Abstract
Background and Aim
This single‐arm, open‐label, multicenter, phase 3 trial evaluated the efficacy and safety of seraprevir, an hepatitis C virus (HCV) nonstructural protein 3/4A (NS3/4A) inhibitor, combined with sofosbuvir for treating Chinese patients with chronic HCV infection without cirrhosis.
Methods
Treatment‐naive or interferon‐experienced adult patients without cirrhosis were treated with a universal, combinational regimen of seraprevir 100 mg, twice daily and sofosbuvir 400 mg, once daily, for 12 or 24 weeks. The primary efficacy endpoint was sustained virologic response at week 12 after treatment (SVR12).
Results
Overall, 205 patients with genotype 1 HCV infection without cirrhosis were enrolled from 23 sites, 202 of whom completed the full treatment and post‐treatment course and 3 discontinued follow‐up. In total, 27 patients (13.2%) were interferon experienced. SVR12 was achieved by 201 out of 205 (98.0% [95% CI, 95.1%, 99.5%]) patients, 100.0% of patients with genotype 1a, and 98.0% of genotype 1b. In the other exploratory study, SVR 12 was achieved by 100% patients with genotype 2 (n = 21), genotype 3 (n = 7), and genotype 6 (n = 8). The majority of adverse events were mild to moderate and transient and did not require a specific medical intervention.
Conclusions
The all‐oral, ribavirin‐free regimen of seraprevir and sofosbuvir is an effective and well‐tolerated treatment option for Chinese patients mono‐infected with HCV, including those with a history of interferon treatment.
Keywords: hepatitis C, safety, seraprevir, sofosbuvir, sustained virologic response
Introduction
Hepatitis C virus (HCV) infection is an important global health concern, with an estimated prevalence of 71 million viremic infections, and only 7% of the infected individuals have received treatment. 1 In China, the prevalence of HCV is approximately 0.43%, and at least 10 million individuals have been estimated to be infected. 2 Antiviral therapy is an essential treatment for patients with chronic HCV infection, as it reportedly halts progression to liver cirrhosis and hepatocellular carcinoma. 3 Thus far, HCV has been classified into seven recognized genotypes on the basis of the sequence of the viral genome. 4 HCV genotype distribution also varies worldwide, whereas HCV genotype 1 is the most prevalent worldwide (49.1%). 5 Genotype 1 is composed of at least 60 subtypes, with differences in nucleotide and acid sequences. The 1a and 1b strains are prominent. 6 Even in mainland China, HCV genotype distribution is highly diverse across the nationwide geographic regions, with genotype 1b being the most dominant in most areas of China (52.2%). 7 Therefore, an accessible, effective, native, genotype 1 HCV treatment regimen is warranted in China from the perspectives of both clinical practice and public health.
Seraprevir (coded as GP205) is an HCV nonstructural protein NS3/4A inhibitor with antiviral activities against HCV genotypes 1b, 1a, and 2a. Compared with telaprevir, seraprevir exerts a much higher antiviral activity, especially in HCV genotype 1b. In early‐phase clinical pharmacology studies, oral seraprevir shows a favorable pharmacokinetic and tolerability profile in healthy participants and high efficacy in patients of genotypes 1b and 2a receiving a 72‐h monotherapy, in which the maximal HCV‐RNA reduction up to 3 log10 IU/mL was observed. In a previous phase IIa study, a standard 12‐week treatment regimen of seraprevir (100 mg twice a day, 200 mg once a day, or 200 mg twice a day) with sofosbuvir (400 mg once a day) resulted in a sustained virologic response (SVR) of 100% among treatment‐naive Chinese patients infected with HCV of genotypes 1b.
The primary objective of this study was to evaluate the efficacy and safety of a 12‐week combined regimen of seraprevir (100 mg twice a day) plus sofosbuvir (400 mg once a day) for Chinese adult patients with HCV mono‐infection, including those with a history of interferon treatment.
Methods
Study design
This single‐arm, open‐label, phase 3 study was conducted at 23 clinical sites in China. This trial is registered with ClinicalTrials.gov (number NCT004001608) and ChinaDrugTrials.org.cn (number CTR20182134). The efficacy and safety of a 12‐week regimen of seraprevir in combination with sofosbuvir in adults with chronic HCV genotype 1 infection were evaluated. The study protocol was approved by the Institutional Review Board or Independent Ethics Committee at each participating site, and the study was designed and conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from all participants before enrolment.
Participants
Eligible patients with at least a 6‐month history of HCV genotype 1 infection who were aged between 18 and 75 years, had a body weight of 40 kg or more, and a plasma HCV‐RNA of 10 000 IU/mL or more were enrolled. Patients were HCV treatment naive (those who had previously used NS3/4A, NS5A, NS4B, or NS5B inhibitors were excluded) and without cirrhosis. The presence of cirrhosis was determined at screening by two ways: a liver biopsy showing cirrhosis (METAVIR F4 or Ishak ≥ 5 within 24 months of enrollment) or FibroScan results indicating cirrhosis (>12.5 kilopascals [kPa] within 6 months of enrollment). Patients co‐infected with human immunodeficiency virus, hepatitis B virus, more than one HCV genotype, and those with alanine aminotransferase or aspartate aminotransferase level more than 10 times the upper limit of normal (ULN), total bilirubin more than two times the ULN, white blood cell count less than 3000/μL, neutrophil granulocytes less than 1500/μL, platelets less than 75 000/μL, creatinine clearance less than 50 mL/min by the Cockcroft–Gault equation, AFP more than 50 ng/mL, albumin less than 3.5 g/dL, or international normalized ratio above 1.5 times the ULN were excluded. Detailed inclusion and exclusion criteria are provided in Table S1.
Procedures
Eligible patients were instructed to self‐administer seraprevir 100 mg (Shanghai Viromedicine Co., Ltd., Shanghai, China) twice a day and sofosbuvir 400 mg (Gilead Sciences, Cork, Ireland) once a day for 12 successive weeks. Their efficacy and safety were evaluated at baseline; weeks 1, 2, 4, 8, and 12 of treatment and weeks 4 and 12 after treatment. HCV‐RNA testing was performed using the COBAS Ampliprep/COBAS Taqman HCV test v2.0 (Roche Molecular Systems, Pleasanton, CA, USA), with a lower limit of quantitation (LLOQ) of 15 IU/mL and an upper limit of quantitation of 108 IU/mL. HCV genotype and subtype were assessed using Sanger sequencing (ABI 3730XL Sequencer; Applied Biosystems; Carlsbad, CA, USA). Blood samples for detection of viral resistance‐associated substitution (RAS) were collected at baseline and at all on‐treatment and post‐treatment study visits. At the time of virologic failure, HCV NS3 and NS5B coding regions were deep sequenced from samples obtained at baseline and at the time of failure. Virologic failure was defined as non‐response (detectable HCV‐RNA at the end of treatment with HCV‐RNA greater than the LLOQ throughout treatment), rebound (>1 log10 increase in HCV‐RNA from nadir while on treatment), breakthrough (on‐treatment HCV‐RNA titer equal to or above LLOQ after previously being < LLOQ twice), and relapse (HCV‐RNA titer equal to or above LLOQ after completion of treatment for patients having achieved virologic response at treatment week 12). 9 Criteria for discontinuation of the study procedures before completion included HCV‐RNA titer equal to or above LLOQ after previously being < LLOQ twice, > 1 log10 increase in HCV‐RNA from nadir value, detectable HCV‐RNA at the week 8 of treatment with HCV‐RNA greater than the LLOQ throughout treatment, and any adverse event (AE) or laboratory abnormality assessed as not appropriate to continue treatment.
Endpoints
Efficacy and safety were assessed in all patients who received at least one dose of the study drug. The primary efficacy outcome variable was SVR12, defined as the proportion of participants with undetectable HCV‐RNA at 12 weeks after the completion of treatment. The secondary efficacy endpoints included the proportions of patients who achieved virologic response at treatment weeks 1, 2, 4, 8, and 12 and at post‐treatment week 4; the proportion of patients who experienced on‐treatment virologic breakthrough at treatment weeks 2, 4, 8, and 12; and the proportions of patients who experienced post‐treatment virologic relapse at post‐treatment weeks 4 and 12.
Safety was monitored at every study visit until post‐treatment week 12. The primary safety was assessed by monitoring AEs, vital signs, physical examinations and clinical laboratory test results, and electrocardiography and upper abdominal ultrasonography scans from the start of treatment up to 12 weeks after the last dose of study drugs. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 22.0 (MedDRA MSSO, McLean, VA, USA) and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. The attribution of causality for any AE to the study drug was at the discretion of the investigator according to a national adverse drug reaction (ADR) vigilance procedure. ADR is defined as any AE that is definitely, probably, or possibly caused by the use of the study drug, as assessed by the investigator.
Statistical analysis
The overall SVR12 for patients receiving seraprevir plus sofosbuvir was conservatively estimated at 97%. A sample size of 171 patients would provide a lower confidence interval of 93.2% at a one‐sided significance level of 0.025. Considering a 20% exclusion rate, the sample size was set at 206 patients.
All analyses were conducted according to the statistical analysis plan. Continuous variables were presented as median (interquartile ranges) or mean (SD), and categorical variables were expressed as numbers and frequencies (%). All efficacy analyses were performed on the intention‐to‐treat population. Point estimates and two‐sided 95% confidence intervals (95% CIs) were calculated using the Clopper–Pearson method for primary and secondary efficacy endpoints. The SVR of patient subsets and the potential effects of genotype (1a and 1b), HCV‐RNA (<800 000 and ≥800 000 IU/mL), and interferon treatment experience on SVR (expressed as odds ratio [OR] and 95% CI) were analyzed using the Pearson χ 2 test or Fisher exact test. Safety results and secondary outcomes were descriptively summarized. A two‐sided P‐value below or equal to 0.05 was considered statistically significant. All statistical summaries and analyses were performed using the SAS software package version 9.4 (SAS Institute Inc., Cary, NC, USA).
Missing HCV‐RNA data for any reason were counted as treatment failure for the full analysis set using the intention‐to‐treat principle. Exploratory efficacy endpoints and safety endpoints were descriptively summarized.
Results
Patient characteristics
Between September 2018 and January 2019, 279 patients were screened; 74 of them were excluded, as they did not meet the eligibility criteria for laboratory tests. Overall, 205 patients were enrolled in this study and treated with seraprevir plus sofosbuvir. All patients completed the 12‐week treatment and additional 12‐week follow‐up visits, except for one patient who withdrew from the treatment at week 1 owing to a serious adverse event (SAE), which was determined as not related to the study drug (myocardial ischemia), another patient who withdrew from the treatment at week 8 owing to failure to achieve a virologic response, and one patient who was lost to post‐treatment week 12 follow‐up owing to virologic relapse at post‐treatment week 4 (Fig. 1).
FIGURE 1.
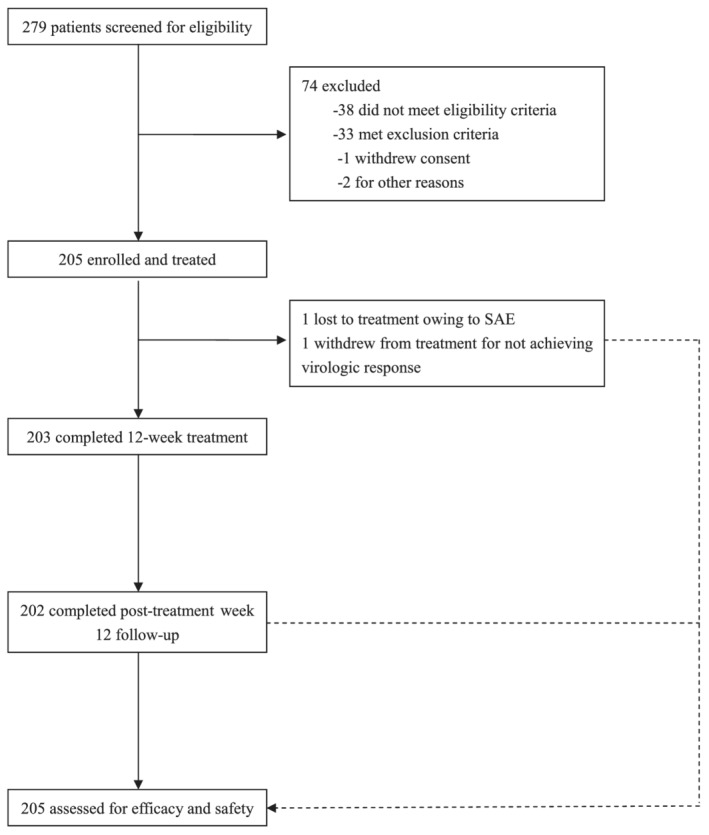
Study flow chart.
Overall, all patients had HCV genotype 1 infection, including genotype 1a (2.4% [n = 5]) and genotype 1b (97.6% [n = 200]). The patients included similar proportions of men and women (45.4% vs 54.6%), with a median age of 47 (range, 24–73) years, and the majority of them were Han Chinese (88.8%) in ethnicity. All patients were seronegative to HBV and HIV; none of them had liver cirrhosis, and the mean value of liver stiffness was 6.52 ± 2.16 kPa at baseline. Most patients had FibroScan value ≤12.5 kPa (99.5%); only one patient had liver stiffness, with a value more than 12.5 kPa. However, liver biopsy demonstrated no cirrhosis. In total, 27 patients (13.2%) had been previously exposed to interferons. None of the patients had a serum creatinine clearance below 50 mL/min (using the Cockcroft–Gault formula), as per the eligibility criteria (Table 1).
TABLE 1.
Patient demographics and baseline characteristics
| Patients (n = 205) | |
|---|---|
| Age, years, median (range) | 47 (24–73) |
| Sex | |
| Male | 93 (45.4%) |
| Female | 112 (54.6%) |
| Ethnicity | |
| Han Chinese | 182 (88.8%) |
| Others | 23 (11.2%) |
| HCV‐RNA genotype | |
| 1a | 5 (2.4%) |
| 1b | 200 (97.6%) |
| HCV‐RNA titer (IU/mL) | |
| < 800 000 | 70 (34.1%) |
| ≥ 800 000 | 135 (65.9%) |
| Liver fibrosis (FibroScan value, kPa) | |
| ≤ 7.3 | 136 (69.7%) |
| > 7.3 and ≤ 9.7 | 40 (20.5%) |
| > 9.7 and ≤ 12.5 | 18 (9.2%) |
| > 12.5 | 1 (0.5%) |
| Previous interferon experience | |
| No | 178 (86.8%) |
| Yes | 27 (13.2%) |
HCV, hepatitis C virus.
Virologic responses
In total, 203 patients had HCV‐RNA less than the lower limit of quantitation by treatment week 8. Of the 205 treated patients, 201 patients achieved the primary endpoint of SVR12 (98.0%; 95% CI [95.1%, 99.5%]). The lower limit of 95% confidence interval of SVR12 was significantly greater than the prespecified goal of 93.2%, meeting the primary efficacy endpoint for this study. Subset analysis by genotype (Table 2) showed that patients with genotype 1a had a SVR12 of 100.0% (95% CI [47.8%, 100.0%]), similar to 98.0% (95% CI [95.0%, 99.5%]) for those with genotype 1b (P > 0.999). No significant difference was observed between patients with HCV‐RNA < 800 000 IU/mL and those with HCV‐RNA ≥ 800 000 IU/mL (97.1% vs 98.5%, P = 0.607), whereas previous exposure to interferon treatment did not significantly affect SVR12 (P > 0.999). Detailed on‐treatment and post‐treatment virologic responses are shown in Table 2.
TABLE 2.
Virologic responses during and after treatment
| Response | Patients (n = 205) |
|---|---|
| Treatment week 2 | 154/205 (75.1% [68.6, 80.9]) |
| Treatment week 4 | 200/205 (97.6% [94.4, 99.2]) |
| Treatment week 8 | 203/205 (99.0% [96.5, 99.9]) |
| Treatment week 12 | 203/205 (99.0% [96.5, 99.9]) |
| Post‐treatment week 4 | 197/205 (99.0% [96.4, 99.0]) |
| Post‐treatment week 12 | 201/205 (98.0% [95.1, 99.5]) |
| HCV‐RNA titer (IU/mL) | |
| < 800 000 | 68/70 (97.1% [90.1, 99.7]) |
| ≥ 800 000 | 133/135 (98.5% [94.8, 99.8]) |
| HCV‐RNA genotype | |
| 1a | 5/5 (100.0% [47.8, 100.0]) |
| 1b | 196/200 (98.0% [95.0, 99.5]) |
| Previous interferon experience | |
| No | 174/178 (97.8% [94.4, 99.4]) |
| Yes | 27/27 (100.0% [87.2, 100.0]) |
HCV, hepatitis C virus.
Virologic failure
Out of 205 patients, four patients (2%) did not achieve SVR12, all of whom had genotype 1b and were naive to interferon treatment. Except one patient who prematurely withdrew from treatment owing to an SAE, three of the patients were eligible for predefined polymorphism sequencing for NS3 and NS5B. One patient experienced on‐treatment virologic failure and withdraw from the treatment at on‐treatment week 8 based on the protocol; this patient had baseline polymorphisms in both NS3 (S122G) and NS5B (C316N). No treatment‐emergent RAS was detected in this patient at the time of virologic failure. The other two patients with virologic failure experienced post‐treatment relapse. One of the patients had baseline polymorphisms in both NS3 (S122G) and NS5B (C316N and D62N) and experienced virologic relapse at post‐treatment week 4. At the time of relapse, the patient had treatment‐emergent substitutions of D168E in NS3. The other patient with a baseline C316N mutation in NS5B had virologic relapse at post‐treatment week 12. No treatment‐emergent RAS was detected in NS3 or NS5B. A detailed description of virologic failure is shown in Table 3.
TABLE 3.
Virologic failure and baseline/treatment‐emergent NS3/NS5B polymorphisms
| NS3 RASs | NS5B RASs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of virologic failure | Sex | Age | Genotype | Baseline HCV‐RNA (IU/mL) | FibroScan (kPa) | On‐/post‐treatment | Baseline | On failure | Baseline | On failure |
| Relapse | Female | 35 | 1b | 1.54E+06 | 5.0 | Post‐treatment week 4 | S122G | S122G D168E | C316N D62N | C316N D62N |
| Relapse | Female | 52 | 1b | 4.68E+05 | 13.0 | Post‐treatment week 12 | No | No | C316N | C316N |
| Lost‐to‐follow‐up | Male | 54 | 1b | 2.88E+06 | 10.2 | On‐treatment week 8 | S122G | S122G | C316N | C316N |
| Premature withdrawal | Male | 57 | 1b | 3.30E+05 | 8.2 | On‐treatment week 1 | ND | ND | ND | ND |
Virologic failure: defined as not achieved SVR12 (sustained virologic response at post‐treatment week 12). One patient with genotype 1b lost to follow‐up at treatment week 8 owing to failure to achieve virologic response. One patient with genotype 1b who prematurely withdrew from treatment at week 1 owing to SAE.
HCV, hepatitis C virus; ND, not done; RAS, resistance‐associated substitution.
Safety data
Adverse events were reported in 143 of the 205 patients (69.8%); one patient discontinued the treatment and withdrew from the study at week 1 owing to an SAE (myocardial ischemia) that was not considered related to the study drug. Seven patients had eight SAEs, namely, pneumonia, gastroenteritis, cerebral infarction, acetabular fracture, periarthritis, edema, pectoralgia, and myocardial ischemia; however, none of these were considered as related to treatment with seraprevir and sofosbuvir. No patient died during or after treatment (Table 4).
TABLE 4.
Adverse events and laboratory abnormalities
| Patients (n = 205) | |
|---|---|
| Any AEs | 143 (69.8%) |
| Any TEAEs | 140 (68.3%) |
| Grade 3 | 10 (4.9%) |
| Grade 4 | 0 (0%) |
| Any serious AEs | 7 (3.4%) |
| Any AEs leading to discontinuation of study drug | 1 (0.5%) |
| Death | 0 (0%) |
| Any TEAE related study drug | 48 (23.4%) |
| Grade 1 | 43 (21.0%) |
| Grade 2 | 5 (2.4%) |
| Grade 3 or 4 | 0 (0%) |
| Any TEAEs or TEAE related study drug ≥ 5% | 0 (0%) |
| Any TEAE related to study drug ≥ 1% | |
| Dizziness | 5 (2.4%) |
| Fatty liver | 2 (1.0%) |
| Nausea | 2 (1.0%) |
| Urinary tract infection | 2 (1.0%) |
| Laboratory abnormalities ≥ 1% | |
| Leucopenia | 8 (3.9%) |
| Neutropenia | 6 (2.9%) |
| Increased creatine kinase | 6 (2.9%) |
| Proteinuria | 5 (2.4%) |
| Hypercholesteremia | 5 (2.4%) |
| Plastocytopenia | 4 (2.0%) |
| Hyperbilirubinemia | 2 (1.0%) |
Data are n (%).
AE, adverse events; TEAEs, treatment‐emergent adverse events.
The AEs reported in ≥ 5% of patients were upper respiratory tract infection (16.6%), urinary infection (7.3%), leucopenia (5.4%), increased creatine kinase (5.4%), and nonalcoholic fatty liver (8.3%). In total, 48 patients (23.4%) experienced AEs related to the study drug; however, none of them were Grade 3 or 4 AEs. No AEs related to the study drug were reported at a frequency of ≥ 5%, and the most frequently reported AEs related to the study drug (≥ 2%) were leucopenia (3.9%), neutropenia (2.9%), plastocytopenia (2.0%), dizziness (2.4%), increased creatine kinase (2.9%), proteinuria (2.4%), and hypercholesterolemia (2.4%). The majority of AEs related to the study drug were transient and required no specific medical intervention. General liver function tests, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), showed a significant trend of normalization throughout the study period. After antiviral treatment, the mean values of ALT and AST were significantly reduced compared with the baseline. General renal function tests, including creatinine and blood urea nitrogen, yielded stable results throughout the on‐treatment and post‐treatment follow‐up periods. FibroScan also showed a trend in improved liver stiffness modulus (LSM); the mean reduction in the value of LSM was 1.10 KPa from the baseline to post‐treatment week 12 (6.53 kPa vs 5.42 kPa), as shown in Figure 2.
FIGURE 2.
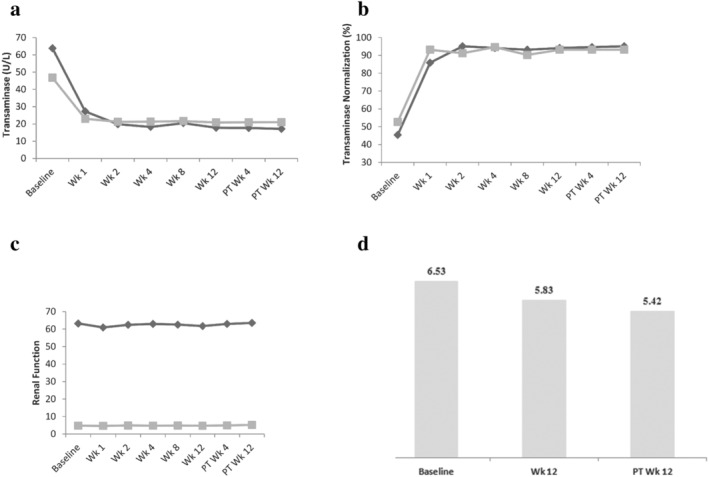
Liver function tests renal function tests, and liver transient elastography. (a) Alanine aminotransferase (ALT) and aspartate aminotransferase (AST); (b) normalization rates of ALT and AST from baseline to post‐treatment week 12; (c) creatinine and blood urea nitrogen (BUN); (d) mean values of liver stiffness modulus (LSM). (a and b)  , ALT;
, ALT;  , AST. (c)
, AST. (c)  , Creatinine (μmol/L);
, Creatinine (μmol/L);  , BUN (mmol/L).
, BUN (mmol/L).
Exploratory study in patients with genotypes 2, 3, and 6
An exploratory study was made to evaluate the efficacy and safety of seraprevir and sofosbuvir in chronic genotype 2, 3, and 6 HCV infection patients (NCT004111367, CRT20190786). Eligible patients with genotypes 2 and 6 were instructed to self‐administer seraprevir 100 mg (Shanghai Viromedicine Co., Ltd., Shanghai, China) twice a day and sofosbuvir 400 mg (Gilead Sciences, Cork, Ireland) once a day for 12 successive weeks, while the patients with genotype 3 for 24 weeks. A total of 36 treatment‐naive patients without cirrhosis were enrolled in this study including genotype 2 (n = 21), genotype 3 (n = 7), and genotype 6 (n = 8) HCV infection. All the patients achieved the primary endpoint of SVR12. AEs were reported in 25 of the 36 patients (86.3%); no SAE was reported. None of the patients experienced grade 3 or 4 TEAEs related to study drug. No patients discontinued or interrupted treatment due to AEs. No patient died during or after treatment. Most of the AEs were mild or moderate, such as upper respiratory tract infection (11.1%), urinary tract infection (11.1%), and increased creatine kinase (5.6%). Most of the AEs did not require a specific medical intervention.
Discussion
In this study, the efficacy and safety of seraprevir and sofosbuvir were evaluated for patients with chronic HCV infection in China. After treatment with seraprevir and sofosbuvir for 12 weeks, SVR12 was achieved in 98% of the patients with genotype 1. The SVR rates were comparable or superior to those associated with other DAA regimens used in patients with genotype 1 HCV infection in Asia. 8 , 10 , 11 , 12 , 13 SVR12 was also high for patients with high viral load (≥ 800 000 IU/mL, 65.9%) and was achieved by 100% of interferon‐experienced patients. The high SVR after treatment with seraprevir plus sofosbuvir showed no significant effect confounded by the HCV genotype, baseline HCV‐RNA titer, previous interferon exposure, or the interactive effects of these factors.
Many DAA regimens have been approved by the China Food and Drug Administration (CFDA), including NS3/4A protease inhibitors, NS5A inhibitors, and NS5B polymerase inhibitors. Asunaprevir, simeprevir, grazoprevir, and danoprevir are the representative NS3/4A protease inhibitors approved by the CFDA for treatment of HCV in combination with other DAAs. As reported in two randomized clinical trials, the SVR12 rates of simeprevir plus sofosbuvir and ribavirin were 92% and 93%, 14 , 15 which were lower than the rate reported by this study. In the C‐CORAL study, SVR12 was achieved by 98.2% of participants with genotype 1b infection who were treated with elbasvir 50 mg/grazoprevir 100 mg for 12 weeks. 9 A similar SVR12 rate was observed in this study. Therefore, seraprevir had comparable or superior efficacy to that of the approved NS3/4A protease inhibitors. Owing to the small number of patients (n = 3) for whom treatment with seraprevir and sofosbuvir failed, the effect of the NS3 or NS5B resistance‐associated variants on virologic response could not be analyzed. In these patients, the common pre‐existing RASs included S122G for NS3 (n = 2) and C316N for NS5B (n = 3). The treatment‐emergent substitution of D168E in NS3 was observed in one of the three patients. This finding was similar to those of the studies on simeprevir and grazoprevir. 9 , 16 S122G was one of the major RASs of simeprevir. 17 However, the most important NS3 mutations related to simeprevir failure are V36M, R155K/G/T, Q80K/R, S122R, and D168A/E/H/V. 18 This substitution of S122G in NS3 in our samples probably has no relation with treatment failure. Meanwhile, the presence of baseline NS3 polymorphisms did not adversely affect SVR12 rates in patients treated with simeprevir and sofosbuvir for 12 weeks. 16 No treatment‐emergent NS5B polymorphism was detected in this study. Similar results were obtained in two other studies on simeprevir. 14 , 16 Previous studies also confirmed that NS5B‐C316N was associated with treatment failure when patients with HCV genotype 1b received sofosbuvir plus ribavirin. 19 , 20 , 21 A previous epidemiologic study confirmed that pre‐existing RASs S122G for NS3 and C316N for NS5B were detected in 82/145 (56.6%) and 129/137 (94.2%) treatment‐naïve patients with genotype 1b. 22 Therefore, pre‐existing NS3 and NS5B polymorphisms might also be prevalent in the patients in this study, although NS3 and NS5B polymorphisms were not genotyped in patients who achieved SVR12. Together with the data from the study on simeprevir, it can be expected that the presence of NS3 polymorphisms may not affect the efficacy of the regimen of seraprevir and sofosbuvir. Treatment with sofosbuvir/velpatasvir for 12 weeks is recommended for patients for whom seraprevir and sofosbuvir treatment failed. 23
The 12‐week treatment with seraprevir plus sofosbuvir showed a generally favorable safety and tolerability profile; most of the treatment‐emergent AEs resolved with ongoing treatment and post‐treatment follow‐up. It is noteworthy that patients showed a trend of improvement in liver disease with respect to liver inflammation and fibrosis, with a generally stable renal function profile, throughout the on‐treatment and post‐treatment follow‐up periods. Overall, the safety profile of this regimen was acceptable and similar to that reported in previous studies on ribavirin‐free, all‐oral DAA regimens.
In China, at least 10 million individuals have been estimated to be infected with HCV2, and genotype 1 is the most predominant subtype. 24 The 69th World Health Assembly has endorsed the global HCV elimination strategy by 2030. China, as the country with the largest number of HCV infections, plays an important role in this aspect. Only 6.7% of the patients in medical institutions received antiviral therapy, although many of the direct‐acting antivirals had been approved in China. A lower income is one of the independent predictors of not receiving antiviral therapy. Up to 32.9% of patients not receiving antiviral treatment have an annual income less than $1610. 25 One study reported that elbasvir/grazoprevir was the most cost‐effective option for treatment of patients with genotype 1 HCV in China. 26 The selling price of elbasvir/grazoprevir is about $930 in China. In the United States, the most cost‐effective regimen is sofosbuvir/velpatasvir, which costs about $1850 in China. Therefore, an effective, accessible, native, and much cheaper HCV treatment regimen remains an unmet medical need in China.
China is a large geographical region with a diverse HCV genotype distribution. The generalizability of the results of this study might, thus, be limited because of the enrolment of all patients with HCV genotype 1 infection. The other exploratory study was made to evaluate the efficacy and safety of seraprevir and sofosbuvir in chronic genotype 2, 3, and 6 HCV infection patients. All the patients achieved the primary endpoint of SVR12. We will further evaluate the efficacy and safety of seraprevir and sofosbuvir for patients with chronic HCV infection of genotypes 2, 3, and 6 in phase 3 clinical trial. The efficacy and safety of seraprevir and sofosbuvir for patients with cirrhosis were not evaluated in this study; thus, they may be not a good choice for this patient population.
In conclusion, the all‐oral, ribavirin‐free regimen of seraprevir and sofosbuvir is a safe and effective treatment for Chinese patients with HCV infection, including those with a history of interferon experience. Therefore, this regimen would be a novel choice of treatment for this patient population.
Supporting information
Table S1. Inclusion and exclusion criteria.
Kong, F. , Wen, X. , Wen, X. , Wang, X. , Wu, G. , Lin, S. , Wang, L. , Xing, H. , Yan, X. , Zheng, S. , Ning, Q. , Wang, Z. , Zhang, L. , Lin, J. , Tong, Z. , Huang, C. , Su, M. , Tong, L. , Jia, J. , Xin, Y. , Zhu, Q. , Wang, J. , Chen, L. , Li, X. , Wu, X. , Niu, D. , Liu, Q. , Wei, W. , Zhang, Y. , Li, G. , and Niu, J. (2021) Seraprevir and sofosbuvir for treatment of chronic hepatitis C virus infection: A single‐arm, open‐label, phase 3 trial. Journal of Gastroenterology and Hepatology, 36: 2375–2382. 10.1111/jgh.15412.
Fei Kong and Xiaoyu Wen contributed equally to this work.
Declaration of conflict of interest: Shanghai Viromedicine Co., Ltd. provided funding for the study and was responsible for study initiation and administrative oversight, data collection, and statistical analyses. Li Chen, Xiaowen Li, Xuegang Wu, and Duan Niu were employees of Shanghai Viromedicine Co., Ltd. when executing this trial. The other authors declare no competing interests.
Contributor Information
Yuexin Zhang, Email: zhangyx3103@163.com.
Guangming Li, Email: lgm177@sina.com.
Junqi Niu, Email: junqiniu@aliyun.com.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon request.
References
- 1. Dhiman RK, Grover GS, Premkumar M. Hepatitis C elimination: a Public Health Perspective. Curr. Treat. Options Gastroenterol. 2019; 17: 367–377. [DOI] [PubMed] [Google Scholar]
- 2. Chen YS, Li L, Cui FQ et al. A sero‐epidemiological study on hepatitis C in China. Zhonghua Liu Xing Bing Xue Za Zhi 2011; 32: 888–891. [PubMed] [Google Scholar]
- 3. Gane E. Future perspectives: towards interferon‐free regimens for HCV. Antivir. Ther. 2012; 17: 1201–1210. [DOI] [PubMed] [Google Scholar]
- 4. Simmonds P, Alberti A, Alter HJ et al. A proposed system for the nomenclature of hepatitis C viral genotypes. Hepatology 1994; 19: 1321–1324. [PubMed] [Google Scholar]
- 5. Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up‐date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 2016; 22: 7824–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andriulli A, Morisco F, Ippolito AM et al. HCV genotype 1 subtypes (1a and 1b): similarities and differences in clinical features and therapeutic outcome. Hepat Int. 2015; 9: 52–57. [DOI] [PubMed] [Google Scholar]
- 7. Chen Y, Yu C, Yin X, Guo X, Wu S, Hou J. Hepatitis C virus genotypes and subtypes circulating in Mainland China. Emerg. Microbes. Infect. 2017; 6: e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wei L, Lim SG, Xie Q et al. Sofosbuvir‐velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single‐arm, open‐label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2019; 4: 127–134. [DOI] [PubMed] [Google Scholar]
- 9. Wei L, Jia JD, Wang FS et al. Efficacy and safety of elbasvir/grazoprevir in participants with hepatitis C virus genotype 1, 4, or 6 infection from the Asia‐Pacific region and Russia: final results from the randomized C‐CORAL study. J. Gastroenterol. Hepatol. 2019; 34: 12–21. [DOI] [PubMed] [Google Scholar]
- 10. Ji F, Wei B, Yeo YH et al. Systematic review with meta‐analysis: effectiveness and tolerability of interferon‐free direct‐acting antiviral regimens for chronic hepatitis C genotype 1 in routine clinical practice in Asia. Aliment. Pharmacol. Ther. 2018; 47: 550–562. [DOI] [PubMed] [Google Scholar]
- 11. Manns M, Pol S, Jacobson IM et al. All‐oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet 2014; 384: 1597–1605. [DOI] [PubMed] [Google Scholar]
- 12. Reddy KR, Bourliere M, Sulkowski M et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology 2015; 62: 79–86. [DOI] [PubMed] [Google Scholar]
- 13. Yee BE, Nguyen NH, Jin M, Lutchman G, Lim JK, Nguyen MH. Lower response to simeprevir and sofosbuvir in HCV genotype 1 in routine practice compared with clinical trials. BMJ Open Gastroenterol. 2016; 3: e000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lawitz E, Sulkowski MS, Ghalib R et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non‐responders to pegylated interferon and ribavirin and treatment‐naive patients: the COSMOS randomised study. Lancet 2014; 384: 1756–1765. [DOI] [PubMed] [Google Scholar]
- 15. Pearlman BL, Ehleben C, Perrys M. The combination of simeprevir and sofosbuvir is more effective than that of peginterferon, ribavirin, and sofosbuvir for patients with hepatitis C‐related Child's class A cirrhosis. Gastroenterology 2015; 148: 762–770. [DOI] [PubMed] [Google Scholar]
- 16. Kwo P, Gitlin N, Nahass R et al. Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1‐infected patients without cirrhosis: OPTIMIST‐1, a phase 3, randomized study. Hepatology 2016; 64: 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobson IM, Dore GJ, Foster GR et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment‐naive patients with chronic hepatitis C virus genotype 1 infection (QUEST‐1): a phase 3, randomised, double‐blind, placebo‐controlled trial. Lancet 2014; 384: 403–413. [DOI] [PubMed] [Google Scholar]
- 18. Sorbo MC, Cento V, Di Maio VC et al. Hepatitis C virus drug resistance associated substitutions and their clinical relevance: update 2018. Drug Resist. Updat. 2018; 37: 17–39. [DOI] [PubMed] [Google Scholar]
- 19. Charlton M, Gane E, Manns MP et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology 2015; 148: 108–117. [DOI] [PubMed] [Google Scholar]
- 20. Lawitz E, Mangia A, Wyles D et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N. Engl. J. Med. 2013; 368: 1878–1887. [DOI] [PubMed] [Google Scholar]
- 21. Donaldson EF, Harrington PR, O'Rear JJ, Naeger LK. Clinical evidence and bioinformatics characterization of potential hepatitis C virus resistance pathways for sofosbuvir. Hepatology 2015; 61: 56–65. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Rao HY, Xie XW, Wei L. Direct‐acting antiviral agents resistance‐associated polymorphisms in Chinese treatment‐naive patients infected with genotype 1b hepatitis C virus. Chin Med J (Engl) 2015; 128: 2625–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hepatitis C guidance 2018 update: AASLD‐IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin. Infect. Dis. 2018; 67: 1477–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, Chen LM, He M. Hepatitis C Virus in mainland China with an emphasis on genotype and subtype distribution. Virol. J. 2017; 14: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bian DD, Zhou HY, Liu S et al. Current treatment status and barriers for patients with chronic HCV infection in mainland China: a national multicenter cross‐sectional survey in 56 hospitals. Medicine (Baltimore) 2017; 96: e7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuen MF, Liu SH, Seto WK et al. Cost‐utility of all‐oral direct‐acting antiviral regimens for the treatment of genotype 1 chronic hepatitis C virus‐infected patients in Hong Kong. Dig. Dis. Sci. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Inclusion and exclusion criteria.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon request.


