Abstract
Spontaneous coronary artery dissection (SCAD) can lead to acute coronary syndrome and sudden cardiac death, particularly in young women. Observational data show that, in SCAD patients, both percutaneous coronary intervention and coronary artery bypass grafting seem to be hampered by higher technical complexity, lower success rates, and worse outcomes. As spontaneous healing is a common occurrence, expert consensus advices medical management of the acute phase, when feasible. We present the case of a young woman with SCAD of left anterior descending artery causing myocardial infarction with ST‐segment elevation. High‐anatomical complexity and unstable conditions of the patient made both medical management and immediate revascularization unfeasible options. Therefore, we decided to implant a percutaneous off‐loading mechanical support device to improve coronary perfusion pressure by unloading the left ventricle and preserve cardiac function, preventing worse complications of acute myocardial infarction. This strategy was successful in stabilizing the patient, until the definitive revascularization treatment became an option.
Keywords: acute myocardial infarction/STEMI, cardiogenic shock, coronary artery disease, coronary bypass grafts, diastolic dysfuntion, ECMO/IABP/tandem/impella, mechanical circulatory support
1. INTRODUCTION
Spontaneous coronary artery dissection (SCAD) is an important cause of acute myocardial infarction in young patients, especially in women. It is defined as a separation of the layers of an epicardial coronary‐artery wall by intramural hemorrhage, with or without an intimal tear; in this setting, acute myocardial infarction is caused by coronary obstruction due to true lumen compression, either by a dissection flap or by propagation of an intramural hematoma. 1 The goal of management in the acute phase is to restore or preserve myocardial perfusion and cardiac function. However, medical management is usually preferred over attempts at revascularization, as spontaneous healing over time is a common occurrence and both percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) are technically challenging and hampered by high rates of short and long‐term adverse events. 2 , 3 We present a case report of a patient who experienced an acute myocardial infarction with ST‐segment elevation (STEMI) caused by a SCAD involving proximal segments of the left anterior descending artery (LAD) for which both percutaneous and surgical revascularization strategies were not viable options in the acute phase.
2. CASE REPORT
A 45‐year‐old female patient with a history of SCAD three years before, was admitted at our emergency department due to chest pain with ST‐elevation in anterior electrocardiogram (EKG) leads (Figure 1, panel A). At index coronary angiography, we found an extensive, flow‐limiting type 2A SCAD involving LAD and diagonal branch 1 (Figure 1, panel B, Supplementary material online, video 1). The left ventricle end‐diastolic pressure (LVEDP) was severely increased, measuring 35 mmHg. Aortic pressure was 160/90 mmHg and heart rate was 75 bpm. During examination, the patient had 8/10 chest pain, persistent ST‐elevation and experienced a cardiac arrest due to ventricular fibrillation promptly cardioverted by DC‐shock.
FIGURE 1.
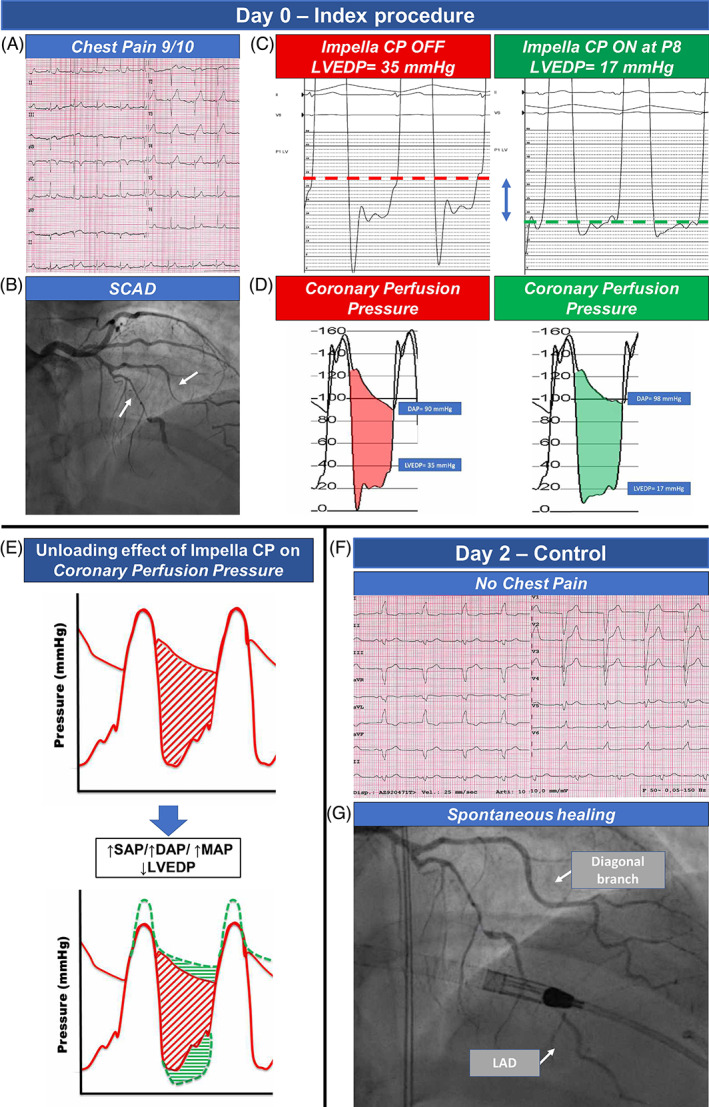
Panel A shows baseline EKG with an ST‐elevation in anterior leads; an extensive flow limiting SCAD involving LAD and diagonal branch was evident at angiography (panel B). LVEDP was increased at baseline (35 mmHg), while it significantly decreased to 17 mmHg during Impella CP unloading (panel C and D). Panel E highlights the beneficial effect of unloading system on myocardial perfusion gradient, through an increase in systolic, diastolic, and mean aortic pressure and a decrease in LVEDP. The unloading therapy allowed a regression of symptoms and ST abnormalities (panel F) and an improved run‐off of the LAD as well as a relief of the SCAD in the diagonal branch (panel G). DBP, diastolic blood pressure; LAD, left anterior descending artery; LVDEP, left ventricle end‐diastolic pressure; MAP, mean arterial pressure; SBP, systolic blood pressure; SCAD, spontaneous coronary artery dissection
Due to patient's unstable conditions and considering the prohibitive risk of coronary occlusion/progression of SCAD with PCI, we requested an emergent cardiac surgery consultation; however, the surgeon refused to perform emergent CABG surgery due to high risk of graft failure, as distal segments of LAD were poorly visible on angiography and possibly involved by SCAD. Therefore, given the absence of other possible therapeutic alternatives, we decided to implant a percutaneous off‐loading mechanical support device (Impella CP, Abiomed, Inc, Danvers, MA) in an effort to achieve the following goals: (1) to significantly reduce the LVEDP (LV unloading), thus improving the coronary perfusion pressure gradient (Figure 1, panel C and D); (2) to reduce ongoing ischemia by minimizing oxygen demand to stabilize the patient and prevent worse complications of acute myocardial infarction, such as further hemodynamic decay and malignant arrhythmias; (3) potentially, to facilitate spontaneous healing by improvement of antegrade coronary flow.
The impact of left ventricle unloading on coronary perfusion gradient is shown in Figure 1, panel E: the increase in systolic, diastolic, and mean aortic pressure and the decrease in LVEDP determined a significant increase of the myocardial perfusion pressure gradient.
At the end of the procedure, the patient was transferred to the Intensive Care Unit, asymptomatic and in stable conditions. The Impella was left in place through a femoral artery access for 48 h, without major vascular or hemorrhagic complications. The patient was then re‐evaluated with coronary angiography that showed a reduction of hematoma compression with gain in lumen diameter only in the diagonal branch, while diffuse narrowing and critical stenosis of LAD were still present; however, we observed an improved run‐off of the vessel with its distal segments now clearly visible. (Figure 1, panel F and G, Supplementary material online, video 2). This allowed the cardiac surgeon to perform a successful elective single CABG with left internal mammary artery on LAD, on the same day. Post‐operative course was regular, and the patient was discharged 13 days after surgery. Post‐operative echocardiogram showed mild systolic left ventricular dysfunction (LVEF 51%) with limited akinetic areas. A 1‐month coronary CT scan showed patency of bypass graft and almost complete healing of LAD and of diagonal branch, with optimal opacification of their distal segments (Figure 2, panel A and B). A longer clinical and instrumental follow‐up will be necessary to evaluate the impact of dissection healing on long‐term patency of the bypass graft.
FIGURE 2.
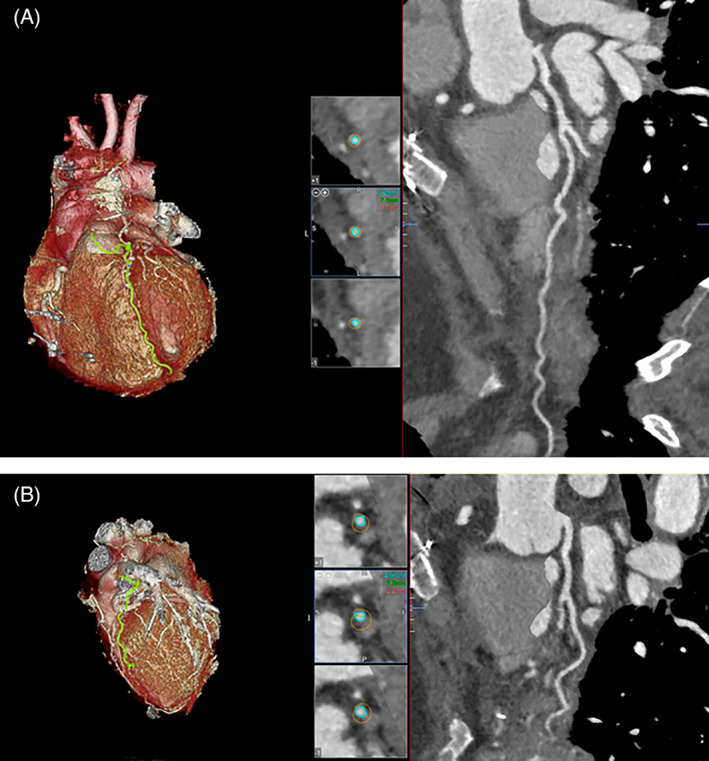
Three dimensional multiplanar reconstruction and volume rendering of 1‐month coronary CT scan, which shows patency of native LAD (panel A) and diagonal branch (panel B) with good opacification of their distal segments. LAD, left anterior descending artery;
3. DISCUSSION
SCAD is an uncommon cause of myocardial infarction, and the optimal approach to treatment is a subject of ongoing study due to its low incidence and conflicting evidence. The therapeutic management is influenced by a major difference between this condition and atherosclerotic myocardial infarction. Instead of plaque rupture or erosion, the peculiar pathophysiological feature of SCAD is medial dissection with or without an intimal tear. 4 Although PCI is the mainstay of guideline‐based revascularization for acute coronary syndromes (ACS), it might be complicated by increased risk of procedural complications, (e.g., extension of the dissection and abrupt vessel occlusion), in the setting of SCAD. Furthermore, at long‐term, the resorption of the intramural hematoma may cause late malapposition of the stent struts with the risk of late target vessel failure. 2 On the other hand, observational data showed that a conservative approach, when feasible, is associated with an high rate of spontaneous vessel healing in the range of 70%–97%. 5 Accordingly, both ESC and AHA expert consensus documents suggest that medical management is preferred over immediate revascularization for patients who are in clinically stable conditions, without anatomical high‐risk features (e.g., left main, proximal LAD or proximal 2‐vessel SCAD) and with adequate distal flow in the target vessel. 2 , 5 Granted that, when revascularization is recommended because of ongoing ischemia or hemodynamic instability of the patient, the decision on how to perform PCI remains very challenging, particularly in case of dissection extending into the distal vessels. 3 Moreover, focusing on the role of CABG, there is an early hazard of adverse outcomes related to technical difficulty in grafting the true lumen, but also the risk of late occlusion because of the spontaneous healing of the vessel resulting in competitive flow. 1
In case of SCAD complicated by cardiogenic shock, the potential advantages of mechanical circulatory support with extracorporeal membrane oxygenation devices as bridge to recovery or heart transplantation have been previously described. 6 , 7 More recently, the percutaneous left ventricular assist device Impella has been increasingly utilized in cardiogenic shock and in high‐risk PCI patients, but data on its use in SCAD patients are very scant. 8 , 9 Sharma et al. described a case series of four patients with SCAD complicated by cardiogenic shock, successfully treated with Impella device on top of immediate percutaneous revascularization, highlighting the potential role of this device in supporting the recovery of left ventricular function in this context. 9
On the contrary, the case we hereby presented is the first one evaluating the use of Impella in a hemodynamically unstable patient with extensive SCAD of the LAD, with ongoing ischemia and judged not eligible for both immediate percutaneous or surgical revascularization. We found that the use of Impella significantly improved the coronary perfusion gradient as assessed by measuring both left ventricle and aortic pressures during unloading (Figure 1, panel C and D). The use of Impella determined improvement in coronary perfusion pressure by means of a significant reduction in LVEDP, and consequent reduction of oxygen demand in the context of reduced coronary flow, with a significative improvement of patient symptoms and hemodynamics until a definitive revascularization treatment became a feasible option. Increased LVEDP is a common finding in STEMI patients undergoing primary PCI, mainly due to decreased LV compliance and to diastolic dysfunction. 10 Furthermore, LVEDP is also a major determinant of coronary perfusion pressure and flow, 11 and has been shown to be a major predictor of adverse in‐hospital and long‐term adverse outcomes 12 , 13 and to be correlated with the extent of myocardial ischemia, reduced myocardial salvage and greater infarct size in STEMI setting. 14
Lastly, no major adverse events linked to Impella usage in the context of SCAD were described, even though, the potential risk of propagating the intramural hematoma in SCAD vessels (due to the necessity for anticoagulation) and a higher risk of peripheral dissection (in patients with concomitant fibromuscular dysplasia or other connective tissue disorders) should be considered. 7 , 9
4. CONCLUSIONS
Although hypothesis generating, our case report explores the possibility of using off‐loading systems in complex SCAD patients presenting with hemodynamic instability for whom revascularization is not an immediate option.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Supporting information
Video 1 Antero‐posterior cranial projection of baseline coronary angiography showing extensive flow limiting SCAD involving LAD and diagonal branch
Video 2 Antero‐posterior cranial projection of control coronary angiography after 2 days of left ventricle unloading with Impella. Improved run‐off of the LAD as well as a gain in lumen diameter of diagonal branch is evident
Tarantini G, Fabris T, Rodinò G, Fraccaro C. Improvement of symptoms and coronary perfusion gradient with mechanical left ventricular unloading in flow‐limiting complex spontaneous coronary artery dissection, without revascularization. Catheter Cardiovasc Interv. 2021;98:E581–E585. 10.1002/ccd.29836
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kim ESH. Spontaneous coronary‐artery dissection. N Engl J Med. 2020;383(24):2358‐2370. 10.1056/NEJMra2001524. [DOI] [PubMed] [Google Scholar]
- 2. Adlam D, Alfonso F, Maas A, Vrints EC. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39(36):3353‐3368. 10.1093/eurheartj/ehy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hayes SN, Tweet MS, Adlam D, et al. Spontaneous Coronary Artery Dissection. Journal of the American College of Cardiology. 2020;76 (8):961–984. 10.1016/j.jacc.2020.05.084. [DOI] [PubMed] [Google Scholar]
- 4. Jackson R et al. Spontaneous coronary artery dissection. JACC: Cardiovasc Imaging. 2019;12(12):2475‐2488. 10.1016/j.jcmg.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 5. Hayes SN et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137(19):35. 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Havakuk O, Goland S, Mehra A, Elkayam EU. Pregnancy and the risk of spontaneous coronary artery dissection: an analysis of 120 contemporary cases. Circ Cardiovasc Interv. 2017;10(3):e004941. 10.1161/CIRCINTERVENTIONS.117.004941 [DOI] [PubMed] [Google Scholar]
- 7. Cox J et al. Acute isolated coronary artery dissection causing massive acute myocardial infarction and leading to unsuccessful coronary bypass, extracorporeal life support, and successful cardiac transplantation. Am J Cardiol. 2020;125(9):3. 10.1016/j.amjcard.2020.01.034. [DOI] [PubMed] [Google Scholar]
- 8. Desai CK, Bhatnagar U, Stys A, Jonsson EO. Iatrogenic propagation of coronary dissection during diagnostic coronary angiography: an uncommon but important procedural consideration. Case Rep. 2017;2017:bcr2017222463. 10.1136/bcr-2017-222463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma S et al. Management of spontaneous coronary artery dissection complicated by cardiogenic shock using mechanical circulatory support with the impella device. Catheter Cardiovasc Interv. 2021;97(1):4. [DOI] [PubMed] [Google Scholar]
- 10. Satıroğlu O, Ciçek Y, Bostan M, Cetin M, Bozkurt EE. Acute change in left ventricle end‐diastolic pressure after primary percutaneous coronary intervention in patients with ST segment elevation myocardial infarction. Am Heart Hosp J. 2010;8(2):E86‐E90. [PubMed] [Google Scholar]
- 11. Goodwill AG, Dick GM, Kiel AM, Tune EJD. Regulation of coronary blood flow. Compr Physiol. 2017;7(2):321‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Planer D et al. Prognostic utility of left ventricular end‐diastolic pressure in patients with ST‐segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2011;108(8):1068‐1074. 10.1016/j.amjcard.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 13. Brienesse SC, Davies AJ, Khan A, Boyle EAJ. Prognostic value of LVEDP in acute myocardial infarction: a systematic review and meta‐analysis. J Cardiovasc Trans Res. 2018;11(1):33‐35. 10.1007/s12265-017-9776-7. [DOI] [PubMed] [Google Scholar]
- 14. Ndrepepa G et al. Relationship of left ventricular end‐diastolic pressure with extent of myocardial ischemia, myocardial salvage and long‐term outcome in patients with ST‐segment elevation myocardial infarction. Catheter Cardiovasc Interv. 2019;93(5):901‐909. 10.1002/ccd.28098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1 Antero‐posterior cranial projection of baseline coronary angiography showing extensive flow limiting SCAD involving LAD and diagonal branch
Video 2 Antero‐posterior cranial projection of control coronary angiography after 2 days of left ventricle unloading with Impella. Improved run‐off of the LAD as well as a gain in lumen diameter of diagonal branch is evident
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


