Abstract
African Swine Fever (ASF) is a highly contagious and fatal viral disease affecting both domestic and wild suids. The virus was introduced to Southeast Asia in early 2019 and has since spread rapidly throughout the region. Although significant efforts have been made to track and diagnose the disease in domestic pigs, very little is known about ASF in free‐ranging wild boar and their potential role in maintaining the disease within Southeast Asia. Through a collaboration between government and non‐government actors in Laos, Viet Nam, and Cambodia, investigations were conducted to (a) characterize the interface between domestic pigs and wild boar, (b) document risk factors for likely ASF spillover into wild boar populations by way of this interface, and (c) determine whether ASF in wild boar could be detected in each country. An extensive overlap between wild boar habitat and domestic pig ranging areas was found around villages bordering forests in all three countries, creating a high‐risk interface for viral spillover between domestic pig and wild boar populations. Fifteen and three wild boar carcasses were detected through passive reporting in Laos and Viet Nam, respectively, in 2019 and early 2020. Four of five carcasses screened in Laos and two of three in Viet Nam were confirmed positive for African swine fever virus using real‐time PCR. There were no confirmed reports of wild boar carcasses in Cambodia. This is the first confirmation of ASF in wild boar in Southeast Asia, the result of a probable viral spillover from domestic pigs, which highlights the importance of early reporting and monitoring of ASF in wild boar to enable the implementation of appropriate biosecurity measures.
Keywords: African swine fever, ASF, livestock–wildlife interface, outbreak, Southeast Asia, spillover, wild boar
1. INTRODUCTION
African swine fever virus (ASFV), the causative agent of an acute haemorrhagic fever of domestic and wild suids, was first identified in East Africa in the early 1900s (Dixon et al., 2019). In the last decade, African swine fever (ASF) has seen a remarkable expansion across Eurasia after an initial re‐introduction in Georgia, followed by a spread through Russia and towards Europe (Sánchez‐Cordón et al., 2018). The dissemination of ASF in Eurasia since 2007 has been marked by the emergence of a new epidemiological pattern involving wild boar (Sus scrofa) infections and environmental persistence of infected boar carcasses, in what has been called the wild boar‐habitat cycle (Chenais et al., 2018). This cycle is possible because of the relatively slow decay of carcasses in temperate and continental climates (Probst et al., 2019), the long persistence of virus in carcasses and other substrates (Mazur‐Panasiuk et al., 2019), and frequent contact between live wild boar and infected carcasses (Cukor et al., 2020; Probst et al., 2017). This has allowed maintenance of ASF in the wild boar independently of domestic pigs and a slow spread across eastern Europe (Podgórski & Śmietanka, 2018), resulting in persistent challenges for ASF control (Chenais et al., 2019; Sánchez‐Vizcaíno et al., 2013).
African swine fever virus was first reported in China in August of 2018 (Wang et al., 2019). It spread rapidly throughout the country and by August 2019 had caused significant pig population losses, both from widespread culling and the virus's high case fatality rate, destabilizing the national and global pig supply chain and trade (Yun, 2020; Zhao et al., 2019). Shortly after its spread throughout China, ASF was detected in Viet Nam, Cambodia and Laos in February, March and June 2019, respectively (OIE World Animal Health Information System, 2020). Although the exact distribution and magnitude of the ASF epizootic in the region are underestimated, available reports suggest a rapid spread which resulted in the depopulation of domestic pigs in many villages (FAO Emergency Prevention System for Animal Health, 2020). The Eurasian wild boar (Sus scrofa) is endemic to forested areas throughout Southeast Asia. Deforestation and over‐hunting have reduced the numbers and diversity of the wild ungulate community, but the resilience of wild boar has allowed them to persist (Gray et al., 2012; Rasphone et al., 2019; Son et al., 2014). Despite their known presence, limited information is available on the distribution, density and ecology of wild boar populations in Southeast Asia (for some existing information see Gray et al., 2012; Guo et al., 2017; Rasphone et al., 2019), and their interface with domestic pigs. While we know that wild boar are susceptible to ASFV (Blome et al., 2013), the virus had yet to be documented in free‐ranging populations in this region. Consequently, it is unknown if they are able to maintain the virus in a wild boar‐habitat cycle in Southeast Asia as they do in eastern Europe, and if they will become a reservoir for domestic pigs.
In the wake of the ASF epizootic in Viet Nam, Cambodia and Laos, we sought to detect the disease in the wild boar population using cost‐effective, risk‐based surveillance methods based on participatory engagement of local actors in each country. We conducted a preliminary assessment of the risk of ASFV transmission at the interface between domestic and wild pigs in several pre‐selected villages located within or near wild pig habitat. The objectives were to characterize the interface between domestic pigs and wild boar to ascertain the risk of viral spillover from domestic pigs to wild boar, and to determine whether ASF transmission to the wild boar population could be detected in each country. Follow‐up site visits and sampling were conducted to identify and document suspected viral spillover.
2. MATERIALS AND METHODS
2.1. Site selection
Investigative teams first identified villages in Laos, Cambodia and Viet Nam with a confirmed ASF outbreak in domestic pigs or with unconfirmed reports of high pig mortality numbers, and in close proximity to forested areas with known or suspected presence of wild boar. These sites were then visited for preliminary assessments of potential interfaces between wild and domestic pigs with risk of ASFV transmission. Based on relevant information obtained from the community members, forest rangers and protected area staff at these sites, additional villages were added to the investigation and visited. Three villages in Houaphanh province in Laos, two in Dong Nai province in Viet Nam and eleven in Ratanakiri province in Cambodia were selected for the assessments which were conducted in July, October/December and April/June 2019, respectively (Figure 1) and mapped using ArcGIS 10.7.1 (ESRI, Redlands, CA, USA). Sites were not selected following a formal risk assessment, but rather were a convenience sample based on local reports received and field team time availability and resources.
FIGURE 1.
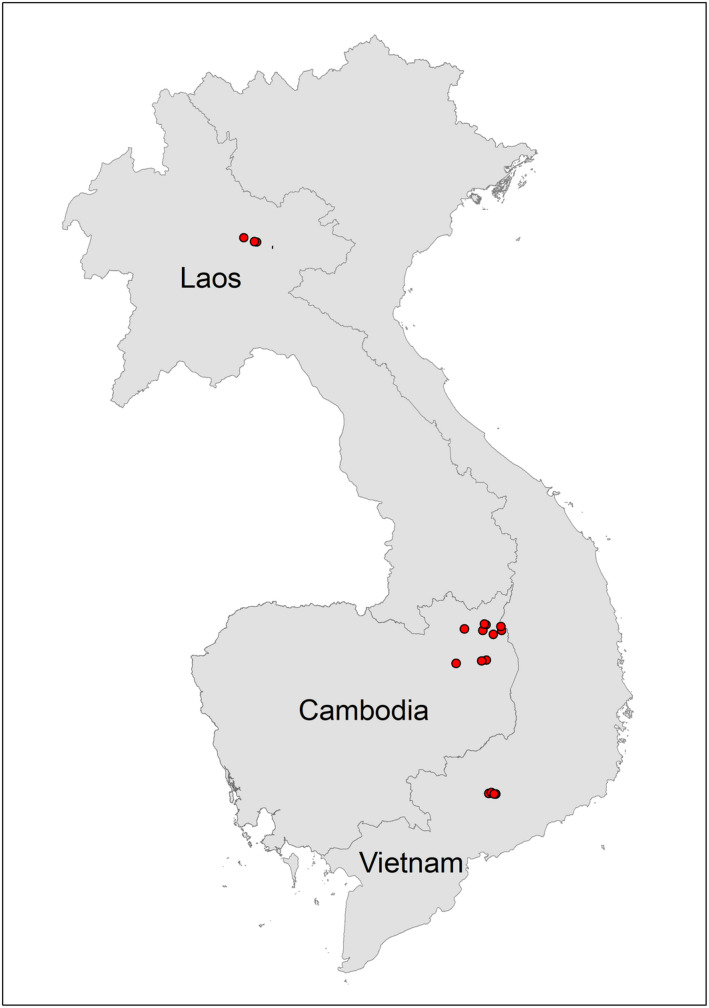
Location of villages visited during investigations in Cambodia, Laos and Viet Nam
2.2. General approach
Investigative field teams performing site visits consisted of central, provincial, and district‐level animal health and environmental sector representatives from local government, as well as field staff and veterinarians from the Wildlife Conservation Society (WCS). In each village, a meeting was convened gathering key informants to discuss ASF and potential transmission at the domestic–wild pig interface. These typically included the village chief (or other village‐level administrative authority), protected area staff, forest rangers, animal health workers, hunters and villagers who experienced recent pig mortalities.
Interviews were open but facilitated to focus on the following themes: (a) locations where pigs are housed, (b) any clinical signs observed, (c) movement of pigs or pork products, (d) number of domestic pig mortalities within the village and location of carcass disposal sites, (e) location of wild boar habitat and population dynamics, (f) location of observed wild boar tracks and droppings, (g) location of observed wild boar in proximity to crops, villages, food waste, and domestic pigs and (h) location of observed wild boar mortalities. Paper maps were used to draw the general outline of the village and surrounding areas, and meeting participants were encouraged to locate their observations on these maps (Figure 2).
FIGURE 2.

A village pig farmer indicates locations of interest on a map during the investigation in Laos
These meetings were typically followed with visits by the field teams to the locations of interest reported by villagers during the meeting. In particular, the teams focused on visiting locations of domestic pig mortalities, signs of wild boar presence (e.g. tracks, faeces) and domestic pig carcass disposal sites to search for signs of scavenging. When time allowed, additional searches for wild boar carcasses were conducted in surrounding forests. If found, wild boar faeces were sampled for ASFV testing. In Cambodia, wild boar meat samples were also collected from hunters in the ASF outbreak villages and in nearby markets.
Following the initial site visits, passive reporting of wild boar carcasses was encouraged in all three countries involving villagers, animal health workers, and local rangers and enforcement authorities, and contact information was provided for submitting these reports. When detected, carcasses were sampled promptly by one or more trained field practitioners depending on their location and proximity to the carcass, and samples were shipped to the investigative team or directly to the country's respective laboratory. Samples taken from wild boar carcasses included swabs or biopsies of muscle tissue, and/or bone marrow. Skeletal remains were sampled by swabbing or extracting bone marrow from long bones.
In Laos, six bone marrow, and five muscle tissue samples from five animals were submitted to the National Animal Health Laboratory. In Viet Nam, four bone marrow samples from three animals, two faecal samples and one leachate sample from a designated domestic pig carcass disposal site were submitted to the Laboratory of the Regional Animal Health Office No. 6 and the Viet Nam National University of Agriculture. In Cambodia, three meat samples obtained from hunters, one meat sample from a local market, and one faecal sample were submitted to the National Animal Health and Production Research Institute. All samples were screened for ASFV using real‐time PCR according to the OIE‐recommended protocol (King et al., 2003) in each of the respective countries.
3. RESULTS/DISCUSSION
In all sites visited in the three countries, information gathered during community meetings was consistent with an ASF outbreak as the cause of high mortality rates of domestic pigs in the area. Mortalities were often described by community members as having started earlier than the first laboratory confirmation of ASFV in their respective countries, as early as two weeks prior in Cambodia, and two months prior in Laos.
3.1. Housing & husbandry
In all sites surveyed by field teams, small‐scale farmers housed their domestic pigs within or in close proximity to wild boar habitat, and the presence of wild boar was confirmed in the surrounding areas and mapped (Figure 3). Similarities in domestic pig housing styles were also noted in all three countries, with frequent reports of the pigs being completely free‐ranging or semi free‐ranging. Furthermore, villages in all countries reported seasonal variation in how often they observe wild boar or evidence of their presence (e.g. crop raiding, tracks, etc.), with harvest season (August to October in Laos and Cambodia; November to April in southern Viet Nam) being the time of year these observations are made most frequently in their fields and around their domestic pig farms. Reports of wild boar foraging in corn and cassava fields were common, and crops from these same fields were often used as the main feed source for domestic pigs in the respective villages. Evidence of cross‐breeding between wild boar and free‐ranging domestic pigs was noted to varying degrees during each investigation, ranging from only one farm in Laos with suspected hybrid piglets, to 4 of 11 villages in Cambodia, to the majority of farms identified in this study in Viet Nam raising domestic–wild pig hybrids. Observations in each country confirmed the occurrence of both direct and indirect interactions between wild and domestic pigs, which could facilitate viral spillover.
FIGURE 3.
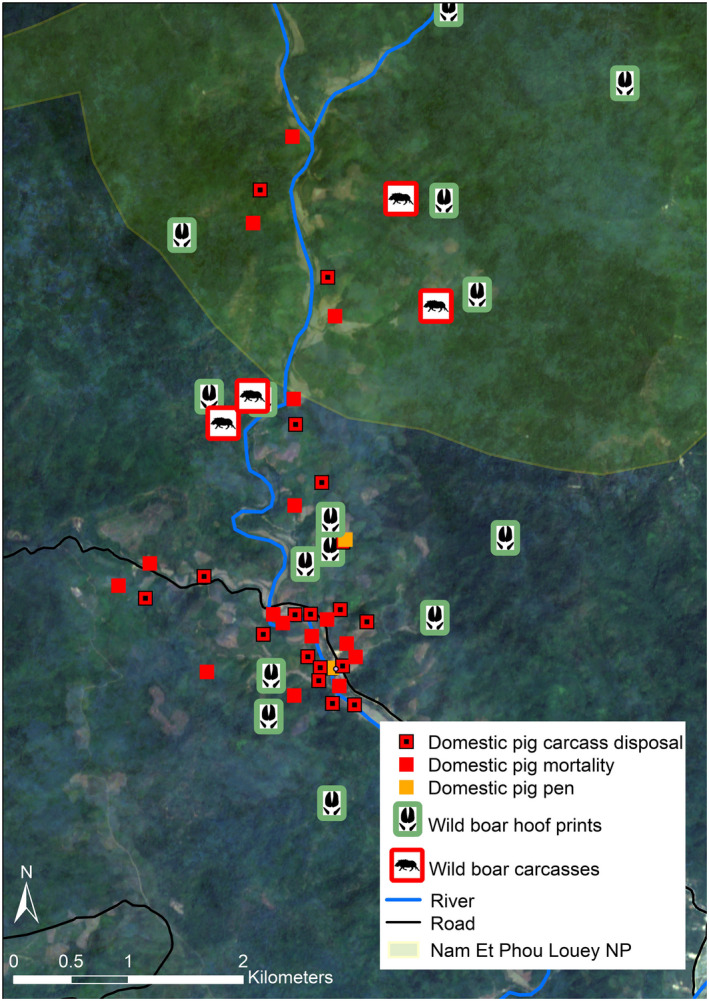
The interface between domestic pigs and wild boar at Namsat Village, Houaphanh Province in Laos. Locations of farms with domestic pig mortalities, domestic pig carcass disposal sites, and detected wild boar hoofprints and carcasses are indicated both within the village and within Nam Et ‐ Phou Louey National Park boundaries
3.2. Carcass disposal and biosecurity practices
In each of the sites visited during these investigations, there were difficulties in managing the large number of domestic pig carcasses resulting from ASF outbreaks or related culling activities which resulted in significant biosecurity challenges. There were also varied methods of domestic pig carcass disposal used in the communities. During the outbreaks, villagers in Laos and Cambodia reported selling potentially infected pigs and pork products from pigs that had died to buyers from other villages. Several incidences of disposing of infected carcasses or entrails in rivers and streams were documented in 2/3 of villages visited by the field team in Laos, and 4/11 villages visited in Cambodia. This may have promoted further spread of ASF as both carcasses and contaminated water reached downstream locations.
Villagers in Laos and Viet Nam also frequently buried carcasses on‐site, sometimes in close proximity to where healthy pigs were being housed (see Figure 3 for an example of the interface in one village). Communities from all three countries also reported that pig carcasses would sometimes be discarded along roads or at the edge of forests, leaving them accessible to other free‐roaming animals for scavenging. Some villagers in Viet Nam and Cambodia reported burning carcasses; however, it is unknown if they were incinerated completely. In Viet Nam, a designated disposal site was noted at the edge of a protected forest considered wild boar habitat and adjacent to agricultural fields where wild boar are known to forage. Carcasses were collected from villages by government officials and buried at this location; however, this was reportedly only done when case numbers from a particular farm were high. This communal disposal site was visited by the investigative team twice, and on both occasions, domestic pig carcasses were noted to be exposed in shallow, uncovered pits. In addition, leachates were observed flowing out from the disposal site and pooling within its vicinity.
In all sites visited across the three countries, limited to no physical barriers were in place to prevent people or animals from accessing infected pig disposal sites. Evidence of this was documented in Viet Nam where tracks of domestic dogs were commonly observed throughout the disposal site. Although no direct evidence of scavenging was noted at the limited number of domestic pig burial sites visited during each country's investigation, nothing was in place to prevent the access of wild boar to infected pig carcasses at these sites. Given the relative persistence of the virus in contaminated environments (Beltran‐Alcrudo et al., 2017), these disposal practices may be long‐term sources of ASFV to both wild and domestic pigs, contributing to onward transmission to other farms and villages, and to viral spillover into wild boar.
3.3. Evidence of spillover
Following the initial investigation in Laos, 15 individual wild boar carcasses in varying stages of composition were discovered and reported between September 2019 and February 2020 from the forest and surrounding villages. Prior to this, no wild boar mortalities had been reported. Nine of the 15 carcasses were sampled, and five of these were screened for ASFV (Table 1). Due to laboratory interruptions and delays from COVID‐19, samples from the remaining carcasses were still awaiting testing at the time of this report. Four of the 5 submitted individuals were confirmed positive for ASFV following testing of bone marrow and/or muscle tissue. Most wild boar carcasses were concentrated at sites with an interface between domestic and wild pigs, and comparatively fewer carcasses were found by rangers patrolling deep within the protected areas.
TABLE 1.
Summary of wild boar specimens positive for ASFV/total specimens tested during outbreak investigations in Laos, Viet Nam, and Cambodia
| Carcass | Faeces | Meat for consumption obtained from hunter or market | Leachate from carcass disposal site | |
|---|---|---|---|---|
| Laos | 4/5 a | ‒ | ‒ | ‒ |
| Viet Nam | 2/3 b | 1/2 | ‒ | 1/1 |
| Cambodia | ‒ | 0/1 | 0/4 | ‒ |
Among carcasses tested, 4/6 bone marrow & 3/5 muscle specimens were positive.
Among carcasses tested, 2/4 bone marrow specimens were positive.
No sample obtained.
In Viet Nam, bone marrow collected from the skeletal remains of a wild boar, wild boar faeces, and leachate from the surrounding substrate at a domestic pig disposal site were screened for ASFV between October and December, 2019 (Table 1). Two of the samples collected from the carcass (one bone marrow sample and one faecal sample) tested positive for the virus. The leachate sample also tested positive, indicating viral contamination of surface water in the vicinity. A short time thereafter, two additional wild boar carcasses found adjacent to the previously visited communal disposal site were reported by protected area staff. Three bone marrow samples were collected from these individuals and screened for ASFV, one of which tested positive. To date, a total of three wild boar carcasses from the same region have been tested for the virus, with two individuals identified as ASFV‐positive.
During the June 2019 investigation in Cambodia, four wild boar meat samples (two dry, one salted and one fresh) were collected from a local market and hunters in villages affected by the ASF outbreak in domestic pigs. Additionally, one wild boar faecal sample found in the forest near the villages was collected. All samples were negative for ASFV (Table 1). There were anecdotal reports of wild boar mortality; however, none could be confirmed as carcasses were disposed of or destroyed before an investigation or sampling could be performed. Villagers and forest rangers could not comment on whether or not they had noticed any changes in the wild boar population before and after the domestic pig ASF outbreak.
These investigations confirm the presence of ASF in wild boar in Laos and Viet Nam, and the first epidemiological evidence suggesting viral spillover from domestic pigs into wild boar in Southeast Asia. ASF has yet to be detected in wild boar in Cambodia. These events were likely a product of the multiple opportunities for close contact between wild and domestic pigs documented in all three countries, a situation that is likely repeated throughout the region.
In each country, wild boar were present in forests surrounding villages with ongoing ASF outbreaks in domestic pigs, and many opportunities for wild and domestic pig interaction were documented due in particular to the common practice of allowing domestic pigs to free‐range. The observed carcass disposal methods of ASF‐suspected domestic pigs, and the limited biosecurity practices overall, add to the multitude of potential transmission pathways. All three countries reported the rapid sale of ASF‐affected meat/animals to other villages, the disposal of ASF‐affected pigs in rivers, and inappropriate burial of affected animals. While greatly increasing the risk for ASF transmission between domestic pigs, these practices may have also led to spillover of ASFV into wild boar.
Mass mortality of domestic pigs due to ASF was recorded in both Laos and Viet Nam for several months before the first wild boar carcasses were noted by any interviewed villagers or officials (Figure 4). Short of phylogenetic analyses, the timeline of events and geographic distribution of wild boar carcasses suggests the virus spilled over from domestic to wild suids, rather than the reverse.
FIGURE 4.

The timeline of ASF detection in domestic pigs in Laos, Cambodia, and Viet Nam, and subsequently wild boar in Laos and Viet Nam
During subsequent visits, villagers in Laos and Viet Nam reported that they experienced less frequent and less severe crop raiding from wild boar during the ASF outbreaks than they had during prior years; however, there is insufficient evidence to conclude on the effects of ASFV on the wild boar population abundance in the area.
It is still unknown whether wild boar can maintain the virus in a wild boar‐habitat cycle in Southeast Asia as is the case in Europe, whether they play an epidemiological role in transmitting the virus back to domestic pigs (‘spillback’), and what the effect is on wild boar populations and broader ecosystems. Answering these questions will be crucial in the planning and implementation of ASF control measures within each country, in predicting how ASF will impact pig farming in the future across Southeast Asia (Vergne et al., 2020), and in anticipating potential conservation consequences. Furthermore, both impacts and mitigation measures may differ from those in Europe where wild boar are known to be abundant throughout the continent and are frequently managed with both supplemental feeding and designated hunting practices. Prompt efforts to identify the extent of ASF infection in wild boar populations, and the ASF transmission pathways from domestic pigs within the Southeast Asia context, are necessary to reduce further impact of ASF on endemic wild species. To minimize the potential spillover of ASFV between domestic and wild suids in both directions, through the movement of animals but also by human behaviour, wild boar should be considered when designing ASF outbreak response and control plans.
These focal investigations in each country were part of a broader effort in Southeast Asia to structure and strengthen wildlife disease surveillance. Despite the limited scale of this study and small numbers of carcasses found, passive reporting proved an effective method of detecting ASF in wild boar. Following these initial investigations, systematic long‐term surveillance to monitor ASF in wild boar, including both passive reporting and targeted study designs, is needed to further investigate the prevalence and potential circulation of ASFV in wild boar throughout Cambodia, Laos, Viet Nam and the rest of Southeast Asia.
CONFLICT OF INTERESTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Food and Agriculture Organization of the United Nations.
ETHICAL APPROVAL
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received from the Wildlife Conservation Society's Institutional Animal Care and Use Committee (IACUC).
ACKNOWLEDGEMENTS
The authors would like to extend their gratitude and appreciation to the villages who gave us their time and welcomed us into their communities during this investigation. Our sincere thanks as well to the national park and protected area rangers who dedicate their days to protecting wildlife and who continue to be on the front lines of wildlife disease surveillance. Thank you as well to all local government field staff as well as technical laboratory staff in each country for assisting in this investigation. In Laos, we would like to thank Manoly Sisavanh (Deputy Country Director, WCS Laos), Santi Saypanya (Country Director, WCS Laos), Dr. Sarah Olson (Associate Director of Epidemiology, WCS Health Program), Phaisouk Phutthapanya (Department of Livestock and Fisheries, Laos), Dr. Phouvong Phommachanh, and Sengsai Phonthasy, Chanthana Senaphanh, and Vilayvan Soukvilay (National Animal Health Laboratory, Laos) for their support in this investigation. In Viet Nam, we extend our gratitude to Hoang Bich Thuy (Country Director, WCS Viet Nam), Nguyen Thi Lan (Viet Nam National University of Agriculture, Hanoi, Viet Nam), Nguyen Manh Diep (Dong Nai Culture and Nature Reserve, Dong Nai province, Viet Nam), Ngo Xuan Hai (Vinh Cuu District Vet Officer, Dong Nai province, Viet Nam), Do Van Minh (Dinh Quan District FPD, Dong Nai province, Viet Nam), Vu Van Phon (Tan Phu Protected Area Management Board, Dong Nai province, Viet Nam), Pham Hong Quan (Department of Animal Health, Hanoi, Viet Nam), Nguyen Van Dung (Dong Nai Forest Protection Department, Dong Nai province, Viet Nam), Bui Van Manh (Dong Nai Sub‐ Department of Animal Health, Dong Nai province, Viet Nam), Nguyen Hoang Hao (Deputy Director, Dong Nai Culture and Nature Reserve, Dong Nai province, Viet Nam), and Nguyen Le Anh Tuan (Director, Tan Phu Protected Area Management Board, Dong Nai province, Viet Nam). In Cambodia, we extend our gratitude to Sereyrotha Ken (Country Director, WCS Cambodia), Hak Makara (National Technical Advisor for Animal Health, FAO Cambodia), Moung Mann (Chief of Animal Health and Production Office, Ratanakiri Province) and Soy Sona (Director of Department of Agriculture, Forestry, and Fisheries, Ratanakiri Province). This regional effort was made possible by support from the Defense Threat Reduction Agency Biological Threat Reduction Program (BTRP) under the United States Department of Defense.
Denstedt E, Porco A, Hwang J, et al. Detection of African swine fever virus in free‐ranging wild boar in Southeast Asia. Transbound Emerg Dis. 2021;68:2669–2675. 10.1111/tbed.13964
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Beltran‐Alcrudo, D. , Arias, M. , Gallardo, C. , Kramer, S. A. , Penrith, M.‐L. &Food and Agriculture Organization of the United Nations . (2017). African swine fever: detection and diagnosis. A manual for veterinarians. Food and Agriculture Organization of the United Nations. [Google Scholar]
- Blome, S. , Gabriel, C. , & Beer, M. (2013). Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Research, 173, 122–130. 10.1016/j.virusres.2012.10.026 [DOI] [PubMed] [Google Scholar]
- Chenais, E. , Depner, K. , Guberti, V. , Dietze, K. , Viltrop, A. , & Ståhl, K. (2019). Epidemiological considerations on African swine fever in Europe 2014–2018. Porcine Health Management, 5, 6. 10.1186/s40813-018-0109-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenais, E. , Ståhl, K. , Guberti, V. , & Depner, K. (2018). Boar‐habitat epidemiologic cycle in African Swine Fever Epizootic. Emerging Infectious Diseases, 24, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukor, J. , Linda, R. , Václavek, P. , Mahlerová, K. , Šatrán, P. , & Havránek, F. (2020). Confirmed cannibalism in wild boar and its possible role in African swine fever transmission. Transboundary and Emerging Diseases, 67(3), 1068–1073. 10.1111/tbed.13468 [DOI] [PubMed] [Google Scholar]
- Dixon, L. K. , Sun, H. , & Roberts, H. (2019). African swine fever. Antiviral Research, 165, 34–41. 10.1016/j.antiviral.2019.02.018 [DOI] [PubMed] [Google Scholar]
- FAO . (2020). ASF situation update ‐ African Swine Fever (ASF). Emergency Prevention System for Animal Health (EMPRES‐AH) [Online].. http://www.FAO.org/ag/againfo/programmes/en/empres/ASF/situation_update.html (accessed January 29, 2020). [Google Scholar]
- Gray, T. N. E. , Phan, C. , Pin, C. , & Prum, S. (2012). Establishing a monitoring baseline for threatened large ungulates in eastern Cambodia. Wildlife Biology, 18, 406–413. 10.2981/11-107 [DOI] [Google Scholar]
- Guo, W. , Cao, G. , & Quan, R.‐C. (2017). Population dynamics and space use of wild boar in a tropical forest, Southwest China. Global Ecology and Conservation, 11, 115–124. 10.1016/j.gecco.2017.04.005 [DOI] [Google Scholar]
- King, D. P. , Reid, S. M. , Hutchings, G. H. , Grierson, S. S. , Wilkinson, P. J. , Dixon, L. K. , Bastos, A. D. S. , & Drew, T. W. (2003). Development of a TaqMan PCR assay with internal amplification control for the detection of African swine fever virus. Journal of Virological Methods, 107, 53–61. 10.1016/s0166-0934(02)00189-1 [DOI] [PubMed] [Google Scholar]
- Mazur‐Panasiuk, N. , Żmudzki, J. , & Woźniakowski, G. (2019). African Swine Fever Virus – persistence in different environmental conditions and the possibility of its indirect transmission. Journal of Veterinary Research, 63, 303–310. 10.2478/jvetres-2019-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE World Animal Health Information System . (2020). [Online] Available at https://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/statusdetail (accessed January 29, 2020). [Google Scholar]
- Podgórski, T. , & Śmietanka, K. (2018). Do wild boar movements drive the spread of African Swine Fever? Transboundary and Emerging Diseases, 65, 1588–1596. 10.1111/tbed.12910 [DOI] [PubMed] [Google Scholar]
- Probst, C. , Gethmann, J. , Amler, S. , Globig, A. , Knoll, B. , & Conraths, F. J. (2019). The potential role of scavengers in spreading African swine fever among wild boar. Scientific Reports, 9, 10.1038/s41598-019-47623-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst, C. , Globig, A. , Knoll, B. , Conraths, F. J. , & Depner, K. (2017). Behaviour of free ranging wild boar towards their dead fellows: Potential implications for the transmission of African swine fever. Royal Society Open Science, 4, 170054. 10.1098/rsos.170054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasphone, A. , Kéry, M. , Kamler, J. F. , & Macdonald, D. W. (2019). Documenting the demise of tiger and leopard, and the status of other carnivores and prey, in Lao PDR’s most prized protected area: Nam Et ‐ Phou Louey. Global Ecology and Conservation, 20, e00766. 10.1016/j.gecco.2019.e00766 [DOI] [Google Scholar]
- Sánchez‐Cordón, P. J. , Montoya, M. , Reis, A. L. , & Dixon, L. K. (2018). African swine fever: A re‐emerging viral disease threatening the global pig industry. The Veterinary Journal, 233, 41–48. 10.1016/j.tvjl.2017.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Vizcaíno, J. M. , Mur, L. , & Martínez‐López, B. (2013). African swine fever (ASF): Five years around Europe. Veterinary Microbiology, 165, 45–50. 10.1016/j.vetmic.2012.11.030 [DOI] [PubMed] [Google Scholar]
- Son, H. N. , Long, L. T. , & Mai, N. T. P. (2014). Biological characterization of Vietnamese native wild boars in central highland (Sus scrofa). TAP CHI SINH HOC, 36, 253–258. 10.15625/0866-7160/v36n2.5124 [DOI] [Google Scholar]
- Vergne, T. , Guinat, C. , & Pfeiffer, D. U. (2020). Undetected circulation of African Swine Fever in Wild Boar, Asia. Emerging Infectious Diseases, 26(10), 2480–2482. 10.3201/eid2610.200608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Gao, L. , Li, Y. , Xu, Q. , Yang, H. , Shen, C. , & Huang, B. (2019). African swine fever in China: Emergence and control. Journal of Biosafety and Biosecurity, 1, 7–8. 10.1016/j.jobb.2019.01.006 [DOI] [Google Scholar]
- Yun, C.‐H. (2020). Unforeseen enemy: African swine fever. Asian‐Australasian Journal of Animal Sciences, 33, 1–3. 10.5713/ajas.2020.0001ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Bai, Z. , & Ma, L. (2019). China needs long‐term solutions for African Swine Fever. Science Bulletin, 64, 1469–1471. 10.1016/j.scib.2019.08.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


