ABSTRACT
A rebound of osteoclast activity during the 2 years after a treatment or prevention of osteoporosis with denosumab (Dmab) leads to an increased risk of vertebral fractures (VFs). We attempted to identify the risk factors for these VF and to examine the protective role of bisphosphonates. For that, 22 specialists in Switzerland provided data of unselected patients, treated with denosumab for osteoporosis or breast cancer without metastases under aromatase inhibitors, who have received at least two injections of Dmab, with at least 1 year of follow‐up after discontinuation. The questionnaire covered separately the periods before, during, and after Dmab treatment, and registered clinical, radiological, and lab data. For the analysis of the risk factors, the main outcomes were the time to the first VF after the treatment, the presence of multiple VFs (MVFs), and the number of VFs. The incidence of VF was 16.4% before, 2.2% during, and 10.3% after the treatment with Dmab. The risk of VF after Dmab discontinuation was associated with an increased risk of non‐vertebral fractures. The pretreatment predictors of the post‐treatment fracture risk were a parental hip fracture and previous VFs. Further risk factors appeared later, such as low total hip bone mineral density (BMD) during and after denosumab, increased bone resorption markers, and the loss of total hip BMD after the denosumab. Treatment with bisphosphonates, especially after Dmab, had a protective effect. Bisphosphonates given before Dmab did not further decrease the risk of VF in cases who got bisphosphonates after Dmab. This study shows that the risk of VF is poorly predictable before the prescription of denosumab. But during and after the treatment, bone resorption markers and BMD have a significant predictive value. Bisphosphonates after the treatment with denosumab are protective against VFs. © 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: OSTEOPOROSIS, FRACTURE RISK ASSESSMENT, STATISTICAL METHODS, ANTIRESORPTIVES, DENOSUMAB
Introduction
Denosumab discontinuation results in a rebound activity of osteoclasts, characterized by an increase in bone turnover markers (BTMs) and a resulting loss of bone mineral density (BMD). Approximately 6 months after the last denosumab injection, BTMs increase rapidly, exceeding baseline values and remaining high before returning to baseline values approximately 30 months after the last injection. BMD gains are lost accordingly with a return to pretreatment baseline values, sometimes incompletely, sometimes even below, 1 to 2 years after stopping denosumab.( 1 , 2 , 3 , 4 , 5 ) The reversibility of this rebound effect was confirmed by the re‐increase of BMD when denosumab was given again,( 1 ) and by a histomorphometric study showing that after 2 years, women who discontinued denosumab had similar levels of bone remodeling compared with untreated postmenopausal women.( 6 )
This rebound effect is associated with an increased risk of fracture. Initially, a post hoc analysis of the FREEDOM phase 3 trial reported a comparable incidence of the rate of vertebral fractures (VFs) in women who discontinued denosumab or placebo.( 7 ) However, the observation time was rather short with a median duration of 14 months after the last denosumab injection.
In 2016 reports appeared in the literature of several cases with multiple VFs (MVFs) after denosumab discontinuation in Switzerland and in Greece.( 5 , 8 , 9 , 10 ) Since 2017, multiple case series from Switzerland, Greece, Spain, and Israel confirmed the risk of MVFs after denosumab discontinuation.( 11 , 12 , 13 , 14 , 15 , 16 ) An observational study of 84 women showed that 11.9% of them had at least one VF, and 7.1% of them at least two FVs, within 18 months after the last denosumab injection.( 3 ) In another observational study of 38 women with the same follow‐up duration, the risk was 10.5% for at least one VF and 2.6% for MVFs.( 4 ) In the thorough analysis of the FREEDOM and FREEDOM Extension trials, 1001 patients discontinuing denosumab or placebo were followed for 9 to 12 months after the last denosumab injection.( 17 ) The estimated annualized risk of VFs after discontinuation was similar in the denosumab and the placebo groups, 7.1% and 8.5%, respectively. Yet, a significantly higher proportion of patients suffered MVFs after denosumab (60.7%) than after placebo (38.7%) discontinuation. In a review of publications including 70 women with MVFs after denosumab discontinuation, the median number of VFs was 5, occurring 7 to 20 months (median 11) after the last denosumab injection.( 18 ) The true incidence of MVFs after denosumab discontinuation is not known because of the types of studies published, the short follow‐up duration,( 3 , 4 , 17 ) and the fact that some of the patients were treated previously with bisphosphonates and/or received an osteoporosis treatment after denosumab discontinuation.( 3 , 4 , 17 , 18 ) Almost all of the fractures described after denosumab discontinuation are VFs. But recently, hip fractures have been reported as well.( 19 )
The question, whether specific factors characterize the patients who presented vertebral fractures after treatment with denosumab, was discussed in several publications. Previous VFs, a high number of denosumab injections, a smaller gain in hip BMD during treatment, and greater loss of hip BMD after denosumab discontinuation were identified as risk factors for VFs after denosumab treatment.( 9 , 11 , 17 ) Vertebroplasty seem to increase the risk for further VFs shortly after the procedure.( 9 , 13 , 14 , 15 , 16 ) Prescribing a bisphosphonate before starting denosumab was recommended for reducing the risk of VFs after denosumab discontinuation, but this remains controversial.( 13 , 20 , 21 )
It was also proposed that denosumab should not be discontinued without consideration of other antiresorptive therapy.( 18 , 22 ) In this context, bisphosphonates are most often recommended. However, cases of MVFs have been reported in patients who received ibandronate or alendronate after stopping denosumab.( 21 , 23 )
Predisposing as well as protective factors for VFs after denosumab discontinuation are not well known. Because their elucidation requires a large number of patients, we decided to conduct a national survey to try to fill this knowledge gap and to devise preventive measures.
Materials and Methods
Selection and survey of patients
For this retrospective chart‐review study, we attempted to contact all physicians in Switzerland specialized in bone diseases and osteoporosis. We identified 39 specialists, among whom 22 of the three linguistic regions of Switzerland agreed to participate. The five university hospitals and most of the major centers participated. The inclusion criteria were: (i) postmenopausal women with osteoporosis or women with breast cancer without metastases undergoing adjuvant therapy with aromatase inhibitors, (ii) who received at least two injections of denosumab, and (iii) who ended denosumab therapy with at least 1 year of follow‐up after the last dose. The exclusion criteria were treatment with corticosteroids for more than 3 months, cancer disease with metastases, existing documents stating that the patient declined participation in observational studies, and insufficient data collected.
Our questionnaire was divided in three parts covering three periods: the first period (before the first denosumab injection); the second one (during denosumab treatment, starting with the first injection of denosumab and ending 6 months after the last injection); and the third one (after, starting 6 months and ending 30 months after the last injection of denosumab). The questionnaire addressed the following information for all three periods: age, risk factors for osteoporosis, vertebral and nonvertebral fractures, BMD and trabecular bone score (TBS), markers of bone turnover (BTMs), and treatments for osteoporosis with dates of beginning and end. Women receiving a bisphosphonate in the rebound period only after the occurrence of a VF were classified as not receiving a bisphosphonate after denosumab discontinuation.
The treatment with denosumab was described by the dates of beginning and end, the number of injections, and the reasons for interruption.
The primary objectives of this survey were: (i) to describe the incidence of VFs, of multiple fractures (MVFs), and the number of vertebral fractures (NVF) after denosumab discontinuation; and (ii) to identify associated risk/protective factors (collected during each of the three periods) for VFs, MVFs, and NVFs. The secondary objective was to describe the evolution of BMD, TBS, and BTMs during the three periods.
In some centers part of the data were collected by two medical students who had access to the records of patients, which were selected by the physicians. Each physician was encouraged to include all patients corresponding to the inclusion criteria but was free to choose all or a subset of the patients of his or her practice.
Data management and security
The data were collected on an online questionnaire and stored on a SPHINX server by the ESOPE (Enquêtes de Satisfaction et d'Opinion des Patients et Employés) team, specialized in carrying out surveys in various fields within the Center for Primary Care and Public Health, University of Lausanne, Switzerland (Unisanté). The SPHINX server is located at the University Hospital, Lausanne, Switzerland CHUV and meets the ISO 27005 and ISO 27001 standards. It is only accessible by the ESOPE team, which meets the ISO 9001:2015 certification. After the data collection, the ESOPE team produced an export in CSV, then encrypted the unique IDs with a dedicated Python script. Once the data set was de‐identified, it was securely (Opentrust MFT) transferred to the main researcher for analysis.
Markers of bone resorption
The values of the bone turnover markers were recorded for each of the three periods. The results were given in absolute values, and the upper limit of the norm for premenopausal women was indicated. Ninety percent of bone resorption markers were measured as beta C‐terminal telopeptides; the remaining 10% were deoxypyridinoline cross‐links, amino‐terminal telopeptides, and exceptionally hydroxy‐proline excretion. Because reference values may differ between markers and laboratories, we expressed all the results in percent of the upper premenopausal normal limit.
Bone mineral density
BMD was measured at lumbar spine, total hip and femoral neck by dual‐energy X‐ray absorptiometry (DXA). The study recorded a first DXA performed during the 12 months before or the first 3 months after the first denosumab injection. The second DXA was recorded before the end of treatment with denosumab, and the third DXA was recorded between 18 and 36 months after the last denosumab injection. These examinations were performed on different DXA machines (70% Hologic [Marlborough, MA, USA], 30% Lunar [GE Healthcare Lunar, Madison, WI, USA]). To pool the results, all BMD values were expressed as T‐scores, and the BMD changes were expressed in percent of T‐scores. This corresponds to standard practice in the literature (eg, references( 1 , 2 , 3 , 6 , 7 , 13 , 17 , 24 , 25 , 26 )).
Fractures
The occurrences of VFs, fractures of femoral neck, pelvis, humerus, wrist, and at least three ribs were recorded during each of the three study periods. The diagnosis of VF was determined on the basis of radiographic evidence by standard X‐ray, MRI, or vertebral morphometry obtained by DXA. VFs on DXA were defined according to the semiquantitative method developed by Genant( 27 ) as a decrease of at least 20% in vertebral height. We used the term “multiple vertebral fractures” (MVFs) in the presence of 2 or more VFs. Hip fractures include fractures of the femoral neck and intertrochanteric fractures.
Funding and ethical considerations
The principal investigator, PB, received an unrestricted grant from Amgen. The total amount of the grant was used to pay: (i) the Biostatistics Consulting Unit of Unisanté (Lausanne, Switzerland) for the database and the statistical analysis; (ii) two medical students who collected part of the data; (iii) a fixed amount to participating physicians or students for each patient included; (iv) cost for the statistical support and administrative costs.
The study was approved by the Research Ethics Committee of the Canton de Vaud (protocol number 2018‐00978).
Statistical methods
Data analysis was performed using the Stata Software (version 15, 2017, Stata Corp, College Station, TX, USA). Data summary quality (unusual values, consistency, etc.) and completeness (missing values) were checked using individual plots (box plot, qq‐plot, histograms). Continuous variables were summarized as mean (SD) and categorical variables as numbers (percent). The rebound effect after stopping treatment was evaluated using three main outcomes: (i) the time (months) to the first VF, (ii) presence of MVFS (yes, no), and (iii) the number of VFs (NVFs; counts). Cox proportional regression, logistic regression, and negative binomial regression were used, respectively, to assess the association between risk factors (age, body mass index [BMI], weight, previous fractures, bisphosphonate prescription, resorption markers, etc.) and the outcomes, using univariable and multivariable analyses. The strength of associations for the three models was measured, respectively, using the hazards ratio (HR), the odds ratio (OR), and the incidence rates ratio (IRR), and their significance was assessed by the corresponding p value. Potential interactions were tested and the form of relationships between continuous variables and outcomes was tested using fractional polynomial models (plausibility of the linearity assumption). Significant predictors (p value <0.2) as well as potential confounders from univariable analyses were included into a backward selection procedure to fit a multivariate model. Standard diagnostic tools were used to check the goodness of fit for each fitted model.
The evolution of BMD, TBS, and BTMs during the three periods were analyzed using a linear mixed‐effects model.
Analysis included only patients for which there are no missing data on the variables of interest (complete case analysis).
Results
The questionnaires were collected from January 17 to November 15, 2019, when the number of patients exceeded 800 and the return became slow. Of the 861 returned questionnaires, 64 had to be excluded because of incomplete data. Thus, 797 female patients were included: Their mean age was 65.3 years (SD = 9.2), mean weight 60.2 kg (SD = 10.7), and mean BMI 23.1 kg/m2 (SD = 4.0); 134 had breast cancer.
The BMD was below −2.5 T‐score at the lumbar spine in 63% and at the femoral neck in 31%.
The average number of denosumab injections given was 5.9 (range 2 to 20) per patient, the average duration of the treatment was 35 months (5 to 120), and the average follow‐up after denosumab discontinuation was 27.5 months (SD = 15.5).
The decision to discontinue the denosumab treatment was taken more often by the physician (61.5%) than by the patient (35.4%) or the dentist (4.3%). The reasons most often invoked were a sufficient increase in BMD (31.9%), the occurrence of side effects (10.0%, one‐third corresponding to musculoskeletal pain), the completion of a course of aromatase inhibitors (6.1% essentially in patients with breast cancer), unsatisfactory therapeutic effectiveness (4.9%), or low compliance (2.4%).
BMD lumbar spine T‐score increased significantly by 26% in average (p < 0.001) during denosumab treatment (measured 36 months on average after the first injection) and decreased significantly (p < 0.001) by 12.4% thereafter (measured 20 months on average after the last denosumab injection). The corresponding changes of BMD T‐score were +11.9% and –6.3% at the femoral neck, and +17.3% and −12.4% at the total hip (p for all <0.001). TBS improved significantly by 3.6% (p < 0.001) during denosumab and did not decline after discontinuation. When only the results from patients who had TBS measurements in all three periods were included (n = 95), the results were very similar (TBS‐1 = 1.221; TBS‐2 = 1.271; TBS‐3 = 1.287; increase by 4.1% [p < 0.0001] during denosumab). TBS in period 3 was not significantly different from TBS in period 2 (p = 0.075) (Fig. 1).
Fig. 1.
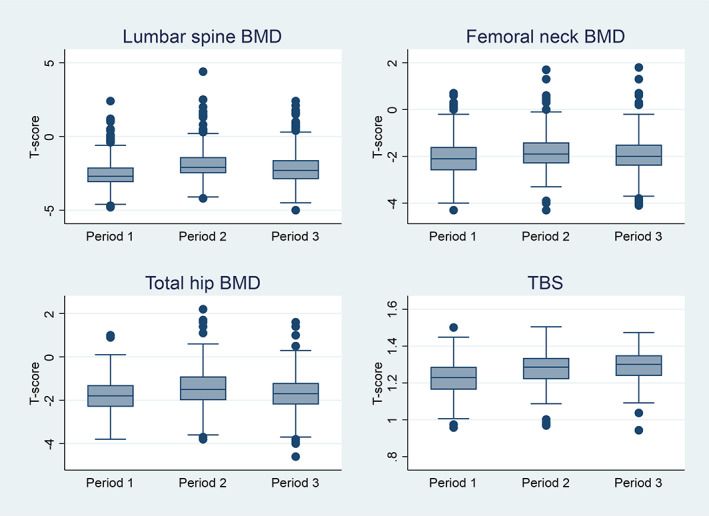
Evolution of bone mineral density (BMD) and trabecular bone score (TBS).
Vertebral fractures
Vertebral fractures were identified by their clinical symptoms and confirmed by MRI in 70% and by X‐rays in 29%. In 1 case, the VF was discovered on an X‐ray.
Before denosumab treatment, 131 women (16.4%) had 243 VFs. During denosumab treatment (over an average of 35 months), 18 women (2.2%, 0.75%/year) had 31 VFs. After the denosumab discontinuation, between 6 and 30 months after the last injection, 82 patients (10.3%, 4.1%/year) presented one or more VFs, altogether 215 VFs, ie, an average of 2.6 per patient (1 to 5) (Figs. 2 and 3). Among fractured patients, 69.5% had MVFs. The first VF occurred on average 13 months (median 12.0) after the last denosumab injection, and 75% of VFs occurred between 6 and 15 months after the last denosumab injection. The fractures concerned all vertebrae, from D1 to L5, with the same distribution before initiation and after discontinuation of denosumab. D12 and L1 were the vertebrae most often fractured before and after denosumab (Fig. 4). The patients with breast cancer did not present significantly more VFs after denosumab discontinuation than the patients who had no breast cancer.
Fig. 2.
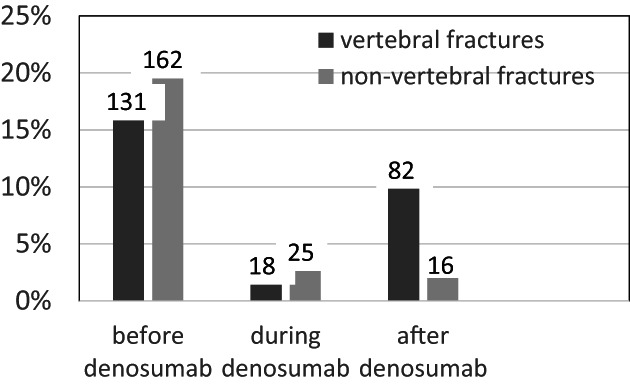
Incidence of vertebral and non‐vertebral fractures in % (and number of cases), reported in the total group of 797 patients.
Fig. 3.
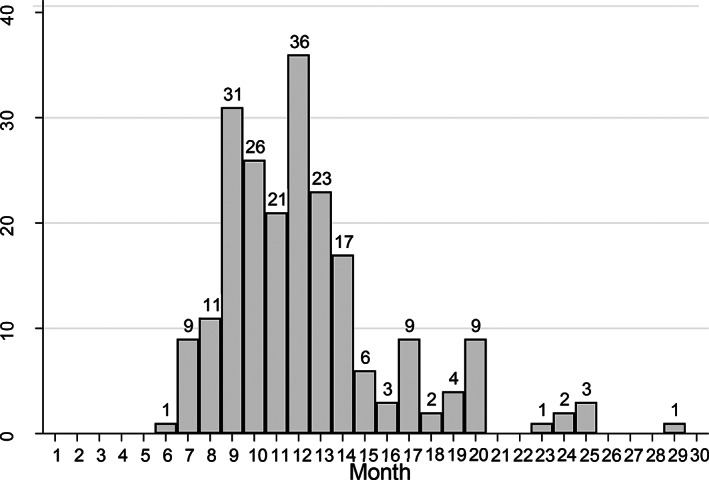
Incidence of vertebral fractures (VFs) 6 to 30 months after the last injection of denosumab (215 VFs in 82 patients).
Fig. 4.
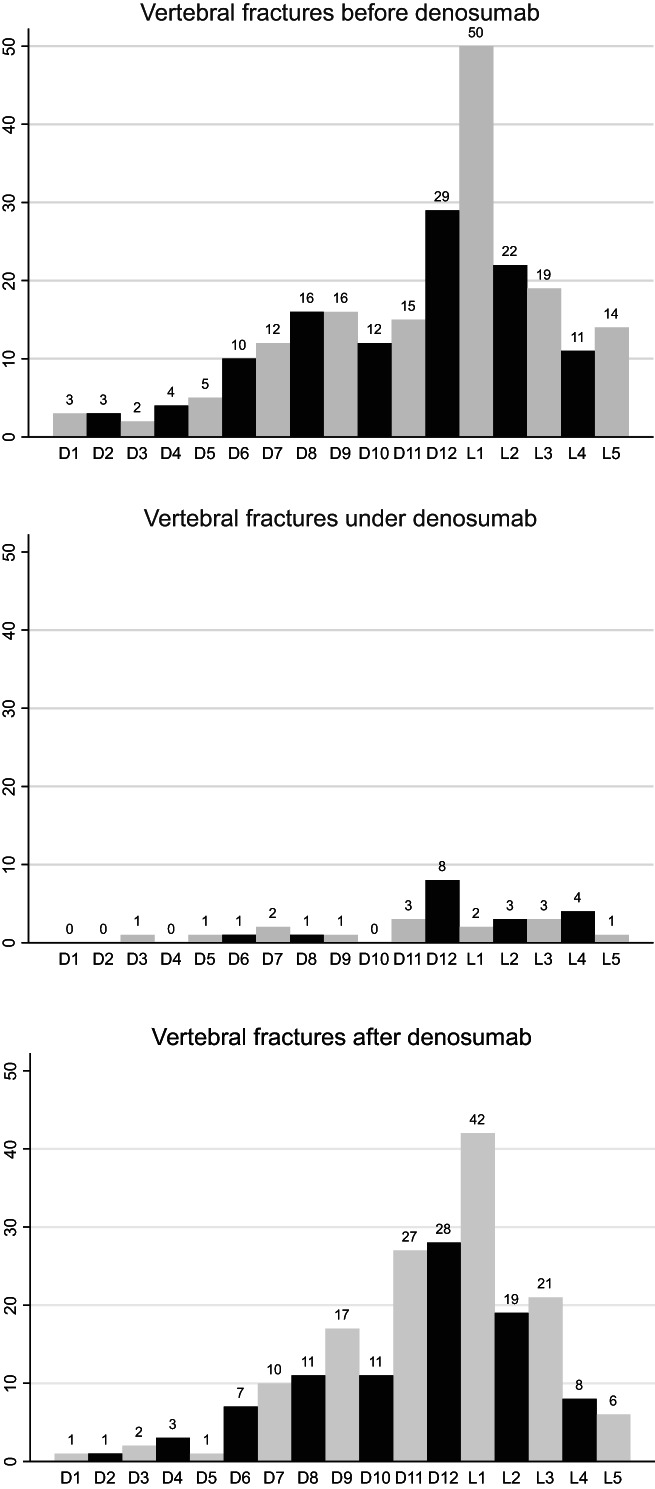
Distribution of vertebral fractures over the vertebral column before, during, and after denosumab treatment.
Non‐vertebral fractures
Non‐vertebral fractures occurred before the treatment with denosumab in 162 patients (20.3%), during the treatment in 25 patients (3.1%), and after the treatment in 16 patients (2.0%) (Fig. 2).
Risk factors
In search of factors that influence significantly the risk of VF in the rebound period, the 82 patients with VFs within 30 months after denosumab discontinuation were compared with the 715 patients who did not have VFs during this period by Cox proportional hazards model.
All data were tested in univariate and multivariate analyses, and results are summarized in Tables 1, 2, and 3. Patients with VF did not differ from patients without VF after denosumab discontinuation in terms of age, alcohol consumption, tobacco use, or diseases known as risk factors for osteoporosis, such as rheumatoid arthritis, diabetes mellitus, chronic renal failure, and chronic inflammatory bowel disease. The number of denosumab injections had no significant influence on VFs occurrence in the rebound period. Parental hip fracture was significantly associated with the risk of VFs (HR = 2.13, p = 0.006, Table 1) and with MVFs (OR = 2.25, p = 0.02, Table 2) in the rebound period but not with the risk of NVFs.
Table 1.
Factors Associated With the Risk of a First Vertebral Fracture (VF) After Denosumab Discontinuation
| Factor | No VF in period 3 mean (SD) or n (%) | VF in period 3 mean (SD) or n (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| HR | p Value | HR | p Value | |||
| Total | 715 (89.7%) | 82 (10.3%) | ||||
| At denosumab initiation (period 1) | ||||||
| Age (years) | 65.27 (9.13) | 65.89 (10.03) | 1.01 | 0.64 | ||
| Body mass index (kg/m2) | 23.08 (3.96) | 23.69 (4.36) | 1.04 | 0.20 | ||
| Weight (kg) | 60.01 (10.59) | 63.04 (11.47) | 1.03 | 0.17 | 1.04 | 0.001 |
| Breast cancer | 117 (16.36%) | 17 (20.73%) | 1.43 | 0.19 | ||
| Parental hip fracture | 83 (11.62%) | 17 (20.73%) | 2.13 | 0.006 | ||
| T‐score lumbar spine | −2.53 (0.99) | −2.58 (0.88) | 0.98 | 0.92 | ||
| T‐score total hip | −1.74 (0.76) | −1.89 (0.76) | 0.76 | 0.15 | ||
| T‐score femoral neck | −2.05 (0.75) | −2.15 (0.86) | 0.81 | 0.28 | ||
| Bone resorption markers a | 0.76 (0.45) | 0.89 (0.57) | 1.62 | 0.30 | ||
| Previous vertebral fracture | 131 (18.32%) | 27 (32.93%) | 1.91 | 0.007 | 2.49 | 0.01 |
| Previous non‐vertebral fracture | 141 (19.72%) | 21 (25.61%) | 1.38 | 0.21 | ||
| Previous bisphosphonates | 355 (59.66%) b | 24 (34.78%) c | 0.35 | <0.0001 | ||
| During denosumab treatment (period 2) | ||||||
| No. of denosumab doses | 5.92 (3.16) | 5.84 (3.30) | 1.00 | 0.96 | ||
| Duration of denosumab treatment (months) | 35.02 (18.94) | 34.45 (19.12) | 1.00 | 0.92 | ||
| Vertebral fractures | 17 (2.38%) | 1 (1.22%) | 0.58 | 0.59 | ||
| Non‐vertebral fractures | 22 (3.08%) | 3 (3.66%) | 1.22 | 0.73 | ||
| T‐score lumbar spine | −1.88 (1.13) | −1.89 (0.89) | 1.00 | 0.98 | ||
| T‐score change period 2–1 | 0.70 (0.62) | 0.68 (0.39) | 1.02 | 0.91 | ||
| T‐score total hip | −1.42 (0.84) | −1.75 (0.72) | 0.61 | 0.004 | 0.39 | 0.001 |
| T‐score change period 2–1 | 0.29 (0.37) | 0.21 (0.31) | 0.46 | 0.16 | ||
| T‐score femoral neck | −1.80 (0.78) | −1.92 (0.78) | 0.81 | 0.29 | ||
| T‐score change period 2–1 | 0.22 (0.44) | 0.28 (0.55) | 1.33 | 0.43 | ||
| After denosumab treatment (period 3) | ||||||
| T‐score lumbar spine | −2.12 (1.17) | −2.37 (1.02) | 0.87 | 0.24 | ||
| T‐score change period 3–2 | −0.25 (0.64) | −0.39 (0.71)) | 0.80 | 0.28 | ||
| T‐score change period 3–1 | 0.41 (0.62) | 0.26 (0.70) | 0.79 | 0.32 | ||
| T‐score total hip | −1.63 (0.80) | 1.88 (0.84) | 0.72 | 0.033 | ||
| T‐score change period 3–2 | −0.18 (0.43) | −0.08 (0.38) | 2.25 | 0.046 | ||
| T‐score change period 3–1 | 0.12 (0.35) | 0.11 (0.42) | 1.00 | 0.99 | ||
| T‐score femoral neck | −1.91 (0.75) | −2.15 (0.74) | 0.65 | 0.015 | ||
| T‐score change period 3–2 | −0.10 (0.42) | −0.15 (0.30) | 0.87 | 0.73 | ||
| T‐score change period 3–1 | 0.10 (0.40) | 0.02 (0.40) | 0.74 | 0.40 | ||
| Bone resorption markers a | 0.66 (0.64) | 1.28 (1.19) | 1.78 | <0.0001 | ||
| Non‐vertebral fractures | 11 (1.54%) | 5 (6.10%) | 3.38 | 0.008 | ||
| Bisphosphonates after denosumab | 487 (68.11%) | 16 (19.51%) | 0.11 | <0.0001 | 0.14 | <0.0001 |
Cox proportional hazards regression model analysis (HR = hazard ratio).
Bone resorption markers are indicated in % of the upper premenopausal normal limit of the given method.
Refers to total 595 (data missing for 120 patients).
Refers to total 69 (data missing for 13 patients).
Table 2.
Factors Associated With the Risk of Multiple Vertebral Fractures (MVFs) After Denosumab Discontinuation
| No MVF in period 3 mean (SD) or n (%) | MVF in period 3 (yes) mean (SD) or n (%) | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| OR | p Value | OR | p Value | |||
| Total | 715 (92.62%) | 57 (7.38%) | ||||
| At denosumab initiation (period 1) | ||||||
| Age (years) | 65.27 (9.13) | 64.71 (10.25) | 0.99 | 0.66 | ||
| Body mass index (kg/m2) | 23.08 (3.96) | 23.18 (4.33) | 1.005 | 0.90 | ||
| Weight (kg) | 60.01 (10.59) | 62.16 (11.07) | 1.02 | 0.26 | ||
| Breast cancer | 117 (16.36%) | 13 (22.81%) | 1.51 | 0.21 | ||
| Parental hip fracture | 83 (11.61%) | 13 (22.81%) | 2.25 | 0.02 | ||
| T‐score lumbar spine | −2.53 (0.99) | −2.68 (0.90) | 0.85 | 0.36 | ||
| T‐score total hip | −1.74 (0.76) | −1.91 (0.81) | 0.74 | 0.21 | ||
| T‐score femoral neck | −2.05 (0.75) | −2.12 (0.94) | 0.87 | 0.56 | ||
| Bone resorption markers a | 0.76 (0.45) | 1.07 (0.64) | 3.16 | 0.03 | ||
| Previous vertebral fracture | 131 (18.32%) | 17 (29.82%) | 1.89 | 0.04 | 5.34 | 0.006 |
| Previous non‐vertebral fracture | 141 (19.72%) | 11 (19.30%) | 0.97 | 0.94 | ||
| Previous bisphosphonates | 355 (59.66%) b | 12 (24.49%) c | 0.22 | <0.0001 | 0.10 | <0.0001 |
| During denosumab treatment (period 2) | ||||||
| No. of denosumab doses | 5.92 (3.16) | 5.69 (3.11) | 0.98 | 0.61 | ||
| Duration of denosumab treatment (months) | 35.02 (18.94) | 34.70 (19.76) | 0.99 | 0.90 | ||
| Vertebral fractures | 17 (2.38) | 1 (1.75) | 0.73 | 0.77 | ||
| Non‐vertebral fractures | 22 (3.08) | 2 (3.51) | 1.14 | 0.86 | ||
| T‐score lumbar spine | −1.88 (1.13) | −2.0 (0.86) | 0.89 | 0.48 | ||
| T‐score change period 2–1 | 0.70 (0.62) | 0.70 (0.36) | 1.004 | 0.99 | ||
| T‐score total hip | −1.42 (0.84) | −1.86 (0.73) | 0.50 | 0.002 | 0.38 | 0.002 |
| T‐score change period 2–1 | 0.29 (0.37) | 0.18 (0.37) | 0.37 | 0.14 | ||
| T‐score femoral neck | −1.80 (0.78) | −1.96 (0.78) | 0.76 | 0.28 | ||
| T‐score change period 2–1 | 0.22 (0.44) | 0.20 (0.38) | 0.87 | 0.81 | ||
| After denosumab treatment (period 3) | ||||||
| T‐score lumbar spine | −2.12 (1.17) | −2.48 (1.06) | 0.74 | 0.05 | ||
| T‐score change period 3–2 | −0.25 (0.64) | −0.43 (0.76) | 0.69 | 0.14 | ||
| T‐score change period 3–1 | 0.41 (0.62) | 0.20 (0.73) | 0.58 | 0.05 | ||
| T‐score total hip | −1.63 (0.80) | −1.94 (0.86) | 0.62 | 0.01 | ||
| T‐score change period 3–2 | −0.18 (0.43) | −0.08 (0.38) | 2.18 | 0.14 | ||
| T‐score change period 3–1 | 0.12 (0.35) | 0.04 (0.43) | 0.54 | 0.25 | ||
| T‐score femoral neck | −1.91 (0.75) | −2.19 (0.75) | 0.58 | 0.01 | ||
| T‐score change period 3–2 | −0.10 (0.42) | −0.18 (0.35) | 0.62 | 0.33 | ||
| T‐score change period 3–1 | 0.10 (0.40) | −0.03 (0.43) | 0.49 | 0.08 | ||
| Bone resorption markers a | 0.66 (0.64) | 1.28 (1.13) | 2.31 | <0.0001 | ||
| Non‐vertebral fractures | 11 (1.54%) | 5 (8.77%) | 6.15 | 0.001 | ||
| Bisphosphonate after denosumab | 487 (68.11%) | 7 (12.28%) | 0.07 | <0.0001 | 0.006 | <0.0001 |
OR = odds ratio.
Logistic regression model analysis.
Bone resorption markers are indicated in % of the upper premenopausal normal limit of the given method.
Refers to total 595 (data missing for 120 patients).
Refers to total 49 (data missing for 8 patients).
Table 3.
Factors Associated With the Number of Vertebral Fractures (NVFs) in the Rebound Period After Denosumab Discontinuation
| Variate | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| IRR | p Value | IRR | p Value | |
| At denosumab initiation (period 1) | ||||
| Age (years) | 0.99 | 0.49 | ||
| Body mass index (kg/m2) | 1.01 | 0.84 | ||
| Weight (kg) | 1.02 | 0.35 | ||
| Breast cancer | 1.35 | 0.45 | ||
| Parental hip fracture | 1.84 | 0.16 | ||
| T‐score BMD lumbar spine | 0.87 | 0.49 | ||
| T‐score BMD total hip | 0.75 | 0.23 | ||
| T‐score BMD femoral neck | 0.87 | 0.52 | ||
| Bone resorption markers a | 2.41 | 0.23 | ||
| Previous vertebral fracture | 1.46 | 0.31 | ||
| Previous non‐vertebral fracture | 1.008 | 0.98 | ||
| Previous bisphosphonate Tx | 0.25 | <0.0001 | 0.34 | 0.004 |
| During denosumab treatment (period 2) | ||||
| No. of denosumab doses | 1.002 | 0.96 | ||
| Duration of denosumab Tx | 1.002 | 0.79 | ||
| Vertebral fractures | 0.41 | 0.43 | ||
| Non‐vertebral fractures | 0.73 | 0.73 | ||
| T‐score BMD lumbar spine | 0.96 | 0.86 | ||
| T‐score change period 2–1 | 1.19 | 0.73 | ||
| T‐score BMD total hip | 0.52 | 0.009 | 0.46 | 0.001 |
| T‐score change period 2–1 | 0.39 | 1.19 | ||
| T‐score BMD femoral neck | 0.84 | 0.48 | ||
| T‐score change period 2–1 | 0.96 | 0.95 | ||
| After denosumab treatment (period 3) | ||||
| T‐score BMD lumbar spine | 0.78 | 0.14 | ||
| T‐score BMD total hip | 1.71 | 0.28 | ||
| T‐score BMD femoral neck | 0.64 | 0.04 | ||
| Bone resorption markers a | 2.01 | 0.001 | ||
| Non‐vertebral fractures | 4.19 | 0.14 | ||
| Bisphosphonates after denosumab | 0.09 | <0.0001 | 0.08 | <0.0001 |
IRR = incidence rate ratio; BMD = bone mineral density; Tx = treatment.
Negative binomial regression model analysis.
Bone resorption markers are indicated in % of the upper premenopausal normal limit of the given method.
The role of fractures
VFs before denosumab treatment were significantly associated with the risk of VFs (HR = 1.91, p = 0.007) and of MVFs (OR = 1.89, p = 0.04) after denosumab discontinuation but not with the number of fractures (IRR = 1.46, p = 0.31). The incidence of fractures occurring during the denosumab treatment was low and was not included in the statistical analysis. VFs were increased in patients who experienced non‐vertebral fractures (HR = 3.38, p = 0.008 and for MVFs OR = 6.15, p = 0.001) after denosumab.
The role of bone mineral density
The role of bone mineral density is indicated in Tables 1, 2, and 3. BMD at any site before denosumab initiation was not associated with the risk of VF after denosumab discontinuation. A higher total hip BMD during denosumab treatment predicted a decreased risk of VFs (HR = 0.61, p = 0.004), MVFs (OR = 0.50, p = 0.002), and NVFs (IRR = 0.52, p = 0.009) after denosumab discontinuation. A higher total hip BMD after denosumab treatment was significantly associated with a decreased risk of VFs (HR = 0.72, p = 0.03) and MVF (OR = 0.62, p = 0.012) after denosumab discontinuation. A higher femoral neck BMD after denosumab treatment was also associated with a decreased risk of MVFs and NVFs after denosumab discontinuation.
We analyzed the influence of changes in BMD on the three sites between periods 1 (before denosumab), 2 (during denosumab), and 3 (after denosumab). The percent loss of BMD at the total hip between periods 2 and 3 after denosumab discontinuation was associated with the risk of VFs during the rebound period (OR = 2.25, p = 0.05).
The role of bone resorption markers
Higher levels of bone resorption markers before the treatment with denosumab were associated with a significantly higher risk of MVFs (OR = 3.164, p = 0.034), while similar trends regarding the risk of VFs or NVFs did not meet statistical significance. On the other hand, when measured after denosumab discontinuation, bone resorption makers had a strong predictive value with respect to VFs, MVFs, and NVFs (all with p ≤ 0.001).
The role of bisphosphonates
Of all the patients (N = 797), 47.55% received bisphosphonates before the treatment with denosumab and 63.1% received them after the treatment (Table 1).
For the patients where detailed data on the treatment with bisphosphonates were available, the outcomes of this treatment were compared between the four following groups: patients who did not receive any bisphosphonates at all, patients who received bisphosphonates both before and after the treatment with denosumab; patients who got bisphosphonates only before denosumab but not after; and patients who got bisphosphonates only after denosumab but not before (Table 4; Fig. 5).
Table 4.
Association Between Bisphosphonates (BP) Given Before and/or After Denosumab (Dmab) and Vertebral Fractures (VFs, MVFs, NVFs) in the Rebound Period (Period 3)
| Bisphosphonate | Risk of VFs | Multiple VFs | No. of VFs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No VF in period 3 | VFs in period 3 | HR | p Value | No MVF in period 3 | MVF in period 3 | OR | p Value | IRR | p Value | |
| No BP (reference) | 70 (63.6%) | 40 (36.4%) | — | — | 70 (67.3%) | 34 (32.7%) | — | — | — | — |
| BP before Dmab only | 120 (87.6%) | 17 (12.4%) | 0.24 | <0.0001 | 120 (92.3%) | 10 (7.7%) | 0.20 | 0.001 | 0.220 | <0.0001 |
| BP after Dmab only | 170 (97.1%) | 5 (2.9%) | 0.042 | <0.0001 | 170 (98.3%) | 3 (1.7%) | 0.06 | <0.0001 | 0.059 | <0.0001 |
| BP before and after | 235 (97.1%) | 7 (2.9%) | 0.048 | <0.0001 | 235 (99.2%) | 2 (0.8%) | 0.03 | <0.0001 | 0.039 | <0.0001 |
VFs = vertebral fractures; MVFs = multiple vertebral fractures; NVFs = number of vertebral fractures; HR = hazard ratio; OR = odds ratio.
Patients who got bisphosphonates after denosumab (period 3) only after having suffered a VF are considered as having received no BP after denosumab. The total number of patients in this analysis is 664, while the study total is 797 patients, because exact information on the treatment with bisphosphonates was missing in 133 cases.
Fig. 5.
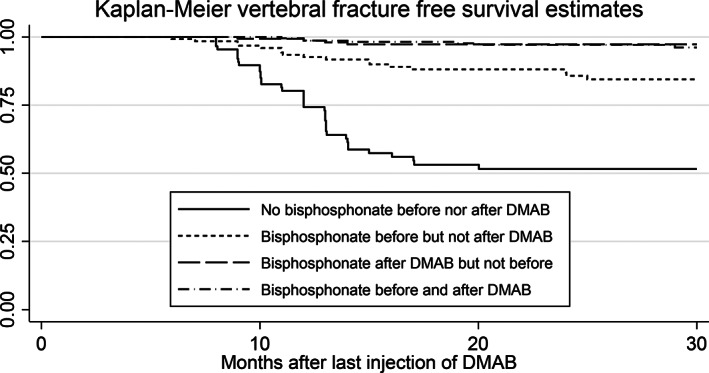
Kaplan–Meier fracture‐free survival curves after denosumab discontinuation; effect of bisphosphonates.
In the patients who got bisphosphonates before the treatment with denosumab, ibandronate was given in 27.3%, alendronate in 25.7%, zoledronate in 7.7%, and risedronate in 4.4%. In 34.9%, the drug was not indicated by the physician. Teriparatide was prescribed in 6.8%. The bisphosphonate mostly given after denosumab treatment was zoledronate (76.5%). Treatment with bisphosphonates before the treatment with denosumab, as well as bisphosphonates given after denosumab discontinuation, were associated with a significantly lower risk of VFs, MVFs, and NVFs. When given before the treatment with denosumab, the protective effect of bisphosphonates was weaker than when given after denosumab (Table 4; Fig. 5).
The marked protective effect of bisphosphonates given after denosumab was not improved by bisphosphonates given before.
The duration of the treatment with bisphosphonates before the treatment of denosumab had no influence on the fracture risk thereafter.
Multivariate analysis
The fitted multivariate Cox proportional model (Table 1) identified four independent risk factors for VF after denosumab discontinuation. Increased weight (HR = 1.04) and previous vertebral fractures (HR = 2.49) increase significantly the risk of VFs, while an increase of the BMD of the total hip during treatment (HR = 0.39) and a prescription of bisphosphonate after denosumab discontinuation (HR = 0.14) played a protective role against VF. For the specific outcome of MVFs (Table 2), four independent factors were retained in the multivariable analysis; previous VFs (OR = 5.34) was significantly associated with high risk of MVFs, while the increase of BMD of the total hip during treatment (OR = 0.38), a treatment with a bisphosphonate before (OR = 0.10), and especially after (OR = 0.006) treatment with denosumab played a protective role against the occurrence of MVFs. For the NVFs (Table 3), a treatment with a bisphosphonate before denosumab treatment (IRR = 0.34), an increase of BMD of the total hip during treatment (IRR = 0.46), and a prescription of bisphosphonate after denosumab discontinuation (IRR = 0.08) were identified as significant independent protective factors to reduce the NVFs.
Discussion
In this large retrospective analysis, the overall incidence of VF after denosumab discontinuation was 10.3%. Bisphosphonates given before denosumab initiation and especially after denosumab discontinuation decrease this risk consistently. We found an already reported decrease of BMD after discontinuation of denosumab.( 3 , 4 , 24 , 28 , 29 , 30 ) This occurred despite the fact that the majority of our patients received bisphosphonates. However, the increase of TBS observed with denosumab persisted after its discontinuation. Because this is the first time that TBS changes are analyzed after denosumab discontinuation, this observation needs further confirmation.
We found the previously reported observation that after denosumab discontinuation, VFs become relatively frequent, while they are rare during the treatment. The incidence of 10.3% is close to the values reported in other studies, but comparisons are difficult because the post‐treatment follow‐ups were short and the number of patients receiving a bisphosphonate after denosumab discontinuation differed from study to study.( 3 , 4 , 7 ) We considered VFs occurring within the 30 months after the last injection of denosumab because this time limit corresponds to the rebound period of osteoclasts.( 1 , 2 )
Compared with the patients who did not receive any bisphosphonate before or after denosumab, the risk of VFs in the patients treated with bisphosphonates before and after denosumab was reduced by 95% (Table 4; Fig. 5). In an early report based on the phase 3 FREEDOM trial,( 7 ) VF incidence was 9.7/100 patient‐years, after adjustment for age and BMD, close to the placebo group, but the median time of observation of the rebound period was short (0.8 years). McClung and colleagues reported a VF incidence of 9.8% within 1 year after cessation of denosumab,( 3 ) whereas another study reported 13.5% during an off‐treatment period of 16 to 20 months.( 4 ) The post hoc analysis of the FREEDOM trial reported 7.1 VFs per 100 patient‐years in a short off‐treatment period of less than 1 year.( 17 ) This risk was similar to that described in the placebo group, but, surprisingly, there was a rebound effect when the placebo was stopped, with a threefold increase in the VF risk.( 31 ) In a large computerized survey, 7.3% of the patients who discontinued denosumab had VFs, but the follow‐up did not include the first 3 months of the rebound period and was restricted to 1 year.( 25 ) As in this study, these authors found that patients with VFs after denosumab discontinuation had a higher risk of non‐vertebral fractures.( 25 )
In this study, 2.2% of the women had VFs during the average 35 months of treatment with denosumab, whereas 10.4% experienced VFs during the 24 months after discontinuation, which corresponds to a sevenfold increase in the annualized risk, rising from 0.75% to 5.15%. In the FREEDOM trial, the increase in risk was of the same magnitude.( 17 ) The number of VFs increased from 31 to 215 during the same monitoring periods, corresponding to a 10‐fold increase of the annualized risk for one VF per patient risk, from 0.013 to 0.13. MVFs represent a major subset of VFs associated with the rebound effect (in 69.5% of our patients). The post hoc analysis of the FREEDOM trial reported a significantly higher proportion of patients suffered MVFs after denosumab (60.7%) than after placebo discontinuation (38.7%). However, this analysis had several limitations, as mentioned above.( 31 )
Our study allowed us to examine a large number of factors that could potentially influence the risk of VF after denosumab discontinuation, as it was done in the post hoc analysis of the FREEDOM trial.( 17 ) The true VF risk attributable to the rebound effect after denosumab discontinuation remains difficult to assess for an individual patient. It depends on the patient's fracture risk profile and is also associated with protective factors (see below). VFs occurring after denosumab discontinuation may also result from the combined risk associated with both the severity of underlying osteoporosis and the rebound phenomenon itself.
Risk factors for rebound vertebral fractures
First, there were no noticeable differences between the patients who presented a VF in the rebound period and those who did not, whether in age and BMI, nor in baseline levels of bone resorption markers or BMD values measured before the treatment.
A parental history of hip fracture was associated with the VF risk after denosumab discontinuation in the univariate analyses and higher weight was associated to the VF risk in the multivariate analyses. This was not reported in other studies, perhaps because of smaller sample size.
Previous VFs were significantly associated with the risk of rebound VFs and of MVFs in the univariate and in the multivariate analyses. For MVFs, the odds ratio exceeded 5 in the multivariate analysis. The same finding was reported in the multivariate analysis of the post hoc analysis of the FREEDOM trial, where previous VFs were the strongest predictor of rebound‐associated VFs (OR = 3.6).( 17 )
The occurrence of non‐vertebral fractures after denosumab treatment increased the risk of MVFs after denosumab more than sixfold. Although rare, such fractures, eg, hip fractures, have to be considered as a serious risk factor for rebound vertebral fractures.
BMD is a main factor of the osteoporotic fracture risk and could have a significant influence on the risk of VFs in the rebound period. But baseline BMD measurements did not have a significant influence, as already observed by others.( 7 , 17 ) Neither did the lumbar spine BMD during and after denosumab treatment influence the VF risk. This may be due to the advanced age of the patients, who often have degenerative retro‐vertebral calcifications.( 32 ) Higher total hip and/or femoral neck BMD, measured during and after denosumab treatment, had some protective effect on the VF during the rebound period. These results need to be confirmed in further studies, as they raise the question if an eventual hip T‐score cut‐off was applied by some physicians for stopping denosumab.
BMD loss at total hip after denosumab discontinuation treatment was associated with the occurrence of VF in the univariate (p 0.046) but not in the multivariate analyses. The same association (OR = 1.2 per 1% annualized loss) was observed in the post hoc analysis of the FREEDOM study.( 17 )
Baseline bone resorption marker levels had no influence, as also reported in the post hoc analysis of the FREEDOM trial.( 17 ) But when measured during the rebound period, these markers were significantly associated with the risk of VF, MVFs, and NVFs. High levels of resorption markers were reported in many patients with MVFs after denosumab discontinuation.( 11 , 14 , 15 , 16 ) They were considered as a risk factor for VFs, but they were not associated with the NVF.( 16 , 18 ) They showed increased osteoclast activity, implying an increase in osteoclastogenesis, which was not found in treatment‐naive osteoporotic women with clinical VFs.( 28 ) Keeping bone resorption markers low with pharmacological treatments after stopping denosumab could reduce or avoid VFs.( 21 )
Benefit of bisphosphonates
Bisphosphonates, given before or after the treatment with denosumab, markedly decreased the risk of VFs in the rebound period. Already in 2017, it was observed that the increase of resorption markers after denosumab treatment was less in patients previously treated with bisphosphonates.( 20 ) According to several authors, previous treatment with bisphosphonates did not protect from VFs after denosumab discontinuation.( 13 , 15 , 21 ) The present study, which analyzed specifically this point in a larger population of patients, showed a significant protective effect of previous treatments with bisphosphonates. However, this effect was much weaker than that of bisphosphonates given after denosumab treatment. It appeared that there was no advantage to give bisphosphonates before the treatment with denosumab, since the effect of bisphosphonates given thereafter was much stronger.
Bisphosphonates are also proposed after denosumab discontinuation to preserve the BMD gained, besides decreasing the risk of VFs. In our study, bisphosphonate prescription (mainly zoledronate) after denosumab discontinuation was associated with a clear decrease of VFs. In small studies, zoledronate given after denosumab discontinuation diminished BMD loss during the rebound period.( 26 , 33 ) It prevented it for 1 year( 34 ) and diminished it partially for a longer period.( 24 ) It also prevented off‐treatment fractures. This was first demonstrated in a small series( 29 ) and then in an observational study of 120 patients.( 30 )
Study limitations and strengths
Several limitations have to be noted in our study. It is a retrospective study, implying non‐recoverable missing information in certain patients, as not all questionnaires were complete. The participant physicians were free about how to select the patients for this study. Most of them conceivably collected consecutive medical records meeting our inclusion criteria, but some inclusion bias cannot be formally excluded. The follow‐up of patients was not standardized, and various intensities of monitoring were probably applied by the practitioners in the study patients. In the absence of a standardized follow‐up protocol, it is possible, although highly improbable, that some VFs were missed. Only osteoporosis specialists were asked to include patients in order to reflect the use of denosumab by experts. Almost but not all Swiss specialized centers participated, and general practitioners were not approached, despite the fact that they can prescribe denosumab. Therefore, the results obtained do not exactly reflect the use and experience of denosumab nationwide. This procedure might also have selected a case mix of osteoporosis and breast cancer patients more severely affected than patients managed by general practitioners. However, these patients are probably more representative of the real‐world use of denosumab than phase 3 prospective clinical trials conducted in a sample of thoroughly selected patients. We are aware that the results of the BMD measures would be more robust had they been standardized by a central quality control, as done in prospective studies. But the retrospective character of the study limited the possibility of such a control, even retrospectively for the 1829 BMD measurements.
The strengths of the study rely on the registration of VFs and of risk factors in three distinct periods: before, during, and after the treatment with denosumab, in order to get the best chances of identifying risk and protective factors for the occurrence of rebound VFs. Another strength relies on the use of three different statistical methods for the three different outcomes (VF, MVF, NVF), which makes the results more robust. We emphasize that the planning of the study, the collection and the analysis of data, as well as the writing of the manuscript were performed without any interference of the sponsor (Amgen Inc).
In conclusion, the VF risk after denosumab discontinuation was close to 10% and was increased in patients who experienced non‐vertebral fractures. Previous VFs were predictors of the post‐treatment fracture risk, as was low total hip BMD achieved on treatment. Bisphosphonates given after denosumab treatment decreased the VF risk. Bisphosphonates given before denosumab were a partially protective factor but did not provide an additional benefit, as long as they were given after.
Disclosures
PB and OL are occasional consultants for Amgen Switzerland.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4335.
Acknowledgments
We thank the physicians who included their patients in the data bank. We also thank M Perriraz of the Department of Epidemiology and Health Systems of the University Institute of General Medicine and Public Health for creating and managing the electronic questionnaire. We thank Amgen Inc. for funding the study. Amgen had no role in the design, conduct, or analysis of the study or the decision to submit the manuscript for publication. The study was compliant with ethical standards.
Authors' roles: Conceptualization: PB, OL, TB, and MF. Data curation: MF and PB. Formal analysis: MF. Funding acquisition: PB. Methodology: MF, PB, TB, OL, and MP. Project administration: MP and PB. Software: MP. Supervision: PB. Validation: MF and PB. Visualization: PB, MF, and OL. Writing initial draft: PB, OL, TB, and MF. Writing—review and editing: PB, OL, TB, and MF.
PB and MF contributed equally to this work.
Data Availability Statement
Data availability The data set is available on request on the Unisanté repository: https://doi.org/10.16909/dataset/24 The data set contains de‐identified medical information and can only be shared on request considering the Federal Act on data protection and the recommendation of the Ethics Committee Vaud, Switzerland.
References
- 1. Miller PD, Bolognese MA, Lewiecki EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long‐term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43(2):222‐229. [DOI] [PubMed] [Google Scholar]
- 2. Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96(4):972‐980. [DOI] [PubMed] [Google Scholar]
- 3. McClung MR, Wagmann RB, Miller PD, Wang A, Lwiecki EM. Observations following discontinuation of long‐term denosumab therapy. Osteoporos Int. 2017;28:1723‐1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zanchetta MB, Boailchuk J, Massari F, Silveira F, Bogado C, Zanchetta JR. Significant bone loss after stopping long‐term denosumab treatment: a post FREEDOM study. Osteoporos Int. 2018;29(1):41‐47. [DOI] [PubMed] [Google Scholar]
- 5. Popp AW, Zysset PK, Lippuner K. Rebound‐associated vertebral fractures after discontinuation of denosumab—from clinic and biomechanics. Osteoporos Int. 2016;27(5):1917‐1921. [DOI] [PubMed] [Google Scholar]
- 6. Brown JP, Dempster DW, Ding B, et al. Bone remodeling in postmenopausal women who discontinued denosumab treatment: off‐treatment biopsy study. J Bone Miner Res. 2011;11:2737‐2744. [DOI] [PubMed] [Google Scholar]
- 7. Brown JP, Roux C, Törring O, et al. Discontinuation of denosumab and associated fracture incidence: analysis from the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial. J Bone Miner Res. 2013;28:746‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aubry‐Rozier B, Gonzalez‐Rodriguez E, Stoll D, Lamy O. Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporos Int. 2016;27(5):1923‐1925. [DOI] [PubMed] [Google Scholar]
- 9. Anastasilakis AD, Makras P. Multiple clinical vertebral fractures following denosumab discontinuation. Osteoporos Int. 2016;27(5):1929‐1930. [DOI] [PubMed] [Google Scholar]
- 10. Polyzos SA, Terpos E. Clinical vertebral fractures following denosumab discontinuation. Endocrine. 2016;54(1):271‐272. [DOI] [PubMed] [Google Scholar]
- 11. Lamy O, Stoll D, Gonzalez‐Rodriguez E, Hans D, Aubry‐Rozier B. Severe rebound‐associated vertebral fractures after denosumab discontinuation: nine clinical cases report. J Clin Endocrinol Metab. 2017;102:354‐358. [DOI] [PubMed] [Google Scholar]
- 12. Anastasilakis AD, Polyzos SA, Makras P, Aubry‐Rozier B, Kaouri S, Lamy O. Clinical features of 24 patients with rebound‐associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res. 2017;32(6):1291‐1296. [DOI] [PubMed] [Google Scholar]
- 13. Tripto‐Shkolnik L, Rouach V, Marcus Y, Rotman‐Pikielny P, Benbassat C, Vered I. Previous treatment with bisphosphonate did not protect from this phenomenon. Vertebral fractures following denosumab discontinuation in patients with prolonged exposure to bisphosphonates. Calcif Tissue Int. 2018;103(1):44‐49. [DOI] [PubMed] [Google Scholar]
- 14. Fernandez FE, Benavent ND, Bonilla HG, et al. Multiple vertebral fractures following discontinuation of denosumab treatment: Ten clinical cases report. 2020;16(6):480‐484. [DOI] [PubMed] [Google Scholar]
- 15. Florez H, Ramirez J, Monegal A, Guanabens N, Peris P. Spontaneous vertebral fractures after denosumab discontinuation: a case collection and review of the literature. Semin Arthrit Rheumat. 2019;49(2):197‐203. [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez‐Rodriguez E, Aubry‐Rozier B, Stoll D, Zaman K, Lamy O. Sixty spontaneous vertebral fractures after denosumab discontinuation in 15 women with early‐stage breast cancer under aromatase inhibitors. Breast Cancer Res Treat. 2020;179(1):153‐159. [DOI] [PubMed] [Google Scholar]
- 17. Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo‐controlled FREEDOM trial and its extension. J Bone Miner Res. 2018;33(2):190‐198. [DOI] [PubMed] [Google Scholar]
- 18. Lamy O, Stoll D, Aubry‐Rozier B, Rodriguez EG. Stopping denosumab. Curr Osteoporos Rep. 2019;17(1):8‐15. [DOI] [PubMed] [Google Scholar]
- 19. Sosa Henriquez M, Gomez de Tejada Romero MJ, Esscudero‐Socorro M, Torregrossa Suau O. Hip fractures following denosumab discontinuation: three clinical case reports. J R Soc Med. 2019;112(11):472‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uebelhart B, Rizzoli R, Ferrari SL. Retrospective evaluation of serum CTX levels after denosumab discontinuation in patients with or without prior exposure to bisphosphonates. Osteoporos Int. 2017;28(9):2701‐2705. [DOI] [PubMed] [Google Scholar]
- 21. Lamy O, Fernandez‐Fernandez E, Monjo‐Henry I, et al. Alendronate after denosumab discontinuation in women previously exposed to bisphosphonates was not effective in preventing the risk of spontaneous multiple vertebral fractures: two case reports. Osteoporos Int. 2019;30(5):1111‐1115. [DOI] [PubMed] [Google Scholar]
- 22. Tsourdi E, Langdahl B, Cohen‐Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11‐17. [DOI] [PubMed] [Google Scholar]
- 23. Anagnostis P, Paschou SA, Gonzalez‐Rodriguez E, et al. Spontaneous vertebral fractures in males with osteoporosis after denosumab discontinuation: a report of two cases. J Clin Rheumatol. 2019. 10.1097/RHU.0000000000000979. [DOI] [PubMed] [Google Scholar]
- 24. Reid IR, Horne AM, Mihov B, Gamble GD. Bone loss after denosumab: only partial protection with zoledronate. Calcif Tissue Int. 2017;101(4):371‐374. [DOI] [PubMed] [Google Scholar]
- 25. Tripto‐Shkolnik L, Fund N, Rouach V, Chodick G, Shalev V, Goldshtein I. Fracture incidence after denosumab discontinuation: real‐world data from a large healthcare provider. Bone. 2020;130:115150. [DOI] [PubMed] [Google Scholar]
- 26. Horne AM, Mihov B, Reid IR. Effect of zoledronate on bone loss after romosozumab/denosumab: 2‐year follow‐up. Calcif Tissue Int. 2019;105:107‐108. [DOI] [PubMed] [Google Scholar]
- 27. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 2009;8:1137‐1148. [DOI] [PubMed] [Google Scholar]
- 28. Anastasilakis AD, Yavropoulou MP, Makras P, et al. Increased osteoclastogenesis in patients with vertebral fractures following discontinuation of denosumab treatment. Eur J Endocrinol. 2017;176(6):677‐683. [DOI] [PubMed] [Google Scholar]
- 29. Lehmann T, Aeberli D. Possible protective effect of switching from denosumab to zoledronic acid on vertebral fractures. Osteoporos Int. 2017;28:3067‐3068. [DOI] [PubMed] [Google Scholar]
- 30. Everts‐Graber J, Reichenbach S, Ziswiler HR, Studer U, Lehmann TA. Single infusion of zoledronate in postmenopausal women following denosumab discontinuation results in partial conservation of bone mass gains. J Bone Miner Res. 2020;35(7):1207‐1215. [DOI] [PubMed] [Google Scholar]
- 31. Lamy O, Gonzalez‐Rodriguez E. Underestimation of vertebral fractures after denosumab discontinuation. J Bone Miner Res. 2018;33:547. [DOI] [PubMed] [Google Scholar]
- 32. Padlina I, Gonzalez‐Rodriguez E, Hans D, et al. The lumbar spine age‐related degenerative disease influences the BMD not the TBS: the osteolaus cohort. Osteoporos Int. 2017;28:909‐915. [DOI] [PubMed] [Google Scholar]
- 33. Anastasilakis AD, Papapoulos SE, Polyzos SA, Appelman‐Dijkstra NM, Makras P. Zoledronate for the prevention of bone loss in women discontinuing denosumab treatment. A prospective 2‐year clinical trial. J Bone Miner Res. 2019;34(12):2220‐2228. [DOI] [PubMed] [Google Scholar]
- 34. Kendler D, Chines A, Clark P, et al. Bone mineral density after transitioning from denosumab to alendronate. J Clin Endocrinol Metab. 2020;105(3):e255‐e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability The data set is available on request on the Unisanté repository: https://doi.org/10.16909/dataset/24 The data set contains de‐identified medical information and can only be shared on request considering the Federal Act on data protection and the recommendation of the Ethics Committee Vaud, Switzerland.


