The NovoSorb® Biodegradable Temporising Matrix (BTM) is a synthetic polyurethane dermal matrix used to reconstruct complex wounds. In this study, we explore a case series of 35 wounds of varying indications and report on outcomes and complications. We found that BTM offers a safe and reliable reconstructive option in challenging wounds that would otherwise require more complex operations.
![]()
Keywords: Biodegradable Temporising Matrix, synthetic dermal matrix, wound reconstruction
Abstract
Background
The NovoSorb® Biodegradable Temporising Matrix (BTM) is a synthetic polyurethane dermal matrix used to reconstruct complex wounds including deep dermal and full‐thickness burns, necrotising fasciitis and free flap donor site. We hope to further explore its potential applications in this series.
Methods
Patients who received BTM application across four centres over an 18‐month period were included. Patients were followed up to assess BTM and graft take, the aesthetic, the return of sensation and complications.
Results
A total of 27 patients with 35 wounds were identified with a range of aetiologies. Thirty‐three wounds had 100% integration of BTM at the time of sealing membrane removal. Seven wounds had partial graft loss that later healed by secondary intention. In two cases, re‐epithelialisation occurred with BTM alone without split‐skin graft.
Conclusion
BTM offers a safe and reliable reconstructive option in challenging wounds that would otherwise require more complex operations.
Introduction
The NovoSorb® Biodegradable Temporising Matrix (BTM) (PolyNovo Biomaterials Pty Ltd., Port Melbourne, VIC, Australia) is a fully synthetic dermal matrix that can be used to reconstruct complex wounds. It consists of a 2‐mm thick NovoSorb biodegradable polyurethane open cell foam covered by a non‐biodegradable sealing membrane. The open cell matrix allows for infiltration of cellular materials and acts as a scaffold for the neo‐dermis. The sealing membrane provides physiological wound closure but also contains small fenestrations to prevent the accumulation underneath the material. 1
The application of BTM involves a two‐stage procedure. 1 In the first stage, the BTM is laid onto a clean wound bed. Cells and blood vessels migrate into the BTM during the integration phase and a vascularised neo‐dermis is formed. Capillary refill can be seen from as early as 2 weeks. The polyurethane matrix is biodegradable and breaks down via hydrolysis. 2 In the second stage, the sealing membrane is removed and a split‐skin graft (SSG) is applied to the neo‐dermis. 1
BTM differs from the traditional SSG in that it helps to replace the natural thickness of the dermis, minimises contracture, prevents tethering to the underlying structures and allows for the rapid temporising of large total body surface area wounds. 3 Unlike other artificial dermal templates that are comprised of allogenic or xenogenic materials, the fully synthetic BTM eliminates the possibility of inter‐species immune rejection or disease transmission and avoids ethical or cultural obstacles. 4
Early in vitro studies confirmed the biocompatibility of BTM. 2 , 5 In vivo studies utilising rats and porcine models demonstrated adequate reconstruction of full‐thickness wounds with a high resistance to wound contracture and an absence of systemic toxic effects. 6 , 7 Comparisons between BTM and Integra® (Life Sciences Corp., Plainsboro, NJ, USA) in animal models highlighted the effectiveness of the BTM in providing a stable and flexible wound reconstruction. 4 , 8
The first use in humans was trialled as a polyurethane foam (NovoPore™, Polynovo) in negative‐pressure wound therapy (NPWT) for pressure ulcers. 9 This showed that short‐term implantation in patients did not cause adverse reactions. Following this, the use of a prototype bilayer device consisting of NovoSorb foam with a non‐biodegradable sealing matrix in free flap donor wounds showed promising results. 10 Further modification of the sealing membrane including the thickness, bonding layer and the introduction of fenestrations produced superior results in subsequent studies. 11
The use of BTM in burns demonstrated that it could successfully treat large total body surface area burns with excellent cosmetic and functional results. 3 , 12 This success led to its use in necrotising soft tissue infections to provide pliable wound coverage. 13 , 14
Here, we report a consecutive case series of 35 wounds describing the use of BTM in a range of challenging wounds which would otherwise require more complex reconstructions.
Methods
The study was a multicentred, prospective case series involving 27 patients with 35 complex wounds from January 2019 to December 2020. This study was approved by the ethics committee of Austin Health (Melbourne) with consents obtained from all patients. Patient demographics, indications for BTM, surgical details and outpatient follow‐up were recorded. Inclusion criteria included complex wounds with (1) exposure of a critical structure such as tendon and bone, (2) failure of previous skin graft and (3) wound bed where the surgeon did not expect a traditional SSG to take. Exclusion criteria included active infection or residual malignancy.
All patients were followed up in the outpatient departments of their respective institutions with a maximum follow‐up of 18 months. Outcomes measured included percentage of BTM take at the time of grafting, percentage of SSG take, sensation and aesthetics as measured by the Patient and Observer Scar Assessment Scale (POSAS). 15
Surgical management included initial debridement to remove all devitalised and infected tissue prior to reconstruction with BTM. In cases of malignancies, oncological clearance was obtained prior to BTM reconstruction.
First stage of reconstruction involved the inset of BTM with either staples or sutures. Quilting staples or sutures were utilised to maximise contact between the BTM and the wound bed. The wound edges were also saucerised in some instances to improve dermal contact. After the application of BTM, Acticoat™ (Smith and Nephew) was applied over the BTM and either secured with NPWT between 50 and 75 mmHg or dressed with gauze, crepe bandage and Mefix® (Mölnlycke Health Care AB, Gothenburg, Sweden) to provide compression. In wounds that involved the limbs or joints, a plaster or orthotic splint was applied for the first post‐operative week. The external dressing was changed once or twice weekly. The BTM was evaluated weekly for integration by assessing for capillary refill. Excess fluid was expressed through the fenestrations before re‐dressing. This continued until the BTM was deemed ready for the second stage, which varied from 2 to 10 weeks.
Second stage of reconstruction involved delamination of the sealing membrane and coverage with SSG. Grafts of less than 0.01 in. were harvested with a dermatome and applied as a sheet or meshed graft depending on the size and location of the wound. Inset was achieved with either glue, sutures or staples. Dressings included a combination of Jelonet™, Bactigras or Xeroform® (Kendall) followed by gauze or foam with crepe bandage or tape. NPWT dressings were used in selected cases. If the wound involved a limb or joint, immobilisation was applied until graft check at 5–7 days post‐operation.
Results
The age of patients ranged from 47 to 95 years, with 19 males and 8 females. The total number of wounds was 35 and included 14 lower limb wounds, 7 upper limb wounds, 12 head and neck wounds, one abdominal wound and one breast wound post‐autologous reconstruction. Follow‐up ranged from 3 to 18 months. The results are summarised in Table 1.
Table 1.
Patient details
| Age | Indication | Complications | Time from BTM inset to SSG | Wound base | % BTM integration at the time of SSG | % SSG take at 1 month | POSAS score (overall, out of 10) | Sensation (out of 10) |
|---|---|---|---|---|---|---|---|---|
| 72/M | Grade IV pressure sores on right calf and right heel with calcaneal osteomyelitis | Nil | 27 days | Muscle | Calf 100% | Calf 100% | Patient calf 7 | Calf 8 |
| Observer calf 3 | ||||||||
| Bone (with history of osteomyelitis) | Heel 100% | Heel 75% | Patient heel 7 | Heel 8 | ||||
| Observer heel 3 | ||||||||
| 61/F | Failed SSG with exposed Achilles tendon in the setting of necrotising fasciitis of the right leg | Nil | 34 days | Muscle and tendon | Calf 100% | Calf 100% | Patient 9 | 6 |
| Observer 3 | ||||||||
| 47/F | Left mastectomy skin flap necrosis and abdominal wound dehiscence in the setting of a DIEP flap | Sinus tract in the abdominal wound | 36 days | Fat | Left breast 100% | Left breast 100% | Patient breast 9 | Left breast 0 (insensate DIEP) |
| Observer breast 3 | ||||||||
| Fat | Abdomen 90% | Abdomen 90% | Patient abdomen 9 | Abdomen 3 | ||||
| Observer abdomen 4 | ||||||||
| 84/M | Delayed reconstruction of vertex and anteiror scalp following excision of SCC and prior radiotherapy | Nil | 52 days | Bone (irradiated) | Vertex 100% |
Vertex 50% |
Patient vertex 10 |
Vertex 3 |
| Observer vertex 4 | ||||||||
| Bone (irradiated) | Anterior scalp 100% | Anterior scalp 70% | Patient anterior scalp 9 | Anterior scalp 4 | ||||
| Observer anterior scalp 5 | ||||||||
| 63/M | Left radial forearm free flap donor site after failed SSG | Nil | Never grafted—healed by secondary intention | Muscle and tendon | 100% | N/A (sealing membrane removed on day 52) |
Patient 4 |
10 |
| Observer 2 | ||||||||
| 64/M | Left fibula free flap donor site after failed SSG with exposed tendon | Nil | 24 days | Muscle and tendon | 100% | 100% | Patient 5 | 10 |
| Observer 1 | ||||||||
| 65/F | Left knee wound breakdown with polymicrobial prosthetic knee joint infection and exposed patella tendon | Nil | 81 days | Muscle and tendon (significant scarring) | 100% | 100% | Patient 8 | 5 |
| Observer 2 | ||||||||
| 66/F | Same patient as above but 1 year later. Second‐stage left knee reconstruction | Nil | 28 days | Fat and paratenon | 100% | 100% | Patient 7 | 5 |
| Observer 5 | ||||||||
| 87/M | Delayed reconstruction following scalp SCC excision and prior radiation | Partial wound breakdown with some exposed bone | 28 days | Bone (irradiated) | 100% | 90% | Patient 9 | 7 |
| Observer 5 | ||||||||
| 75/F | Right leg BCC with signficiant ulceration | Failed initial skin graft | 46 days | Fat | 100% | 100% (second SSG) | Patient 2 | 8 |
| Observer 2 | ||||||||
| 95/M | Grade III right heel pressure sore | Nil | Never grafted—healed by secondary intention | Fat | 100% | N/A | Patient 4 | 6 |
| Observer 1 | ||||||||
| 73/M | Right hand dorsum traumatic degloving with exposed extensor tendon | Nil | 29 days | Tendon | 100% | 100% | Patient 3 | 7 |
| Observer 2 | ||||||||
| 68/M | Scalp SCC excision with burring of the outer table | BTM failed to integrate, no infection | N/A | Bone | 0% | Trans‐position flap used | N/A (10) | N/A (0) |
| 69/F | Left hand dorsum wound post excision of SCC with failed SSG and exposed tendon | Nil | 49 days | Tendon | 100% | 100% | Patient 1 | 10 |
| Observer 2 | ||||||||
| 95/M | Left pre‐auricular SCC re‐excision | Nil | 15 days | Muscle | 100% | 100% | Patient 7 | 8 |
| Observer 2 | ||||||||
| 62/F | Left dorsal foot wound breakdown with exposed tendon post arthrodesis | Complete graft failure—left to heal by secondary intention | 40 days | Tendon | 100% | 0% | Patient 8 | 6 |
| Observer 7 | ||||||||
| 75/M | Right hand middle and ring finger traumatic wounds with exposed extensor tendons | Nil | 24 days | Tendon | 100% | 100% | Patient 4 | 9 |
| Observer 4 | ||||||||
| 93/M | Nasal dorsum wound post BCC excision with failed full‐thickness skin graft | Nil | 26 days | Perichondrium | 100% | 100% | Patient 1 | 8 |
| Observer 2 | ||||||||
| 91/M | Scalp SCC excision with burring of the outer table | Nil | 39 days | Bone | 100% | 50% | Patient 9 | 0 |
| Observer 8 | ||||||||
| 42/M | Right wrist traumatic wound with exposed extensor tendons | Nil | 36 days | Muscle and tendon | 100% | 100% | Patient 3 | 7 |
| Observer 3 | ||||||||
| 74/M | Failed SSG post left heel melanoma excision | Nil | 43 days | Fat | 100% | 100% | Patient 5 | 6 |
| Observer 4 | ||||||||
| 89/M | Right nasal sidewall infiltrative BCC requiring multiple re‐excisions | Nil | 43 days | Perichondrium | 100% | 100% | Patient 4 | 5 |
| Observer 2 | ||||||||
| 59/M | Excision of ulcerative BCC right thigh BCC with muslce involvement | Fall with haematoma | 41 days | Muscle | 100% | 100% | Patient 6 | 9 |
| Observer 3 | ||||||||
| 70/F | Excision of multiple SCCs in right brow, left brow, nasal dorsum and right hand dorsum | Nil | 31 days | Muscle | Right brow 100% | Right brow 100% | Patient right brow 6 | Right brow 4 |
| Observer right brow 5 | ||||||||
| Muscle | Left brow 100% | Left brow 100% | Patient left brow 6 | Left brow 4 | ||||
| Observer left brow 5 | ||||||||
| Perichondrium | Nasal dorsum 100% | Nasal dorsum 100% | Patient nasal dorsum 9 | Nasal dorsum 3 | ||||
| Observer nasal dorsum 8 | ||||||||
| Tendon | Right hand 100% | Right hand 100% | Patient right hand 4 | Right hand 5 | ||||
| Observer right hand 4 | ||||||||
| 82/M | Excision of left pre‐tibial and left medial ankle SCCs with exposed periosteum | Nil | 30 days | Periosteum | Left pre‐tibial 100% | Left pre‐tibial 100% | Patient left pre‐tibial 1 | Left pre‐tibial 8 |
| Observer left pre‐tibial 2 | ||||||||
| Periosteum | Left medial ankle 100% | Left medial ankle 100% | Patient left medial ankle 1 | Left medial ankle 8 | ||||
| Observer left medial ankle 2 | ||||||||
| 86/F | Soft tissue defect following open left distal fibula fracture and open reduction with internal fixation | Nil | 44 days | Fat and muscle | 100% | 90% | Patient 6 | 7 |
| Observer 4 | ||||||||
| 76/M | Right dorsal hand degloving with exposed extensor tendons | Nil | 27 days | Paratenon | 100% | 100% | Patient 4 | 5 |
| Observer 3 | ||||||||
| 81/M | Excision of large scalp SCC with exposed calvarium | Nil | 25 days | Bone | 100% | 100% | Patient 3 | 3 |
| Observer 2 |
BCC, basal cell carcinoma; BTM, Biodegradable Temporising Matrix; DIEP, deep inferior epigastric perforator; ICU, intensive care unit; POSAS, Patient and Observer Scar Assessment Scale; SCC, squamous cell carcinoma; SSG, split‐skin graft.
Thirty‐three wounds had 100% integration of BTM at the time of second‐stage reconstruction. In one of the cases, the BTM failed to integrate over exposed calvarium despite an absence of haematoma or infection. This patient subsequently had a transposition flap. The other case had an incomplete integration of the BTM with 10% loss. The SSG was taken over the 90% BTM, with the rest of the wound healed by secondary intention. Seven patients had partial graft loss after the second‐stage reconstruction, which all healed by secondary intention.
The POSAS scale was used to evaluate the scars of each patient. A mean ± standard deviation overall patient score of 5.67 ± 2.82 out of 10 was observed, with one representing normal skin and 10 being very different to normal skin. A mean overall observer score from an independent plastic surgery is of 3.63 ± 2.04.
Light touch sensation was measured in all patients with an average score of 5.86 ± 2.72 out of 10, with 10 being full sensation. However, one patient had BTM applied over an insensate deep inferior epigastric perforator flap and was excluded.
The following is a further discussion of four example cases.
Case 1
A 72‐year‐old gentleman presented with right calcaneal osteomyelitis secondary to a pressure injury after a complicated valvular heart surgery. The calcaneal pressure wound was treated with multiple debridement including partial calcanectomy and NPWT dressings. Vascular supply of the foot was optimised with angiography and stenting. Intraoperative bone samples grew Proteus and Staphylococcus aureus and were treated with prolonged intravenous antibiotics. The patient requested limb preservation. BTM was applied over the residual calcaneum and a separate posterior calf pressure ulcer. There was full integration of the BTM, and the wound was successfully grafted 4 weeks later. Follow‐up at 18 months post‐grafting (Fig. 1) showed adequate and durable soft tissue coverage with no recurrent wound breakdown.
Fig 1.
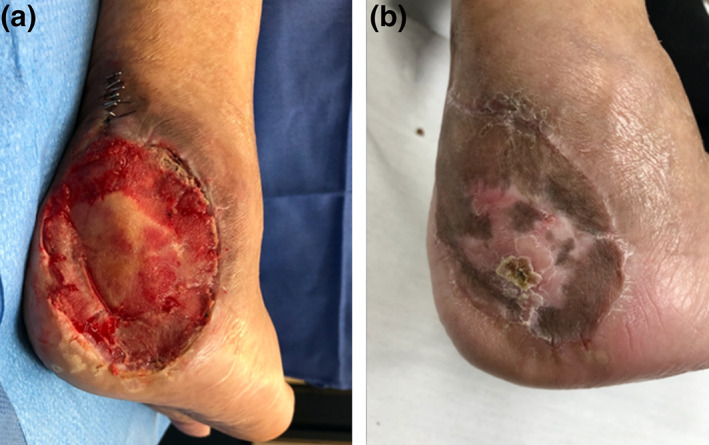
Right calcaneal pressure sore with osteomyelitis reconstructed—(a) with Biodegradable Temporising Matrix after delamination. (b) Result at 18 months post‐operatively.
Case 2
A 64‐year‐old lady presented with an infected and dehisced knee wound with underlying prosthesis. She had a previous left total knee replacement with gradually worsening range of motion following an intra‐medullary nail fixation of a femur fracture of the ipsilateral side. An arthrotomy and manipulation under anaesthesia was performed to release scar tissue but she presented 1 month later with wound dehiscence and infected prosthesis. She underwent multiple debridement and the prosthesis was replaced with an antibiotic spacer. Intraoperative samples grew Pseudomonas, Escherichia coli, Bacteroides and Staphylococcus epidermidis which were treated with a prolonged course of intravenous antibiotics. The antibiotic spacer was covered with a heavily scarred patella tendon temporised with NPWT. BTM was applied over the patella tendon. After 10 weeks, an SSG was applied. Follow‐up at 12 months demonstrates complete healing of the wound (Fig. 2b).
Fig 2.
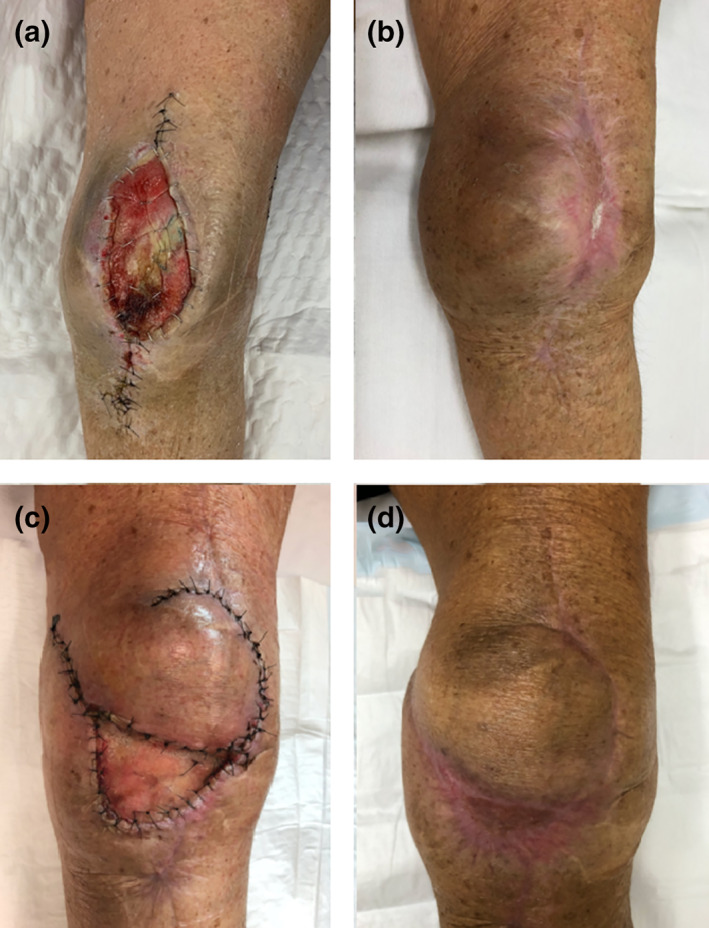
Left knee wound dehiscence with underlying metalware. (a) Reconstructed with Biodegradable Temporising Matrix (BTM) over scarred patella tendon (b) at 12 months post‐reconstruction, (c) second reconstruction with a local perforator flap and BTM to the donor site and (d) at 6 months post‐reconstruction.
The spacer was removed at 12 months and replaced with a new prosthesis. The wound was closed primarily but she again developed a wound dehiscence just distal to the previous BTM site at 4 weeks after the operation. The wound was debrided, and a local perforator flap was used to cover the wound and BTM was applied to the donor defect. The donor defect was grafted at 8 weeks. At 6 months follow‐up, the wound remained stable (Fig. 2c).
Case 3
A 65‐year‐old gentleman underwent a left radial free forearm flap for a maxillary squamous cell carcinoma defect. The initial SSG to his forearm donor site failed. The wound was debrided leaving the flexor carpi radialis and palmaris longus tendons exposed. Intraoperative samples grew mixed skin flora. BTM was applied and, interestingly, the wound almost completely re‐epithelialised at 4 weeks. A decision was made to allow it to heal without an SSG with the sealing membrane removed at day 52 post‐operatively. Follow‐up at 12 months showed no recurrence of wound breakdown.
Case 4
A 93‐year‐old gentlemen presented with a failed full‐thickness skin graft after the excision of a nasal dorsum squamous cell carcinoma. Biopsy of a lesion on the left nasal alar confirmed a separate basal cell carcinoma (BCC). The nasal dorsum wound and the left nasal alar BCC were excised en bloc and resurfaced with BTM. The BTM was well integrated and delaminated at 4 weeks. An SSG was applied and at 8 weeks follow‐up the graft showed excellent aesthetic result (Fig. 3).
Fig 3.
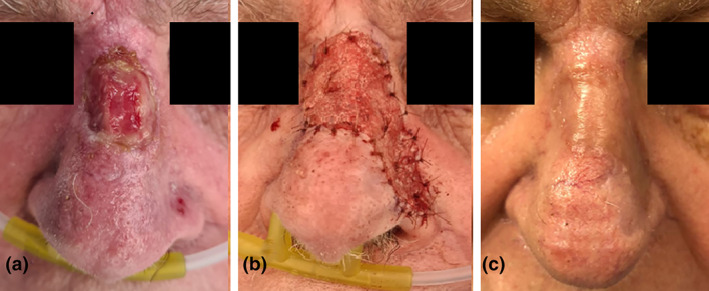
(a) Failed full‐thickness skin graft on the dorsum of the nose with a separate foci of basal cell carcinoma (BCC) on the left nasal alar. (b) Debridement and excision of left nasal alar BCC en bloc and resurface with Biodegradable Temporising Matrix (c) at 6 months follow‐up.
Discussion
BTM has demonstrated its reliability and versatility in the reconstruction of complex wounds in patient with multiple comorbidities. Most cases were successfully grafted at 3–4 weeks post‐operatively, ranging from 2 to 10 weeks. Furthermore, the dermal matrix was robust enough to heal in the setting of partial graft loss.
Advantages of BTM include the ability to convert a wound bed into a surface suitable for skin graft, such as exposed bone or tendon. In the cases of exposed tendons, this also allows preservation of the tendon function. In patients with multiple morbidities, it provides a robust and simple reconstructive option for complex wounds. The operation can be performed under local or regional anaesthesia and has an overall low complication profile and donor site morbidity.
In two of the cases, there was complete re‐epithelisation of the wound without the need for a skin graft. To our knowledge, this has yet to be reported in the literature. This offers valuable insight into what BTM is capable of as a dermal matrix and could provide the basis for future research.
The sensory regeneration of the wounds reconstructed with BTM can significantly influence the patients' quality of life after the reconstruction. This is particularly important when BTM is used in the weight‐bearing portion of the lower limb. A sensate reconstruction can maintain function and also help in preventing further injury to the area. In our cases, early results are promising, with most patients regaining partial sensation over the majority of their wounds. This may provide further benefits over more complex reconstructions. Further studies with longer follow‐up and more in‐depth sensory assessment would be beneficial.
The POSAS score also suggest overall good aesthetic result with BTM reconstruction. It matches the thickness of most defects without the need for further revision or debulking.
Disadvantages of BTM include potential failure to integrate especially in cases of borderline vascularity or infection, as well as the staged nature of the reconstruction. These limitations are, however, common to all dermal matrices. Prior radiation, especially in the scalp, poses high risk of failure to integrate in our series. Four patients with scalp malignancies that involved burring of the outer table and radiotherapy had either partial graft failure or, in one case, complete failure of integration of BTM. This may form the basis of future studies.
Conclusion
BTM has been shown to be effective in burns, necrotising fasciitis and free flap donor wounds. Our series suggest it provides reliable and robust reconstructive options in a wide range of complex wound, especially when local and free tissue transfer are not a suitable option.
Author contributions
Henry Li: Conceptualization; data curation; formal analysis; investigation; methodology; software; validation; writing‐original draft; writing‐review & editing. Pelicia Lim: Data curation; investigation; resources. Edward Stanley: Investigation; methodology; resources. Geoffrey Lee: Data curation; investigation; resources. Sandra Lin: Data curation; investigation; resources. Derek Neoh: Resources; supervision. Julian Liew: Resources; supervision. Sally Ng: Conceptualization; investigation; methodology; project administration; resources; supervision; validation; writing‐original draft; writing‐review & editing.
Conflict of interest
There are no conflicts of interest to disclose except that author Julian Liew holds minor shares in the PolyNovo.
Data availability statement
The data used in this study is kept in the respective hospital's departmental database and medical records.
Acknowledgement
The authors thank Tim Barker from Polynovo for the provision of the product specifics.
H. Li MBBS; P. Lim MD; E. Stanley MBBS; G. Lee MBBS; S. Lin MD; D. Neoh MBBS, FRACS; J. Liew MBBS, FRACS; S. K.‐H. Ng MBBS, FRACS.
References
- 1. Greenwood J. The evolution of acute burn care–retiring the split skin graft. Ann R Coll Surg Engl. 2017;99:432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greenwood J, Li A, Dearman B, Moore TG. Evaluation of NovoSorb novel biodegradable polymer for the generation of a dermal matrix part 1: in‐vitro studies. Wound Pract Res. 2010;18:14. [Google Scholar]
- 3. Greenwood JE, Schmitt BJ, Wagstaff MJ. Experience with a synthetic bilayer Biodegradable Temporising Matrix in significant burn injury. Burns Open. 2018;2:17–34. [Google Scholar]
- 4. Cheshire PA, Herson MR, Cleland H, Akbarzadeh S. Artificial dermal templates: a comparative study of NovoSorb Biodegradable Temporising Matrix (BTM) and Integra(R) Dermal Regeneration Template (DRT). Burns. 2016;42:1088–96. [DOI] [PubMed] [Google Scholar]
- 5. Li A, Dearman BL, Crompton KE, Moore TG, Greenwood JE. Evaluation of a novel biodegradable polymer for the generation of a dermal matrix. J Burn Care Res. 2009;30:717–28. [DOI] [PubMed] [Google Scholar]
- 6. Greenwood J, Li A, Dearman B, Moore TG. Evaluation of NovoSorb novel biodegradable polymer for the generation of a dermal matrix part 2: in‐vivo studies. Wound Pract Res. 2010;18:24. [Google Scholar]
- 7. Dearman BL, Li A, Greenwood JE. Optimization of a polyurethane dermal matrix and experience with a polymer‐based cultured composite skin. J Burn Care Res. 2014;35:437–48. [DOI] [PubMed] [Google Scholar]
- 8. Greenwood JE, Dearman BL. Comparison of a sealed, polymer foam biodegradable temporizing matrix against Integra(R) dermal regeneration template in a porcine wound model. J Burn Care Res. 2012;33:163–73. [DOI] [PubMed] [Google Scholar]
- 9. Wagstaff MJD, Driver S, Coghlan P, Greenwood JE. A randomized, controlled trial of negative pressure wound therapy of pressure ulcers via a novel polyurethane foam. Wound Repair Regen. 2014;22:205–11. [DOI] [PubMed] [Google Scholar]
- 10. Wagstaff MJ, Schmitt BJ, Coghlan P, Finkemeyer JP, Caplash Y, Greenwood JE. A biodegradable polyurethane dermal matrix in reconstruction of free flap donor sites: a pilot study. Eplasty. 2015;15:e13. [PMC free article] [PubMed] [Google Scholar]
- 11. Wagstaff MJ, Schmitt BJ, Caplash Y, Greenwood JE. Free flap donor site reconstruction: a prospective case series using an optimized polyurethane biodegradable temporizing matrix. Eplasty. 2015;15:e27. [PMC free article] [PubMed] [Google Scholar]
- 12. Greenwood JE, Wagstaff MJ, Rooke M, et al. Reconstruction of extensive calvarial exposure after major burn injury in 2 stages using a biodegradable polyurethane matrix. Eplasty. 2016;16:e17. [PMC free article] [PubMed] [Google Scholar]
- 13. Wagstaff MJ, Salna IM, Caplash Y, Greenwood JE. Biodegradable Temporising Matrix (BTM) for the reconstruction of defects following serial debridement for necrotising fasciitis: a case series. Burns Open. 2019;3:12–30. [Google Scholar]
- 14. Sreedharan S, Morrison E, Cleland H, Ricketts S, Bruscino‐Raiola F. Biodegradable temporising matrix for necrotising soft tissue infections: a case report. Aust J Plast Surg. 2019;2:106–9. [Google Scholar]
- 15. Draaijers LJ, Tempelman FR, Botman YA, Tuinebreijer WE, Middelkoop E, Kreis RW, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004;113:1960–5, discussion 1966–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study is kept in the respective hospital's departmental database and medical records.


