Abstract
Background
Detection of galactomannan (GM) from bronchoalveolar lavage fluid (BALF) or serum is broadly used for diagnosis of invasive aspergillosis (IA), although the sensitivity of GM from serum is lower in non‐neutropenic patients. We evaluated the Aspergillus galactomannan Lateral Flow assay (LFA) with digital readout from serum in a mixed cohort of patients.
Methods
We performed a retrospective two‐centre study evaluating the LFA from serum of patients with clinical suspicion of IA obtained between 2015 and 2021 at the University of California San Diego and the Medical University of Graz. The sensitivity and specificity was calculated for proven/probable aspergillosis versus no aspergillosis. Correlation with same‐sample GM was calculated using Spearman correlation analysis and kappa statistics.
Results
In total, 122 serum samples from 122 patients were analysed, including proven IA (n = 1), probable IA or coronavirus‐associated pulmonary aspergillosis (CAPA) (n = 27), and no IA/CAPA/non‐classifiable (n = 94). At a 0.5 ODI cut‐off, the sensitivity and specificity of the LFA was 78.6% and 80.5%. Spearman correlation analysis showed a strong correlation between serum LFA ODI and serum GM ODI (ρ 0.459, p < .0001). Kappa was 0.611 when both LFA and GM were used with a 0.5 ODI cut‐off, showing substantial agreement (p < .001).
Discussion
The LFA with digital read out from serum showed good performance for the diagnosis of probable/proven aspergillosis, with substantial agreement to GM from serum. Like the LFA from BALF, the LFA from serum may serve as a more rapid test compared to conventional GM, particularly in settings where GM is not readily available.
Keywords: Aspergillus galactomannan Lateral Flow assay (LFA), COVID‐19, SARS‐CoV2, galactomannan, haematologic malignancy, HIV, intensive care unit, respiratory diseases, serum, solid organ transplant recipients
1. INTRODUCTION
Invasive aspergillosis (IA) continues to emerge as a major cause of morbidity and mortality, including among non‐traditional risk groups such as critically ill patients in the intensive care unit (ICU), 1 including those with severe coronavirus disease 2019 (COVID‐19) infection. 2
Although microscopy and culture are the gold standard for the diagnosis of IA, sensitivity is limited 3 and therefore the detection of the fungal cell wall component galactomannan (GM) from bronchoalveolar lavage fluid (BALF) or serum 4 is commonly used (sensitivity 78% and specificity 85% at 0.5 cut‐off, sensitivity 71% and specificity 90% at 1.0 cut‐off 4 ), although the sensitivity of GM from serum is low in non‐neutropenic patients and those with SARS‐CoV‐2 associated pulmonary aspergillosis (CAPA). 2 , 5 , 6 Furthermore, the sensitivity of GM from blood declines in those on antifungal prophylaxis or treatment. 5 , 7 , 8 Other molecular tests such as polymerase chain reaction lack standardisation, have variable diagnostic performance across studies and settings, with declining performance in those on mould‐active prophylaxis. 9 Thus, there is a need for improved diagnostics from blood for IA.
The performance of the IMMY sōna Aspergillus GM Lateral Flow assay (LFA; IMMY) with manual readout in BALF demonstrated a sensitivity and specificity of 77% and 81%, respectively. 10 , 11 , 12 , 13 A recent multicentre study in a mixed cohort of patients found a sensitivity of 74% and specificity of 83% with digital readout from BALF at an optical density cut‐off (ODI) of 1.5, with stable test performance across centres and patient groups. 14 In serum, the LFA with digital readout was evaluated in three studies focusing exclusively on patients with haematologic malignancy, with one study reporting a sensitivity of 49% and a specificity of 95%, 15 and two reporting higher sensitivities of 96.9% 16 and 90.9% 17 with specificities between 90% and 96% at an ODI of ≥0.5. However, evaluation of the LFA test with digital readout from serum outside the haematologic malignancy setting is lacking.
We performed a retrospective two‐centre study evaluating the LFA with digital readout from serum in a mixed cohort of patients.
2. METHODS
A total of 122 serum samples from 122 patients with various underlying diseases with clinical suspicion of IA and a GM ordered between 2015 and 2021 at the University of California San Diego (UCSD), United States, and the Medical University of Graz, Austria, were retrospectively analysed.
Aspergillus disease was classified according to the revised European Organization for Research and Treatment of Cancer (EORTC)/Mycoses Study Group (MSG) criteria, 18 and for those with severe COVID‐19 as the only risk factor according to the European Confederation of Medical Mycology (ECMM)/International Society for Human and Animal Mycoses criteria. 6 Importantly, the EORTC/MSG criteria are only applicable to patients with cancer, transplant recipients and other severely immunocompromised patients. 1 Patients who did not fall into either of these categories but who had clinical, radiological and mycological evidence of IA were categorised as non‐classifiable.
GM (Platelia Aspergillus Ag ELISA; Bio‐Rad Laboratories) was routinely and prospectively performed in the majority of samples at each participating centre before samples were stored at −70°C for up to 5 years. Between January 2020 and April 2021 these serum samples where thawed, vortexed, and tested with the LFA according to the manufacturer's instructions, as previously described. 14 Test lines intensities were first read by naked eye by two investigators blinded to disease classification and then by an automated cube reader that was included with the test kits and displayed in ODI. 14
Statistical analyses were performed using SPSS 25 (SPSS Inc.). For continuous data, including LFA and GM ODIs, receiver operating characteristic curve analyses were performed and area under the curve (AUC) values were presented including 95% confidence intervals (95% CI) for the outcomes proven/probable aspergillosis (vs. no aspergillosis) in the overall study cohort and sub‐cohorts. LFA ODI cut‐offs of 0.5 ODI and 1.0 ODI were compared by calculating sensitivity and specificity for aspergillosis (ie fulfilling criteria of probable or proven) versus no aspergillosis (exclusion of cases that were non‐classifiable). Correlation between serum GM and LFA ODIs was calculated using Spearman rho correlation analysis due to the non‐normal distributions as well as Kappa statistics. Two‐sided p < 0.05 was taken as cut‐off for statistical significance. The study protocol and all study‐related procedures were approved by the Human Research Protections Program at UCSD (IRB project no. 171104), and the Medical University of Graz (EC no. 23‐343).
3. RESULTS
Of 122 serum samples, 50 were from the UCSD and 72 from the Medical University of Graz. The LFA produced valid results in all samples. One patient fulfilled criteria of proven IA, 27 probable IA or CAPA, and 87 did not fulfil criteria for IA or CAPA. Seven patients were non‐classifiable.
The majority of patients were male (77/122, 63% vs. 45/122, 37% female) and median age was 57 years (range 22–88). Underlying haematological malignancy was present in 55/122 patients (45%), 24/122 (20%) had chronic pulmonary diseases, 8/122 (7%) were solid organ transplant (SOT) recipients, 5/122 (4%) had liver cirrhosis, 3/122 human immunodeficiency virus infection, 2/122 had autoimmune disease requiring systemic corticosteroid treatment, and 2/122 had a solid malignant tumour (Table 1). Lastly, 59/122 (48%) were in the ICU for severe COVID‐19 infection (including 6 with underlying haematological malignancy, of which 5/6 did not have CAPA); of these 59 patients, 28 had no other underlying disease predisposing them for IA other than COVID‐19 at the time the sample was obtained. Sixty‐four percent (18/28) of those with probable/proven IA/CAPA and 43% (40/94) of patients without IA/CAPA died.
TABLE 1.
Demographic data and underlying diseases of the study
| Haematological malignancy (n = 55) | Other traditional underlying diseases predisposing for IA but no haematological malignancy (n = 39) | COVID‐19 acute respiratory failure but no other underlying disease predisposing for IA (n = 28) | |
|---|---|---|---|
| Age (median, IQR) | 55 (48–65) | 57 (49–69) | 60 (54–76) |
| Female sex (n, %) | 22 (44%) | 11 (28%) | 12 (43%) |
| COVID‐19 acute respiratory failure (n, %) | 6 (11%) | 25 (64%) | 28 (100%) |
| Structural pulmonary disease (n, %) | 2 (4%) | 23 (59%) | 0 |
| Solid organ transplantation (n, %) | 1 (2%) | 7 (18%) | 0 |
| Child Pugh C liver cirrhosis (n, %) | 0 | 5 (13%) | 0 |
| HIV (n, %) | 0 | 3 (8%) | 0 |
| Autoimmune disease with systemic corticosteroids (n, %) | 0 | 2 (5%) | 0 |
| Solid malignant tumour (n, %) | 0 | 2 (5%) | 0 |
| Overall mortality (n, %) | 27 (49%) | 16 (41%) | 15 (54%) |
| 30‐day mortality (n, %) | 15 (27%) | 12 (31%) | 13 (46%) |
| Proven or probable IA/CAPA (n, %) | 20 (36%) | 6 (15%) | 2 (7%) |
Abbreviations: CAPA, COVID associated pulmonary aspergillosis; COVID‐19, coronavirus disease 2019; HIV, human immunodeficiency virus; IA, invasive aspergillosis; ICU, intensive care unit; IQR, interquartile range.
The visual read out performance varied between the investigators with sensitivities of 67.9% and 78.6% and specificities of 70.1% and 67.8% for differentiating probable/proven disease versus no aspergillosis. Performance improved when using the automated read out at the recommended 0.5 ODI cut‐off, with a sensitivity of 78.6% (22/28) and a specificity of 80.5% (70/87). When increasing the cut‐off to 1.0 ODI, the LFA had a decreased sensitivity (14/28, 50%) but increased specificity (84/87, 96.6%). Overall results and those for subgroups are displayed in Table 2.
TABLE 2.
Sensitivity and Specificity for the Serum Lateral Flow Assay with automated read out for diagnosing proven/probable invasive aspergillosis or COVID‐19 associated pulmonary aspergillosis versus no IA in the Overall Study Cohort and Subgroups. Evaluation of Different Optical Density Index (ODI) cut‐offs
| LFA Cut‐off/patient group | 0.5 ODI | 1.0 ODI | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| Overall | 79% (22/28) | 80% (70/87) | 50% (14/28) | 97% (84/87) |
| Haematological malignancy | 85% (17/20) | 72% (23/32) | 55% (11/20) | 97% (31/32) |
| Other traditional underlying diseases predisposing for IA but no haematological malignancy | 83% (5/6) | 76% (22/29) | 50% (3/6) | 93% (27/29) |
| COVID‐19 acute respiratory failure but no other underlying disease predisposing for IA | 0% (0/2) | 96% (25/26) | 0% (0/2) | 100% (26/26) |
Abbreviations: COVID‐19, coronavirus disease 2019; IA, invasive aspergillosis; LFA, lateral flow assay; ODI, optical density index.
Receiver operating characteristic curve analysis showed an AUC of 0.809 (95% CI 0.698‐0.921) for the serum LFA for differentiating probable/proven disease versus no aspergillosis (Figure 1). The serum LFA tended to be more discriminatory in patients with underlying haematological malignancies (AUC 0.853, 95% CI 0.750–0.977; n = 20 with probable/proven disease) versus those without haematological malignancy (AUC 0.677, 95% CI 408–975; n = 8 with probable/proven disease; p = 0.12 Hanley McNeil). When excluding those with COVID‐19 as the only risk factor for IA/CAPA and analysing all non‐haematological malignancy patients with other underlying predisposing disease (total n = 39), AUC was 0.810 (95% CI 0.554–1.000). Only two cases of CAPA were observed among the remaining 28 patients with COVID‐19 in whom COVID‐19 infection was the sole risk factor for CAPA.
FIGURE 1.
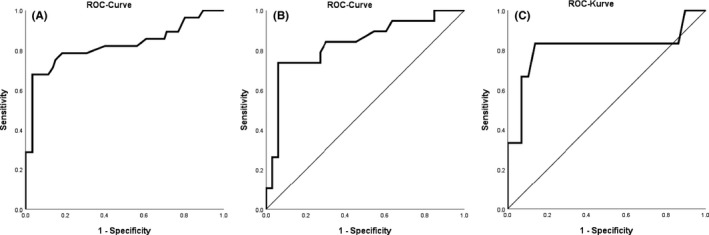
Receiver Operating Characteristics Analysis Curves for Serum Lateral Flow Assay for Diagnosing Proven/Probable Invasive Aspergillosis or COVID‐19 associated pulmonary aspergillosis versus no aspergillosis in the overall Study Cohort and Subgroups. A overall study population, B Haematological Malignancy, C Other traditional underlying diseases predisposing for IA but no haematological malignancy
Same‐day serum GM ODI were available in 111/122 samples and AUC of serum GM ODI was 0.915 (95% CI 0.851–0.979; AUC for serum LFA ODI in those samples 0.830, 95% CI 0.719–0.941) for differentiating probable/proven IA/CAPA versus no IA/CAPA. Among those with probable/proven aspergillosis where same‐day serum GM gave a positive result (≥0.5 ODI), serum LFA sensitivity was 91.3% (21/23, 0.5 ODI cut‐off), while sensitivity was 20% (1/5) in those with probable/proven disease where same‐day serum GM was negative. Spearman correlation analysis showed a strong correlation between serum LFA ODI and serum GM ODI (rho 0.459, p < 0.0001; Figure 2). Kappa was 0.611 when both LFA and GM were used with a 0.5 ODI cut‐off, showing substantial agreement (p < .001).
FIGURE 2.
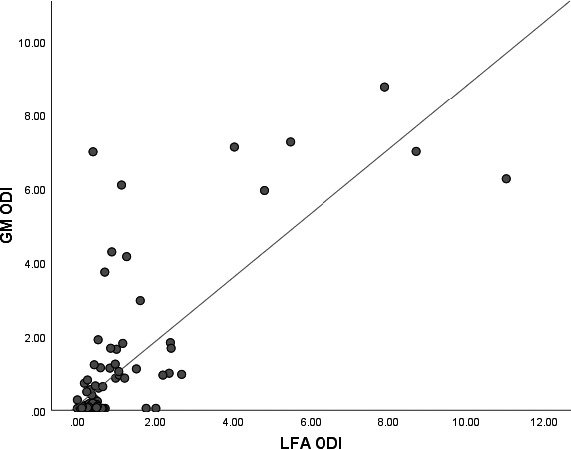
Scatter blots showing correlation between serum galactomannan (GM) and Lateral Flow Assay (LFA) Optical Density Indexes (ODIs) in the study population with both tests from the same serum sample (n = 115)
Thirty‐eight per cent (46/122) were receiving mould‐active antifungal prophylaxis or treatment at the time of serum sampling but this did not impact discriminatory power of the LFA (AUC 0.900, 95%CI 0.795–1.000, n = 13 with probable/proven disease).
4. DISCUSSION
We evaluated the LFA from serum in a mixed patient cohort and found that when used with the automated reader the LFA from serum showed a strong correlation with serum GM and a good performance for diagnosing probable/proven aspergillosis. At a 0.5 ODI cut‐off the overall sensitivity and specificity was 78.6% and 80.5%, respectively, in differentiating probable/proven disease versus no aspergillosis with similar performance in patients without haematological malignancies (eg, 83.3% sensitivity and 75.9% specificity in those with other established risk factors for IA but without haematologic malignancy), which represented 55% of our study cohort. Previously the LFA with automated read out was evaluated exclusively in serum samples from patients with haematological malignancies, where two studies found higher sensitivity and specificity 16 , 17 and one a lower sensitivity, compared to our findings. 14
The serum LFA has several advantages over GM ELISA testing, including that it allows for single sample testing, requires minimal laboratory equipment so can potentially be performed at a lower cost than ELISA testing, and has shorter turn‐around times, making it an attractive option for smaller centres that don't test GM in house. It has also shown promise in combination with other diagnostic tests and immunological markers. 3
Coronavirus disease 2019 associated fungal infections, in particular aspergillosis, 19 , 20 mucormycosis 21 , 22 and candidiasis, 23 have recently emerged as important complications in patients with COVID‐19 associated acute respiratory failure. Our study enrolled a total of 59 patients with COVID‐19 associated acute respiratory failure, however more than half had also other underlying diseases that traditionally predispose for IA, and among the remaining 28 where severe COVID‐19 was the only risk factor, only two fulfilled criteria of probable or proven CAPA. While a single‐centre study showed a sensitivity and specificity of 60% and 73% for the LFA from tracheal aspiration with a 2.0 ODI cut‐off in diagnosing CAPA, 24 larger and preferably multicentre studies are needed to further evaluate the LFA in patients with CAPA, preferably in same‐day respiratory and serum samples. This is particularly important given the limited sensitivity of serum GM for CAPA diagnosis. 2 , 25
The results of the LFA from serum at a 0.5 ODI cut‐off in our mixed cohort are slightly superior to those of a recent multicentre study evaluating the LFA from BALF in a mixed cohort of patients, where the sensitivity was 74% and specificity 83% at an ODI of 1.5. 10 In contrast to BALF, where a higher ODI cut‐off may be warranted to increase test performance, a 0.5 ODI cut‐off may be optimal in serum samples, mirroring similar findings for GM. This is not surprising given the strong correlation and substantial agreement between serum LFA ODI and serum GM ODI found in our study and other studies before. 10 , 15 Finally, we found the automated reader increased the reliability and test performance compared to manual read.
Our study has several limitations including the low number of proven cases of aspergillosis. In addition, this study was performed with banked serum samples that were tested after they were stored and frozen. Prospective validation of the LFA in a clinical setting to determine whether the rapid turnaround time of the LFA informs clinical decision‐making would be helpful. Finally, considering the significant correlation with GM, there is a risk of selection bias leading to an overestimation of the LFA performance, as GM was used for classification of the majority of cases.
Nevertheless, our findings are significant in that they come from the only multicentre study to evaluate the LFA in serum, and the only study in a mixed cohort of patients. Like the LFA from BALF, the LFA from serum may serve a role as a more rapid test, compared to the conventional GM, for the diagnosis of aspergillosis, particularly in settings where GM testing is not readily available.
TRANSPARENCY DECLARATION
Aspergillus GM LFAs were provided by IMMY, Norman, Oklahoma, USA. They had no role in the study design, data collection, analysis, interpretation, decision to publish, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
CONFLICT OF INTEREST
MH received research funding from Gilead, Astellas, Scynexis, F2G and Pfizer. JDJ received research funding from Astellas, F2G and Pfizer. ES received congress support from Amgen, Incyte, Jazz, Janssen, Novartis, Roche and Takeda, participated in advisory boards of Amgen and Celgene, and received honoraria from Amgen and Novartis. All other authors declare no conflict of interest for this study.
AUTHOR CONTRIBUTION
Martin Hoenigl: Conceptualization (lead); Data curation (equal); Formal analysis (lead); Funding acquisition (lead); Investigation (lead); Methodology (equal); Project administration (lead); Supervision (lead); Validation (lead); Writing‐original draft (equal). Matthias Egger: Data curation (supporting); Methodology (supporting); Project administration (supporting); Writing‐review & editing (supporting). Johannes Boyer: Investigation (supporting); Methodology (supporting); Project administration (supporting); Writing‐review & editing (supporting). Eduard Schulz: Investigation (supporting); Visualization (supporting); Writing‐review & editing (supporting). Juergen Prattes: Formal analysis (supporting); Investigation (equal); Methodology (equal); Project administration (equal); Validation (equal); Writing‐original draft (equal). Jeffrey D Jenks: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Project administration (equal); Validation (equal); Writing‐original draft (equal).
ACKNOWLEDGEMENT
The authors wish to acknowledge Marina Linhofers support as research coordinator.
Hoenigl M, Egger M, Boyer J, Schulz E, Prattes J, Jenks JD. Serum Lateral Flow assay with digital reader for the diagnosis of invasive pulmonary aspergillosis: A two‐centre mixed cohort study. Mycoses. 2021;64:1197–1202. 10.1111/myc.13352
Juergen Prattes and Jeffrey D. Jenks shared senior authorship.
Funding information
The work was supported by an investigator initiated research study from Pfizer (no. 60278533). The study was also supported by NIH (no. UL1TR001442). LFA tests were provided by IMMY diagnostics.
Contributor Information
Martin Hoenigl, Email: hoeniglmartin@gmail.com.
Juergen Prattes, Email: juergen.prattes@medunigraz.at.
DATA AVAILABILITY STATEMENT
Data is available on request.
REFERENCES2
- 1. Bassetti M, Azoulay E, Kullberg B‐J, et al. EORTC/MSGERC definitions of invasive fungal diseases: summary of activities of the Intensive Care Unit Working Group. Clin Infect Dis. 2021;72(Supplement_2):S121‐S127. [DOI] [PubMed] [Google Scholar]
- 2. Arastehfar A, Carvalho A, van de Veerdonk FL, et al. COVID‐19 Associated Pulmonary Aspergillosis (CAPA)‐from immunology to treatment. J Fungi (Basel). 2020;6(2):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heldt S, Prattes J, Eigl S, et al. Diagnosis of invasive aspergillosis in hematological malignancy patients: performance of cytokines, Asp LFD, and Aspergillus PCR in same day blood and bronchoalveolar lavage samples. J Infect. 2018;77(3):235‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leeflang MMG, Debets‐Ossenkopp YJ, Wang J, et al. Galactomannan detection for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst Rev. 2015;2015(12):Cd007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jenks JD, Nam HH, Hoenigl M. Invasive aspergillosis in critically ill patients: review of definitions and diagnostic approaches. Mycoses. 2021. 10.1111/myc.13274. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID‐19‐associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21(6):e149‐e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis. 2005;40(12):1762‐1769. [DOI] [PubMed] [Google Scholar]
- 8. Eigl S, Hoenigl M, Spiess B, et al. Galactomannan testing and Aspergillus PCR in same‐day bronchoalveolar lavage and blood samples for diagnosis of invasive aspergillosis. Med Mycol. 2017;55(5):528‐534. [DOI] [PubMed] [Google Scholar]
- 9. Egger M, Jenks JD, Hoenigl M, Prattes J. Blood Aspergillus PCR: the good, the bad, and the ugly. J Fungi (Basel). 2020;6(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jenks JD, Hoenigl M. Point‐of‐care diagnostics for invasive aspergillosis: nearing the finish line. Expert Rev Mol Diagn. 2020;20(10):1009‐1017. [DOI] [PubMed] [Google Scholar]
- 11. Mercier T, Dunbar A, de Kort E, et al. Lateral flow assays for diagnosing invasive pulmonary aspergillosis in adult hematology patients: a comparative multicenter study. Med Mycol. 2020:58(4):444‐452. [DOI] [PubMed] [Google Scholar]
- 12. Jenks JD, Mehta SR, Taplitz R, Law N, Reed SL, Hoenigl M. Bronchoalveolar lavage Aspergillus galactomannan lateral flow assay versus Aspergillus‐specific lateral flow device test for diagnosis of invasive pulmonary aspergillosis in patients with hematological malignancies. J Infect. 2019;78(3):249‐259. [DOI] [PubMed] [Google Scholar]
- 13. Jenks JD, Mehta SR, Taplitz R, Aslam S, Reed SL, Hoenigl M. Point‐of‐care diagnosis of invasive aspergillosis in non‐neutropenic patients: Aspergillus Galactomannan Lateral Flow Assay versus Aspergillus‐specific Lateral Flow Device test in bronchoalveolar lavage. Mycoses. 2019;62(3):230‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jenks JD, Prattes J, Frank J, et al. Performance of the bronchoalveolar lavage fluid Aspergillus Galactomannan Lateral Flow Assay with cube reader for diagnosis of invasive pulmonary aspergillosis: a multicenter cohort study. Clin Infect Dis. 2020. 10.1093/cid/ciaa1281. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mercier T, Guldentops E, Lagrou K, Maertens J. Prospective evaluation of the turbidimetric β‐D‐glucan assay and two lateral flow assays on serum in invasive aspergillosis. Clin Infect Dis. 2021;72(9):1577‐1584. [DOI] [PubMed] [Google Scholar]
- 16. White PL, Price JS, Posso R, Cutlan‐Vaughan M, Vale L, Backx M. Evaluation of the performance of the IMMY sona Aspergillus galactomannan lateral flow assay when testing serum to aid in diagnosis of invasive aspergillosis. J Clin Microbiol. 2020;58(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Serin I, Dogu MH. Serum Aspergillus galactomannan lateral flow assay for the diagnosis of invasive aspergillosis: a single‐centre study. Mycoses. 2021;64(6):678–683. [DOI] [PubMed] [Google Scholar]
- 18. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367‐1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salmanton‐García J, Sprute R, Stemler J, et al. COVID‐19‐associated pulmonary aspergillosis, March–August 2020. Emerg Infect Dis. 2021;27(4):1077‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoenigl M. Invasive fungal disease complicating COVID‐19: when it rains it pours. Clin Infect Dis. 2020. 10.1093/cid/ciaa1342. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rudramurthy SM, Hoenigl M, Meis JF, et al. ECMM/ISHAM recommendations for clinical management of COVID‐19 associated mucormycosis in low‐ and middle‐income countries. Mycoses. 2021. 10.1111/myc.13335. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoenigl M, Seidel D, Carvalho A, et al. The emergence of COVID‐19 associated mucormycosis: analysis of cases from 18 countries. SSRN Electron J. 2021. 10.2139/ssrn.3844587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arastehfar A, Carvalho A, Nguyen MH, et al. COVID‐19‐Associated Candidiasis (CAC): an underestimated complication in the absence of immunological predispositions? J Fungi (Basel). 2020;6(4):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roman‐Montes CM, Martinez‐Gamboa A, Diaz‐Lomelí P, et al. Accuracy of galactomannan testing on tracheal aspirates in COVID‐19‐associated pulmonary aspergillosis. Mycoses. 2021;64(4):364‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prattes J, Wauters J, Giacobbe DR, Lagrou K, Hoenigl M, ECMM‐CAPA Study Group . Diagnosis and treatment of COVID‐19 associated pulmonary apergillosis in critically ill patients: results from a European confederation of medical mycology registry. Intensive Care Med. 2021. 10.1007/s00134-021-06471-6. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available on request.


