Abstract
Objective
The aim of this study was to determine whether a Mediterranean‐style, ketogenic diet mobile health application (app) with breath acetone biofeedback is superior to a calorie‐restricted, low‐fat diet app in promoting weight loss.
Methods
Participants (n = 155) with overweight/obesity (mean [SD]: age 41 [11] years, BMI = 34 [5] kg/m2, 71% female) were randomized to one of the interventions delivered entirely via app. Participants received a wireless scale and were instructed to take daily weight measurements. A third‐party laboratory collected blood samples at baseline and 12 weeks.
Results
Weight loss at 12 weeks was greater in the ketogenic (−5.6 kg; 95% CI: −6.7 kg to −4.5 kg) compared with the low‐fat group (−2.5 kg; 95% CI: −3.6 kg to −1.4 kg) (between‐group difference: −3.1 kg; 95% CI: −4.6 kg to −1.5 kg; p < 0.001). Weight loss at 24 weeks indicated durability of the effect (between‐group difference: −5.5 kg; 95% CI: −8.3 kg to −2.8 kg; p < 0.001). Secondary/exploratory outcomes of hemoglobin A1c and liver enzymes were improved to a greater extent in the ketogenic diet group (p < 0.01).
Conclusions
Among adults with overweight/obesity, a ketogenic diet app with breath acetone biofeedback was superior to a calorie‐restricted diet app at promoting weight loss in a real‐world setting.
Study Importance.
What is already known?
-
►
Ketogenic diets have been demonstrated to be effective for weight loss; however, concerns remain regarding potential adverse effects of a ketogenic diet high in (saturated) fat on cardiovascular risk markers.
-
►
Mobile technology now makes delivery of dietary interventions easily accessible and scalable; and breath acetone has been suggested to be viable biofeedback for monitoring fat loss, specifically during a low‐carbohydrate, ketogenic diet.
What does this study add?
-
►
A self‐managed, Mediterranean‐based ketogenic diet intervention delivered entirely remotely via an app and paired with a breath acetone biofeedback device was superior in achieving weight loss at 12 and 24 weeks compared with a low‐fat, calorie‐restricted comparator arm.
-
►
The ketogenic diet intervention improved hemoglobin A1c and liver enzymes to a greater extent than the low‐fat, calorie‐restricted diet intervention, with no detrimental outcomes in other risk factors, including lipids and lipoproteins.
How might these results change the direction of research or the focus of clinical practice?
-
►
Our findings highlight the opportunity to successfully implement a ketogenic diet remotely.
-
►
App‐based interventions may help aid implementation and scalability of ketogenic diets to promote weight loss and improve metabolic health.
INTRODUCTION
Low‐carbohydrate or ketogenic diets can be effective for promoting weight loss and improving metabolic health (1). On a ketogenic diet, when carbohydrate intake is kept very low, increased delivery of free fatty acids to the liver leads to their conversion to ketone bodies (β‐hydroxybutyrate, acetoacetate, and acetone), which can then be used as an alternative fuel source for tissues such as brain and heart.
Mobile health (mHealth) applications (apps) now make delivery of dietary interventions easily accessible and widely scalable (2, 3). However, traditional ketogenic diets require a considerable amount of knowledge about the macronutrient content of different foods and careful self‐monitoring of ketosis to be successful (4, 5). It is therefore important to examine whether hands‐off mHealth technology can be used to facilitate a ketogenic diet intervention to promote weight loss under free‐living conditions.
Keyto (Keyto Inc., San Francisco, California) is a scalable, comprehensive, app‐based weight loss program incorporating a Mediterranean‐style ketogenic diet paired with a breath acetone biofeedback device. The app combines resources (e.g., recipes, searchable database, meal plans) with self‐monitoring in the form of an accompanying breath acetone sensor to help individuals learn about how different foods impact their level of ketosis. The program emphasizes a Mediterranean‐style ketogenic diet, encouraging intake of low‐carbohydrate foods that are high in monounsaturated and omega‐3 polyunsaturated fatty acids without concomitant increases in the intake of foods rich in saturated fatty acids. This was designed in response to concerns that traditional ketogenic diets high in saturated fats might have adverse effects on cardiovascular risk markers (6).
The purpose of this pragmatic randomized trial was to test the efficacy of the Keyto diet app and breath acetone biofeedback device compared with the WW diet app (WW, Inc., New York, New York). The WW app was chosen as an evidence‐based weight loss intervention effective at reducing weight and cardiovascular risk (7, 8), such that the ketogenic diet app and biofeedback device could be tested against a well‐known and well‐studied active comparator. We hypothesized greater weight loss for individuals randomized to the ketogenic diet app paired with a breath acetone biofeedback device compared with the calorie‐restricted, low‐fat diet app.
METHODS
Design
This study examines the prespecified primary and secondary outcomes at 12 weeks from baseline using a two‐group pragmatic randomized trial. Using a virtual hands‐off design, participants were mailed study materials and they interacted with study staff via email, text messages, or phone calls only. The study was conducted with approval from the University of British Columbia’s clinical research ethics board, and all participants provided written informed consent digitally prior to data collection. The clinical trial was registered on ClinicalTrials.gov (NCT04165707) and the protocol published (DERR1‐10.2196/19053) (9).
Participants
A total of 155 participants with overweight/obesity (BMI 27 to 43 kg/m2) from the state of California were recruited between December 1, 2019, and August 11, 2020, and randomized to one of the interventions. Key exclusion criteria were pregnancy, current smoker, diabetes diagnosis, history of heart attack, bariatric surgery, eating disorder, losing or gaining more than 5% body weight in past 6 months, and currently following a low‐carbohydrate or ketogenic diet (9). The original published protocol aimed to recruit 144 participants per the a priori sample size calculation in order to detect a clinically meaningful 5% difference in weight loss with 80% power and α = 0.5 with two groups and two time points (baseline and primary outcome at 12 weeks), assuming a mean body mass of 100 kg with a standard deviation of 15 kg and a correlation among repeated measures of r = 0.75 (9). To account for an expected increase in dropout rate due to the challenges of the COVID‐19 pandemic, the trial steering committee decided to increase enrollment to 155 participants on July 28, 2020. Randomization schedule was maintained by a third‐party, password‐protected website using variable permuted block sizes and stratified by sex (male, female) and age (18 to 40, 41 to 64 years).
Interventions
At the start of the study, participants received a phone call to provide assistance with setting up of study devices and a brief introduction to their respective diet app and eating plan. Following that, both interventions were delivered entirely via a mobile app with no in‐person meetings or dietary counseling outside of the app’s framework. The Mediterranean‐style ketogenic diet app, paired with a breath acetone biofeedback device, uses a traffic light system to recommend consumption of foods (ad libitum, cautiously, avoid) according to the amount of net carbohydrates based on nutritional information gathered from the United States Department of Agriculture food database. The app emphasizes avoidance of refined carbohydrates, and priority is placed on fats from plant‐ (e.g., olive oil, avocado, nuts) and fish‐based (e.g., salmon) sources that fit the Mediterranean guidelines (10) and that are indicated with a “Heart First” badge. Participants in the trial were encouraged to prioritize foods that fall in this category. The app is paired with a biofeedback device that measures breath acetone levels as a proxy for level of nutritional ketosis. The breath acetone device is roughly the size of a pen, consisting of a gas sensor using a semiconducting metal oxide core selective for acetone, with each sensor individually calibrated with acetone standard gas during the production process (11). Participants were instructed to use the device three times daily, with their level of ketosis provided within the paired app on a scale of 0 (lowest) to 6+ (highest). In case of a lower score (0 to 3), participants were instructed to further restrict carbohydrate intake, whereas in case of a higher score (4 or higher), participants were encouraged to continue with their current dietary habits. The calorie‐restricted, low‐fat diet app intervention uses a points‐based tracking system and requires participants to record their food and beverage intake in the app, which assigns a points value to each item. The points value is based on caloric content of each food item, with protein content lowering and saturated fat and sugar increasing the value. Based on baseline anthropometrics, a daily points value is calculated, which participants are instructed not to exceed. Furthermore, the app contains so‐called zero‐point low‐calorie foods that can be consumed ad libitum and that do not count toward the daily points allotment (12). Both apps provided similar ancillary resources, including recipes, a food search option, and a social support function, as detailed previously (9).
Weight measurements
Participants received an iHealth Lite Bluetooth scale (Model Lina H2; iHealth, Mountain View, California) via mail and they were instructed to measure their weight daily. Weight measurements were automatically uploaded to the iHealth cloud where they could be accessed by the researchers. Weight loss at 12 weeks was the preregistered primary outcome.
Secondary and exploratory outcomes
At baseline and at 12 weeks, a fasted blood sample was obtained by a third‐party laboratory (Quest Diagnostics, Secaucus, New Jersey) for measuring metabolic and cardiovascular risk markers. Participants completed weekly and monthly survey measures as manipulation checks of adherence and intervention fidelity. Surveys were sent via email and they included the Automated Self‐Administered 24‐Hour Dietary Recall (13), the Pittsburgh Sleep Quality Index (14), and questionnaires about food attitude, diet adherence, physical activity (15), and cravings, mood, and energy. We also explored the durability of the effect on weight loss by assessing the change in body mass at 24 weeks using available data from the Bluetooth‐ and cloud‐connected weigh scales.
Statistical analysis
The primary objective of this trial was to test the superiority of the ketogenic diet app with breath acetone biofeedback, compared with the calorie‐restricted, low‐fat diet app, for promoting weight loss after 12 weeks. Blinded data were analyzed on an intention‐to‐treat basis. We used constrained baseline longitudinal analysis via a linear mixed model with fixed effects for time point (baseline and postintervention), stratified allocation factors, and the interaction between time point and dietary intervention group and random effects for participants. Effect estimates with 95% CI for the between‐group differences from the model are reported as the main analyses of interest. Effect estimates for within‐group changes over time are also reported. Statistical significance was established at an α level of 0.05. Baseline weight was the weight recorded on the first day of the trial or, if no weight was logged on that day, the closest weight measurement to 8 AM on the start date of the trial. Postintervention weight was calculated as the average (mean) weight of the final (i.e., 12th) week of the intervention phase. Sensitivity analysis on the primary outcome was performed in a similar manner using a linear mixed model with all available daily weight measures (as opposed to using only baseline and postintervention weight for the primary analysis). Secondary end points were analyzed similarly. Bonferroni‐adjusted critical α was used for secondary outcomes to account for multiple comparisons. No α adjustments were applied to exploratory outcomes. To explore the impacts of the different diets independent of weight loss, we reran the linear mixed model on secondary and exploratory outcomes that were statistically significant with baseline weight and change in body mass included as additional fixed effects. The statistical analysis plan is presented in the online Supporting Information.
RESULTS
Participants
Of the 155 randomized participants, 116 (75%) completed the 12‐week intervention; 60 (78%) and 56 (72%) participants were retained in the Mediterranean‐style ketogenic diet app paired with breath acetone biofeedback group and in the calorie‐restricted, low‐fat diet app group, respectively (Figure 1). Basic baseline characteristics are shown in Table 1. Socioeconomic participant characteristics are presented in Supporting Information Table S1.
FIGURE 1.
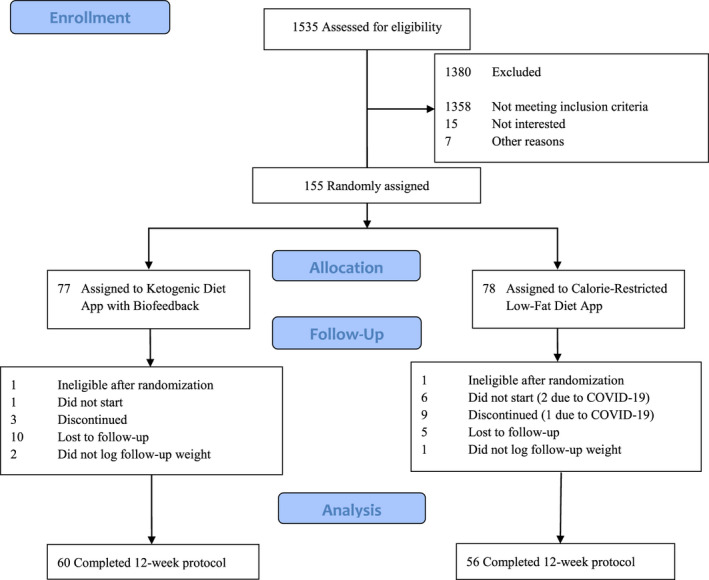
CONSORT flow diagram describing the process of determining participant eligibility, enrollment, random assignment, and data analysis. CONSORT, Consolidated Standards of Reporting Trials [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Participant baseline characteristics
| Total | Ketogenic diet app with biofeedback | Calorie‐restricted, low‐fat diet app | |
|---|---|---|---|
| N | 155 | 77 | 78 |
| Age, mean (SD), y | 41 (11) | 42 (11) | 41 (11) |
| Female, n (%) | 110 (71) | 55 (71) | 55 (71) |
| Male, n (%) | 45 (29) | 22 (29) | 23 (29) |
| Weight, mean (SD), kg | 94.4 (16.0) | 94.7 (17.1) | 94.1 (14.7) |
| BMI, mean (SD) | 33.5 (4.7) | 33.5 (4.7) | 33.6 (4.7) |
| Secondary outcomes a | |||
| HbA1c (%) | 5.4 (0.5) | 5.4 (0.6) | 5.4 (0.5) |
| HbA1c (mmol/mol) | 35.6 (5.9) | 36.0 (6.2) | 34.2 (6.0) |
| Glucose (mmol/L) | 5.4 (1.1) | 5.4 (1.4) | 5.3 (0.8) |
| Insulin (pmol/L) | 76 (61) | 76 (52) | 76 (68) |
| HOMA‐IR (a.u.) b | 3.2 (3.2) | 3.1 (2.5) | 3.2 (3.7) |
| hs‐CRP (mg/L) | 4.4 (4.2) | 4.6 (4.2) | 4.2 (4.1) |
| Total cholesterol (mmol/L) | 5.2 (1.0) | 5.1 (1.0) | 5.3 (1.1) |
| HDL cholesterol (mmol/L) | 1.4 (0.3) | 1.4 (0.3) | 1.4 (0.4) |
| Cholesterol/HDL ratio | 4.0 (1.1) | 4.0 (1.1) | 3.9 (1.1) |
| LDL cholesterol (mmol/L) c | 3.2 (0.8) | 3.1 (0.8) | 3.2 (0.9) |
| Non‐HDL cholesterol (mmol/L) d | 3.8 (1.0) | 3.8 (1.0) | 3.9 (1.0) |
| Triglycerides (mmol/L) | 1.7 (1.1) | 1.7 (1.0) | 1.6 (1.2) |
| LDL particle number (nmol/L) | 1,396 (335) | 1,378 (343) | 1,413 (327) |
| Small LDL particles (nmol/L) | 258 (138) | 258 (132) | 258 (125) |
| Medium LDL particles (nmol/L) | 290 (102) | 283 (103) | 298 (101) |
| LDL particle size (nm) | 21.7 (0.7) | 21.7 (0.7) | 21.8 (0.6) |
| Large HDL particles (nmol/L) | 5,597 (1,308) | 5,460 (1,120) | 5,734 (1,467) |
| Apolipoprotein B (g/L) | 1.0 (0.2) | 1.0 (0.2) | 1.0 (0.2) |
| Lipoprotein (a) (nmol/L) | 53 (73) | 56 (79) | 51 (67) |
| Exploratory outcomes a | |||
| Albumin (g/L) | 44 (3) | 43 (3) | 44 (3) |
| Globulin (g/L) | 27 (3) | 26 (3) | 28 (3) |
| Total bilirubin (µmol/L) | 10.3 (6.8) | 10.3 (3.4) | 12.0 (8.6) |
| ALP (U/L) | 67 (19) | 67 (19) | 66 (18) |
| AST (U/L) | 22 (19) | 22 (23) | 22 (15) |
| ALT (U/L) | 25 (19) | 25 (18) | 26 (20) |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HbA1c, hemoglobin A1c; HDL cholesterol, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; hs‐CRP, high‐sensitivity C‐reactive protein; LDL cholesterol, low‐density lipoprotein cholesterol.
All markers assessed in a fasted state. Data presented as mean (SD).
Calculated as: fasting insulin (μIU/mL) * fasting glucose (mmol/L) / 22.5.
Calculated with Martin‐Hopkins Formula (28).
Calculated as: total cholesterol – HDL cholesterol.
Weight loss
There was a decrease in body mass in the ketogenic diet app with biofeedback group (−5.6 kg; 95% CI: −6.7 kg to −4.5 kg) and in the calorie‐restricted, low‐fat diet app group (−2.5 kg; 95% CI: −3.6 kg to −1.4 kg) (Figure 2). The difference in weight change between groups was statistically significant (−3.1 kg; 95% CI: −4.6 kg to −1.5 kg; p < 0.001) (Table 2). Sensitivity analysis including all daily body weight measures during the 12‐week intervention period resulted in a similar between‐group treatment effect (−2.8 kg; 95% CI: −4.2 kg to −1.3 kg; p < 0.001). Based on available data from 73 participants at 24 weeks (n = 42 in the ketogenic diet app group, n = 31 in the calorie‐restricted, low‐fat diet group), there was a decrease in body mass in the ketogenic diet app with biofeedback group (−8.4 kg; 95% CI: −10.2 kg to −6.6 kg) and in the calorie‐restricted, low‐fat diet app group (−2.9 kg; 95% CI: −5.0 kg to −0.8 kg). This exploratory analysis showed a persistent difference in weight loss between groups (−5.5 kg; 95% CI: −8.3 kg to −2.8 kg; p < 0.001) (Table 2), which was confirmed by sensitivity analysis including all daily body weight measures available during the 24 weeks (−4.9 kg; 95% CI: −7.3 kg to −2.6 kg; p < 0.001) (Supporting Information Figure S1).
FIGURE 2.
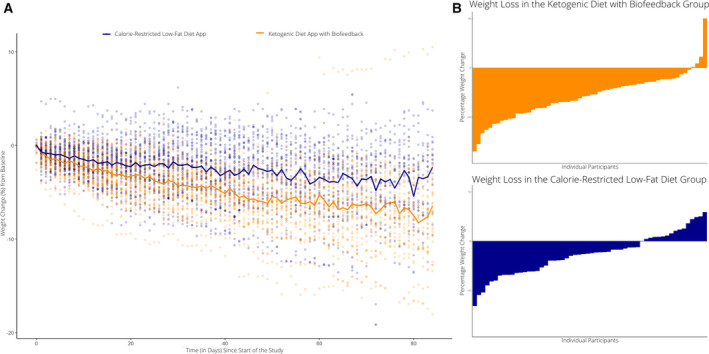
Weight change at 12 weeks in ketogenic diet app with biofeedback and calorie‐restricted, low‐fat diet app groups including individual participant data points. (A) Individual change in body weight (calculated as daily percent change from baseline based on measurements recorded from an at‐home Bluetooth scale) are shown for each participant over time throughout the duration of the study. Daily mean values over time for each group are represented in solid lines (orange, ketogenic diet app with biofeedback; blue, calorie‐restricted, low‐fat diet app). (B) Waterfall plots showing percent weight change from baseline for each participant in the ketogenic diet app with biofeedback group (top) and calorie‐restricted, low‐fat diet app group (bottom) [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Effect estimates for 12‐week changes in body mass (primary outcome) and blood markers (secondary/exploratory outcomes)
| Marker a | Ketogenic diet app with biofeedback (n = 77) | Calorie‐restricted, low‐fat diet app (n = 78) | Difference between groups | p value b |
|---|---|---|---|---|
| Primary outcome | ||||
| Body mass at 12 weeks (kg) | −5.6 (−6.7 to −4.5) | −2.5 (−3.6 to −1.4) | −3.1 (−4.6 to −1.5) | <0.001 |
| Secondary outcomes | ||||
| Body mass at 24 weeks (kg) | −8.4 (−10.2 to −6.6) | −2.9 (−5.0 to −0.8) | −5.5 (−8.3 to −2.8) | <0.001 |
| HbA1c (%) | −0.2 (−0.3 to −0.1) | 0.0 (−0.1 to 0.1) | −0.2 (−0.3 to −0.1) | <0.001 |
| HbA1c (mmol/mol) | −2.2 (−3.0 to −1.4) | 0.0 (−0.9 to 0.9) | −2.2 (−3.4 to −1.0) | <0.001 |
| Glucose (mmol/L) | −0.1 (−0.3 to 0.1) | 0.0 (−0.2 to 0.2) | −0.1 (−0.3 to 0.2) | 0.69 |
| Insulin (% change) c | −23 (−36 to −8) | −16 (−31 to 2) | −9 (−29 to 17) | 0.48 |
| HOMA‐IR (% change) c , d | −25 (−38 to −9) | −17 (−32 to 3) | −10 (−31 to 18) | 0.44 |
| hs‐CRP (% change) c | −5 (−21 to 15) | 1 (−18 to 25) | −6 (−29 to 24) | 0.66 |
| Total cholesterol (mmol/L) | 0.1 (−0.1 to 0.2) | −0.1 (−0.3 to 0.1) | 0.2 (−0.1 to 0.4) | 0.13 |
| HDL cholesterol (mmol/L) | 0.0 (−0.1 to 0.0) | 0.0 (−0.1 to 0.0) | 0.0 (−0.1 to 0.1) | 0.96 |
| Cholesterol/HDL ratio | 0.2 (0.0 to 0.4) | 0.0 (−0.2 to 0.2) | 0.2 (−0.1 to 0.5) | 0.18 |
| LDL cholesterol (mmol/L) e | 0.1 (0.0 to 0.3) | 0.0 (−0.2 to 0.1) | 0.1 (−0.1 to 0.4) | 0.19 |
| Non‐HDL cholesterol (mmol/L) f | 0.1 (−0.1 to 0.2) | −0.1 (−0.3 to 0.1) | 0.2 (−0.1 to 0.4) | 0.18 |
| Triglycerides (% change) c | −15 (−25 to 5) | −13 (−23 to −1) | −3 (−18 to 15) | 0.75 |
| LDL particle number (nmol/L) | 62 (−18 to 142) | 60 (−27 to 146) | 3 (−110 to 115) | 0.96 |
| Small LDL particles (% change) c | −1 (−10 to 9) | 1 (−9 to 12) | −2 (−15 to 12) | 0.74 |
| Medium LDL particles (nmol/L) | 10 (−16 to 35) | 6 (−21 to 34) | 4 (−32 to 39) | 0.84 |
| LDL particle size (nm) | 0.2 (0.1 to 0.4) | 0.1 (−0.1 to 0.2) | 0.2 (0.0 to 0.3) | 0.07 |
| Large HDL particles (nmol/L) | 296 (−13 to 606) | 24 (−311 to 358) | 272 (−162 to 707) | 0.22 |
| Apolipoprotein B (g/L) | 0.0 (0.0 to 0.1) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.1) | 0.33 |
| Lipoprotein (a) (% change) c | 3 (−7 to 15) | 7 (−5 to 21) | −4 (−18 to 13) | 0.64 |
| Exploratory outcomes | ||||
| Albumin (g/L) | 0 (−1 to 0) | −1 (−1 to 0) | 0 (0 to 1) | 0.24 |
| Globulin (g/L) | −1 (−2 to 0) | 1 (0 to 1) | −2 (−2 to −1) | 0.001 |
| Total bilirubin (% change) c | −5.6 (−13.2 to 2.7) | 3.3 (−5.7 to 13.3) | −8.6 (−19.1 to 3.1) | 0.14 |
| ALP (U/L) | −6 (−8 to −4) | 1 (−2 to 3) | −7 (−10 to −4) | <0.001 |
| AST (% change) c | −6 (−14 to 2) | 4 (−5 to 15) | −10 (−21 to 2) | 0.1 |
| ALT (% change) c | −15 (−25 to −4) | 7 (−6 to 22) | −21 (−33 to −6) | 0.009 |
All data are presented as (within‐group or between‐group) effect estimates (95% CI). Effect estimates are based on intention‐to‐treat analyses and included all participants that had a baseline or a follow‐up value (n = 146 for body mass and n = 151 for blood analyses). Bonferroni‐adjusted critical α of 0.003 is used for secondary outcomes.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HbA1c, hemoglobin A1c; HDL cholesterol, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL cholesterol, low‐density lipoprotein cholesterol.
All markers were assessed in a fasted state.
Data analyzed via constrained longitudinal data analysis (cLDA) using a linear mixed model.
Variable was log‐transformed; interpret effect estimates as percent change.
Calculated as: fasting insulin (μIU/mL) * fasting glucose (mmol/L) / 22.5.
Calculated with Martin–Hopkins Formula (28).
Calculated as: total cholesterol – HDL cholesterol.
Dietary intake
There was no significant difference in self‐reported energy intake between intervention groups across the 12 weeks (16 kcal; 95% CI: −68 kcal to 101 kcal; P = 0.71). The ketogenic diet app with biofeedback group reported consuming significantly less carbohydrates and significantly more total fat, saturated fat, and monounsaturated fat compared with the calorie‐restricted, low‐fat diet app group. There was no significant difference in self‐reported intake of polyunsaturated fat between the intervention groups. Full analyses of self‐reported dietary intake data are presented in Supporting Information Table S2.
Metabolic and cardiovascular blood markers
There was a significantly greater decrease in hemoglobin A1c (HbA1c) at 12 weeks in the ketogenic diet app with biofeedback group as compared with the calorie‐restricted, low‐fat diet app group (−0.2%; 95% CI: −0.3 % to −0.1%; p < 0.001). Similarly, there was a significant difference in change in alanine aminotransferase (−21%; 95% CI: −33% to −6%; p = 0.009), alkaline phosphatase (−7.0 U/L; 95% CI: −10.0 U/L to −4.0 U/L; p < 0.001), and globulin (−0.2 g/dL; 95% CI: −0.2 g/dL to −0.1 g/dL; p = 0.001) at 12 weeks. These between‐group differences remained significant in exploratory analyses controlling for baseline weight and change in weight in the model as presented in Supporting Information Table S3. There were no other differences between groups in assessed blood markers of cardiometabolic risk at 12 weeks (Table 2).
Manipulation checks
When considering participants who completed the 12‐week protocol, compliance with daily weight measurements was ~68% (~5 d/wk) in the ketogenic diet app with biofeedback group and ~60% (~4 d/wk) in the calorie‐restricted, low‐fat diet app group, with no difference between groups (p = 0.17). Average self‐reported dietary adherence (indicated on a 5‐point Likert scale measured weekly) was moderate in both groups; Mann–Whitney U test revealed significantly higher adherence in the ketogenic diet app with biofeedback group (2.6 [0.8]) than in the calorie‐restricted, low‐fat diet app group (2.1 [0.8]). All survey measures are presented in Supporting Information Table S4.
DISCUSSION
The Mediterranean‐style ketogenic diet app paired with a breath acetone biofeedback device was found to result in superior weight loss than the calorie‐restricted, low‐fat diet app, with secondary outcomes revealing greater improvements in important markers of cardiometabolic health. Although ketogenic diets have gained widespread popularity because of perceived efficacy for weight loss and glucose control, potential concerns regarding negative effects on blood lipid profiles need to be weighed against benefits (16). Furthermore, there is inconclusive evidence about the real‐world effectiveness of such diets, particularly when implemented via apps, in free‐living conditions and without significant human intervention (17).
Our findings in this pragmatic, virtual, app‐based trial provide evidence to address these concerns. Consistent with our primary hypothesis, those randomized to the ketogenic diet app and biofeedback group had significantly greater weight loss when compared with those in the calorie‐restricted, low‐fat diet app group at 12 weeks. Additionally, the ketogenic diet intervention group showed greater improvements in markers of glycemic control (HbA1c) and an important marker of hepatocyte damage associated with nonalcoholic steatohepatitis (alanine aminotransferase) at 12 weeks. These effects remained statistically significant after accounting for differences in change in body mass, suggesting that the Mediterranean‐based ketogenic diet app intervention may improve select metabolic markers independent of weight loss. There was also higher reported dietary adherence, and there were no differences observed between groups in blood lipids, lipoprotein fractions (including low‐density lipoprotein cholesterol [LDL] particle number or size), or self‐reported energy intake. Therefore, an app‐based ketogenic diet intervention with biofeedback based on breath acetone monitoring of ketosis appears to be effective for weight loss and improving metabolic health (potentially independent of weight loss) over the course of 12 weeks in a real‐world setting.
The mechanisms leading to greater weight loss in the ketogenic diet app group despite no significant difference in self‐reported energy intake between groups are unclear. Previous research suggests that energy intake may be overreported on low‐carbohydrate diets during the initial months of the dietary intervention (18) or that diets lower in carbohydrates may increase total energy expenditure (19). However, estimation of energy intake in our study was based on self‐report, and future research is needed to understand these findings. Additionally, our trial was unable to separate the effects of individual app components (e.g., recipes, group support, breath acetone biofeedback device) on outcomes, and future research aiming to identify the relation between the different intervention components could help clarify which are the most important contributors to weight loss and improvements of cardiometabolic health.
There is mixed evidence regarding the relative effectiveness of low‐carbohydrate diets compared with those restricted in fat and/or calories in promoting weight loss. In a recent umbrella review, Churuangsuk et al. showed a superior effect of low‐carbohydrate diets compared with control interventions in lower quality meta‐analyses, whereas the authors found no difference between dietary interventions in high‐quality meta‐analyses (20). Critically, and in contrast to many of the studies in the literature employing a more structured, high‐contact design, our trial examined the real‐world application of two hands‐off mHealth applications that promote weight loss via two different types of diet. Even though our fully remote app‐based study design involved no on‐site visits or in‐person counseling, we observed comparable weight loss with other high‐contact ketogenic/low‐carbohydrate diet studies that include in‐person center visits, group counseling, and/or individualized nutrition support (8, 21, 22). Conversely, the calorie‐restricted, low‐fat diet group in our study achieved slightly less weight loss than observed in previous, more structured, hands‐on trials (~3.5 to 5 kg (8, 21, 23) vs. ~2.5 kg in our trial) that involved in‐person group meetings and/or nutritional counseling. Overall, the app‐based ketogenic diet program with a breath acetone biofeedback device was superior to the calorie‐restricted, low‐fat diet program in promoting weight loss, providing evidence for the efficacy of this app‐based ketogenic diet intervention in a hands‐off, real‐world setting that was comparable with more intensive in‐person studies.
In contrast to many studies reporting on low‐carbohydrate ketogenic diet interventions (24), we did not detect an increase in total or LDL cholesterol when compared with the comparator group. Likewise, we did not observe a difference between groups in number of LDL particles or the important cardiovascular risk marker, apolipoprotein B. These results are supported by the findings of Fuehrlein et al., who showed that a ketogenic diet high in polyunsaturated fatty acids (as compared with saturated fatty acids) did not adversely affect levels of total and LDL cholesterol (25). Meta‐analytic evidence presented by Mensink et al. reported that an isoenergetic substitution of saturated and trans monounsaturated fatty acids for carbohydrates raises, whereas an exchange of carbohydrates with cis poly‐ or monounsaturated fatty acids lowers LDL cholesterol (26). Our data extend these previous findings by providing evidence that an app promoting a Mediterranean‐style ketogenic diet emphasizing monounsaturated and polyunsaturated fatty acids over saturated fatty acids may be a viable option to provide metabolic and weight loss benefits without potential negative effects on blood lipids and associated cardiovascular risk.
Although a strength of the study includes its real‐world, hands‐off intervention, the weight measurements were self‐administered on an at‐home wireless scale, which may not have been as accurate as if they were obtained through clinician‐assessment. Similarly, the dietary intake data were limited by relying on self‐report at discrete time points, which can introduce bias (27) and limit accuracy (18).
It is likely that not every participant in the ketogenic diet with biofeedback group achieved and maintained nutritional ketosis. As such, weight loss findings should be interpreted as being the result of an app and biofeedback‐based app that promotes the ketogenic diet under free‐living conditions rather than the effects of a strict ketogenic diet per se. Despite this inevitable consequence of the pragmatic study design, robust weight loss differences were observed. Furthermore, the primary end‐phase of this study was at 12 weeks. Exploratory analysis of change in body mass at 24 weeks suggests the durability of the effect, but findings should not be generalized beyond that point until planned longer‐term secondary analyses can be performed. Similarly, findings should not be generalized beyond the current study population, which was composed of generally healthy adults with overweight and obesity.
Although our virtual trial was designed prior to the COVID‐19 outbreak, most of this study occurred during the pandemic. To account for an expected increase in dropout, we decided to increase target enrollment as specified in the statistical analysis plan (see online Supporting Information). Participants generally reported minor negative impacts of the pandemic on their ability to stick with their program (Supporting Information Table S4) and were mostly able to complete the study despite any challenges posed.
CONCLUSION
In this 12‐week randomized clinical trial, dietary advice to follow an app‐based Mediterranean‐style ketogenic diet resulted in greater weight loss compared with the advice to follow an app‐based, standard calorie‐restricted, low‐fat diet. We found select biomarkers of glycemic control and hepatic function to be improved in the ketogenic diet group; however, there were no significant differences between groups for the majority of cardiometabolic health markers assessed. This suggests that an app‐based ketogenic diet with breath acetone biofeedback is superior to a calorie‐restricted, low‐fat diet app intervention to achieve weight loss and improve metabolic health in a real‐world setting. Future studies should aim to evaluate the long‐term effectiveness of such an intervention and its potential role in treatment of obesity‐related diseases.
CONFLICT OF INTEREST
JPL is chief scientific officer for the Institute for Personalized Therapeutic Nutrition, a not‐for‐profit organization promoting a food‐first approach to treating and preventing chronic disease. JPL holds shares in Metabolic Insights Inc., a for‐profit company developing a saliva insulin monitor. EJW is an equity holder at Keyto and Virta Health. DAL is employed as a consultant for Keyto. All other authors have no conflicts to declare.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov Identifier NCT04165707.
Supporting information
Supplementary Material
Acknowledgments
Data collected for this study, including individual deidentified participant data and a data dictionary defining each field in the set, will be made available to others with an academic affiliation upon reasonable request from the corresponding author. The published study protocol is available (9).
Falkenhain K, Locke SR, Lowe DA, et al. Keyto app and device versus WW app on weight loss and metabolic risk in adults with overweight or obesity: A randomized trial. Obesity (Silver Spring). 2021;29:1606–1614. 10.1002/oby.23242
Funding information
This work was supported by the Canadian Institutes of Health Research (MSH‐141980), Michael Smith Foundation for Health Research (MSFHR 16890), and Mitacs (IT15608).
REFERENCES
- 1. Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very‐low‐carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang E, Abrahamson K, Liu PJ, Ahmed A. Can mobile technology improve weight loss in overweight adults? A systematic review. West J Nurs Res. 2020;42(9):747‐759. [DOI] [PubMed] [Google Scholar]
- 3. Dounavi K, Tsoumani O. Mobile health applications in weight management: a systematic literature review. Am J Prev Med. 2019;56(6):894‐903. [DOI] [PubMed] [Google Scholar]
- 4. Burke LE, Wang J, Sevick MA. Self‐monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson JC. Measuring breath acetone for monitoring fat loss: review. Obesity (Silver Spring). 2015;23(12):2327‐2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirkpatrick CF, Bolick JP, Kris‐Etherton PM, et al. Review of current evidence and clinical recommendations on the effects of low‐carbohydrate and very‐low‐carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019;13(5):689‐711.e1. [DOI] [PubMed] [Google Scholar]
- 7. Gudzune KA, Doshi RS, Mehta AK, et al. Efficacy of commercial weight‐loss programs. Ann Intern Med. 2015;162(7):501‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction. JAMA. 2005;293(1):43‐53. [DOI] [PubMed] [Google Scholar]
- 9. Locke SR, Falkenhain K, Lowe DA, et al. Comparing the Keyto app and device with Weight Watchers’ WW app for weight loss: protocol for a randomized trial. JMIR Res Protoc. 2020;9(8):e19053. doi: 10.2196/19053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Estruch R, Ros E, Salas‐Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279‐1290. [DOI] [PubMed] [Google Scholar]
- 11. Keyto . The science behind Keyto, part one – how the Keyto breath sensor works. https://getkeyto.com/science‐behind‐keyto‐how‐the‐breath‐sensor‐works/. Accessed May 8, 2021.
- 12. Weight Watcher's WW . SmartPoints: Starter Guide. Accessed May 8, 2021. https://www.weightwatchers.com/us/how‐it‐works/smartpoints
- 13. Subar AF, Kirkpatrick SI, Mittl B, et al. The Automated Self‐Administered 24‐Hour Dietary Recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet. 2012;112(8):1134‐1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(8):193‐213. [DOI] [PubMed] [Google Scholar]
- 15. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141‐146. [PubMed] [Google Scholar]
- 16. Mansoor N, Vinknes KJ, Veierød MB, Retterstøl K. Effects of low‐carbohydrate diets v. low‐fat diets on body weight and cardiovascular risk factors: a meta‐analysis of randomised controlled trials. Br J Nutr. 2016;115(3):466‐479. [DOI] [PubMed] [Google Scholar]
- 17. Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low‐carbohydrate diet for obesity. N Engl J Med. 2003;348(21):2082‐2090. [DOI] [PubMed] [Google Scholar]
- 18. Guo J, Robinson JL, Gardner CD, Hall KD. Objective versus self‐reported energy intake changes during low‐carbohydrate and low‐fat diets. Obesity (Silver Spring). 2019;27(3):420‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ludwig DS, Dickinson SL, Henschel B, Ebbeling CB, Allison DB. Do lower‐carbohydrate diets increase total energy expenditure? An updated and reanalyzed meta‐analysis of 29 controlled‐feeding studies. J Nutr. 2020;151(3):482‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Churuangsuk C, Kherouf M, Combet E, Lean M. Low‐carbohydrate diets for overweight and obesity: a systematic review of the systematic reviews. Obes Rev. 2018;19(12):1700‐1718. [DOI] [PubMed] [Google Scholar]
- 21. Truby H, Baic S, deLooy A, et al. Randomised controlled trial of four commercial weight loss programmes in the UK: initial findings from the BBC “diet trials”. BMJ. 2006;332:1309‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Samaha FF, Iqbal N, Seshadri P, et al. A low‐carbohydrate as compared with a low‐fat diet in severe obesity. N Engl J Med. 2003;348(21):2074‐2081. [DOI] [PubMed] [Google Scholar]
- 23. Jolly K, Lewis A, Beach J, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. BMJ. 2011;343:d6500. doi: 10.1136/bmj.d6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dong T, Guo M, Zhang P, Sun G, Chen B. The effects of low‐carbohydrate diets on cardiovascular risk factors: a meta‐analysis. PLoS One. 2020;15(1):e0225348. doi: 10.1371/journal.pone.0225348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fuehrlein BS, Rutenberg MS, Silver JN, et al. Differential metabolic effects of saturated versus polyunsaturated fats in ketogenic diets. J Clin Endocrinol Metab. 2004;89(4):1641‐1645. [DOI] [PubMed] [Google Scholar]
- 26. Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta‐analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146‐1155. [DOI] [PubMed] [Google Scholar]
- 27. Park Y, Dodd KW, Kipnis V, et al. Comparison of self‐reported dietary intakes from the Automated Self‐Administered 24‐h recall, 4‐d food records, and food‐frequency questionnaires against recovery biomarkers. Am J Clin Nutr. 2018;107(1):80‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low‐density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310(19):2061‐2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


