Abstract
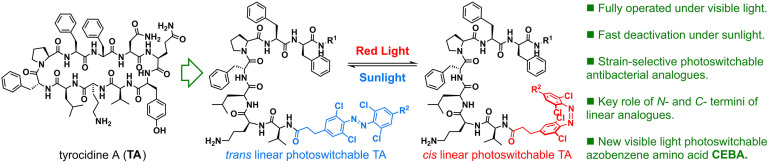
The introduction of a novel tetra‐ortho‐chloroazobenzene amino acid (CEBA) has enabled photoswitching of the antimicrobial activity of tyrocidine A analogues by using exclusively visible light, granting spatiotemporal control under benign conditions. Compounds bearing this photoswitchable amino acid become active upon irradiation with red light, but quickly turn‐off upon exposure to other visible light wavelengths. Critically, sunlight quickly triggers isomerisation of the red light‐activated compounds into their original trans form, offering an ideal platform for self‐deactivation upon release into the environment. Linear analogues of tyrocidine A were found to provide the best photocontrol of their antimicrobial activity, leading to compounds active against Acinetobacter baumannii upon isomerisation. Exploration of their N‐ and C‐termini has provided insights into key elements of their structure and has allowed obtaining new antimicrobials displaying excellent strain selectivity and photocontrol.
Keywords: antibiotics, azobenes, peptidomimetics, photochemistry, tyrocidine A
A novel photoswitchable amino acid has provided access to tyrocidine A analogues fully operated under visible light. This has led to linear analogues that have shown good photoswitchability and strain selectivity against A. baumannii and S. pyogenes clinical strains by using different analogues. Activated cis compounds turn back to their deactivated trans form upon short exposure times to sunlight, paving the way to self‐deactivating antibacterials with minor evolutionary pressure on the environment.
Antibiotic resistance is an increasing concern worldwide. Accumulation of antibiotics in the environment increases the evolutionary pressure on microorganisms, which evolve and adapt at a great pace. Moreover, the number of new antibiotic classes entering the pipeline has been alarmingly low during the last decades. [1] Although new antimicrobials are still needed, a change of paradigm on how we fight bacterial infections is on high demand as new drugs are bound to suffer from the emergence of resistances as well. [2] On this regard, gaining the ability to switch drugs on and off offers a unique opportunity to avoid the release of active drugs into the environment, thus reducing the chances of resistance arising and disseminating. In particular, visible light can be used as a clean and harmless stimulus to modulate the structure of a molecule and thus, its biological profile. [3]
Natural antimicrobial peptides play a crucial role as first line of defence against pathogenic bacteria. [4] These are produced by a plethora of microorganisms, but also by complex animals, including humans. [5]
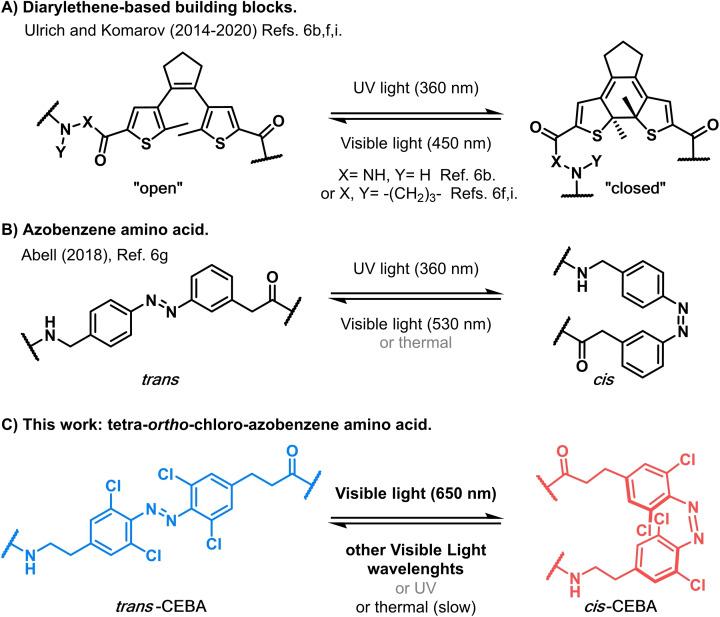
Previous works have shown how the introduction of photoswitches into small molecules can allow control over the activity of antimicrobial compounds, but only recently has the control of antibacterial activity with visible light been reported. [6] In this context, a photoswitchable amino acid fully operated under visible light is a coveted goal in order to gain control over the structure of peptides under harmless conditions.[ 7 , 8 ] Tetra‐ortho‐chloro‐azobenzenes can be isomerised efficiently under red or green light into their cis form and will quickly return to their trans isomer under other visible light sources unless kept in the dark. Importantly, the use of harmful UV light is avoided. This feature offers a unique platform for safe in situ activation of drugs, also through living tissue, and subsequent deactivation upon release into the environment after their therapeutic use, once exposed to sunlight.
The pioneering work of Feringa and co‐workers showed that photocontrol of antibacterial activity to fight the appearance of resistances is possible. [6a] Short after, Ulrich and Komarov reported the use of visible light to trigger the antimicrobial activity of a gramicidin S photoswitchable peptidomimetic, which could be deactivated upon irradiation with UV light thanks to a diaryl‐ethane photoswitch (Figure 1A).[ 6b , 6f , 6i ] Other works have also used gramicidin S as a platform to study the photocontrol of antibacterial activity in cyclic peptidomimetics (Figure 1B). [6g] However, all reports on the photocontrol of peptidomimetic's antibacterial activity require the use of UV light for one of the isomerisations. In this context, we embarked on the design of a photoswitchable amino acid building block that could be operated with visible light for both trans→cis and cis→trans isomerisations, resulting in CEBA (tetra‐ortho‐chloro‐diethylelene‐azobenzene Amino acid) (Figure 1C). Importantly, it was crucial to achieve fast cis→trans upon exposure to sunlight and other white light sources in order to achieve self‐deactivation of the compounds upon release into the environment.
Figure 1.
Photoswitchable building blocks used to photocontrol the antibacterial activity of tyrocidines’ and gramicidins’ analogues.
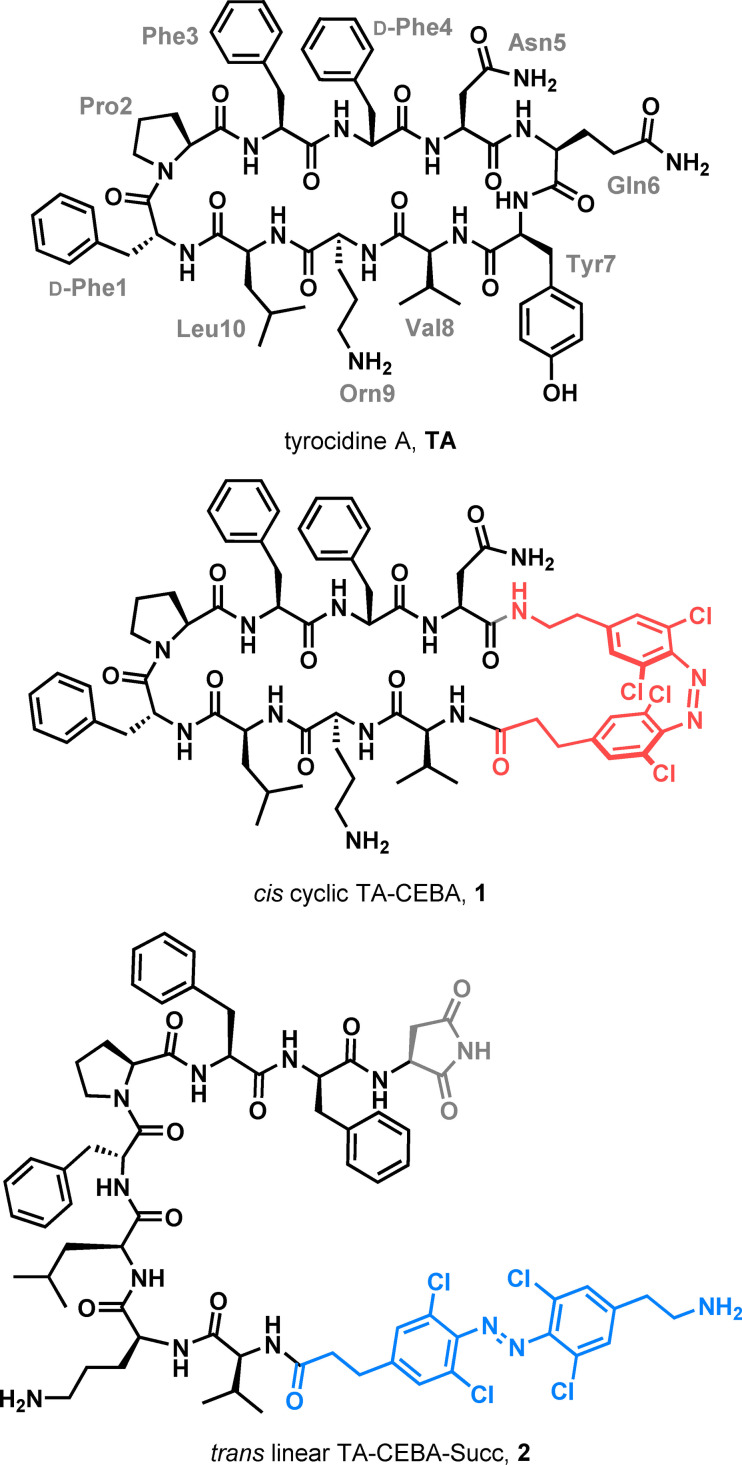
Tyrocidine A is a cyclic decapeptide with antimicrobial activity originally found along gramicidin in tyrothricin, a mixture of peptide antibiotics isolated from Brevibacillus brevis. Like gramicidin, tyrocidine is primarily active against Gram‐positive bacteria. Modification of its structure has granted access to analogues with improved biological profiles such as an expanded panel of susceptible bacterial strains and higher therapeutic indexes. [9] However, its precise mechanism of action has remained elusive. [10]
We hypothesized that modification of tyrocidine A's structure by introducing our tetra‐ortho‐chloro‐azobenzene amino acid (CEBA) in substitution of . Gln6 and Tyr7 would distort the natural peptide's β‐hairpin secondary structure while in its trans configuration. However, upon isomerisation under red light, the cis form would become the major isomer and re‐establish the biologically active secondary structure of the natural product (Figure 2).
Figure 2.
Tyrocidine A and examples of cyclic and linear visible light active photoswitchable analogues described in this work.
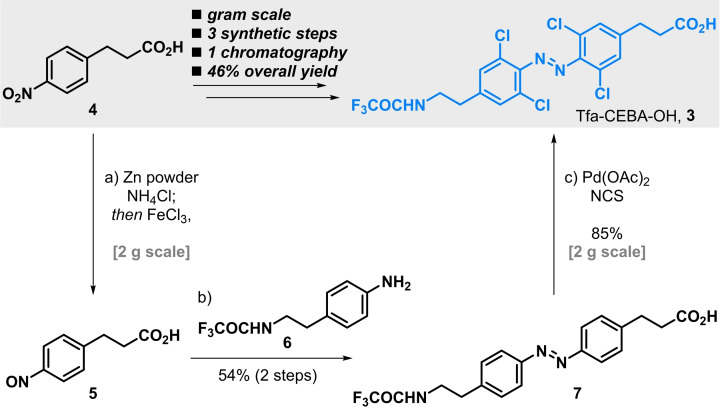
Azobenzene amino acid 3 possesses the required features and its synthesis proved to be reliable and easily scalable, offering a useful route to this building block (Scheme 1). First, 3‐(4‐nitrophenyl)propanoic acid 4 was turned into the corresponding nitroso derivative 5 and upon condensation with aniline 6, the azobenzene scaffold was set, yielding amino acid 7. Subsequent palladium‐catalysed chlorination gave the desired amino acid photoswitch 3 in very good yield.[ 11 , 12 , 13 ] Overall, Tfa‐CEBA‐OH (3) was obtained in 46 % yield in just three steps, needing only one chromatographic purification at the end of the synthesis.
Scheme 1.
Synthesis of tetra‐ortho‐chloro‐azobenzene amino acid 3 (Tfa‐CEBA‐OH). Reaction conditions: a) Zn powder (2.5 equiv), NH4Cl (1.5 equiv), H2O, 2‐methoxyethanol, 0 °C to rt, 17 h; then FeCl3 (3.0 equiv), EtOH, H2O, 0 °C, 1.5 h. b) 6 (1 equiv), AcOH, rt, 18 h, 54 % (2 steps). c) Pd(OAc)2, NCS (6 equiv), AcOH, 140 °C, 19 h, 85 %. Tfa=trifluoroacetyl; NCS=N‐chlorosuccinimide.
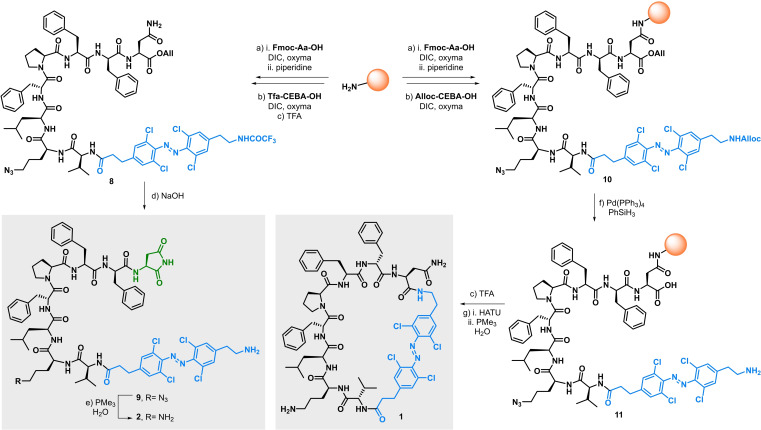
At this stage, we were ready to synthesize a photoswitchable analogue of tyrocidine A using solid phase peptide synthesis (SPPS) starting from Gln5 anchored from its amide side chain to the resin (Scheme 2). Elongation of the peptide was performed uneventfully and TFA‐CEBA‐OH (3) was introduced last, prior to acidic cleavage to produce 8. Subsequent basic hydrolysis in solution did not yield the expected N‐ and C‐ termini unprotected peptide, but a product with the same m/z as the target photoswitchable cyclic tyrocidine A analogue. Further analysis and mechanistic studies revealed that basic conditions had triggered cyclisation of the Asn residue side chain onto the allyl ester to form succinimide 9, whose unanticipated structure prompted us to study it further and carry on with the remaining deprotection step. [14] Thus, the azide group in 9 was reduced to yield the corresponding amine 2 via a mild Staudinger reduction. [15] Alternatively, and in order to obtain the desired cyclic analogue 1, The photoswitch was introduced as its allyl carbamate analogue Alloc‐CEBA‐OH, which was obtained quantitatively from 3 in a two‐step, one‐pot operation. [14] Once peptide 10 was ready, both termini were deprotected under Pd catalysis to yield 11. At this stage, conditions were screened to optimise cyclisation of the peptide, which proved to be very reagent and solvent dependent. [14] In the end, the cyclised product was most efficiently obtained in solution, and a one‐pot Staudinger reduction was performed last to reveal the free amine in 1.
Scheme 2.
Synthesis of linear and cyclic Visible Light photoswitchable tyrocidine A analogues 1 and 2. Reaction conditions: a) see Supporting Information for details on manual and microwave‐assisted SPPS methods. b) Alloc‐CEBA‐OH or Tfa‐CEBA‐OH (2.3 equiv), DIC (2.5 equiv), oxyma (2.5 equiv), DMF, rt, 16 h. c) TFA/CH2Cl2 (95 : 5), rt, 5×45 min. d) NaOH (3.5 equiv), THF, MeOH, H2O, rt. e) PMe3 (3 equiv), H2O, THF, 0 °C to rt. f) Pd(PPh3)4 (10 mol %), PhSiH3 (20 equiv), CH2Cl2, rt, 15 min. g) HATU (2 equiv), DIPEA (6 equiv), DMF, rt, 16 h; then PMe3 (5 equiv), H2O, 0 °C to rt. Alloc=Allyloxycarbonyl; DIC=N,N’‐diisopropylcarbodiimide; oxyma=ethyl cyanohydroxyiminoacetate; DMF=dimethylformamide; TFA=trifluoroacetic acid; THF=tetrahydrofuran HATU=1‐[bis(dimethylamino)methylene]‐1H‐1,2,3‐triazolo[4,5‐b]pyridinium 3‐oxide hexafluorophosphate; DIPEA=diisopropylethylamine.
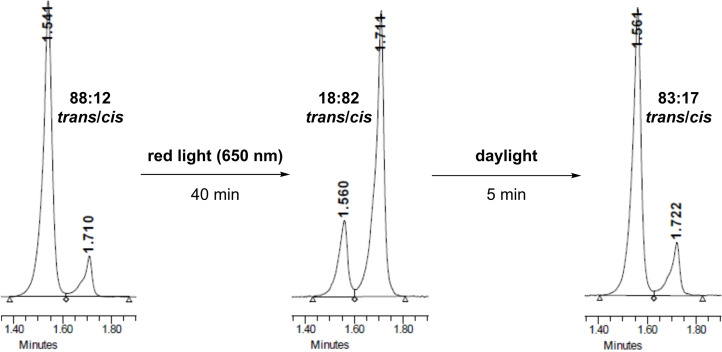
Photoisomerisation of the compounds was studied using 1 and 2 as models for cyclic and linear compounds, respectively. A kinetic profile of trans to cis isomerisation under red light (650 nm) was recorded and in both cases a virtually identical behaviour was observed. [14] Upon exposure to red light, the amount of cis isomer reaches a plateau after ca. 40 min, indicating the minimum time required to achieve the highest ratio of cis isomer under these conditions. The reverse process was also studied using daylight to irradiate the samples, as it would be the ideal source of light to trigger deactivation of the compounds. [14] To our delight, less than three minutes were required for the opposite isomerisation, highlighting the viability of the proposed idea of self‐deactivation upon release into the environment (Figure 3). Importantly, the same behaviour was observed for all compounds described in this work.
Figure 3.
Photoisomerisation of 2. UPLC analysis of sequential trans→cis and cis→trans isomerisation of 2.
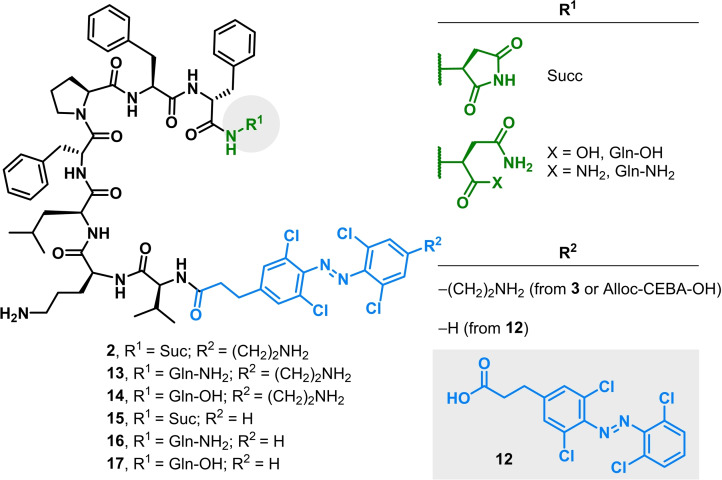
With 1 and 2 in our hands, we carried out some preliminary antibacterial activity assays, which showed a more interesting biological profile for 2 (see below). This finding prompted us to investigate further the role of the N‐ and C‐termini moieties present in this linear analogue. Thus, using the same synthetic approach, we prepared a small library of linear peptides with modifications on both termini. These included substitution of the photoswitchable amino acid CEBA for a photoswitchable carboxylic acid analogue without the 2‐aminoethyl tail (12) and substitution of the Gln‐derived succinimide moiety for the corresponding glutamine carboxylic acid or glutamine amide (Figure 4).
Figure 4.
Library of linear photoswitchable analogues of tyrocidine A.
With all the analogues in hand, we next tested them against a panel of Gram‐positive and Gram‐negative bacterial strains (see the complete table in the Supporting Information). To our surprise, cyclic analogue 1 displayed a lack of photocontrol against all strains tested and its biological profile was very similar to TA, with the exception of A. baumannii (Table 1). Very interestingly, linear analogue 2 also had a similar profile, although photoswitchability against A. baumannii (both a susceptible ‐ATCC 19606‐ and a colistin‐resistance strain ‐CR17‐) was now observed and at least a 3‐fold decrease in the MIC of the compound was observed upon activation with red light. Substitution of the Gln‐derived succinimide for the corresponding glutamine amide (13) or carboxylic acid (14) had completely opposite effects on the compounds’ activity against A. baumannii, showing lower MICs for analogue 13 (8–16 μg mL−1) than analogue 14 (>64 μg mL−1) in either photostationary state, whereas they behaved very similarly against the rest of the panel. Removal of the 2‐aminoethyl tail from CEBA (analogues 15–17) drastically changed the biological profile of the peptides, as they lost activity against most strains, including Gram‐positive bacteria. However, upon irradiation at 650 nm, an increased susceptibility was observed for compounds 16 and 17 against S. pyogenes. In particular, 17 displayed great selectivity against this strain, whereas it remained inactive against all the other ones tested during this study.
Table 1.
Selected in vitro antibacterial activities of TA and its photoswitchable analogues.[a]
|
|
TA |
1 |
2 |
13 |
14 |
15 |
16 |
17 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
NoIrr |
Irr[b] |
NoIrr |
Irr[b] |
NoIrr |
Irr[b] |
NoIrr |
Irr[b] |
NoIrr |
Irr[b] |
NoIrr |
Irr[b] |
NoIrr |
Irr[b] |
NoIrr |
Irr[b] |
|
Bacillus subtilis [c] |
4 |
4 |
4 |
8 |
2 |
2 |
1 |
2 |
4 |
16 |
>64 |
>64 |
64 |
64 |
>64 |
>64 |
|
Streptococcus pyogenes 016[c] |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
8 |
4 |
>64 |
64 |
>64 |
16 |
>64 |
16 |
|
Escherichia coli ATCC 25922[d] |
>64 |
>64 |
>64 |
>64 |
>64 |
64 |
>64 |
32 |
>64 |
>64 |
>64 |
>64 |
>64 |
>64 |
>64 |
>64 |
|
Acinetobacter baumannii ATCC 19606[d] |
16 |
16 |
>64 |
>64 |
64 |
8 |
8 |
8 |
>64 |
>64 |
>64 |
>64 |
>64 |
>64 |
>64 |
>64 |
|
Acinetobacter baumannii CR17[d] |
32 |
16 |
>64 |
>64 |
>64 |
32 |
16 |
8 |
>64 |
>64 |
>64 |
>64 |
>64 |
>64 |
>64 |
>64 |
[a] Measured as minimum inhibitory concentration (MIC) in μg mL−1. [b] Plates were irradiated at 650 nm for 40 min before incubation in the dark. [c] Gram‐positive strain. [d] Gram‐negative strain.
Depending on the compound, differences were also observed in terms of their haemolytic effect. [14] TA, 2, 13 and 15 were less haemolytic in their non‐irradiated state compared to the irradiated one, whereas compounds 1, 14, 16 and 17 showed the opposite effect. In the case of compound 17, which showed good antibacterial activity against S. pyogenes in the irradiated state, a high IC50 value (173 μg mL−1) was also observed (low haemolytic effect), which can likely be attributed to its lack of a net positive charge under the assay conditions and is an optimistic starting point for therapeutic purposes.
In conclusion, we have developed an efficient synthesis of the novel tetra‐ortho‐chloro‐photoswitchable azobenzene amino acid (CEBA) that can be incorporated into peptidic sequences to grant photocontrol using exclusively visible light. This has led to the development of 2, a photoswitchable tyrocidine A linear analogue with a C‐terminal succinimide moiety that can be activated under red light to become active against a clinically relevant Gram‐negative strain such as A. baumannii. Importantly, the compound quickly becomes inactive against this pathogenic strain upon exposure to sunlight, enabling self‐immolation upon release to the environment. Taking into consideration the available data for the linear analogue 17, selectivity and photocontrol have been observed against S. pyogenes, achieving the highest IC50 value in both photostationary states out of all the analogues tested. To confirm their antimicrobial effect against this species, further studies increasing the number of S. pyogenes strains should be conducted. In addition, the decreased toxicity (haemolytic assay) observed in some of the non‐irradiated compounds is an important added value of this strategy that may also contribute to preserving the ecosystem and should be considered in further studies. We hope that these findings will help advancing in the development of more efficient strain selective and self‐deactivating photoswitchable antimicrobials that help diminish the increasing threat of antibiotic resistance.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
We thank AGAUR and Generalitat de Catalunya for a Beatriu de Pinós fellowship to X.J.B. We acknowledge support from the Spanish Ministry of Science and Innovation through the “Centro de Excelencia Severo Ochoa 2019–2023” Program (CEX2018‐000806‐S), and support from the Generalitat de Catalunya through the CERCA Program. Supported by Plan Nacional de I+D+i 2013–2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0010) ‐ co‐financed by European Development Regional Fund “A way to achieve Europe”, Operative program Intelligent Growth 2014–2020. We would also like to thank Prof. Pau Gorostiza and Dr. Rossella Castagna for lending us their 650 nm LED lamp.
X. Just-Baringo, A. Yeste-Vázquez, J. Moreno-Morales, C. Ballesté-Delpierre, J. Vila, E. Giralt, Chem. Eur. J. 2021, 27, 12987.
Dedicated to Prof. Mercedes Álvarez on her retirement
References
- 1.
- 1a.World Health Organization. Worldwide country situation analysis: Response to antimicrobial resistance, April 2015; http://apps.who.int/iris/bitstream/10665/163468/1/9789241564946_eng.pdf?ua=1&ua=1;
- 1b. Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., et al. Lancet Infect. Dis. 2018, 18, 318–327; [DOI] [PubMed] [Google Scholar]
- 1c. Theuretzbacher U., Outterson K., Engel Al., Karlén A., Nat. Rev. Microbiol. 2020, 18, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For reviews on alternative approaches to tackle antibacterial resistance, see:
- 2a. Liu Y., Li R., Xiao X., Wang Z., Crit. Rev. Microbiol. 2019, 45, 301–304; [DOI] [PubMed] [Google Scholar]
- 2b. Huang C.-H., Hsieh Y.-H., Powers Z. M., Kao C.-Y., Int. J. Mol. Sci. 2020, 21, 1061. [Google Scholar]
- 3.For selected reviews on photopharmacology, see:
- 3a. Velema W. A., Szymanski W., Feringa B. L., J. Am. Chem. Soc. 2014, 136, 2178–2191; [DOI] [PubMed] [Google Scholar]
- 3b. Broichhagen J., Frank J. A., Trauner D., Acc. Chem. Res. 2015, 48, 1947–1960; [DOI] [PubMed] [Google Scholar]
- 3c. Lerch M. M., Hansen M. J., van Dam G. M., Szymanski W., Feringa B. L., Angew. Chem. Int. Ed. 2016, 55, 10978–10999; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 11140–11163; [Google Scholar]
- 3d. Hüll K., Morstein J., Trauner D., Chem. Rev. 2018, 118, 10710–10747; [DOI] [PubMed] [Google Scholar]
- 3e. Fuchter M. J., J. Med. Chem. 2020, 63, 11436–11447. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Ageitos J. M., Sánchez-Pérez A., Calo-Mata P., Villa T. G., Biochem. Pharmacol. 2017, 133, 117–138; [DOI] [PubMed] [Google Scholar]
- 4b. Huan Y., Kong Q., Mou H., Yi H., Front. Microbiol. 2020, 11, 582779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zipperer A., Konnerth M. C., Laux C., Berscheid A., Janek D., Weidenmaier C., Burian M., Schilling N. A., Slavetinsky C., Marschal M., Willmann M., Kalbacher H., Schittek B., Brötz-Oesterhelt H., Grond S., Peschel A., Krismer B., Nature 2016, 535, 511–516. [DOI] [PubMed] [Google Scholar]
- 6.For reports on photoswitchable antibiotics, see:
- 6a. Velema W. A., van der Berg J. P., Hansen M. J., Szymanski W., Driessen A. J. M., Feringa B. L., Nat. Chem. 2013, 5, 924–928; [DOI] [PubMed] [Google Scholar]
- 6b. Babii O., Afonin S., Berditsch M., Reißer S., Mykhailiuk P. K., Kubyshkin V. S., Steinbrecher T., Ulrich A. S., Komarov I. V., Angew. Chem. Int. Ed. 2014, 53, 3392–3395; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 3460–3463; [Google Scholar]
- 6c. Hu Y., Marlow J. B., Ramanathan R., Zou W., Tiew H. G., Pottage M. J., Bansal V., Tabor R. F., Wilkinson B. L., Aust. J. Chem. 2015, 68, 1880–1884; [Google Scholar]
- 6d. Velema W. A., Hansen M. J., Lerch M. M., Driessen A. J. M., Szymanski W., Feringa B. L., Bioconjugate Chem. 2015, 26, 2592–2597; [DOI] [PubMed] [Google Scholar]
- 6e. Wegener M., Hansen M. J., Drieseen A. J. M., Szymanski W., Feringa B. L., J. Am. Chem. Soc. 2017, 139, 17979–17986; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6f. Babii O., Afonin S., Ishchenko A. Y., Schober T., Negelia A. O., Tolstanova G. M., Garmanchuk L. V., Ostapchenko L. I., Komarov I. V., Ulrich A. S., J. Med. Chem. 2018, 61, 10793–10813; [DOI] [PubMed] [Google Scholar]
- 6g. Yeoh Y. Q., Yu J., Polyak S. W., Horsley J. R., Abell A. D., ChemBioChem 2018, 19, 2591–2597; [DOI] [PubMed] [Google Scholar]
- 6h. Cheng H.-B., Li X., Kwon N., Fang Y., Baek G., Yoon J., Chem. Commun. 2019, 55,12316–12319; [DOI] [PubMed] [Google Scholar]
- 6i. Afonin S., Babii O., Reuter A., Middel V., Takamiya M., Strähle U., Komarov I. V., Ulrich A. S., Beilstein J. Org. Chem. 2020, 16, 39–49; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6j. Yeoh Y. Q., Horsley J. R., Yu J., Polyak S. W., Jovcevski B., Abell A. D., ChemMedChem 2020, 15, 1505–1508. [DOI] [PubMed] [Google Scholar]
- 7.To the best of our knowledge, only one visible light operated azobenzene amino acid has been reported to date: Albert L., Peñalver A., Djokovic N., Werel L., Hoffarth M., Ruzic D., Xu J., Essen L.-O., Nikolic K., Dou Y., Vázquez O., ChemBioChem 2019, 20, 1417–1429. [DOI] [PubMed] [Google Scholar]
- 8.For reviews on photoswitchable peptides, see:
- 8a. Ciardelli F., Bronco S., Pieroni O., Pucci A. in Molecular Switches Chapter 13: Photoswitchable Polypeptides, 2nd Ed. (B. L. Feringa Edd., Browne W. R.), Wiley-VCH Verlag GmbH, 2011, pp. 321–360; [Google Scholar]
- 8b. Beharry A. A., Woolley G. A., Chem. Soc. Rev. 2011, 40, 4422–4437; [DOI] [PubMed] [Google Scholar]
- 8c. Szymanski W., Beierle J. M., Kistemaker H. A. V., Velema W. A., Feringa B. L., Chem. Rev. 2013, 113, 6114–6178; [DOI] [PubMed] [Google Scholar]
- 8d. Kitzig S., Thilemann M., Cordes T., Rück-Braun K., ChemPhysChem 2016, 17,1252–1263; [DOI] [PubMed] [Google Scholar]
- 8e. Alvert L., Vázquez O., Chem. Commun. 2019, 55, 10192–10213; [DOI] [PubMed] [Google Scholar]
- 8f. Peddie V., Abell A. D., J. Photochem. Photobiol. C 2019, 40, 1–20. [Google Scholar]
- 9.For selected reports on tyrocidine A's SAR, see:
- 9a. Kohli R. M., Walsh C. T., Burkart M. D., Nature 2002, 418, 658–661; [DOI] [PubMed] [Google Scholar]
- 9b. Bu X., Wu X., Xie G., Guo Z., Org. Lett. 2002, 4, 2893–2895; [DOI] [PubMed] [Google Scholar]
- 9c. Qin C., Zhong X., Bu X., Ng N. L. J., Guo Z., J. Med. Chem. 2003, 46, 4830–4833; [DOI] [PubMed] [Google Scholar]
- 9d. Marques M. A., Citron D. M., Wang C. C., Bioorg. Med. Chem. 2007, 15, 6667–6677; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9e. Xiao Q., Pei D., J. Med. Chem. 2007, 50, 3132–3137; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9f. Munyuki G., Jackson G. E., Venter G. A., Kövér K. E., Szilágyi L., Rautenbach M., Spathelf B. M., Bhattacharya B., van der Spoel D. Biochemistry 2013, 52, 44, 7798–7806; [DOI] [PubMed] [Google Scholar]
- 9g. Leussa A. N.-N., Rautenbach M., Chem. Biol. Drug Des. 2014, 84, 543–557; [DOI] [PubMed] [Google Scholar]
- 9h. Cameron A. J., Edwards P. J. B., Harjes E., Sarojini V., J. Med. Chem. 2017, 60, 9565–9574. [DOI] [PubMed] [Google Scholar]
- 10.For recent reports on tyrocidine A's mechanism of action, see:
- 10a. Loll P. J., Upton E. C., Nahoum V., Economou N. J., Cocklin S., Biochim. Biophys. Acta 2014, 1838, 1199–1207; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b. Wenzel M., Rauntenbach M., Vosloo J. A., Siersma T., Aisenbrey C. H. M., Zaitseva E., Laubscher W. E., van Rensburg W., Behrends J. C., Bechinger B., Hamoen L. W., mBio 2018, 9, e00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.For the tetra-ortho-halogenation of azobenzenes, see:
- 11a. Konrad D. B., Frank J. A., Trauner D., Chem. Eur. J. 2016, 22, 4364–4368; [DOI] [PubMed] [Google Scholar]
- 11b. Nguyen T. H. L., Gigant N., Joseph D., ACS Catal. 2018, 8, 1546–1579; [Google Scholar]
- 11c. Poonthiyil V., Reise F., Despras G., Lindhorst T. K., Eur. J. Org. Chem. 2018, 6241–6248; [Google Scholar]
- 11d. Liu Q., Luo X., Wei S., Wang Y., Zhu J., Liu Y., Quan F., Zhang M., Xia C., Tetrahedron Lett. 2019, 60, 1715–1719. [Google Scholar]
- 12.For selected alternative methods to access tetra-ortho-halogenated azobenzenes, see:
- 12a. Bléger D., Schwarz J., Brouwer A. M., Hecht S., J. Am. Chem. Soc. 2012, 134, 20597–20600; [DOI] [PubMed] [Google Scholar]
- 12b. Samanta S., Beharry A. A., Sadovsi O., McCormik T. M., Babalhavaeji A., Tropepe V., Woolley G. A., J. Am. Chem. Soc. 2013, 135, 9777–9784; [DOI] [PubMed] [Google Scholar]
- 12c. Hansen M. J., Lerch M. M., Szymanski W., Feringa B. L., Angew. Chem. Int. Ed. 2016, 55, 13514–13518; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 13712–13716. [Google Scholar]
- 13.For recent reports on the properties of tetra-ortho-halogenated azobenzenes, see:
- 13a. Lameijer L. N., Budzak S., Simeth N. A., Hansen M. J., Feringa B. L., Jacquemin D., Szymanski W., Angew. Chem. Int. Ed. 2020, 59, 21663–21670; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 21847–21854; [Google Scholar]
- 13b. Konrad D. B., Savasci G., Allmendinger L., Trauner D., Ochsenfeld C., Ali A. M., J. Am. Chem. Soc. 2020, 142, 6538–6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.See Supporting Information. Blood samples were purchased from Zenbio (https://www.zen-bio.com/products/serum/human-blood-products.php).
- 15. Janssen A. P. A., van der Vliet D., Bakker A. T., Jiang M., Grimm S. H., Campiani G., Butini S., van der Stelt M., ACS Chem. Biol. 2018, 13, 2406–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information