Abstract
Rifaximin is an oral nonsystemic antibiotic with minimal gastrointestinal absorption and broad‐spectrum antibacterial activity covering both gram‐positive and gram‐negative organisms. Rifaximin is currently used worldwide in patients with cirrhosis for preventing recurrent HE because its efficacy and safety have been proven by large randomized clinical trials. In the last decade, experimental and clinical evidence suggest that rifaximin could have other beneficial effects on the course of cirrhosis by modulating the gut microbiome and affecting the gut‐liver axis, which in turn can interfere with major events of the pathophysiological cascade underlying decompensated cirrhosis, such as systemic inflammatory syndrome, portal hypertension, and bacterial infections. However, the use of rifaximin for prevention or treatment of other complications, including spontaneous bacterial peritonitis or other bacterial infections, is not accepted because evidence by clinical trials is still very weak. The present review deals in the first part with the potential impact of rifaximin on pathogenic mechanisms in liver diseases, whereas in the second part, its clinical effects are critically discussed. It clearly emerges that, because of its potential activity on multiple pathogenic events, the efficacy of rifaximin in the prevention or management of complications other than HE deserves to be investigated extensively. The results of double‐blinded, adequately powered randomized clinical trials assessing the effect of rifaximin, alone or in combination with other drugs, on hard clinical endpoints, such as decompensation of cirrhosis, acute‐on‐chronic liver failure, and mortality, are therefore eagerly awaited.
Abbreviations
- ACLF
acute‐on‐chronic liver failure
- BDL
bile duct ligation
- CHE
covert hepatic encephalopathy
- hPXR
human pregnane X receptor
- LPS
lipopolysaccharide
- MELD
Model for End‐Stage Liver Disease
- PXR
pregnane X receptor
- RCT
randomized controlled trial
- SBP
spontaneous bacterial peritonitis
- TLR4
toll‐like receptor 4
More than four decades ago, an association between HE and abnormalities in ammonia metabolism was observed.( 1 ) The association between circulating unmetabolized ammonia level and HE was weak. However, because production of ammonia is closely related to the composition of gut microbiota, the idea emerged that oral administration of minimally absorbed antibiotics that were effective against ammonia‐producing bacteria could help treat or prevent HE.( 2 ) Neomycin, which is active against urease‐producing bacteria, has been one of the first minimally absorbed antibiotics to be used, and controlled trials showed it had similar efficacy compared with lactulose.( 3 ) However, because neomycin is slightly absorbed, there were concerns about nephrotoxicity and ototoxicity during long‐term treatment. Ten years ago, a seminal controlled trial showed that rifaximin, another minimally absorbed antibiotic, was superior to placebo to prevent recurrent HE.( 4 ) It rapidly became apparent that beyond HE, rifaximin could have beneficial effects on the course of cirrhosis through interactions with the gut‐liver axis.( 5 , 6 , 7 ) This review focuses on rifaximin in the management of HE in cirrhosis but also on other documented or potential targets of rifaximin, including systemic inflammatory response syndrome, portal hypertension, and bacterial infections.
Pharmacological Characteristics of Rifaximin
Rifaximin is a semisynthetic, water‐insoluble, rifamycin‐based nonsystemic antibiotic with very low gastrointestinal absorption and good antibacterial activity.( 8 ) Compared with rifampicin, it contains an extra pyrido‐imidazole ring to reduce systemic absorption that is less than 1% after oral administration( 8 , 9 ) (Fig. 1). Nevertheless, it is important to note that rifaximin plasma concentrations are not negligible in patients with cirrhosis, particularly in those with moderate‐to‐severe liver function impairment (Child B or C patients).( 10 ) There are some published reports on potential muscle toxicity in cirrhosis in patients receiving rifaximin in combination with simvastatin 40 mg/day.( 11 ) However, the possibility of these systemic muscle effects of rifaximin seems remote because in studies with large numbers of patients with cirrhosis, no muscle or other systemic adverse events have been reported (see later). The slight increase in systemic exposure to rifaximin in subjects with cirrhosis should be interpreted in the context of its low systemic availability as well as the rifaximin safety data in cirrhosis. Therefore, no dosage adjustment in patients with advanced cirrhosis is recommended.
FIG. 1.
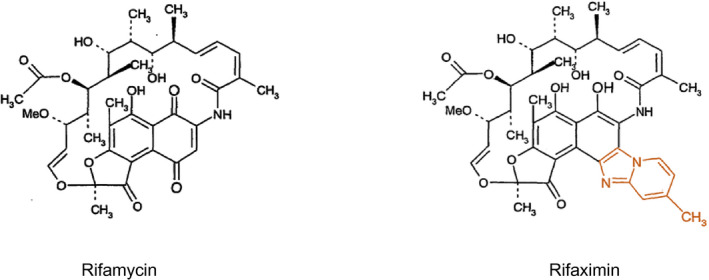
Molecular structure of rifaximin and rifampicin.
Both experimental and clinical data show that rifaximin has a broad‐spectrum antibacterial action covering gram‐positive and gram‐negative aerobic and anaerobic bacteria.( 8 , 12 , 13 ) Rifaximin elicits its antimicrobial properties by binding the beta‐subunit of the bacterial DNA‐dependent RNA polymerase and thus inhibiting bacterial RNA synthesis. It has the advantage of low microbial resistance and few systemic adverse events and is safe in all patient populations.( 8 , 12 , 13 )
Being virtually nonabsorbed, bioavailability of rifaximin within the gastrointestinal tract is high with intraluminal and fecal drug concentrations largely exceeding the minimal inhibitory concentration values observed in vitro against a wide range of pathogenic organisms. The gastrointestinal tract represents, therefore, the primary therapeutic target of rifaximin.( 8 , 9 , 12 , 13 )
Rifaximin has been shown to modify the gut microbiome. However, changes in overall gut microbiome composition have shown to be relatively sparse, and the effects on microbiome have been described to be mediated also by rifaximin‐induced changes in bile acid composition and modulation of microbiome function (see later).( 14 , 15 )
Effects of Rifaximin on Pathogenic Mechanisms of Chronic Liver Diseases and Cirrhosis
Impact on Liver Fibrosis and Portal Hypertension
Antibiotics have been reported to protect against alcohol‐associated liver disease in rat models,( 16 ) whereas others report aggravation of fibrosis in a carbon tetrachloride/alcohol rat model.( 17 ) In a bile‐duct‐ligated (BDL) model, rifaximin attenuated fibrosis and portal hypertension,( 18 ) and more recently, rifaximin was shown to prevent alcohol‐induced liver injury in a murine obesity model, whereby pathological changes in the intestinal microbiota signature that were induced by chronic ethanol feeding were partly reversed by rifaximin.( 19 )
Given rifaximin’s ability to selectively target the gut microbiota,( 20 ) decreased liver fibrosis in preclinical models,( 18 ) and a favorable long‐term safety profile, it follows that rifaximin may be a useful intervention in reducing fibrogenesis driven by gut microbiota components.( 21 )
From the established relationship between portal pressure and systemic inflammation,( 22 ) it follows that rifaximin, through modulation of fibrosis and gut microbiota, could be expected to lower portal pressure. Indeed, in an observational study, Vlachogiannakos et al.( 23 ) showed that rifaximin significantly lowered portal pressure at 4 weeks, associated with decreased plasma endotoxin levels. In contrast, another randomized study in stable cirrhosis with ascites (mean Model for End‐Stage Liver Disease [MELD] 11.7), showed no reduction in portal pressure after 4 weeks of rifaximin and no improvement in renal function or major effects on inflammatory markers and gut microbiota composition.( 15 , 24 ) The reason(s) for the different results of these studies is not known, but it is possible that unnoticed active alcohol use, differences in liver disease severity, and/or duration of treatment may explain differences across studies. Although the dynamic component of portal hypertension and markers of bacterial translocation can change relatively quickly, structural effects, such as reduction of fibrosis, are slow, and treatment duration of at least 12‐24 months is probably necessary to allow histological changes.
Of interest, a controlled study assessing the combination of rifaximin with propranolol showed a greater impact on portal pressure reduction and necessitated a lower mean propranolol dose compared with propranolol alone.( 25 ) A mechanistic explanation forwarded for the observed beneficial effects on portal pressure and fibrosis is provided by a study in BDL toll‐like receptor 4 (TLR4) mutant mice, showing that rifaximin modulates TLR4 activation by lipopolysaccharides (LPS), thus resulting downstream in less fibronectin generation from activated stellate cells and endothelial cells.( 18 )
Gut‐Liver Axis and Systemic Inflammation
Patients with cirrhosis experience alterations in their gut‐liver axis: an altered gut microbiome, increased intestinal permeability, and lower gut luminal primary bile acid levels may contribute to increased bacterial translocation.( 26 ) As mentioned before, rifaximin is minimally absorbed and is thought to act locally in the gut; therefore, rifaximin might exert modulating effects on elements of the gut‐liver axis.
In vitro, rifaximin enhances intestinal epithelial layer homeostasis through activation of human pregnane X receptor (PXR), leading to inhibition of NF‐κB–dependent inflammatory pathways including TNF‐α, IL‐6, IL‐8, and IL‐10 secretion and by induction of biotransformation enzymes phase 1 (e.g., cytochrome P450 3A4) or phase 2 (e.g., glutathione‐S‐transferase A1).( 27 , 28 , 29 ) Bacterial attachment and internalization in enterocytes is also affected by rifaximin. In addition, rifaximin increases transepithelial electrical resistance and viability of human colonic tumor‐derived Caco‐2 cells.( 30 ) Rifaximin also decreases toxicity PXR dependently caused by Clostridium difficile toxin A via the TLR4 pathway in Caco‐2 cells( 30 ) (Fig. 2).
FIG. 2.
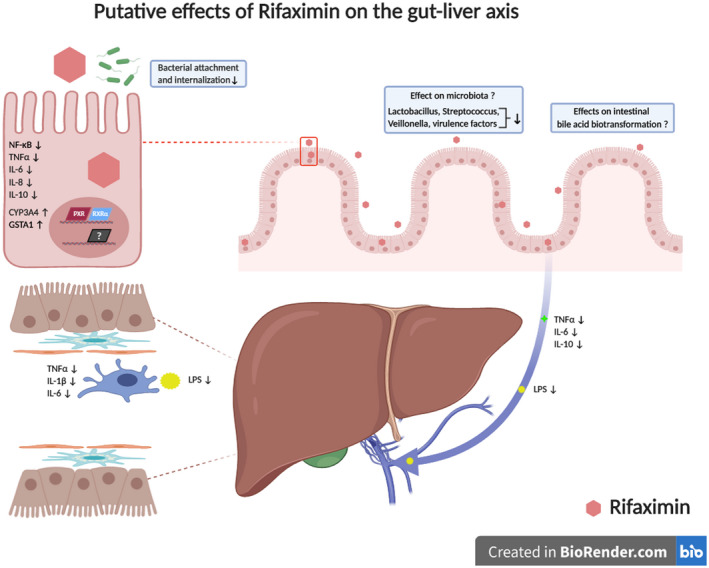
Putative effects of rifaximin on the gut‐liver axis. Rifaximin was shown to increase intestinal epithelial homeostasis by PXR‐dependent mechanisms. Among them, inhibition of NF‐κB; decrease of TNF‐α, IL‐6, IL‐8, and IL‐10 secretion; and induction of biotransformation phase 1 and 2 enzyme activities (e.g., cytochrome P450 3A4 [CYP3A4], glutathione S‐transferase alpha 1 [GSTA‐1]) are notable. Subtle changes in intestinal microbiome composition and lowering of virulence factors have also been observed. Effects of rifaximin on bacterial bile acid biotransformation are yet unclear. Rifaximin led to normalization of serum LPS binding protein levels and thereby lowering of the proinflammatory state in the liver. Figure created with Biorender.com. Abbreviation: RXR, retinoid X receptor.
Rodent models expressing human PXR (hPXR) were established to investigate PXR‐dependent effects of rifaximin in vivo, because rifaximin is a human, but not rodent, PXR agonist. Induction of biotransformation enzymes by rifaximin was confirmed in these hPXR mice.( 28 , 29 ) Notably, favorable effects of rifaximin were also observed in mice without hPXR. Serum LPS binding protein levels were markedly enhanced after BDL in wild‐type mice and tended to be normalized after rifaximin treatment, which is, again, an effect mediated by TLR4.( 18 )
Effects of rifaximin on synthesis and metabolism of bile acids and their enterohepatic circulation are yet unclear. A reduction in the secondary/primary fecal bile acids ratio after 8 weeks of rifaximin treatment was observed in 6 patients with cirrhosis.( 14 ) The relevance of this finding, however, is yet unclear because plasma bile acid composition was not affected in another study in 38 patients with cirrhosis after 90 days of rifaximin treatment.( 31 )
The effects of rifaximin on systemic inflammatory markers are not extensively studied in patients with cirrhosis. IL‐6, IL‐10, and/or TNF‐α levels in plasma were decreased in patients with NAFLD, alcohol‐associated cirrhosis, and cirrhosis with HE, respectively, with treatment periods ranging from 4 to 12 weeks.( 31 , 32 , 33 ) In contrast, no effects of rifaximin on systemic cytokine levels were observed in patients with cirrhosis and ascites after 4 weeks.( 15 ) In patients with NAFLD, rifaximin also improved insulin resistance, serum glucose, aspartate aminotransferase, alanine aminotransferase, and gamma‐glutamyl transpeptidase after 6 months.( 32 )
In contrast to patients with cirrhosis or NAFLD, rifaximin showed no effects on IL‐6 and IL‐10 mRNA levels in peripheral blood mononuclear cells, serum CD14 levels, or LPS in 40 patients with common variable immunodeficiency after 2 weeks.( 34 ) This suggests that the systemic inflammatory status may be regarded as a prerequisite for the beneficial anti‐inflammatory effects of rifaximin on patients with cirrhosis or NAFLD.
Given the barely detectable quantities of rifaximin in the systemic circulation, local effects in the gut have to be assumed as the basis for the beneficial effects of rifaximin in patients with liver diseases. The human intestinal epithelium may be regarded as the key player mediating effects of rifaximin in patients with cirrhosis. Experimental studies are underway exploring effects of rifaximin on human enterocyte transport, metabolism, and detoxification function. It is yet unclear whether rifaximin exerts its effects exclusively via PXR‐dependent mechanisms or also PXR‐independently. Further in‐depth molecular characterization of rifaximin’s effects on systemic inflammatory activity via its effects on the enterocyte and, thereby, the gut‐liver axis in patients with cirrhosis is clearly needed.
Gut Microbiome
The human gut microbiome is assumed to play a role in many diseases.( 35 , 36 ) Because the microbiome comprises trillions of microbial cells with high metabolic activity, it is likely that induced changes by specific treatments may be complex and prone to bias of interpretation.( 35 , 36 ) Indeed, the liver is the first organ encountering microbial products that cross the gut epithelial barrier; therefore, it is likely to be affected by the gut microbiome and its changes in many ways.( 37 ) Especially in end‐stage cirrhosis, bacterial translocation seems to play a decisive role in different complications, including acute‐on‐chronic liver failure (ACLF).( 26 , 38 )
Different studies have demonstrated that the effect of rifaximin on the gut microbiome is limited on phylum, class, order, and family level( 7 , 39 ) regardless of the part of the gastrointestinal tract that the sample was taken from, which is quite unexpected for a minimally absorbed antibiotic.( 40 ) Interestingly, other antibiotics, such as norfloxacin, that are recommended as prophylactic therapy in decompensated cirrhosis( 41 ) may have a stronger effect on the microbiome composition than rifaximin, at least in experimental cirrhosis.( 42 )
The question that arises is how rifaximin interacts with microbiome to improve HE and, potentially, other complications of cirrhosis. Maybe the very subtle changes of the microbiome composition (Lactobacillus, Streptococcus, Veillonella) are sufficient to reduce hyperammonemia and endotoxemia in cirrhosis.( 39 , 43 , 44 ) Those changes were consistent for Veillonella species only in the cohorts cited. The underlying mechanisms are still under investigation, but older studies have demonstrated that rifaximin strongly changes the metabolism of the host with increased circulating saturated and unsaturated fatty acids, which are associated with altered microbiome linkages with those metabolites.( 39 ) This observation in humans is paralleled by another study in mice, which demonstrates that rifaximin decreased small‐intestinal glutaminase and increased cecal glutamine content and probably thereby decreased ammonia production.( 45 ) Along these lines, a recent study showed that rifaximin can impact phage‐Streptococcus linkages, especially those that produce ammonia.( 46 ) However, the evidence so far is based either on cross‐sectional studies or longitudinal smaller studies, with diverging results not only for the host effects but also for the microbiome effects. We therefore hope the ongoing larger, longitudinal, and multicenter studies within the H2020 projects GALAXY, LIVERHOPE, and MICROB‐PREDICT may lead to better understanding of the mechanisms of action and possible stratification of patients who may or may not benefit from rifaximin treatment.
Effects of Rifaximin on Prevention and Management of Complications of Cirrhosis
Rifaximin in HE
Given the alterations in gut‐liver‐brain axis in HE, gut‐focused interventions have been the mainstay of therapy.( 47 ) Lactulose and lactitol are first‐line treatments, but they can be associated with adverse events that can limit their acceptance.( 48 ) Rifaximin, being an orally available, minimally absorbed, and safe medication, has been studied in all aspects of HE.
Although rifaximin was used off‐label for HE therapy across several European countries, the widespread use and labeling for HE specifically increased after the U.S. Food and Drug Administration approval based on the pivotal randomized controlled trial (RCT) by Bass et al. in 2010.( 4 ) Table 1 shows a summary of the experience with rifaximin in HE, and details of prominent trials are provided in Supporting Table S1.
TABLE 1.
Summary of Studies Reporting the Use of Rifaximin for Treatment or Prevention of HE in Cirrhosis
| Type of Study | Number of Trials (Sample Size) | Results |
|---|---|---|
| Short‐term therapy studies (5‐30 days) | ||
| Rifaximin vs. placebo | 1 (93) | Asterixis improved only with rifaximin. PSE index, mental status, and intellectual function improved similarly in both groups. |
| Rifaximin 200 mg vs. 400 mg vs. 800 mg per day | 1 (54) | PSE index improved only in 400‐mg and 800‐mg groups. |
| Rifaximin vs. other antibiotics | 7 (227) | Ammonia improved more with rifaximin than neomycin (1 RCT) or similarly in both (6 RCTs). PSE index improved similarly in both groups (1 RCT). Intellectual function or mental status improved similarly in both groups (5 RCTs). Asterixis improved faster with rifaximin than with neomycin (1 RCT). |
| Rifaximin vs. nonabsorbable disaccharides | 6 (448) | Higher ammonia improvement with rifaximin (3 RCTs) or similarly in both groups (3 RCTs) |
| Long‐term studies (3‐6 months cyclical) | ||
| Rifaximin vs. nonabsorbable disaccharides | 2 (80) | Ammonia and mental status improved with both trials with all strategies compared with baseline. Higher improvement in PSE index, EEG, and mental status with rifaximin. In the second study, rifaximin ± lactitol did better than lactitol alone with mental status. |
| Rifaximin vs. neomycin | 1 (60) | Improvement in psychometric/neurophysiologic tests, mental status, and ammonia were similar across both groups. |
| Inpatient use | ||
| Rifaximin + lactulose vs. lactulose alone | 1 (120) | Higher HE reversal and lower death in group given rifaximin and lactulose compared with lactulose alone |
| Prevention of recurrence | ||
| Rifaximin vs. placebo | 1 (299) | Reduction in recurrent HE episodes and hospitalization in the rifaximin group with significantly higher improvement in neurophysiological, quality of life, and ammonia in the rifaximin group. In both groups, 91% of patients were on lactulose. |
| Real‐world and open‐label rifaximin experience | ||
| Open‐label extension and precomparison/postcomparison | 2 (474) | Open‐label extension and addition of new patients on rifaximin was associated with continued reduction in HE‐related and all‐cause hospitalization, and conversion of patients from placebo to rifaximin further reduced HE episodes. |
| Evaluation of health care systems after rifaximin introduction | 5 (760) | Reduction in mean hospitalizations, readmissions, and length of stay |
Adapted from Bajaj and Riggio 2010.(53) PSE index: a composite score for HE consisting of 100 × (mental status [Conn score] × 3 + asterixis grade × 1 + NCT grade × 1 + ammonia grade × 1 + EEG grade × 1 [if available]); it is not widely used at this time. EEG, electroencephalogram; PSE, portosystemic encephalopathy.
Covert HE Therapy
Covert HE (CHE) is very common and can be associated with poor survival, overt HE development, and psychosocial impact. Double‐blind RCTs have shown that rifaximin is better than placebo with respect to improvement in cognition, quality of life, and driving capability.( 48 , 49 ) Although the mechanism is unclear, changes in microbial function and immune features and enhanced brain activation have been found. The European Association for the Study of the Liver/American Association for the Study of Liver Diseases guidelines recommend therapy for CHE on a case‐by‐case basis.( 44 ) However, rifaximin therapy for CHE is not currently cost‐effective, even to prevent major outcomes, such as traffic crashes.( 50 )
Inpatient Therapy for HE
The four prongs for the therapy of HE in patients who are hospitalized include (a) caring for the patient who is unconscious, (b) evaluating other causes of altered mental status, (c) identifying and treating precipitating factors, and (d) starting empiric therapy.( 47 ) All these processes need to occur simultaneously. The first‐line empiric therapy in most countries remains lactulose. The role of rifaximin in this setting is unclear.( 47 )
Prior smaller‐scale trials have shown that rifaximin may be useful in reducing blood ammonia and asterixis compared with selected therapies, such as neomycin and nonabsorbable disaccharides. One large open‐label study evaluated rifaximin compared with lactulose with clinical outcomes centered on survival and mental status recovery.( 51 ) In that study, rifaximin therapy was better than lactulose in inpatient outcomes. These results need to be replicated in other centers. Rifaximin also has not been directly compared with L‐ornithine L‐aspartate therapy, which in some studies has shown improvement in mental status.( 52 )
Until now, there is a trend toward off‐label use of rifaximin in inpatients, but it is most likely a continuation of rifaximin that they were already on prior to hospitalization. The use of rifaximin on discharge of patients with HE is a major quality indicator of therapy because it is associated with a lower risk of HE recurrence (see the following).
Prevention of Recurrence
The pivotal trial by Bass et al. randomized 299 patients with multiple HE episodes into receiving daily rifaximin 550 mg bis in die (twice a day) versus placebo over 6 months.( 4 ) The primary endpoint was breakthrough hospitalizations because of HE, and secondary endpoints included prevention of hospitalizations with HE. More than 90% of patients were on lactulose at baseline, which was continued throughout the trial. In those randomized to rifaximin, there was a significant reduction in breakthrough events and hospitalizations over the 6 months. The number needed to treat was 4 and 9 for the above two outcomes, respectively. However, most participants had MELD score <19, and the initial usage was focused on these patients with >2 episodes. This trial followed smaller‐scale trials from Europe in which long‐term cyclical use was studied with good outcomes. However, the newer formulation’s ability to use it twice a day and drug agency approval meant that it could be prescribed for patients to prevent HE.( 53 )
“Real‐World” Use of Rifaximin
Open‐label experiences and evaluation of a placebo‐assigned group that was subsequently given rifaximin showed continued reduction in HE‐related episodes, even outside the clinical trial setting.( 54 ) Studies focused on changes in resource utilization before rifaximin compared with after rifaximin showed that it can lead to reduction in HE episodes and hospital admissions.
Barriers to Rifaximin Therapy
Cost and lack of awareness remain major issues to rifaximin therapy. Even in tertiary care centers, 12.5% of patients who were admitted with HE were subsequently discharged without HE‐specific medications, which translated directly into readmissions.( 55 ) Given the expense of hospitalizations, studies have shown that rifaximin is cost‐effective for prevention of recurrence, but greater education of providers, patients, and caregivers is needed.( 56 )
Given its track record of acceptance by patients and efficacy in prevention of HE, further trials that evaluate the role of rifaximin in CHE and inpatient therapy of HE are needed, especially in combination or head‐to‐head against other established therapies.
Effects of Rifaximin on Bacterial Infections
Based on its pharmacological characteristics and the putative effects on pathogenetic mechanisms of cirrhosis, rifaximin is considered by many hepatologists to be the logical alternative to quinolones or other systemic antibiotics for the prophylaxis of spontaneous bacterial peritonitis (SBP) and potentially of non‐SBP bacterial infections, with the belief that rifaximin is active on a broader range of intestinal gram‐positive and gram‐negative bacteria, is safer, and carries a low risk of inducing resistance to antibiotics.
Unfortunately, after more than a decade of clinical research, a conclusive indication on the use of rifaximin in prevention of bacterial infections cannot currently be given. Almost all the published data are related to primary or secondary prophylaxis for SBP. In general, retrospective studies have shown that the use of rifaximin is associated with lower incidence of SBP and other complications of the disease.( 57 , 58 ) Conversely, prospective observational studies have yielded less consistent results.( 59 ) Only a few RCTs have been performed so far, in Egypt and South Arabia, comparing the efficacy of rifaximin versus norfloxacin; in these studies, an advantage was seen in favor of rifaximin given for primary or secondary SBP( 60 , 61 ); in the former study, rifaximin was also given alternated to norfloxacin and compared with norfloxacin alone.( 60 ) However, the strength of these positive results is undermined by some methodological drawbacks.
To overcome the limitations inherent to each single study, several systematic reviews and meta‐analysis assessing both the observational cohort studies and the RCTs have been performed. Again, results suggest that only low‐quality evidence supports the superiority of rifaximin over norfloxacin or other systemic antibiotics for either primary or secondary SBP prophylaxis.( 6 , 62 , 63 ) In all these studies, the high heterogeneity in terms of patient inclusion criteria, type and modalities of administration of the comparative prophylactic therapies, primary and secondary endpoints, and the moderate‐to‐high risk of methodological biases related to randomization, blinding, attrition, and intention‐to‐treat analysis preclude the possibility to reach solid conclusions.( 6 , 62 , 63 )
Effects of Rifaximin on Other Complications of Cirrhosis
Several studies have assessed the impact of rifaximin in prevention of complications of cirrhosis other than HE and bacterial infections( 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 ) (Table 2). Data are not univocal and are often burdened by serious methodological issues. Taking this into consideration, it is suggested that, by reducing serum levels of proinflammatory mediators linked to intestinal bacterial translocation, rifaximin may decrease portal hypertension and improve systemic hemodynamics (increasing systemic vascular resistances and mean arterial pressure) and renal function (improving glomerular filtration rate and urinary sodium excretion).( 23 , 24 , 25 , 66 , 70 ) Hence, valuable clinical benefits have been associated with rifaximin, including a reduction in the incidence of decompensation of cirrhosis, all‐cause hospitalizations and readmissions, variceal bleeding, and acute kidney injury, including hepatorenal syndrome, with a decreased risk of renal replacement therapy.( 58 , 64 , 65 , 67 , 68 , 69 , 72 ) A better control of difficult‐to‐treat/refractory ascites was also shown when rifaximin was added to treatment.( 70 , 71 ) A reduction in mortality was suggested in some studies.( 51 , 58 , 65 , 70 , 71 ) Although promising, these data must be confirmed by well‐conducted, large RCTs.
TABLE 2.
Summary of Studies Evaluating the Effect of Rifaximin on Complications of Cirrhosis Other Than HE and Bacterial Infections
| Reference | Study Design | Patient Population | Main Outcomes |
|---|---|---|---|
| Assem et al. 2016( 60 ) | Prospective randomized, open‐label, comparative multicenter study | 239 patients with cirrhosis with ascites randomized to 3 groups: rifaximin (550 mg bid), norfloxacin, or alternating rifaximin/norfloxacin | Primary outcome: incidence of SBP |
| Overall, 10 patients developed HRS (6 patients [7.6%] in the norfloxacin group, 2 [2.4%] in the rifaximin group and 2 [2.5%] in the combined group; P > 0.05). | |||
| HRS was the main cause of mortality. | |||
| Sharma et al. 2013( 51 ) | RCT | 120 patients with overt HE randomized into 2 groups: lactulose + rifaximin (1,200 mg/day) vs. lactulose + placebo | Primary outcome: reversal of HE |
| Addition of rifaximin was associated with the following: | |||
| ‐ Reduced mortality (24% vs. 49%, P < 0.05), with no differences in HRS‐related and gastrointestinal bleeding–related deaths | |||
| Dong et al. 2016( 64 ) | Retrospective study | 88 patients on rifaximin (550 mg bid) for ≥90 days vs. 88 matched controls | Primary outcomes: incidence of AKI and HRS |
| Rifaximin reduced the following: | |||
| ‐ The incidence rate ratio of AKI (IRR, 0.71; 95% CI, 0.54‐0.94) and HRS (IRR, 0.21; 95% CI, 0.06‐0.70) | |||
| ‐ The requirement of RRT (5.7% vs. 15.9%; OR, 0.23; 95% CI, 0.07‐0.74) | |||
| Vlachogiannakos et al. 2013( 65 ) | Prospective, nonrandomized case‐control study | 23 patients with known hemodynamic response (HVPG) to short‐term rifaximin treated with long‐term rifaximin (1,200 mg/day) vs. 46 matched controls | Primary outcomes: survival, variceal bleeding, HE, SBP, HRS |
| Rifaximin therapy was as follows: | |||
| ‐ An independent negative predictor of variceal bleeding (RH, 0.246; 95% CI, 0.069‐0.870; P = 0.03) | |||
| ‐ The only factor associated with lower probability of HRS (RH, 0.110; 95% CI, 0.012‐0.973; P = 0.047) | |||
| ‐ Predictor of 5‐year survival (RH for mortality, 0.258; 95% CI, 0.075‐0.891; P = 0.032) | |||
| Kimer et al. 2017( 24 ) | Double‐blind RCT | 54 stable outpatients with cirrhosis and ascites with HVPG ≥ 10 mm Hg randomized to rifaximin 550 mg bid (n = 36) or placebo bid (n = 18) for 4 weeks | Primary outcomes: hepatic and systemic hemodynamics, renal function |
| No effect of rifaximin on the following: | |||
| ‐ HVPG (P = 0.94) | |||
| ‐ PRA (P = 0.12) or other vasoactive hormones (P = NS) | |||
| ‐ GFR (P = 0.14) | |||
| Kalambokis et al. 2012( 66 ) | Open‐label, prospective, single‐center pilot study | 13 patients with alcohol‐associated cirrhosis and ascites treated with rifaximin (1,200 mg/day) for 4 weeks | Primary outcomes: systemic hemodynamics and renal function |
| Rifaximin: | |||
| ‐ increased MAP (P = 0.05) in keeping with increased SVR (P = 0.01), decreased PRA (P = 0.02), and decreased CO (P = 0.02) | |||
| ‐ improved renal function, consistent with increase in GFR (P = 0.006) and urinary sodium excretion (P = 0.03) | |||
| ‐ decreased plasma endotoxin (P = 0.005), IL‐6 (P = 0.01), and TNF‐α (P = 0.02) levels | |||
| Vlachogiannakos et al. 2009( 23 ) | Prospective study | 30 patients with alcohol‐associated cirrhosis and ascites treated with rifaximin (1,200 mg/day) for 28 days | Primary outcomes: hepatic hemodynamics |
| Rifaximin: | |||
| ‐ decreased HVPG (P < 0.0001) | |||
| ‐ increased MAP (P < 0.05) | |||
| ‐ reduced plasma endotoxin levels both in systemic (P < 0.0001) and splanchnic circulation (P < 0.0001) | |||
| Lim et al. 2017( 25 ) | Open‐label RCT | 73 patients with HVPG ≥ 12 mm Hg randomized to propranolol monotherapy (n = 54) or rifaximin (1,200 mg/day) + propranolol (n = 19) for 3 months | Primary outcome: HVPG response rate |
| The combination therapy achieved the following: | |||
| ‐ a significant decline of HVPG (P = 0.016) | |||
| ‐ higher HVPG response rate than propranolol alone (87% vs. 56%; P = 0.034). | |||
| ‐ higher rates of reduction of LPS (P = 0.009) and LPS binding protein (P = 0.002) compared with propranolol monotherapy | |||
| Salehi et al. 2019( 67 ) | Retrospective cohort study | 101 patients with HE: 66 treated with rifaximin vs. 35 naïve | Primary outcome: all‐cause emergency hospital admissions |
| Rifaximin therapy achieved the following: | |||
| ‐ reduced all‐cause admissions (P = 0.037) | |||
| ‐ reduced admissions for complications of ascites, including HRS (P = 0.008) and variceal bleeding (P = 0.026) | |||
| ‐ increased time to hospital readmission (P = 0.040) | |||
| Kang et al. 2017( 58 ) | Retrospective cohort study | 1,042 patients with previous HE: 621 patients with HCC (173 receiving rifaximin 1,200 mg/day + lactulose, 448 controls receiving lactulose alone) and 421 without HCC (145 rifaximin + lactulose, 276 lactulose alone) | Primary outcome: overall survival |
| Rifaximin was associated with lower risk of variceal bleeding in the following: | |||
| ‐ Entire cohort (HR, 0.520; 95% CI, 0.349‐0.773; P = 0.001) | |||
| ‐ Non‐HCC cohort (aHR, 0.425; 95% CI, 0.220‐0.821; P = 0.011) | |||
| Rifaximin was associated with a nonsignificant trend toward lower risk of HRS in the following: | |||
| ‐ Non‐HCC cohort (HR, 0.595; 95% CI, 0.334‐1.060; P = 0.078) | |||
| Rifaximin was associated with a lower risk of death in the following: | |||
| ‐ Entire cohort (HR, 0.702; 95% CI, 0.504‐0.978; P = 0.036) | |||
| ‐ Non‐HCC cohort (aHR, 0.697; 95% CI, 0.510‐0.954; P = 0.024) | |||
| Flamm et al. 2018( 68 ) | Post hoc analysis of a placebo‐controlled multicenter RCT | 299 patients with cirrhosis and HE in remission, randomized to rifaximin 550 mg bid (n = 140) or placebo (n = 159) for 6 months | Primary outcome of RCT: time to a breakthrough episode of overt HE |
| In patients with MELD ≥ 12 and INR ≥ 1.2, rifaximin was associated with the following: | |||
| ‐ Reduced risk of any first complication (HR, 0.41; 95% CI, 0.25‐0.67; P < 0.001) | |||
| ‐ Nonsignificant trend for reduction in the overall risk of non‐HE complications, including variceal bleeding and AKI/HRS (HR, 0.46; 95% CI, 0.18‐1.17; P = 0.10) | |||
| In patients with MELD <12 and INR <1.2, rifaximin was associated with the following: | |||
| ‐ Nonsignificant trend for reduction in the risk of any complication (HR, 0.26; 95% CI, 0.06‐1.20; P = 0.06) | |||
| Ibrahim et al. 2017( 69 ) | RCT | 80 patients with decompensated cirrhosis randomized to rifaximin 550 mg bid for 12 weeks (n = 40) or no treatment (n = 40) | Primary outcome: incidence of HRS |
| ‐ Lower incidence of HRS in the rifaximin group (5% vs. 22.5%; P = 0.048) | |||
| ‐ Being in the control group was a predictor of HRS at univariate analysis but not at multivariate analysis (OR, 3.01; 95% CI, 0.46‐19.52; P = 0.249) | |||
| Hanafy and Hassaneen 2016( 70 ) | RCT | 600 patients with refractory or rapidly recurrent ascites randomized to diuretic therapy + midodrine 5 mg tid + rifaximin 550 mg bid (n = 400) or diuretic therapy alone (n = 200) | Primary outcomes: control of ascites |
| Rifaximin + midodrine was associated with the following: | |||
| ‐ Improvement in hemodynamics, as expressed by increase in MAP and reduction in PRA and aldosterone (P < 0.05 for all variables) | |||
| ‐ Improvement in renal function, as assessed by serum creatinine and BUN decrease, serum sodium increase, 24‐hour urine output and urinary sodium excretion increase (P < 0.05 for all variables) | |||
| ‐ Better control of ascites, as expressed by a reduction in tapping of ascites (P < 0.0001), weight loss (P < 0.0001), higher rates of complete disappearance of ascites (80% vs. 15%; P < 0.0001) | |||
| ‐ Improvement in overall survival (19.6 vs. 11.6 months; P < 0.0001) | |||
| Lv et al. 2020( 71 ) | Prospective observational study | 75 patients with ascites receiving SMT (n = 25) or SMT + rifaximin 200 mg qid for 3 to 4 weeks (n = 50) | Primary outcomes: control of ascites and survival |
| Rifaximin was associated with the following: | |||
| ‐ Better control of ascites, as shown by enhanced response to diuretic therapy (P = 0.009) and more marked weight loss (P = 0.011) | |||
| ‐ Increased survival (HR, 2.53; 95% CI, 1.01‐6.38; P = 0.048) |
Abbreviations: aHR, HR adjusted for Child‐Pugh class; AKI, acute kidney injury; bid, bis in die (twice a day); BUN, blood urea nitrogen; CO, cardiac output; GFR, glomerular filtration rate; HRS, hepatorenal syndrome; INR, international normalized ratio; IRR, incidence rate ratio; MAP, mean arterial pressure; PRA, plasma renin activity; qid, quater in die (four times a day); RH, relative hazard; RRT, renal replacement therapy; SMT, standard medical therapy; SVR, systemic vascular resistance; tid, ter in die (three times a day).
Side Effects
In the rifaximin data sheet, dizziness, headache, constipation, abdominal pain, diarrhea, flatulence, nausea, rectal tenesmus, vomiting, and pyrexia are listed as frequent side effects (>1/100 to <1/10).
When used in HE, rifaximin side effects have been mild and infrequent. In a review article including the pivotal prophylaxis trial, rifaximin was well tolerated, and adverse events were similar to placebo group.( 4 , 73 ) In the pivotal study, there were two cases of infection by C. difficile versus none in the control group. In the extension study, the frequency of this infection did not increase and was reported in 6 patients.( 74 ) All these patients presented predisposing factors for this infection. Postmarketing studies also described cases of C. difficile infection.( 75 ) When rifaximin was used in prophylaxis of SBP, few side effects were observed. In a study, no side effects were described,( 57 ) and in others, they were uncommon, including abdominal pain, flatulence, nausea, dizziness, and headache.( 60 , 61 )
It has been suggested that rifaximin may favor the muscular toxicity of statins in cirrhosis.( 11 ) This possibility seems unlikely because only three cases of rifaximin rhabdomyolysis have been described in a postmarketing database,( 75 ) and it has not been observed in patients with cirrhosis treated with low doses of simvastatin (20 mg/day) together with rifaximin.( 76 )
Future Directions
Currently, the only accepted indication for rifaximin in cirrhosis is prevention of recurrent HE.( 46 ) Use of rifaximin for other indications, such as prevention of bacterial infections, including SBP, is not supported by strong evidence; therefore, rifaximin should not be used in clinical practice for indications other than HE (Table 3). Nonetheless, because of its complex effects in gut‐liver axis and modulating gut microbiome, the potential efficacy of rifaximin in prevention or management of complications of cirrhosis other than HE deserves to be investigated extensively. Ideally, studies should be double‐blinded, with rifaximin alone or in combination with other drugs, include large patient populations, and aimed at hard clinical endpoints, particularly prevention of decompensated cirrhosis, bacterial infections, and ACLF. Some of these studies are underway, and results are eagerly awaited (Table 4).
TABLE 3.
Recommendations for Use of Rifaximin in the Management of Complications of Cirrhosis in Clinical Practice
| Recommended | Recommended on a Case‐by‐Case Basis | Needs Further Research and Therefore Not Recommended Currently |
|---|---|---|
| Prevention of recurrence of HE | CHE (treatment not cost‐effective) | Inpatient therapy of episodes of overt HE |
| Prevention of SBP recurrence | ||
| Prevention of other complications of cirrhosis |
TABLE 4.
Studies Reported in clinicaltrials.gov Investigating the Use of Rifaximin in Cirrhosis
| Endpoints | Number of Studies |
|---|---|
| Prevention of complications of cirrhosis | |
| SBP | 4 |
| CHE | 3 |
| Decompensated cirrhosis | 2 |
| HE in patients with transjugular intrahepatic portosystemic shunts | 2 |
| ACLF* | 1 |
| Variceal bleeding | 1 |
| Renal failure | 1 |
| Persistent HE | 1 |
| Other | |
| Portal pressure | 2 |
| Systemic inflammation | 1 |
| B‐cell dysregulation | 1 |
| Portal vein thrombosis | 1 |
| Quality of life | 1 |
Associated with simvastatin.
Conclusions
In recent years, the paradigm on pathophysiology of decompensation of cirrhosis has deeply changed. It is now clear that decompensated cirrhosis is characterized by sustained proinflammatory and pro‐oxidant milieu resulting from the systemic spread of pathogen‐associated molecular patterns (PAMPs) and damage‐associated molecular patterns from gut and diseased liver, respectively. The deep alterations in their gut‐liver axis favoring translocation of PAMPs as well as viable bacteria have been the rationale for the use of rifaximin in prevention of several complications of cirrhosis. Rather than having a bactericidal effect, rifaximin seems to have direct effects on bacterial function and virulence. Confirming this, its use results in very little change in stool microbiome in cirrhosis. In addition, rifaximin does not appear to increase antibiotic resistance rates. Therefore, rifaximin represents an antibiotic strategy that may prevent infections and other complications of cirrhosis without development of multidrug‐resistant bacteria. However, beyond the prevention of recurrent HE, there are currently no other recommended indications for its use in cirrhosis. Indeed, several studies have already shown a potential favorable impact of rifaximin on several steps in pathophysiology of decompensation. Accordingly, beneficial effects of rifaximin have been described. Unfortunately, most of these data are not the result of high‐quality RCTs but rather of not‐controlled “real‐world” studies, meta‐analysis, or systematic reviews. This can explain why, up to now, the results are not so univocal and consistent as to be translated into recommendations.
Author Contributions
P.G., P.C., E.P., and E.S. all participated to the conception, design and coordination of the review. All the authors contributed to drafting the article and revising it critically for important intellectual content and gave the final approval of the version to be published.
Supporting information
Table S1
Acknowledgment
The Liverhope Consortium investigators: Hospital Clinic de Barcelona and IDIBAPS: Adrià Juanola, Laura Napoleone, Marta Carol, Emma Avitabile, Ann Ma Thu, Marta Cervera, Martina Pérez, Ana Belén Rubio‐Garcia, Alicia Ardiaca, Adriana Vives, Judit Pich University of Barcelona, School of Medicine and Health Sciences: Núria Fabrellas University of Bologna: Giacomo Zaccherini; Maria Teresa Chiappa Hospital Vall d’Hebron: César Jiménez, Ester Palacio University of Torino: Daniela Campion, Tommaso Lanzillotti University of Padova: Salvatore Piano, Gea Nicolao Goethe‐University Frankfurt: Frank Uschner, Sabine Graf_Dirmeier Hospital Beaujon: Claire Francoz, Olivier Roux, Vanessa Esnault Amsterdam University Medical Centers, location AMC : Jeltje Helder Royal Free Hospital: Marites Aban, Konstantin Kazankov ELPA: Marko Korenjak Mayo Clinic Rochester: Patrick Kamath University Hospital of Alberta: Juan G. Abraldes Evotec: Hugh Watson. We thank Roser Poblet and Nicki van Berckel for their work in the preparation of the manuscript.
Supported by a H2020 Grant – LIVERHOPE – project number: 731875. The work described has been funded by a H2020 Grant – LIVERHOPE – project number: 731875. P.G. has been funded by grant numbers PI16/00043 and PI20/00529, and E.S. is funded by PI18/00727, both of which are integrated in the Plan Nacional I+D+I and cofunded by ISCIII‐Subdirección General de Evaluación and European Regional Development Fund FEDER. E.P., E.S., P.G., and other members of the LIVERHOPE Consortium have been funded by a grant from AGAUR 2017SGR‐01281.
Potential conflict of interest: Dr. Angeli is on the speakers’ bureau and received grants from Behring. He advises Ferring and Biovie. Dr. Caraceni advises and is on the speakers’ bureau for Grifols. He is on the speakers’ bureau for and received grants from Octapharma. He advises Mallinckrodt. He is on the speakers’ bureau for Kedrion. Dr. Vargas advises Promethere and is on the speakers’ bureau for Intercept. Dr. Alessandria consults for Alfa Sigma. Dr. Krag advises and is on the speakers’ bureau for Norgie. Dr. Bajaj advises Norgine and received grants from Bausch Health. Dr. Gines advises and received grants from Grifols, Gilead, and Mallinckrodt. He advises Novartis.
Contributor Information
Pere Ginès, Email: pgines@clinic.cat.
for the Liverhope Consortium:
Adrià Juanola, Laura Napoleone, Marta Carol, Emma Avitabile, Ann Ma Thu, Marta Cervera, Martina Pérez, Ana Belén Rubio‐Garcia, Alicia Ardiaca, Adriana Vives, Judit Pich, Núria Fabrellas, Giacomo Zaccherini, Maria Teresa Chiappa, César Jiménez, Ester Palacio, Daniela Campion, Tommaso Lanzillotti, Salvatore Piano, Gea Nicolao, Frank Uschner, Sabine Graf_Dirmeier, Claire Francoz, Olivier Roux, Vanessa Esnault, Jeltje Helder, Marites Aban, Konstantin Kazankov, Marko Korenjak, Patrick Kamath, Juan G. Abraldes, and Hugh Watson
References
- 1. Macbeth WA, Kass EH, McDermott WV Jr. Treatment of hepatic encephalopathy by alteration of intestinal flora with lactobacillus acidophilus. Lancet 1965;1:399‐403. [DOI] [PubMed] [Google Scholar]
- 2. Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med 1997;337:473‐479. [DOI] [PubMed] [Google Scholar]
- 3. Conn HO, Leevy CM, Vlahcevic ZR, Rodgers JB, Maddrey WC, Seeff L, et al. Comparison of lactulose and neomycin in the treatment of chronic portal‐systemic encephalopathy. A double‐blind controlled trial. Gastroenterology 1977;72:573‐583. [PubMed] [Google Scholar]
- 4. Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010;362:1071‐1081. [DOI] [PubMed] [Google Scholar]
- 5. Bajaj JS, Khoruts A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J Hepatol 2020;72:1003‐1027. [DOI] [PubMed] [Google Scholar]
- 6. Goel A, Rahim U, Nguyen LH, Stave C, Nguyen MH. Systematic review with meta‐analysis: rifaximin for the prophylaxis of spontaneous bacterial peritonitis. Aliment Pharmacol Ther 2017;46:1029‐1036. [DOI] [PubMed] [Google Scholar]
- 7. Bajaj JS. Review article: Potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis. Aliment Pharmacol Ther 2016;43(Suppl 1):11‐26. [DOI] [PubMed] [Google Scholar]
- 8. Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: Pharmacology and clinical potential. Chemotherapy 2005;51(Suppl 1):36‐66. [DOI] [PubMed] [Google Scholar]
- 9. Huang DB, DuPont HL. Rifaximin – A novel antimicrobial for enteric infections. J Infect 2005;50:97‐106. [DOI] [PubMed] [Google Scholar]
- 10. Xifaxan (rifaximin) tablets, for oral use [package insert]. Bridgewater, NJ: Salix Pharmaceuticals; 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021361s023lbl.pdf. [Google Scholar]
- 11. Cacciottolo TM, Kingdon A, Alexander GJ. Rifaximin is largely safe and well tolerated but caution is necessary when taken with statins. Clin Gastroenterol Hepatol 2014;12:1765. [DOI] [PubMed] [Google Scholar]
- 12. Ojetti V, Lauritano EC, Barbaro F, Migneco A, Ainora ME, Fontana L, et al. Rifaximin pharmacology and clinical implications. Expert Opin Drug Metab Toxicol 2009;5:675‐682. [DOI] [PubMed] [Google Scholar]
- 13. DuPont HL. Review article: The antimicrobial effects of rifaximin on the gut microbiota. Aliment Pharmacol Ther 2016;43(Suppl 1):3‐10. [DOI] [PubMed] [Google Scholar]
- 14. Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol 2013;58:949‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kimer N, Pedersen JS, Tavenier J, Christensen JE, Busk TM, Hobolth L, et al. Rifaximin has minor effects on bacterial composition, inflammation, and bacterial translocation in cirrhosis: a randomized trial. J Gastroenterol Hepatol 2018;33:307‐314. [DOI] [PubMed] [Google Scholar]
- 16. Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long‐term exposure to ethanol. Gastroenterology 1995;108:218‐224. [DOI] [PubMed] [Google Scholar]
- 17. Plummer JL, Ossowicz CJ, Whibley C, Ilsley AH, Hall PD. Influence of intestinal flora on the development of fibrosis and cirrhosis in a rat model. J Gastroenterol Hepatol 2000;15:1307‐1311. [PubMed] [Google Scholar]
- 18. Zhu Q, Zou L, Jagavelu K, Simonetto DA, Huebert RC, Jiang ZD, et al. Intestinal decontamination inhibits TLR4 dependent fibronectin‐mediated cross‐talk between stellate cells and endothelial cells in liver fibrosis in mice. J Hepatol 2012;56:893‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitagawa R, Kon K, Uchiyama A, Arai K, Yamashina S, Kuwahara‐Arai K, et al. Rifaximin prevents ethanol‐induced liver injury in obese KK‐A(y) mice through modulation of small intestinal microbiota signature. Am J Physiol Gastrointest Liver Physiol 2019;317:G707‐G715. [DOI] [PubMed] [Google Scholar]
- 20. Descombe JJ, Dubourg D, Picard M, Palazzini E. Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int J Clin Pharmacol Res 1994;14:51‐56. [PubMed] [Google Scholar]
- 21. Madsen BS, Trebicka J, Thiele M, Israelsen M, Arumugan M, Havelund T, et al. Antifibrotic and molecular aspects of rifaximin in alcoholic liver disease: Study protocol for a randomized controlled trial. Trials 2018;19:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mookerjee RP. Acute‐on‐chronic liver failure: The liver and portal haemodynamics. Curr Opin Crit Care 2011;17:170‐176. [DOI] [PubMed] [Google Scholar]
- 23. Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, et al. Intestinal decontamination improves liver haemodynamics in patients with alcohol‐related decompensated cirrhosis. Aliment Pharmacol Ther 2009;29:992‐999. [DOI] [PubMed] [Google Scholar]
- 24. Kimer N, Pedersen JS, Busk TM, Gluud LL, Hobolth L, Krag A, et al. Rifaximin has no effect on hemodynamics in decompensated cirrhosis: A randomized, double‐blind, placebo‐controlled trial. Hepatology 2017;65:592‐603. [DOI] [PubMed] [Google Scholar]
- 25. Lim YL, Kim MY, Jang YO, Baik SK, Kwon SO. Rifaximin and propranolol combination therapy is more effective than propranolol monotherapy for the reduction of portal pressure: An open randomized controlled pilot study. Gut Liv 2017;11:702‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albillos A, de Gottardi A, Rescigno M. The gut‐liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol 2020;72:558‐577. [DOI] [PubMed] [Google Scholar]
- 27. Mencarelli A. Inhibition of NF‐κβ by a PXR‐dependent pathway mediates counter‐regulatory activities of rifaximin on innate immunity in intestinal epithelial cells. Eur J Pharmacol 2011;668:317‐324. [DOI] [PubMed] [Google Scholar]
- 28. Ma X, Shah YM, Guo GL, Wang T, Krausz KW, Idle JR, et al. Rifaximin is a gut‐specific human pregnane X receptor activator. J Pharmacol Exp Ther 2007;322:391‐398. [DOI] [PubMed] [Google Scholar]
- 29. Cheng J, Shah YM, Ma X, Pang X, Tanaka T, Kodama T, et al. Therapeutic role of rifaximin in inflammatory bowel disease: Clinical implication of human pregnane X receptor activation. J Pharmacol Exp Ther 2010;335:32‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Esposito G, Nobile N, Gigli S, Seguella L, Pesce M, d'Alessandro A, et al. Rifaximin improves Clostridium difficile toxin A‐induced toxicity in Caco‐2 cells by the PXR‐dependent TLR4/MyD88/NF‐κB pathway. Front Pharmacol 2016;7:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel VC, Shawcross DL, McPhail MJL. Scientific report: A placebo controlled single centre double blind randomised trial to investigate the efficacy of RIFaximin versus placebo in improving SYStemic inflammation and neutrophil malfunction in patients with cirrhosis and chronic hepatic encephalopathy. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2013‐004708‐20/results. Published December 5, 2018.
- 32. Abdel‐Razik A, Mousa N, Shabana W, Refaey M, Elzehery R, Elhelaly R, et al. Rifaximin in nonalcoholic fatty liver disease: Hit multiple targets with a single shot. Eur J Gastroenterol Hepatol 2018;30:1237‐1246. [DOI] [PubMed] [Google Scholar]
- 33. Kalambokis GN, Mouzaki A, Rodi M, Tsianos EV. Rifaximin improves thrombocytopenia in patients with alcoholic cirrhosis in association with reduction of endotoxaemia. Liver Int 2012;32:467‐475. [DOI] [PubMed] [Google Scholar]
- 34. Jørgensen SF, Macpherson ME, Bjørnetrø T, Holm K, Kummen M, Rashidi A, et al. Rifaximin alters gut microbiota profile, but does not affect systemic inflammation ‐ A randomized controlled trial in common variable immunodeficiency. Sci Rep 2019;9:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35. Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, et al. Extensive impact of non‐antibiotic drugs on human gut bacteria. Nature 2018;555:623‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmidt TSB, Raes J, Bork P. The human gut microbiome: From association to modulation. Cell 2018;172:1198‐1215. [DOI] [PubMed] [Google Scholar]
- 37. Trebicka J, Reiberger T, Laleman W. Gut‐liver axis links portal hypertension to acute‐on‐chronic liver failure. Visc Med 2018;34:270‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute‐on‐chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426‐1437.e9. [DOI] [PubMed] [Google Scholar]
- 39. Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One 2013;8:e60042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schulz C, Schutte K, Vilchez‐Vargas R, Vasapolli R, Malfertheiner P. Long‐term effect of rifaximin with and without lactulose on the active bacterial assemblages in the proximal small bowel and faeces in patients with minimal hepatic encephalopathy. Dig Dis 2019;37:161‐169. [DOI] [PubMed] [Google Scholar]
- 41. European Association for the Study of the Liver . EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406‐460. [DOI] [PubMed] [Google Scholar]
- 42. Gomez‐Hurtado I, Gimenez P, Garcia I, Zapater P, Frances R, Gonzalez‐Navajas JM, et al. Norfloxacin is more effective than Rifaximin in avoiding bacterial translocation in an animal model of cirrhosis. Liver Int 2018;38:295‐302. [DOI] [PubMed] [Google Scholar]
- 43. Zeng X, Tang XJ, Sheng X, Ni W, Xin HG, Chen WZ, et al. Does low‐dose rifaximin ameliorate endotoxemia in patients with liver cirrhosis: A prospective study. J Dig Dis 2015;16:665‐674. [DOI] [PubMed] [Google Scholar]
- 44. Kawaguchi T, Suzuki F, Imamura M, Murashima N, Yanase M, Mine T, et al. Rifaximin‐altered gut microbiota components associated with liver/neuropsychological functions in patients with hepatic encephalopathy: An exploratory data analysis of phase II/III clinical trials. Hepatol Res 2019;49:404‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang DJ, Kakiyama G, Betrapally NS, Herzog J, Nittono H, Hylemon PB, et al. Rifaximin exerts beneficial effects independent of its ability to alter microbiota composition. Clin Transl Gastroenterol 2016;7:e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bajaj JS, Sikaroodi M, Shamsaddini A, Henseler Z, Santiago Rodríguez T, Acharya C, et al. Interaction of bacterial metagenome and virome in patients with cirrhosis and hepatic encephalopathy. Gut 2020. Sept 30. 10.1136/gutjnl-2020-322470. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47. Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715‐735. [DOI] [PubMed] [Google Scholar]
- 48. Sidhu SS, Goyal O, Mishra BP, Sood A, Chhina RS, Soni RK. Rifaximin improves psychometric performance and health‐related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial). Am J Gastroenterol 2011;106:307‐316. [DOI] [PubMed] [Google Scholar]
- 49. Bajaj JS, Heuman DM, Wade JB, Gibson DP, Saeian K, Wegelin JA, et al. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology 2011;140:478‐487.e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bajaj JS, Pinkerton SD, Sanyal AJ, Heuman DM. Diagnosis and treatment of minimal hepatic encephalopathy to prevent motor vehicle accidents: A cost‐effectiveness analysis. Hepatology 2012;55:1164‐1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double‐blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol 2013;108:1458‐1463. [DOI] [PubMed] [Google Scholar]
- 52. Butterworth RF, Kircheis G, Hilger N, McPhail MJW. Efficacy of l‐Ornithine l‐Aspartate for the treatment of hepatic encephalopathy and hyperammonemia in cirrhosis: Systematic review and meta‐analysis of randomized controlled trials. J Clin Exp Hepatol 2018;8:301‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bajaj JS, Riggio O. Drug therapy: Rifaximin. Hepatology 2010;52:1484‐1488. [DOI] [PubMed] [Google Scholar]
- 54. Hudson M, Schuchmann M. Long‐term management of hepatic encephalopathy with lactulose and/or rifaximin: a review of the evidence. Eur J Gastroenterol Hepatol 2019;31:434‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bajaj JS, O'Leary JG, Tandon P, Wong F, Kamath PS, Biggins SW, et al. Targets to improve quality of care for patients with hepatic encephalopathy: Data from a multi‐centre cohort. Aliment Pharmacol Ther 2019;49:1518‐1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jesudian AB, Ahmad M, Bozkaya D, Migliaccio‐Walle K. Cost‐effectiveness of rifaximin treatment in patients with hepatic encephalopathy. J Manag Care Spec Pharm 2020;26:750‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hanouneh MA, Hanouneh IA, Hashash JG, Law R, Esfeh JM, Lopez R, et al. The role of rifaximin in the primary prophylaxis of spontaneous bacterial peritonitis in patients with liver cirrhosis. J Clin Gastroenterol 2012;46:709‐715. [DOI] [PubMed] [Google Scholar]
- 58. Kang SH, Lee YB, Lee JH, Nam JY, Chang Y, Cho H, et al. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patient experiencing hepatic encephalopathy. Aliment Pharmacol Ther 2017;46:845‐855. [DOI] [PubMed] [Google Scholar]
- 59. Lutz P, Parcina M, Bekeredjian‐Ding I, Nischalke HD, Nattermann J, Sauerbruch T, et al. Impact of rifaximin on the frequency and characteristics of spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites. PLoS One 2014;9:e93909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Assem M, Elsabaawy M, Abdelrashed M, Elemam S, Khodeer S, Hamed W, et al. Efficacy and safety of alternating norfloxacin and rifaximin as primary prophylaxis for spontaneous bacterial peritonitis in cirrhotic ascites: A prospective randomized open‐label comparative multicenter study. Hepatol Int 2016;10:377‐385. [DOI] [PubMed] [Google Scholar]
- 61. Elfert A, Abo Ali L, Soliman S, Ibrahim S, Abd‐Elsalam S. Randomized‐controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol 2016;28:1450‐1454. [DOI] [PubMed] [Google Scholar]
- 62. Soni H, Kumar‐M P, Sharma V, Bellam BL, Mishra S, Mahendru D, et al. Antibiotics for prophylaxis of spontaneous bacterial peritonitis: Systematic review & Bayesian network meta‐analysis. Hepatol Int 2020;14:399‐413. [DOI] [PubMed] [Google Scholar]
- 63. Komolafe O, Roberts D, Freeman SC, Wilson P, Sutton AJ, Cooper NJ, et al. Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis in people with liver cirrhosis: A network meta‐analysis. Cochrane Database Syst Rev 2020;1:CD013125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dong T, Aronsohn A, Gautham Reddy K, Te HS. Rifaximin decreases the incidence and severity of acute kidney injury and hepatorenal syndrome in cirrhosis. Dig Dis Sci 2016;61:3621‐3626. [DOI] [PubMed] [Google Scholar]
- 65. Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long‐term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol 2013;28:450‐455. [DOI] [PubMed] [Google Scholar]
- 66. Kalambokis GN, Mouzaki A, Rodi M, Pappas K, Fotopoulos A, Xourgia X, et al. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol‐related cirrhosis and ascites. Clin Gastroenterol Hepatol 2012;10:815‐818. [DOI] [PubMed] [Google Scholar]
- 67. Salehi S, Tranah TH, Lim S, Heaton N, Heneghan M, Aluvihare V, et al. Rifaximin reduces the incidence of spontaneous bacterial peritonitis, variceal bleeding and all‐cause admissions in patients on the liver transplant waiting list. Aliment Pharmacol Ther 2019;50:435‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Flamm SL, Mullen KD, Heimanson Z, Sanyal AJ. Rifaximin has the potential to prevent complications of cirrhosis. Ther Adv Gastroenterol 2018;11:1756284818800307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ibrahim ES, Alsebaey A, Zaghla H, Moawad Abdelmageed S, Gameel K, Abdelsameea E. Long‐term rifaximin therapy as a primary prevention of hepatorenal syndrome. Eur J Gastroenterol Hepatol 2017;29:1247‐1250. [DOI] [PubMed] [Google Scholar]
- 70. Hanafy AS, Hassaneen AM. Rifaximin and midodrine improve clinical outcome in refractory ascites including renal function, weight loss, and short‐term survival. Eur J Gastroenterol Hepatol 2016;28:1455‐1461. [DOI] [PubMed] [Google Scholar]
- 71. Lv XY, Ding HG, Zheng JF, Fan CL, Li L. Rifaximin improves survival in cirrhotic patients with refractory ascites: A real‐world study. World J Gastroenterol 2020;26:199‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kamal F, Khan MA, Khan Z, Cholankeril G, Hammad TA, Lee WM, et al. Rifaximin for the prevention of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis: A systematic review and meta‐analysis. Eur J Gastroenterol Hepatol 2017;29:1109‐1117. [DOI] [PubMed] [Google Scholar]
- 73. Scott LJ. Rifaximin: A review of its use in reducing recurrence of overt hepatic encephalopathy episodes. Drugs 2014;74:2153‐2160. [DOI] [PubMed] [Google Scholar]
- 74. Mullen KD, Sanyal AJ, Bass NM, Poordad FF, Sheikh MY, Frederick RT, et al. Rifaximin is safe and well tolerated for long‐term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol 2014;12:1390‐1397.e2. [DOI] [PubMed] [Google Scholar]
- 75. RIFAXIMIN‐EIR (Extended Intestinal Release) 400 mg tablets [investigator’s brochure]. Covington, LA: Alfasigma; 2016. [Google Scholar]
- 76. Pose E, Napoleone L, Amin A, Campion D, Jimenez C, Piano S, et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE‐SAFETY): A randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Gastroenterol Hepatol 2020;5:31‐41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


