Abstract
Background
Annexin A1, a member of the Annexin superfamily, has been shown to play a vital role in a broad range of molecular and cellular processes. This study aims to explore the relationship between the Annexin A1 expression and the clinical response to cisplatin, docetaxel and 5‐fluorouracil (TPF) as induction chemotherapy in patients with oral squamous cell carcinoma (OSCC).
Methods
This study recruited two hundred thirty‐two patients from a III/IVA OSCC trial. Immunohistochemistry was used to assess the level of Annexin A1 expression. Overexpression and knockdown methods in HB96, HN4 and CAL27 cell lines were used to assess the role of Annexin A1 in the neoplastic cellular response to chemotherapy.
Results
We found that reduced expression of Annexin A1 conferred a prognostic benefit from induction chemotherapy based on the TPF drug combination in patients with moderately/poorly differentiated disease. Using an in vitro model, we found that low Annexin A1 enhanced cellular proliferation by activating the EGFR/AKT signalling pathway and inhibiting p27 expression. Furthermore, low Annexin A1 initiated a significant decrease in cell viability after treatment with TPF agents. In addition, downregulation of Annexin A1 promoted apoptosis induced by docetaxel, cisplatin and 5‐fluorouracil, and upregulation of Annexin A1 inhibited apoptosis.
Conclusion
Annexin A1 may be of prognostic value in patients with locally advanced OSCC who are managed with TPF chemotherapy, as low Annexin A1 promotes chemosensitivity to TPF chemotherapy in oral cancer cells via enhanced caspase‐dependent apoptosis.
Keywords: Annexin A1, biomarker, chemotherapy, oral squamous cell carcinoma
1. INTRODUCTION
Oral squamous cell carcinoma (OSCC) is one of the most prevalent malignancies in the head and neck. 1 Despite several efforts towards improving the diagnosis, treatment and rehabilitation of patients with this cancer, the 5‐year survival rate remains approximately 50%–60%. 2 Induction chemotherapy has been explored as a viable approach to improve the treatment outcomes of OSCC patients. The recommended combination of chemotherapeutic agents for planned induction chemotherapy in patients with locally advanced OSCC of the head and neck is docetaxel, cisplatin and 5‐fluorouracil (TPF). This is based on the findings of two randomized phase III trials (TAX323 and TAX324) where patients placed on this therapeutic regimen had improved treatment outcomes. 3 , 4 To highlight the contribution of TPF induction chemotherapy in OSCC patients managed with surgical intervention, we reported the findings of a randomized, phase III trial (NCT01542931) in patients with locally advanced OSCC. 5 Our findings, consistent with the DeCIDE and PARADIGM trials, did not support an overall significant survival advantage in the study participants. Rather, the studies suggested that induction chemotherapy should not be universally incorporated into the surgical or nonsurgical management of patients with OSCC. However, induction chemotherapy using the TPF combination might result in improved outcomes in a molecularly specified subset of patients, as some possible prognostic biomarkers, including Annexin A1, have been identified. 6 , 7
Annexin A1, a member of the Annexin superfamily and a calcium‐dependent phospholipid‐binding protein, has a defined function in cellular proliferation, membrane organization and trafficking, cellular differentiation and cell signal transduction. 8 Previous investigations have shown that reduced Annexin A1 expression is associated with poor cellular differentiation 9 and poor response to induction chemotherapy. 10 Notably, Annexin A1 expression was also shown to be strongly associated with overall patient survival, and reduced expression of Annexin A1 could serve as a biomarker for predicting short‐term (2‐year follow‐up) treatment outcomes after induction chemotherapy intervention in patients with moderately or poorly differentiated OSCC. 7 However, it has yet to be clarified whether Annexin A1 has a similar predictive capability for the survival of patients with advanced OSCC at a long‐term follow‐up of 5 years.
As we reported earlier, OSCC patients with reduced expression of Annexin A1 benefit from TPF induction chemotherapy. This improved response might be dependent on the level of Annexin A1 expression, and the sensitivity of malignant squamous cells to TPF induction chemotherapy agents, which is based on previous studies that reported that reduced Annexin A1 expression induces a significant increase in colon cancer cell vulnerability to 5‐FU. 11 Similarly, reduced expression of Annexin A1 has been shown to enhance sensitivity to anticancer chemotherapy in multidrug‐resistant tumour cells. 12 However, the effect of the level of Annexin A1 expression on the cellular response to chemotherapy in the management of oral cancer has yet to be fully elucidated. To assess this effect of Annexin A1, we explored the effect of the level of Annexin A1 expression on the sensitivity of oral carcinoma cell lines to TPF chemotherapy.
2. MATERIALS AND METHODS
2.1. Patients
Two hundred thirty‐two out of two hundred and fifty‐six OSCC patients managed for stage III and IVA in the TPF trial (NCT01542931) were recruited for this study. The TPF trial evaluated the benefit of induction chemotherapy using the TPF combination compared with the standard treatment in patients with locally advanced OSCC. 5 After treatment, patients were reviewed every quarter during the first 2 years, biannually during the subsequent 3–5 years, and annually thereafter until the patient was lost to follow up or expired. Ethical approval was obtained from the Shanghai Ninth Peoples' Hospital IRB (approval number [2016]105), and all participants endorsed an informed consent form.
2.2. Immunohistochemical analysis
Pretreatment biopsied tissues, which were formalin‐fixed and paraffin‐embedded, were processed for Annexin A1 immunohistochemical staining. Sections were incubated overnight at 4°C with a rabbit Annexin A1 polyclonal antibody (1:150) and subsequently stained using a 3,3′‐diaminobenzidine detection kit (Dako Cytomation). Two pathologists who had no knowledge of the treatment group performed a microscopic examination of the processed slides. The expression index of Annexin A1 was assessed according to the quantity of cells stained. The following criteria were used to categorize the expression index: negative, absence of cell staining; weak positive, less than 50% of cells stained; and strongly positive, equal or greater than 50% of cells stained. Tumours with weak positive or negative expression indices were categorized as having low expression of Annexin A1, while tumours with a strong positive expression index were categorized as having high expression of Annexin A1.
2.3. Cell cultures
The HB96, HN4 and CAL27 cell lines were used in this study. The HB96 cell line was derived from a cellular carcinogenesis model of OSCC. 13 CAL27 was sourced from ATCC (Manassas, USA), while HN4 was a gift from the National Institutes of Health of the United States of America. These three cell lines were cultured in DMEM (Gibco, USA, product code: C11995500BT) supplemented with 1% penicillin‐streptomycin (Beyotime Biotechnology, China, product code: ST488) and 10% foetal bovine serum (Foundation GEMINI, USA, product code: 900–108) and maintained in a humidified environment of 5% CO2 at 37°C.
2.4. Annexin A1 RNA interference gene transfection
We designed two sets of short hairpin RNA oligonucleotides and a negative control oligonucleotide (Sangon Biotech), shown in Table S1, to construct retroviral vectors. We cloned these oligonucleotides into the pSIREN‐Puro plasmid, transferred these plasmids into 293T cells and collected the supernatant of virus filtered through a 4 µm filter. To construct stable Annexin A1‐downregulated cell lines, HB96 and HN4 cells were transfected with virus supernatant and screened with puromycin (Life Technologies, Inc.), which was added to the medium at a concentration of 0.5 µg/ml. Real‐time PCR and Western blotting were performed to detect Annexin A1 expression in HB96 and HN4 cells infected with virus.
2.5. Annexin A1 gene transfection
We obtained the pMSCVpuro‐hANXA1 (Annexin A1 overexpression) retroviral vector and a blank pMSCV puro plasmid from Qihe Bio (China, Shanghai). The blank plasmid was used as a control. CAL27 cells were transfected with the retroviral vector and screened with puromycin (Life Technologies, Inc.), which was added to the medium at a 1 µg/ml concentration. Real‐time PCR and Western blotting were performed to detect Annexin A1 expression in CAL27 cells infected with virus.
2.6. Cytotoxicity assay
A 96‐well plate was seeded with 1.5 × 103 cells per well. Cells in each well were incubated in 100 μl medium without penicillin‐streptomycin and glutamine for 8–12 hr before exposure to a series of concentrations of cisplatin (CDDP) [0, 0.02, 0.05, 0.16, 0.49 and 1.48 µg/ml], docetaxel [0, 0.14, 0.41, 1.23, 3.70 and 11.11 nM] or 5‐fluorouracil (5‐FU) [0, 0.11, 0.33, 0.99, 2.96 and 8.89 µg/ml] for 72 hr. The supernatant of each well was collected and mixed with 100 µl CKK8 (Dojindo Laboratories) solution in fresh medium at a concentration of 10%. Finally, the 96‐well plates were incubated for an additional 2 hr at 37°C. Uptake at 450 nm using a SpectraMax M3 (Molecular Devices) signifies cell viability. This procedure was performed 3 times for quality control.
2.7. Real‐time PCR assay
Total RNA was extracted on ice from cultured cells at 80% confluence using TRIzol (Invitrogen, USA) in accordance with the manufacturer's protocol. Two micrograms of total RNA were reverse transcribed into cDNA with a reverse transcription kit (Takara Bio Inc.). Real‐time PCR was performed in a Step One Plus Real‐Time PCR System. We designed Annexin A1 gene and β‐actin primers, which are shown in Table S2. The relative Annexin A1 mRNA level compared with level of the internal control gene β‐actin was calculated according to the method. All samples were tested in duplicate, and the relative measure of expression of each target gene was performed in triplicate.
2.8. Western blotting and antibodies
Total protein was processed from the retrieved cells. All procedures were performed on ice. The extracted proteins (15 µg/lane) were separated using 10–12% SDS‐PAGE and then electrophoretically transferred onto 0.22 µm polyvinylidene difluoride (PVDF) membranes (Millipore) using a wet transfer system. The membranes were blocked with blocking buffer for 1 hr and subsequently incubated overnight with primary antibody at 4°C, which was followed by incubation at room temperature for 1 hr with a secondary antibody conjugated to horseradish peroxidase (HRP) to enhance chemiluminescence detection. Finally, the PVDF membranes were scanned using an enhanced chemiluminescence detection system (Amersham Imager 600). The β‐actin expression level served as an internal control. The primary antibodies are shown in Table S3.
2.9. Statistical analysis
The primary outcome variable of this study was survival rate. The other outcome measures were local disease control and patient safety. Overall survival (OS) was assessed from the date of recruitment into the clinical trial to the date of death. Locoregional recurrence‐free survival (LRFS), disease‐free survival (DFS) and distant metastasis‐free survival (DMFS) were assessed from the date of recruitment into the clinical trial to the date of detection of locoregional recurrence, disease recurrence or distant metastasis/death from any cause.
Survival analysis was performed using the log‐rank test and Kaplan‐Meier method. A Cox proportional hazards model was employed to calculate the hazard ratios (HRs). Statistically significant differences were assessed using Student's paired t test and one‐way ANOVA. All tests were two‐tailed at a significance level of ≤0.05. Data analysis was performed using PASW Statistics for Windows, Version 18.0. (Chicago, USA: SPSS Inc. 2009).
3. RESULTS
3.1. Annexin A1 expression and patient outcomes
Of the 232 patients from the trial who participated in this study, 127 were in the control group, and 105 were in the experimental group. The median follow‐up duration was 80 months. Patients treated with induction chemotherapy consisting of the TPF combination showed a slight survival benefit compared with those who received the traditional treatment of surgical intervention followed by postoperative radiotherapy, as demonstrated by the OS (p = 0.109), DFS (p = 0.139), LRFS (p = 0.240) and DMFS (p = 0.095) data; however, the observed differences were not statistically significant. Low expression of Annexin A1 was detected in 96 patients (40 in the experimental group and 56 in the control group). Patients with a low level of Annexin A1 expression demonstrated no obvious benefit from receiving the TPF induction chemotherapy protocol with regard to OS (p = 0.185), DFS (p = 0.131), LRFS (p = 0.226) and DMFS (p = 0.165). High Annexin A1 expression was detected in 136 specimens (65 in the experimental group and 71 in the control group), and patients with high Annexin A1 expression also exhibited no obvious benefit from TPF induction chemotherapy with regard to OS (p = 0.293), DFS (p = 0.444), LRFS (p = 0.561) and DMFS (p = 0.267).
In patients with low levels of Annexin A1 expression, a subgroup analysis according to the clinical characteristics found that patients with moderately or poorly differentiated disease received no benefit from induction chemotherapy consisting of the TPF combination in terms of DMFS (69.7% vs. 51.1%, p = 0.063) (Figure 1A). For patients with a high level of Annexin A1 expression, a subgroup analysis based on clinical characteristics did not reveal a survival benefit from TPF induction chemotherapy in patients with moderately or poorly differentiated disease (DMFS, 52.5% vs. 40.7%, p = 0.302) (Figure 1B).
FIGURE 1.
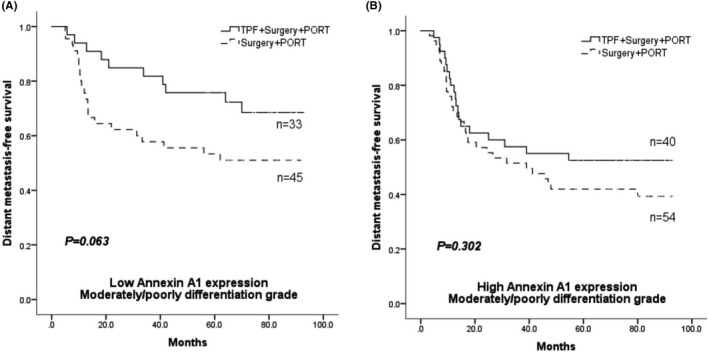
Distant metastasis‐free survival (DMFS) in patients with moderately/poorly differentiated OSCC; Low Annexin A1 expression group (A) and high Annexin A1 expression group (B)
3.2. Decreased Annexin A1 enhances cell proliferation by regulating p27 expression via the EGFR/AKT signalling pathway in vitro
HB96 and HN4 cells transfected with two sets of shRNAs showed better downregulation of Annexin A1 compared with cells transfected with shRNA‐NC (Figure 2A,B and Figure 2E, F). HB96 and HN4 cells with low Annexin A1 expression exhibited significantly increased proliferative activity compared with the control (Figure 2C and Figure 2G). Following decreased expression of Annexin A1 in HB96 and HN4 cells, the levels of EGFR, PDK1 and AKT phosphorylation increased significantly, and p27 expression decreased compared with the corresponding levels in parental cells (Figure 2D, H).In contrast, decreased levels of EGFR, PDK1 and AKT phosphorylation and p27 overexpression were observed in Annexin A1‐overexpressing CAL27 cells compared with control cells (Figure S1).
FIGURE 2.
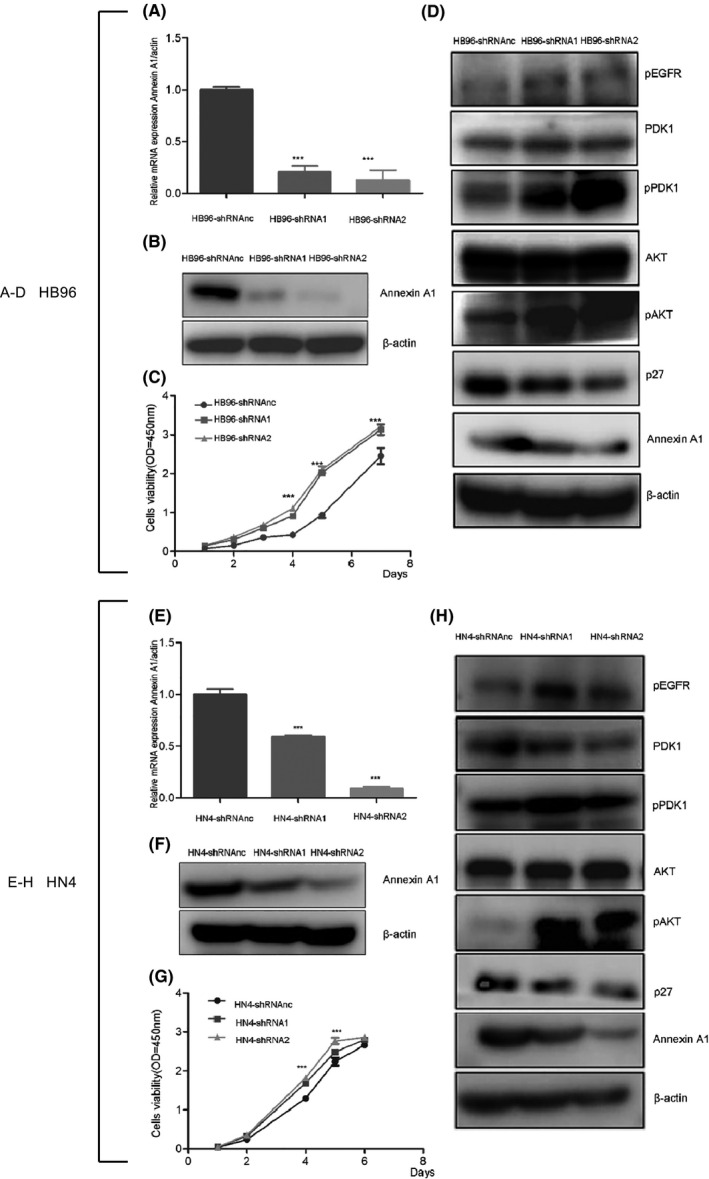
Low Annexin A1 expression enhanced cell proliferation by regulating p27 expression. The expression of Annexin A1 mRNA was analysed using real‐time PCR in HB96 (A) and HN4 (E) cells transfected with retroviral vectors containing shRNA1, shRNA2 or shRNAnc. The expression of Annexin A1 in HB96 (B) and HN4 (F) cells transfected with retroviral vectors containing shRNA1, shRNA2 or shRNAnc was assessed by Western blotting. The proliferation of HB96 (C) and HN4 (G) cell lines following shRNA transfection was detected using a CCK‐8 kit at 450 nm. The expression of pEGFR, PDK1, pPDK1, AKT, pAKT and p27 in HB96 (D) and HN4 (H) cells transfected with shRNA retroviral vectors was detected by Western blotting (*p < 0.05, **p < 0.01, ***p < 0.001).
3.3. Decreased Annexin A1 increases the cytotoxicity of docetaxel, cisplatin and 5‐FU in OSCC in vitro
We used a CCK‐8 assay to determine the viability of cells in which Annexin A1 was downregulated after treatment with docetaxel, cisplatin and 5‐fluorouracil. As shown in Figure 3, TPF chemotherapy drugs decreased the survival rate of HB96 and HN4 cells, regardless of shRNA transfection. Interestingly, cell viability was significantly lower in Annexin A1‐downregulated cells than in cells transfected with shRNA‐NC. We analysed the effect of Annexin A1 overexpression on sensitivity to TPF drugs, and the results showed that Annexin A1‐overexpressing cells had a higher survival rate than control cells (Figure S2).
FIGURE 3.
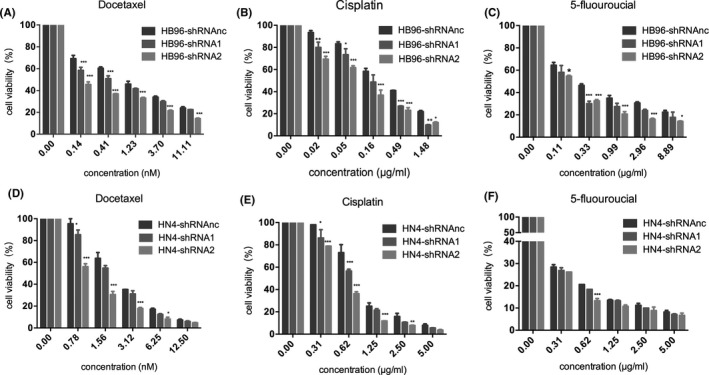
Decreased Annexin A1 increased the cytotoxicity of TPF chemotherapy in OSCC cell lines. (A‐C): Decreased viability of HB96 cells was observed after treatment with docetaxel, cisplatin and 5‐fluorouracil for 72 hr. Following transfection of HB96 cells with shRNA1 and shRNA2, cell viability was decreased significantly compared with HB96 cells transfected with shRNAnc; (D‐F): Decreased viability of HB96 cells was observed after treatment with docetaxel, cisplatin and 5‐fluorouracil for 72 hr. Similar to the findings in HB96 cells, the viability of HN4 cells transfected with shRNA1 and shRNA2 was decreased significantly compared with that of HN4 cells transfected with shRNAnc (*p < 0.05, **p < 0.01, ***p < 0.001)
3.4. Decreased Annexin A1 increases the cytotoxicity of chemotherapeutic agents through Caspase‐dependent apoptosis
Western blotting analysis demonstrated that TPF chemotherapy agents induced activation of PARP and caspase 3; clearly, an increased expression level of cleaved PARP and cleaved caspase 3, which are the predominant active fragments of PARP protein and caspase 3, respectively, was observed compared with the no dose group. Remarkably, increased expression of cleaved PARP and cleaved caspase 3 in HB96 and HN4 cells (Figure 4) with Annexin A1 downregulation was seen compared with control cells. In agreement with the aforementioned results, Annexin A‐overexpressing CAL27 cells had lower expression of cleaved PARP and cleaved caspase 3 than cells transfected with empty vector (Figure S3).
FIGURE 4.
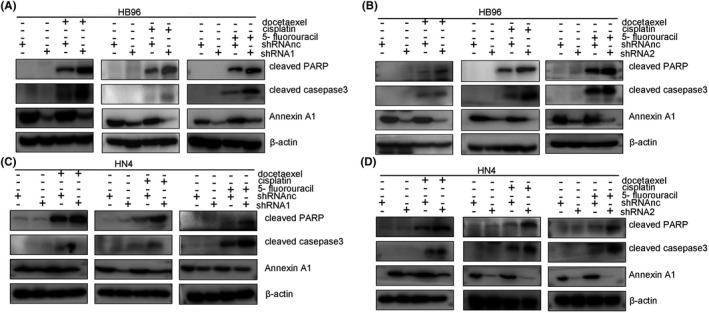
Decreased levels of Annexin A1 expression enhanced apoptosis induced by TPF chemotherapy in oral cancer cell lines. After transfection of HB96 cells with shRNA1 (A) and shRNA2 (B), the expression levels of cleaved PARP and cleaved caspase 3 were increased significantly following treatment of the cells with docetaxel (5 nM), cisplatin (2 µg/ml) and 5‐fluorouracil (2 µg/ml) for 72 hr. After transfection of HN4 cells with shRNA1 (C) and shRNA2 (D), the expression of cleaved PARP and cleaved caspase 3 was increased significantly in cells treated with docetaxel (5 nM), cisplatin (2 µg/ml) and 5‐fluorouracil (2 µg/ml) for 72 hr; a similar response was also noted in CAL27 cells
4. DISCUSSION
In the present study, a retrospective analysis was performed to explore the effect of Annexin A1 on the response to TPF induction chemotherapy in patients with locally advanced OSCC over a long‐term follow‐up of 5 years. This study did not demonstrate an obvious correlation between the level of Annexin A1 expression and clinical response to TPF induction chemotherapy in patients with OSCC, which is similar to a previous report, which focused on a short‐term follow‐up. 7 However, in the subgroup analysis, we found that lower expression of Annexin A1 was beneficial in the administration of TPF induction chemotherapy in patients with poorly or moderately pathologically differentiated disease according to DMFS. This study demonstrates the potential of Annexin A1 to predict the prognosis of OSCC patients, which is similar to the report by Lin et al, 14 who reported that Annexin A1 nuclear expression was associated with an increased recurrence rate and decreased overall survival. Low expression of Annexin A1 has the potential to be a biomarker used to categorize patients who may be sensitive to chemotherapy. However, the mechanism responsible for the improved sensitivity to TPF chemotherapy in some subgroups of patients with low expression of Annexin A1 is still unclear.
Annexin A1, also known as lipocortin 1, was initially described as a member of the Annexin superfamily that is present in the inflammatory corpuscle; it is also a glucocorticoid‐regulated anti‐inflammatory protein. 15 This protein has been shown to play a significant role in a broad range of molecular and cellular processes, including kinase activities in signal transduction, modulation of phospholipase A2, extracellular matrix integrity, cytoskeletal maintenance, differentiation, tissue growth and blood coagulation. 16 In different cancers, the expression of Annexin A1 may fluctuate. According to the literature, decreased Annexin A1 levels accelerate tumorigenesis in head and neck SCC, 17 as cells with low levels of Annexin A1 expression show increased proliferative activity. 18 In this study, we found that the EGFR/AKT signalling pathway was significantly activated and that p27 expression decreased as Annexin A1 expression decreased. The role of p27 in imposing the G1 restriction point is consequent upon its inhibitory binding to CDK2/cyclin E and other CDK/cyclin complexes. 19 Therefore, decreased p27 expression promotes the progression of oral cancer cells into S phase of the cell cycle, which results in tumour development and progression.
Annexin A1 is also known as the substrate of the activated EGF receptor at Tyr21, 20 and the Annexin A1/S100A11 complex plays a vital role in the degradation of activated EGFR through vesicular transportation. 21 In the present study, we found that EGFR phosphorylation is increased under conditions of low Annexin A1 expression and that downstream molecules, including PDK1 and AKT, are activated. Knock down of Annexin A1 decreases the expression of p27 but does not increase the expression of cyclin D1 (data not shown), which is contrary to what has been reported in some previous studies. 22 Our study demonstrated that decreased Annexin A1 may deregulate p27 protein expression through the EGFR/AKT signalling pathway.
Annexin A1 has a role in regulating resistance to some chemotherapeutic drugs. 23 However, the function of Annexin A1 in tumour development and drug resistance is not consistent. Annexin A1 is overexpressed in some multidrug‐resistant cancer cells, 24 , 25 while some reports have shown contrary results. 12 In our studies, increased chemosensitivity of oral cancer cells to TPF was observed in HB96 and HN4 cell lines with Annexin A1 downregulation. The results indicated that low Annexin A1 expression could boost the chemosensitivity of oral cancer cells to docetaxel, cisplatin and 5‐fluorouracil. Moreover, we found that chemotherapeutic agents induced cytotoxic processes including necrosis and apoptosis. This is consistent with previous studies, 26 , 27 which showed that oral cancer cells could be induced to undergo caspase‐dependent apoptosis when treated with appropriate concentrations of chemotherapeutic agents, for example docetaxel, CDDP and 5‐FU. Additionally, the cytotoxicity of TPF chemotherapy may be linked to the cell cycle status of cancer cells, as we found that low Annexin A1 expression can promote the progression of oral cancer from G1 phase to S phase by inhibiting p27 expression. Furthermore, we also analysed the enhancing effect of Annexin A1 expression on caspase‐dependent apoptosis induced by TPF and found that HB96 and HN4 cells with low Annexin A1 expression expressed higher cleaved PARP and cleaved caspase 3 levels compared with parental cells.
The mechanism by which Annexin A1 expression influences chemosensitivity and the apoptotic response to chemotherapeutic drugs is still unclear. In this study, we explored the mechanism of caspase‐dependent apoptosis. However, the extrinsic and intrinsic apoptosis pathways are both dependent on caspases. It is, therefore, necessary to further explore which apoptosis pathway is induced by TPF chemotherapy. In this study, we demonstrated that Annexin A1 is a potential biomarker that could be used to predict prognosis, screen target groups and categorize patients who may derive a prognostic benefit from TPF induction chemotherapy. In addition, we clarified the mechanism by which Annexin A1 regulates cancer progression through the EGFR/AKT signalling pathway. This may provide a new treatment strategy in which biomarkers for TPF chemotherapy and targeted therapy are combined.
5. CONCLUSION
These results suggest that Annexin A1 may be of prognostic value in a subgroup of patients treated with TPF chemotherapy, as low Annexin A1 promotes chemosensitivity to TPF chemotherapy agents in oral cancer cells by enhancing caspase‐dependent apoptosis.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
wenwen sun: Data curation. tongchao zhao: Formal analysis. Timothy O ALADELUSI: Writing‐review & editing. wutong ju: Software. dongwang zhu: Investigation; Writing‐original draft. zhong laiping: Resources. zhiyuan zhang: Supervision.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/jop.13221.
Supporting information
Fig S1
Fig S2
Fig S3
Table S1‐3
Supplementary Material
ACKNOWLEDGEMENT
This study was supported by research grants 81602370 and 81972525 from the National Natural Science Foundation of China.
Sun W, Zhao T, Aladelusi TO, et al. Decreased Annexin A1 expression enhances sensitivity to docetaxel, cisplatin and 5‐fluorouracil combination induction chemotherapy in oral squamous cell carcinoma. J Oral Pathol Med. 2021;50:795–802. 10.1111/jop.13221
Wenwen Sun, Tongchao Zhao and Timothy O Aladelusi have contributed equally to this work.
Contributor Information
Laiping Zhong, Email: zhonglaiping9th@126.com.
Dongwang Zhu, Email: zhudongwang@yeah.net.
REFERENCES
- 1. Parkin DM, Bray FJ, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74. [DOI] [PubMed] [Google Scholar]
- 2. Guo T, Califano JA. Molecular biology and immunology of head and neck cancer. Surg Oncol Clin N Am. 2015;24:397‐407. 10.1016/j.soc.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695‐1704. 10.1056/NEJMoa071028 [DOI] [PubMed] [Google Scholar]
- 4. Posner Marshall R, Hershock Diane M, Blajman Cesar R, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;1705‐1715. 10.1056/NEJMoa070956 [DOI] [PubMed] [Google Scholar]
- 5. Zhong LP, Zhang CP, Ren GX, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up‐front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31:744‐751. 10.1200/JCO.2012.43.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhong LP, Zhu DW, William WN, et al. Elevated cyclin D1 expression is predictive for a benefit from TPF induction chemotherapy in oral squamous cell carcinoma patients with advanced nodal disease. Mol Cancer Ther. 2013;12:1112‐1121. 10.1158/1535-7163.MCT-12-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu DW, Liu Y, Yang X, et al. Low Annexin A1 expression predicts benefit from induction chemotherapy in oral cancer patients with moderate or poor pathologic differentiation grade. BMC Cancer. 2013;13:301. 10.1186/1471-2407-13-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim LH, Pervaiz S. Annexin 1: the new face of an old molecule. FASEB J. 2007;21:968‐975. 10.1096/fj.06-7464rev [DOI] [PubMed] [Google Scholar]
- 9. Foo SL, Yap G, Cui J, Lim LHK. Annexin‐A1 ‐ a blessing or a curse in cancer? Trends Mol Med. 2019;25:315‐327. 10.1016/j.molmed.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 10. Chuthapisith S, Bean BE, Cowley G, et al. Annexins in human breast cancer: possible predictors of pathological response to neoadjuvant chemotherapy. Eur J Cancer. 2009;45:1274‐1281. 10.1016/j.ejca.2008.12.026 [DOI] [PubMed] [Google Scholar]
- 11. Onozawa H, Saito M, Saito K, et al. Annexin A1 is involved in resistance to 5‐FU in colon cancer cells. Oncol Rep. 2017;37:235‐240. 10.3892/or.2016.5234. [DOI] [PubMed] [Google Scholar]
- 12. Yu S, Meng Q, Hu H, Zhang M. Correlation of ANXA1 expression with drug resistance and relapse in bladder cancer. D ‐ NLM: PMC4203166 OTO ‐ NOTNLM. [PMC free article] [PubMed]
- 13. Zhong LP, Pan HY, Zhou XJ, et al. Characteristics of a cancerous cell line, HIOEC‐B(a)P‐96, induced by benzo(a)pyrene from human immortalized oral epithelial cell line. Arch Oral Biol. 2008;53:443‐452. 10.1016/j.archoralbio.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 14. Lin CY, Jeng YM, Chou HY, et al. Nuclear localization of annexin A1 is a prognostic factor in oral squamous cell carcinoma. J Surg Oncol. 2008;97:544‐550. 10.1002/jso.20992 [DOI] [PubMed] [Google Scholar]
- 15. Lima KM, Vago JPA, Caux TRA, et al. The resolution of acute inflammation induced by cyclic AMP is dependent on annexin A1. [DOI] [PMC free article] [PubMed]
- 16. Ying X, Ouyang C, Huang W, Tang Y, Fu W, Cheng A. Annexin A1 can inhibit the in vitro invasive ability of nasopharyngeal carcinoma cells possibly through Annexin A1/S100A9/Vimentin interaction. PLoS ONE. 2017;12:e0174383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han G, Lu K, Huang J, et al. Effect of Annexin A1 gene on the proliferation and invasion of esophageal squamous cell carcinoma cells and its regulatory mechanisms. Int J Mol Med. 2017;39:357‐363. 10.3892/ijmm.2016.2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu DW, Yang X, Yang CZ, et al. Annexin A1 down‐regulation in oral squamous cell carcinoma correlates to pathological differentiation grade. Oral Oncol. 2013;49:542‐550. 10.1016/j.oraloncology.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 19. Lloyd RV, Erickson LA, Jin L, et al. p27kip1: a multifunctional cyclin‐dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. D'Acunto CW, Gbelcova H, Festa M, Ruml T. The complex understanding of Annexin A1 phosphorylation. Cell Signal. 2014;26:173‐178. 10.1016/j.cellsig.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 21. Eden ER, Sanchez‐Heras E, Tsapara A, Sobota A, Levine TP, Futter CE. Annexin A1 tethers membrane contact sites that mediate ER to endosome cholesterol transport. Dev Cell. 2016;37:473‐483. 10.1016/j.devcel.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alldridge LC, Bryant CE. Annexin 1 regulates cell proliferation by disruption of cell morphology and inhibition of cyclin D1 expression through sustained activation of the ERK1/2 MAPK signal. Exp Cell Res. 2003;290:93‐107. [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Serfass L, Roy M‐O, Wong J, Bonneau A‐M, Georges E. Annexin‐I expression modulates drug resistance in tumor cells. Biochem Biophys Res Comm. 2004;314:565‐570. 10.1016/j.bbrc.2003.12.117 [DOI] [PubMed] [Google Scholar]
- 24. Zhu F, Wang Y, Zeng S, Fu X, Wang L, Cao J. Involvement of annexin A1 in multidrug resistance of K562/ADR cells identified by the proteomic study. OMICS. 2009;13:467‐476. 10.1089/omi.2009.0046 [DOI] [PubMed] [Google Scholar]
- 25. Wang C, Xiao Q, Li YW, et al. Regulatory mechanisms of annexin‐induced chemotherapy resistance in cisplatin resistant lung adenocarcinoma. Asian Pac J Cancer Prev. 2014;15:3191‐3194. [DOI] [PubMed] [Google Scholar]
- 26. Shigeishi H, Biddle A, Gammon L, et al. Elevation in 5‐FU‐induced apoptosis in Head and Neck Cancer Stem Cells by a combination of CDHP and GSK3β inhibitors. J Oral Pathol Med. 2015;44:201‐207. [DOI] [PubMed] [Google Scholar]
- 27. Iida S, Shimada J, Sakagami H. Cytotoxicity induced by docetaxel in human oral squamous cell carcinoma cell lines. Vivo (Athens, Greece). 2013;27:321‐332. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Table S1‐3
Supplementary Material


