Abstract
Aims
N‐chlorotaurine (NCT) is a body‐own mild oxidizing antiseptic that can be applied topically as a well‐tolerated anti‐infective at many body sites. The objective of this study was to demonstrate its activity against representative nosocomial multidrug‐resistant bacteria.
Methods and Results
The bactericidal activity of NCT was tested in quantitative killing assays against a panel of multiresistant Gram‐positive and Gram‐negative clinical isolates. N‐chlorotaurine (1%, 55 mmol l−1) reduced the number of CFU of strains of methicillin‐resistant Staphylococcus aureus, linezolid‐resistant Staphylococcus epidermidis, vancomycin‐resistant, and linezolid‐ and vancomycin‐resistant Enterococcus faecium, 3MRGN and 4MRGN Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae by at least 2 log10 steps after 15 min and completely or nearly to the detection limit after 30 min at pH 7·1 and 37°C.
Conclusion
The activity of NCT against these clinical isolates is similar to that against non‐resistant ATCC strains and therefore not influenced by antibiotic resistance. This can be explained by the oxidizing and chlorinating mechanism of action of NCT, which leads to an attack of multiple targets in the microorganisms.
Significance and Impact of the Study
The bactericidal spectrum of NCT is not restricted by resistance against antibiotics. Therefore, it can be used against resistant strains, too.
Keywords: anti‐infective, antiseptic, chloramines, multiresistant bacteria, N‐chlorotaurine, resistance
Introduction
Antimicrobial resistance is an important cause of morbidity and mortality worldwide with an estimated 67 000 infections and 330 000 deaths in the European Union/European Economic Area (ECDC 2019) and more than 2·8 million infections and 35 000 deaths in the United States each year (CDC 2019). Emergence of antimicrobial resistance has been identified, in 2019, as one of the ten major global health threats by the World Health Organization. Overuse and misuse of antibiotics both in human medicine and agriculture are estimated as reasons (Gajdács and Albericio 2019). Carbapenem‐resistant Acinetobacter and Enterobacterales are classified as urgent threats. Extended‐spectrum β‐lactamase (ESBL)‐producing Enterobacterales, multidrug‐resistant Pseudomonas aeruginosa, vancomycin‐resistant enterococci (VRE) and methicillin‐resistant Staphylococcus aureus (MRSA) are defined as serious threats in the Antibiotic Resistance Threats report 2019 of the Centers for Disease Control and Prevention (CDC 2019). Due to the lack of effective antimicrobial agents, antibiotic resistant infections are often difficult, or sometimes even impossible to treat. Thus, there is a need for alternative strategies to combat infections with multidrug‐resistant bacteria.
Measures of hygiene and antibiotic stewardship are attempts to keep resistance rates low or to avoid it. An additional strategy is to apply antiseptics as an alternative or supplement to antibiotics, whenever topical therapy of infections is possible. There are several advantages of antiseptics compared to antibiotics. Their unspecific mechanism of action is completely different so that in general resistance does not play a role (Dong et al. 2017). The microbicidal activity is broader and markedly stronger (Dong et al. 2017). They have higher activity against biofilms, and they inactivate microbial toxins (Cole et al. 2008; Schultz et al. 2017). A problem restricting their application to non‐sensitive body regions and to a short period of about one week in general, is the higher cytotoxicity of antiseptics due to the unspecific mechanism of action (Schultz et al. 2017).
However, this may be overcome by using body‐own (endogenous) substances with mild, but sufficient microbicidal activity. A typical representative is N‐chlorotaurine (Cl‐HN‐CH2‐CH2‐SO3H) from the class of oxidants and group of chloramines (R‐NHCl compounds) (Grisham et al. 1984), which can be synthesized as sodium salt (NCT, Cl‐HN‐CH2‐CH2‐SO3Na) (Gottardi and Nagl 2010). It is part of innate immunity, produced from hypochlorite (HOCl) and taurine within activated human granulocytes and monocytes (Grisham et al. 1984), and it is involved in the defence against microbes as well as in termination of inflammation (Marcinkiewicz and Kontny 2014). Due to its oxidizing and chlorinating mechanism of action, it has microbicidal activity against all kinds of micro‐organisms (bacteria, viruses, fungi, protozoa, worm larvae) (Gottardi and Nagl 2010). In parallel, it downregulates proinflammatory mediators, cytokines and chemokines (Marcinkiewicz and Kontny 2014). The mild reactivity of NCT is the reason for its high tolerability by human tissue so that it can be applied topically in sufficient concentration (usually 1%, 55 mmol l−1) and repeatedly to regions all over the human body including sensitive ones, such as skin ulcers, mucous membranes, the eye, the ear, paranasal sinuses, the urinary bladder, organ abscesses, bone joints and others (Gottardi and Nagl 2010).
Due to the oxidizing and chlorinating mechanism of action of NCT, it can be expected that it is bactericidal against Gram‐positive and Gram‐negative bacteria irrespective of their resistance against antibiotics. It was the aim of this study to prove the microbicidal activity of NCT against a panel of such microorganisms.
Materials and methods
NCT and other chemicals
N‐chlorotaurine (NCT) (molecular weight 181·57 g mol−1, lot 2018‐11‐06) was prepared as crystalline sodium salt (Cl‐HN‐CH2‐CH2‐SO3 − Na+) from taurine and chloramine T in our laboratory at pharmaceutical grade, as reported (Gottardi and Nagl 2010), stored at minus 20°C, and freshly dissolved in sterile phosphate buffer (0·1 mol l−1) to a concentration of 1% for each experiment. The chemical structure is shown in Fig. 1. Identity and purity were verified by spectrophotometric analysis (peak at 251·5 nm), infrared spectrometry (band at 3260 cm−1), and mass spectrometry, and the content of active chlorine by iodometric titration (maximum chlorine content 19·52%) (Gottardi and Nagl 2002). The sodium salt can be stored at 20°C for about 2 weeks, at 2–4°C for 1 year, at −20°C or lower for at least 2 years, so that cooling is necessary for long‐term storage. Solutions can be stored up to 1 year at 2–4°C and 2 weeks at room temperature protected from UV light. Phosphate buffer (0·1 mol l−1), and tryptic soy broth were from Merck (Darmstadt, Germany), l‐histidine from Serva (Heidelberg, Germany), and dl‐methionine from Sigma‐Aldrich (Steinheim, Germany).
Figure 1.
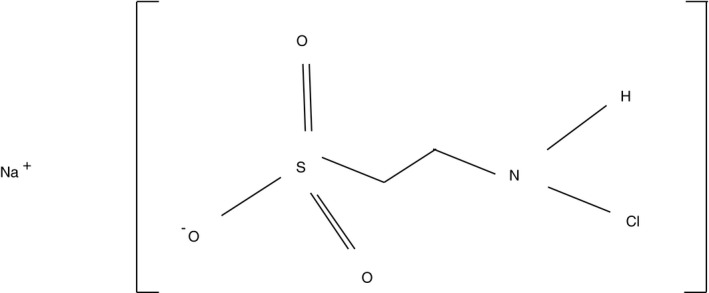
Chemical structure of N‐chlorotaurine sodium salt.
Bacteria
Multiresistant clinical isolates deep frozen for storage at the Institute of Hygiene and Medical Microbiology were used. All strains were grown on Columbia blood agar at 37°C. For the tests, colonies from the agar were grown overnight for 16 h at 37°C in tryptic soy broth, centrifuged at 1800 g for 10 min and washed twice in 0·9% NaCl. The washed suspensions contained 5 × 108 to 1 × 109 colony forming units (CFU) per ml.
The following isolates were tested separately. Methicillin‐resistant S. aureus (MRSA), three isolates; linezolid‐resistant Staphylococcus epidermidis (LRS), three isolates; vancomycin‐resistant Enterococcus faecium (VRE), three isolates; linezolid‐ and vancomycin‐resistant E. faecium (LVRE), two isolates; three multidrug‐resistant Gram‐negative (MRGN) ESBL Escherichia coli, three isolates; 4MRGN E. coli, three isolates; 3MRGN P. aeruginosa, three isolates; 4MRGN P. aeruginosa, three isolates; 3MRGN Acinetobacter baumannii, two isolates; 4MRGN A. baumannii, three isolates; 3MRGN Klebsiella pneumoniae, two isolates; 4MRGN K. pneumoniae, three isolates. For the 3 and 4 MRGN classification, the KRINKO guidelines of the Robert Koch Institute were followed (KRINKO 2012).
The isolates originated from swabs from the skin or mucosa (14/33, 42·4%), from urine (13/33, 39·4%), from sputum (3/33, 9·1%), and from blood cultures (3/33, 9·1%).
Quantitative killing assays
To 3·96 ml prewarmed 1% NCT solution in phosphate buffer at pH 7·1, 40 µl of the bacterial suspension (5 × 108 to 1 × 109 CFU per ml) was added and vortexed. Therefore, the final test suspensions contained 5 × 106 to 1 × 107 CFU per ml. After different incubation times at 37°C (5, 15, 30, 60 min), aliquots of 100 µl were removed and mixed with 900 µl of 1% methionine/1% histidine in distilled water to inactivate NCT. Aliquots of this suspension were spread onto Mueller‐Hinton agar plates in duplicate (50 µl each) using an automatic spiral plater (model WASP 2; Don Whitley, Shipley, UK). The detection limit was 100 CFU per ml, taking into account both plates and the previous 10‐fold dilution in the inactivating solution. Plates were grown for 24 h at 37°C before colonies were counted. Plates with no growth or only a low CFU count were grown for up to 5 days to detect bacteria attenuated but not killed by the treatment. Controls in 0·1 mol l−1 phosphate buffer were performed without NCT. Inactivation controls, where NCT was mixed with its inactivator 1% methionine and 1% histidine immediately before addition of pathogens at low CFU counts, showed full survival of bacteria as known from previous studies (Böttcher et al. 2017; Gruber et al. 2017).
Statistics
The data are presented as mean values and standard deviations (SD) of at least three independent experiments. Student’s unpaired t test in case of two groups, or one‐way analysis of variance and Tukey’s multiple‐comparison test in case of more than two groups, were used to test for a difference between the test and control groups. A P value of <0·05 was considered significant for all tests. Calculations were done with the GraphPad Prism 7.00 software (GraphPad Inc., La Jolla, CA).
To gain an improved survey on the microbicidal activity of NCT against the different strains, the recently introduced Integral Method was used, which transforms the whole killing curve (log10 CFU per ml vs time) into one value of ‘Bactericidal Activity (BA, log10 CFU per ml per min)’ (Gottardi et al. 2015). The method is based on the area below the killing curve, which is calculated by addition of the areas of trapezoids between the single time points of incubation and transformed into an orthogonal triangle with the same area. Its hypotenuse forms with the abscissa the angle α, whose tangent, tgα = y/2x, represents the sought average BA. The ‘Specific Bactericidal Activity’ as an intrinsic measure of the strength of activity was obtained by division by the concentration of NCT (SBA = BA/0·055 mol l−1) (Gottardi et al. 2015). The method allows an expanded statistical analysis, particularly between killing curves with small differences.
Results
N‐chlorotaurine demonstrated its typical bactericidal activity against all multidrug‐resistant test strains. In Fig. 2, four representative killing curves are depicted. The CFU per ml of all strains were reduced by at least 2 log10 steps after 15 min and completely or nearly to the detection limit after 30 min.
Figure 2.
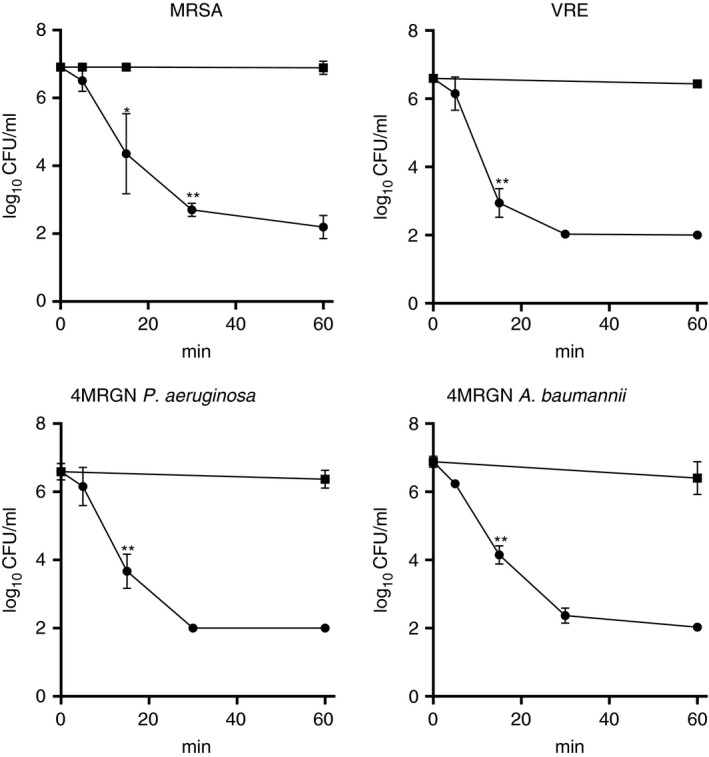
Bactericidal activity of 1% NCT against different multidrug‐resistant clinical isolates at pH 7·1 and 37°C. ‐●‐ NCT; ‐■‐ phosphate buffer without NCT (control); mean values ± SD of three independent experiments with three different strains. *P < 0·05, **P < 0·01 vs control. The detection limit was 2 log10 CFU per ml.
An overview is provided by the BA and SBA values calculated with the Integral Method (Table 1). The higher the value, the higher the bactericidal activity. The advantage of the Integral Method is the possibility to reliably compare whole killing curves of the different strains. The descending order, starting with the highest susceptibility, was K. pneumoniae>E. coli, P. aeruginosa, E. faecium (VRE)>S. aureus, S. epidermidis, A. baumannii and E. faecium (LVRE). These differences between the species were statistically significant.
Table 1.
Bactericidal activity of 1% NCT, calculated with the integral method
| Bacterial strain | BA | SD | SBA | SD |
|---|---|---|---|---|
| LRS | 0·1174 | 0·0075 | 2·13 | 0·14 |
| MRSA | 0·1276 | 0·0086 | 2·32 | 0·16 |
| VRE | 0·1916 | 0·0158 | 3·48 | 0·29 |
| LVRE | 0·1386 | 0·0087 | 2·52 | 0·16 |
| 3MRGN Escherichia coli | 0·1780 | 0·0138 | 3·24 | 0·25 |
| 4MRGN E. coli | 0·2082 | 0·0166 | 3·78 | 0·30 |
| 3MRGN Pseudomonas aeruginosa | 0·1913 | 0·0162 | 3·48 | 0·29 |
| 4MRGN P. aeruginosa | 0·1659 | 0·0154 | 3·02 | 0·28 |
| 3MRGN Acinetobacter baumannii | 0·1092 | 0·0095 | 1·99 | 0·18 |
| 4MRGN A. baumannii | 0·1498 | 0·0118 | 2·72 | 0·22 |
| 3MRGN Klebsiella pneumoniae | 0·2840 | 0·0225 | 5·16 | 0·41 |
| 4MRGN K. pneumoniae | 0·3126 | 0·0295 | 5·68 | 0·54 |
BA, bactericidal activity (log10 CFU per min); SBA, specific bactericidal activity (BA mol−1); SD, standard deviation; LRS, linezolid‐resistant S. epidermidis; MRSA, methicillin‐resistant S. aureus; VRE, vancomycin‐resistant E. faecium; LVRE, linezolid‐ and vancomycin‐resistant E. faecium; MRGN, multidrug‐resistant Gram‐negative.
Mean values of BA and SD of three to nine independent experiments.
The variability of single strains within the same species was generally smaller and significant only between VRE and LVRE, between 3 and 4MRGN E. coli, and between 3 and 4MRGN A. baumannii.
Discussion
Resistance of bacteria against antibiotics is one of the emerging problems in the field of infectious diseases and chronic diseases where pathogens play a role, for instance chronic obstructive pulmonary disease. Whenever topical therapy comes into question, antiseptics should be considered as an alternative. Our study clearly demonstrates the broad‐spectrum bactericidal activity of a mild active chlorine compound irrespective of antibiotic resistance.
The absence of resistance against NCT can be explained easily by the oxidizing and chlorinating mechanism of action (Gottardi et al. 2013), which leads to multiple sites of attack within pathogens (Arnitz et al. 2006). Similarly, their virulence factors are chemically inactivated as shown with toxins and enzymes of E. coli, S. aureus, Aspergillus fumigatus and Candida albicans (Gottardi et al. 2013). Micro‐organisms upregulate anti‐oxidative and salvage mechanisms in the presence of NCT, such as heat‐shock proteins, proteins associated with ribosomes, transcription and translation (Arnitz et al. 2006; Sheehan et al. 2019). These pathways may be effective against physiological micromolar concentrations of oxidants created by leukocytes during inflammation. However, because of its mild activity and high tolerability, NCT can be applied therapeutically in 1000‐fold higher concentration (55 mmol l−1) than the natural one (about 20–50 µmol l−1, (Grisham et al. 1984)). In this range, even repeated exposure to NCT and other chloramines does not induce resistance (Nagl and Gottardi 1996; D'Lima et al. 2012).
Basically, the activity of NCT was in a similar range against all test bacteria in this study. When the susceptibility of defined, non‐multiresistant test strains of S. aureus (ATCC 25923, ATCC 6538), E. coli (ATCC 11229), P. aeruginosa (ATCC 27853) to NCT is compared with that of the multiresistant clinical isolates of this study, similar killing curves and BA values can be found (Martini et al. 2012; Gottardi et al. 2015; Gruber et al. 2017). It is true that statistically significant differences can be seen, but they are small and represent a typical inter‐strain and inter‐species variability against NCT. The reason for this variability must be considered in differences in the speed of penetration of active chlorine through the covers of micro‐organisms into the cytosolic compartment. Oxidation of the surface resulting in a chlorine cover removes virulence of bacteria and fungi, but does not kill them (Gottardi and Nagl 2005). Thereby, active chlorine from NCT is attached to the surface of pathogens within seconds to a minute. Such incubation of bacteria and fungi for sublethal time in NCT leads to a lag of their regrowth, a so‐called postantibiotic (postantiseptic) effect. As a consequence, these attenuated micro‐organisms have been shown to lose their potency to kill mice and moth larvae respectively (Nagl et al. 1999; Lackner et al. 2015). It is assumed that the postantiseptic effect contributes to therapeutic effects of NCT in vivo, too.
In previous studies, we throughout found a higher susceptibility of E. coli and P. aeruginosa to NCT than S. aureus (Martini et al. 2012; Gottardi et al. 2015; Gruber et al. 2017). Also in this study, the same order occurred with multiresistant strains. The difference between bacterial species seems to be higher than that between single strains within a species, irrespective of their resistance to antibiotics. The data of this study do not support any relevant influence of different mechanisms of antibiotic resistance on the susceptibility to NCT.
In any case, the extent of variability between bacterial species can be regarded as irrelevant for clinical efficacy of NCT, similar to stronger active chlorine compounds like chloramine T and hypochlorous acid (Gottardi et al. 2013). Although not being the main endpoint, an influence of the bacterial species on the efficacy of NCT was not evident in previous clinical studies, for instance in crural ulcers, conjunctivitis, and otitis (for review see (Gottardi and Nagl 2010)). It has to be considered that the activity of NCT, in contrast to other active halogen compounds and other antiseptics, is not decreased but enhanced in the presence of organic matter (for instance body fluids, purulent exudation) (Gottardi and Nagl 2010; Gottardi et al. 2014; Gruber et al. 2017) due to transfer of the active chlorine to ammonium and formation of the more lipophilic and higher microbicidal monochloramine in equilibrium (Gottardi et al. 2007). This reaction outmatches chlorine consumption and formation of high molecular weight chloramines with lower activity (Gottardi and Nagl 2010; Gottardi et al. 2014; Gruber et al. 2017), both of which occur to a relatively low extent because of the low reactivity of NCT.
A further consequence of this is the high tolerability of NCT, allowing its application to sensitive body regions, for instance to the eye, the urinary bladder and organ abscesses (Gottardi and Nagl 2010). In the last few years, also inhalation became a topic of interest, whereby indications such as chronic bronchitis and cystic fibrosis are well conceivable (Arnitz et al. 2018). Early treatment of infective exacerbations in these diseases could attenuate these causative episodes of worsening and delay the progression of loss of lung function. Considering the resistance problem with antibiotics (Gajdács and Urbán 2019), topical therapy with a well‐tolerated antiseptic appears to be an interesting alternative or additive. Several indications are near at hand in this regard. Cleansing of chronic, infected wounds and soft tissue infections is a significant, not satisfactorily solved problem. Decontamination of carriers of multiresistant bacteria with NCT on different body sites on skin and mucosa is conceivable. Further examples are chronic and recurrent urinary tract infections, lung infections and organ abscesses (Gottardi and Nagl 2010; Arnitz et al. 2018). The advantage of NCT as an active chlorine compound is the absence of resistance development because of its multiple targets, the absence of systemic distribution and systemic adverse effects, and the good topical tolerability as an endogenous, mild oxidant (Gottardi and Nagl 2010; Gottardi et al. 2013).
The present study was done at a neutral pH for standardized comparability. At sites of infection, the pH may decrease. This is no matter of concern since the bactericidal activity of NCT increases at acidic pH. This can be explained by formation of the protonated form of NCT, NCTH+, which more easily comes into reaction with nucleophilic agents such as amino groups (Gottardi and Nagl 2002).
To decrease the danger and burden of multiresistant pathogens is one of the great goals announced by international health organizations such as the World Health Organization, the Center for Disease Control, and the European Center for Disease Control. Usage of antiseptics instead of antibiotics for topical treatment of localized infections is a logical strategy to fight antibiotic resistance and should be pursued in general. Our study clearly confirms that multiresistance against antibiotics does not even influence the bactericidal activity of a mild antiseptic from the class of active chlorine compounds with both high tissue tolerability and sufficient activity in exudates.
Conflict of Interest
No conflict of interest declared.
Ethics approval
Not applicable.
Acknowledgements
The authors thank Andrea Windisch and Elisa Frezzini for excellent technical assistance. This study was supported by the Austrian Cystic Fibrosis self‐regulating community ‘Mukoviscidose HILFE Oberösterreich’, CF Austria (Cystische Fibrose Hilfe Österreich), Mukoviszidose Hilfe Wien, Niederösterreich and Nördliches Burgenland. All funders provided financial support for consumption laboratory materials needed for the performance of the study. There was no influence of the funders on the concept, publication or other parts of the study.
References
- Arnitz, R. , Sarg, B. , Ott, H.W. , Neher, A. , Lindner, H. and Nagl, M. (2006) Protein sites of attack of N‐chlorotaurine in Escherichia coli . Proteomics 6, 865–869. [DOI] [PubMed] [Google Scholar]
- Arnitz, R. , Stein, M. , Bauer, P. , Lanthaler, B. , Jamnig, H. , Scholl‐Bürgi, S. , Stempfl‐Al‐Jazrawi, K. , Ulmer, H. et al. (2018) Tolerability of inhaled N‐chlorotaurine in humans – a double‐blind randomized phase I clinical study. Ther Adv Respir Dis 12, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher, B. , Sarg, B. , Lindner, H.H. and Nagl, M. (2017) Inactivation of microbicidal active halogen compounds by sodium thiosulphate and histidine/methionine for time‐kill assays. J Microbiol Methods 141, 42–47. [DOI] [PubMed] [Google Scholar]
- CDC (2019) Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: CDC. [Google Scholar]
- Cole, K.D. , Gaigalas, A. and Almeida, J.L. (2008) Process monitoring the inactivation of ricin and model proteins by disinfectants using fluorescence and biological activity. Biotechnol Prog 24, 784–791. [DOI] [PubMed] [Google Scholar]
- D'Lima, L. , Friedman, L. , Wang, L. , Xu, P. , Anderson, M. and Debabov, D. (2012) No decrease in susceptibility to NVC‐422 in multiple‐passage studies with methicillin‐resistant Staphylococcus aureus, S. aureus, Pseudomonas aeruginosa, and Escherichia coli . Antimicrob Agents Chemother 56, 2753–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, A. , Wang, Y.J. , Gao, Y. , Gao, T. and Gao, G. (2017) Chemical insights into antibacterial N‐halamines. Chem Rev 117, 4806–4862. [DOI] [PubMed] [Google Scholar]
- ECDC . (2019) Surveillance of Antimicrobial Resistance in Europe 2018. Solna, Sweden: ECDC. [Google Scholar]
- Gajdács, M. and Albericio, F. (2019) Antibiotic resistance: from the bench to patients. Antibiotics 8, 129– 10.3390/antibiotics8030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdács, M. and Urbán, E. (2019) Prevalence and antibiotic resistance of Stenotrophomonas maltophilia in respiratory tract samples: a 10‐year epidemiological snapshot. Health Serv Res Manag Epidemiol 6, 10.1177/2333392819870774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi, W. , Arnitz, R. and Nagl, M. (2007) N‐chlorotaurine and ammonium chloride: an antiseptic preparation with strong bactericidal activity. Int J Pharm 335, 32–40. [DOI] [PubMed] [Google Scholar]
- Gottardi, W. , Debabov, D. and Nagl, M. (2013) N‐chloramines: a promising class of well‐tolerated topical antiinfectives. Antimicrob Agents Chemother 57, 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi, W. , Klotz, S. and Nagl, M. (2014) Superior bactericidal activity of N‐bromine compounds compared to their N‐chlorine analogues can be reversed under protein load. J Appl Microbiol 116, 1427–1437. [DOI] [PubMed] [Google Scholar]
- Gottardi, W. and Nagl, M. (2002) Chemical properties of N‐chlorotaurine sodium, a key compound in the human defence system. Arch Pharm Pharm Med Chem 335, 411–421. [DOI] [PubMed] [Google Scholar]
- Gottardi, W. and Nagl, M. (2005) Chlorine covers on living bacteria: the initial step in antimicrobial action of active chlorine compounds. J Antimicrob Chemother 55, 475–482. [DOI] [PubMed] [Google Scholar]
- Gottardi, W. and Nagl, M. (2010) N‐chlorotaurine, a natural antiseptic with outstanding tolerability. J Antimicrob Chemother 65, 399–409. [DOI] [PubMed] [Google Scholar]
- Gottardi, W. , Pfleiderer, J. and Nagl, M. (2015) The Integral Method, a new approach to quantify bactericidal activity. J Microbiol Methods 115, 71–78. [DOI] [PubMed] [Google Scholar]
- Grisham, M.B. , Jefferson, M.M. , Melton, D.F. and Thomas, E.L. (1984) Chlorination of endogenous amines by isolated neutrophils. J Biol Chem 259, 10404–10413. [PubMed] [Google Scholar]
- Gruber, M. , Moser, I. , Nagl, M. and Lackner, M. (2017) Bactericidal and fungicidal activity of N‐chlorotaurine is enhanced in cystic fibrosis sputum medium. Antimicrob Agents Chemother 61, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRINKO (2012) Hygiene measures for infection or colonization with multidrug resistant gram‐negative bacilli. Commission recommendation for hospital hygiene and infection prevention (KRINKO) at the Robert Koch Institute (RKI). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 55, 1311–1354. [DOI] [PubMed] [Google Scholar]
- Lackner, M. , Binder, U. , Reindl, M. , Gönül, B. , Fankhauser, H. , Mair, C. and Nagl, M. (2015) N‐chlorotaurine exhibits fungicidal activity against therapy‐refractory Scedosporium species and Lomentospora prolificans . Antimicrob Agents Chemother 59, 6454–6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz, J. and Kontny, E. (2014) Taurine and inflammatory diseases. Amino Acids 46, 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini, C. , Hammerer‐Lercher, A. , Zuck, M. , Jekle, A. , Debabov, D. , Anderson, M. and Nagl, M. (2012) Antimicrobial and anticoagulant activity of N‐chlorotaurine (NCT), N, N‐dichloro‐2,2‐dimethyltaurine (NVC‐422) and N‐monochloro‐2,2‐dimethyltaurine (NVC‐612) in human blood. Antimicrob Agents Chemother 56, 1979–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagl, M. and Gottardi, W. (1996) Enhancement of the bactericidal efficacy of N‐chlorotaurine by inflammation samples and selected N‐H compounds. Hyg Med 21, 597–605. [Google Scholar]
- Nagl, M. , Hengster, P. , Semenitz, E. and Gottardi, W. (1999) The postantibiotic effect of N‐chlorotaurine on Staphylococcus aureus. Application in the mouse peritonitis model. J Antimicrob Chemother 43, 805–809. [DOI] [PubMed] [Google Scholar]
- Schultz, G. , Bjarnsholt, T. , James, G.A. , Leaper, D.J. , McBain, A.J. , Malone, M. , Stoodley, P. , Swanson, T. et al. (2017) Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen 25, 744–757. 10.1111/wrr.12590. [DOI] [PubMed] [Google Scholar]
- Sheehan, G. , Nagl, M. and Kavanagh, K. (2019) Exposure to N‐chlorotaurine induces oxidative stress responses in Aspergillus fumigatus . J Med Microbiol 68, 279–288. [DOI] [PubMed] [Google Scholar]


