Abstract
Background and Purpose
Neurosteroids influence neuronal function and have multiple promising clinical applications. Direct modulation of postsynaptic neurotransmitter receptors by neurosteroids is well characterized, but presynaptic effects remain poorly understood. Here, we report presynaptic glutamate release potentiation by neurosteroids pregnanolone and pregnanolone sulfate and compare their mechanisms of action to phorbol 12,13‐dibutyrate (PDBu), a mimic of the second messenger DAG.
Experimental Approach
We use whole‐cell patch‐clamp electrophysiology and pharmacology in rat hippocampal microisland cultures and total internal reflection fluorescence (TIRF) microscopy in HEK293 cells expressing GFP‐tagged vesicle priming protein Munc13‐1, to explore the mechanisms of neurosteroid presynaptic modulation.
Key Results
Pregnanolone sulfate and pregnanolone potentiate glutamate release downstream of presynaptic Ca2+ influx, resembling the action of a phorbol ester PDBu. PDBu partially occludes the effect of pregnanolone, but not of pregnanolone sulfate. Calphostin C, an inhibitor that disrupts DAG binding to its targets, reduces the effect PDBu and pregnanolone, but not of pregnanolone sulfate, suggesting that pregnanolone might interact with a well‐known DAG/phorbol ester target Munc13‐1. However, TIRF microscopy experiments found no evidence of pregnanolone‐induced membrane translocation of GFP‐tagged Munc13‐1, suggesting that pregnanolone may regulate Munc13‐1 indirectly or interact with other DAG targets.
Conclusion and Implications
We describe a novel presynaptic effect of neurosteroids pregnanolone and pregnanolone sulfate to potentiate glutamate release downstream of presynaptic Ca2+ influx. The mechanism of action of pregnanolone, but not of pregnanolone sulfate, partly overlaps with that of PDBu. Presynaptic effects of neurosteroids may contribute to their therapeutic potential in the treatment of disorders of the glutamate system.
Keywords: glutamate, Munc13‐1, neurosteroid, phorbol ester, pregnanolone, presynaptic
Abbreviations
- BAPTA‐AM
1,2‐bis(2‐aminophenoxy)ethane‐N,N,N′,N′‐tetraacetic acid tetrakis (acetoxymethyl ester)
- NBQX
2,3‐dioxo‐6‐nitro‐1,2,3,4‐tetrahydrobenzo[f]quinoxaline‐7‐sulfonamide
- PDBu
phorbol 12,13‐dibutyrate
- SERCA
sarcoplasmic/endoplasmic reticulum Ca2+ ATPase
- TIRF
total internal reflection fluorescence
- TTX
tetrodotoxin
What is already known
Neurosteroids directly modulate postsynaptic GABAA and NMDA receptors.
Some neurosteroids, such as pregnenolone sulfate, potentiate glutamate release by enhancing presynaptic Ca2+ influx.
What does this study add
Pregnanolone and pregnanolone sulfate potentiate glutamate release acting downstream of presynaptic Ca2+ influx.
Mechanism of action of pregnanolone partly overlaps with that of phorbol esters.
What is the clinical significance
NMDA receptor‐modulating neurosteroids may be useful in treating disorders of the glutamatergic system.
Presynaptic effects may contribute to neurosteroid therapeutic advantage.
1. INTRODUCTION
It has been recognized since the 1980s that certain steroids, termed neurosteroids (Corpechot et al., 1981), may be produced locally in the brain and act rapidly to influence neuronal physiology. Subsequent research has revealed an intriguing complexity of neurosteroid signalling—one type of neurosteroid often influences multiple molecular targets and a single target is often modulated by multiple neurosteroids, sometimes in opposite directions.
Best characterized is the ability of neurosteroids to directly modulate postsynaptic neurotransmitter receptors. At inhibitory synapses, metabolites of progesterone, such as allopregnanolone and pregnanolone, strongly potentiate GABAA receptors (Harrison et al., 1987; Majewska et al., 1986; Puia et al., 1990), producing sedative, anxiolytic and antidepressant effects (Belelli & Lambert, 2005; Selye, 1941)—the line of research culminating in the recent Food and Drug Administration (FDA) approval for allopregnanolone (brexanolone) as a treatment for post‐partum depression (Meltzer‐Brody et al., 2018). Interestingly, sulfation at the C3 carbon of the steroid skeleton reverses the direction of GABAA receptor modulation. Thus pregnanolone sulfate and pregnenolone sulfate, the sulfate of the progesterone precursor pregnenolone, are GABAA receptor inhibitors (Majewska et al., 1988; Majewska & Schwartz, 1987; Park‐Chung et al., 1999).
At excitatory synapses, neurosteroid modulation primarily targets NMDA‐type ionotropic glutamate receptors: pregnanolone sulfate is an NMDA receptor inhibitor (Park‐Chung et al., 1994; Petrovic et al., 2005), whereas pregnenolone sulfate has a mixed effect, with potentiation predominating (Horak et al., 2004; Wu et al., 1991). NMDA receptor modulation by neurosteroids may have clinical potential in the treatment of diseases associated with the dysfunction of the glutamate system. NMDA receptor inhibition by neurosteroids can be neuroprotective against excitotoxic neurodegeneration caused by excessive NMDA receptor activation (Rambousek et al., 2011; Vyklicky et al., 2016); conversely, positive modulation of NMDA receptors by potentiating neurosteroids could compensate for insufficient NMDA receptor function associated with conditions such as schizophrenia (Vyklicky et al., 2018).
GABAA and NMDA receptor‐modulating neurosteroids are thus poised to powerfully influence the balance of excitation and inhibition in neuronal networks. Importantly, some of these compounds also have presynaptic effects, particularly on the release of glutamate. Metabolites of progesterone, including allopregnanolone, may enhance glutamate release by potentiating GABAA receptors located in presynaptic terminal membrane (Kim et al., 2011; Ruiz et al., 2010). It is thought that Cl− current through presynaptic GABAA receptors is depolarizing, enhancing presynaptic Ca2+ influx and increasing glutamate release. NMDA receptor‐potentiating neurosteroid pregnenolone sulfate has also been shown to potentiate glutamate release in a variety of preparations, targeting metabotropic σ1 receptors (Meyer et al., 2002), presynaptic NMDA receptors (Mameli et al., 2005) or transient receptor potential channels (Drews et al., 2014; Lee et al., 2010; Zamudio‐Bulcock et al., 2011; Zamudio‐Bulcock & Valenzuela, 2011) to increase presynaptic Ca2+ influx and enhance glutamate release.
Here, we use patch‐clamp electrophysiology in hippocampal microisland cultures to describe the potentiation of synaptic glutamate release by NMDA receptor‐inhibiting neurosteroid pregnanolone sulfate and we report for the first time a similar effect for the nonsulfated pregnanolone. In contrast to presynaptic signalling by allopregnanolone and pregnenolone sulfate, we show that pregnanolone sulfate and pregnanolone enhance glutamate release downstream of presynaptic Ca2+ influx. Interestingly, the mechanism of action of pregnanolone, but not of pregnanolone sulfate, resembles that of phorbol esters, mimics of an endogenous lipid second messenger DAG. Our data suggest that neurosteroid presynaptic regulation of glutamate signalling is more complex than previously appreciated and needs to be considered in any study of neurosteroid physiological functions or potential clinical applications.
2. METHODS
2.1. Animals
All animal protocols were in accordance with the guidelines of the European Union Directive 2010/63/EU and approved by the Animal Care and Use Committee of the Institute of Physiology, Czech Academy of Sciences. Animal studies are reported in compliance with the ARRIVE guidelines (Percie du Sert et al., 2020) and with the recommendations made by the British Journal of Pharmacology (Lilley et al., 2020). Newborn (P0–P2) male albino Wistar rats bred by the Institute of Physiology, Czech Academy of Sciences, were used to prepare primary hippocampal microisland cultures and primary hippocampal mass cultures as described below. Given the relevance of sex as a biological variable, cultures derived from animals of one sex (male), rather than mixed cultures combining cells from male and female animals, were used to allow for potential future sex‐based comparisons.
2.2. Primary hippocampal microisland cultures
Primary hippocampal microisland cultures were prepared according to the protocol of Burgalossi et al. (2012). Briefly, glass coverslips (Glaswarenfabrik Karl Hecht) were coated with 0.15% agarose (Serva) and stamped with an array of microdots (~200 μm in diameter, spaced ~200 μm apart) of growth‐permissive substrate containing 0.1‐mg·ml−1 poly‐d‐lysine (Sigma) and 0.2‐mg·ml−1 collagen (Serva). P0–P2 male Wistar rat pups were killed by decapitation and cerebral cortices and hippocampi were dissected. Primary cortical astrocyte cultures were prepared and grown for 12 days in astrocyte growth medium consisting of DMEM with GlutaMAX (Gibco/Invitrogen), 10% FBS (Gibco/Invitrogen) and pen/strep (Sigma) at 37°C in 5% CO2. Subsequently, astrocytes were trypsinized, plated on the microdot‐stamped coverslips at a density of ~6000 cells per cm2 and allowed to grow to confluence (2 days). Further astrocyte growth was limited by replacing the astrocyte growth medium with neuronal medium consisting of Neurobasal A medium with B27 Supplement and GlutaMAX (Gibco/Invitrogen) and pen/strep (Sigma). Primary hippocampal cultures were prepared with cells plated at a low density of ~300 cells per cm2 on top of the confluent astrocyte microislands in the neuronal medium and grown at 37°C in 5% CO2. This protocol yields a large proportion of microislands containing a solitary hippocampal neuron forming autapses.
2.3. Primary hippocampal mass cultures
Astrocytes were cultured as described above for 12 days, then trypsinized, plated at a density of ~6000 cells per cm2 on glass coverslips coated with 0.1‐mg·ml−1 poly‐d‐lysine (Sigma) and 0.2‐mg·ml−1 collagen (Serva) and allowed to grow to confluence (2 days). Primary hippocampal cultures were prepared from P0–P2 male Wistar rat pups as described above, with cells plated at a density of 50,000 cells per cm2 on top of the confluent astrocyte layer in the neuronal medium and grown at 37°C in 5% CO2. At 4 days in vitro, the mass cultures were treated with 5‐μM cytosine β‐d‐arabinofuranoside (Sigma) for 24 h to inhibit excessive glial cell proliferation.
2.4. Electrophysiology
Whole‐cell voltage‐clamp recordings of autaptic excitatory postsynaptic currents (EPSCs) were obtained at room temperature at a holding potential of −70 mV from solitary excitatory hippocampal neurons at 10–15 days in vitro. Patch electrodes (4–6 MΩ) were fabricated from thick‐walled borosilicate glass capillaries (Science Products GmbH) using a P‐1000 micropipette puller (Sutter Instruments). Intracellular solution contained the following (in mM): 125 gluconic acid, 15 KCl, 10 HEPES, 5 EGTA, 0.5 CaCl2, 2 ATP Mg salt, 0.3 GTP Na salt and 10 creatine phosphate, pH adjusted to 7.2 with KOH. Standard extracellular solution contained (in mM): 160 NaCl, 2.5 KCl, 10 glucose, 10 HEPES, 2 CaCl2 and 1 MgCl2, pH adjusted to 7.3 with NaOH. NMDA receptor‐mediated currents were recorded in extracellular solution with MgCl2 omitted. In some experiments, Ca2+‐free extracellular solution was used (in mM): 160 NaCl, 2.5 KCl, 10 glucose, 10 HEPES, 0 CaCl2, 1 MgCl2 and 1 EGTA, pH adjusted to 7.3 with NaOH. Bicuculline methochloride (10 μM) was always included in the extracellular solution to block GABAA receptors. AMPA receptor‐mediated EPSCs were isolated by recording in the absence of added glycine and in the presence of Mg2+ at −70 mV. NMDA receptor‐mediated EPSCs were recorded at −70 mV in Mg2+‐free extracellular solution in the presence of 10‐μM glycine, 5‐μM strychnine and 1‐μM 2,3‐dioxo‐6‐nitro‐1,2,3,4‐tetrahydrobenzo[f]quinoxaline‐7‐sulfonamide (NBQX). Agonist‐evoked NMDA receptor‐mediated whole‐cell currents were recorded under identical conditions but with 0.5‐μM tetrodotoxin (TTX) added to the extracellular solution.
A voltage‐clamped solitary autaptic neuron does not fire spontaneous action potentials; therefore, we considered spontaneous AMPA receptor‐mediated EPSCs recorded under our conditions to correspond to miniature EPSCs (mEPSCs); TTX was not used (Basu et al., 2007; Mennerick & Zorumski, 1995). We also recorded AMPA receptor‐mediated mEPSCs from primary hippocampal mass cultures at 12–15 days in vitro in the presence of 0.5‐μM TTX. The average baseline amplitude of mass‐culture mEPSCs in TTX was not different from the average baseline amplitude of spontaneous autaptic EPSCs in the absence of TTX, supporting the assumption that spontaneous autaptic EPSCs in our experiments correspond to mEPSCs. Autaptic evoked EPSCs (eEPSCs) were triggered by 1‐ms depolarization to +10 mV every 15 s. Typically, paired‐pulse stimulation with a 50‐ms interpulse interval was used to evoke a pair of eEPSCs every 15 s. While recording eEPSCs (but not mEPSCs), series resistance (<20 MΩ) was ≥80% compensated. EPSC data were acquired with a MultiClamp 700B amplifier (Axon Instruments/Molecular Devices), sampled at 20 kHz, filtered at 2 kHz and analysed offline using pClamp software (Molecular Devices; RRID:SCR_011323). Agonist‐evoked whole‐cell currents were recorded using Axopatch 200B amplifier (Axon Instruments/Molecular Devices), sampled at 5 kHz and filtered at 2 kHz. Figures display individual sweeps recorded under the conditions indicated; in traces of eEPSCs, stimulus artefacts have been removed.
Solutions were applied using a custom‐built microprocessor‐controlled multibarrel fast perfusion system. For NMDA receptor‐mediated agonist‐evoked currents, brief synaptic‐like agonist application was achieved by stepping between two outer barrels containing extracellular solution without the agonist across a middle barrel containing 1‐mM glutamate. Stock solutions of steroids and other drugs were prepared in DMSO and added to the extracellular solution; the final total DMSO concentration (0.3%) was equal in control and in test solutions. We have confirmed that this DMSO concentration has no effect on any of the synaptic parameters studied. Stock solutions of pregnanolone sulfate, pregnenolone sulfate and pregnanolone in DMSO were prepared fresh on the day of the recording; other stock solutions in DMSO were kept in aliquots at −20°C.
2.5. HEK293 cell culture
HEK293 cells (ATCC; RRID:CVCL_0045) were maintained in Opti‐MEM I (Gibco/Invitrogen) supplemented with 5% FBS (PAN‐Biotech) at 37°C in 5% CO2. Cells were transiently transfected with pEGFP‐N1 expression vectors containing GFP‐tagged Munc13‐1 wild‐type (WT) or GFP‐tagged Munc13‐1(H567K) (a generous gift from Prof Nils Brose, Max Planck Institute of Experimental Medicine, Göttingen, Germany) using MATra‐A Reagent (IBA). Following transfection, cells were grown in poly‐l‐lysine‐coated #1 glass‐bottom dishes (glass thickness ~0.15 mm; Cellvis).
2.6. Total internal reflection fluorescence microscopy
Total internal reflection fluorescence (TIRF) images were acquired ~24 h after transfection from live cells at room temperature in standard extracellular solution, using a LEICA DMi8 microscope controlled by LAS X software (Leica Microsystems). The system was equipped with a HC PLAPO 63×/1.47 oil objective and a Leica DFC 9000 GTC camera. TIRF was excited by a 488‐nm laser line using a QSP‐T filter cube in 90‐nm TIRF mode. For each experiment, 3–10 regions of interest containing transfected cells were imaged repeatedly every 2–5 min. After a 30‐min baseline recording, a tested compound was bath applied for 40 min. Fluorescence was analysed offline using ImageJ software (NIH, MD, USA; RRID:SCR_003070).
2.7. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018).
2.7.1. Experimental design and data normalization
Evoked or miniature EPSCs or agonist‐evoked currents were first recorded under baseline conditions and then, in the same cell, in the presence of the tested drug (applied for 2 min, unless stated otherwise), followed in most cases by a period of washout. To compare eEPSC, mEPSC or agonist‐evoked current parameters during baseline versus in the presence of a drug, we used absolute parameter values for statistical comparisons; for each cell, we compared the average of values over the 2‐min period of baseline recording immediately prior to drug application with the average of values over the last 1 min of recording in the presence of the drug. For display purposes and to compare the size of an effect of a given drug under different conditions, each parameter was normalized to its mean baseline value (the average of values over the 2‐min period of baseline recording immediately prior to drug application) for the given cell. In case of paired‐pulse ratios, absolute values were used in statistical comparisons; normalized values are sometimes also presented for easier comparison. TIRF signal intensities were normalized for each cell by the cell's mean baseline fluorescence (the average of values over the 30‐min baseline recording).
Data being compared (i.e. drug effect under control vs. experimental conditions or for WT vs. mutant genotype) were collected on the same days in random order, with an effort to keep group sizes similar (slightly unbalanced sample sizes are due to days with an odd number of successful recordings); n refers to the number of cells tested and the values are given in figures or figure legends. Sample sizes were not estimated prospectively; post hoc power analysis confirmed that sample sizes in the range used were appropriate to detect meaningful differences in our experiments. Blinding was not feasible for two reasons: first, different drugs often required different handling during solution preparation and second, in many cases, solutions or drug effects were clearly distinguishable.
2.7.2. Statistical analysis
Data in figures are presented as mean ± SEM. Statistical analysis was performed with SigmaPlot 10.0 or 14.0 (RRID:SCR_003210). Data distribution was assessed using the Shapiro–Wilk normality test. We used parametric statistics (paired Student's t‐test or ANOVA) if data distribution was normal and non‐parametric statistics (Wilcoxon signed‐rank test or Mann–Whitney rank‐sum test) if data did not have a normal distribution. Post hoc tests were conducted only if F in ANOVA achieved P ≤ 0.05. Groups were subjected to statistical analysis only with sample sizes n ≥ 5, where n is the number of independent values. P ≤ .05 was considered statistically significant throughout the study.
2.8. Materials
Pregnanolone (3α‐hydroxy‐5β‐pregnan‐20‐one) was from Sigma. Pregnanolone sulfate (20‐oxo‐5β‐pregnan‐3α‐yl 3‐sulfate) and pregnenolone sulfate (20‐oxo‐pregn‐5‐en‐3β‐yl 3‐sulfate) were prepared as pyridinium salts, as described previously (Stastna et al., 2009, for pregnanolone sulfate; Krausova et al., 2018, for pregnenolone sulfate; method based on Arnostova et al., 1992). Bicuculline methochloride, TTX, NBQX, phorbol 12,13‐dibutyrate (PDBu), thapsigargin, bisindolylmaleimide I (GF109203X) and calphostin C were obtained from Tocris. 1,2‐Bis(2‐aminophenoxy)ethane‐N,N,N′,N′‐tetraacetic acid tetrakis (acetoxymethyl ester) (BAPTA‐AM) was purchased from Invitrogen. Reagents for the preparation of extracellular and intracellular solution, and glutamate, glycine, strychnine and ionomycin were from Sigma or AnalaR. Cell culture media and solutions were from Gibco/Invitrogen and Serva. Molecular biology reagents were from Biolife and Geneaid.
2.9. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
3. RESULTS
3.1. Pregnanolone sulfate and pregnanolone potentiate spontaneous and evoked synaptic glutamate release
We used whole‐cell voltage‐clamp recording of AMPA receptor‐mediated EPSCs in solitary excitatory hippocampal neurons in microisland cultures to characterize the influence of several endogenous neurosteroids on glutamate release. First, we examined spontaneous glutamate release reflected in the frequency of mEPSCs (see Section 2). We observed that neurosteroid pregnanolone sulfate at 100 μM (Figure 1a) rapidly and reversibly potentiated the frequency of mEPSCs ~3‐fold, while slightly decreasing mEPSC amplitude (Table 1), the latter being consistent with modest inhibition of postsynaptic AMPA/kainate receptors by pregnanolone sulfate (Park‐Chung et al., 1994). Because sulfation at the C3 carbon of the steroid skeleton is an important structural feature with consequences for neurosteroid modulatory actions, we also tested nonsulfated pregnanolone. Remarkably, pregnanolone at 10 μM (Figure 1b) also robustly and reversibly increased mEPSC frequency ~3.5‐fold, with a slightly slower time course; the amplitude of mEPSCs was unchanged (Table 1). Both neurosteroids (100‐μM pregnanolone sulfate and 10‐μM pregnanolone) also potentiated mEPSC frequency in hippocampal mass cultures ~2.5‐fold, not significantly different from their effects in microisland cultures.
FIGURE 1.
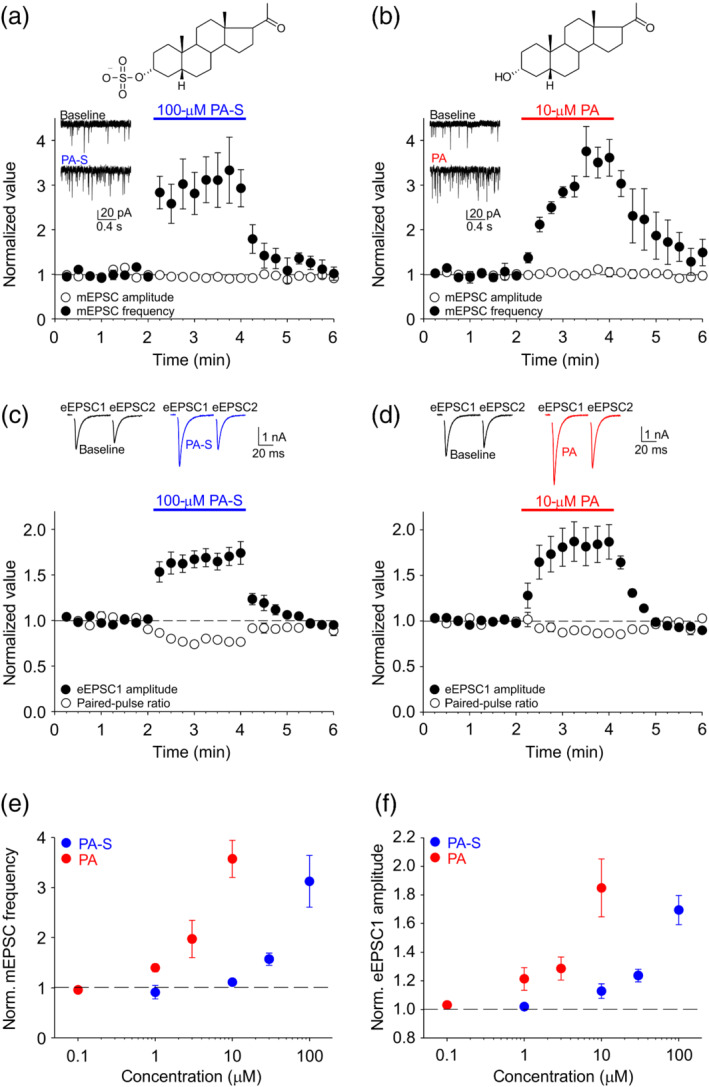
Neurosteroids pregnanolone sulfate (PA‐S) and pregnanolone (PA) potentiate spontaneous and evoked synaptic glutamate release in hippocampal microisland cultures. (a) Top, structure of PA‐S. Bottom, plot of mean ± SEM (n = 6) normalized mEPSC frequency and mEPSC amplitude before, during and after the application of 100‐μM PA‐S for 2 min (period of PA‐S application is indicated by the blue bar). Inset, sample mEPSC traces recorded during baseline and in the presence of PA‐S. (b) Top, structure of PA. Bottom, plot of mean ± SEM (n = 5) normalized mEPSC frequency and mEPSC amplitude before, during and after the application of 10‐μM PA for 2 min (period of PA application is indicated by the red bar). Inset, sample traces recorded during baseline and in the presence of PA. (c) Top, sample eEPSC traces recorded during baseline and in the presence of 100‐μM PA‐S. Bottom, plot of mean ± SEM (n = 7) normalized eEPSC1 amplitude and paired‐pulse ratio before, during and after the application of 100‐μM PA‐S for 2 min (period of PA‐S application is indicated by the blue bar). (d) Top, sample eEPSC traces recorded during baseline and in the presence of 10‐μM PA. Bottom, plot of mean ± SEM (n = 7) normalized eEPSC1 amplitude and paired‐pulse ratio before, during and after the application of 10‐μM PA for 2 min (period of PA application is indicated by the red bar). (e) Mean ± SEM normalized mEPSC frequency in the presence of the indicated steroid concentrations; n = 5–8. (f) Mean ± SEM normalized eEPSC1 amplitude in the presence of the indicated steroid concentrations; n = 5–7
TABLE 1.
Raw current amplitudes and paired‐pulse ratios at baseline and in the presence of pregnanolone sulfate (PA‐S) or pregnanolone (PA)
| Treatment | Parameter | Baseline | Steroid | P value (n) |
|---|---|---|---|---|
| AMPAR mEPSC | ||||
| PA‐S (100 μM) | Amplitude | 22.86 ± 2.65 pA | 21.14 ± 2.82 pA | <0.05 a (6) |
| Frequency | 3.76 ± 1.88 Hz | 11.37 ± 6.32 Hz | <0.05 b (6) | |
| PA (10 μM) | Amplitude | 23.65 ± 1.94 pA | 23.90 ± 2.39 pA | >0.05 a (5) |
| Frequency | 6.08 ± 1.58 Hz | 21.88 ± 6.08 Hz | <0.05 a (5) | |
| AMPAR eEPSC | ||||
| PA‐S (100 μM) | Amplitude | 3111 ± 707 pA | 5239 ± 1374 pA | <0.05 a (7) |
| Paired‐pulse ratio | 0.80 ± 0.06 | 0.62 ± 0.05 | <0.05 a (7) | |
| PA (10 μM) | Amplitude | 3841 ± 542 pA | 6640 ± 839 pA | <0.05 b (7) |
| Paired‐pulse ratio | 0.83 ± 0.02 | 0.73 ± 0.02 | <0.05 a (7) | |
| NMDAR current | ||||
| PA‐S (100 μM) | eEPSC amplitude | 3697 ± 1014 pA | 3424 ± 820 pA | >0.05 a (7) |
| Glu‐evoked amplitude | 7849 ± 584 pA | 4784 ± 532 pA | <0.05 a (8) | |
| PA (10 μM) | eEPSC amplitude | 4811 ± 1057 pA | 6123 ± 1274 pA | <0.05 a (8) |
| Glu‐evoked amplitude | 6479 ± 1451 pA | 5104 ± 1264 pA | <0.05 a (6) | |
Note: Values presented are mean ± SEM.
Abbreviations: AMPAR, AMPA receptor; eEPSC, evoked excitatory postsynaptic current; Glu, glutamate; mEPSC, miniature excitatory postsynaptic current; NMDAR, NMDA receptor; PA‐S, pregnanolone sulfate; PA, pregnanolone.
Groups compared by Student's paired t‐test.
Groups compared by Wilcoxon signed‐rank test.
We next triggered unclamped action potentials in the patched neurons by delivering 1‐ms step depolarizations to +10 mV; such action potentials induce autaptic eEPSCs in the same neuron. We delivered two depolarizations 50 ms apart every 15 s and monitored the amplitudes of the eEPSCs as well as paired‐pulse ratio = eEPSC1/eEPSC2, a parameter largely determined by presynaptic release properties (Mennerick & Zorumski, 1995). As shown in Figure 1c and Table 1, 100‐μM pregnanolone sulfate rapidly and reversibly potentiated eEPSC amplitude and decreased the paired‐pulse ratio. Similarly (Figure 1d and Table 1), 10‐μM pregnanolone robustly and reversibly potentiated eEPSC amplitude and decreased the paired‐pulse ratio, modestly but significantly. Together, these results argue that pregnanolone sulfate and pregnanolone act presynaptically to enhance spontaneous and evoked glutamate release at excitatory hippocampal synapses.
We performed a dose–response analysis of the presynaptic effects of pregnanolone sulfate and pregnanolone, recording autaptic mEPSCs and eEPSCs in the presence of 1‐ to 100‐μM pregnanolone sulfate or 0.1‐ to 10‐μM pregnanolone (Figure 1e,f). The effects were likely not saturated at the highest concentrations tested, but concentrations >100 μM for pregnanolone sulfate and >10 μM for pregnanolone could not be reliably dissolved. We found that pregnanolone was ~10‐fold more potent than pregnanolone sulfate at potentiating both the mEPSC frequency and the eEPSC amplitude. For each steroid, the effects on mEPSCs and eEPSCs had a similar dose dependence and a similar time course, suggesting that, for a given steroid, a common mechanism may underlie the modulation of spontaneous and evoked release.
In contrast to AMPA receptor‐mediated eEPSCs that primarily reflect neurosteroid presynaptic effects, because postsynaptic AMPA receptor modulation by neurosteroids is minimal (Park‐Chung et al., 1994; Table 1), NMDA receptor‐mediated eEPSCs would likely be influenced both by the presynaptic enhancement of glutamate release and by the postsynaptic modulation of NMDA receptor function (Park‐Chung et al., 1994; Petrovic et al., 2005). To examine the relative contribution of presynaptic versus postsynaptic mechanisms of neurosteroid modulation of NMDA receptor‐mediated synaptic transmission, we compared neurosteroid effects on NMDA receptor eEPSCs (presumably reflecting presynaptic and postsynaptic modulation) with neurosteroid effects on NMDA receptor‐mediated whole‐cell currents evoked by fast synaptic‐like glutamate application (postsynaptic modulation only). As shown in Figure 2a and Table 1, pregnanolone sulfate (100 μM) strongly reduced the amplitude of NMDA receptor‐mediated responses to fast glutamate application, consistent with its inhibitory effect on NMDA receptors (Vyklicky et al., 2016). In contrast, pregnanolone sulfate had no consistent effect on peak NMDA receptor‐mediated eEPSC, suggesting that the presynaptic enhancement of glutamate release compensates for the postsynaptic NMDA receptor inhibition. In the case of pregnanolone (10 μM; Figure 2b and Table 1), we observed a moderate reduction of NMDA receptor‐mediated responses to fast glutamate application, but a potentiation of NMDA receptor‐mediated eEPSCs, suggesting that, for pregnanolone, the presynaptic modulation is dominant. Together, these data confirm robust enhancement of glutamate release by pregnanolone sulfate and pregnanolone in cultured hippocampal neurons.
FIGURE 2.
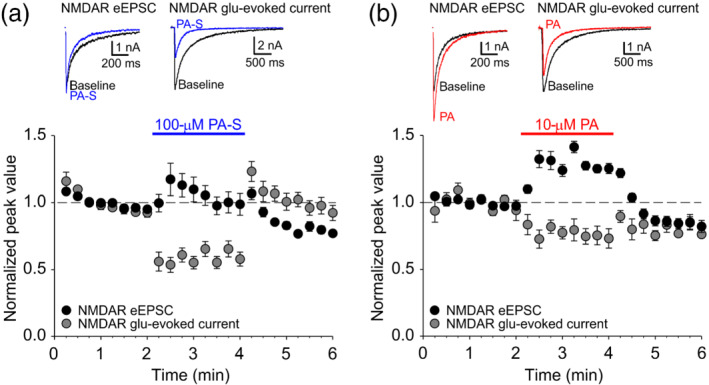
Neurosteroid modulation of NMDA receptor (NMDAR)‐mediated eEPSCs combines presynaptic glutamate (glu) release potentiation and postsynaptic NMDA receptor inhibition. (a) Top left, sample NMDA receptor‐mediated eEPSC traces recorded at baseline (black) and in the presence of 100‐μM PA‐S (blue). Top right, sample traces of NMDA receptor‐mediated responses to fast glutamate application recorded at baseline (black) and in the presence of 100‐μM pregnanolone sulfate (PA‐S;blue). Bottom, plot of mean ± SEM normalized peak eEPSC amplitude (n = 7) and peak glutamate‐evoked current amplitude (n = 8) before, during and after the application of 100‐μM PA‐S (blue bar). (b) Top left, sample NMDA receptor‐mediated eEPSC traces recorded at baseline (black) and in the presence of 10‐μM PA (red). Top right, sample traces of NMDA receptor‐mediated responses to fast glutamate application recorded at baseline (black) and in the presence of 10‐μM pregnanolone (red). Bottom, plot of mean ± SEM normalized peak eEPSC amplitude (n = 8) and peak glutamate‐evoked current amplitude (n = 6) before, during and after the application of 100‐μM PA‐S (red bar)
3.2. Pregnanolone sulfate and pregnanolone potentiate glutamate release independently of presynaptic Ca2+ influx or Ca2+ release from internal stores
Presynaptic effects of neurosteroids allopregnanolone or pregnenolone sulfate are consistently eliminated by manipulations that interfere with Ca2+ influx into the presynaptic terminal (Kim et al., 2011; Lee et al., 2010; Meyer et al., 2002; Zamudio‐Bulcock & Valenzuela, 2011). To test whether the presynaptic effect of pregnanolone sulfate and pregnanolone may rely on presynaptic Ca2+ influx, we recorded AMPA receptor‐mediated EPSCs in extracellular solution containing 0‐mM Ca2+ and 1‐mM EGTA. First, we performed a series of controls to confirm that the removal of extracellular Ca2+ selectively interferes with processes known to depend on Ca2+ influx. As shown in Figure 3a, evoked release was completely abolished in the Ca2+‐free extracellular solution, whereas spontaneous release was reduced approximately by half, consistent with the role of Ca2+ in spontaneous release (Groffen et al., 2010). The application of Ca2+ ionophore ionomycin (2 μM for 1 min; Capogna et al., 1996) in control extracellular solution robustly increased mEPSC frequency and as expected, this effect was significantly smaller in Ca2+‐free extracellular solution (Figure 3b). Further, we tested PDBu, a compound that potentiates release by modulating vesicle release machinery downstream of presynaptic Ca2+ influx (Capogna et al., 1997; Lou et al., 2005). Accordingly, the application of 100‐nM PDBu increased mEPSC frequency ~6‐fold and a similar increase was observed even in the absence of extracellular Ca2+ (Figure 3b).
FIGURE 3.
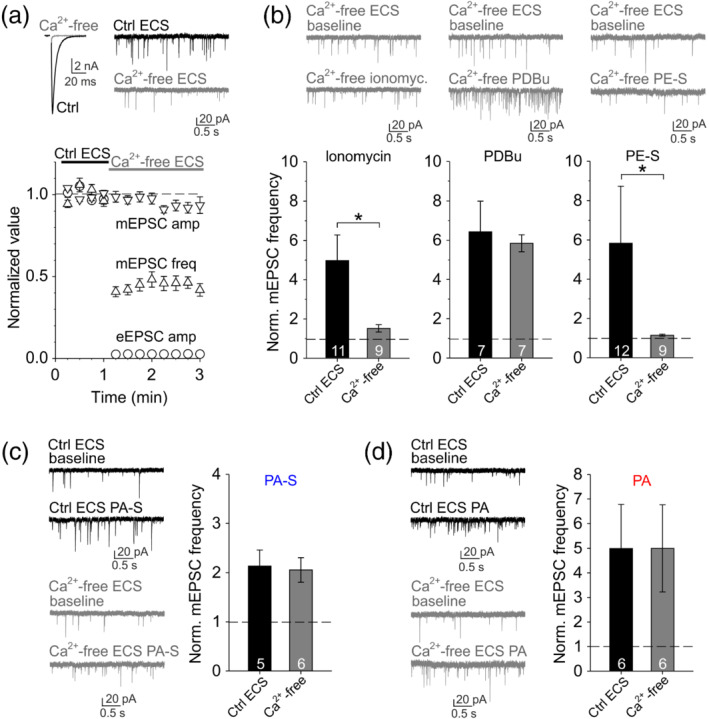
Pregnanolone sulfate (PA‐S) and pregnanolone (PA) potentiate glutamate release downstream of presynaptic Ca2+ influx. (a) Top left, sample AMPA receptor‐mediated eEPSC traces recorded in control extracellular solution (Ctrl ECS) containing 2‐mM Ca2+ or in Ca2+‐free extracellular solution (Ca2+‐free ECS) containing nominally 0‐mM Ca2+ and 1‐mM EGTA. Top right, sample AMPA receptor‐mediated mEPSC traces recorded in control ECS or in Ca2+‐free ECS. Bottom, plot of mean ± SEM normalized eEPSC amplitude (n = 5) and mEPSC frequency and amplitude (n = 17) recorded in control ECS followed by Ca2+‐free ECS as indicated. (b) Top, sample mEPSC traces recorded in Ca2+‐free ECS before and during the application of 2‐μM ionomycin (left), 100‐nM PDBu (middle) or 30‐μM pregnenolone sulfate, PE‐S (right). Bottom left, plot of mean ± SEM (n values are indicated in the figure) normalized mEPSC frequency in the presence of 2‐μM ionomycin recorded in control ECS or in Ca2+‐free ECS. *P <.05 , Mann–Whitney rank‐sum test. Bottom middle, plot of mean ± SEM (n values as indicated) normalized mEPSC frequency in the presence of 100‐nM PDBu recorded in control ECS or in Ca2+‐free ECS. Bottom right, plot of mean ± SEM (n values as indicated) normalized mEPSC frequency in the presence of 30‐μM PE‐S recorded in control ECS or in Ca2+‐free ECS. *P <.05 , Mann–Whitney rank‐sum test. (c) Left, sample mEPSC traces recorded before and in the presence of 100‐μM PA‐S in control ECS or in Ca2+‐free ECS as indicated. Right, mean ± SEM (n values as indicated) normalized mEPSC frequency in the presence of 100‐μM PA‐S in control ECS or in Ca2+‐free ECS. (d) Left, sample mEPSC traces recorded before and in the presence of 10‐μM PA in control ECS or in Ca2+‐free ECS as indicated. Right, mean ± SEM (n values as indicated) normalized mEPSC frequency in the presence of 10‐μM PA in control ECS or in Ca2+‐free ECS
Next, we examined the role of presynaptic Ca2+ influx in neurosteroid effects. When applying 30‐μM pregnenolone sulfate in control extracellular solution, we observed a rather variable increase in mEPSC frequency, which, however, was significantly smaller in Ca2+‐free extracellular solution (Figure 3b). This confirms previous reports that presynaptic effects of neurosteroid pregnenolone sulfate are dependent on presynaptic Ca2+ influx (Lee et al., 2010; Meyer et al., 2002; Zamudio‐Bulcock & Valenzuela, 2011). Surprisingly, in contrast to the effect of pregnenolone sulfate, neurosteroids pregnanolone sulfate (100 μM) and pregnanolone (10 μM) both potentiated mEPSC frequency equally in control and in Ca2+‐free extracellular solution (Figure 3c,d), suggesting that their presynaptic action is downstream of presynaptic Ca2+ influx.
An alternative source of presynaptic Ca2+ that could play a role in neurosteroid‐induced potentiation of glutamate release is the system of presynaptic Ca2+ stores—endoplasmic reticulum‐related organelles that use sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) pumps to sequester cytoplasmic Ca2+ and can release it to modulate neurotransmitter release (Emptage et al., 2001), including in response to neurosteroid pregnenolone sulfate (Lee et al., 2010). To prevent a potential increase in presynaptic Ca2+ concentration regardless of the source, we preincubated cells with a cell‐permeable Ca2+ chelator BAPTA‐AM (10 μM) for 15 min before recording. Applying a SERCA pump inhibitor thapsigargin (2 μM) as a positive control, we observed ~3‐fold increase in mEPSC frequency (Figure 4a), presumably due to impaired Ca2+ clearance leading to presynaptic Ca2+ accumulation (Emptage et al., 2001). As expected, BAPTA‐AM preincubation prevented the thapsigargin‐induced mEPSC frequency increase. In contrast, pregnanolone sulfate (100 μM) and pregnanolone (10 μM) increased mEPSC frequency even following BAPTA‐AM preincubation (Figure 4b,c), suggesting that the effect of these neurosteroids on glutamate release does not require an elevation of presynaptic Ca2+—whether coming from the extracellular space or from the internal stores.
FIGURE 4.
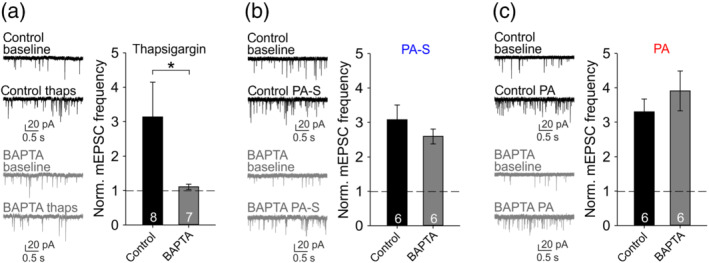
Potentiation of glutamate release by pregnanolone sulfate (PA‐S) or pregnanolone (PA) does not require increased presynaptic Ca2+ concentration. (a) Left, sample mEPSC traces recorded before and in the presence of 2‐μM thapsigargin after preincubation of cells in control extracellular solution (ECS) or in 10‐μM BAPTA‐AM as indicated. Right, mean ± SEM (n values as indicated) normalized mEPSC frequency in the presence of 2‐μM thapsigargin after control ECS or BAPTA‐AM preincubation. *P <.05, Mann–Whitney rank‐sum test. (b) Left, sample mEPSC traces recorded before and in the presence of 100‐μM PA‐S after preincubation of cells in control ECS or in 10‐μM BAPTA‐AM as indicated. Right, mean ± SEM (n values as indicated) normalized mEPSC frequency in the presence of 100‐μM PA‐S after control ECS or BAPTA‐AM preincubation. (c) Left, sample mEPSC traces recorded before and in the presence of 10‐μM PA after preincubation of cells in control ECS or in 10‐μM BAPTA‐AM as indicated. Right, mean ± SEM (n values as indicated) normalized mEPSC frequency in the presence of 10‐μM PA after control ECS or BAPTA‐AM preincubation
3.3. Presynaptic effects of pregnanolone sulfate and pregnanolone compared with PDBu
Phorbol esters, including PDBu, are a family of lipophilic compounds known to mimic the actions of a lipid second messenger DAG by binding to DAG‐sensitive C1 domains of target proteins (Brose & Rosemund, 2002; Newton, 2018). Our observation that neurosteroids pregnanolone sulfate and pregnanolone resemble PDBu in their presynaptic action downstream of Ca2+ influx (Figure 3) suggested that these neurosteroids may potentiate glutamate release via a mechanism related to phorbol ester/DAG signalling. We recorded mEPSCs and eEPSCs in the presence of 1‐nM to 1‐μM PDBu (Figure 5a). Similar to our results with pregnanolone sulfate and pregnanolone, PDBu potently and robustly enhanced both spontaneous and evoked glutamate release. It has been proposed that the same mechanism downstream of Ca2+ influx mediates PDBu‐induced enhancement of both spontaneous and evoked release (Basu et al., 2007; Lou et al., 2005). Because previous pharmacological characterization of presynaptic phorbol ester action has largely focused on eEPSCs (Hori et al., 1999; Lou et al., 2008; Wierda et al., 2007), we turned to eEPSCs in our subsequent experiments. First, we selected a subsaturating concentration of 10‐nM PDBu, which closely matched 10‐μM pregnanolone and 100‐μM pregnanolone sulfate in terms of the effect on mEPSC frequency and on eEPSC amplitude. Remarkably, pregnanolone sulfate consistently depressed paired‐pulse ratio more strongly than did either pregnanolone or PDBu (Figure 5b). Comparison of the effects on absolute eEPSC amplitudes and paired‐pulse ratio is shown in Figure 5c.
FIGURE 5.
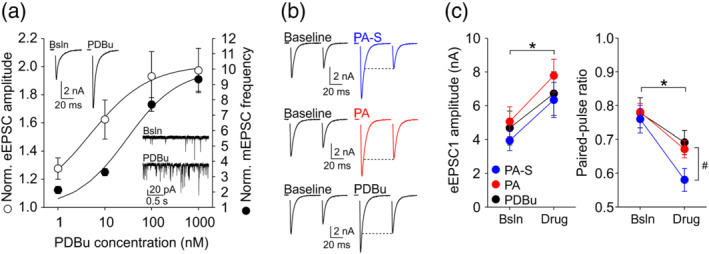
Pregnanolone sulfate (PA‐S) depresses paired‐pulse ratio more strongly than pregnanolone (PA) or phorbol 12,13‐dibutyrate (PDBu). (a) Dose–response analysis of the potentiation of synaptic glutamate release by PDBu. Plot shows mean ± SEM normalized eEPSC amplitude (empty symbols) and normalized mEPSC frequency (filled symbols) in the presence of the indicated PDBu concentrations; n = 4–7. Curves are fits to the data according the equation: normalized value of the parameter = minimum value (set to 1) + [maximum value − minimum value]∕[1 + (EC50/PDBu concentration) h ], where h represents the Hill coefficient and EC50 represents PDBu concentration inducing a half‐maximal effect. For eEPSC amplitude, the fit gave the maximal normalized eEPSC amplitude of 2.01, EC50 = 4.52 nM and h = 0.68. For mEPSC frequency, the fit gave the maximal normalized mEPSC frequency of 9.95, EC50 = 29.92 nM and h = 0.79. Insets show representative eEPSC and mEPSC traces recorded during baseline (Bsln) and in the presence of 100‐nM PDBu. (b) Representative eEPSC traces recorded before and during the application of 100‐μM PA‐S (top), 10‐μM PA (middle) or 10‐nM PDBu (bottom). Note the strong paired‐pulse depression in the presence of PA‐S. (c) Left, mean ± SEM eEPSC1 amplitude before and in the presence of 100‐μM PA‐S (n = 13), 10‐μM PA (n = 12) or 10‐nM PDBu (n = 13). *P <.05, two‐way repeated measures ANOVA (one‐factor repetition), main effect of modulator application. Right, mean ± SEM paired‐pulse ratio before and in the presence of the indicated treatments from the same cells as on the left. *P <.05, two‐way repeated measures ANOVA (one‐factor repetition), main effect of modulator application; # P <.05, interaction between modulator application and experiment (i.e., the type of modulator applied)
If the presynaptic mechanism of action of neurosteroids pregnanolone and/or pregnanolone sulfate overlaps with that of PDBu, a saturating concentration of PDBu should occlude further EPSC potentiation by the steroids. As shown in Figure 5a, a 10‐fold increase in PDBu concentration from 100 nM to 1 μM induced no further increase in eEPSC amplitude, suggesting that the potentiation of evoked release is saturated at 100‐nM PDBu. Thus, we proceeded to compare the increase in eEPSC amplitude induced by each steroid when applied either alone or in the presence of 100‐nM PDBu. As shown in Figure 6a–c, pregnanolone sulfate alone potentiated eEPSCs by 56 ± 6% of baseline and when applied to the same cells in the presence of PDBu, it still potentiated eEPSCs robustly, by 71 ± 14% of the pre‐PDBu baseline. In contrast, whereas pregnanolone alone potentiated eEPSCs by 62 ± 8%, its effect was significantly smaller in the presence of PDBu, with eEPSCs potentiated only by 41 ± 11% of the pre‐PDBu baseline (Figure 6d–f). It is unlikely that the diminished effect of pregnanolone in the presence of PDBu is due to a ceiling effect, given that the effects of pregnanolone sulfate and PDBu (presumably involving distinct mechanisms) are fully additive. Our results support the notion that the mechanism of action of pregnanolone, unlike pregnanolone sulfate, may partly overlap with that of PDBu.
FIGURE 6.
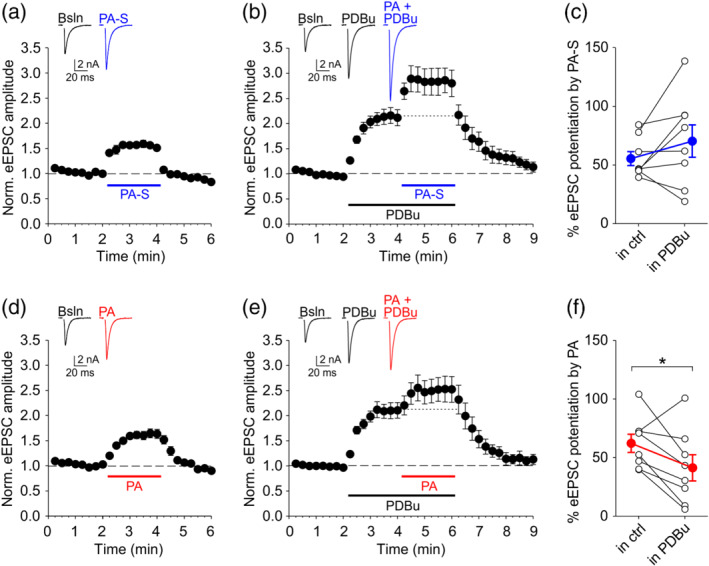
Phorbol 12,13‐dibutyrate (PDBu) partially occludes eEPSC potentiation by pregnanolone (PA) but not by pregnanolone sulfate (PA‐S). (a) Mean ± SEM normalized eEPSC amplitude before and in the presence of 100‐μM PA‐S applied alone (as indicated). Inset shows representative traces recorded during baseline (Bsln) and in PA‐S as indicated. (b) Mean ± SEM normalized eEPSC amplitude from the same cells as in (a), recorded before and in the presence of 100‐nM PDBu and in the presence of 100‐μM PA‐S applied together with 100‐nM PDBu (as indicated). Inset shows representative traces recorded in indicated conditions. Traces in (a) and (b) are from the same cell. (c) Plot of % eEPSC potentiation by 100‐μM PA‐S applied either alone or in the presence of 100‐nM PDBu. Empty symbols show data from individual experiments. Blue‐filled symbols show mean ± SEM (n = 8). (d) Mean ± SEM normalized eEPSC amplitude before and in the presence of 10‐μM PA applied alone (as indicated). Inset shows representative eEPSC traces. (e) Mean ± SEM normalized eEPSC amplitude from the same cells as in (d), recorded before and in the presence of 100‐nM PDBu and in the presence of 10‐μM PA applied together with 100‐nM PDBu (as indicated). Inset shows representative traces recorded in indicated conditions. Traces in (d) and (e) are from the same cell. (f) Plot of % eEPSC potentiation by 10‐μM PA applied either alone or in the presence of 100‐nM PDBu. Empty symbols show data from individual experiments. Red‐filled symbols show mean ± SEM (n = 8). *P <.05, paired Student;s t‐test
3.4. Calphostin C, a competitive blocker of phorbol ester/DAG binding to target C1 domains, inhibits glutamate release potentiation by pregnanolone, but not by pregnanolone sulfate
Literature suggests two main presynaptic targets for phorbol ester/DAG signalling: PKC and/or Munc13 vesicle priming proteins (Betz et al., 1998; Hori et al., 1999; Lou et al., 2008; Rhee et al., 2002). In either case, the interaction is mediated by a phorbol ester/DAG‐binding C1 domain of the target protein. We used a pharmacological approach to explore possible mechanisms of action of pregnanolone sulfate and pregnanolone compared with PDBu. We used bisindolylmaleimide I, a selective PKC inhibitor that interacts with the ATP‐binding site of the kinase catalytic domain or calphostin C, an inhibitor that interacts with the DAG‐binding C1 domains of either PKC or Munc13‐1 (Betz et al., 1998; Hori et al., 1999). Cells were patched and incubated for ≥15 min with control extracellular solution, 2‐μM bisindolylmaleimide I or 1‐μM calphostin C before the application of 100‐nM PDBu or one of the steroids in the continued presence of either control extracellular solution or the inhibitor.
As shown in Figure 7a,b, PDBu‐induced eEPSC potentiation was significantly reduced by either calphostin C or bisindolylmaleimide I. In contrast, as shown in Figure 7c,d, eEPSC potentiation by pregnanolone sulfate was unaffected by either inhibitor. Interestingly, the effect of PA on eEPSC amplitude was significantly reduced calphostin C, but not by bisindolylmaleimide I (Figure 7e,f). Our results thus support some involvement of PKC in the PDBu effect in our preparation and confirm an interaction between PDBu and a phorbol ester/DAG‐binding C1 domain of either PKC or another target protein. Interestingly, it appears that neurosteroid pregnanolone binds to a phorbol ester/DAG‐binding C1 domain of some presynaptic target other than PKC to potentiate glutamate release.
FIGURE 7.
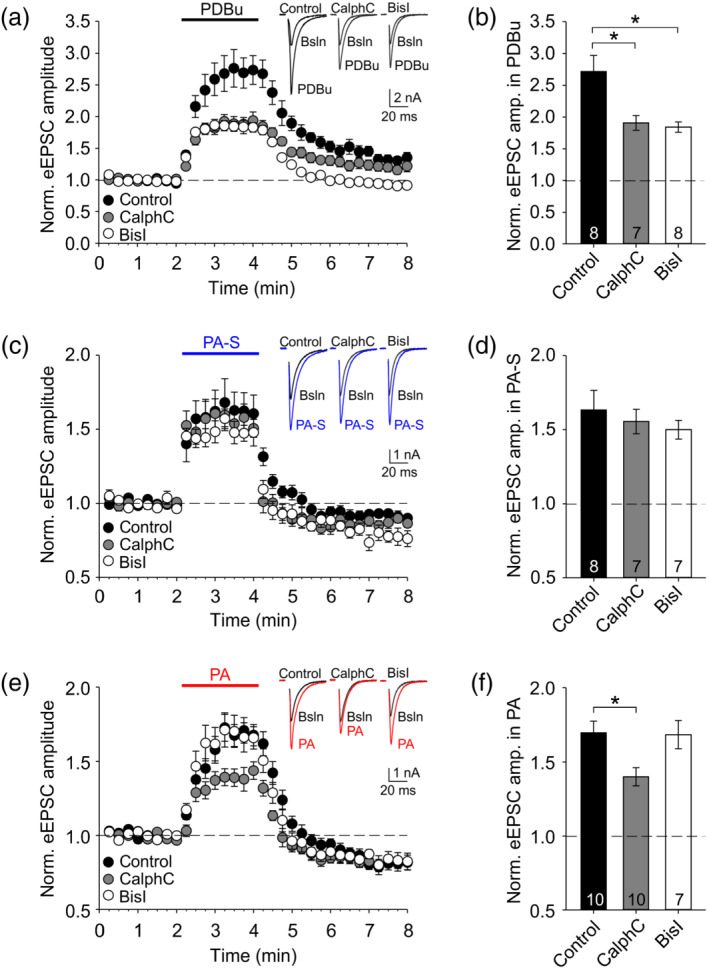
Calphostin C, a blocker of phorbol ester/DAG binding to C1 domains, inhibits eEPSC potentiation by pregnanolone (PA), but not by pregnanolone sulfate (PA‐S). (a) Mean ± SEM normalized eEPSC amplitude from experiments where 100‐nM PDBu was applied after a 15‐min pretreatment and in the continued presence of control extracellular solution (ECS), calphostin C (CalphC; 1 μM) or bisindolylmaleimide I (BisI; 2 μM). Inset shows representative eEPSC traces at baseline (Bsln) and in PDBu from indicated experiments. (b) Summary plot of mean ± SEM eEPSC amplitudes in the presence of 100‐nM PDBu in the indicated inhibitors; n values are as shown. One‐way ANOVA p <.05; *Significant Holm–Sidak post hoc comparison versus control. (c) Mean ± SEM normalized eEPSC amplitude from experiments where 100‐μM PA‐S was applied after a 15‐min pretreatment and in the continued presence of control ECS, calphostin C (1 μM) or bisindolylmaleimide I (2 μM). Inset shows representative eEPSC traces from indicated experiments. (d) Summary plot of mean ± SEM eEPSC amplitudes in the presence of 100‐μM PA‐S in the indicated inhibitors; n values are as shown. (e) Mean ± SEM normalized eEPSC amplitude from experiments where 10‐μM PA was applied after a 15‐min pretreatment and in the continued presence of control ECS, calphostin C (1 μM) or bisindolylmaleimide I (2 μM). Inset shows representative eEPSC traces from indicated experiments. (f) Summary plot of mean ± SEM eEPSC amplitudes in the presence of 10‐μM PA in the indicated inhibitors; n values are as shown. One‐way ANOVA P <.05; *Significant Holm–Sidak post hoc comparison versus control
3.5. Neurosteroids fail to translocate GFP‐tagged Munc13‐1 to the plasma membrane in HEK293 cells
The ability of calphostin C but not bisindolylmaleimide I to inhibit the presynaptic effect of pregnanolone (Figure 7e,f) pointed to Munc13 as a possible target of pregnanolone action. Proteins of the Munc13 family are essential for vesicle docking/priming and their function is enhanced by phorbol esters or by endogenous DAG (Basu et al., 2007; Betz et al., 1998; Xu et al., 2017); the importance of the DAG–Munc13 interaction is underscored by the fact that mice with a single amino acid substitution in Munc13‐1(H567K) that disrupts phorbol ester/DAG binding to Munc13‐1 die within hours after birth (Rhee et al., 2002). To test for a potential direct interaction between pregnanolone (and/or pregnanolone sulfate) and the phorbol ester/DAG‐binding C1 domain of Munc13‐1 (the isoform important for glutamate release from excitatory synapses; Augustin et al., 1999), we examined the ability of pregnanolone or pregnanolone sulfate to translocate GFP‐tagged Munc13‐1 from cytosol to the plasma membrane in HEK293 cells, an effect well documented for phorbol esters (Andrews‐Zwilling et al., 2006; Betz et al., 1998). We transfected HEK293 cells with constructs encoding either GFP‐tagged WT Munc13‐1 or GFP‐tagged phorbol ester‐insensitive Munc13‐1(H567K). We used TIRF microscopy in live HEK293 cells to monitor the intensity of GFP fluorescence in the immediate vicinity of the plasma membrane before and after treatment with PDBu or one of the neurosteroids (Figure 8).
FIGURE 8.
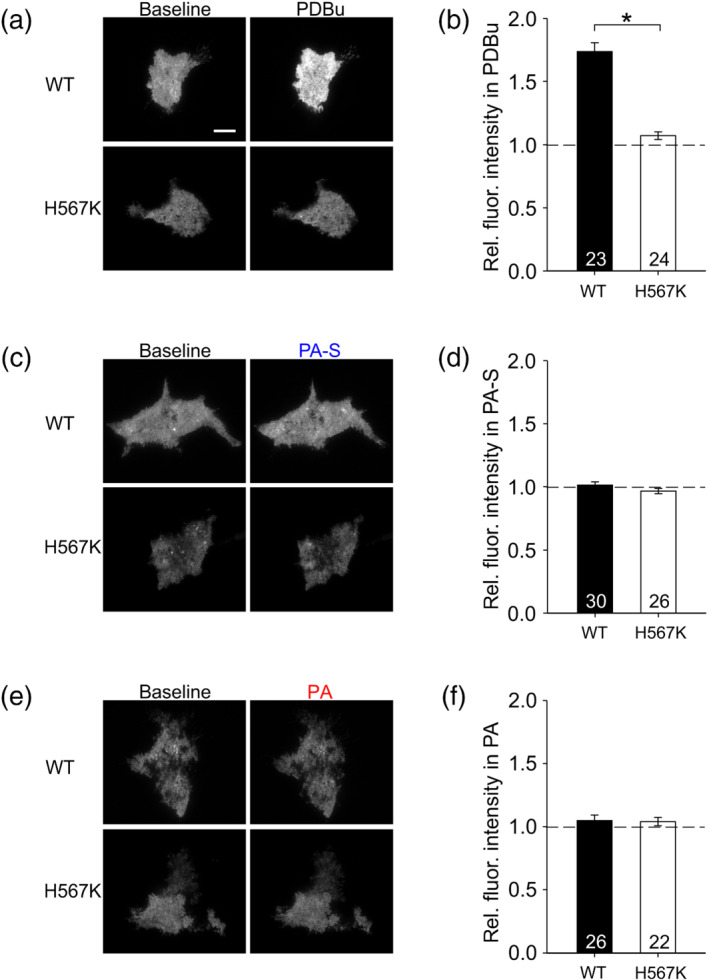
Neurosteroids pregnanolone sulfate (PA‐S) and pregnanolone (PA) fail to translocate GFP‐tagged Munc13‐1 to the plasma membrane in HEK293 cells. (a) Representative images of TIRF signal from HEK293 cells expressing GFP‐tagged Munc13‐1 WT or Munc13‐1(H567K) during baseline and in the presence of 100‐nM PDBu. Scale bar corresponds to 10 μm and applies to all images in (a), (c) and (e). (b) Mean ± SEM normalized TIRF intensity in 100‐nM PDBu in cells expressing Munc13‐1 WT or Munc13‐1(H567K). *P <.05, Mann–Whitney rank‐sum test; n values are indicated in the figure. (c) Representative images of TIRF signal from HEK293 cells expressing GFP‐tagged Munc13‐1 WT or Munc13‐1(H567K) during baseline and in the presence of 100‐μM PA‐S. (d) Mean ± SEM normalized TIRF intensity in 100‐μM PA‐S in cells expressing Munc13‐1 WT or Munc13‐1(H567K); n values are indicated. (e) Representative images of TIRF signal from HEK293 cells expressing GFP‐tagged Munc13‐1 WT or Munc13‐1(H567K) during baseline and in the presence of 10‐μM PA. (f) Mean ± SEM normalized TIRF intensity in 10‐μM PA in cells expressing Munc13‐1 WT or Munc13‐1(H567K); n values are indicated
As expected, 100‐nM PDBu applied for 40 min robustly increased the TIRF signal in cells expressing WT Munc13‐1, but not in cells expressing the phorbol ester‐insensitive Munc13‐1(H567K) (Figure 8a,b). A similar experiment with 100‐μM pregnanolone sulfate showed no effect of the steroid on membrane‐associated GFP fluorescence either in WT Munc13‐1‐expressing cells or in cells expressing the Munc13‐1(H567K) mutant (Figure 8c,d). Based on our observation that calphostin C, an inhibitor of phorbol ester/DAG binding to C1 domains of target proteins, partly blocks glutamate release potentiation by pregnanolone (Figure 7e,f), we hypothesized that pregnanolone might induce Munc13‐1 translocation to the membrane in HEK293 cells, similar to the effect of PDBu. However, we observed no effect of 10‐μM pregnanolone on membrane‐associated GFP fluorescence either in WT Munc13‐1‐expressing cells or in cells expressing the Munc13‐1(H567K) mutant (Figure 8e,f). Thus, our TIRF imaging experiments found no evidence of neurosteroid‐induced translocation of the vesicle priming protein Munc13‐1 in HEK293 cells. Together, our data suggest that neurosteroid pregnanolone may potentiate glutamate release by influencing Munc13‐1 function indirectly or by interacting with some other C1 domain‐containing DAG target.
4. DISCUSSION
We describe novel presynaptic modes of action of GABAA/NMDA receptor‐modulating neurosteroids pregnanolone sulfate and pregnanolone to potentiate spontaneous and evoked synaptic glutamate release in primary hippocampal cultures from male rats. In contrast to reported presynaptic effects of other neurosteroids such as pregnenolone sulfate, pregnanolone sulfate and pregnanolone potentiate glutamate release downstream of presynaptic Ca2+ influx. Interestingly, the effects of pregnanolone sulfate and pregnanolone appear distinct; the mechanism of action of pregnanolone, but not of pregnanolone sulfate, partially overlaps with that of a phorbol ester PDBu. Our findings suggest the possibility that the neurosteroid pregnanolone signals via a phorbol ester/DAG‐binding C1 domain of a presynaptic target protein to enhance the release of glutamate from excitatory synapses.
In comparing the effects of pregnanolone and PDBu, multiple observations suggest that their mechanisms of action may overlap, but are not the same. First, the occlusion of the pregnanolone‐induced eEPSC potentiation by PDBu, although significant, is incomplete (Figure 6), suggesting that pregnanolone potentiates release in part through an independent mechanism. Second, although the potentiation of eEPSCs by PDBu is reduced by two different PKC inhibitors calphostin C and bisindolylmaleimide I, the effect of pregnanolone is inhibited by calphostin C but unaffected by bisindolylmaleimide I (Figure 7). These data support a role of PKC in the PDBu‐induced presynaptic potentiation of hippocampal excitatory synapses (Wierda et al., 2007) and confirm the involvement of a phorbol ester/DAG‐binding C1 domain, of PKC and/or another target protein, in PDBu signalling. Pregnanolone effect, however, seems to involve an interaction with a C1 domain of a target protein other than PKC. Third, our TIRF imaging experiment (Figure 8) clearly demonstrates that PDBu interacts with a phorbol ester/DAG‐binding C1 domain of Munc13‐1 protein and translocates it into the plasma membrane in HEK293 cells (Andrews‐Zwilling et al., 2006; Betz et al., 1998), but we found no evidence of a similar direct interaction between Munc13‐1 and neurosteroid pregnanolone. Together, our results suggest that pregnanolone and PDBu share the ability to robustly potentiate synaptic glutamate release via a signalling process involving a DAG‐sensitive C1 domain of a target protein, but exactly how neurosteroid pregnanolone achieves this is still unclear.
The observation that pregnanolone treatment is not sufficient to translocate Munc13‐1 to the membrane in HEK293 cells does not rule out the possibility that pregnanolone may influence Munc13‐1 function in neurons. At synapses, a significant portion of Munc13‐1 and Munc13‐2 resides in association with membranes (Brose et al., 1995). Munc13‐1 contains several structural features that likely cooperate in anchoring the protein to the plasma membrane: the DAG‐binding C1 domain is found near the C2B domain that binds phosphatidylinositol 4,5‐biphosphate‐containing membranes in a Ca2+‐dependent manner and the region is rich in basic residues that may interact with the membrane even in the absence of DAG or elevated Ca2+ (Liu et al., 2016; Xu et al., 2017); thus, it is conceivable that neurosteroid pregnanolone could interact with Munc13‐1 already associated with presynaptic plasma membrane. Another possibility consistent with our findings is that pregnanolone could enhance endogenous DAG production in neurons and thus activate Munc13‐1 (and/or other DAG‐sensitive targets) indirectly. This would account for the observed similarity between pregnanolone and PDBu effects and might explain the inability of pregnanolone to translocate Munc13‐1 to the membrane in HEK293 cells that presumably lack some necessary upstream signalling component(s). Alternatively, pregnanolone may interact with a C1 domain‐containing DAG‐sensitive target protein other than PKC or Munc13‐1 (Brose & Rosemund, 2002; Newton, 2018).
Specific presynaptic mechanism(s) of neurosteroid action could have important implications in the context of short‐term and long‐term synaptic plasticity. Although pregnanolone sulfate and pregnanolone both enhance glutamate release, their effects on synaptic plasticity could differ. We find that pregnanolone sulfate has a stronger effect on paired‐pulse depression than pregnanolone or PDBu (Figure 5), suggesting that pregnanolone sulfate may produce a particularly strong short‐term depression of high‐frequency input. Short‐term plasticity is believed to optimize information transfer within neuronal circuits (Rotman et al., 2011) and recent modelling studies propose that short‐term depression enhances the capacity of circuits to accommodate long‐term synaptic plasticity (Hiratani & Fukai, 2014; Zeng et al., 2019). Changes in short‐term and long‐term plasticity have been implicated in various neuropathologies associated with altered glutamatergic transmission, including neurodevelopmental disorders such as schizophrenia and autism (Crabtree & Gogos, 2014), and neurodegenerative disorders such as Alzheimer's disease (Chakroborty et al., 2019). It will be important to determine how the modulation of synaptic plasticity by neurosteroids may affect their therapeutic potential.
Interestingly, some mechanisms of neuromodulation may differ between the sexes and this may be particularly important in the context of neurosteroids (e.g., Jain et al., 2019). Despite recent clinical advances (Meltzer‐Brody et al., 2018), physiological roles of neurosteroids in males and females are poorly understood. Here, we studied single‐sex (male) rather than mixed‐sex cultures, so as not to lose information about sex as a biological variable. Potential sex differences in presynaptic modulation by pregnanolone sulfate and pregnanolone should be explicitly addressed in future studies.
For any given neurosteroid, its presynaptic and postsynaptic effects presumably combine to exert corresponding synaptic, circuit‐level and behavioural changes. The mechanism of antidepressant action of GABAA receptor‐potentiating nonsulfated neurosteroids, such as allopregnanolone, is still not completely clear (Zorumski et al., 2019). Although the GABAA receptor modulation is probably key for the neurosteroid therapeutic effect, affective disorders have been associated with impaired GABAergic as well as glutamatergic synaptic transmission (Duman et al., 2019) and a contribution of increased presynaptic glutamate release to neurosteroid modulation of mood and anxiety cannot be ruled out.
The specific combinations of synaptic effects of sulfated neurosteroids could be particularly beneficial. Due to use‐dependent NMDA receptor inhibition, pregnanolone sulfate and its synthetic analogues are neuroprotective against excitotoxic neurodegeneration (Rambousek et al., 2011). Importantly, pregnanolone sulfate analogue pregnanolone hemipimelate is remarkably selective for blocking tonic but not synaptic NMDA receptor activation in vitro and is neuroprotective without inducing psychomimetic side effects in vivo (Vyklicky et al., 2016). The preservation of synaptic transmission may be in part due to presynaptic glutamate release potentiation compensating for postsynaptic NMDA receptor inhibition, as shown here for pregnanolone sulfate. Further, our present finding that the presynaptic potentiation by pregnanolone sulfate does not involve presynaptic Ca2+ influx or Ca2+ release from internal stores is relevant, because a compound elevating intracellular Ca2+ concentration might be less compatible with neuroprotection. On the other hand, presynaptic effects of NMDA receptor‐potentiating neurosteroids, such as pregnenolone sulfate, could strongly amplify their ability to compensate for insufficient NMDA receptor function (Vyklicky et al., 2018) associated with schizophrenia or other neurodevelopmental disorders. Thus, the presynaptic dimension of neurosteroid action, far from representing an irrelevant or an undesirable side effect, may actually contribute to neurosteroid therapeutic advantage.
AUTHOR CONTRIBUTIONS
T.S. and L.V. designed the research. T.S., M.K., M.C.S. and D.H. performed the electrophysiological experiments and analysis. J.K. and B.H.K. performed the TIRF microscopy experiments. E.K. and H.C. synthesized the steroids. T.S. wrote the manuscript. All authors discussed the results and commented on the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis and Animal Experimentation and as recommended by funding agencies, publishers and other organizations engaged with supporting research.
ACKNOWLEDGEMENTS
This work was supported by Czech Science Foundation (Grantová agentura České republiky): 16‐03913Y; Technology Agency of the Czech Republic (Technologická agentura České republiky): TN01000013; European Regional Development Fund: PharmaBrain CZ.02.1.01/0.0/0.0/16_025/0007444; Research Project of the Czech Academy of Sciences (Akademie věd České republiky): RVO: 67985823 and RVO: 61388963; and BIOCEV—Biotechnology and Biomedicine Centre of the Academy of Sciences and Charles University in Vestec, project supported from European Regional Development Fund. We thank Magda Kuntosova for excellent technical assistance and Prof Nils Brose, Max Planck Institute of Experimental Medicine, Göttingen, Germany, for a generous gift of the GFP‐tagged Munc 13‐1 WT and GFP‐tagged Munc 13‐1(H567K) expression vectors.
Smejkalova, T. , Korinek, M. , Krusek, J. , Hrcka Krausova, B. , Candelas Serra, M. , Hajdukovic, D. , Kudova, E. , Chodounska, H. , & Vyklicky, L. (2021). Endogenous neurosteroids pregnanolone and pregnanolone sulfate potentiate presynaptic glutamate release through distinct mechanisms. British Journal of Pharmacology, 178(19), 3888–3904. 10.1111/bph.15529
Funding information BIOCEV—Biotechnology and Biomedicine Centre of the Academy of Sciences and Charles Univeristy in Vestec; European Regional Development Fund, Grant/Award Number: PharmaBrain CZ.02.1.01/0.0/0.0/16_025/0007444; Czech Science Foundation (Grantová agentura České republiky), Grant/Award Number: 16‐03913Y; Research Project of the Czech Academy of Sciences (Akademie věd České republiky), Grant/Award Numbers: RVO: 61388963, RVO: 67985823; Technology Agency of the Czech Republic (Technologická agentura České republiky), Grant/Award Number: TN01000013
Contributor Information
Tereza Smejkalova, Email: tereza.smejkalova@fgu.cas.cz.
Ladislav Vyklicky, Email: ladislav.vyklicky@fgu.cas.cz.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , CGTP Collaborators , Aldrich, R. W. , Becirovic, E. , Biel, M. , Catterall, W. A. , Conner, A. C. , … Zhu, M. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176(S1), S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Zwilling, Y. S. , Kawabe, H. , Reim, K. , Varoqueaux, F. , & Brose, N. (2006). Binding to Rab3A‐interacting molecule RIM regulates the presynaptic recruitment of Munc13‐1 and ubMunc13‐2. Journal of Biological Chemistry, 281(28), 19720–19731. 10.1074/jbc.M601421200 [DOI] [PubMed] [Google Scholar]
- Arnostova, L. M. , Pouzar, V. , & Drasar, P. (1992). Synthesis of the sulfates derived from 5α‐cholestane‐3β,6α‐diol. Steroids, 57, 1991–1993. [DOI] [PubMed] [Google Scholar]
- Augustin, I. , Rosenmund, C. , Südhof, T. C. , & Brose, N. (1999). Munc13‐1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature, 400(6743), 457–461. 10.1038/22768 [DOI] [PubMed] [Google Scholar]
- Basu, J. , Betz, A. , Brose, N. , & Rosenmund, C. (2007). Munc13‐1 C1 domain activation lowers the energy barrier for synaptic vesicle fusion. Journal of Neuroscience, 27(5), 1200–1210. 10.1523/JNEUROSCI.4908-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli, D. , & Lambert, J. J. (2005). Neurosteroids: Endogenous regulators of the GABAA receptor. Nature Reviews Neuroscience, 6(7), 565–575. 10.1038/nrn1703 [DOI] [PubMed] [Google Scholar]
- Betz, A. , Ashery, U. , Rickmann, M. , Augustin, I. , Neher, E. , Südhof, T. C. , Rettig, J. , & Brose, N. (1998). Munc13‐1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron, 21(1), 123–136. 10.1016/S0896-6273(00)80520-6 [DOI] [PubMed] [Google Scholar]
- Brose, N. , Hofmann, K. , Hata, Y. , & Südhof, T. C. (1995). Mammalian homologues of Caenorhabditis elegans unc‐13 gene define novel family of C2‐domain proteins. Journal of Biological Chemistry, 270(42), 25273–25280. 10.1074/jbc.270.42.25273 [DOI] [PubMed] [Google Scholar]
- Brose, N. , & Rosemund, C. (2002). Move over protein kinase C, you've got company: Alternative cellular effectors of diacylglycerol and phorbol esters. Journal of Cell Science, 115(23), 4399–4411. 10.1242/jcs.00122 [DOI] [PubMed] [Google Scholar]
- Burgalossi, A. , Jung, S. Y. , Man, K.‐n. M. , Nair, R. , Jockusch, W. J. , Wojcik, S. M. , Brose, N. , & Rhee, J.‐S. (2012). Analysis of neurotransmitter release mechanisms by photolysis of caged Ca2+ in an autaptic neuron culture system. Nature Protocols, 7(7), 1351–1365. 10.1038/nprot.2012.074 [DOI] [PubMed] [Google Scholar]
- Capogna, M. , Gähwiler, B. H. , & Thompson, S. M. (1996). Presynaptic inhibition of calcium‐dependent and ‐independent release elicited with ionomycin, gadolinium, and α‐latrotoxin in the hippocampus. Journal of Neurophysiology, 75(5), 2017–2028. 10.1152/jn.1996.75.5.2017 [DOI] [PubMed] [Google Scholar]
- Capogna, M. , McKinney, A. R. , O'Connor, V. , Gähwiler, B. H. , & Thompson, S. M. (1997). Ca2+ or Sr2+ partially rescues synaptic transmission in hippocampal cultures treated with botulinum toxin A and C, but not tetanus toxin. Journal of Neuroscience, 17(19), 7190–7202. 10.1523/jneurosci.17-19-07190.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakroborty, S. , Hill, E. S. , Christian, D. T. , Helfrich, R. , Riley, S. , Schneider, C. , Kapecki, N. , Mustaly‐Kalimi, S. , Seiler, F. A. , Peterson, D. A. , West, A. R. , Vertel, B. M. , Frost, W. N. , & Stutzmann, G. E. (2019). Reduced presynaptic vesicle stores mediate cellular and network plasticity defects in an early‐stage mouse model of Alzheimer's disease. Molecular Neurodegeneration, 14(1), 1–21. 10.1186/s13024-019-0307-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot, C. , Robel, P. , Axelson, M. , Sjövall, J. , & Baulieu, E. E. (1981). Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proceedings of the National Academy of Sciences of the United States of America, 78, 4704–4707. 10.1073/pnas.78.8.4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree, G. , & Gogos, J. A. (2014). Synaptic plasticity, neural circuits and the emerging role of altered short‐term information processing in schizophrenia. Frontiers in Synaptic Neuroscience, 6(OCT), 1–27. 10.3389/fnsyn.2014.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Sobey, C. G. , Stanford, S. C. , Teixeira, M. M. , Wonnacott, S. , & Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews, A. , Mohr, F. , Rizun, O. , Wagner, T. F. J. , Dembla, S. , Rudolph, S. , Lambert, S. , Konrad, M. , Philipp, S. E. , Behrendt, M. , Marchais‐Oberwinkler, S. , Covey, D. F. , & Oberwinkler, J. (2014). Structural requirements of steroidal agonists of transient receptor potential melastatin 3 (TRPM3) cation channels. British Journal of Pharmacology, 171(4), 1019–1032. 10.1111/bph.12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman, R. S. , Sanacora, G. , & Krystal, J. H. (2019). Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron, 102(1), 75–90. 10.1016/j.neuron.2019.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage, N. J. , Reid, C. A. , & Fine, A. (2001). Calcium stores in hippocampal synaptic boutons mediate short‐term plasticity, store‐operated Ca2+ entry, and spontaneous transmitter release. Neuron, 29(1), 197–208. 10.1016/S0896-6273(01)00190-8 [DOI] [PubMed] [Google Scholar]
- Groffen, A. J. , Martens, S. , Arazola, R. D. , Cornelisse, L. N. , Lozovaya, N. , de Jong, A. P. H. , Goriounova, N. A. , Habets, R. L. P. , Takai, Y. , Borst, J. G. , Brose, N. , & Mcmahon, H. T. (2010). Doc2b is a high affinity Ca2+ sensor for spontaneous neurotransmitter release. Science, 327(5973), 1614–1618. 10.1126/science.1183765.Doc2b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, N. L. , Majewska, M. D. , Harrington, J. W. , & Barker, J. L. (1987). Structure‐activity relationships for steroid interaction with the gamma‐aminobutyric acidA receptor complex. Journal of Pharmacology and Experimental Therapeutics, 241(1), 346–353. [PubMed] [Google Scholar]
- Hiratani, N. , & Fukai, T. (2014). Interplay between short‐ and long‐term plasticity in cell‐assembly formation. PLoS ONE, 9(7), e101535. 10.1371/journal.pone.0101535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak, M. , Vlcek, K. , Petrovic, M. , Chodounska, H. , & Vyklicky, L. (2004). Molecular mechanism of pregnenolone sulfate action at NR1/NR2B receptors. Journal of Neuroscience, 24(46), 10318–10325. 10.1523/JNEUROSCI.2099-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori, T. , Takai, Y. , & Takahashi, T. (1999). Presynaptic mechanism for phorbol ester‐induced synaptic potentiation. Journal of Neuroscience, 19(17), 7262–7267. 10.1523/jneurosci.19-17-07262.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, A. , Huang, G. Z. , & Woolley, C. S. (2019). Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus. Journal of Neuroscience, 39(9), 1552–1565. 10.1523/JNEUROSCI.1897-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B. G. , Cho, J. H. , Choi, I. S. , Lee, M. G. , & Jang, I. S. (2011). Modulation of presynaptic GABAA receptors by endogenous neurosteroids. British Journal of Pharmacology, 164(6), 1698–1710. 10.1111/j.1476-5381.2011.01491.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausova, B. , Slavikova, B. , Nekardova, M. , Hubalkova, P. , Vyklicky, V. , Chodounska, H. , Vyklicky, L. , & Kudova, E. (2018). Positive modulators of the N‐methyl‐d‐aspartate receptor: Structure–activity relationship study of steroidal 3‐hemiesters. Journal of Medicinal Chemistry, 61(10), 4505–4516. 10.1021/acs.jmedchem.8b00255 [DOI] [PubMed] [Google Scholar]
- Lee, K. H. , Cho, J. H. , Choi, I. S. , Park, H. M. , Lee, M. G. , Choi, B. J. , & Jang, I. S. (2010). Pregnenolone sulfate enhances spontaneous glutamate release by inducing presynaptic Ca2+‐induced Ca2+ release. Neuroscience, 171(1), 106–116. 10.1016/j.neuroscience.2010.07.057 [DOI] [PubMed] [Google Scholar]
- Lilley, E. , Stanford, S. C. , Kendall, D. E. , Alexander, S. P. , Cirino, G. , Docherty, J. R. , George, C. H. , Insel, P. A. , Izzo, A. A. , Ji, Y. , Panettieri, R. A. , Sobey, C. G. , Stefanska, B. , Stephens, G. , Teixeira, M. , & Ahluwalia, A. (2020). ARRIVE 2.0 and the British Journal of Pharmacology: Updated guidance for 2020. British Journal of Pharmacology, 177(16), 3611–3616. 10.1111/bph.15178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Seven, A. B. , Camacho, M. , Esser, V. , Xu, J. , Trimbuch, T. , Quade, B. , Su, L. , Ma, C. , Rosenmund, C. , & Rizo, J. (2016). Functional synergy between the Munc13 C‐terminal C1 and C2 domains. eLife, 5(MAY2016), 1–27. 10.7554/eLife.13696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou, X. , Korogod, N. , Brose, N. , & Schneggenburger, R. (2008). Phorbol esters modulate spontaneous and Ca2+‐evoked transmitter release via acting on both Munc13 and protein kinase C. Journal of Neuroscience, 28(33), 8257–8267. 10.1523/JNEUROSCI.0550-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou, X. , Scheuss, V. , & Schneggenburger, R. (2005). Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature, 435(7041), 497–501. 10.1038/nature03568 [DOI] [PubMed] [Google Scholar]
- Majewska, M. D. , Harrison, N. L. , Schwartz, R. D. , Barker, J. L. , & Paul, S. M. (1986). Steroid hormone metabolites are barbiturate‐like modulators of the GABA receptor. Science, 232(4753), 1004–1007. 10.1126/science.2422758 [DOI] [PubMed] [Google Scholar]
- Majewska, M. D. , Mienville, J. M. , & Vicini, S. (1988). Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neuroscience Letters, 90(3), 279–284. 10.1016/0304-3940(88)90202-9 [DOI] [PubMed] [Google Scholar]
- Majewska, M. D. , & Schwartz, R. D. (1987). Pregnenolone‐sulfate: An endogenous antagonist of the γ‐aminobutyric acid receptor complex in brain? Brain Research, 404(1–2), 355–360. 10.1016/0006-8993(87)91394-1 [DOI] [PubMed] [Google Scholar]
- Mameli, M. , Carta, M. , Partridge, L. D. , & Valenzuela, C. F. (2005). Neurosteroid‐induced plasticity of immature synapses via retrograde modulation of presynaptic NMDA receptors. Journal of Neuroscience, 25(9), 2285–2294. 10.1523/JNEUROSCI.3877-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer‐Brody, S. , Colquhoun, H. , Riesenberg, R. , Epperson, C. N. , Deligiannidis, K. M. , Rubinow, D. R. , Li, H. , Sankoh, A. J. , Clemson, C. , Schacterle, A. , Jonas, J. , & Kanes, S. (2018). Brexanolone injection in post‐partum depression: Two multicentre, double‐blind, randomised, placebo‐controlled, phase 3 trials. The Lancet, 392(10152), 1058–1070. 10.1016/S0140-6736(18)31551-4 [DOI] [PubMed] [Google Scholar]
- Mennerick, S. , & Zorumski, C. F. (1995). Paired‐pulse modulation of fast excitatory synaptic currents in microcultures of rat hippocampal neurons. The Journal of Physiology, 488(1), 85–101. 10.1113/jphysiol.1995.sp020948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, D. A. , Carta, M. , Partridge, L. D. , Covey, D. F. , & Valenzuela, C. F. (2002). Neurosteroids enhance spontaneous glutamate release in hippocampal neurons. The Journal of Biological Chemistry, 277(32), 28725–28732. 10.1074/jbc.M202592200 [DOI] [PubMed] [Google Scholar]
- Newton, A. C. (2018). Protein kinase C: Perfectly balanced. Critical Reviews in Biochemistry and Molecular Biology, 53(2), 208–230. 10.1080/10409238.2018.1442408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park‐Chung, M. , Wu, F. S. , & Farb, D. H. (1994). 3α‐Hydroxy‐5β‐pregnan‐20‐one sulfate: A negative modulator of the NMDA‐induced current in cultured neurons. Molecular Pharmacology, 46(1), 146–150. [PubMed] [Google Scholar]
- Park‐Chung, M. , Malayev, A. , Purdy, R. H. , Gibbs, T. T. , & Farb, D. H. (1999). Sulfated and unsulfated steroids modulate γ‐aminobutyric acidA receptor function through distinct sites. Brain Research, 830(1), 72–87. 10.1016/S0006-8993(99)01381-5 [DOI] [PubMed] [Google Scholar]
- Percie du Sert, N. , Hurst, V. , Ahluwalia, A. , Alam, S. , Avey, M. T. , Baker, M. , Browne, W. J. , Clark, A. , Cuthill, I. C. , Dirnagl, U. , Emerson, M. , Garner, P. , Holgate, S. T. , Howells, D. W. , Karp, N. A. , Lazic, S. E. , Lidster, K. , MacCallum, C. J. , Macleod, M. , … Würbel, H. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biology, 18(7), e3000410. 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic, M. , Sedlacek, M. , Horak, M. , Chodounska, H. , & Vyklický, L. (2005). 20‐Oxo‐5β‐pregnan‐3α‐yl sulfate is a use‐dependent NMDA receptor inhibitor. Journal of Neuroscience, 25(37), 8439–8450. 10.1523/JNEUROSCI.1407-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia, G. , Santi, M. R. , Vicini, S. , Pritchett, D. B. , Purdy, R. H. , Paul, S. M. , Seeburg, P. H. , & Costa, E. (1990). Neurosteroids act on recombinant human GABAA receptors. Neuron, 4(5), 759–765. https://doi.org/10.1016/0896-6273(90)90202-Q, 10.1016/0896-6273(90)90202-Q [DOI] [PubMed] [Google Scholar]
- Rambousek, L. , Bubenikova‐Valesova, V. , Kacer, P. , Syslova, K. , Kenney, J. , Holubova, K. , Najmanova, V. , Zach, P. , Svoboda, J. , Stuchlik, A. , Chodounska, H. , Kapras, V. , Adamusova, E. , Borovska, J. , Vyklicky, L. , & Vales, K. (2011). Cellular and behavioural effects of a new steroidal inhibitor of the N‐methyl‐d‐aspartate receptor 3α5β‐pregnanolone glutamate. Neuropharmacology, 61(1–2), 61–68. 10.1016/j.neuropharm.2011.02.018 [DOI] [PubMed] [Google Scholar]
- Rhee, J. S. , Betz, A. , Pyott, S. , Reim, K. , Varoqueaux, F. , Augustin, I. , Hesse, D. , Südhof, T. C. , Takahashi, M. , Rosenmund, C. , & Brose, N. (2002). β phorbol ester‐ and diacylglycerol‐induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell, 108(1), 121–133. 10.1016/S0092-8674(01)00635-3 [DOI] [PubMed] [Google Scholar]
- Rotman, Z. , Deng, P. Y. , & Klyachko, V. A. (2011). Short‐term plasticity optimizes synaptic information transmission. Journal of Neuroscience, 31(41), 14800–14809. 10.1523/JNEUROSCI.3231-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz, A. , Campanac, E. , Scott, R. S. , Rusakov, D. A. , & Kullmann, D. M. (2010). Presynaptic GABAA receptors enhance transmission and LTP induction at hippocampal mossy fiber synapses. Nature Neuroscience, 13(4), 431–438. 10.1038/nn.2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye, H. (1941). Anesthetic effect of steroid hormones. Proceedings of the Society for Experimental Biology and Medicine, 46(1), 116–121. 10.3181/00379727-46-11907 [DOI] [Google Scholar]
- Stastna, E. , Chodounska, H. , Pouzar, V. , Kapras, V. , Borovska, J. , Cais, O. , & Vyklicky, L. (2009). Synthesis of C3, C5, and C7 pregnane derivatives and their effect on NMDA receptor responses in cultured rat hippocampal neurons. Steroids, 74(2), 256–263. 10.1016/j.steroids.2008.11.011 [DOI] [PubMed] [Google Scholar]
- Vyklicky, V. , Krausova, B. , Cerny, J. , Ladislav, M. , Smejkalova, T. , Kysilov, B. , Korinek, M. , Danacikova, S. , Horak, M. , Chodounska, H. , Kudova, E. , & Vyklicky, L. (2018). Surface expression, function, and pharmacology of disease‐associated mutations in the membrane domain of the human GluN2B subunit. Frontiers in Molecular Neuroscience, 11(April), 1–20. 10.3389/fnmol.2018.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyklicky, V. , Smejkalova, T. , Krausova, B. , Balik, A. , Korinek, M. , Borovska, J. , Horak, M. , Chvojkova, M. , Kleteckova, L. , Vales, K. , Cerny, J. , Nekardova, M. , Chodounska, H. , Kudova, E. , & Vyklicky, L. (2016). Preferential inhibition of tonically over phasically activated NMDA receptors by pregnane derivatives. Journal of Neuroscience, 36(7), 2161–2175. 10.1523/JNEUROSCI.3181-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierda, K. D. B. , Toonen, R. F. G. , de Wit, H. , Brussaard, A. B. , & Verhage, M. (2007). Interdependence of PKC‐dependent and PKC‐independent pathways for presynaptic plasticity. Neuron, 54(2), 275–290. 10.1016/j.neuron.2007.04.001 [DOI] [PubMed] [Google Scholar]
- Wu, F. S. , Gibbs, T. T. , & Farb, D. H. (1991). Pregnenolone sulfate: A positive allosteric modulator at the N‐methyl‐d‐aspartate receptor. Molecular Pharmacology, 40(3), 333–336. [PubMed] [Google Scholar]
- Xu, J. , Camacho, M. , Xu, Y. , Esser, V. , Liu, X. , Trimbuch, T. , Pan, Y. Z. , Ma, C. , Tomchick, D. R. , Rosenmund, C. , & Rizo, J. (2017). Mechanistic insights into neurotransmitter release and presynaptic plasticity from the crystal structure of Munc13‐1 C1C2BMUN. eLife, 6(FEB2017), 1–27. 10.7554/eLife.22567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio‐Bulcock, P. A. , Everett, J. , Harteneck, C. , & Valenzuela, C. F. (2011). Activation of steroid‐sensitive TRPM3 channels potentiates glutamatergic transmission at cerebellar Purkinje neurons from developing rats. Journal of Neurochemistry, 119(3), 474–485. 10.1111/j.1471-4159.2011.07441.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio‐Bulcock, P. A. , & Valenzuela, C. F. (2011). Pregnenolone sulfate increases glutamate release at neonatal climbing fiber‐to‐Purkinje cell synapses. Neuroscience, 175, 24–36. 10.1016/j.neuroscience.2010.11.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, G. , Huang, X. , Jiang, T. , & Yu, S. (2019). Short‐term synaptic plasticity expands the operational range of long‐term synaptic changes in neural networks. Neural Networks, 118, 140–147. 10.1016/j.neunet.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Zorumski, C. F. , Paul, S. M. , Covey, D. F. , & Mennerick, S. (2019). Neurosteroids as novel antidepressants and anxiolytics: GABA‐A receptors and beyond. Neurobiology of Stress, 11(September), 100196. 10.1016/j.ynstr.2019.100196 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


