Abstract
Purpose
Xanthomatous hypophysitis (XHP) is an extremely rare form of primary hypophysitis for which there is a lack of clinical experience. A comprehensive understanding of its clinical characteristics, diagnosis and treatment is needed.
Methods
Here, we report a case study and conduct a systematic review of XHP. Thirty-six cases were included, and their clinical manifestations, endocrine assessment, imaging features, treatment and follow-up data were collected and analyzed.
Results
The mean age at diagnosis was 39.1 years, and females were predominant (75.0%). The most common symptom was headache (68.6%), and 66.7% of female patients presented menstrual disorders. The most common pituitary dysfunction was growth hormone (GH) deficiency. More than half of patients exhibited central diabetes insipidus (CDI). The majority of patients had an imaging presentation of a cystic lesion with peripheral enhancement. Pituitary stalk thickening was observed in half of the patients. Total lesion resection was achieved in 57.1% of cases. The recurrence rate after partial resection and biopsy was significantly higher than that after total lesion resection (57.1% vs. 0.0%, P = 0.0147). The most common pituitary hormone abnormalities to resolve after surgery were hyperprolactinemia (100.0%) and GH deficiency (91.7%). The typical pathological feature was inflammatory infiltration of foamy histiocytes, which showed positivity for CD68.
Conclusion
Diagnosis of XHP is difficult when relying on clinical symptoms and imaging features. Therefore, surgical histopathology is necessary. Based on the available evidence, total lesion resection is recommended for treatment. However, the long-term prognosis for this rare disease remains unclear.
Keywords: xanthomatous hypophysitis, clinical characteristics, pathological examination, surgery, recurrence
Introduction
Hypophysitis is a rare disease characterized by lymphocytic infiltration of the pituitary gland, which can be divided into primary and secondary types (1, 2). Primary hypophysitis is the most common type, including several heterogeneous forms (1, 2). XHP is thought to be the rarest subtype of primary hypophysitis, and there is a lack of clinical experience with this disease (2). In this study, we report a case of XHP in our center and review all of the reported cases in previous literature. The objective of this study is to summarize the clinical characteristics and to bring an up-to-date understanding of XHP to provide evidence for the diagnosis, differential diagnosis and treatment of this rare disease.
Methods
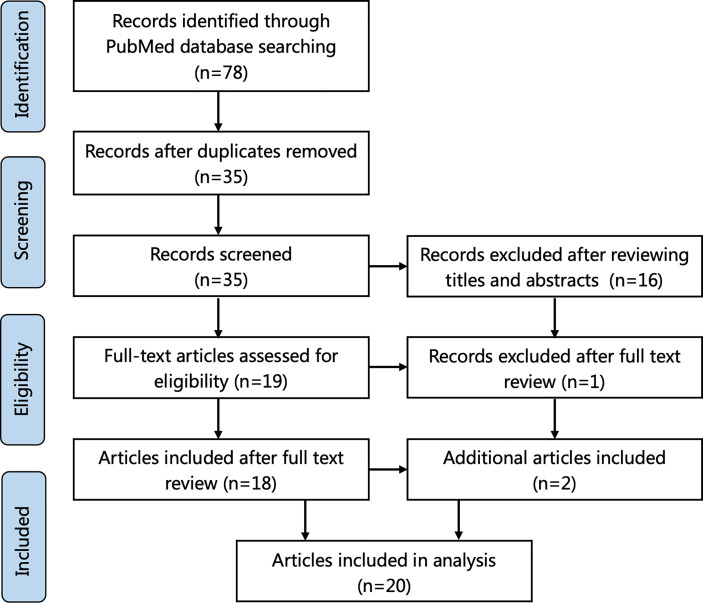
A review of the literature was performed in accordance with PRISMA guidelines. The terms “xanthomatous hypophysitis” and “xanthomatous AND hypophysitis” were searched using the online database PubMed until May 2021. Cases whose diagnosis of XHP was confirmed by histological examination were included. Review articles without new cases were excluded. The relevant adequate cases mentioned in the references of these articles were also considered. The search flowchart is shown in Figure 1 . lf total of 35 applicable cases reported in 20 articles were finally included.
Figure 1.
Flowchart of literature selection using the PRISMA guidelines.
Information on clinical symptoms, laboratory results, radiological features, surgery and postoperative conditions was extracted from these articles. Continuous data are expressed as the means ± standard deviations; categorical variables are summarized as frequencies and percentages and were analyzed using the χ2 test or Fisher’s exact test. The data were analyzed using GraphPad Prism, version 8 (GraphPad Software, San Diego, California, USA).
Results
Case Report
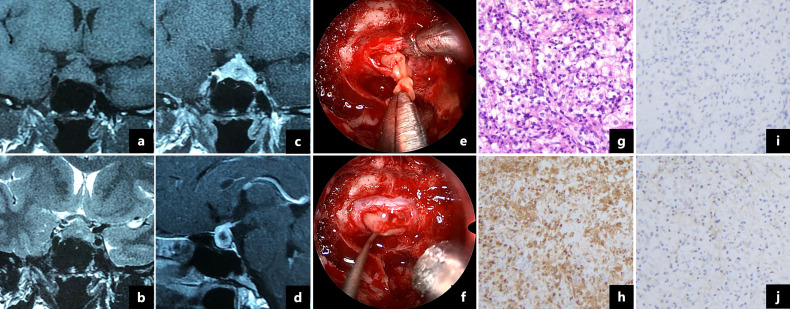
A 36-year-old woman presented with a 5-month history of headaches, polyuria and polydipsia and a 2-month history of amenorrhea. Her daily water intake was 3500-5000 ml. A water deprivation test indicated central diabetes insipidus (CDI). The results of the pituitary hormone test are shown in Table 1 . Her anti-peroxidase antibody (>600 IU/mL, normal range<34 IU/mL) and anti-thyroglobulin antibody (730 IU/mL, normal range<115 IU/mL) were high. Thyroid gland ultrasonography showed a normal volume with diffuse heterogeneous background echotexture of both thyroid glands. A visual field test showed partial bilateral temporal defects. A pituitary magnetic resonance imaging (MRI) scan revealed a 1.7×1.2 cm cystic lesion occupying the sella turcica with mainly peripheral enhancement ( Figures 2A–D ). Desmopressin (0.05 mg) was administered orally three times per day for DI as hormone replacement therapy. Endoscopic transsphenoidal surgery was performed and displayed a soft yellowish lesion with pus-like discharge ( Figures 2E, F ). The lesion was totally removed. The pathologic examination showed inflammatory infiltration of normal pituicytes by foamy histiocytes and lymphocytes, with fibrosis, local cysts and necrosis. Immunohistochemistry of the foamy histiocytes indicated strong staining for CD68 ( Figures 2G–J ).
Table 1.
The results of the pituitary hormone test in our case.
| Test projects | Normal range | Before surgery | After surgery (1 months) | After surgery (6 months)* |
|---|---|---|---|---|
| GH (ng/mL) | <2.0 | 3.1 | 1.5 | 1.8 |
| IGF-1 (ng/mL) | 115-320 | 352 | 288 | 209 |
| Morning cortisol (μg/dL) | 4.0-22.3 | 7.4 | 7.0 | 10.7 |
| ACTH (pg/mL) | 0-46 | 19.3 | 29.6 | 15.7 |
| TSH (μIU/mL) | 0.38-4.34 | 3.015 | 14.827 | 2.624 |
| Free T3 (pg/mL) | 1.80-4.10 | 2.61 | 3.49 | 2.58 |
| Free T4 (ng/dL) | 0.81-1.89 | 0.78 | 1.19 | 1.13 |
| LH (IU/L) | 2.12-10.89 | 3.23 | 3.35 | 6.27 |
| FSH (IU/L) | <10 | 6.79 | 5.80 | 8.45 |
| E2 (pg/mL) | 22-115 | <15 | 74 | 25 |
| P (ng/ml) | 0.38-2.28 | 0.27 | 6.18 | 0.49 |
| T (ng/mL) | 0.10-0.75 | 0.31 | <0.1 | 0.20 |
| PRL (ng/mL) | <30.0 | 67.1 | 21.0 | 29.2 |
*The hormones were measured on the third day of the patient’s menstrual cycle.
GH, growth hormone; IGF-1, insulin-like growth factor-1; ACTH, adrenocorticotropic hormone; TSH, thyroid-stimulating hormone; T3, triiodothyronine; T4, thyronine; LH, luteinizing hormone; FSH, follicle-stimulating hormone; E2, estradiol; P, progesterone; T, testosterone; PRL, prolactin.
Figure 2.
Magnetic resonance imaging showed a 1.7×1.2 cm cystic lesion with a thickened pituitary stalk and mainly peripheral enhancement (A–D). Intraoperative pictures revealed a soft yellow lesion (E) with pus-like fluid (F). Pathologic examination showed that the pituitary gland was infiltrated by foamy histocytes and lymphocytes (G, ×200). The foamy cells were immunopositive for CD68 (H, ×200) and immunonegative for CD1a (I, ×200) and S-100 protein (J, ×200).
The patient was discharged with no surgical complications. Postoperatively, her headaches and visual field defects resolved. The dose of desmopressin decreased during follow-up, and levothyroxine was administered because of autoimmune thyroiditis. The patient’s menstruation resumed three months after surgery. At the 6-month follow-up, desmopressin was discontinued, and the pituitary hormones were within the normal range ( Table 1 ).
Literature Review
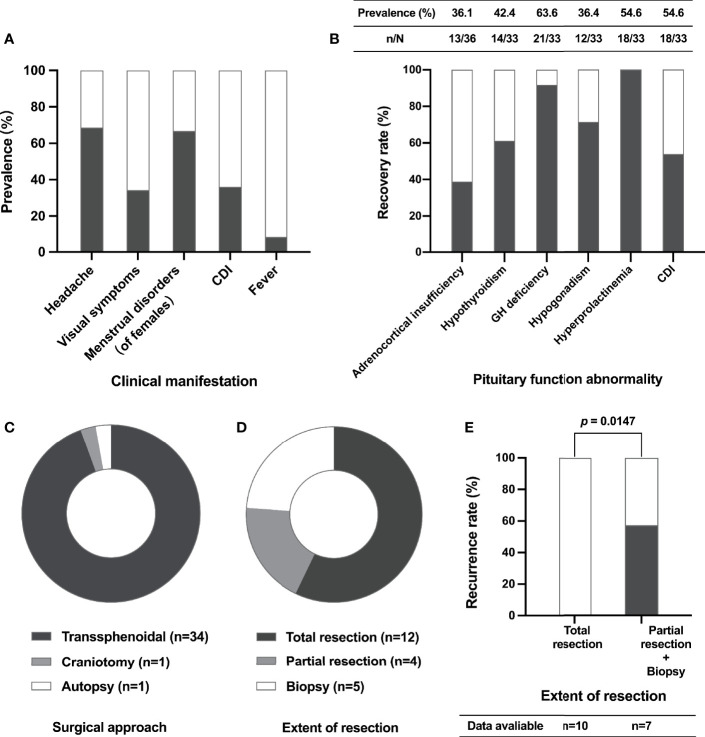
There were 36 cases of XHP, including 35 in previous literature (3–22) and one in our center (summarized in Table 2 ). The mean age at diagnosis was 39.1 years (range 12-72), and 27 (75.0%) patients were female. The most common symptoms were headache (68.6%), visual impairment (34.3%) and diabetes insipidus (DI) (36.1%). A total of 66.7% of female patients presented menstrual disorders, and three (8.3%) patients presented fever ( Figure 3A ). In addition, apoplectic events occurred in two patients (10, 13). Interestingly, five patients had autoimmune disease, including two who had autoimmune thyroiditis (12), one who had rheumatoid arthritis (13), one who had ulcerative colitis (9) and one who had both rheumatoid arthritis and Sjogren’s syndrome (16).
Table 2.
Review of 36 cases of xanthomatous hypophysitis.
| Case | Reference | Sex/Age (years) | MRI presentation | Gross appearance | Pathologic findings | IHC | Treatment | Recurrence | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Folkerth et al., 1998 (3) | F/30 | Cystic sellar mass | Fibrous capsule with “purulent” material | Foamy histiocytes and lymphocytes | CD68(+), CD1a(-), S-100(-) | TSS | N | 8 |
| 2 | F/30 | Sellar and suprasellar mass with ring enhancement | Soft lesion with white material | Foamy histiocytes and lymphocytes | CD68(+), CD1a(-), S-100(-) | TSS | N | 9 | |
| 3 | F/12 | Sellar mass with peripheral enhancement, PST | Cystic lesion containing pus-like fluid | Foamy histiocytes and lymphocytes | CD68(+), CD1a(-), S-100(-) | TSS+HRT | NA | NA | |
| 4 | Deodhare et al., 1999 (4) | F/43 | Intrasellar and suprasellar lesion, PST | Cystic lesion containing pus-like fluid | Foamy histiocytes, lymphocytes, and fibrosis | CD68(+), CD1a(-), S-100(-) | TSS+HRT | N | 2 |
| 5 | Cheung et al., 2001 (5) | F/32 | Cystic lesion, PST | Cyst with fibrous wall containing orange material | Foamy histiocytes, lymphocytes, hemosiderin and fibrosis | NA | Surgery | N | 24 |
| 6 | Tashiro et al., 2002 (6) | F/43 | NA | NA | Foamy histiocytes and lymphocytes | S-100(-) | Autopsy | — | — |
| 7 | F/72 | NA | NA | Foamy histiocytes and lymphocytes | S-100(-) | Surgery | NA | NA | |
| 8 | Burt et al., 2003 (7) | M/29 | Pituitary mass extending into suprasellar cistern with peripheral enhancement | Tense cystic lesion containing yellow fluid | Foamy histiocytes, lymphocytes, cholesterol clefts, and multinucleated giant cells | CD68(+), CD1a(-) | TSS+HRT | N | 18 |
| 9 | M/26 | Lobulated intrasellar and suprasellar mass | Greenish-tinged partially cystic thick-walled lesion containing thick brown fluid | Foamy histiocytes, lymphocytes, hemosiderin, cholesterol clefts, and fibrosis | CD68(+), CD1a(-) | TCS+HRT | N | 8 | |
| 10 | Gutenberg et al., 2006 (8) | 3F/ Average age: 29.0 ± 2.7 | Mild enhancing well-defined intrasellar mass, PST (1/3) | NA | NA | Surgery+HRT | NA | NA | |
| 11 | |||||||||
| 12 | |||||||||
| 13 | M/41 | ||||||||
| 14 | Aste et al., 2010 (9) | F/31 | Solid lesion with necrotic core and hemorrhagic spot, PST | Cystic lesion containing pus-like fluid | Foamy histiocytes, lymphocytes, cholesterol clefts, multinucleated giant cells | CD68(+) | TSS+HRT | N | 8 |
| 15 | Haas et al., 2012 (10) | M/57 | Cystic lesion with peripheral ring enhancement | Emergence of a thick yellow fluid | Foamy histiocytes and lymphocytes | CD68(+) | TSS+HRT | N | 6 |
| 16 | Niyazoglu et al., 2012 (11) | F/39 | Sella lesion with a necrotic/cystic core | NA | Foamy histiocytes | CD68(+), CD1a(-), S-100(-) | TSS+HRT | N | 3 |
| 17 | Joung et al., 2013 (12) | F/36 | Cystic lesion with peripheral enhancement, PST | Friable, encapsulated mass with moderate vascularity | Foamy histiocytes | CD68(+), CD1a(-), S-100(-) | TSS+HRT+High-dose glucocorticoid therapy | Y | 10 |
| 18 | Hanna et al., 2015 (14) | F/69 | Pituitary mass with peripheral enhancement, PST | Red-tan colored tissue; fibrous and with a firm rubbery texture, consisting of friable red tissue | Histiocytes and lymphoid infiltrate | CD68(+), CD1a(-), S-100(-) | TSS+HRT→TSS+HRT+RT | Y | 3 |
| 19 | Tang et al., 2015 (15) | F/33 | Solid sellar and suprasellar mass surrounding the left cavernous, PST | Solid lesion with rich blood supply and tough texture | Foamy histiocytes, lymphoplasmacytes and fibrosis | CD68(+), S-100(+), CD1a(-) | TSS | NA | NA |
| 20 | Gopal Kothandapani et al., 2015 (13) | F/14 | Pituitary mass with heterogeneous enhancement and central necrosis | NA | Necrosis, cholesterol clefts, fibrosis and hemosiderin | NA | TSS+HRT | N | 6 |
| 21 | F/21 | Pituitary lesion with suprasellar extension | NA | Necrosis, cholesterol clefts, fibrosis, multinucleated giant cells and hemosiderin | CD68(+) | TSS+HRT | N | 6 | |
| 22 | M/67 | Cystic pituitary mass | NA | NA | NA | TSS→TSS+High-dose steroids and methotrexate | Y | 12 | |
| 23 | Oishi et al., 2016 (16) | F/72 | Multicystic lesion extending toward the hypothalamus, PST | Soft yellowish lesion along with the pituitary stalk | Infiltrating foamy cells with lymphocytes | CD68(+), CD1a(-), S-100(-) | TSS+HRT | NA | NA |
| 24 | Duan et al., 2017 (17) | 5F, 2M/ Average age: 43.3 ± 18.3 | NA | NA | Foamy macrophages and multinucleated giant cells | NA | TSS | NA | NA |
| 25 | Suprasellar extension | Cystic component with thick yellowish fluid | |||||||
| 26 | Suprasellar extension, compression of the optic chiasm and extension into the left cavernous sinus | Cystic component | |||||||
| 27 | Suprasellar extension, PST | Cystic component with a thick yellowish fluid | |||||||
| 28 | Suprasellar extension, compression of the optic chiasm | Mucinous and speck-like | |||||||
| 29 | Suprasellar extension | Cystic component with yellowish mucous material | |||||||
| 30 | Suprasellar extension, compression of the optic chiasm | Hemorrhagic cystic component | |||||||
| 31 | Lin et al., 2017 (18) | F/41 | Sellar lesion with peripheral ring enhancement, PST | Friable yellow hypovascular mass | Foamy histiocytes and lymphocytes | CD68(+) | TSS | N | 15 |
| 32 | Singh et al., 2018 (19) | M/18 | Heterogenous enhancing mass, PST | Yellow mass with irregular necrotic tissue and thick, yellow fluid pus | Foamy macrophages | CD68(+) | TSS+HRT | NA | NA |
| 33 | Kini et al., 2019 (21) | F/55 | Sellar mass with suprasellar extension | NA | Foamy histiocytes and lymphoplasmacytic infiltrate with interspersed fibrosis and hyalinization | CD68(+) | TSS | NA | NA |
| 34 | Imga et al., 2019 (20) | M/27 | Sellar mass with heterogenous enhancement, PST | NA | NA | NA | TSS | N | 28 |
| 35 | Mathkour et al., 2020 (22) | F/45 | Cystic lesion with suprasellar extension | Mass with thick yellow colloidal material | Macrophagocytic infiltration of the adenohypophysis | CD68(+) | TSS→TSS | Y | 108 |
| 36 | Our case | F/36 | Pituitary mass with peripheral enhancement, PST | Tense cystic lesion with yellowish pus-like fluid | Inflammatory infiltration by foamy histiocytes and lymphocytes, with fibrosis, cyst and necrosis | CD68(+), CD1a(-), S-100(-) | TSS+HRT | N | 6 |
MRI, magnetic resonance imaging; IHC, immunohistochemistry; F, female; M, male; NA, not available; PST, pituitary stalk thickening; TSS, transsphenoidal surgery; TCS, transcranial surgery; HRT, hormone replacement therapy; RT, radiation therapy; N, no; Y, yes.
Figure 3.
Clinical characteristics, surgical aspects and recurrence of XHP cases. The prevalence of different clinical manifestations of XHP patients (A); the prevalence of pituitary function abnormalities before surgery (table) and the recovery rate of pituitary function after surgery (histogram) (B); the composition of different surgical approaches (C); the composition of different extents of lesion resection (D); the relationship between recurrence and extent of resection (E). CDI, central diabetes insipidus; GH, growth hormone.
Data on anterior pituitary function before surgery were available in 33 cases ( Figure 3B ). Hypogonadotropic hypogonadism, adrenocortical insufficiency, central hypothyroidism and growth hormone (GH) deficiency occurred in 63.6%, 54.6%, 54.6% and 36.4% of patients, respectively. In addition, 42.4% of patients had hyperprolactinemia.
The MRI presentation of patients with XHP is shown in Table 2 . Based on the available data, the MRI scan showed a cystic mass with ring or peripheral enhancement in almost all of these patients. The mean maximum diameter of these lesions was 16.3 ± 4.8 mm (data available in 20 patients). In addition, pituitary stalk thickening (PST) was shown in 50.0% of patients.
A total of 35 patients underwent surgery, including one who received craniotomy and 34 (97.14%) who received transsphenoidal surgery ( Figure 3C ). Based on the available data, total lesion resection was achieved in 57.14% of patients ( Figure 3D ). New-onset transient DI was recorded in 3 patients, and rhinorrhea appeared in one patient. Follow-up data were available in 17 patients. The median follow-up time was 8.0 (4.5, 16.5) months, ranging from 2.0 to 108.0 months. Notably, the recurrence rate of patients who underwent partial lesion resection and biopsy was significantly higher than that of patients who received total resection (57.1% vs. 0.0%, P = 0.0147) ( Figure 3E ). Of the four patients who developed recurrence after surgery, one received high-dose glucocorticoid therapy, and the mass showed reduction three months later (12); one patient suffered recurrence again after repeat surgery, and radiation therapy was given (14); one patient was treated with high-dose steroids, but the prognosis was unknown (13); and one patient underwent reoperation and achieved total tumor resection with no recurrence during the 9-year follow-up (22).
The most common pituitary hormone abnormality to recover is hyperprolactinemia (100.0%), followed by GH deficiency (91.7%), hypogonadism (71.4%) and hypothyroidism (61.1%) postoperatively. Recovery of adrenocortical insufficiency was only noted in 38.9% of patients. In addition, 53.8% of patients with CDI were cured through surgery ( Figure 3B ).
The pathological results were available for 30 cases. The descriptions of “foamy histocytes”, “lymphocytes”, “fibrosis”, “hemosiderin” and “necrosis” appeared in 20 (66.7%), 17 (56.7%), 8 (26.7%), 4 (13.3%), 4 (13.3%), and 3 (10.0%) cases, respectively. The immunohistochemical results were available for 26 cases. CD68 status results were reported in 19 cases, and all showed positivity. Of the 11 cases reporting CD1a results and 12 cases reporting S-100 results, 100.0% and 91.7% showed negativity, respectively ( Table 2 ).
Discussion
Based on the histopathologic features, primary hypophysitis can be divided into five categories: lymphocytic hypophysitis (LHP), granulomatous hypophysitis (GHP), IgG-4 hypophysitis (IgG4HP), XH and necrotizing hypophysitis (NHP) (1, 23). The first two are main forms, and the last three are rare variants (1). As the most common form of hypophysitis, LHP predominantly occurs in females, especially during pregnancy (2, 24). Approximately 20-50% of cases with LHP have been shown to have other autoimmune diseases (25). Similar to patients with XHP, headaches (48.0%) and visual defects (40.0%) are the most frequent complaints in patients with LHP, and 70.0% have anterior pituitary dysfunction (26). In addition, CDI occurs in up to 70.0% of cases with LHP (26) but is relatively uncommon (36.1%) in XHP. GHP is the second most common subtype of primary hypophysitis and shows a female predilection (23, 27). Headaches and visual changes present in 61.0% and 40.2% of cases with GHP, but CDI only occurs in 26.8% of these patients (27). Unlike other subtypes of primary hypophysitis, IgG4HP is usually found in the context of IgG4-related disease and mainly occurs in males and older patients (mean age: 64.2 years) (23, 28). A total of 52.4%, 26.2% and 17.9% of patients with IgG4HP have panhypopituitarism, anterior hypopituitarism and CDI, respectively (28). In addition, NHP is the rarest subtype of primary hypophysitis and is thought to be a severe form of one of the other subtypes of hypophysitis (23). Because XHP has similar clinical presentations to other subtypes of hypophysitis, it is difficult to differentiate these disease types by symptoms alone.
Interestingly, five patients with XHP had other autoimmune diseases. Thus, there are some views that an autoimmune phenomenon may participate in the pathogenesis, and XHP is thought to be part of a systemic autoimmune condition (12, 13). In addition, XHP may develop as a local reaction to hemorrhage, necrosis and inflammation, but the pathogenesis remains unclear (13, 23).
MRI is an important tool in the diagnosis of pituitary diseases that can provide some clues for differential diagnosis. A finding in our study was that PST occurred in half of cases with GHP. PST can be seen in a variety of diseases (29, 30). A retrospective study that summarized 321 patients with PST in our center, showed that the etiologies of PST included neoplastic (75.2%), inflammatory (13.1%), and congenital diseases (11.7%) (30). It is not difficult to identify benign tumors such as craniopharyngioma, pituitary adenoma and meningioma and Rathke cleft cyst by MRI because these diseases usually have typical imaging features (31). And age, medical history, and laboratory examination help identify germ cell tumors and metastatic tumors (32). Besides, histiocytosis such as Langerhans cell histiocytosis (LCH) and Erdheim-Chester disease (ECD) can also affect the pituitary stalk, and diabetes insipidus is found in a quarter of patients with LCH and ECD (33, 34). However, both LCH and ECD can affect multiple systems (33, 34) and long-bone involvement is considered to be a hallmark of ECD (34). Therefore, multi-organ radiological screening and appropriate histology are sufficient for differential diagnosis (33, 34).
In our study, we found that most XHPs manifest as cystic sellar lesions with peripheral enhancement on MRI. However, this performance is also seen in both LHP and NHP (24). Thus, the differential diagnosis of hypophysitis by radiological examination is still a challenge. In this situation, biopsy seems to be unavoidable to make a definitive diagnosis. As one of the largest pituitary centers in China, we have performed hundreds of biopsy procedures on patients with PST or CDI in the last few years (30, 32). According to our experience, transsphenoidal biopsy is a safe and effective way to diagnose these indistinguishable sellar lesions (32). Therefore, for sellar lesions that are difficult to identify by clinical manifestations, laboratory examinations and radiological features, biopsy surgery should be considered, especially in experienced pituitary centers. According to previous descriptions in the literature, the typical gross presentation of XHP is a cystic lesion with yellowish pus-like fluid ( Table 2 ). In addition, based on our intraoperative findings, the lesion had a thick wall, and the texture was tough.
The common feature of primary hypophysitis is inflammatory infiltration of lymphocytes and plasma cells into the pituitary gland, but the individual features among different subtypes differ except LHP (1). The diagnosis of GHP can be confirmed by the presence of multinucleated giant cells, and IgG4HP is characterized by IgG4-positive plasma cells (1, 2, 23). The typical pathological feature of XHP is foamy histiocyte infiltration, which can be marked by CD68 glycoprotein on immunohistochemistry ( Table 2 ). Since Langerhans cells stain positive for CD1a, S-100 and CD207 (33), the absence of CD1a and S-100 helps to rule out LCH. Thus, histopathology remains the gold-standard diagnostic criterion for different forms of primary hypophysitis and histiocytosis.
Unlike LHP, which usually presents as a self-resolving process and has a good response to glucocorticoid therapy (35), clinical experience in the treatment of XHP is extremely limited. Although Joung et al. (12) reported a patient with recurrent XHP whose mass decreased after high-dose glucocorticoid therapy, and another patient showed less response to steroid therapy (14). In addition, tentative bromocriptine therapy failed to reduce the volume of the mass (5). Our study found that the recurrence rate was significantly lower in patients who underwent total lesion resection than in those who underwent partial resection or biopsy. In addition to the adrenocortical axis, anterior pituitary function can improve obviously, and more than half of patients with CDI can be cured through surgery. Therefore, surgical resection should be considered, especially for patients suffering from mass effects and pituitary dysfunction, and total mass resection is recommended. In addition, close follow-up and routine assessment of pituitary function are essential, and hormone replacement therapy should be given postoperatively when necessary (2, 23). Nevertheless, there were still two problems in the treatment of XHP. On the one hand, the lack of follow-up data does not allow for an accurate assessment of the long-term recurrence of XHP after surgery, resulting in a lack of best evidence for the treatment. On the other hand, the gross appearance of XHP—as well as the tough texture of the mass— is unfamiliar to surgeons, which leads some patients to undergo only partial resection or biopsy. Therefore, we have summarized the characteristics of XHP to increase surgeons’ awareness of this rare disease.
There were some limitations in our study. First, due to the rarity of XHP, selection bias was intrinsic to this investigation. Second, the majority of data were extracted from descriptions in previous literature, and this may have influenced the accuracy and integrity of the results. Third, due to the heterogeneity of surgical techniques in different centers and the incompleteness of follow-up data, the optimal treatment and long-term prognosis for XHP is still unclear. Therefore, the establishment of a database of rare diseases and close follow-up should be emphasized to provide evidence for guiding the diagnosis and treatment of these rare diseases.
Conclusion
XHP is an extremely rare form of primary hypophysitis. Due to the similarity of its clinical manifestations and radiological findings to those of other forms of primary hypophysitis, the diagnosis and differential diagnosis of XHP are difficult to reach without a pathological examination. According to our study, pituitary dysfunction can be improved through surgery, and patients who undergo total lesion resection have a relatively low recurrence rate. Thus, surgery is both a diagnostic and a therapeutic method. However, because of the limited data, the real prognosis of this rare disease is still unclear.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
YY, HZ, and JZ: conception and design. JZ, ZW, and YZ: development of methodology. JZ, ZW, XL, JL, and SJ: acquisition and analysis of data. JZ, ZW, and YY: writing, review, and revision of manuscript. WW and JF: technical and material support. YY, HZ, LD, and KD: study supervision. All authors have read and approved the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Chinese Academy of Medical Sciences Innovation Fund (CAMS-2016-I2M-1-002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Fukuoka H. Hypophysitis. Endocrinol Metab Clin North Am (2015) 44(1):143–9. doi: 10.1016/j.ecl.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 2. Joshi MN, Whitelaw BC, Carroll PV. Mechanisms in Endocrinology: Hypophysitis: Diagnosis and Treatment. Eur J Endocrinol (2018) 179(3):R151–r163. doi: 10.1530/eje-17-0009 [DOI] [PubMed] [Google Scholar]
- 3. Folkerth RD, Price DL, Jr., Schwartz M, Black PM, De Girolami U. Xanthomatous Hypophysitis. Am J Surg Pathol (1998) 22(6):736–41. doi: 10.1097/00000478-199806000-00011 [DOI] [PubMed] [Google Scholar]
- 4. Deodhare SS, Bilbao JM, Kovacs K, Horvath E, Nomikos P, Buchfelder M, et al. Xanthomatous Hypophysitis: A Novel Entity of Obscure Etiology. Endocr Pathol (1999) 10(3):237–41. doi: 10.1007/bf02738885 [DOI] [PubMed] [Google Scholar]
- 5. Cheung CC, Ezzat S, Smyth HS, Asa SL. The Spectrum and Significance of Primary Hypophysitis. J Clin Endocrinol Metab (2001) 86(3):1048–53. doi: 10.1210/jcem.86.3.7265 [DOI] [PubMed] [Google Scholar]
- 6. Tashiro T, Sano T, Xu B, Wakatsuki S, Kagawa N, Nishioka H, et al. Spectrum of Different Types of Hypophysitis: A Clinicopathologic Study of Hypophysitis in 31 Cases. Endocr Pathol (2002) 13(3):183–95. doi: 10.1385/ep:13:3:183 [DOI] [PubMed] [Google Scholar]
- 7. Burt MG, Morey AL, Turner JJ, Pell M, Sheehy JP, Ho KK. Xanthomatous Pituitary Lesions: A Report of Two Cases and Review of the Literature. Pituitary (2003) 6(3):161–8. doi: 10.1023/b:pitu.0000011177.43408.56 [DOI] [PubMed] [Google Scholar]
- 8. Gutenberg A, Hans V, Puchner MJ, Kreutzer J, Brück W, Caturegli P, et al. Primary Hypophysitis: Clinical-Pathological Correlations. Eur J Endocrinol (2006) 155(1):101–7. doi: 10.1530/eje.1.02183 [DOI] [PubMed] [Google Scholar]
- 9. Aste L, Bellinzona M, Meleddu V, Farci G, Manieli C, Godano U. Xanthomatous Hypophysitis Mimicking a Pituitary Adenoma: Case Report and Review of the Literature. J Oncol (2010) 2010:1–5. doi: 10.1155/2010/195323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haas AV, Smith TW. Xanthomatous Hypophysitis: An Unusual Case of a Sellar Mass. Neurological Bulletin. (2012) 4(1):41–4. doi: 10.7191/neurol_bull.2012.1034 [DOI] [Google Scholar]
- 11. Niyazoglu M, Celik O, Bakkaloglu DV, Oz B, Tanriöver N, Gazioglu N, et al. Xanthomatous Hypophysitis. J Clin Neurosci (2012) 19(12):1742–4. doi: 10.1016/j.jocn.2011.08.041 [DOI] [PubMed] [Google Scholar]
- 12. Joung JY, Jeong H, Cho YY, Huh K, Suh YL, Kim KW, et al. Steroid Responsive Xanthomatous Hypophysitis Associated With Autoimmune Thyroiditis: A Case Report. Endocrinol Metab (Seoul). (2013) 28(1):65–9. doi: 10.3803/EnM.2013.28.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gopal-Kothandapani JS, Bagga V, Wharton SB, Connolly DJ, Sinha S, Dimitri PJ. Xanthogranulomatous Hypophysitis: A Rare and Often Mistaken Pituitary Lesion. Endocrinol Diabetes Metab Case Rep (2015) 25(1):1–9. doi: 10.1530/edm-14-0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanna B, Li YM, Beutler T, Goyal P, Hall WA. Xanthomatous Hypophysitis. J Clin Neurosci (2015) 22(7):1091–7. doi: 10.1016/j.jocn.2015.01.019 [DOI] [PubMed] [Google Scholar]
- 15. Tang F, Liu H, Zhou S, Liu J, Xiao E, Tan C. MRI Manifestation of Xanthomatous Hypophysitis: A Case Report and Review of the Literature. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2015) 40(2):228–32. doi: 10.11817/j.issn.1672-7347.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 16. Oishi M, Hayashi Y, Fukui I, Kita D, Miyamori T, Nakada M. Xanthomatous Hypophysitis Associated With Autoimmune Disease in an Elderly Patient: A Rare Case Report. Surg Neurol Int (2016) 7(Suppl 16):S449–53. doi: 10.4103/2152-7806.185773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duan K, Asa SL, Winer D, Gelareh Z, Gentili F, Mete O. Xanthomatous Hypophysitis Is Associated With Ruptured Rathke’s Cleft Cyst. Endocr Pathol (2017) 28(1):83–90. doi: 10.1007/s12022-017-9471-x [DOI] [PubMed] [Google Scholar]
- 18. Lin W, Gao L, Guo X, Wang W, Xing B. Xanthomatous Hypophysitis Presenting With Diabetes Insipidus Completely Cured Through Transsphenoidal Surgery: Case Report and Literature Review. World Neurosurg. (2017) 1041051:e7–1051.e13. doi: 10.1016/j.wneu.2017.05.156 [DOI] [PubMed] [Google Scholar]
- 19. Singh K, Kanodia AK, Ross P, Torgersen A, Maclean J, Leese G, et al. Xanthomatous Hypophysitis Causing Hypogonadotropic Hypogonadism Resulting in Delayed Presentation of Slipped Capital Femoral Epiphysis. Br J Neurosurg. (2018) 19:1–4. doi: 10.1080/02688697.2018.1525482 [DOI] [PubMed] [Google Scholar]
- 20. Imga NN, Yildirim AE, Baser OO, Berker D. Clinical and Hormonal Characteristics of Patients With Different Types of Hypophysitis: A Single-Center Experience. Arch Endocrinol Metab (2019) 63(1):47–52. doi: 10.20945/2359-3997000000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kini H, Rao R, Pai M. Xanthomatous Hypophysitis: A Rare Case Report With Review of Literature. Indian J Pathol Microbiol (2019) 62(3):448–50. doi: 10.4103/ijpm.Ijpm_319_18 [DOI] [PubMed] [Google Scholar]
- 22. Mathkour M, Zeoli T, Werner C, Scullen T, Garces J, Keen J, et al. Recurring Primary Xanthomatous Hypophysitis Behaving Like Pituitary Adenoma: Additional Case and Literature Review. World Neurosurg (2020) 138:27–34. doi: 10.1016/j.wneu.2020.02.055 [DOI] [PubMed] [Google Scholar]
- 23. Gubbi S, Hannah-Shmouni F, Verbalis JG, Koch CA. Hypophysitis: An Update on the Novel Forms, Diagnosis and Management of Disorders of Pituitary Inflammation. Best Pract Res Clin Endocrinol Metab (2019) 33(6):101371. doi: 10.1016/j.beem.2019.101371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caranci F, Leone G, Ponsiglione A, Muto M, Tortora F, Muto M, et al. Imaging Findings in Hypophysitis: A Review. Radiol Med (2020) 125(3):319–28. doi: 10.1007/s11547-019-01120-x [DOI] [PubMed] [Google Scholar]
- 25. Naran J, Can AS. Lymphocytic Hypophysitis. In: StatPearls. Treasure Island (FL: StatPearls Publishing LLC; (2021). StatPearls Publishing Copyright © 2021. [Google Scholar]
- 26. Wang S, Wang L, Yao Y, Feng F, Yang H, Liang Z, et al. Primary Lymphocytic Hypophysitis: Clinical Characteristics and Treatment of 50 Cases in a Single Centre in China Over 18 Years. Clin Endocrinol (Oxf) (2017) 87(2):177–84. doi: 10.1111/cen.13354 [DOI] [PubMed] [Google Scholar]
- 27. Hunn BH, Martin WG, Simpson S, Jr., McLean CA. Idiopathic Granulomatous Hypophysitis: A Systematic Review of 82 Cases in the Literature. Pituitary (2014) 17(4):357–65. doi: 10.1007/s11102-013-0510-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shikuma J, Kan K, Ito R, Hara K, Sakai H, Miwa T, et al. Critical Review of IgG4-Related Hypophysitis. Pituitary (2017) 20(2):282–91. doi: 10.1007/s11102-016-0773-7 [DOI] [PubMed] [Google Scholar]
- 29. Salvatori R. The Differential Diagnosis of Pituitary Stalk Thickening. Endocr Pract (2019) 25(6):616–8. doi: 10.4158/ep-2019-0070 [DOI] [PubMed] [Google Scholar]
- 30. Zhou X, Zhu H, Yao Y, Lian X, Feng F, Wang L, et al. Etiological Spectrum and Pattern of Change in Pituitary Stalk Thickening: Experience in 321 Patients. J Clin Endocrinol Metab (2019) 104(8):3419–27. doi: 10.1210/jc.2018-02297 [DOI] [PubMed] [Google Scholar]
- 31. Adams NC, Farrell TP, O’Shea A, O’Hare A, Thornton J, Power S, et al. Neuroimaging of Central Diabetes Insipidus-When, How and Findings. Neuroradiology (2018) 60(10):995–1012. doi: 10.1007/s00234-018-2072-7 [DOI] [PubMed] [Google Scholar]
- 32. Wang Z, Zhu J, Yao Y, Zhu H, Deng K, Lu L, et al. Clinical and Pathological Features of 124 Patients With Indistinguishable Sellar Lesions and Central Diabetes Insipidus. J Clin Neurosci (2020) 80:215–22. doi: 10.1016/j.jocn.2020.08.001 [DOI] [PubMed] [Google Scholar]
- 33. Monsereenusorn C, Rodriguez-Galindo C. Clinical Characteristics and Treatment of Langerhans Cell Histiocytosis. Hematol Oncol Clin North Am (2015) 29(5):853–73. doi: 10.1016/j.hoc.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 34. Haroche J, Cohen-Aubart F, Amoura Z. Erdheim-Chester Disease. Blood (2020) 135(16):1311–8. doi: 10.1182/blood.2019002766 [DOI] [PubMed] [Google Scholar]
- 35. Gubbi S, Hannah-Shmouni F, Stratakis CA, Koch CA. Primary Hypophysitis and Other Autoimmune Disorders of the Sellar and Suprasellar Regions. Rev Endocr Metab Disord (2018) 19(4):335–47. doi: 10.1007/s11154-018-9480-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.