Abstract
The American Thoracic Society Sleep Core Curriculum updates clinicians on important sleep topics, presented during the annual meeting, and appearing in summary here. This year’s sleep core theme is sleep-disordered breathing and its management. Topics range from pathophysiological mechanisms for the association of obstructive sleep apnea (OSA) and metabolic syndrome, surgical modalities of OSA treatment, comorbid insomnia and OSA, central sleep apnea, and sleep practices during a pandemic. OSA has been associated with metabolic syndrome, independent of the role of obesity, and the pathophysiology suggests a role for sleep fragmentation and intermittent hypoxia in observed metabolic outcomes. In specific patient populations, surgical treatment modalities for OSA have demonstrated large reductions in objective disease severity compared with no treatment and may facilitate adherence to positive airway pressure treatment. Patient-centered approaches to comorbid insomnia and sleep apnea include evaluating for both OSA and insomnia simultaneously and using shared-decision making to determine the order and timing of positive airway pressure therapy and cognitive behavioral therapy for insomnia. The pathophysiology of central sleep apnea is complex and may be due to the loss of drive to breathe or instability in the regulatory pathways that control ventilation. Pandemic-era sleep practices have evolved rapidly to balance safety and sustainability of care for patients with sleep-disordered breathing.
Keywords: obstructive sleep apnea, central sleep apnea, insomnia, upper airway surgery, metabolic syndrome
Metabolic Consequences of Untreated Obstructive Sleep Apnea
Chenjuan Gu and Jonathan C. Jun
Obstructive sleep apnea (OSA) is a highly prevalent disorder. It is well established that obesity predisposes individuals to developing OSA and that untreated OSA may exacerbate metabolic dysfunction and obesity (1).
Laboratory OSA Models
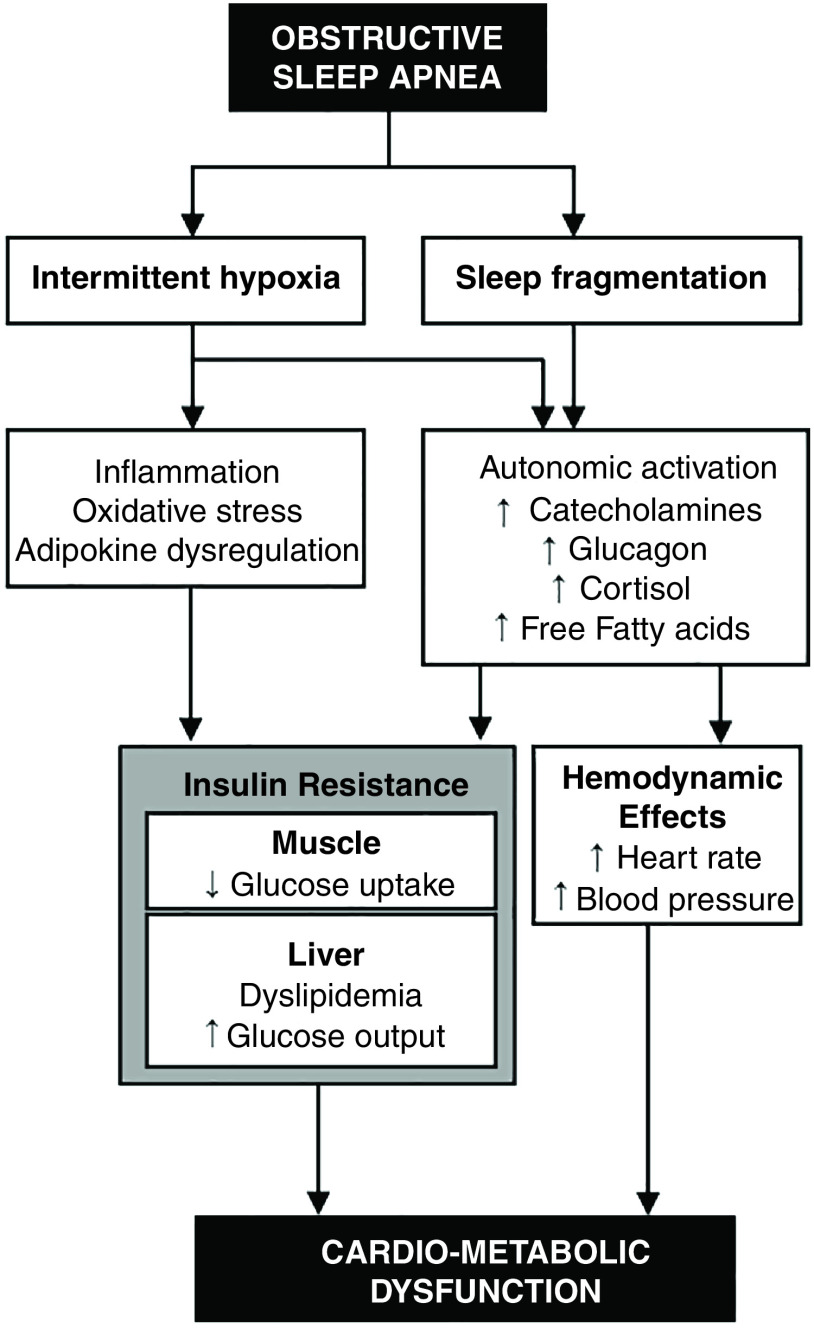
Intermittent hypoxia and sleep fragmentation are two hallmarks of OSA, and potential mediators of changes in metabolism. In laboratory experiments, intermittent hypoxia exposure induced insulin resistance in mice and healthy humans, whereas sleep fragmentation impaired both insulin-dependent and insulin-independent glucose disposal (1). A leading hypothesis contends that intermittent hypoxia leads to tissue oxidative stress, inflammation, and the dysregulation of adipokines. In addition, sleep fragmentation and intermittent hypoxia (via stimulation of arterial chemoreceptors) activate the sympathetic nervous system and the hypothalamus–pituitary–adrenal axis (1). In turn, autonomic activation can lead to insulin resistance and cardiometabolic dysfunction (Figure 1). Autonomic activation stimulates the release of counterregulatory hormones (e.g., cortisol, catecholamines, and glucagon) and mobilizes fatty acids from adipose tissues, which in turn inhibit insulin action in tissues.
Figure 1.
Mechanisms underlying metabolic impacts of obstructive sleep apnea. ↑ = increase; ↓ = decrease; HPA = hypothalamus–pituitary–adrenal; SNS = sympathetic nervous system.
Observational Studies of OSA and Metabolic Dysfunction
Several observational studies have revealed a close relationship between OSA and metabolic dysfunction, independent of obesity. OSA increased the odds of metabolic syndrome, a clustering of clinical features made up of abdominal obesity, hyperglycemia, hypertension, and atherogenic dyslipidemia (2). Patients with OSA exhibited a greater prevalence and incident risk of type 2 diabetes mellitus and worse glycemic control than OSA-free populations (3). Some studies also reported a more atherogenic lipid profile in patients with OSA (1). Most of these studies only adjusted for obesity in terms of the body mass index (BMI), although visceral obesity results in a greater predisposition to developing OSA and metabolic dysfunction (1). Thus, these studies clearly identify OSA as a marker of metabolic dysfunction but cannot establish causation.
Treatment Effects on Metabolic Outcomes
Meta-analyses of studies in patients with type 2 diabetes mellitus revealed no overall effect of continuous positive airway pressure (CPAP) on glycemic control (4). However, CPAP improved insulin sensitivity in certain groups, such as patients with prediabetes and those with insulin resistance (5, 6). Regarding dyslipidemia, a meta-analysis revealed decreased total cholesterol after CPAP but revealed no effect on other lipid levels (7). It is important to note that CPAP adherence in most of these studies was relatively low (∼2.4 to 6.2 h/night), perhaps explaining the limited effectiveness of CPAP in altering metabolic outcomes. However, CPAP improved glucose metabolism in settings of directly observed adherence (6, 8). Similarly, CPAP withdrawal for 3 nights increased plasma free fatty acids, glucose, and cortisol in CPAP-compliant patients with severe OSA (9). These short-term CPAP efficacy data indicate that OSA has the potential to induce harmful metabolic changes. However, the applicability of these studies to the long-term health of the general population with OSA remains unclear. Although OSA treatment might be expected to improve daytime energy and activity, leading to weight loss, it was actually observed that CPAP promoted modest weight gain (10). Further research is needed to clarify the effects of CPAP on the metabolic outcomes of OSA.
References
- 1.Younas H, Gu C, Rathore A, Jun JC, Polotsky VY. In: Richard A, Johnston BTS, editors. Cambridge, MA: Academic Press; 2019. Metabolic syndrome and sleep apnea: a bidirectional relationship; pp. 169–200. [Google Scholar]
- 2. Xu S, Wan Y, Xu M, Ming J, Xing Y, An F, et al. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulm Med. 2015;15:105. doi: 10.1186/s12890-015-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pamidi S, Tasali E. Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neurol. 2012;3:126. doi: 10.3389/fneur.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu B, Ma C, Chaiard J, Shi C. Effect of continuous positive airway pressure on glucose metabolism in adults with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath. 2018;22:287–295. doi: 10.1007/s11325-017-1554-x. [DOI] [PubMed] [Google Scholar]
- 5. Abud R, Salgueiro M, Drake L, Reyes T, Jorquera J, Labarca G. Efficacy of continuous positive airway pressure (CPAP) preventing type 2 diabetes mellitus in patients with obstructive sleep apnea hypopnea syndrome (OSAHS) and insulin resistance: a systematic review and meta-analysis. Sleep Med. 2019;62:14–21. doi: 10.1016/j.sleep.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 6. Pamidi S, Wroblewski K, Stepien M, Sharif-Sidi K, Kilkus J, Whitmore H, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes: a randomized controlled trial. Am J Respir Crit Care Med. 2015;192:96–105. doi: 10.1164/rccm.201408-1564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu H, Yi H, Guan J, Yin S. Effect of continuous positive airway pressure on lipid profile in patients with obstructive sleep apnea syndrome: a meta-analysis of randomized controlled trials. Atherosclerosis. 2014;234:446–453. doi: 10.1016/j.atherosclerosis.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 8. Mokhlesi B, Grimaldi D, Beccuti G, Abraham V, Whitmore H, Delebecque F, et al. Effect of one week of 8-hour nightly continuous positive airway pressure treatment of obstructive sleep apnea on glycemic control in type 2 diabetes: a proof-of-concept study. Am J Respir Crit Care Med. 2016;194:516–519. doi: 10.1164/rccm.201602-0396LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chopra S, Rathore A, Younas H, Pham LV, Gu C, Beselman A, et al. Obstructive sleep apnea dynamically increases nocturnal plasma free fatty acids, glucose, and cortisol during sleep. J Clin Endocrinol Metab. 2017;102:3172–3181. doi: 10.1210/jc.2017-00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Benseñor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258–264. doi: 10.1136/thoraxjnl-2014-205361. [DOI] [PubMed] [Google Scholar]