin‐angiotensin system represents a central compensatory mechanism for systemic vasodilatation, as reflected by the close association between Na and PRC in our cohort. Given that systemic vasodilatation itself is merely a consequence of PH and systemic inflammation, dilutional hyponatremia represents a phenomenon that occurs far downstream of a pathophysiological cascade, whereas vWF‐Ag and CRP might more directly reflect the disease‐driving pathophysiological mechanisms acting upstream. This might explain why we observed a similar overall model performance when CRP and vWF‐Ag were added to either MELD or MELD‐Na (Supporting Fig. S7).
Recent evidence documented that 90‐day mortality is similar in ACLF patients with severe bacterial infections or alcohol‐associated hepatitis as a precipitating event,( 49 ) whereas patients with severe bacterial infection had considerably higher CRP levels. In addition, there were also important between‐group differences in variables that contribute to the MELD score, with higher serum creatinine in those with severe bacterial infection and (as expected) higher bilirubin levels in those with alcohol‐associated hepatitis, although MELD/MELD‐Na did not show major differences. Accordingly, even in patients at the same risk of mortality, individual parameters may show considerable discrepancies in different clinical contexts (e.g., severe bacterial infection vs. alcohol‐associated hepatitis), which supports the use of a prognostic model that captures a broad spectrum of prognostic factors/distinct pathophysiological mechanisms. However, despite this theoretical benefit, it seems essential to evaluate/validate such a model in different clinical contexts (e.g., acute decompensation and ACLF with different precipitating events), before being applied in clinical practice.
Besides the retrospective design of our analyses, our study suffers from weaknesses that have to be considered when interpreting our results. The most significant potential bias is that vWF‐Ag—and to a lesser extent CRP—were assessed only in a subgroup of patients listed for LT. To address this potential limitation, we assessed the differences of our exploration cohort between evaluated patients and remaining patients listed for LT during the same period of time and did not observe any significant differences in baseline characteristics, suggesting that included patients were representative of the overall population of patients listed for LT. We further want to point out that we were able to validate our findings in an independent validation cohort from another continent, demonstrating not only the robustness of our findings, but also indicating that they may be generalizable to the majority of patients listed for LT in the Western world.
Within the present study, we were able to document that CRP and vWF‐Ag, though inter‐related, seem to reflect multiple pathophysiological processes associated with worse outcome in patients with ESLD awaiting LT. This might explain why both parameters increased the predictive value of MELD‐Na, but their combined integration into the MELD‐Na score was particularly powerful. If future larger analyses are able to validate the improved predictive value of the combination of CRP and vWF‐Ag with MELD‐Na, this combined score could eventually allow for a more accurate waitlist prioritization of patients at highest risk of mortality and thus increased survival in patients awaiting LT.
Authors Contributions
All listed authors have (1) made substantial contributions to conception and design, acquisition of data, and/or its analysis/interpretation; (2) participated in drafting the article or revising it critically for important intellectual content; and (3) given final approval of the version to be published.
Supporting information
Supplementary Material
Potential conflict of interest: Dr. Trauner consults for, is on the speakers’ bureau for, and received grants from Gilead, Intercept, and MSD. He consults for and received grants from Albireo and Falk. He consults for BioMX, Boehringer Ingelheim, Genfit, Janssen, Novartis, Phenex, and Regulus. He is on the speakers’ bureau for Falk Foundation. He received grants from CymaBay, Takeda, and AbbVie. Dr. Simbrunner received travel grants from Gilead and AbbVie.
REFERENCES
Author names in bold designate shared co‐first authorship
- 1. Jochmans I, van Rosmalen M, Pirenne J, Samuel U. Adult liver allocation in eurotransplant. Transplantation 2017;101:1542‐1550. [DOI] [PubMed] [Google Scholar]
- 2. Györi GP, Silberhumer GR, Rahmel A, de Vries E, Soliman T, Zehetmayer S, et al. Impact of dynamic changes in MELD score on survival after liver transplantation—a Eurotransplant registry analysis. Liver Int 2016;36:1011‐1017. [DOI] [PubMed] [Google Scholar]
- 3. Goldberg D, French B, Trotter J, Shetty K, Schiano T, Reddy KR, et al. Underreporting of liver transplant waitlist removals due to death or clinical deterioration: results at four major centers. Transplantation 2013;96:211‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver‐transplant waiting list. N Engl J Med 2008;359:1018‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goudsmit BFJ, Putter H, Tushuizen ME, de Boer J, Vogelaar S, Alwayn IPJ, van Hoek B, et al. Validation of the Model for End‐stage Liver Disease sodium (MELD‐Na) score in the Eurotransplant region. Am J Transplant 2021;21:229‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagai S, Chau LC, Schilke RE, Safwan M, Rizzari M, Collins K, et al. Effects of allocating livers for transplantation based on Model for End‐Stage Liver Disease‐Sodium scores on patient outcomes. Gastroenterology 2018;155:1451‐1462 e3. [DOI] [PubMed] [Google Scholar]
- 7. Kwong AJ, Goel A, Mannalithara A, Kim WR. Improved posttransplant mortality after share 35 for liver transplantation. Hepatology 2018;67:273‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nicolas CT, Nyberg SL, Heimbach JK, Watt K, Chen HS, Hathcock MA, et al. Liver transplantation after share 35: impact on pretransplant and posttransplant costs and mortality. Liver Transpl 2017;23:11‐18. [DOI] [PubMed] [Google Scholar]
- 9. Petrowsky H, Rana A, Kaldas FM, Sharma A, Hong JC, Agopian VG, et al. Liver transplantation in highest acuity recipients: identifying factors to avoid futility. Ann Surg 2014;259:1186‐1194. [DOI] [PubMed] [Google Scholar]
- 10. Atiemo K, Skaro A, Maddur H, Zhao L, Montag S, VanWagner L, et al. Mortality risk factors among patients with cirrhosis and a low Model for End‐Stage Liver Disease Sodium score (≤15): an analysis of liver transplant allocation policy using aggregated electronic health record data. Am J Transplant 2017;17:2410‐2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferlitsch M, Reiberger T, Hoke M, Salzl P, Schwengerer B, Ulbrich G, et al. Von Willebrand factor as new noninvasive predictor of portal hypertension, decompensation and mortality in patients with liver cirrhosis. Hepatology 2012;56:1439‐1447. [DOI] [PubMed] [Google Scholar]
- 12. Mandorfer M, Schwabl P, Paternostro R, Pomej K, Bauer D, Thaler J, et al. Von Willebrand factor indicates bacterial translocation, inflammation, and procoagulant imbalance and predicts complications independently of portal hypertension severity. Aliment Pharmacol Ther 2018;47:980‐988. [DOI] [PubMed] [Google Scholar]
- 13. Györi GP, Pereyra D, Rumpf B, Hackl H, Köditz C, Ortmayr G, et al. The von Willebrand factor facilitates MELD‐independent risk stratification on the waiting list for liver transplantation. Hepatology 2020;72:584‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, et al. C‐reactive protein predicts short‐term mortality in patients with cirrhosis. J Hepatol 2012;56:1299‐1304. [DOI] [PubMed] [Google Scholar]
- 15. Di Martino V, Coutris C, Cervoni JP, Dritsas S, Weil D, Richou C, et al. Prognostic value of C‐reactive protein levels in patients with cirrhosis. Liver Transpl 2015;21:753‐760. [DOI] [PubMed] [Google Scholar]
- 16. Cervoni JP, Amorós À, Bañares R, Luis Montero J, Soriano G, Weil D, et al. Prognostic value of C‐reactive protein in cirrhosis: external validation from the CANONIC cohort. Eur J Gastroenterol Hepatol 2016;28:1028‐1034. [DOI] [PubMed] [Google Scholar]
- 17. Turco L, Garcia‐Tsao G, Magnani I, Bianchini M, Costetti M, Caporali C, et al. Cardiopulmonary hemodynamics and C‐reactive protein as prognostic indicators in compensated and decompensated cirrhosis. J Hepatol 2018;68:949‐958. [DOI] [PubMed] [Google Scholar]
- 18. Praktiknjo M, Monteiro S, Grandt J, Kimer N, Madsen JL, Werge MP, et al. Cardiodynamic state is associated with systemic inflammation and fatal acute‐on‐chronic liver failure. Liver Int 2020;40:1457‐1466. [DOI] [PubMed] [Google Scholar]
- 19. Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al.; PREDICT STUDY group of the EASL‐CLIF Consortium . The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol 2020;73:842‐854. [DOI] [PubMed] [Google Scholar]
- 20. Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for End‐Stage Liver Disease (MELD) and allocation of donor livers. Gastroenterology 2003;124:91‐96. [DOI] [PubMed] [Google Scholar]
- 21. Graziadei I, Zoller H, Fickert P, Schneeberger S, Finkenstedt A, Peck‐Radosavljevic M, et al. Indications for liver transplantation in adults : Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr 2016;128:679‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reiberger T, Schwabl P, Trauner M, Peck‐Radosavljevic M, Mandorfer M. Measurement of the hepatic venous pressure gradient and transjugular liver biopsy. J Vis Exp 2020;(160). 10.3791/58819. [DOI] [PubMed] [Google Scholar]
- 23. O’Leary JG, Wong F, Reddy KR, Garcia‐Tsao G, Kamath PS, Biggins SW, et al. Gender‐specific differences in baseline, peak, and delta serum creatinine: the NACSELD experience. Dig Dis Sci 2017;62:768‐776. [DOI] [PubMed] [Google Scholar]
- 24. Somsouk M, Kornfield R, Vittinghoff E, Inadomi JM, Biggins SW. Moderate ascites identifies patients with low model for end‐stage liver disease scores awaiting liver transplantation who have a high mortality risk. Liver Transpl 2011;17:129‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heuman DM, Abou‐Assi SG, Habib A, Williams LM, Todd Stravitz R, Sanyal AJ, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology 2004;40:802‐810. [DOI] [PubMed] [Google Scholar]
- 26. Kaiser T, Kinny‐Köster B, Gnewuch C, Karailieva D, Kiehntopf M, Kessler A, et al. Limited comparability of creatinine assays in patients with liver cirrhosis and their impact on the MELD score. Pract Lab Med 2017;8:41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruiz‐del‐Arbol L, Achecar L, Serradilla R, Rodriguez‐Gandia MA, Rivero M, Garrido E, et al. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension, and a normal creatinine. Hepatology 2013;58:1732‐1741. [DOI] [PubMed] [Google Scholar]
- 28. Sarkar M, Watt KD, Terrault N, Berenguer M. Outcomes in liver transplantation: does sex matter? J Hepatol 2015;62:946‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Angermayr B, Luca A, König F, Bertolini G, Ploner M, Gridelli B, et al. Aetiology of cirrhosis of the liver has an impact on survival predicted by the Model of End‐stage Liver Disease score. Eur J Clin Invest 2009;39:65‐71. [DOI] [PubMed] [Google Scholar]
- 30. Starlinger P, Pereyra D, Haegele S, Braeuer P, Oehlberger L, Primavesi F, et al. Perioperative von Willebrand factor dynamics are associated with liver regeneration and predict outcome after liver resection. Hepatology 2018;67:1516‐1530. [DOI] [PubMed] [Google Scholar]
- 31. Yilmaz VT, Dincer D, Avci AB, Cetinkaya R. Significant association between serum levels of von Willebrand Factor (vWF) antigen with stages of cirrhosis. Eurasian J Med 2015;47:21‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maieron A, Salzl P, Peck‐Radosavljevic M, Trauner M, Hametner S, Schöfl R, et al. Von Willebrand Factor as a new marker for non‐invasive assessment of liver fibrosis and cirrhosis in patients with chronic hepatitis C. Aliment Pharmacol Ther 2014;39:331‐338. [DOI] [PubMed] [Google Scholar]
- 33. Horvatits T, Drolz A, Roedl K, Herkner H, Ferlitsch A, Perkmann T, et al. Von Willebrand factor antigen for detection of hepatopulmonary syndrome in patients with cirrhosis. J Hepatol 2014;61:544‐549. [DOI] [PubMed] [Google Scholar]
- 34. Hugenholtz GC, Adelmeijer J, Meijers JC, Porte RJ, Stravitz RT, Lisman T. An unbalance between von Willebrand factor and ADAMTS13 in acute liver failure: implications for hemostasis and clinical outcome. Hepatology 2013;58:752‐761. [DOI] [PubMed] [Google Scholar]
- 35. La Mura V, Reverter JC, Flores‐Arroyo A, Raffa S, Reverter E, Seijo S, et al. Von Willebrand factor levels predict clinical outcome in patients with cirrhosis and portal hypertension. Gut 2011;60:1133‐1138. [DOI] [PubMed] [Google Scholar]
- 36. Schwarzer R, Reiberger T, Mandorfer M, Kivaranovic D, Hametner S, Hametner S, et al. The von Willebrand Factor antigen to platelet ratio (VITRO) score predicts hepatic decompensation and mortality in cirrhosis. J Gastroenterol 2020;55:533‐542. [DOI] [PubMed] [Google Scholar]
- 37. Semmler G, Binter T, Kozbial K, Schwabl P, Hametner‐Schreil S, Zanetto A, et al. Non‐invasive risk stratification after HCV eradication in patients with advanced chronic liver disease. Hepatology 2021;73:1275‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Herbig BA, Diamond SL. Pathological von Willebrand factor fibers resist tissue plasminogen activator and ADAMTS13 while promoting the contact pathway and shear‐induced platelet activation. J Thromb Haemost 2015;13:1699‐1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Razdan K, Hellums JD, Kroll MH. Shear‐stress‐induced von Willebrand factor binding to platelets causes the activation of tyrosine kinase(s). Biochem J 1994;302(Pt. 3):681‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carnevale R, Raparelli V, Nocella C, Bartimoccia S, Novo M, Severino A, et al. Gut‐derived endotoxin stimulates factor VIII secretion from endothelial cells. Implications for hypercoagulability in cirrhosis. J Hepatol 2017;67:950‐956. [DOI] [PubMed] [Google Scholar]
- 41. Praktiknjo M, Trebicka J, Carnevale R, Pastori D, Queck A, Ettorre E, et al. Von Willebrand and factor VIII portosystemic circulation gradient in cirrhosis: implications for portal vein thrombosis. Clin Transl Gastroenterol. 2020;11:e00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gyoeri GP, Pereyra D, Braunwarth E, Ammann M, Jonas P, Offensperger F, et al. The 3‐60 criteria challenge established predictors of postoperative mortality and enable timely therapeutic intervention after liver resection. Hepatobiliary Surg Nutr 2019;8:111‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huo TI, Lin HC, Lee FY, Hou MC, Lee PC, Wu JC, et al. Occurrence of cirrhosis‐related complications is a time‐dependent prognostic predictor independent of baseline model for end‐stage liver disease score. Liver Int 2006;26:55‐61. [DOI] [PubMed] [Google Scholar]
- 44. La Mura V, Nicolini A, Tosetti G, Primignani M. Cirrhosis and portal hypertension: the importance of risk stratification, the role of hepatic venous pressure gradient measurement. World J Hepatol 2015;7:688‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arroyo V, Moreau R, Jalan R. Acute‐on‐chronic liver failure. N Engl J Med 2020;382:2137‐2145. [DOI] [PubMed] [Google Scholar]
- 46. Llach J, Ginès P, Arroyo V, Rimola A, Titó L, Badalamenti S, et al. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology 1988;94:482‐487. [DOI] [PubMed] [Google Scholar]
- 47. Solà E, Kerbert AJC, Verspaget HW, Moreira R, Pose E, Ruiz P, et al. Plasma copeptin as biomarker of disease progression and prognosis in cirrhosis. J Hepatol 2016;65:914‐920. [DOI] [PubMed] [Google Scholar]
- 48. Jalan R, Pavesi M, Saliba F, Amorós A, Fernandez J, Holland‐Fischer P, et al. The CLIF Consortium Acute Decompensation score (CLIF‐C ADs) for prognosis of hospitalised cirrhotic patients without acute‐on‐chronic liver failure. J Hepatol 2015;62:831‐840. [DOI] [PubMed] [Google Scholar]
- 49. Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol 2020;20:33772‐33777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


