Abstract
To generate three‐dimensional tissue in vitro, promoting vasculogenesis in cell aggregates is an important factor. Here, we found that ultrasound promoted vasculogenesis of human umbilical vein endothelial cells (HUVECs). Promotion of HUVEC network formation and lumen formation were observed using our method. In addition to morphological evaluations, protein expression was quantified by western blot assays. As a result, expression of proteins related to vasculogenesis and the response to mechanical stress on cells was enhanced by exposure to ultrasound. Although several previous studies have shown that ultrasound may promote vasculogenesis, the effect of ultrasound was unclear because of unregulated ultrasound, the complex culture environment, or two‐dimensional‐cultured HUVECs that cannot form a lumen structure. In this study, regulated ultrasound was propagated on three‐dimensional‐monocultured HUVECs, which clarified the effect of ultrasound on vasculogenesis. We believe this finding may be an innovation in the tissue engineering field.
Keywords: mechanotransduction, tissue engineering, ultrasound, vasculogenesis
Travelling wave was propagated on three‐dimensional‐monocultured human umbilical vein endothelial cells (HUVECs) in our device, which clarified the effect of ultrasound on vasculogenesis. In our study, promotion of HUVEC network formation and lumen formation were observed due to the travelling wave. In addition to morphological evaluations, protein expression was quantified, and expression of proteins related to vasculogenesis and the response to mechanical stress on cells was enhanced by exposure to ultrasound.
1. INTRODUCTION
Tissue engineering generates tissues and organs for regenerative medicine, drug testing, and biomedical research (Esch et al., 2015; Imashiro & Shimizu, 2021). Currently, many studies of engineering three‐dimensional (3D) tissue in vitro have been reported. However, a lack of intrinsic vascularity and transport systems to nourish tissues limits their survival because of the nutrient and oxygen deficiency as well as waste products from the tissue itself (Sakaguchi et al., 2013). Some methods to generate 3D tissue employ animals as a living reactor to fabricate vascular networks have been reported (Shimizu et al., 2006). However, these in vivo methods have practical limitations for most applications (Sakaguchi et al., 2013). Using a 3D scaffold is also effective to develop vascular networks, but excessive connective tissue formation or insufficient cell seeding in scaffolds may occur (Asakawa et al., 2010). Thus, to engineer beneficial 3D tissue, promotion of vasculogenesis in cell aggregates in vitro without a scaffold is important.
To regulate in vitro vasculogenesis, three main factors—chemical stimulus, positional, and mechanical stimulus factors—have been studied (dela Paz et al., 2012; Kang et al., 2018; Martino et al., 2015; Newman et al., 2011; Takehara et al., 2015). Regulating by chemical stimulus is the most conventional and common strategy. Chemical factors for vasculogenesis have been identified and used to promote vasculogenesis in many studies. To regulate by a positional factor, a 3D scaffold with hollow structures and 3D bioprinting technology have been developed (Seto et al., 2010; Skylar‐Scott et al., 2019; Zhu et al., 2017). However, to fabricate 3D tissue without a scaffold, there are very few methods for regulation by a positional factor. Recently, mechanical stimulus was found to regulate vasculogenesis (Price et al., 2010). Medium flow exerting shear stress on two‐dimensional (2D)‐cultured endothelial cells promotes their elongation (Sasamoto et al., 2005). Further, when endothelial cells cultured in 3D tubular scaffold were exposed to medium flow, the vessel‐like structure with the scaffold is easily realized (X. Y. Wang et al., 2015). A tensile force on 3D‐cultured endothelial cells in scaffold regulates the orientation of vasculogenesis (Rosenfeld et al., 2016). These studies indicate the potential of mechanical stimulus to regulate vasculogenesis. However, no method can regulate vasculogenesis in 3D tissue without a scaffold. Cell aggregates cannot be exposed to tensile force, and simple medium flow for mechanical stimulation of cells cannot be effectively delivered to cells in an aggregate. It is therefore necessary to add mechanical stimulus to endothelial cells inside of aggregates to regulate vascularization without a scaffold.
As a tool that delivers mechanical stimuli into the deep parts of 3D objects, ultrasound has demonstrated its biocompatibility in echo and ultrasound applications for bioengineering (Ding et al., 2014; Imashiro et al., 2020; Nakao et al., 2018; Teague & Sharma, 1991). In fact, studies have reported that exposure to ultrasound at the location of ischemia in vivo induces angiogenesis, but the factor promoting vascular structures is unclear (Hanawa et al., 2014; Ogata et al., 2017). Ultrasound propagating into bodies causes many phenomena, and there are many possible pathways to regulate cell functions in vivo because of the many cell types. Thus, it is unclear whether ultrasound in endothelial cells is truly critical to promote vasculogenesis. To clarify the effect of ultrasound on vasculogenesis, in vitro studies have been performed (Gollmann‐Tepeköylü et al., 2018). Although some of them have reported that ultrasound exposure regulates protein expression of 2D‐cultured endothelial cells, 3D culture is needed for vasculogenesis. Furthermore, ultrasound behaviors, such as reflection or attenuation resulting in generation of standing wave or acoustic streaming and temperature increases, were not considered in most previous studies (Huang et al., 2015). Standing wave has distributions of pressure amplitude and vibration displacement amplitude (Nakao et al., 2019), and medium flow and temperature increase can affect cell function. These can be additional factors for cells, adding to acoustic waves itself. Thus, a direct effect of ultrasound on vasculogenesis has not been demonstrated. Therefore, the potential of ultrasound to promote vasculogenesis is difficult to confirm based on previous studies. To investigate the potential of ultrasound to promote vasculogenesis, it is necessity to develop an experimental system in which travelling ultrasound waves with homogenous intensity are propagated on 3D‐monocultured endothelial cells. To avoid secondary effects, such as temperature increment and acoustic streaming and to understand the pure effect of ultrasound itself, a travelling ultrasound wave with a tone burst excitation should be employed. To realize these conditions, endothelial cells embedded in a collagen gel were exposed to travelling ultrasound waves without a temperature rise or medium flow in our study. In this system, human umbilical vein endothelial cells (HUVECs) were used as typical endothelial cells, and MHz ultrasound was used to avoid reflection. As a result, 24 h of ultrasound exposure to HUVECs promoted a network structure and even formed a lumen structure. This study is the first report of observing a lumen structure of HUVECs with this simple and rapid culture method (Newman et al., 2011). Furthermore, protein quantification suggested the mechanism of the promotion of vascularization by ultrasound. Based on these results, ultrasound has the potential to promote vascularization.
2. METHODS
2.1. Development of the experimental device
To apply homogeneous travelling ultrasound waves on cells, the device shown in Figure 1a and b was developed. As an ultrasound transducer, a piezoelectric transducer (thickness: 2 mm, diameter: 28 mm, C‐213; Fuji Ceramics Corporation) was used. For 3D cell culture, HUVECs were seeded in a 4 mg/ml collagen gel (IAC‐50; Koken Co., Ltd.) into a culture chamber fabricated with PDMS rubber (SYLGARD 184 Silicone Elastomer Kit; DuPont Toray Specialty Materials Kabushiki Kaisha) as shown in Figure 1c. For effective propagation of ultrasound to the cells in the culture chamber, water was introduced into the gap between the transducer and culture chamber as a coupling liquid. Furthermore, an ultrasound absorber consisting of silicone rubber (KE‐1316; Shin‐Etsu Chemical Co., Ltd.) was fabricated for attenuation of the ultrasound to achieve travelling waves. These were set in an acrylic case. The detailed dimensions of this device are shown in Figure S1.
Figure 1.

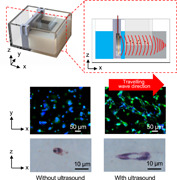
Experimental system for propagation of acoustic stimulus on cells. Overview (a) and side view (b) of the device propagating ultrasound on cells. Schematic method to seed cells in a collagen gel for three‐dimensional (3D) cell culture (c). Schematic image showing the experimental plan (d) [Color figure can be viewed at wileyonlinelibrary.com]
For the cell experiment demonstrating our research concept, the procedures shown in Figure 1d were performed. The culture chamber was sterilized in an autoclave before cell seeding. The seeded cells were cultured for 24 h before exposure to ultrasound. A sample cultured with ultrasound exposure was called the test sample, and a sample without ultrasound was called the control sample. To propagate ultrasound on cells, the gap between the chamber and piezoelectric transducer was filled with water. In this system, 96% vibration amplitude of the ultrasound transmitted on the culture chamber should have propagated into the culture chamber (see Supporting Information Note 1 for the calculation) (Kurashina et al., 2019).
2.2. Measurement of vibration
To determine the resonance frequency of the transducer in our device, the frequency characteristics of the AC input on the transducer were evaluated by an impedance analyzer (FRA5097; NF Corporation). To evaluate the frequency characteristics, the conditions of the cell experiment were reproduced. A laser Doppler vibrometer (CLV‐3D, PI Polytech) and an oscilloscope (TBS2074, Tektronix) were used to measure the vibration amplitude distribution. For stable measurements of vibration distribution shown in normalized value, a continuous AC input was used and the jig was placed in the air. Farther, since the average mode of the oscilloscope was used, the data shown was averaged from 64 measurement trials. The other conditions were similar to those in the cell experiment, although the device was set at room temperature. Further, to measure the absolute value of the vibration amplitude at one point of (y, z) = (5, 1), the jig was placed in a water in the acrylic case and tone burst wave mentioned later was used.
2.3. Preparation of cells
HUVECs (C2519A; Lonza) were used in experiments. The cells were cultured in endothelial growth medium and supplements (CC‐3162; Lonza) with a 50 mg/ml Gentamicin Sulfate Solution (11980‐14; Nacalai Tesque, Inc.) at 37°C in a humidified atmosphere with 5% CO2. Cell passaging was performed by trypsinization in 0.05% trypsin‐EDTA (25300062; Thermo Fisher Scientific).
To reconstitute a pre‐gel collagen solution, 50 mM sodium hydroxide, 200 mM HEPES, and 260 mM sodium hydrogen carbonate were mixed to prepare a reconstitution buffer. The reconstitution buffer, ×10 Hanks balanced salt solution and acid‐solubilized collagen solution (IAC‐50), was then mixed on ice at a volume ratio of 1:1:8 to reconstitute the collagen pre‐gel solution. In this step, cells were included in the solution at 7 × 106 cells/ml. Then, 90 µl of the prepared suspension was introduced into the culture chamber, and 3 ml of the medium was introduced.
2.4. Cell staining
Live cells and nuclei were stained with calcein‐AM and Hoechst 33342 (B2261, Sigma‐Aldrich), respectively. The samples were washed three times with phosphate‐buffered saline (PBS), and a 2 µl/ml calcein and 5 µl/ml Hoechst solution diluted with PBS was applied. After 40 min of incubation, the samples were washed with PBS three times. Then, the cells were fixed with 4% paraformaldehyde (15710; Microscopy Sciences) and washed three times with PBS. Samples were washed for 5 min. Image analysis was carried out using ImageJ that is freely available in the public domain.
For cross‐sectional observation, samples were fixed in 4% paraformaldehyde and routinely processed into 7‐µm‐thick paraffin‐embedded sections. HE staining was performed using conventional methods.
2.5. Glucose consumption assay
Glucose consumptions of control and test samples were monitored after the experiment. The glucose concentration in the medium was determined by a glucose assay kit (GAHK‐20; Sigma‐Aldrich), following the manufacturer's protocol.
2.6. Western blot assay
Pellets of HUVECs were lysed in 50 µl SDS‐PAGE sample buffer at 75°C for 5 min. The resulting samples (10 µl/lane for glyceraldehyde 3‐phosphate dehydrogenase [GAPDH] or 20 µl/lane for β‐actin, CD29, vascular endothelial growth factor [VEGF], and stromal cell‐derived factor‐1 [SDF‐1]) were resolved on an SDS‐PAGE gel (Tris–HCl gel: 10% for GAPDH, β‐actin, and CD29 and 15% for VEGF and SDF‐1). Then, the resolved proteins were transferred onto polyvinylidene fluoride membranes. The membranes were gently incubated with Blocking One (Nacalai Tesque) for 30 min. Then, the membranes were incubated with primary antibodies (1000‐fold dilution of a mouse anti‐human GAPDH monoclonal antibody [60004‐1‐lg; Proteintech], 500‐fold dilution of a rabbit anti‐human CD29 polyclonal antibody [GTX50784; GeneTex], 500‐fold dilution of a rabbit anti‐human VEGF‐A polyclonal antibody [R30265; NSJ Bioreagents], 500‐fold dilution of a rabbit anti‐human SDF‐1 polyclonal antibody [#41422; Signalway Antibody], and 1000‐fold dilution of a rabbit anti‐mouse β‐actin polyclonal antibody [#4967; Cell Signaling Technology]) at 4°C for 18 h. The membranes were washed in TBS containing 0.05% Tween 20 (TBS‐T) for 10 min three times. The membranes were then incubated with secondary antibodies (10,000‐fold dilution of HRP‐conjugated anti‐rabbit IgG, Perkin‐Elmer and 5000‐fold dilution of HRP‐conjugated anti‐mouse IgG, Sigma Aldrich) for 30 min. After washing with TBS‐T three times, the membranes were treated with chemiluminescent reagent (Immobilon Western, Millipore). Analysis of the membranes was carried out using an image analyzer (Fluoro Chem Q, Protein Simple). The band density was normalized to the GAPDH band and expressed as the quantity relative to the control sample. This protocol is from a previous work (Nakao et al., 2019).
2.7. Statistical analysis
For comparisons of datasets, unpaired Student's t tests were used. p values are stated in figure legends.
3. RESULTS
3.1. Evaluation of mechanical stimulus in cells
Mechanical stimulus in cells was estimated using our method. To apply mechanical vibration stimulus on cells effectively, the resonance frequency of the ultrasonic transducer was measured as shown in Figure S2 and identified as 1.004 MHz. Thus, we used this frequency as the driving frequency of our device.
Distributions of the vibration amplitude of the surface of the transducer and ultrasound absorber were measured as shown in Figure 2. Figure 2 shows that homogeneous vibration was induced on the surface of the transducer, and the average vibration displacement was 10.42 × 10−2 µmpp with an input voltage of 30 Vpp. To input AC voltage into the ultrasound transducer, a function generator (WF1946B; NF Corporation) and amplifier (LZY‐22+; Mini‐Circuits; Brooklyn) were used in our study. Therefore, homogeneous ultrasound was propagated from the surface of the transducer. Furthermore, the vibration amplitude of the surface of the ultrasound absorber was negligible compared with the transducer. The attenuation of the ultrasound in the culture chamber and ultrasound reflection on the inner wall of the culture chamber were minor (see Supporting Information Note 1) (Domingo, 2008). Thus, the transmitted ultrasound was attenuated in the ultrasound absorber, and the cells in the culture chamber were exposed to homogeneous travelling waves. The vibration amplitude and frequency of the ultrasound travelling on cells were approximately 10.0 × 10−2 µmpp and 1.004 MHz, respectively.
Figure 2.
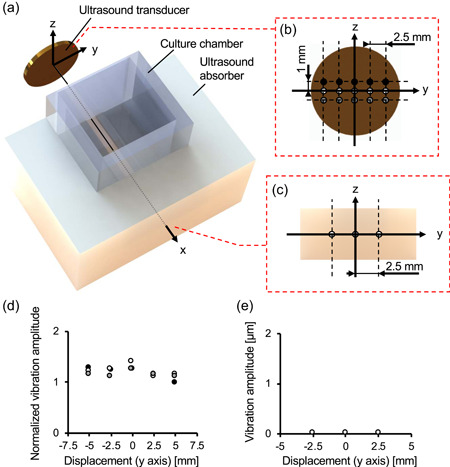
Experimental system for propagation of acoustic stimulus on cells. Overview (a) and side view (b) of the device propagating ultrasound on cells. Schematic method to seed cells in a collagen gel for three‐dimensional (3D) cell culture (c). Vibration distribution of the transducer (d) and ultrasound absorber (e) are shown. The vibration amplitude was normalized to the minimum value of 8.68 × 10−2 µmpp (d). The color of the plots in (b) and (d) and in (c) and (e) corresponds to each other [Color figure can be viewed at wileyonlinelibrary.com]
The temperature variation of the medium in the culture chamber was maintained at 36–38°C, which was appropriate for cell culture during the experiments as shown in Figure S3. To suppress a temperature increase, an input signal was applied to the transducer intermittently with a burst ratio of 10% and burst period of 0.01 s (Tauchi et al., 2019). We also evaluated medium flow that can be generated by ultrasound, but no medium flow was observed in our experimental system by introducing particles with diameter <20 µm (19096‐2; Cosmo Bio Co., Ltd.) into culture media as tracer particles to observe possible flow.
3.2. Morphology of HUVECs
Cytoplasm and nuclei of live cells were stained after experiments, and fluorescence images were obtained at the regions shown in Figure S4 in each experiment. Because homogeneous travelling waves were propagated on every cell, the cell in each imaged area was exposed to the same intensity of ultrasound. Figure 3a shows a typical image of cells in each sample. As shown in this figure, the test sample with ultrasound exposure had cells adhering to each other, which formed vascular networks, whereas many single cells were observed in control samples without ultrasound application. To quantify the formed vascular networks, the average number of cells forming one vascular network was determined as shown in Figure 3b (see Figure S5 for raw data). In the test sample, networks of HUVECs were promoted significantly. We further measured the length of the long axis of a single cell in each sample as shown in Figure 3c. As a result, even single cells in the test sample showed significant elongation compared with the control.
Figure 3.
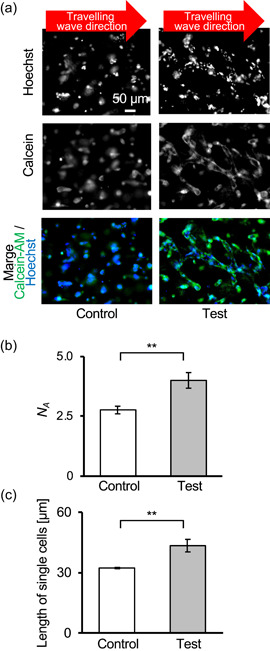
Morphology of cells with acoustic stimulus. Immunofluorescence image of cells (a). The positions where images were taken are shown in Figure S4. The average number of cells, N A, building each vascular network (b) (N = 3, **p < .01, mean ± SD). Note that N is the number of experiments, and each experiment included several cells. Raw data of each experiment are shown in Figure S5. Average length of single cells in each sample (c) (N = 3, **p < .01, mean ± SD). Note that N is the number of experiments, and each experiment included several cells [Color figure can be viewed at wileyonlinelibrary.com]
To confirm whether the formed vascular networks had a lumen structure, a cross‐section image of each sample was obtained as shown in Figure 4. These images were typical for each sample, which showed that only the test sample had a lumen structure in the vascular network. Other cross‐section images were shown in Figure S6.
Figure 4.

Hematoxylin–eosin (HE)‐stained sections of control (a) and test (b) samples. A lumen structure was only found in the test sample [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Glucose consumption
Figure 5a shows glucose consumption of each sample. No significant difference was observed between test and control samples, although there was a trend that the test samples had higher glucose consumption. Furthermore, the number of cells was estimated as shown Figure 5b. Although no significant difference was observed in the number of cells under both conditions, more cells tended to be observed in the test samples. Therefore, ultrasound did not affect cell glucose consumption, which is one of the major indexes for cell metabolism (Anggayasti et al., 2020).
Figure 5.
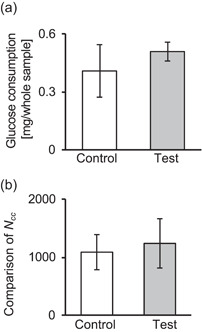
Glucose consumption (a) in culture medium and comparison of the number of cells, N cc, (b) in images of each sample. N cc was normalized to the control (mean ± SD, N = 4)
3.4. Protein assay
The expression of CD29, glyceraldehyde 3‐phosphate dehydrogenase (GAPDH), β‐actin, VEGF‐A, and stromal cell‐derived factor (SDF)−1 were quantified as shown in Figure 6. Expression of GAPDH was stable under each condition, which demonstrated the applicability of using GAPDH as a loading control. However, expression of other proteins was increased significantly with ultrasound, although β‐actin showed had no statistical difference. CD29, VEGF‐A, and SDF‐1 are related to vasculogenesis, while CD29 and β‐actin are used as indexes for mechanotransduction (Maniotis et al., 1997; Moore et al., 2012; Shih et al., 2011; Yeh et al., 2017). CD29 is located on the cell membrane and acts as a collagen receptor(Chen et al., 2014; Kamata et al., 1994). CD29 also plays an important role in vascularization (S. Li et al., 2017). VEGF‐A and SDF‐1 are cytokines that promote vascularization, and SDF‐1 suppresses apoptosis (Brandimarti et al., 2004). β‐Actin is a cytoskeleton protein that mainly drives migration and many other cellular activities (Haeger et al., 2015; Jiang et al., 2013). GAPDH is related to cell metabolism and commonly used as a loading control for protein analysis.
Figure 6.
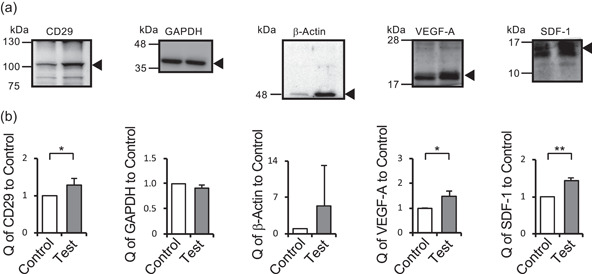
Comparison of protein expression. Control and test samples were lysed in SDS‐PAGE sample buffer, and proteins were analyzed by western blot analysis. Arrows indicate target bands (a). Relative protein quantities (Q) of CD29, GAPDH, β‐actin, VEGF‐A, and SDF‐1 were measured using their band densities on western blots (b). Protein quantities were normalized to the band density of GAPDH and expressed as the quantity relative to the control (mean ± SD, N = 3, **p < .01, *p < .05). Western blots were cropped for clarity. Uncropped western blots are shown in Figure S7
4. DISCUSSION
The main purpose of this study was demonstration of the promoting effect of ultrasound on vasculogenesis. We developed a device in which travelling waves were dominantly propagated on 3D‐monocultured HUVECs and showed the effects of ultrasound on not only morphology, but also protein expression. Promotion of HUVEC network formation and even lumen formation was observed using our method in this simple culture environment. To form a lumen, it has been believed that coculture or a tubular scaffold is needed, but we chose this simple condition to evaluate the potential of ultrasound to promote vasculogenesis, leading to excellent results. Thus, ultrasound has the potential to promote vascularization of endothelial cells. Furthermore, we did not optimize the ultrasound conditions in our study, suggesting that the potential of ultrasound may be higher than demonstrated.
Several factors in ultrasound may promote vasculogenesis, such as acoustic streaming, heat generation, diffusion of chemical species, deformation, and acoustic pressure. Acoustic streaming and heat generation were not found in this study. Thus, the diffusion rate should not have been enhanced, because temperature is a critical factor regulating diffusion (H. Li et al., 1997). In terms of deformation, the wavelength of the travelling wave in our study was approximately 1.5 mm, which is far larger than the cellular size. Thus, the cells could sense the homogeneous tensile force (Moon et al., 2008). Based on the wavelength of 1.5 mm and vibration amplitude of 0.01 µmpp, cells were exposed to 0.0007% stretch, which is negligible compared with previous studies using several tens of percent stretch to examine mechanotransduction (Ali et al., 2004; Clark et al., 2002). Thus, we believe the acoustic pressure was dominant in our study. In previous research, it has been already demonstrated that if endothelial cells were exposed to the mechanical stress such as shear stress, tube formation was promoted (X. Y. Wang et al., 2015). Further, the mechanism that the endothelial cells enhance their function in the reaction to the mechanical stress were reported (Radel & Rizzo, 2005). Since acoustic pressure was demonstrated as a dominant factor, we argue that the mechanism of enhanced vasculogenesis with ultrasound was similar to the previous research. To the best of our knowledge, no study has examined the effect of acoustic pressure on vasculogenesis, because there has been no device in which cells can be exposed to a regulated travelling wave. When mentioning pressure caused by ultrasound, there are acoustic pressure (pressure variation in the medium) and radiation pressure (time‐averaged acoustic force). The acoustic pressure and the radiation pressure were obtained as 0.67 MPa and 0.335 nPa (see Supporting Information Notes 2 and 3). Since travelling wave of cyclic incident wave was dominant, cells should be exposed to directional cyclic pressure and the acoustic pressure was dominant (Johnson et al., 2016). Further, In the previous study, although the studied cell type was not HUVECs, cell differentiation was promoted in a range from 0.1 to 10 MPa (Elder & Athanasiou, 2009). This may indicate that the acoustic pressure delivered into the culture chamber had reasonable value, while the radiation pressure maybe minor in our set up. The acoustic pressure in our study was larger than the reported threshold of occurring cavitation, which should give cells damages. Since burst input was utilized, we assumed there was no cavitation (Mancia et al., 2017). However, we do not have enough proof of no cavitation, which may have possibility to promote vasculogenesis by changing microenvironment of cells (X. Wang et al., 2018). Investigation of the effect to cavitation on vasculogenesis may be an interesting topic for a future work. Although the burst period of 0.01 s is different from blood pulsation in humans, both of them have only one force direction. Here, realizing an in vitro mechanical condition similar to in vivo conditions is a basic and traditional strategy of cell culture. Although HUVECs were cultured in a collagen gel in this study, ultrasound is only a tool to realize this kind of cyclic and directional force on cells inside of a scaffold‐free tissue.
The mechanism connecting acoustic pressure and promoted vascularization is important. There are several reports that mention the mechanism of vasculogenesis promoted by force on cells, such as medium flow and tensile force. Although the mechanisms to generate force on cells and in a culture environment are different, there should be a common regulatory mechanism in the present study and studies using medium flow. In the previous studies, the mechanism through which mechanical stress such as force on cells is transduced into a biochemical reaction is called “mechanotransducion.” To confirm whether mechanotransduction was related to our results, we quantified protein expression. As shown in Figure 6, proteins related to the development of vessel structures, such as VEGF and SDF‐1, were upregulated, both of which are hormones. In a previous study, their production and receptors were increased by mechanical stimulus, which is consistent with our study (Gollmann‐Tepeköylü et al., 2018; Mahajan et al., 2017). Furthermore, the mechanosenser protein CD29 was upregulated. CD29 also has a role in vasculogenesis, and integrin α1β1, α2β1, α3β1, α4β1, and α5β1 regulate the formation of vessel structures. Thus, an increase of CD29 (integrin β1) expression should promote vasculogenesis. Although no statistical difference was found, expression of β‐actin driving cell migration tended to be enhanced. In one of the classic pathways of mechanotransduction, mechanical stimulus on CD29 is transmitted via β‐actin to activate cell migration, which is a very important step to develop vessel structures (Gardel et al., 2008; Plotnikov et al., 2012). Furthermore, increased expression of β‐actin indicated suppression of apoptosis. During static culture without any mechanical stimulus, HUVECs tend to undergo apoptosis in vitro after diminishment of β‐actin, while blood flow provides mechanical stimulation to maintain vessel homeostasis (dela Paz et al., 2012; Pinto et al., 2019). In previous studies, even if vessel structures were formed, they collapsed during culture. This is because adding mechanical stimulus was impossible during culture because perfusion culture can only be allowed in a perfect vessel structure with a flawlessly connected lumen that has not been developed stably. As shown in Figure 5b, the number of cells in the test sample tended to be higher than that in the control. Additionally, upregulated SDF‐1 suggested suppression of apoptosis in test samples due to the mechanical stimulation. GAPDH used as the loading control showed stable expression. This protein is related to metabolism, and Figure 5a shows that glucose consumption as an index of cell metabolism was stable. Thus, it is reasonable to use GAPDH as a loading control, which supports the validity of our protein quantification. Thus, ultrasound promoted vasculogenesis by mechanotransduction. Based on the results of protein assays, not only vasculogenesis, but also angiogenesis may be promoted by ultrasound exposure, which should raise the value of our study. This is because protein expression related to vasculogenesis and angiogenesis was upregulated and apoptosis was suppressed, although vasculogenesis and angiogenesis are different phenomena (Kim et al., 2013).
We demonstrated that ultrasound exposure to HUVECs is effective for vasculogenesis, and acoustic pressure may be the most dominant factor among the many factors in ultrasound. It is true that previous report realized perfusable vascular networks with more physiological conditions. However, because we demonstrated that ultrasound on endothelial cells promotes vasculogenesis using a simple experimental system, we believe ultrasound has a huge potential to regulate vasculogenesis and will become one of the most important tools for tissue engineering. Furthermore, because ultrasound with a proper intensity is biocompatible in vitro and in vivo, our present method can also be used in vivo. Ultrasound can be propagated to specific areas in vivo and even deep parts of tissues, which reinforces the potential of ultrasound for vasculogenesis in vivo (Inui et al., 2019; Van Velthoven et al., 2016). This should be helpful for regenerative therapy of ischemic areas. Regardless of in vivo or in vitro, it is worthwhile to combine ultrasound with other methods suggested in previous studies (Itai et al., 2019; Kang et al., 2018; Sekine et al., 2013). Further, although we used travelling wave in this study, it should be interesting to investigate the effect of standing wave on vasculogenesis as a future work. In conclusion, our novel finding may be a breakthrough in tissue engineering and regenerative therapy.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Chikahiro Imashiro, Tetsuya Azuma, and Kenjiro Takemura designed the study. Chikahiro Imashiro, Tetsuya Azuma, Hiroaki Onoe, and Kenjiro Takemura fabricated the system. Chikahiro Imashiro, Tetsuya Azuma, Shun Itai, Taiki Kuribara, and Kenjiro Totani conducted cell experiments and assays. Chikahiro Imashiro, Shun Itai, Taiki Kuribara, Hiroaki Onoe, and Kenjiro Takemura wrote the manuscript with input from all authors.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This study was supported by JSPS KAKENHI Grant Numbers 16H04259, 17KK0119, 17H07081, 18J12482, and 20J00337 and the Fukuda Foundation for Medical Technology. C. Imashiro and S. Itai are JSPS research fellows. The authors thank to Prof. Tatsuya Shimizu at Tokyo Women's Medical University and Prof. Katsuhisa Sakaguchi at Waseda University for the helpful advice.
Imashiro, C. , Azuma, T. , Itai, S. , Kuribara, T. , Totani, K. , Onoe, H. , & Takemura, K. (2021). Travelling ultrasound promotes vasculogenesis of three‐dimensional‐monocultured human umbilical vein endothelial cells. Biotechnology and Bioengineering, 118, 3760–3769. 10.1002/bit.27852
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available.
REFERENCES
- Ali, M. H. , Pearlstein, D. P. , Mathieu, C. E. , Schumacker, P. T. , & (2004). Mitochondrial requirement for endothelial responses to cyclic strain: Implications for mechanotransduction. American Journal of Physiology: Lung Cellular and Molecular Physiology, 287(3), 486–496. 10.1152/ajplung.00389.2003 [DOI] [PubMed] [Google Scholar]
- Anggayasti, W. L. , Imashiro, C. , Kuribara, T. , Totani, K. , & Takemura, K. (2020). Low‐frequency mechanical vibration induces apoptosis of A431 epidermoid carcinoma cells. Engineering in Life Sciences, 20(7), 232–238. 10.1002/elsc.201900154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa, N. , Shimizu, T. , Tsuda, Y. , Sekiya, S. , Sasagawa, T. , Yamato, M. , Fukai, F. , & Okano, T. (2010). Pre‐vascularization of in vitro three‐dimensional tissues created by cell sheet engineering. Biomaterials, 31(14), 3903–3909. 10.1016/J.BIOMATERIALS.2010.01.105 [DOI] [PubMed] [Google Scholar]
- Brandimarti, R. , Khan, M. Z. , Fatatis, A. , & Meucci, O. (2004). Regulation of cell cycle proteins by chemokine receptors: A novel pathway in human immunodeficiency virus neuropathogenesis. Journal of Neurovirology, 10(Suppl. 1), 108–112. 10.1080/753312761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Li, C. , & Wang, S. (2014). Periodic heat shock accelerated the chondrogenic differentiation of human mesenchymal stem cells in pellet culture. PLOS One, 9(3), 91561. 10.1371/journal.pone.0091561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, C. B. , McKnight, N. L. , & Frangos, J. A. (2002). Strain and strain rate activation of G proteins in human endothelial cells. Biochemical and Biophysical Research Communications, 299(2), 258–262. 10.1016/S0006-291X(02)02628-1 [DOI] [PubMed] [Google Scholar]
- Ding, X. , Peng, Z. , Lin, S. C. , Geri, M. , Li, S. , Li, P. , Chen, Y. , Dao, M. , Suresh, S. , & Huang, T. J. (2014). Cell separation using tilted‐angle standing surface acoustic waves. Proceedings of the National Academy of Sciences, 111, 12992–12997. 10.1073/pnas.1413325111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo, M. C. (2008). Overview of channel models for underwater wireless communication networks. Physical Communication, 1(3), 163–182. 10.1016/j.phycom.2008.09.001 [DOI] [Google Scholar]
- Elder, B. D. , & Athanasiou, K. A. (2009). Hydrostatic pressure in articular cartilage tissue engineering: From chondrocytes to tissue regeneration. Tissue Engineering—Part B: Reviews, 15(1), 43–53. 10.1089/ten.teb.2008.0435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch, E. W. , Bahinski, A. , & Huh, D. (2015). Organs‐on‐chips at the frontiers of drug discovery. Nature Reviews Drug Discovery, 14(4), 248–260. 10.1038/nrd4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel, M. L. , Sabass, B. , Ji, L. , Danuser, G. , Schwarz, U. S. , & Waterman, C. M. (2008). Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. Journal of Cell Biology, 183(6), 999–1005. 10.1083/jcb.200810060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollmann‐Tepeköylü, C. , Lobenwein, D. , Theurl, M. , Primessnig, U. , Lener, D. , Kirchmair, E. , Mathes, W. , Graber, M. , Pölzl, L. , An, A. , Koziel, K. , Pechriggl, E. , Voelkl, J. , Paulus, P. , Schaden, W. , Grimm, M. , Kirchmair, R. , & Holfeld, J. (2018). Shock wave therapy improves cardiac function in a model of chronic ischemic heart failure: Evidence for a mechanism involving VEGF signaling and the extracellular matrix. Journal of the American Heart Association, 7(20), 010025. 10.1161/JAHA.118.010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeger, A. , Wolf, K. , Zegers, M. M. , & Friedl, P. (2015). Collective cell migration: Guidance principles and hierarchies. Trends in Cell Biology, 25(9), 556–566. 10.1016/j.tcb.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Hanawa, K. , Ito, K. , Aizawa, K. , Shindo, T. , Nishimiya, K. , Hasebe, Y. , Tuburaya, R. , Hasegawa, H. , Yasuda, S. , Kanai, H. , & Shimokawa, H. (2014). Low‐intensity pulsed ultrasound induces angiogenesis and ameliorates left ventricular dysfunction in a porcine model of chronic myocardial ischemia. PLOS One, 9(8), 1–11. 10.1371/journal.pone.0104863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. J. , Shi, Y. Q. , Li, R. L. , Hu, A. , Lu, Z. Y. , Weng, L. , Wang, S. Q. , Han, Y. P. , Zhang, L. , Li, B. , Hao, C. N. , & Duan, J. L. (2015). Angiogenesis effect of therapeutic ultrasound on HUVECs through activation of the PI3K–Akt–eNOS signal pathway. American Journal of Translational Research, 7(6), 1106–1115. Retrieved from. www.ajtr.org. [PMC free article] [PubMed] [Google Scholar]
- Imashiro, C. , Hirano, M. , Morikura, T. , Fukuma, Y. , Ohnuma, K. , Kurashina, Y. , Miyata, S. , & Takemura, K. (2020). Detachment of cell sheets from clinically ubiquitous cell culture vessels by ultrasonic vibration. Scientific Reports, 10(1), 1–11. 10.1038/s41598-020-66375-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imashiro, C. , & Shimizu, T. (2021). Fundamental technologies and recent advances of cell‐sheet‐based tissue engineering. International Journal of Molecular Sciences, 22(1), 1–18. 10.3390/ijms22010425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui, T. , Kurashina, Y. , Imashiro, C. , & Takemura, K. (2019). Method of localized removal of cells using a bolt‐clamped Langevin transducer with an ultrasonic horn. Engineering in Life Sciences, 19(8), 575–583. 10.1002/elsc.201800173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itai, S. , Tajima, H. , & Onoe, H. (2019). Double‐layer perfusable collagen microtube device for heterogeneous cell culture. Biofabrication, 11(1), 015010. 10.1088/1758-5090/aaf09b [DOI] [PubMed] [Google Scholar]
- Jiang, J. , Li, L. , He, Y. , & Zhao, M. (2013). Collective cell migration: Implications for wound healing and cancer invasion. Burns & Trauma, 1(1), 21–26. 10.4103/2321-3868.113331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K. A. , Vormohr, H. R. , Doinikov, A. A. , Bouakaz, A. , Shields, C. W. , López, G. P. , & Dayton, P. A. (2016). Experimental verification of theoretical equations for acoustic radiation force on compressible spherical particles in traveling waves. Physical Review E, 93(5), 053109. 10.1103/PhysRevE.93.053109 [DOI] [PubMed] [Google Scholar]
- Kamata, T. , Puzon, W. , & Takada, Y. (1994). Identification of putative ligand binding sites within I domain of integrin α2β1 (VLA‐2, CD49b/CD29). Journal of Biological Chemistry, 269(13), 9659–9663. 10.1074/jbc.271.31.19008 [DOI] [PubMed] [Google Scholar]
- Kang, B. , Shin, J. , Park, H.‐J. , Rhyou, C. , Kang, D. , Lee, S.‐J. , Yoon, Y. S. , Cho, S. W. , & Lee, H. (2018). High‐resolution acoustophoretic 3D cell patterning to construct functional collateral cylindroids for ischemia therapy. Nature Communications, 9(1), 5402. 10.1038/s41467-018-07823-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Lee, H. , Chung, M. , & Jeon, N. L. (2013). Engineering of functional, perfusable 3D microvascular networks on a chip. Lab on a Chip, 13(8), 1489–1500. 10.1039/c3lc41320a [DOI] [PubMed] [Google Scholar]
- Kurashina, Y. , Imashiro, C. , Hirano, M. , Kuribara, T. , Totani, K. , Ohnuma, K. , Friend, J. , & Takemura, K. (2019). Enzyme‐free release of adhered cells from standard culture dishes using intermittent ultrasonic traveling waves. Communications Biology, 2(1), 393. 10.1038/s42003-019-0638-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Ohdaira, E. , & Ide, M. (1997). Effect of ultrasound on driving force of diffusion dialysis. Japanese Journal of Applied Physics, Part 1: Regular Papers and Short Notes and Review Papers, 36(5 Suppl. B), 3138–3139. 10.1143/jjap.36.3138 [DOI] [Google Scholar]
- Li, S. , Nih, L. R. , Bachman, H. , Fei, P. , Li, Y. , Nam, E. , Dimatteo, R. , Carmichael, S. T. , Barker, T. H. , & Segura, T. (2017). Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nature Materials, 16(9), 953–961. 10.1038/nmat4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan, K. D. , Nabar, G. M. , Xue, W. , Anghelina, M. , Moldovan, N. I. , Chalmers, J. J. , & Winter, J. O. (2017). Mechanotransduction effects on endothelial Cell Proliferation via CD31 and VEGFR2: Implications for Immunomagnetic Separation. Biotechnology Journal, 12(9), 10.1002/biot.201600750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia, L. , Vlaisavljevich, E. , Xu, Z. , & Johnsen, E. (2017). Predicting tissue susceptibility to mechanical cavitation damage in therapeutic ultrasound. Ultrasound in Medicine and Biology, 43(7), 1421–1440. 10.1016/j.ultrasmedbio.2017.02.020 [DOI] [PubMed] [Google Scholar]
- Maniotis, A. J. , Chen, C. S. , & Ingber, D. E. (1997). Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Sciences of the United States of America, 94(3), 849–854. 10.1073/pnas.94.3.849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino, M. M. , Brkic, S. , Bovo, E. , Burger, M. , Schaefer, D. J. , Wolff, T. , Gürke, L. , Briquez, P. S. , Larsson, H. M. , Gianni‐Barrera, R. , Hubbell, J. A. , & Banfi, A. (2015). Extracellular matrix and growth factor engineering for controlled angiogenesis in regenerative medicine. Frontiers in Bioengineering and Biotechnology, 3(April), 1–8. 10.3389/fbioe.2015.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, D. G. , Christ, G. , Stitzel, J. D. , Atala, A. , & Yoo, J. J. (2008). Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Engineering. Part A, 14(4), 473–482. 10.1089/tea.2007.0104 [DOI] [PubMed] [Google Scholar]
- Moore, S. W. , Zhang, X. , Lynch, C. D. , & Sheetz, M. P. (2012). Netrin‐1 attracts axons through FAK‐dependent mechanotransduction. Journal of Neuroscience, 32(34), 11574–11585. 10.1523/JNEUROSCI.0999-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao, M. , Imashiro, C. , Kuribara, T. , Kurashina, Y. , Totani, K. , & Takemura, K. (2019). Formation of large scaffold‐free 3‐D aggregates in a cell culture dish by ultrasound standing wave trapping. Ultrasound in Medicine and Biology, 45(5), 1306–1315. 10.1016/j.ultrasmedbio.2019.01.013 [DOI] [PubMed] [Google Scholar]
- Nakao, M. , Kurashina, Y. , Imashiro, C. , & Takemura, K. (2018). A method for collecting single cell suspensions using an ultrasonic pump. IEEE Transactions on Biomedical Engineering, 65(1), 224–231. 10.1109/TBME.2017.2699291 [DOI] [PubMed] [Google Scholar]
- Newman, A. C. , Nakatsu, M. N. , Chou, W. , Gershon, P. D. , & Hughes, C. C. W. (2011). The requirement for fibroblasts in angiogenesis: Fibroblast‐derived matrix proteins are essential for endothelial cell lumen formation. Molecular Biology of the Cell, 22(20), 3791–3800. 10.1091/mbc.E11-05-0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata, T. , Ito, K. , Shindo, T. , Hatanaka, K. , Eguchi, K. , Kurosawa, R. , Kagaya, Y. , Monma, Y. , Ichijo, S. , Taki, H. , Kanai, H. , & Shimokawa, H. (2017). Low‐intensity pulsed ultrasound enhances angiogenesis and ameliorates contractile dysfunction of pressure‐overloaded heart in mice. PLOS One, 12(9), 1–17. 10.1371/journal.pone.0185555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dela Paz, N. G. , Walshe, T. E. , Leach, L. L. , Saint‐Geniez, M. , & D'Amore, P. A. (2012). Role of shear‐stress‐induced VEGF expression in endothelial cell survival. Journal of Cell Science, 125(4), 831–843. 10.1242/jcs.084301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, T. S. , Fernandes, C. J. , Da, C. , da Silva, R. A. , Gomes, A. M. , Vieira, J. C. S. , Padilha, P. , de De, M. , & Zambuzzi, W. F. (2019). c‐Src kinase contributes on endothelial cells mechanotransduction in a heat shock protein 70‐dependent turnover manner. Journal of Cellular Physiology, 234(7), 11287–11303. 10.1002/jcp.27787 [DOI] [PubMed] [Google Scholar]
- Plotnikov, S. V. , Pasapera, A. M. , Sabass, B. , & Waterman, C. M. (2012). Force fluctuations within focal adhesions mediate ECM‐rigidity sensing to guide directed cell migration. Cell, 151(7), 1513–1527. 10.1016/j.cell.2012.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, G. M. , Wong, K. H. K. , Truslow, J. G. , Leung, A. D. , Acharya, C. , & Tien, J. (2010). Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials, 31(24), 6182–6189. 10.1016/j.biomaterials.2010.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radel, C. , & Rizzo, V. (2005). Integrin mechanotransduction stimulates caveolin‐1 phosphorylation and recruitment of Csk to mediate actin reorganization. American Journal of Physiology—Heart and Circulatory Physiology, 288(5), 936–945. 10.1152/ajpheart.00519.2004 [DOI] [PubMed] [Google Scholar]
- Rosenfeld, D. , Landau, S. , Shandalov, Y. , Raindel, N. , Freiman, A. , Shor, E. , Blinder, Y. , Vandenburgh, H. H. , Mooney, D. J. , & Levenberg, S. (2016). Morphogenesis of 3D vascular networks is regulated by tensile forces. Proceedings of the National Academy of Sciences of the United States of America, 113(12), 3215–3220. 10.1073/pnas.1522273113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi, K. , Shimizu, T. , Horaguchi, S. , Sekine, H. , Yamato, M. , Umezu, M. , & Okano, T. (2013). In vitro engineering of vascularized tissue surrogates. Scientific Reports, 3, 1316. 10.1038/srep01316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasamoto, A. , Nagino, M. , Kobayashi, S. , Naruse, K. , Nimura, Y. , & Sokabe, M. (2005). Mechanotransduction by integrin is essential for IL‐6 secretion from endothelial cells in response to uniaxial continuous stretch. American Journal of Physiology—Cell Physiology, 288(5 57‐5), 1012–1022. 10.1152/ajpcell.00314.2004 [DOI] [PubMed] [Google Scholar]
- Sekine, H. , Shimizu, T. , Sakaguchi, K. , Dobashi, I. , Wada, M. , Yamato, M. , Kobayashi, E. , Umezu, M. , & Okano, T. (2013). In vitro fabrication of functional three‐dimensional tissues with perfusable blood vessels. Nature Communications, 4, 1399. 10.1038/ncomms2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto, Y. , Inaba, R. , Okuyama, T. , Sassa, F. , Suzuki, H. , & Fukuda, J. (2010). Engineering of capillary‐like structures in tissue constructs by electrochemical detachment of cells. Biomaterials, 31(8), 2209–2215. 10.1016/J.BIOMATERIALS.2009.11.104 [DOI] [PubMed] [Google Scholar]
- Shih, Y. R. V. , Tseng, K. F. , Lai, H. Y. , Lin, C. H. , & Lee, O. K. (2011). Matrix stiffness regulation of integrin‐mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. Journal of Bone and Mineral Research, 26(4), 730–738. 10.1002/jbmr.278 [DOI] [PubMed] [Google Scholar]
- Shimizu, T. , Sekine, H. , Yang, J. , Isoi, Y. , Yamato, M. , Kikuchi, A. , Kobayashi, E. , & Okano, T. (2006). Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. The FASEB Journal, 20(6), 708–710. 10.1096/fj.05-4715fje [DOI] [PubMed] [Google Scholar]
- Skylar‐Scott, M. A. , Uzel, S. G. M. , Nam, L. L. , Ahrens, J. H. , Truby, R. L. , Damaraju, S. , & Lewis, J. A. (2019). Biomanufacturing of organ‐specific tissues with high cellular density and embedded vascular channels. Science Advances, 5(9), 2459. 10.1126/sciadv.aaw2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara, H. , Sakaguchi, K. , Kuroda, M. , Muraoka, M. , Itoga, K. , Okano, T. , & Shimizu, T. (2015). Controlling shape and position of vascular formation in engineered tissues by arbitrary assembly of endothelial cells. Biofabrication, 7(4), 45006. 10.1088/1758-5090/7/4/045006 [DOI] [PubMed] [Google Scholar]
- Tauchi, H. , Imashiro, C. , Kuribara, T. , Fujii, G. , Kurashina, Y. , Totani, K. , & Takemura, K. (2019). Effective and intact cell detachment from a clinically ubiquitous culture flask by combining ultrasonic wave exposure and diluted trypsin. Biotechnology and Bioprocess Engineering, 24(3), 536–543. 10.1007/s12257-018-0491-2 [DOI] [Google Scholar]
- Teague, S. M. , & Sharma, M. K. (1991). Detection of paradoxical cerebral echo contrast embolization by transcranial Doppler ultrasound. Stroke, 22(6), 740–745. 10.1161/01.STR.22.6.740 [DOI] [PubMed] [Google Scholar]
- Van Velthoven, R. , Aoun, F. , Marcelis, Q. , Albisinni, S. , Zanaty, M. , Lemort, M. , Peltier, A. , & Limani, K. (2016). A prospective clinical trial of HIFU hemiablation for clinically localized prostate cancer. Prostate cancer and prostatic diseases, 19(1), 79–83. 10.1038/pcan.2015.55 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Zhao, D. , Phan, D. , Liu, J. , Chen, X. , Yang, B. , Hughes, C. , Zhang, W. , & Lee, A. P. (2018). A hydrostatic pressure‐driven passive micropump enhanced with siphon‐based autofill function. Lab on a Chip, 18(15), 2167–2177. 10.1039/c8lc00236c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. Y. , Pei, Y. , Xie, M. , Jin, Z. H. , Xiao, Y. S. , Wang, Y. , Zhang, L. N. , Li, Y. , & Huang, W. H. (2015). An artificial blood vessel implanted three‐dimensional microsystem for modeling transvascular migration of tumor cells. Lab on a Chip, 15(4), 1178–1187. 10.1039/c4lc00973h [DOI] [PubMed] [Google Scholar]
- Yeh, Y. C. , Ling, J. Y. , Chen, W. C. , Lin, H. H. , & Tang, M. J. (2017). Mechanotransduction of matrix stiffness in regulation of focal adhesion size and number: Reciprocal regulation of caveolin‐1 and β1 integrin. Scientific Reports, 7(1), 15008. 10.1038/s41598-017-14932-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W. , Qu, X. , Zhu, J. , Ma, X. , Patel, S. , Liu, J. , Wang, P. , Lai, C. S. , Gou, M. , Xu, Y. , Zhang, K. , & Chen, S. (2017). Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials, 124, 106–115. 10.1016/j.biomaterials.2017.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are openly available.


