Summary
Microclimatic effects (light, temperature) are often neglected in phenological studies and little information is known about the impact of resource availability (nutrient and water) on tree’s phenological cycles.
Here we experimentally studied spring and autumn phenology in four temperate trees in response to changes in bud albedo (white‐painted vs black‐painted buds), light conditions (nonshaded vs c. 70% shaded), water availability (irrigated, control and reduced precipitation) and nutrients (low vs high availability).
We found that higher bud albedo or shade delayed budburst (up to +12 d), indicating that temperature is sensed locally within each bud. Leaf senescence was delayed by high nutrient availability (up to +7 d) and shade conditions (up to +39 d) in all species, except oak. Autumn phenological responses to summer droughts depended on species, with a delay for cherry (+7 d) and an advance for beech (−7 d).
The strong phenological effects of bud albedo and light exposure reveal an important role of microclimatic variation on phenology. In addition to the temperature and photoperiod effects, our results suggest a tight interplay between source and sink processes in regulating the end of the seasonal vegetation cycle, which can be largely influenced by resource availability (light, water and nutrients).
Keywords: bud albedo, budburst, drought, environmental stressors, leaf senescence, light, microclimate, phenology
Introduction
The phenological responses of plants to environmental cues play a prominent role in shaping species’ distribution ranges (Chuine & Beaubien, 2001; Körner et al., 2016) and Earth’s climate (Richardson et al., 2013). Over recent years, a profusion of studies based on ground observations or remote sensing have documented phenological shifts in response to global warming, consistently showing earlier occurrence of spring phenophases, such as leaf‐out or flowering, and, in some cases, later autumn phenophases, such as fruit maturation or leaf senescence (Garonna et al., 2016; Piao et al., 2019). Changes in phenology have a major impact on the global carbon balance, and earlier leaf‐out timing has been shown to compensate for the increasing carbon loss in summer due to more severe and prolonged drought (Wolf et al., 2016) but earlier phenology may also accelerate and amplify drought in early summer as plants begin to take up water earlier (Ma et al., 2016; Xu et al., 2020; Meier et al., 2021). For these reasons, increasing efforts have been made to include phenological models in global models of species distribution and forest carbon balance (Delpierre et al., 2016; Zohner et al., 2020). Phenological models are still however unable to accurately predict the progression of winter dormancy, which is essential for predicting the beginning of bud development in spring (Basler, 2016; Chuine et al., 2016; Wang et al., 2020), as well as the time of leaf senescence in autumn (Liu et al., 2020). A striking example is that simplistic spring phenology models that ignore chilling and photoperiod often perform similarly compared with more complex phenological models that include these cues (Fu et al., 2012; Basler, 2016). This contrasts with numerous experimental observations of temperate and boreal perennial plants, which have long shown that chilling and photoperiod play a significant role in dormancy release and bud development (e.g. Coville, 1920; Wareing, 1953; Murray et al., 1989; Heide, 1993; Rousi & Pusenius, 2005; Viherä‐Aarnio et al., 2006).
A major limitation of plant physiological and phenological studies carried out under natural conditions is that they usually use the temperature recorded at standard weather stations as an approximation of the temperature perceived by the plant. The microclimate in forests or near buds can largely deviate from 2‐m height air temperature (for example buds of seedlings and saplings are close to the ground whereas buds of adult trees can be at a height of 30 m with more exposure to wind and solar radiation) and even more from standard air temperature measured outside of the forest (De Frenne et al., 2019). Yet, microclimatic conditions have a huge effect on plant performance, and changes in microclimate have even been shown to outweigh macroclimate effects on plant community composition (Zellweger et al., 2020). In fact, there is evidence that the temperature triggering cell growth in the apical meristems of the buds is directly sensed within each individual bud, likely to be by the meristems themselves, as experimentally shown for species used in horticulture such as Cucumis sativus L. (Savvides et al., 2016). Similarly, daylength is perceived at the individual bud level by phytochromes within the leaf primordia (Zohner & Renner, 2015). When plants are growing in an open area, meristem temperature is generally higher compared with standard air temperature during the day and lower during the night, especially during bright days and clear nights due to shortwave and longwave radiative forcing and cooling, respectively (Savvides et al., 2013). More accurate microclimatic records reflecting the actual meristem temperature are therefore necessary to improve models of plant phenology and the associated physiological processes.
The timing of autumn leaf senescence is strongly regulated by autumn temperature and photoperiod in many temperate tree species (Keskitalo et al., 2005; Vitasse et al., 2009; Fu et al., 2018). However, spring and summer photosynthesis (Zani et al., 2020), CO2 concentration (Sigurdsson, 2001), soil nutrient status (Weih & Karlsson, 1999; Sigurdsson, 2001; Estiarte & Peñuelas, 2015; Fu et al., 2019a, 2019b,2019a, 2019b) and water availability (Xie et al., 2015; Arend et al., 2016a, 2016b,2016a, 2016b) can have a large influence as well. These factors have often been studied in situ, not accounting for micro‐environmental heterogeneities, but they have rarely been studied under experimental conditions (but see e.g. Arend et al., 2016b; Fu et al., 2019a, 2019b,2019a, 2019b; Zani et al., 2020), complicating conclusive results about their respective effects and interactions. In addition, inconsistent results have been found for the progress of leaf senescence in response to moderate drought, hot spells and nutritional status under natural conditions in different species (Estiarte & Peñuelas, 2015; Xie et al., 2018; Chen et al., 2020; Mariën et al., 2021), underscoring the importance of controlled experiments (but see Fu et al., 2019a, 2019b,2019a, 2019b; Zani et al., 2020).
Recently, leaf senescence timing of temperate trees has been proposed to be regulated by sink limitation of photosynthesis, which has been experimentally demonstrated under contrasting light conditions, temperature and CO2 levels (Zani et al., 2020). The hypothesis that photosynthesis is regulated by the strength of the carbon sink (i.e. the use of photoassimilates for growth) has been first formulated by Boussingault (1868). Accordingly, at the end of the season when tree primary and secondary growth ceases, there is an increasing imbalance between the production of carbohydrates (source) and their use for growth (sink). During this period, carbohydrates generally accumulate faster in leaves and other organs, even though they can be, to some extent, actively regulated by the plant (Dietze et al., 2014; Gilson et al., 2014). This excess of carbohydrates at a time when growth demand is limited could lead to a downregulation of the photosynthetic genes and accelerate the induction of leaf senescence (Paul & Foyer, 2001). In addition, environmental stress, such as limited water, high solar radiation or extreme temperature, has been shown to accelerate leaf senescence of temperate trees (Gallé et al., 2007). By interacting with endogenous factors (e.g. hormones), these environmental stressors can induce degradation of chlorophyll and photosystems, leading to a decline in the capacity to dissipate excess excitation energy in chloroplasts and, in turn, the accumulation of reactive oxygen species (ROS) and the acceleration of leaf senescence (Juvany et al., 2013). ROS concentration increases during drought‐induced leaf senescence (Munné‐Bosch & Alegre, 2004), but the ability to recover after such stress depends on the species and can be high as for example in pubescent oak (Gallé et al., 2007). As such, the sensitivity of leaf senescence to environmental stress appears to depend on species’ resistance strategies and the severity of the stressor, which can lead to contrasting results among co‐existing trees (e.g. delay rather than an advance of leaf senescence under moderate stress, see Xie et al., 2018). The regulation of leaf senescence therefore appears to result from a complex balance between sink and source strength and stress responses, which needs to be explored under controlled conditions to understand and forecast phenological changes under continued global warming.
Here we experimentally assessed the effects of light (‘sun’, 100% of photosynthetically active radiation (PAR) vs ‘shade’, c. 30% PAR) and bud albedo (white vs black‐painted buds) on budburst timing and the effect of light, soil water availability (irrigated, control and reduced precipitation) and soil nutrients (low vs high) on leaf senescence timing of 2–4‐yr‐old temperate trees (Fagus sylvatica L., Fraxinus excelsior L., Prunus avium L. and Quercus robur L.). We aimed to address the following questions:
To what extent is leaf‐out regulated by microclimatic conditions, that is does high bud albedo and shade delay leaf‐out at the individual level?
Do source–sink feedbacks and/or stress responses explain the effects of nutrient availability, solar radiation and soil moisture on autumn leaf senescence? Specifically, does elevated sink strength (high nutrients) lead to delayed senescence, does increased light availability (elevated photosynthesis) advance senescence, and how does water availability interact with these patterns?
Are the different responses among species related to their tolerance to drought or shade?
Assuming that bud meristems are the temperature‐sensitive part (‘thermometer’) of the plant, we expected that white‐painted buds and shade would delay budburst as a result of lower temperature experienced by buds. We expected earlier senescence under full sun conditions because carbohydrate reserves would be faster accumulated according to the sink‐limitation hypothesis, and/or due to higher oxidative stress damaging the photosystems (photo‐oxidative stress hypothesis), especially for shade intolerant species. Furthermore, we expected delayed leaf senescence under elevated nutrient availability as a result of increased sink strength, which may compensate the cost of maintaining leaves alive (Paul & Foyer, 2001). Finally, we expected a mixed response of the timing of leaf senescence to drought depending on species‐specific sensitive to drought.
Materials and Methods
Study species
We investigated microclimate and nutrient effects on leaf phenology of four species: Prunus avium L., Fraxinus excelsior L., Fagus sylvatica L. and Quercus robur L. For clarity and brevity, we refer from this point forwards to each species by its common name, that is cherry, ash, beech and oak, respectively. These species were selected due to their large variation in spring and autumn phenology and their differences in shade and drought tolerance. In the study area at the juvenile life‐stage, cherry and ash are amongst the first tree species to flush in spring and senesce in autumn, whereas beech and oak are rather late‐flushing and late‐senescing species (Vitasse et al., 2013 and see Supporting Information Fig. S1). Beech is the most shade‐tolerant species followed by cherry, ash and oak, whereas oak and cherry are more drought tolerant than ash and beech (see shade and drought tolerance indexes extracted from Niinemets & Valladares, 2006 in Table S1). Seedlings of each species except ash were purchased at a local nursery (Wiler, 455 m asl, 47°09′N, 7°33′°E) and came from local forests (see details in Table S1). The ash seedlings were taken from a forest near the experimental site (Lenzburg, 400 m asl, 47°24′N, 8°09′E) and were directly transplanted into the experimental boxes on 15 November 2018. Seedlings were 2‐ to 4‐yr‐old and c. 47 cm tall (see Table S1 for more details).
Experimental design and treatments
The experiment took place in a common garden at WSL Research Institute in north‐eastern Switzerland (47°21′38″N, 8°27′16″E; 550 m asl; mean annual temperature 9.3°C, mean annual precipitation 1134 mm, MeteoSwiss station Fluntern, 1981–2010). The design consisted of 54 wooden containers (1 m × 1 m and 0.5 m deep) arranged in groups of three, which was the unit for climate manipulation (called from this point forwards ‘plot’; see Fig. S1). The 18 plots, containing each three containers, were then arranged in three rows (six plots per row), considered as blocks in the experimental design, to account for possible microclimatic heterogeneity, that is each treatment was replicated three times. Only the two outer containers were used per plot, which are from this point forwards referred to as mesocosms (n = 36). The central container was filled with soil but left without any plants (see Fig. S1). Each mesocosm was filled with a mixture of quartz sand, fibric peat, expanded schist and pumice, and the bottom of each mesocosm was covered by a permeable plastic foil to avoid water retention and ensure a good drainage after rainfall. This mixture was designed to be nutrient poor and sandy to facilitate soil nutrient and moisture manipulation by adding fertiliser and water, respectively (Zhang et al., 2020). On 15 November 2018, 20 seedlings were planted in each study mesocosm (four rows of five individuals), mixing and alternating two species per mesocosm. To ensure homogenous plant height and minimise competition for light, ash and cherry, and oak and beech respectively were planted together (cherry and ash were slightly taller than oak and beech, see Table S1). In total, 720 seedlings (4 species × 10 replicates × 6 treatments × 3 blocks) were planted and monitored for phenology and growth. Six treatments were used to analyse spring and autumn phenology, of which four treatments were used to test their effect on both spring and autumn phenology (Table 1). In the ‘sun treatment’, trees were exposed to full sun (100% PAR). In the ‘shade treatment’, trees were exposed to shade conditions, using a shading net that intercepted c. 70.3 ± 2.1% PAR (mean ± SE, PARmesocosm/PARambient × 100; measured on four different days in February and September 2019 between 13:30 and 15:30 under either sunny or cloudy conditions in all three blocks using a Li‐Cor Li189 quantum PAR light sensor). In the ‘drought’ treatment, natural rainfall was intercepted, using a roof with plastic channels that removed c. 50% of the ambient precipitation (using V‐shaped plastic channels mounted upwards at c. 2.5 m from ground above the plants and covering c. 50% of the mesocosm surface; see picture in Fig. S1). The ‘control‐drought’ treatment served as control for the drought treatment, using the same roof infrastructure as used in the drought treatment, but that allowed almost 100% precipitation throughfall (using V‐shaped plastic channels mounted downwards). Because the soil moisture between the drought and the drought‐control treatment differed significantly during the summer but not before budburst in which it remained relatively high (80–100% of the field capacity; Fig. S2a), these two treatments were only used to study the effects on autumn senescence. As additional budburst treatments, we modified the albedo of the buds by painting half of the buds of the plants either in black (low albedo treatment, called from this point forwards ‘black’ treatment) or in white (high albedo treatment, called from this point forwards ‘white’ treatment), using tinting dispersion paints (Schöner Wohnen Vollton‐ & Abtönfarbe) applied on 23 January 2019 (see photographs in Fig. S1). No potential deleterious impact of the paint was detected as the leaves emerged normally and were growing as much as the ones that originated from the nonpainted buds. According to the manufacturer, the painting does not contain relevant persistent, bioaccumulative and toxic substances. Plants with buds painted in white or black were kept in the same mesocosm, which reduced the replicates to 5 instead of 10 per block compared with the other treatments. After leaf‐out, the shade, sun, drought and control‐drought treatments were maintained through the growing season. To test the effect of nutrient and water availability on leaf senescence, we added two additional treatments after leaf‐out. In the ‘water’ treatment, the mesocosms were watered regularly, at least every week from 6 June to 24 October 2019 (25 times). Each mesocosm of the ‘high‐moisture’ treatment was watered manually for 2 min (two times for 1 min with a 5 min break in‐between) using a spray lance, which emitted 30 l water min−1. During a heatwave in June 2019, all mesocosms were watered manually for 5 s (6, 24 and 28 June 2019) to prevent mortality. In the ‘nutrient’ treatment, we added a substantial amount of slow‐release fertiliser (30 g of Gesal Floranid slow‐release lawn fertiliser in a granule form (composition 20% N, 5% P2O5, 8% K2O)) on 24 May and 29 July, by spreading the granules evenly on the surface of the mesocosms. Because the soil was extremely poor in nutrients, we added 5 g of this fertiliser to all the other mesocosms on 24 May 2019. These two treatments were assigned randomly to mesocosms that previously contained the black and white treatment plants (same conditions as the full sun treatment). Table 1 summarises the different treatments used during the spring and autumn phenology monitoring in 2019.
Table 1.
Summary of the different treatments applied and their starting date to test their effect on the timing of budburst and/or leaf senescence.
| Treatments | Treatment period | Tested impact on | |
|---|---|---|---|
| Budburst | Leaf senescence | ||
| Full sun | Since 15 November 2018 | ||
| Full sun + low bud albedo (black‐painted buds) | 23 January 2019 to leaf‐out | ||
| Full sun + high bud albedo (white‐painted buds) | 23 January 2019 to leaf‐out | ||
| Full sun + fertilisation | 24 May 2019 and 29 July 2019 | ||
| Full sun + irrigation | 6 June 2019 to 24 October 2019 (25 times) | ||
| Shade (shading net intercepting 70% of incoming radiation) | Since 24 January 2019 | ||
| Drought (roof intercepting 50% of the rainfall) | Since 24 January 2019 | a | |
| Control drought (roof with rain shelter turned upside down) | Since 24 January 2019 | a | |
The effect of drought vs control drought was not analysed for budburst timing as no significant difference in the soil moisture was observed between the two treatments before budburst.
Microclimatic measurements
Soil moisture was recorded in every mesocosm at 30 min intervals using EC‐5 soil moisture sensors (Decagon, Pullman, WA, USA) measuring volumetric soil water content. Because these sensors are rather sensitive to differences in soil compaction, we standardised the records of each sensor by the value obtained after irrigating the mesocosm at saturation on 21 November 2019 (using the mean value between 06:00 h and 10:00 h on the following day, that is c. 14 h after the irrigation). Therefore, soil moisture is given as % of full saturation (field capacity), which accounts for absolute deviation among the sensors and provides a standardised comparison among the treatments.
Air temperature was recorded in each plot every hour using EL‐USB‐2+ sensors (Lascar Electronics, Salisbury, UK) covered by a radiation shield (TFA Dostmann GmbH, Wertheim, Germany) from 25 January 2019 until December 2019. Additionally, air temperature at a height of 2 m was also recorded outside of the plots under an aluminium radiation shield every 30 min. The second half of February 2019 was particularly warm, with daily maximum temperatures consistently being above the long‐term average (Fig. S3). The last frost days with temperature down to −1.5°C occurred on 5–7 May (day of the year (DOY) 125–127), when all species had already leafed out (Fig. S3), but only slight frost damage was observed on beech seedlings. Two marked warm spells occurred at the end of June to beginning of July with daily maximum temperature higher than 35°C during a consecutive 7 d (DOY 180–186; Fig. S3) and at the end of July (DOY 209–211; Fig. S3). No frost occurred in autumn before leaf senescence reached 50% for any of the species (first autumnal frost on 13 November, DOY 317; Fig. S3).
Bud temperature was recorded in the following year (2020) from 1 January until species‐specific budburst by inserting the needle (0.3 mm diameter and 13 mm long) of a thermocouple probe inside buds (Thermocouple Probe Model HYP1 ©OMEGA, Omega Engineering Inc., Norwalk, CT, USA; Fig. S1). Bud temperature was recorded for two individuals from different blocks for each of the four species and for each of the following treatments: black‐painted buds, white‐painted buds, shade, full sun. All the 32 thermocouples (4 species × 2 replicates × 4 treatments) were connected to a datalogger that recorded bud temperature every 10 min. Some thermocouple probes were disconnected from the buds due to a storm that occurred on 6 March 2020 and were inserted again in the respective buds 4 d later on 10 March. We discarded all records between these two dates. We averaged the temperature of the two replicates for each species and treatment and computed the daily minimum, mean and maximum values. Additionally, air temperature at plant canopy height was recorded every 10 min with the same thermocouple probes protected from direct solar radiation with a custom‐fabricated radiation shield with several layers of carton recovered by aluminium foils (see details in Frei et al., 2020). This latter measurement was used as a reference to compare bud and air temperature in Fig. 1(b).
Fig. 1.
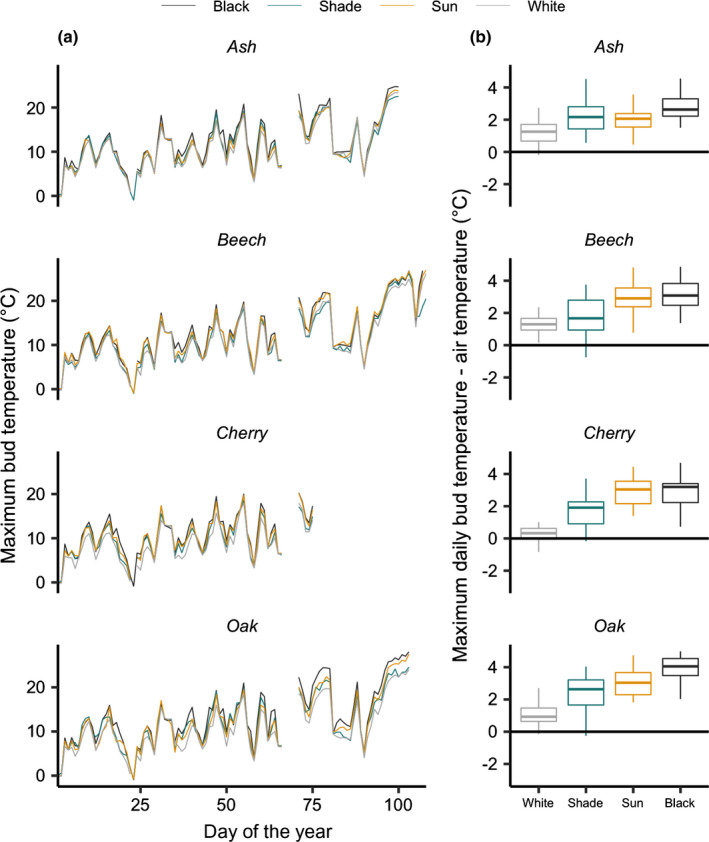
Daily maximum temperatures recorded within the bud from January 2020 until species‐specific budburst. (a) Daily maximum temperatures recorded within the buds for the different species and treatments. (b) Boxplots (first quartile, median and third quartile ± minimum and maximum values within 1.5 times the interquartile range) of the difference between daily maximum bud and air temperature on days with solar radiation reaching up at least 400 W m−2 from 26 January to budburst between 12 and 16 h. This includes 26, 48, 53 and 58 d for cherry, ash, oak and beech, respectively.
Phenology monitoring, growth measurements and soil inorganic nitrogen
Bud development and leaf senescence were monitored for all 720 individual seedlings in spring and autumn 2019. Bud development in spring was monitored by the same observer weekly or twice a week during warmer periods, from 15 February until 24 May, that is when the last individual unfolded its leaves. Bud development was monitored using a four‐stage categorical scale (Vitasse, 2013): stage 0 (dormant bud), no bud development visible; stage 1 (bud swelling), buds swollen and/or elongating; stage 2 (budburst), bud scales open and leaves partially visible; stage 3 (leaf‐out), leaves fully emerged from the buds but still folded, crinkled or pendant, depending on species; stage 4 (leaf unfolded), at least one leaf fully unfolded. For each tree, the day of year when the first bud reached the respective stage was recorded. The stages were estimated by linear interpolation when necessary (i.e. when a given stage occurred in between two monitoring dates).
For leaf senescence in autumn, we evaluated the percentage of coloured or fallen leaves for every seedling according to the method developed in Vitasse et al. (2009) on a weekly basis from 23 August to 29 November. As a proxy for the beginning, middle and end of the leaf senescence process, for each individual tree, we computed the date when 25%, 50% and 75% of leaves were either coloured or had fallen using linear interpolation between two monitoring dates.
Measurements of seedling height and diameter were conducted at 2 cm above plant collar on all individuals before budburst and after leaf fall in spring and autumn 2019, respectively, using a graduated pole and an electronic caliper. We estimated the above‐ground biomass using the allometric equation provided by Annighöfer et al. (2016) as follows:
with AGB = above‐ground biomass (g); RCD = root collar diameter (cm); H = height (cm); and b1 and β1 and β2 = species‐specific coefficients as provided in Table 4 from Annighöfer et al. (2016).
We computed the biomass increment during the growing season 2019 by subtracting the AGB estimated in spring 2019 from the AGB estimated in autumn 2019. These biomass increment measurements were used to characterise plant responses to the different treatments and to interpret the leaf senescence observations.
We measured extractable inorganic N by sampling soil in each mesocosm using a soil corer at 10 cm depth (missing three samples for each mesocosm on 3 September 2019). We used ion exchange by adding KCl solution to extract nitrate and ammonium from the soil (Table S2).
Data analysis and statistics
The progress of bud development in spring and leaf coloration in autumn was modelled by using generalised additive mixed models (GAMMs) with a binomial distribution using the R package gamm4 v.0.2‐5. Bud development stages (0–4) in spring were transformed to fit a 0–1 range by dividing each stage by 4 to apply the binomial distribution. The values of the stages were then back transformed for the visualisation of the graph. For each species, the models included a smoothing spline with four degrees of freedom for the DOY with the treatment as a factor modulating the spline and the block as a grouping variable for the random intercept with individuals nested inside the block. The fitted GAMMs with the associated means and confidence intervals are shown. The GAMMs were used to get the overall time course of bud development in spring and leaf senescence in autumn depending on the treatments. We assessed the effect of the treatments on the time of budburst and leaf senescence across species and within species with linear mixed effect models using the lme function of the R package nlme v.3.1‐149 focusing on the DOY corresponding to stage 2 in spring (budburst) and to 50% senescence in autumn. We used block as random effect and treatments as fixed effects. Estimated marginal means were extracted from the model with the associated 95% confidence intervals. Post‐hoc tests using Tukey’s honest significant difference (HSD) tests were performed to test for significant differences between the control plants and the corresponding treatment. Analyses of stage 3 and stage 4 of spring bud development and of 25% and 75% autumn senescence yielded similar results and are therefore not shown. All data analyses and statistics were performed using R v.4.0.2 (R Core Team, 2020).
Results
Treatment effects on annual growth increment
Additional irrigation significantly increased growth for oak (+43%) and only marginally for ash (+39%; Table 2). Biomass increment was lower under shade conditions compared with the control for all species, but this was significant for cherry only (−37%) and marginally significant for beech (−19%; Table 2). No significant effect was found for the drought treatment for any species (Table 2), suggesting that the reduced soil moisture was not impairing growth further than in the control drought. In none of the species did the nutrient‐addition treatment lead to significantly increased growth compared with the control (Table 2).
Table 2.
Estimated above‐ground biomass increment in 2019 (g dry weight, mean ± 1SE) for each treatment and species.
| Cherry | Ash | Oak | Beech | |
|---|---|---|---|---|
| Control (full sun) | 9.02 ± 0.92 | 1.21 ± 0.16 | 5.66 ± 0.51 | 7.94 ± 0.71 |
| Shade | 5.72 ± 0.4** | 1.03 ± 0.13 | 4.74 ± 0.45 | 6.44 ± 0.52* |
| Water | 9.08 ± 1.00 | 1.68 ± 0.18* | 8.07 ± 0.60** | 8.73 ± 0.75 |
| Nutrient | 9.85 ± 1.09 | 1.23 ± 0.19 | 6.63 ± 0.65 | 7.78 ± 0.81 |
| Control drought | 8.95 ± 0.83 | 0.93 ± 0.13 | 4.88 ± 0.41 | 7.77 ± 0.53 |
| Drought | 7.86 ± 0.75 | 0.86 ± 0.13 | 5.00 ± 0.35 | 7.52 ± 0.50 |
The biomass increment was computed using an allometric equation based on height and diameter with species‐specific parameters provided by Annighöfer et al. (2016) (see details in the Materials and Methods section). Asterisks indicate significant differences between a given treatment and its corresponding control (i.e. full sun for the treatment shade, water and nutrient and control drought for the treatment drought), tested with a mixed effect ANOVA with block as random effect and treatment as a fixed effect: **, P < 0.01; *, P < 0.1.
Effect of bud albedo and light intensity on bud temperature
Bud temperature recorded in 2020 from January to budburst showed similar minimum temperature between the shade, full sun and white or black‐painted buds, irrespective of species (the mean difference ranged within 0.4°C for the different species from 1 January to DOY 75; Fig. S4). However, the daily maximum temperature was warmer in black‐painted buds and in buds fully exposed to sun than in white or shaded buds (Fig. 1a), especially during bright days. When selecting the days when solar radiation reached at least 400 W m−2 from 1 January until the budburst of each species (from 26 to 58 d depending on the species), daily maximum temperature recorded in black‐painted buds was on average 3.1°C (ash), 3.2°C (beech), 3.3°C (cherry) and 4.6°C (oak) warmer than air temperature, whereas white‐painted buds were only 0.2–1.3°C warmer than air temperature (Fig. 1b). Bud temperature measured in the shade was slightly warmer than the temperature of the white‐painted buds and generally cooler than buds fully exposed to sun (Fig. 1).
Effect of bud albedo and light intensity on bud development
Spring phenology significantly varied among species (Table 3), with cherry generally being the first species to leaf‐out (budburst DOY 75.7 ± 1.1; mean ± SE), followed by ash (DOY 102.8 ± 5.7), oak (DOY 111.6 ± 3.2) and beech (DOY 125.3 ± 7.4), irrespective of the treatment (Figs 2a, S3).
Table 3.
Summary of the fixed‐terms of the mixed effect models testing the effects of species, treatments and their interactions on the budburst and leaf senescence dates (DOY), while accounting for the block effect as random effect.
| Treatments | Budburst | Leaf senescence | ||||
|---|---|---|---|---|---|---|
| df | F | P | df | F | P | |
| Bud albedo (white/black/control) | ||||||
| Species | 3 | 899.0 | < 0.0001 | – | – | |
| Treatments | 2 | 24.8 | < 0.0001 | – | – | |
| Species × Treatments | 6 | 2.0 | 0.063 | – | – | |
| Shade (shade/sun) | ||||||
| Species | 3 | 656.9 | < 0.0001 | 3 | 103.1 | < 0.0001 |
| Treatments | 1 | 55.9 | < 0.0001 | 1 | 221.2 | < 0.0001 |
| Species × Treatments | 3 | 5.6 | 0.001 | 3 | 48.8 | < 0.0001 |
| Nutrients (nutrient/control) | ||||||
| Species | – | – | – | 3 | 400.8 | < 0.0001 |
| Treatments | – | – | – | 1 | 5.9 | 0.016 |
| Species × Treatments | – | – | – | 3 | 0.97 | 0.410 |
| Water (water/control) | ||||||
| Species | – | – | – | 3 | 331.3 | < 0.0001 |
| Treatments | – | – | – | 1 | 15.87 | 0.0001 |
| Species × Treatments | – | – | – | 3 | 18.07 | < 0.0001 |
| Drought effect (drought/control drought) | ||||||
| Species | – | – | – | 3 | 198.3 | < 0.0001 |
| Treatments | – | – | – | 1 | < 0.1 | 0.925 |
| Species × Treatments | – | – | – | 3 | 3.8 | 0.011 |
Budburst, stage 2; leaf senescence, 50% of leaves coloured or fallen.
Fig. 2.
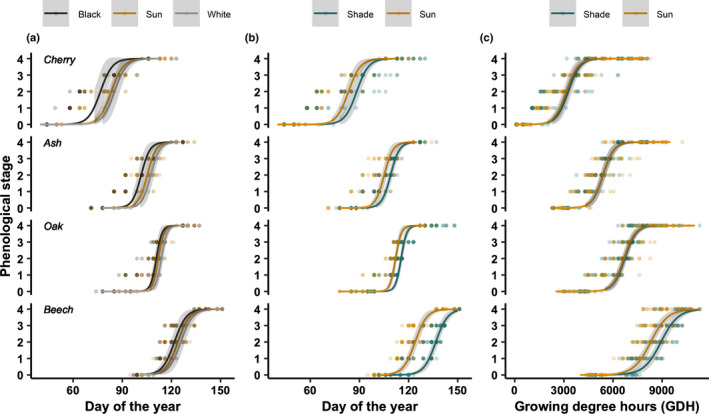
Bud development progress from closed buds (stage 0) to unfolded leaves (stage 4) in spring 2019 for seedlings with black‐painted and white‐painted buds compared with control seedlings (a), and for seedlings under shade condition compared with the control (sun) using either day of the year (b) or growing degree hours recorded within the plot (c) as x‐axis. Lines represent predictions from GAMMs using block as a random factor and a binomial distribution; 95% confidence intervals are represented in shaded areas.
Bud albedo (white‐painted vs black‐painted buds) consistently affected the time of budburst across all species (Table 3). Seedlings with black‐painted buds started bud development significantly earlier than seedlings with white‐painted buds (Fig. 2a), especially for early flushing species (budburst cherry: −10.6 d; ash: −7.5 d; oak: −4.1 d; and beech: −4.3 d; Fig. 3a). Seedlings with no painting buds started bud development later than seedlings with black‐painted buds and slightly earlier than seedlings with white‐painted buds (Figs 2a, 3a).
Fig. 3.
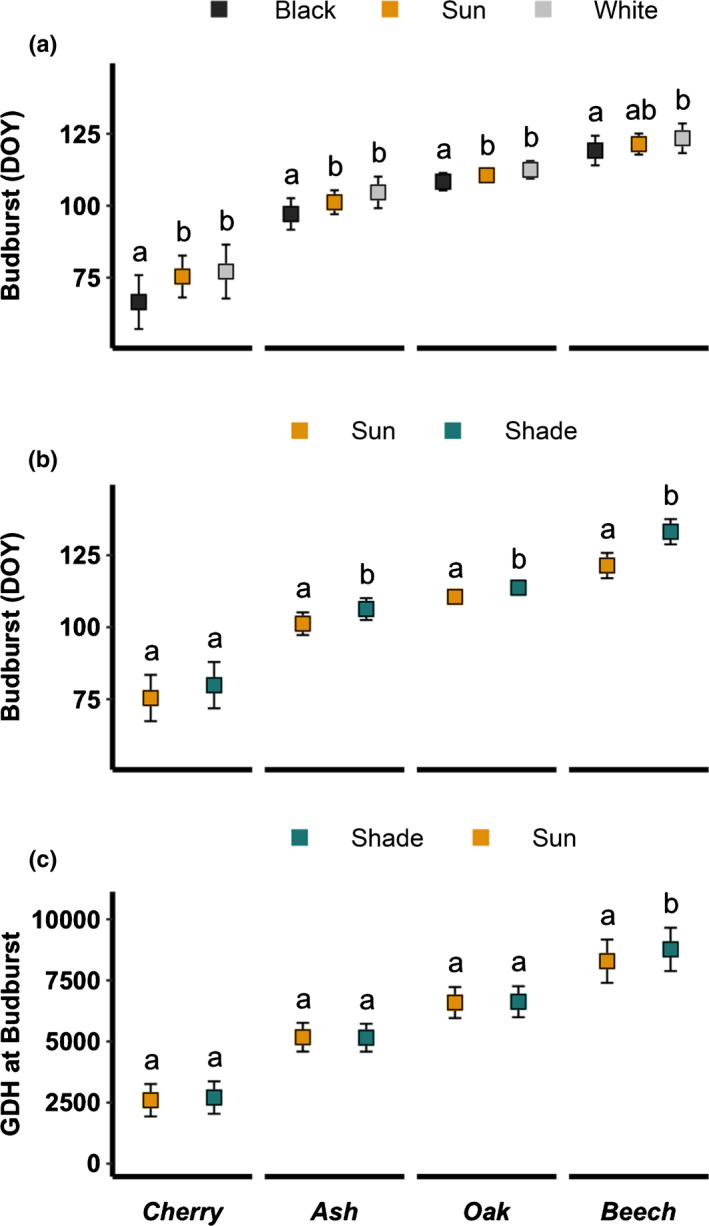
Budburst date (stage 2, day of the year (DOY)) (a, b) and growing degree hours (GDH) at budburst (c). Values correspond to the marginal mean estimates of the mixed effect ANOVA with blocks as random factor. The error bars correspond to the confidence intervals at 95%. The models were performed separately for each species and pairs of treatments shown in each panel. For each species, different letters among treatments indicate significant differences (post‐hoc Tukey’s tests at α = 0.05).
Lower solar radiation significantly affected spring bud development, with later bud development under shaded conditions for all species (Table 3; Fig. 2b). Specifically, budburst was delayed by 4.5 d for cherry (not significant), 5.1 d for ash, 3.2 d for oak and 11.8 d for beech (Fig. 3b). These delays could be explained by lower temperatures in the shade treatments compared with the controls (Figs 3c, S5). Indeed, when accounting for this difference by accumulating GDH (growing degree hours accumulated above 5°C), all species required a similar amount of GDH until budburst (Figs 3c, S5a). Only the late‐leafing beech required 483 additional GDH to budburst under shade conditions (Figs 3c, S5a).
Effect of nutrients and irrigation on leaf senescence
Irrigation and nutrients had a significant effect on leaf senescence, but the effect differed among species as shown by the significant interaction between species and treatments (Table 3). Only in oak did irrigation have a significant effect on leaf senescence (Fig. 4a), with an advance of 19.8 d compared with the control based on the 50% leaf senescence stage (Fig. 5b). Except for oak, nutrients tended to delay the date of 50% leaf senescence compared with the control treatment (Fig. 4a), but this delay was significant for beech only (+6.5 d).
Fig. 4.
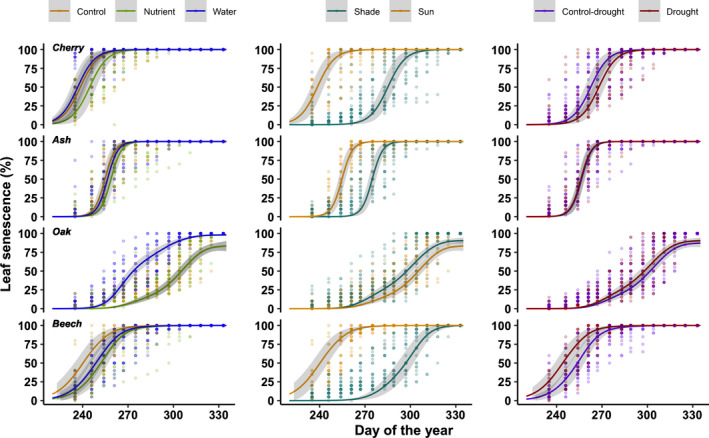
Progress of leaf senescence in autumn 2019 within the different treatments. Lines represent the predictions from GAMMs using block as a random factor and a binomial distribution; 95% confidence intervals are represented in shaded areas.
Fig. 5.
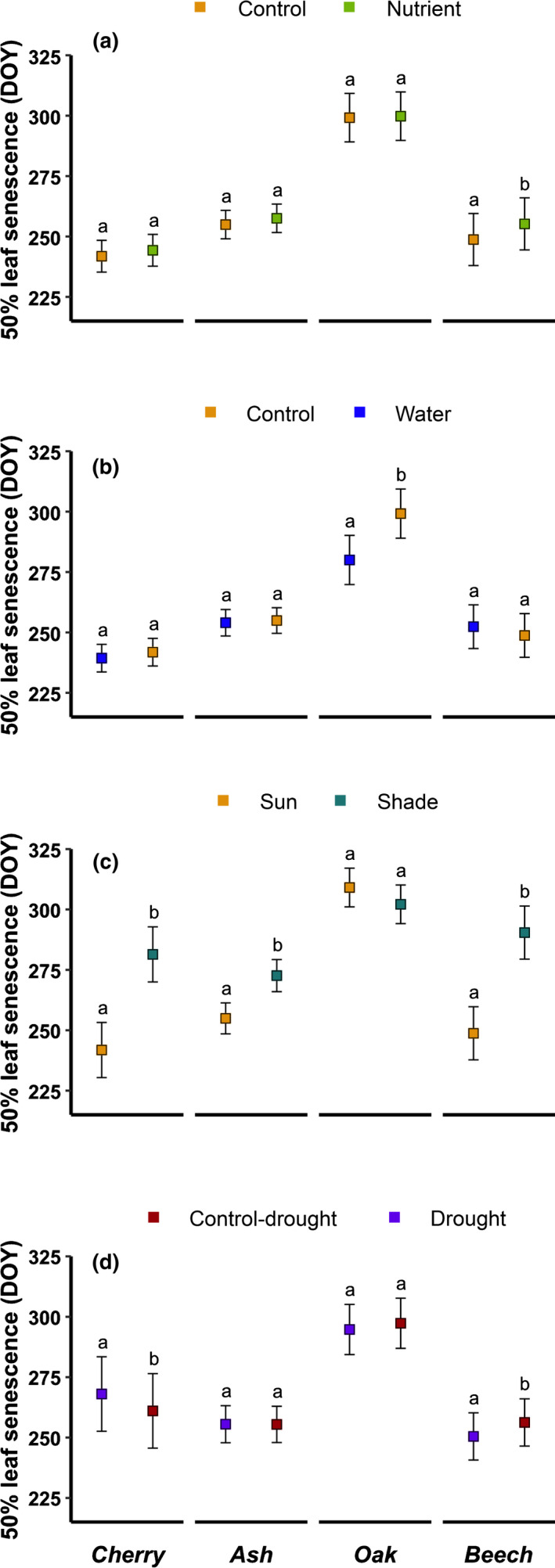
Date of leaf senescence at 50% rate estimated from the mixed effect models using block as random effect for the different treatments (a, nutrient; b, irrigation; c, shade; d, drought) in comparison with their corresponding control. Values correspond to the marginal mean estimates of the mixed effect ANOVA with blocks as random factor. The error bars correspond to the confidence intervals at 95%. The models were performed separately for each species and pairs of treatments shown in each panel. For each species, different letters among treatments indicate significant differences (post‐hoc Tukey’s tests at α = 0.05).
Effect of light intensity on leaf senescence
Leaf senescence was strongly delayed under shaded conditions for all species except oak in which a slight insignificant advance was found (Table 3; Figs 4b, 5c). Shade conditions delayed senescence by +39.6 d, +17.7 d and +44.8 d for cherry, ash and beech, respectively, whereas air temperature measured under a radiation shield was slightly cooler in the shade treatment (Fig. S6). For oak, 50% leaf senescence occurred 5.5 d earlier under shade conditions (not significant; Fig. 4c) and 75% leaf senescence occurred 7.0 d earlier (P = 0.011).
Effect of reduced precipitation on leaf senescence
Overall, the drought treatment had no significant effect on leaf senescence across species (Table 2). However, the species‐specific analysis showed that lower soil moisture during summer significantly delayed leaf senescence of cherry by +7.0 d and significantly advanced senescence of beech by −7.1 d (Figs 4c, 5d). No effect was found for the two other species (Figs 4c, 5d).
Discussion
Our experimental study shows that the microclimate in which seedlings are growing significantly affects leaf phenology both in spring and in autumn. The albedo treatment, in which we painted buds to modify heat reflectance, demonstrates that temperature is sensed at the bud level: black‐painted buds with lower albedo and higher maximum bud temperature during bright days showed earlier bud development relative to white‐painted and unpainted buds. Moreover, a cooler microclimate induced by the shading nets significantly delayed budburst timing of all species. This delay was mainly explained by growing‐degree‐day accumulation, whereby the shaded and control plants required similar warming sums until budburst.
Regarding autumn phenology, reduced light intensity led to reduced biomass increment in all species (but significant for cherry and beech only) and strongly delayed leaf senescence for all species but oak. The magnitude of such delay exceeds the interannual variability observed over several decades in Switzerland for common tree species or for beech in France (Delpierre et al., 2009; Bigler & Vitasse, 2021; Meier et al., 2021). This suggests that the phenological cycles of understory trees are strongly affected by the shade imposed by overstory trees and that trees growing under low light compensate for the reduced photosynthetic assimilation by extending their growing season. In addition, our results showed that higher water availability can strongly advance leaf senescence when it significantly increases growth rate (as found for oak). Overall, nutrients and water availability had only little effect on growth that might also explain their limited impact on leaf senescence timing.
Bud temperature as the main driver of budburst timing
Our study showed that black‐painted buds started their development earlier than nonpainted or white‐painted buds, suggesting that bud albedo affects internal physiological processes by influencing the temperature of bud tissues. Temperature records within buds show that black‐painted buds are up to 3.6°C warmer than white‐painted buds during bright days (mean difference for oak when solar radiation was more than 400 W m−2). The discrepancy in the time of budburst between black and white buds was more pronounced for early (cherry, ash) than for late‐flushing species (oak, beech). Because of the nonlinear response of bud development to spring warming temperatures, lower bud albedo early in spring may lead to a strongly increased accumulation of temperature relevant for bud cell growth, whereas this increase in effective heat sums might be less pronounced later in spring when days are already warm. In addition, early flushing species might be more sensitive to temperature increases than late‐flushing species for which more pronounced photoperiod and/or chilling requirements may limit responsiveness to spring warming (Fu et al., 2019a, 2019b,2019a, 2019b; Montgomery et al., 2020). Our results further demonstrate that lower radiation induced by the shading net substantially changed the microclimate of the buds, delaying bud development of beech by 12 d, which roughly correspond to half of the interannual variability that can be observed over several decades (Meier et al., 2021). The slower accumulation of growing degree days under shaded conditions fully explained the discrepancy in budburst timing between shaded and control plots, for all species except beech, which overall confirms that growing degree days/hours is a good method to predict budburst of temperate trees in regions where chilling is not limiting (Vitasse et al., 2018). However, the remaining discrepancy found for beech suggests that, in addition to temperature and photoperiod (Vitasse & Basler, 2013), light intensity may play a direct role in the regulation of bud development of European beech, as also suggested in a previous study analysing the partial correlation of leaf‐out timing of European trees and insolation (Fu et al., 2015a, 2015b,2015a, 2015b). Alternatively, this species may have a different threshold above which temperature is accumulated or may respond nonlinearly to forcing temperature. Further experiments controlling both light intensity and bud temperature are needed to clarify the potential effect of solar radiation on European beech phenology.
Bud temperature differs from air temperature measured by standard weather stations, that is under ventilated and shaded conditions. Under clear sky conditions, buds are heating up during the day by shortwave radiation and cooling down during the night by losses of heat from the atmosphere to the outer space through longwave radiation (radiative cooling). We therefore suggest that standard weather stations may substantially underestimate bud temperature during the day, when solar radiation is high and overestimate minimum temperature during the night when the sky is clear. Our temperature data recorded inside buds clearly show this effect for daily maximum temperature. Because sky brightness has been shown to have substantially increased since the 1980s in Europe, especially in spring (Sanchez‐Lorenzo et al., 2015; Pfeifroth et al., 2018), the discrepancy between standard air temperature and bud temperature may have substantially increased over recent decades. This may introduce a significant bias in phenology modelling, for example in the estimation of spring phenological sensitivity to temperature, which has been suggested to have decreased since the 1980s (Fu et al., 2015a, 2015b,2015a, 2015b). Our study calls for more investigations on how bud temperature differs from standard air temperature, depending on other important climatic factors such as solar radiation or wind.
Factors affecting leaf senescence
Leaf senescence is assumed to occur when the cost of maintaining active leaves outweighs the benefits of photosynthesis and is seen as a strategy for reabsorbing nutrients from the leaves and reallocating them throughout the plant (Kikuzawa, 1991; Estiarte & Peñuelas, 2015). It has long been thought that the leaf senescence process of temperate trees is mainly triggered by the decrease in temperature and photoperiod during autumn (Delpierre et al., 2009; Liu et al., 2020). However, recently, other factors have been suggested to influence leaf senescence, such as temperature during the growing season (Liu et al., 2019, 2018), water (Liu et al., 2019) and nutrient availability (Weih, 2009; Fu et al., 2019a, 2019b,2019a, 2019b), summer drought stress (Schuldt et al., 2020) and light conditions (Wingler et al., 2006). These factors can have a direct effect on leaf senescence by inducing a stress response or affect leaf senescence indirectly by modulating the plant source–sink relationship (Paul & Foyer, 2001), which plays a prominent role in the senescence process (Zani et al., 2020). The relative effects of these drivers often depend on the species. For example, severe drought has been shown to hasten leaf senescence of temperate trees at low elevations (Hwang et al., 2014; Xie et al., 2015), which might be a strategy to reduce transpiration (Munné‐Bosch & Alegre, 2004) and avoid xylem embolism (Bréda et al., 2006) or the direct consequence of vessel cavitation under extreme severe drought as observed for European beech in central Europe during the summer 2018 (Schuldt et al., 2020; Wohlgemuth et al., 2020). Our results also showed earlier senescence under drought compared with the control‐drought plots for beech and delayed senescence in the irrigated plots. By contrast, we found the opposite pattern for oak with significantly earlier senescence and elevated growth in the irrigated plots and slightly delayed senescence in the drought treatments. We expected drought to affect carbon sink and source strength and therefore senescence timing. However, the drought treatment led to significantly lower soil moisture content during late summer only, whereas the difference with the control was negligible at the beginning of the growing season until mid‐July, that is when most of the growth may have already occurred. Accordingly, no differences in the estimated biomass increment was found between the drought and control treatment after the growing season. It is widely recognised that severe droughts in spring strongly affect growth rate (Vitasse et al., 2019; Bose et al., 2021) and may therefore affect leaf senescence timing due to lesser carbon supply and growth. More investigations should be conducted to determine the seasonal effects of drought on leaf senescence. Opposite responses of leaf senescence to moderate drought stress were also found for temperate trees in the north‐eastern United States, with a delay in ash, maples and birches and an advance in oak and beech (Xie et al., 2018). The heterogeneity among microenvironments could partly explain this pattern when leaf senescence is studied in situ. Here, we rule out the possibility of different microenvironments and attribute these opposite responses to species‐specific physiological features/characteristics (e.g. tolerance to drought or shade). For instance beech is a shade‐tolerant species with rather low tolerance to drought, whereas pedunculate, sessile and pubescent oaks are more tolerant to drought (Gallé et al., 2007; Rubio‐Cuadrado et al., 2018; Vitasse et al., 2019), and capable of maintaining photosynthesis when leaf water potential is low (Raftoyannis & Radoglou, 2002). The species‐level variation in the responses to drought might be the result of differences in the relative importance of stress responses vs sink limitation. Reduced water availability can decrease photosynthetic activity, at least in drought‐intolerant species, which in turn delays the senescence process by delaying saturation of a trees’ annual carbon sink (Zani et al., 2020). Conversely, drought might have a direct effect on leaf senescence, whereby intense droughts cause a stress reaction, increasing ROS concentration in leaves or even hydraulic failure leading to precocious leaf senescence.
Increased nutrient availability has generally been shown to delay leaf senescence. For instance, high nutrient availability was found to delay leaf senescence in Populus trichocarpa in Iceland (Sigurdsson, 2001) and in seedlings of Aesculus hippocastanum and beech (Fu et al., 2019a, 2019b,2019a, 2019b). By contrast, under low nutrient availability, elevated CO2 concentration was found to accelerate growth cessation in Populus trichocarpa (Sigurdsson, 2001). Sigurdsson (2001) suggested that under elevated CO2 or under low nutrient availability, there is an imbalance between carbon and nitrogen sources which alters autumn phenology. Other studies have suggested that a potassium deficiency may lead to earlier leaf senescence (Wang et al., 2012; Pan et al., 2017), probably because, in addition to the negative impacts on photosynthesis, K deficiency hinders the export of sucrose from the leaves through the phloem (Cakmak, 2005). Senescence, therefore, appears to be largely driven by the interaction between nutrient, particularly nitrogen, and carbon supply (Paul & Foyer, 2001). High nutrients will therefore allow trees to maintain source activity (photosynthesis) for longer time and shed their leaves later in the year. However, no significant increase in biomass was found in our experiment for the fertilised treatments, which may explain the insignificant delays in leaf senescence observed for beech, cherry and ash in this treatment. It is possible that the additional fertiliser was not yet absorbed by the trees, or only partially, as suggested by the high nitrate concentration remaining in the soil at the end of the season (25 times higher in the fertiliser treatment than in the control for oak/beech and c. nine times higher for cherry/ash, see Table S2).
We found that reduced light availability (reduction of PAR by c. 70%) strongly delayed leaf senescence of ash, cherry and beech by 18, 39 and 42 d along with a reduction of biomass increment of 15%, 37% and 19%, respectively. This can also be explained by the sink‐limitation hypothesis (Wingler et al., 2006; Wingler & Roitsch, 2008; Dox et al., 2020). Shaded conditions over summer led to reduced carbon uptake (i.e. lower biomass increment) due to lower photosynthetic activity (Sevillano et al., 2016), and subsequently delayed the senescence process. This result was also found for common sunflower and beans (Ono et al., 2001) or for European beech and the Japanese spiraea (Zani et al., 2020). It remains an open question to which degree sink limitation operates at the leaf, branch or whole‐plant level. Given that the source/sink control of leaf senescence appears to be largely driven by leaf‐level nitrogen to carbon ratios (Paul & Foyer, 2001), localised effects on leaf senescence can be expected. This agrees with observations that, under natural conditions, the upper part of the canopy, which is more exposed to full light, shows earlier senescence than more shaded parts of the tree (Gressler et al., 2015).
In addition, photo‐oxidative stress might drive early senescence under high light. Photo‐oxidative stress occurs when light‐energy absorption exceeds the capacity for light utilisation: an excess of photons may lead to nonphotochemical quenching and oxidative stress by an accumulation of ROS (Müller et al., 2001). This may lead to photoinhibition (Long et al., 1994) and can accelerate the process of senescence (Munné‐Bosch & Alegre, 2004; Juvany et al., 2013; Pintó‐Marijuan & Munné‐Bosch, 2014). Photo‐oxidative stress might, therefore, play a role, especially in species adapted to grow under the canopy shade at juvenile age such as beech. The absence of a response to light or drought in oak could be related to its tolerance to high solar radiation (Valladares et al., 2002) and its ability to efficiently dissipate an excess of energy and degrade ROS under photo‐oxidative stress, as demonstrated for pubescent oak (Gallé et al., 2007).
Overall, in addition to the well known effects of temperature and photoperiod on the regulation of leaf senescence timing, our results suggest a tight interplay between source and sink processes in regulating the end of the seasonal vegetation cycle, which can be largely influenced by light, water and nutrient availability. More experiments will be necessary to fully untangle the relative contribution of direct effects of solar radiation on leaf senescence in relation to the indirect effects mediated through sugar and nutrient availability.
Conclusion
This study demonstrates the importance of microclimatic conditions, especially solar radiation, in regulating the timing of budburst in spring and leaf senescence in autumn. While our experiment shows that light availability mainly affects spring budburst through modification of bud temperatures, in European beech, light intensity and/or quality may directly affect budburst as the delayed budburst under shaded conditions could not be fully explained by the local temperature recorded beneath the shading net. A potential avenue to improve phenological predictions will therefore be to quantify how bud and leaf temperatures differ from standard air temperature depending on other meteorological factors, such as solar radiation and wind. Light availability also had a large effect on autumn senescence, with a considerable delay of leaf senescence under shaded conditions during the growing season found for all species except oak along with a reduction of growth. This delay under low light can be explained by the sink‐limitation hypothesis, whereby leaf senescence is tightly linked to photosynthate and nutrient supply. Oxidative stress under high light conditions may further drive this trend in late successional and shade‐tolerant species sensitive to heat and drought, such as European beech. The results provide important insights into the roles of sink limitation and drought stress in mediating autumn phenology and call for a more accurate representation of microclimate to improve phenological predictions.
Author contributions
YV, FB and BM planned and designed the experiment. YV conducted the experiment with the field assistance of RK and MGW. CMZ and YHF helped in the interpretation of the results. YV analysed the data and drafted the manuscript with substantial inputs from all co‐authors.
Supporting information
Fig. S1 Picture showing the experimental infrastructure used in the study.
Fig. S2 Soil moisture of the different treatments during the experiment.
Fig. S3 Air temperature at 2 m height during the experiment.
Fig. S4 Daily minimum temperatures recorded within the bud from January 2020 until species‐specific budburst in white, black, shade or fully exposed buds.
Fig. S5 Growing degree hours above 5°C from 1 February to budburst in the shade and sun treatment.
Fig. S6 Air temperature recorded at canopy height during summer 2019 in the shade and sun treatment.
Table S1 Seed material used in the experiment.
Table S2 Amount of total nitrogen (N), nitrate (NO3 −) and ammonium (NH4 +) in the soil for each of the mesocosms at the end of the growing season (3 September 2019).
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
We thank U. Wasem for setting up the experiment. We thank M. Haeni and J. Gisler for their help in setting all the devices for the microclimatic records. We are grateful to T. Wohlgemuth for providing the experimental facilities at WSL. This project was supported by the SwissForestLab (research grant SFL‐17 P2) and by the Federal Office for the Environment FOEN. YV and MGW are supported by the Swiss National Science Foundation SNF (research grant 315230_192712).
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
- Annighöfer P, Ameztegui A, Ammer C, Balandier P, Bartsch N, Bolte A, Coll L, Collet C, Ewald J, Frischbier N et al. 2016. Species‐specific and generic biomass equations for seedlings and saplings of European tree species. European Journal of Forest Research 135: 313–329. [Google Scholar]
- Arend M, Gessler A, Schaub M. 2016a. The influence of the soil on spring and autumn phenology in European beech. Tree Physiology 36: 78–85. [DOI] [PubMed] [Google Scholar]
- Arend M, Sever K, Pflug E, Gessler A, Schaub M. 2016b. Seasonal photosynthetic response of European beech to severe summer drought: limitation, recovery and post‐drought stimulation. Agricultural and Forest Meteorology 220: 83–89. [Google Scholar]
- Basler D. 2016. Evaluating phenological models for the prediction of leaf‐out dates in six temperate tree species across central Europe. Agricultural and Forest Meteorology 217: 10–21. [Google Scholar]
- Bigler C, Vitasse Y. 2021. Premature leaf discoloration of European deciduous trees is caused by drought and heat in late spring and cold spells in early fall. Agricultural and Forest Meteorology 307: 108492. [Google Scholar]
- Bose AK, Scherrer D, Camarero JJ, Ziche D, Babst F, Bigler C, Bolte A, Dorado‐Liñán I, Etzold S, Fonti P et al. 2021. Climate sensitivity and drought seasonality determine post‐drought growth recovery of Quercus petraea and Quercus robur in Europe. Science of the Total Environment 784: 147222. [DOI] [PubMed] [Google Scholar]
- Boussingault JB. 1868. Agronomie: chimie agricole et physiologie. Paris, France: Mallet Bachelier. [Google Scholar]
- Bréda N, Huc R, Granier A, Dreyer E. 2006. Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long‐term consequences. Annals of Forest Science 63: 625–644. [Google Scholar]
- Cakmak I. 2005. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. Journal of Plant Nutrition and Soil Science 168: 521–530. [Google Scholar]
- Chen L, Hänninen H, Rossi S, Smith NG, Pau S, Liu Z, Feng G, Gao J, Liu J. 2020. Leaf senescence exhibits stronger climatic responses during warm than during cold autumns. Nature Climate Change 10: 777–780. [Google Scholar]
- Chuine I, Beaubien EG. 2001. Phenology is a major determinant of tree species range. Ecology Letters 4: 500–510. [Google Scholar]
- Chuine I, Bonhomme M, Legave J‐M, García de Cortázar‐Atauri I, Charrier G, Lacointe A, Améglio T. 2016. Can phenological models predict tree phenology accurately in the future? The unrevealed hurdle of endodormancy break. Global Change Biology 22: 3444–3460. [DOI] [PubMed] [Google Scholar]
- Coville FV. 1920. The influence of cold in stimulating the growth of plants. Proceedings of the National Academy of Sciences, USA 6: 434–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Frenne P, Zellweger F, Rodríguez‐Sánchez F, Scheffers BR, Hylander K, Luoto M, Vellend M, Verheyen K, Lenoir J. 2019. Global buffering of temperatures under forest canopies. Nature Ecology & Evolution 3: 744–749. [DOI] [PubMed] [Google Scholar]
- Delpierre N, Dufrene E, Soudani K, Ulrich E, Cecchini S, Boe J, Francois C. 2009. Modelling interannual and spatial variability of leaf senescence for three deciduous tree species in France. Agricultural and Forest Meteorology 149: 938–948. [Google Scholar]
- Delpierre N, Vitasse Y, Chuine I, Guillemot J, Bazot S, Rutishauser T, Rathgeber CBK. 2016. Temperate and boreal forest tree phenology: from organ‐scale processes to terrestrial ecosystem models. Annals of Forest Science 73: 5–25. [Google Scholar]
- Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R. 2014. Nonstructural carbon in woody plants. Annual Review of Plant Biology 65: 667–687. [DOI] [PubMed] [Google Scholar]
- Dox I, Gričar J, Marchand LJ, Leys S, Zuccarini P, Geron C, Prislan P, Mariën B, Fonti P, Lange H et al. 2020. Timeline of autumn phenology in temperate deciduous trees. Tree Physiology 40: 1001–1013. [DOI] [PubMed] [Google Scholar]
- Estiarte M, Peñuelas J. 2015. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: effects on nutrient proficiency. Global Change Biology 21: 1005–1017. [DOI] [PubMed] [Google Scholar]
- Frei ER, Schnell L, Vitasse Y, Wohlgemuth T, Moser B. 2020. Assessing the effectiveness of in‐situ active warming combined with open top chambers to study plant responses to climate change. Frontiers in Plant Science 11: 539584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YH, Campioli M, Van Oijen M, Deckmyn G, Janssens IA. 2012. Bayesian comparison of six different temperature‐based budburst models for four temperate tree species. Ecological Modelling 230: 92–100. [Google Scholar]
- Fu YH, Piao S, Delpierre N, Hao F, Hänninen H, Geng X, Peñuelas J, Zhang X, Janssens IA, Campioli M. 2019a. Nutrient availability alters the correlation between spring leaf‐out and autumn leaf senescence dates. Tree Physiology 39: 1277–1284. [DOI] [PubMed] [Google Scholar]
- Fu YH, Piao S, Delpierre N, Hao F, Hänninen H, Liu Y, Sun W, Janssens IA, Campioli M. 2018. Larger temperature response of autumn leaf senescence than spring leaf‐out phenology. Global Change Biology 24: 2159–2168. [DOI] [PubMed] [Google Scholar]
- Fu YH, Piao S, Vitasse Y, Zhao H, De Boeck HJ, Liu Q, Yang H, Weber U, Hänninen H, Janssens IA. 2015a. Increased heat requirement for leaf flushing in temperate woody species over 1980–2012: effects of chilling, precipitation and insolation. Global Change Biology 21: 2687–2697. [DOI] [PubMed] [Google Scholar]
- Fu YH, Zhang X, Piao S, Hao F, Geng X, Vitasse Y, Zohner C, Penuelas J, Janssens IA. 2019b. Daylength helps temperate deciduous trees to leaf‐out at the optimal time. Global Change Biology 25: 2410–2418. [DOI] [PubMed] [Google Scholar]
- Fu YH, Zhao H, Piao S, Peaucelle M, Peng S, Zhou G, Ciais P, Huang M, Menzel A, Peñuelas J et al. 2015b. Declining global warming effects on the phenology of spring leaf unfolding. Nature 526: 104–107. [DOI] [PubMed] [Google Scholar]
- Gallé A, Haldimann P, Feller U. 2007. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytologist 174: 799–810. [DOI] [PubMed] [Google Scholar]
- Garonna I, Jong R, Schaepman ME. 2016. Variability and evolution of global land surface phenology over the past three decades (1982–2012). Global Change Biology 22: 1456–1468. [DOI] [PubMed] [Google Scholar]
- Gilson A, Barthes L, Delpierre N, Dufrêne É, Fresneau C, Bazot S. 2014. Seasonal changes in carbon and nitrogen compound concentrations in a Quercus petraea chronosequence. Tree Physiology 34: 716–729. [DOI] [PubMed] [Google Scholar]
- Gressler E, Jochner S, Capdevielle‐Vargas RM, Morellato LPC, Menzel A. 2015. Vertical variation in autumn leaf phenology of Fagus sylvatica L. in southern Germany. Agricultural and Forest Meteorology 201: 176–186. [Google Scholar]
- Heide OM. 1993. Daylength and thermal time responses of budburst during dormancy release in some northern deciduous trees. Physiologia Plantarum 88: 531–540. [DOI] [PubMed] [Google Scholar]
- Hwang T, Band LE, Miniat CF, Song C, Bolstad PV, Vose JM, Love JP. 2014. Divergent phenological response to hydroclimate variability in forested mountain watersheds. Global Change Biology 20: 2580–2595. [DOI] [PubMed] [Google Scholar]
- Juvany M, Müller M, Munné‐Bosch S. 2013. Photo‐oxidative stress in emerging and senescing leaves: a mirror image? Journal of Experimental Botany 64: 3087–3098. [DOI] [PubMed] [Google Scholar]
- Keskitalo J, Bergquist G, Gardestrom P, Jansson S. 2005. A cellular timetable of autumn senescence. Plant Physiology 139: 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuzawa K. 1991. A cost‐benefit‐analysis of leaf habit and leaf longevity of trees and their geographical pattern. American Naturalist 138: 1250–1263. [Google Scholar]
- Körner C, Basler D, Hoch G, Kollas C, Lenz A, Randin C, Vitasse Y, Zimmermann NE. 2016. Where, why and how? Explaining the low temperature range limits of temperate tree species. Journal of Ecology 104: 1076–1088. [Google Scholar]
- Liu G, Chen X, Fu Y, Delpierre N. 2019. Modelling leaf coloration dates over temperate China by considering effects of leafy season climate. Ecological Modelling 394: 34–43. [Google Scholar]
- Liu G, Chen X, Zhang Q, Lang W, Delpierre N. 2018. Antagonistic effects of growing season and autumn temperatures on the timing of leaf coloration in winter deciduous trees. Global Change Biology 24: 3537–3545. [DOI] [PubMed] [Google Scholar]
- Liu Q, Piao S, Campioli M, Gao M, Fu YH, Wang K, He Y, Li X, Janssens IA. 2020. Modeling leaf senescence of deciduous tree species in Europe. Global Change Biology 26: 4104–4118. [DOI] [PubMed] [Google Scholar]
- Long SP, Humphries S, Falkowski PG. 1994. Photoinhibition of photosynthesis in nature. Annual Review of Plant Biology 45: 633–662. [Google Scholar]
- Ma S, Pitman AJ, Lorenz R, Kala J, Srbinovsky J. 2016. Earlier green‐up and spring warming amplification over Europe. Geophysical Research Letters 43: 2011–2018. [Google Scholar]
- Mariën B, Dox I, De Boeck HJ, Willems P, Leys S, Papadimitriou D, Campioli M. 2021. Does drought advance the onset of autumn leaf senescence in temperate deciduous forest trees? Biogeosciences 18: 3309–3330. [Google Scholar]
- Meier M, Vitasse Y, Bugmann H, Bigler C. 2021. Phenological shifts induced by climate change amplify drought for broad‐leaved trees at low elevations in Switzerland. Agricultural and Forest Meteorology 307: 108485. [Google Scholar]
- Montgomery RA, Rice KE, Stefanski A, Rich RL, Reich PB. 2020. Phenological responses of temperate and boreal trees to warming depend on ambient spring temperatures, leaf habit, and geographic range. Proceedings of the National Academy of Sciences, USA 117: 10397–10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P, Li X‐P, Niyogi KK. 2001. Non‐photochemical quenching. A response to excess light energy. Plant Physiology 125: 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné‐Bosch S, Alegre L. 2004. Die and let live: leaf senescence contributes to plant survival under drought stress. Functional Plant Biology 31: 203–216. [DOI] [PubMed] [Google Scholar]
- Murray MB, Cannell MGR, Smith RI. 1989. Date of budburst of fifteen tree species in Britain following climatic warming. Journal of Applied Ecology 26: 693–700. [Google Scholar]
- Niinemets U, Valladares F. 2006. Tolerance to shade, drought, and waterlogging of temperate Northern Hemisphere trees and shrubs. Ecological Monographs 76: 521–547. [Google Scholar]
- Ono K, Nishi Y, Watanabe A, Terashima I. 2001. Possible mechanisms of adaptive leaf senescence. Plant Biology 3: 234–243. [Google Scholar]
- Pan Y, Lu Z, Lu J, Li X, Cong R, Ren T. 2017. Effects of low sink demand on leaf photosynthesis under potassium deficiency. Plant Physiology and Biochemistry 113: 110–121. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Foyer CH. 2001. Sink regulation of photosynthesis. Journal of Experimental Botany 52: 1383–1400. [DOI] [PubMed] [Google Scholar]
- Pfeifroth U, Sanchez‐Lorenzo A, Manara V, Trentmann J, Hollmann R. 2018. Trends and variability of surface solar radiation in Europe based on surface‐and satellite‐based data records. Journal of Geophysical Research: Atmospheres 123: 1735–1754. [Google Scholar]
- Piao S, Liu Q, Chen A, Janssens IA, Fu Y, Dai J, Liu L, Lian X, Shen M, Zhu X. 2019. Plant phenology and global climate change: current progresses and challenges. Global Change Biology 25: 1922–1940. [DOI] [PubMed] [Google Scholar]
- Pintó‐Marijuan M, Munné‐Bosch S. 2014. Photo‐oxidative stress markers as a measure of abiotic stress‐induced leaf senescence: advantages and limitations. Journal of Experimental Botany 65: 3845–3857. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [WWW document] URL http://www.R‐project.org/. [Google Scholar]
- Raftoyannis Y, Radoglou K. 2002. Physiological responses of beech and sessile oak in a natural mixed stand during a dry summer. Annals of Botany 89: 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AD, Keenan TF, Migliavacca M, Ryu Y, Sonnentag O, Toomey M. 2013. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agricultural and Forest Meteorology 169: 156–173. [Google Scholar]
- Rousi M, Pusenius J. 2005. Variations in phenology and growth of European white birch (Betula pendula) clones. Tree Physiology 25: 201–210. [DOI] [PubMed] [Google Scholar]
- Rubio‐Cuadrado Á, Camarero JJ, del Río M, Sánchez‐González M, Ruiz‐Peinado R, Bravo‐Oviedo A, Gil L, Montes F. 2018. Drought modifies tree competitiveness in an oak‐beech temperate forest. Forest Ecology and Management 429: 7–17. [Google Scholar]
- Sanchez‐Lorenzo A, Wild M, Brunetti M, Guijarro JA, Hakuba MZ, Calbó J, Mystakidis S, Bartok B. 2015. Reassessment and update of long‐term trends in downward surface shortwave radiation over Europe (1939–2012). Journal of Geophysical Research: Atmospheres 120: 2015JD023321. [Google Scholar]
- Savvides A, Dieleman JA, van Ieperen W, Marcelis LF. 2016. A unique approach to demonstrating that apical bud temperature specifically determines leaf initiation rate in the dicot Cucumis sativus . Planta 243: 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvides A, Van Ieperen W, Dieleman JA, Marcelis LF. 2013. Meristem temperature substantially deviates from air temperature even in moderate environments: is the magnitude of this deviation species‐specific? Plant, Cell & Environment 36: 1950–1960. [DOI] [PubMed] [Google Scholar]
- Schuldt B, Buras A, Arend M, Vitasse Y, Beierkuhnlein C, Damm A, Gharun M, Grams TEE, Hauck M, Hajek P et al. 2020. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic and Applied Ecology 45: 86–103. [Google Scholar]
- Sevillano I, Short I, Grant J, O’Reilly C. 2016. Effects of light availability on morphology, growth and biomass allocation of Fagus sylvatica and Quercus robur seedlings. Forest Ecology and Management 374: 11–19. [Google Scholar]
- Sigurdsson BD. 2001. Elevated [CO2] and nutrient status modified leaf phenology and growth rhythm of young Populus trichocarpa trees in a 3‐year field study. Trees 15: 403–413. [Google Scholar]
- Valladares F, Chico J, Aranda I, Balaguer L, Dizengremel P, Manrique E, Dreyer E. 2002. The greater seedling high‐light tolerance of Quercus robur over Fagus sylvatica is linked to a greater physiological plasticity. Trees 16: 395–403. [Google Scholar]
- Viherä‐Aarnio A, Häkkinen R, Junttila O. 2006. Critical night length for bud set and its variation in two photoperiodic ecotypes of Betula pendula . Tree Physiology 26: 1013–1018. [DOI] [PubMed] [Google Scholar]
- Vitasse Y. 2013. Ontogenic changes rather than difference in temperature cause understory trees to leaf out earlier. New Phytologist 198: 149–155. [DOI] [PubMed] [Google Scholar]
- Vitasse Y, Basler D. 2013. What role for photoperiod in the bud burst phenology of European beech. European Journal of Forest Research 132: 1–8. [Google Scholar]
- Vitasse Y, Bottero A, Cailleret M, Bigler C, Fonti P, Gessler A, Lévesque M, Rohner B, Weber P, Rigling A et al. 2019. Contrasting resistance and resilience to extreme drought and late spring frost in five major European tree species. Global Change Biology 25: 3781–3792. [DOI] [PubMed] [Google Scholar]
- Vitasse Y, Hoch G, Randin CF, Lenz A, Kollas C, Scheepens JF, Körner C. 2013. Elevational adaptation and plasticity in seedling phenology of temperate deciduous tree species. Oecologia 171: 663–678. [DOI] [PubMed] [Google Scholar]
- Vitasse Y, Porte AJ, Kremer A, Michalet R, Delzon S. 2009. Responses of canopy duration to temperature changes in four temperate tree species: relative contributions of spring and autumn leaf phenology. Oecologia 161: 187–198. [DOI] [PubMed] [Google Scholar]
- Vitasse Y, Schneider L, Rixen C, Christen D, Rebetez M. 2018. Increase in the risk of exposure of forest and fruit trees to spring frosts at higher elevations in Switzerland over the last four decades. Agricultural and Forest Meteorology 248: 60–69. [Google Scholar]
- Wang H, Wu C, Ciais P, Peñuelas J, Dai J, Fu Y, Ge Q. 2020. Overestimation of the effect of climatic warming on spring phenology due to misrepresentation of chilling. Nature Communications 11: 4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li B, Du M, Eneji AE, Wang B, Duan L, Li Z, Tian X. 2012. Mechanism of phytohormone involvement in feedback regulation of cotton leaf senescence induced by potassium deficiency. Journal of Experimental Botany 63: 5887–5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing PF. 1953. Growth studies in woody species V. Photoperiodism in dormant buds of Fagus sylvatica . Physiologia Plantarum 6: 692–706. [Google Scholar]
- Weih M. 2009. Genetic and environmental variation in spring and autumn phenology of biomass willows (Salix spp.): effects on shoot growth and nitrogen economy. Tree Physiology 29: 1479–1490. [DOI] [PubMed] [Google Scholar]
- Weih M, Karlsson PS. 1999. The nitrogen economy of mountain birch seedlings: implications for winter survival. Journal of Ecology 87: 211–219. [Google Scholar]
- Wingler A, Purdy S, MacLean JA, Pourtau N. 2006. The role of sugars in integrating environmental signals during the regulation of leaf senescence. Journal of Experimental Botany 57: 391–399. [DOI] [PubMed] [Google Scholar]
- Wingler A, Roitsch T. 2008. Metabolic regulation of leaf senescence: interactions of sugar signalling with biotic and abiotic stress responses. Plant Biology 10: 50–62. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth T, Kistler M, Aymon C, Hagedorn F, Gessler A, Gossner MM, Queloz V, Vögtli I, Wasem U, Vitasse Y et al. 2020. Früher Laubfall der Buche während der Sommertrockenheit 2018: Resistenz oder Schwächesymptom? Schweizerische Zeitschrift für Forstwesen 171: 257–269. [Google Scholar]
- Wolf S, Keenan TF, Fisher JB, Baldocchi DD, Desai AR, Richardson AD, Scott RL, Law BE, Litvak ME, Brunsell NA. 2016. Warm spring reduced carbon cycle impact of the 2012 US summer drought. Proceedings of the National Academy of Sciences, USA 113: 201519620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wang X, Silander JA. 2015. Deciduous forest responses to temperature, precipitation, and drought imply complex climate change impacts. Proceedings of the National Academy of Sciences, USA 112: 13585–13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wang X, Wilson AM, Silander JA Jr. 2018. Predicting autumn phenology: how deciduous tree species respond to weather stressors. Agricultural and Forest Meteorology 250: 127–137. [Google Scholar]
- Xu X, Riley WJ, Koven CD, Jia G, Zhang X. 2020. Earlier leaf‐out warms air in the north. Nature Climate Change 10: 370–375. [Google Scholar]
- Zani D, Crowther TW, Mo L, Renner SS, Zohner CM. 2020. Increased growing‐season productivity drives earlier autumn leaf senescence in temperate trees. Science 370: 1066–1071. [DOI] [PubMed] [Google Scholar]
- Zellweger F, De Frenne P, Lenoir J, Vangansbeke P, Verheyen K, Bernhardt‐Römermann M, Baeten L, Hédl R, Berki I, Brunet J et al. 2020. Forest microclimate dynamics drive plant responses to warming. Science 368: 772. [DOI] [PubMed] [Google Scholar]
- Zhang Y‐L, Moser B, Li M‐H, Wohlgemuth T, Lei J‐P, Bachofen C. 2020. Contrasting leaf trait responses of conifer and broadleaved seedlings to altered resource availability are linked to resource strategies. Plants 9: 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohner CM, Mo L, Pugh TA, Bastin JF, Crowther TW. 2020. Interactive climate factors restrict future increases in spring productivity of temperate and boreal trees. Global Change Biology 26: 4042–4055. [DOI] [PubMed] [Google Scholar]
- Zohner CM, Renner SS. 2015. Perception of photoperiod in individual buds of mature trees regulates leaf‐out. New Phytologist 208: 1023–1030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Picture showing the experimental infrastructure used in the study.
Fig. S2 Soil moisture of the different treatments during the experiment.
Fig. S3 Air temperature at 2 m height during the experiment.
Fig. S4 Daily minimum temperatures recorded within the bud from January 2020 until species‐specific budburst in white, black, shade or fully exposed buds.
Fig. S5 Growing degree hours above 5°C from 1 February to budburst in the shade and sun treatment.
Fig. S6 Air temperature recorded at canopy height during summer 2019 in the shade and sun treatment.
Table S1 Seed material used in the experiment.
Table S2 Amount of total nitrogen (N), nitrate (NO3 −) and ammonium (NH4 +) in the soil for each of the mesocosms at the end of the growing season (3 September 2019).
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


