Abstract
Aortic sinus aneurysms are mainly congenital malformations that can involve the left, right, and noncoronary sinus. Rupture of the noncoronary sinus aneurysms is rare, and its mechanisms and complications are still imperfectly known due to the rarity of this condition. A case of multiple organ dysfunction caused by a ruptured noncoronary sinus aneurysm has been reported.
Keywords: aortic sinus aneurysm, congenital disease, echocardiography, multiple organ dysfunction
1. INTRODUCTION
Aneurysms of the sinus of Valsalva (ASV) are rare, and most cases are congenital. It is suspected that aneurysmal development results from the lack of elastic fibers and smooth muscle cells in the wall of the sinus of Valsalva, eventually leading to its rupture. 1 , 2 , 3 Most nonruptured ASV are asymptomatic and are detected incidentally. Rupture may create aortic‐cardiac shunts, potentially resulting in progressive congestive heart failure, acute chest pain and dyspnea, or even cardiac arrest. 4
Here, we present a case of rupture of a giant ASV into the right atrium, leading to multiple organ dysfunction.
1.1. Case report
A 26‐year‐old woman presented to the hospital because of cough, expectoration, nausea, and vomiting during the last 3 days. She reported no chest tightness or shortness of breath. She had been unsuccessfully self‐treated with unspecified traditional Chinese medicine.
The patient had no history of developmental abnormalities or cardiopulmonary disease. On admission, physical examination showed body temperature 36.5°C, heart rate 107 beats per minute, breathing rate 21 per minute, and blood pressure 128/59 mmHg. The patient had normal mental status, stable breathing with coarse breathing sounds in both lungs, reduced breath sounds in both lower lung fields, regular heart beats without pathological murmur at auscultation, jugular vein distension, mild tenderness in the right upper abdomen, rebound pain, but a negative Murphy's sign. The remainder of the physical examination was within normal limits. Laboratory exams showed increased NT‐proBNP, ALT, AST, prothrombin time (PT) ratio, and lactic acid (Table 1). Because of the respiratory symptoms and jugular vein dilation, a chest computed tomography (CT) examination was performed and showed right lung texture thickening and right pleural effusion. No obvious abnormality was found on echocardiography. The patient received inotropic treatment, magnesium isoglycyrrhizinate injection, and antibiotic therapy.
TABLE 1.
Laboratory examination results
| Variable | Units | Normal range | D–2 | D0 | D + 3 |
|---|---|---|---|---|---|
| ALT | (U/L) | (9‐52) | 349 | 4505 | 1122 |
| AST | (U/L) | (14‐36) | 241 | 6740 | 394 |
| Total bilirubin | (μmol/L) | (3‐22) | 19.41 | 48.51 | 166.53 |
| Creatinine | (μmol/L) | (31‐133) | 70.4 | 185 | 58.6 |
| Urea nitrogen | (mmol/L) | (2.5‐7.1) | 8.1 | 13.9 | 7.3 |
| PT ratio | (INR) | (0.8‐1.2) | 1.62 | 3.64 | 1.46 |
| PT percentage | Activity (%) | (80‐200) | 52 | 19 | 61 |
| Platelet | (×109/L) | (125‐350) | 257 | 269 | 96 |
| Lactic acid | (mmol/L) | (0.5‐1.6) | 1.7 | 11 | 2.8 |
Notes: D–2: 2 days before the surgery; D0: the day of surgery; D + 3: the third day after surgery.
Two days after treatment initiation, the patient showed symptoms of anxiety, pallor, dry mouth, sweating, slight cyanosis of limbs, and chills. Electrocardiogram (ECG) revealed that her heart rate was 111 beats per minute with sinus rhythm and no ST segment anomaly. Her breathing rate was 25 per minute, and blood pressure was 81/47 mmHg. The jugular vein distension was obvious, and a Grade 3/6 systolic murmur was heard in the mitral area. A rough Grade 4 continuous murmur was heard at the third and fourth intercostal spaces on the right edge of the sternum. In addition, the patient's urine output decreased every hour. The patient's liver function tests showed marked deterioration (Table 1).
Echocardiography was reiterated because of dilated jugular veins and elevated NT‐proBNP. It showed enlargement of the left atrium, right atrium, and right ventricle, but not of the left ventricle. The noncoronary aortic sinus was enlarged to 26 × 19 mm, bulging into the right atrium, with a thin wall, multiple ruptures, and a groove of about 7.7 mm opening into the right atrium. An additional 3 mm breach was seen near the opening of the coronary sinus, with part of the blood flowing into the noncoronary aortic sinus. The breach gave way to a continuous left to right blood flow with a 4.4 m/s peak velocity and a 76 mmHg peak pressure gradient. The interventricular septum and left ventricular free wall thickness was normal, and there was mild tricuspid regurgitation with mild pulmonary arterial hypertension (estimated systolic pulmonary artery pressure of 44 mmHg). Mild mitral regurgitation was noted, and the aortic valve appeared normal. There was no sign of pericardial effusion, but a 84 mm large pleural effusion, confirmed by chest CT (Figure 1). These findings led to the diagnosis of congenital heart disease, rupture of ASV (rupture of the noncoronary sinus into the right atrium), aortic‐right atrial shunt, left atrium enlargement, right atrial enlargement, right ventricle enlargement, tricuspid regurgitation, mitral regurgitation, mild pulmonary hypertension, and pleural effusion (Figure 2).
FIGURE 1.
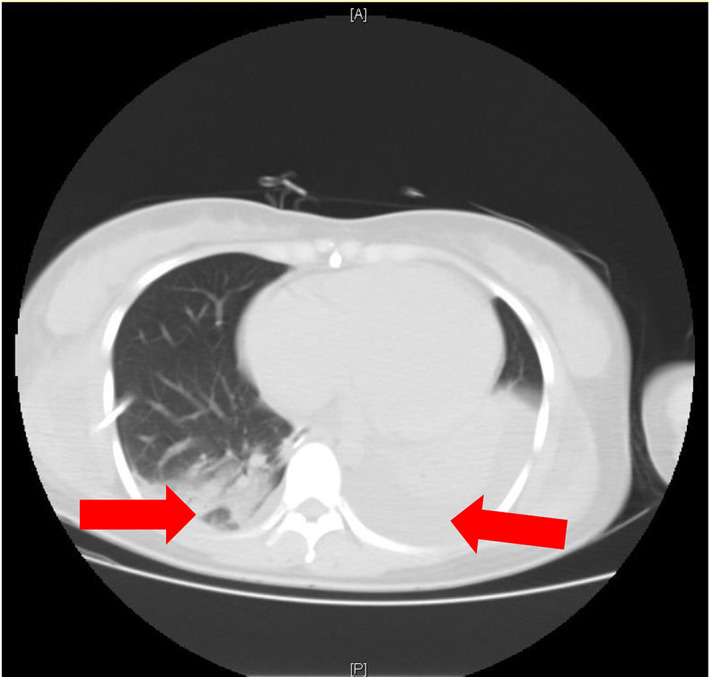
Chest CT showing bilateral pleural effusion. The right pleural effusion had been drained, but a small amount of liquid was still visible. The left pleural effusion was still present (arrow)
FIGURE 2.
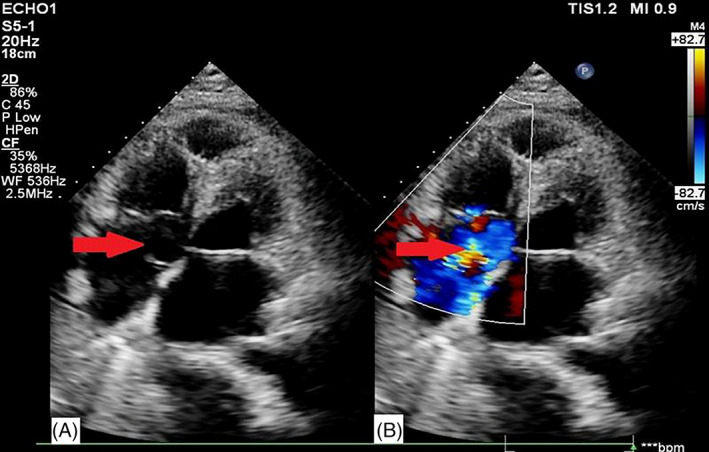
Echocardiographic examination. A, The aorta noncoronary sinus bulged into the right atrium because of a 26 cm × 19 mm aneurysm, ruptured into the right atrium (arrow). B, Color Doppler imaging showed left to right turbulent blood flow through the rupture breach (arrow)
Open cardiac surgery was immediately performed, and confirmed ruptured aortic sinus aneurysm and tricuspid regurgitation. The patient underwent repair of the aortic sinus and of the tricuspid valve. Liver, kidney, and routine blood tests showed significant improvement 3 days after the operation (Table 1). Two weeks after the operation, echocardiography yielded normal images and signals, and blood test were normal. The patient recovered and was discharged from the hospital to rehabilitation.
2. DISCUSSION
ASV are rare and are either congenital or acquired. They have a higher incidence rate in males than in females, and in individuals aged 20‐40 years old. 5 Most ASV originate from the right coronary sinus, followed by the noncoronary sinus, and finally the left coronary sinus. Formation of an ASV occurs because of the congenital lack of continuity of aortic media and annulus fibrosus, and this structural defect results in dysfunction of fibrous tissue connection with the aortic annulus, eventually forming a weak area. Echocardiography plays an increasingly important role in the diagnosis and postoperative evaluation of ruptured ASVs. It can reveal the location, shape, size, rupture status, and complications of ASVs, as well as related hemodynamic changes. 6 , 7 The impact of long‐term high‐velocity blood flow is such that ASVs gradually grow, and may rupture because of sepsis or severe cough, among other reasons. 8 , 9 , 10 Symptoms of ASV rupture depend on the speed and the size of rupture, as well as on the specific heart cavity penetrated. The right coronary sinus aneurysms have the higher rate of rupture. ASV rupture can penetrate any cardiac cavity, mainly in right ventricle and right atrium. When rupture occurs into the right heart cavity, blood follows the pressure gradient and flows from the aorta into the right ventricle. The hemodynamic consequences are comparable to those of a patent ductus arteriosus, with increased blood flow in the pulmonary circulation, and increased right ventricular load, resulting in right ventricle enlargement, pulmonary hypertension, and eventually right heart failure. When rupture occurs into the right atrium, causing its expansion, blood pressure increases, hampering flow from the superior and inferior vena cava, and leading to right heart failure. ASV may co‐exist with cardiac malformations such as a ventricular septal defect. 11 , 12 , 13
The typical sign of ASV rupture is a heart murmur. It generally presents as a continuous two‐stage heart murmur between the third and fourth ribs on the left edge of the sternum, and there may be a tremor of Grade 3 or higher. There may also be signs of right heart failure such as a jugular vein engorgement and hepatomegaly.
Our patient was a young woman with no abnormal medical history. She had been frequently working night shifts, with strong physical efforts, which could have induced chronic cardiac overload. At admission, the clinical presentation was nonspecific, and the first echocardiographic examination may have missed some minor signs related to the ASV and/or ongoing rupture. A sudden increase in intrathoracic pressure caused by a cough may have led to the rupture of an aortic noncoronary sinus aneurysm into the right atrium, inducing a sudden increase in central venous pressure, and leading to acute right heart failure, with subsequent liver and kidney dysfunction. Liver failure was prominent, as shown by continued increase in transaminase and total bilirubin levels, and decreased production of coagulation factors (abnormal PT ratio and percentage). These disorders disappeared progressively after surgical repair, as did renal function troubles. Preoperative arterial blood gas analysis demonstrated increasing lactic acid, consistent with sympathetic overstimulation in the context of cardiac failure, with compromised tissue perfusion, all of which also improved after surgery.
3. CONCLUSION
Aortic sinus aneurysm rupture can induce heart, liver, and kidney dysfunction or even failure, and detailed echocardiographic examination, in such a nonspecific setting, may play a decisive role. Surgery is urgent and mandatory in the presence of ruptured aortic sinus aneurysm. In the present case, surgical repair allowed rapid and complete improvement of the clinical status.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Song J, Pan X. Multiple organ dysfunction caused by a ruptured aortic sinus aneurysm: A case report. J Clin Ultrasound. 2021;49:799–802. 10.1002/jcu.22991
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ring WS. Congenital heart surgery nomenclature and database project: aortic aneurysm, sinus of Valsalva aneurysm, and aortic dissection. Ann Thorac Surg. 2000;69:S147. [DOI] [PubMed] [Google Scholar]
- 2. Edwards JE, Burchell HB. The pathological anatomy of deficiencies between the aortic root and the heart, including aortic sinus aneurysms. Thorax. 1957;12:125‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoey ET, Gulati GS, Singh S, et al. The role of multi‐modality imaging for sinus of Valsalva aneurysms. Int J Cardiovasc Imaging. 2012;28:1725‐1738. [DOI] [PubMed] [Google Scholar]
- 4. Bricker AO, Avutu B, Mohammed TL, et al. Valsalva sinus aneurysms: findings at CT and MR imaging. Radiographics. 2010;30:99‐110. [DOI] [PubMed] [Google Scholar]
- 5. Sinha SK, Khanna NN, Razi M, et al. Safety and feasibility of Transcatheter interruption of ruptured sinus of Valsalva aneurysm using the cocoon duct Occluder: immediate results and mid‐term follow‐up. Cardiol Res. 2017;8:154‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng TO , Yang YL, Xie MX, et al. Echocardiographic diagnosis of sinus of Valsalva aneurysm: a 17‐year (1995‐2012) experience of 212 surgically treated patients from one single medical center in China. Int J Cardiol. 2014;173:33‐39. [DOI] [PubMed] [Google Scholar]
- 7. Xiangmei Y, Ming W, Yanli Z, et al. Significance of color Dopple ultrasound in rupture of aortic Sinusal aneurysm and in its complications. Chinese J Ultrasound Med. 2008;24:755. [Google Scholar]
- 8. Troupis JM, Nasis A, Pasricha S, Patel M, Ellims AH, Seneviratne S. Sinus valsalva aneurysm on cardiac CT angiography: assessment and detection. J Med Imaging Radiat Oncol. 2013;57:444‐447. [DOI] [PubMed] [Google Scholar]
- 9. Jung SH, Yun TJ, Im YM, et al. Ruptured sinus of Valsalva aneurysm: transaortic repair may cause sinus of Valsalva distortion and aortic regurgitation. J Thorac Cardiovasc Surg. 2008;135:1153‐1158. [DOI] [PubMed] [Google Scholar]
- 10. Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68:1573‐1577. [DOI] [PubMed] [Google Scholar]
- 11. Tipoo Sultan FA, Basir N, Fatimi S. Aneurysm of sinus of Valsalva.J Coil Physicians Pak.2011, 21:173. [PubMed] [Google Scholar]
- 12. Sakakibara S, Konno S. Congenital aneurysm of the sinus of Valsalva: anatomy and classification. Am Heart J. 1962;63:405. [DOI] [PubMed] [Google Scholar]
- 13. Masuyama K, Nakamura H, Okamoto N, et al. Unusual case of rupture of right sinus of Valsalva aneurysm into the left ventricle. Circ J. 2017;81:577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


