Abstract
Domoic acid (DA) and saxitoxin (STX)‐producing algae are present in Alaskan seas, presenting exposure risks to marine mammals that may be increasing due to climate change. To investigate potential increases in exposure risks to four pagophilic ice seal species (Erignathus barbatus, bearded seals; Pusa hispida, ringed seals; Phoca largha, spotted seals; and Histriophoca fasciata, ribbon seals), this study analyzed samples from 998 seals harvested for subsistence purposes in western and northern Alaska during 2005–2019 for DA and STX. Both toxins were detected in bearded, ringed, and spotted seals, though no clinical signs of acute neurotoxicity were reported in harvested seals. Bearded seals had the highest prevalence of each toxin, followed by ringed seals. Bearded seal stomach content samples from the Bering Sea showed a significant increase in DA prevalence with time (logistic regression, p = .004). These findings are consistent with predicted northward expansion of DA‐producing algae. A comparison of paired samples taken from the stomachs and colons of 15 seals found that colon content consistently had higher concentrations of both toxins. Collectively, these results suggest that ice seals, particularly bearded seals (benthic foraging specialists), are suitable sentinels for monitoring HAB prevalence in the Pacific Arctic and subarctic.
Keywords: domoic acid, exposure risks, harmful algal blooms, marine mammals, saxitoxin
1. INTRODUCTION
1.1. Changing ocean conditions
Arctic and subarctic seas are experiencing dramatic changes in the persistence, extent, and quality of sea ice due to changing weather patterns and warming ocean temperatures. This is particularly true in the Alaskan Arctic (N. R. Bates et al., 2014; Stevenson & Lauth, 2019) where inputs of Pacific water advected through the Bering Strait are fresher, warmer, and higher in volume (Hu et al., 2012) and where upwelling‐favorable winds have also increased (Pickart et al., 2013). Warmer air temperatures and consequently larger negative air‐sea heat fluxes have compounded conditions, leading to earlier snowmelt and elevated radiative forcing (Bintanja & van der Linden, 2013; Johannessen et al., 2004; Stone et al., 2002; Turner & Overland, 2009). These changes have affected the ecology and biogeography of species at multiple trophic levels (Capotondi et al., 2012; Stevenson & Lauth, 2019; Tremblay & Gagnon, 2009), and as a result, many temperate organisms are predicted to increase their distribution into or increase their numbers within Arctic waters. In the context of impacts to human, wildlife, and ecosystem health, D. M. Anderson et al. (2018) argue that one of the most significant emerging threats is the expansion of harmful algal bloom (HAB) species, particularly diatoms of the genus Pseudo‐nitzschia and the dinoflagellate Alexandrium catenella that produce the potent neurotoxins domoic acid (DA) and saxitoxin (STX), respectively.
1.2. Health effects of harmful algal blooms
Harmful algal blooms of DA‐producing Pseudo‐nitzschia and STX‐producing Alexandrium species are common throughout the temperate world oceans and cause adverse human and wildlife health impacts and mortality. In humans, acute exposure leads to neurologic illnesses known as amnesic shellfish poisoning, caused by DA (S. S. Bates, 2000; S. S. Bates & Trainer, 2006; Berman & Murray, 2002; Perl et al., 1990; Todd, 1993), and paralytic shellfish poisoning, caused by the suite of paralytic shellfish toxins (PSTs) including STX (Etheridge, 2010; Usup et al., 1994). Both toxins accumulate in filter‐feeding marine organisms and are transferred through food webs with significant health consequences to animals at multiple trophic levels (Cembella & Desbiens, 1994; Kvitek et al., 2008; Lefebvre, Bargu, et al., 2002; Lefebvre et al., 2010; Lefebvre, Silver, et al., 2002; Scholin et al., 2000; White, 1980, 1981). Domoic acid exposure causes illness, stranding, and death in seabirds and marine mammals (Fritz et al., 1992; Gulland et al., 2005; Peery et al., 2006; Work et al., 1993). Persistent effects of recurrent DA exposures also lead to long‐term neurotoxic effects and epilepsy in California sea lions (Zalophus californianus; Cook et al., 2015; Goldstein et al., 2008). Exposures to STX also cause illness and death in marine mammals, although less frequently than those reported for DA. However, STX has been documented to cause massive kills of fish and invertebrates (Shumway, 1990; White, 1980, 1981), and has been linked to a mass mortality of humpback whales (Megaptera novaeangliae) off the eastern U.S. coast of Cape Cod, Massachusetts (Geraci et al., 1989). Together, these algal toxins result in significant economic losses in coastal communities relying on commercial and recreational seafood harvesting (C. R. Anderson et al., 2010; D. M. Anderson et al., 2000; Shumway, 1990; Trainer et al., 2007).
1.3. Marine mammal exposure to harmful algal bloom toxins
In the last two decades, almost half of the marine mammal unusual mortality events in the contiguous U.S. have been attributable to algal toxin exposure (Flewelling et al., 2005; Gulland & Hall, 2007; Landsberg et al., 2014; Scholin et al., 2000), and there is concern that wildlife exposure to HAB toxins may be growing. Domoic acid is known to be particularly common on the west coast of the contiguous U.S., where the first documented marine mammal DA poisoning event occurred in Monterey Bay, California, in 1998. During this event, several hundred California sea lions exhibited seizures and/or died over a short period due to consumption of DA‐contaminated anchovies (Gulland, 2000; Lefebvre et al., 1999; Scholin et al., 2000). Since then, dozens to hundreds of sea lions have been affected annually in coastal California (Bargu et al., 2010). In 2015, DA‐induced seizures were first observed in sea lions north of California in Long Beach, Washington, during the largest recorded Pseudo‐nitzschia bloom in coastal waters of North America (McCabe et al., 2016). This bloom was linked to a warm water anomaly that affected oceanic waters northward into the Gulf of Alaska, providing evidence for a potential northward expansion of conditions favorable for Pseudo‐nitzschia growth (Zhu et al., 2017). Saxitoxin has been a marine mammal health concern since suspected poisonings in the late 1980s affected humpback whales in New England and sea otters (Enhydra lutris) in Alaska (DeGange & Vacca, 1989; Geraci et al., 1989; Landsberg et al., 2014). In a recent analysis of HAB events on the Pacific coast of Canada from 1988 to 2017, it was found that STX events occurred on the Canadian Pacific coast with regularity, while DA events occurred infrequently (McKenzie et al., 2021). Algal toxins have been reported in Alaskan Arctic marine mammals; however, algal toxin exposure has not been definitively linked to morbidity and mortality events in the region, and few data exist regarding these events in Alaskan pagophilic seal species (Lefebvre et al., 2016).
1.4. Ice seal exposure to harmful algal bloom toxins
Bearded (Erignathus barbatus), ringed (Pusa hispida), spotted (Phoca largha), and ribbon (Histriophoca fasciata) seals represent critical components of the Pacific Arctic and subarctic marine ecosystems. Collectively referred to as ice seals due to the integral role that ice plays as a substrate for pupping, nursing, and molting, these seals are an important subsistence resource for coastal Alaska Native communities in western and northern Alaska (Nelson et al., 2019). They are also an important component of the Arctic marine ecosystem. In December of 2012, NOAA Fisheries listed ringed and bearded seals as threatened under the Endangered Species Act, citing climate change and resultant sea ice declines as significant threats to the seals' survival (U.S. Federal Register, 2012a, 2012b). Previous analyses of gastrointestinal (GI) samples collected during 2006–2013 detected DA in all four of these ice seal species, and STX in all species except ribbon seals (Lefebvre et al., 2016). As environmental conditions in western and northern Alaska continue to transition, the potential for HAB toxins to increase in prevalence and concentration in the Bering and Chukchi Seas is an increasing health threat for ice seals (D. M. Anderson et al., 2018; Laidre et al., 2015). The objective of this study was to quantify DA and STX prevalence and assess temporal trends therein in four ice seal species in the Bering, Chukchi, and Beaufort Seas. Gastrointestinal samples were collected during 2005–2019 in partnership with coastal Alaska Native communities that harvest ice seals for subsistence purposes (Nelson et al., 2019).
2. METHODS
2.1. Collection of gastrointestinal samples from harvested ice seals
During 2005–2019, samples were collected from ice seals harvested for subsistence purposes between May and September from coastal communities along the coast of the Bering, Chukchi, and Beaufort Seas (Figure 1). Information collected included age, sex, length, girth, blubber thickness, and date and location of harvest. General health assessments for body condition and signs of neurotoxicity were noted by samplers and harvesters. Locations in the Bering Strait and southward were considered to be in the Bering Sea, locations north of the Bering Strait and south of Utqiaġvik were considered to be in the Chukchi Sea, and Utqiaġvik was considered to be in the Beaufort Sea (Logerwell et al., 2011, 2018; Moore & Stabino, 2015; Woodgate et al., 2015).
FIGURE 1.
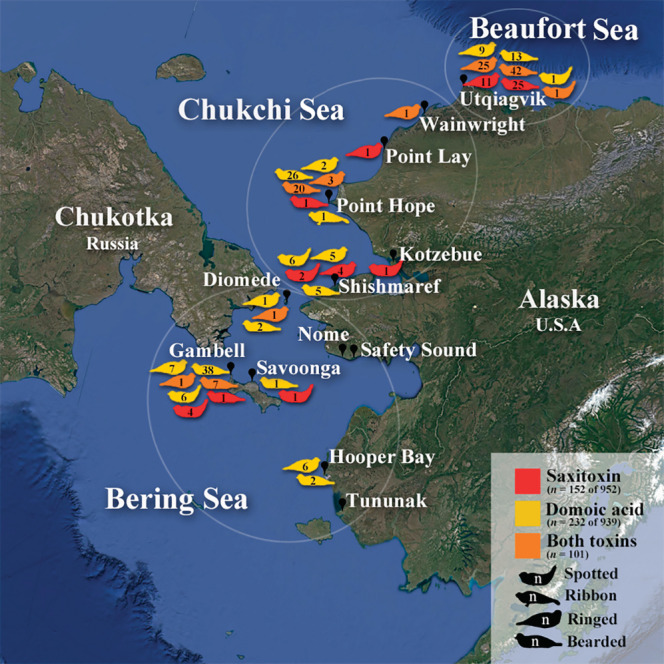
Harvest locations (black pins) are shown within circles indicating regional classifications (Bering, Chukchi, and Beaufort Seas). Next to each harvest location, icons represent the number of each seal species that tested positive for DA (yellow), STX (red), and both toxins (orange). Map generated in Google Earth.
In the field, whole stomachs were collected in Ziploc bags and shipped frozen to laboratories where they were stored at −20°C until they were subsampled. In the laboratory, stomachs were thawed, and 5 ml of semiliquid content was removed and placed in centrifuge tubes with screw caps before being refrozen. Samples removed from stomachs will hereafter be referred to as “stomach contents.” Samples were also collected from the rectum during routine postmortem examination as part of the North Slope Borough Department of Wildlife Management ice seal health monitoring program in Utqiaġvik, Alaska. These samples were stored in 55 cc centrifuge tubes with screw caps and frozen at −20°C. Samples removed from the rectum will hereafter be referred to as “colon contents.” All samples were shipped to the Northwest Fisheries Science Center's Wildlife Algal‐Toxin Research and Response Network (WARRN‐West) laboratory (NOAA Fisheries, Seattle, Washington) for algal toxin testing.
2.2. Quantification of domoic acid (DA) and saxitoxin (STX)
Toxins were extracted from stomach and colon contents via standard procedures using a 1:3 volume:volume ratio of sample to extraction solvent (Lefebvre et al., 2016). Extraction solvent was 50% methanol for all DA samples, and for 591 STX samples; extraction solvent was 80% ethanol for all other STX samples. Differences in STX concentrations quantified from 50% methanol and 80% ethanol extractions were not found to be statistically significant in n = 8 marine mammal GI samples and are therefore not expected to influence trend analyses (data not shown). Final extracts were further diluted 50‐fold for stomach contents and 100‐fold for colon contents in dilution buffer prior to DA quantification and 50‐fold for both stomach contents and colon contents in dilution buffer prior to STX quantification (Lefebvre et al., 2016). These minimum dilutions were chosen to eliminate matrix effects (Frame & Lefebvre, 2013). Samples and solvent were mixed for 1 min, homogenized for 60 s (Omni ES homogenizer), and centrifuged for 20 min at 3,100 rcf (max) at 4°C (Sorvall RC 5C Plus centrifuge). Finally, supernatant was filtered through a spin filter (Millipore Ultra‐Free MC‐GV centrifugal filters) spun at 13,870 rcf (max) for 3 min in a desktop centrifuge (Fisher Scientific accuSpin Micro 17). All extracts thus obtained were stored at 4°C prior to analysis. Concentrations of DA and STX equivalents in nanograms/gram (ng/g) were quantified in extracts using commercially available enzyme‐linked immunosorbent assay (ELISA) kits for DA (Biosense) and for STX equivalents (Abraxis) as per kit instructions. Detection limits for DA in sample material were 4 ng/g for colon contents and 2 ng/g for stomach content. The detection limit for STX in all sample material was 3 ng/g.
It must be noted that the Abraxis STX ELISA kit was specifically designed to detect STX and has limited cross‐reactivity with other PST congeners (as listed in the Abraxis product documents). As such, STX concentrations reported here underestimate total potential PST presence. In the absence of data regarding the PST congener profiles in ice seal GI contents, it is difficult to estimate the magnitude of this underestimation. Future studies will include HPLC analyses to characterize the suite of PSTs present in marine mammal tissues as part of our continued research on the trophic transfer of algal toxins in Arctic and subarctic food webs and will be useful for better total PST exposure estimates.
2.3. Analysis of trends
Temporal trends in each HAB toxin during 2012–2019 were assessed for bearded seals only and the Bering and Chukchi Seas only, due to sample size limitations for the other three ice seal species and the Beaufort Sea. For consistency, only samples from stomach contents were analyzed for trends. Furthermore, samples were restricted to those collected from May to September, when toxins are expected to be present. First, we examined trends in the prevalence or probability of detection for each HAB toxin. We modeled the probability of occurrence for each toxin using logistic regression. Detections were coded as having a value of 1 and nondetections were coded as having a value of 0. Second, given that a toxin was detected, we examined the trends in the concentration of each toxin using simple linear regression. All analyses were performed using the statistical program R (R Core Team, 2018).
3. RESULTS
Samples were analyzed for the HAB toxins DA and STX from 998 ice seals representing four seal species. Sample collection locations in the Bering, Chukchi, and Beaufort Seas are shown in Figure 1. Sex ratios for all species sampled were approximately 1:1, and all age classes (pup, subadult, and adult) were represented for each species.
3.1. Toxin prevalence and maximum concentrations in ice seals
Both DA and STX were detected in all regions sampled (Bering, Chukchi, and Beaufort Seas). Bearded seals had the highest prevalence of DA (46%), followed by ringed (21%), spotted (5%), and ribbon seals (4%) (Table 1). Although bearded seals had the highest DA prevalence, ringed seals had the highest DA concentration recorded (1,740 ng DA/g) followed by bearded seals (1,353 ng DA/g) (Table 1). Maximum DA concentrations in spotted and ribbon seals were two orders of magnitude lower at 90 and 33 DA ng/g, respectively. Bearded seals also had the highest prevalence of STX (24%), followed closely by ringed seals (18%). Saxitoxin was only detected in 4% of spotted seals and was not detected in any of the ribbon seals sampled (Table 1). Bearded seals had the highest STX concentration (464 ng STX equivalents/g) followed by ringed (180 ng STX equivalents/g) and spotted seals (66 ng STX equivalents/g). Prevalence of co‐occurrence (detectable levels of both DA and STX in the same individual) were highest in bearded (17%) and ringed seals (12%) (Table 1).
TABLE 1.
Prevalence of domoic acid (DA) and saxitoxin (STX) in gastrointestinal samples by species. Maximum concentrations did not reach regulatory limits for either DA (regulatory limit = 20,000 ng DA/g shellfish a ) or STX (regulatory limit = 800 ng STX equivalents/g shellfish a ).
| Species | Collection years | n DA positive/n DA tested (%DA positive) | n STX positive/n STX tested (%STX positive) | n DA and STX positive (%co‐occurrence) | Maximum DA concentration (ng/g) | Maximum STX concentration (ng/g) |
|---|---|---|---|---|---|---|
| Bearded seal, Erignathus barbatus | 2005–2019 |
157/344 (46%) |
96/404 (24%) |
69 (17%) |
1,353 | 464 |
| Ringed seal, Pusa hispida | 2005–2019 |
61/289 (21%) |
47/263 (18%) |
31 (12%) |
1,740 | 180 |
| Spotted seal, Phoca largha | 2005–2016 |
14/268 (5%) |
9/257 (4%) |
1 (0%) |
90 | 66 |
| Ribbon seal, Histriophoca fasciata | 2008–2016 |
1/28 (4%) |
0/28 (0%) |
0 (0%) |
33 | 0 |
Note: n = number of animals.
Regulatory limit units have been converted to match those reported in the table above.
3.2. Temporal trends of toxin prevalence in bearded seals
The large number of stomach‐content samples and the greater geographic span of collection locations for bearded seals allowed for the use of logistic regression to test for temporal trends in toxin prevalence in the Bering and Chukchi seas (Table 2). The temporal trend for increasing DA in the Bering Sea was the only significant trend (Figure 2, Table 2; p = .004). The logistic regression model estimates for the probability of DA presence in 2012 and 2019 were 5% [0%, 22%] and 94% [63%, 99%], respectively (Table 2 and Figure 2a). The empirical proportions of DA presence were 0% in 2012 and 100% in 2019 (Figure 2a), providing evidence that the regression model accurately describes the trend. No significant trends in the prevalence of STX were observed over the surveyed period (Figure 2).
TABLE 2.
Proportion of bearded seal stomach content samples collected in the Bering Sea that were found to have domoic acid (DA) by year and fitted logistic regression probabilities by year with 95% confidence intervals (CI). Fewer than three samples were collected in 2018 from the Bering Sea, therefore it was excluded from analysis.
| Year | Samples collected | Samples positive for DA | Proportion positive for DA | Logistic regression estimates of DA probability [95% CI] |
|---|---|---|---|---|
| 2012 | 4 | 0 | 0 | 0.05 [0.01, 0.22] |
| 2013 | 7 | 1 | 0.14 | 0.10 [0.03, 0.30] |
| 2014 | 8 | 4 | 0.50 | 0.20 [0.09, 0.40] |
| 2015 | 14 | 2 | 0.14 | 0.37 [0.22, 0.54] |
| 2016 | 6 | 3 | 0.50 | 0.57 [0.36, 0.75] |
| 2017 | 3 | 3 | 1.00 | 0.75 [0.46, 0.91] |
| 2018 | NA | NA | NA | 0.87 [0.55, 0.97] |
| 2019 | 5 | 5 | 1.00 | 0.94 [0.63, 0.99] |
FIGURE 2.
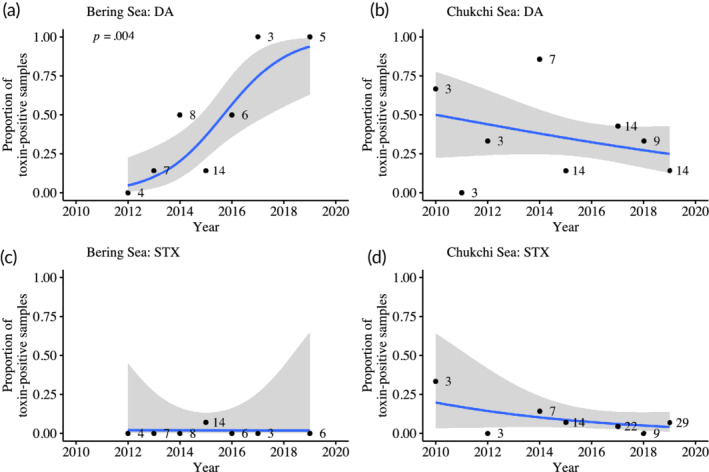
The proportion of bearded seal stomach content samples with detectable concentrations of domoic acid (DA) (a, b) and saxitoxin (STX) (c, d) from May–September in the Bering (a, c) and Chukchi (b, d) Seas by year. Sample size is listed to the right of each corresponding data point. Lines represent logistic regressions comparing presence/absence of toxin over the years, and shaded areas represent associated 95% confidence intervals. The only significant trend (p = .004) was in the Bering Sea (a).
3.3. Comparison of toxin concentrations in stomach and colon content samples
To determine if DA and STX concentrations were consistent throughout the GI tract, we compared samples from the same individual at two GI tract locations (stomach and colon) in a subset of bearded (n = 10) and ringed (n = 5) seals. Domoic acid concentrations were higher in colon content samples compared to corresponding stomach content samples in 9 of 10 bearded seals and 5 of 5 ringed seals (Table 3). In one bearded seal and two ringed seals, stomach content samples were below detection limits (BDL) for DA, but colon content ranged from 12 to 1,293 ng/g (Table 3). The findings for STX concentrations were even more dramatic. Saxitoxin was BDL in stomach content samples from all 15 seals sampled, however, 8 of 10 bearded seals had detectable concentrations in colon content, as did 4 of 5 ringed seals (Table 3).
TABLE 3.
Comparison of toxin concentrations detected in samples from two gastrointestinal tract locations (stomach and colon) collected simultaneously in 15 seals.
| Animal ID | Species | DA concentration (ng/g) | STX concentration (ng/g) | ||
|---|---|---|---|---|---|
| Stomach content | Colon content | Stomach content | Colon content | ||
| 2012BS07 | Bearded seal | 2 | 4 | BDL a | 8 |
| 09BS2 | Bearded seal | 10 | 156 | BDL | 10 |
| 09BS20 | Bearded seal | 7 | 23 | BDL | BDL |
| 09BS21 | Bearded seal | BDL | 12 | BDL | 15 |
| 09BS22 | Bearded seal | 138 | 887 | BDL | BDL |
| 09BS3 | Bearded seal | 3 | 7 | BDL | 3 |
| 09BS4 | Bearded seal | 3 | 11 | BDL | 6 |
| 09BS7 | Bearded seal | 5 | BDL | BDL | 8 |
| 09BS8 | Bearded seal | 6 | 136 | BDL | 108 |
| 09BS9 | Bearded seal | 8 | 12 | BDL | 23 |
| 09RS8 | Ringed seal | 7 | 15 | BDL | 180 |
| 2011RS2 | Ringed seal | 6 | 19 | BDL | 29 |
| 2015‐RS‐10 | Ringed seal | 7 | 113 | BDL | 6 |
| 2015RS12 | Ringed seal | BDL | 142 | BDL | 4 |
| 2015RS13 | Ringed seal | BDL | 1,293 | BDL | BDL |
Note: For each seal, the highest toxin concentration is in bold.
BDL = below detection limits.
4. DISCUSSION
Results from this study confirm previous findings that ice seals are regularly exposed to DA and STX in the Bering, Chukchi, and Beaufort Seas (Lefebvre et al., 2016) (Figure 2, Table 1). The maximum DA concentration reported here (1,740 ng DA/g in ringed seal feces) is an order of magnitude higher than the maximum concentration of DA previously reported (127 ng DA/g in ringed seal feces; Lefebvre et al., 2016). The maximum STX concentration reported here (464 ng STX equivalents/g in bearded seal feces) was also higher than the maximum STX concentration previously reported (172 ng STX equivalents/g in ringed seal feces). However, these new maximum values are still well below the seafood safety regulatory limits for humans for both toxins (Table 1).
4.1. Diet and algal toxin prevalence in ice seals
Algal toxin accumulation and prevalence in ice seals occurs through diet. Bearded seals, primarily benthic foragers (Table 4), had the highest prevalence of both DA (46%) and STX (24%) of the four species examined (Table 1). Ringed seals, primarily pelagic fish and invertebrate consumers (Table 4), had the second highest prevalence of DA (21%) and STX (18%; Table 1). Toxin prevalence was lower in the spotted and ribbon seal species, for which pelagic fish are a large part of the diet (5% and 4% for DA and STX in spotted seals, respectively, and 4% and 0% for DA and STX in ribbon seals, respectively; Tables 4 and 1). In general, filter‐feeding species (benthic and pelagic) accumulate higher concentrations of algal toxins than particulate feeding species due to the direct consumption of algae (Lefebvre, Silver, et al., 2002). A study comparing DA levels in anchovies and sardines collected simultaneously during a toxic Pseudo‐nitzschia bloom in Monterey, California revealed that anchovies had significantly higher toxin levels than sardines (Lefebvre, Silver, et al., 2002). Although both anchovies and sardines can feed on phytoplankton and zooplankton via filter‐feeding or particulate/selective feeding modes (Loukashkin, 1970; Radovich, 1952), comparative mouth morphology and feeding behavior suggests that anchovies feed more generally on diatoms, whereas sardines likely target zooplankton, thereby accumulating Pseudo‐nitzschia secondarily or in lower quantities (Lefebvre, Silver, et al., 2002). Additionally, during toxic Alexandrium blooms, benthic shellfish can accumulate high concentrations of STX via both direct consumption of vegetative algal cells and via consumption of benthic cysts of Alexandrium spp. from disturbed sediments, allowing for exposure to occur even in the absence of vegetative blooms in surface waters (Persson et al., 2006). Abundant Alexandrium cyst beds are present in the sediments of the Chukchi Sea and the eastern Bering Sea (Natsuike et al., 2013). This is consistent with the higher toxin levels and prevalence observed here in bearded seals that primarily consume benthic prey (e.g., flatfish, sculpins, shrimp, crab, gastropods, and clams) and ringed seals that consume filter‐feeding invertebrates and planktivorous fish, compared to spotted and ribbon seals that primarily feed on particulate‐consuming pelagic fish (Table 4). In a previous study, Pacific walruses (Odobenus rosmarus divergens), the most benthic‐dependent feeding pinnipeds in the Bering and Chukchi Seas, had the highest toxin concentrations and prevalence for both DA and STX, further suggesting that benthic prey may be the most significant route for exposure (Lefebvre et al., 2016). The fact that planktivorous‐fish‐consuming ringed seals had the maximum concentrations of both DA and STX reported in previous studies and the maximum STX concentration reported in this study provides further evidence that planktivorous fish are potent vectors of algal toxins.
TABLE 4.
Primary known prey species for bearded, ringed, spotted, and ribbon seals.
| Species | Feeding preferences | Invertebrate prey | Fish prey | References |
|---|---|---|---|---|
|
Bearded seals (Erignathus barbatus) |
Benthic fish and invertebrates |
Bivalves Gastropods Cephalopods Isopods Amphipods Shrimps Crabs Echiurids Polychaetes |
Pelagic Arctic cod (Boreogadus saida) Saffron cod (Eleginus gracilis) Benthic Sculpins (Cottidae) Snailfish (Liparidae) Pricklebacks (Stichaeidae) Pacific sand lance (Ammodytes hexapterus) Flatfish (Pleuronectidae) |
Antonelis et al., 1994; Crawford et al., 2015; Lowry et al., 1980a; ADF&G, unpublished data |
|
Ringed seal (Pusa hispida) |
Pelagic fish and invertebrates |
Mysids Amphipods Shrimp |
Pelagic Arctic cod (Boreogadus saida) Saffron cod (Eleginus gracilis) Walleye pollock (Gadus chalcogramma) Rainbow smelt (Osmerus mordax) Benthic Sculpins (Cottidae) |
Crawford et al., 2015; Dehn et al., 2007; Johnson et al., 1966; Lowry et al., 1980b; ADF&G, unpublished data |
|
Spotted seal (Phoca largha) |
Pelagic fish | Not a significant dietary component |
Pelagic Arctic cod (Boreogadus saida) Saffron cod (Eleginus gracilis) Pacific herring (Clupea pallasi) Capelin (Mallotus villosus) Rainbow smelt (Osmerus mordax) |
Bukhtiyarov et al., 1984; Lowry & Frost, 1981; ADF&G, unpublished data |
|
Ribbon seals (Histriophoca fasciata) |
Pelagic fish and invertebrates |
Shrimp Octopus |
Pelagic Arctic cod (Boreogadus saida) Saffron cod (Eleginus gracilis) Walleye pollock (Gadus chalcogramma) |
Dehn et al., 2007; Frost & Lowry, 1980; ADF&G, unpublished data |
4.2. Comparison of toxin concentrations in stomach vs. colon contents
Colon content samples consistently had higher toxin levels than corresponding stomach content samples for both DA and STX (Table 3). Multiple factors may influence this distribution pattern, including less water content, potential absorption and reabsorption patterns, and that colon content represents more than one stomach's worth of digested material. Regardless, sampling colon contents enhances the ability to detect toxins and is preferable for monitoring toxin prevalence in marine mammals. These results suggest that our previous analyses (Lefebvre et al., 2016) greatly underestimated the prevalence of DA and STX in seals and other marine mammals where stomach content was analyzed. Future monitoring efforts should collect and analyze colon content samples for better estimates of prevalence and concentration even though results will not be directly comparable to past stomach content analysis.
4.3. Temporal trends of toxin prevalence in bearded seals
The significant temporal trend for DA prevalence in bearded seals from 2012 to 2019 reported above in the Bering Sea (Figure 2a) is consistent with a northward expansion of warmer ocean conditions that are favorable for Pseudo‐nitzschia growth (D. M. Anderson et al., 2018; McCabe et al., 2016). In 2015, a strong link was made between anomalously warm ocean conditions along the U.S. West Coast and Canada, and the development of the largest DA‐producing Pseudo‐nitzschia bloom ever recorded. During this coast‐wide bloom, Pseudo‐nitzschia australis thrived north of its typical range in the warm water that spanned the northeast Pacific (McCabe et al., 2016). Unprecedented levels of DA were found in the northeast Pacific Ocean food web causing coast‐wide closures of commercial and recreational fisheries for clams, mussels, Dungeness crab, rock crab, anchovy, and sardine from May to November (McCabe et al., 2016). Unfortunately, concurrent phytoplankton samples were not obtained in the Gulf of Alaska or the Bering Sea, however, warmer ocean conditions were also reported in those regions (McCabe et al., 2016). In fact, sea surface temperature data from the Bering Sea show a significant warming trend of 0.22°C ± 0.10°C per decade during 1966–2018 (Danielson et al., 2020). Although increasing DA was not observed in bearded seals harvested farther north in the Chukchi Sea, continued northern expansion and increases in Pseudo‐nitzschia may eventually reach the Chukchi Sea. Additionally, changes in ice seal behavior and regional feeding patterns in response to changing ocean conditions may influence toxin prevalence in the future.
4.4. Exposure risks for ice seals
Official regulatory limits are 20 μg DA/g (equivalent to 20,000 ng DA/g) shellfish and 80 μg STX equivalents/100 g (equivalent to 800 ng/g) shellfish (Table 1) (Wekell et al., 2004). Regulatory limits were established in seafood for the protection of human health to prevent amnesic shellfish poisoning and paralytic shellfish poisoning from DA and STX, respectively (Wekell et al., 2004). All values reported here were below the seafood safety regulatory limits for both toxins (Table 1). Although the concentrations in prey that would be toxic to marine mammals are unknown, regulatory limits can be used as estimates for concentrations in prey that could be harmful to mammalian species.
While some values reported here fall within the range of toxin concentrations quantified in fecal and GI samples from stranded California sea lions diagnosed with acute DA toxicosis (Lefebvre et al., 2016), those levels in sea lions were highly variable (i.e., ranging from 0.001 μg/g to well above seafood safety regulatory limits of >20,000 ng/g; Figure 2 in Lefebvre et al., 2016) and are not a reliable proxy for actual doses of toxin consumed. Consequently, secondary signs of excitotoxicity such as seizures, ataxia, and head weaving are necessary for a positive clinical diagnosis of DA poisoning in marine mammals (Scholin et al., 2000). No clinical signs of DA‐induced excitotoxicity or STX‐induced paralysis were reported for these seals by the hunters who harvested them. This suggests that algal toxins may not yet be a significant health threat to ice seals, but raises valid concerns about future exposure risks with continued ocean warming as a result of continuing sea ice loss. Because warmer ocean temperatures foster increased harmful algal growth, and Arctic and subarctic regions are undergoing rapid rates of ocean warming, concern for increasing impacts of harmful algal toxins on important marine resources is high (D. M. Anderson et al., 2018). Such impacts are of particular concern for communities where there is a substantial reliance on marine mammals as a food resource (D. M. Anderson et al., 2018; Braund & Associates, 2018; Garlich‐Miller & Burn, 1999; MacCracken et al., 2017; Nelson et al., 2019).
4.5. Summary
Ice seals (i.e., bearded, ringed, spotted, and ribbon seals) are regularly exposed to both DA and STX in the Bering, Chukchi, and Beaufort Seas. Colon content samples are more sensitive indicators for DA and STX prevalence than stomach content samples and should be used in future monitoring efforts. Nonetheless, stomach content analyses in bearded seals were sufficient to identify a significant increase in DA prevalence from 0% in 2012 to 100% in 2019 in the Bering Sea, consistent with warming ocean conditions fostering a northward expansion and increase of Psuedo‐nitzschia spp. Differences found in toxin prevalence and concentration among ice seal species are most likely due to diet differences, with filter feeding benthic prey and planktivorous fish likely presenting the greatest exposure risks for ice seals. Observable health impacts for the harvested seals sampled in this study were not reported by hunters. However, consequences of chronic low‐level exposure are of concern, as is the possibility that toxin concentrations may increase to harmful levels as Alaskan waters continue to respond to the continuing reduction in seasonal sea ice coverage. Ice seals in general, and bearded seals in particular, can be valuable sentinels for changes in DA and STX prevalence in Pacific Arctic and subarctic marine ecosystems.
AUTHOR CONTRIBUTIONS
Alicia Hendrix: Conceptualization; data curation; formal analysis; investigation; methodology; software; validation; visualization; writing‐original draft; writing‐review & editing. Kathi Lefebvre: Conceptualization; data curation; funding acquisition; methodology; project administration; resources; supervision; validation; writing‐original draft; writing‐review & editing. Lori Quakenbush: Conceptualization; funding acquisition; investigation; methodology; project administration; resources; writing‐original draft; writing‐review & editing. Anna Bryan: Conceptualization; funding acquisition; investigation; methodology; project administration; resources; writing‐original draft; writing‐review & editing. Raphaela Stimmelmayr: Conceptualization; funding acquisition; investigation; methodology; project administration; resources; writing‐original draft; writing‐review & editing. Gay Sheffield: Conceptualization; funding acquisition; investigation; methodology; project administration; resources; writing‐original draft; writing‐review & editing. Gabriel Wisswaesser: Data curation; investigation; writing‐review & editing. Maryjean Willis: Data curation; project administration; writing‐review & editing. Emily Bowers: Data curation; investigation; visualization; writing‐review & editing. Preston Kendrick: Data curation; investigation; writing‐review & editing. Elizabeth Frame: Data curation; investigation; writing‐review & editing. Thomas Burbacher: Funding acquisition; project administration; supervision; writing‐review & editing. David Marcinek: Funding acquisition; project administration; supervision; writing‐review & editing.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
ACKNOWLEDGMENTS
This project was made possible by the willingness of hunters to contribute samples from their harvest, by the support of their communities, local governments, and Tribal Councils, and by the Ice Seal Committee. We would like to express our gratitude to the communities of Gambell, Hooper Bay, Kotzebue, Little Diomede, Nome, Point Hope, Point Lay, Safety Sound, Savoonga, Shishmaref, Tununak, Utqiaġvik, and Wainwright. Without their contribution this study would have not been possible. We thank Mark Nelson, Louise Biderman, Ryan Adam, and college interns from the Alaska Department of Fish and Game (ADF&G) and Cyd Hans, Rita Acker, Olive Kanayurak, and Frances Olemaun from the North Slope Borough Department of Wildlife Management (NSB DWM) for sample collection and processing. John Citta of ADF&G provided guidance regarding statistical analysis. We also thank Don Anderson of Woods Hole Oceanographic Institution for help in editing the manuscript.
This paper is a result of research funded by the National Oceanic and Atmospheric Administration (NOAA) National Centers for Coastal Ocean Science Competitive Research Program under award number NA20NOS4780195 to the Northwest Fisheries Science Center (NWFSC to K.A.L.) and Woods Hole Oceanographic Institution (to Don Anderson). This is ECOHAB publication number ECO973. Funding for this project was also provided by NOAA's Office of Protected Resources Species of Concern grant (SOC Office of Protective Resources 2009SOC‐ice seals to K.A.L. and E.F.), by the Marine Mammal Commission, by NOAA's National Marine Fisheries Service Projects NA05NMF4391187, NA08NMF4390544, NA11NMF4390200, NA16NMF4720079, and NA16NMF4390029, by NOAA/NWFSC/Wildlife Algal Toxins Research and Response Network (WARRN‐West), by a substantial grant from the Coastal Impact Assistance Program (F12AF01265), by qualified outer continental shelf oil and gas revenues, by the NSB DWM, by the National Institutes of Health (NIH) R01s ES021930 and ES030319 (to D.J.M. and K.A.L.), and by the National Science Foundation (NSF) R01s OCE‐1314088 and OCE‐1839041 (to D.J.M. and K.A.L.). This work was supported in part by the UW NIEHS sponsored Environmental Pathology/Toxicology Training Program (EP/T) Training Grant (NIEHS T32ES007032). All sample collection from subsistence harvested ice seals was performed under the following permits: National Marine Fisheries Service (NMFS) research permit numbers 358‐1787, 15324, and 20466, issued to ADF&G, and 814‐1899‐00; 814‐1899‐01; 814‐1899‐02; 814‐1899‐03; 814‐1899‐04; 17350‐00; 17350‐01, 17350‐02, and 21386 issued to NSB DWM.
Hendrix AM, Lefebvre KA, Quakenbush L, et al. Ice seals as sentinels for algal toxin presence in the Pacific Arctic and subarctic marine ecosystems. Mar Mam Sci. 2021;37:1292–1308. 10.1111/mms.12822
Funding information Marine Mammal Commission; National Institute of Environmental Health Sciences, Grant/Award Numbers: ES021930, ES030319, T32ES007032; National Oceanic and Atmospheric Administration, Grant/Award Numbers: 2009SOC‐ice seals, F12AF01265, NA05NMF4391187, NA08NMF4390544, NA11NMF4390200, NA16NMF4390029, NA16NMF4720079, NA20NOS4780195; National Science Foundation, Grant/Award Numbers: OCE‐1314088, OCE‐1839041; North Slope Borough Department of Wildlife Management; Qualified outer continental shelf oil and gas revenues
REFERENCES
- Anderson, C. R. , Sapiano, M. R. P. , Prasad, M. B. K. , Long, W. , Tango, P. J. , Brown, C. W. , & Murtugudde, R. (2010). Predicting potentially toxigenic Pseudo‐nitzschia blooms in the Chesapeake Bay. Journal of Marine Systems, 83(3–4), 127–140. 10.1016/j.jmarsys.2010.04.003 [DOI] [Google Scholar]
- Anderson, D. M. , Hoagland, P. , Kaoru, Y. , & White, A. W. (2000). Estimated annual economic impacts from harmful algal blooms (HABs) in the United States (WHOI‐2000‐11). Woods Hole Oceanographic Institution. [Google Scholar]
- Anderson, D. M. , Richlen, M. L. , & Lefebvre, K. A. (2018). Harmful algal blooms in the Arctic. In Osborne E., Richter‐Menge J., & Jeffries M. (Eds.), Arctic report card 2018 (pp. 81–87). https://arctic.noaa.gov/Portals/7/ArcticReportCard/Documents/ArcticReportCard_full_report2018.pdf [Google Scholar]
- Antonelis, G. A. , Melin, S. R. , & Bukhtiyarov, Y. A. (1994). Early Spring feeding habits of bearded seals (Erignathus barbatus) in the central Bering Sea, 1981. Arctic, 47(1), 74–79. [Google Scholar]
- Bargu, S. , Silver, M. , Goldstein, T. , Roberts, K. , & Gulland, F. (2010). Complexity of domoic acid‐related sea lion strandings in Monterey Bay, California: foraging patterns, climate events, and toxic blooms. Marine Ecology Progress Series, 418, 213–222. 10.3354/meps08816 [DOI] [Google Scholar]
- Bates, N. R. , Garley, R. , Frey, K. E. , Shake, K. L. , & Mathis, J. T. (2014). Sea‐ice melt CO2–carbonate chemistry in the western Arctic Ocean: Meltwater contributions to air–sea CO2 gas exchange, mixed‐layer properties and rates of net community production under sea ice. Biogeosciences, 11(23), 6769–6789. 10.5194/bg-11-6769-2014 [DOI] [Google Scholar]
- Bates, S. S. (2000). Domoic‐acid‐producing diatoms: Another genus added! Journal of Phycology, 36(6), 978–983. 10.1046/j.1529-8817.2000.03661.x [DOI] [Google Scholar]
- Bates, S. S. , & Trainer, V. L. (2006). The ecology of harmful diatoms. In Graneli E. & Turner J. T. (Eds.), Ecology of harmful algae (pp. 81–93). Springer. 10.1007/978-3-540-32210-8_7 [DOI] [Google Scholar]
- Berman, F. W. , & Murray, T. F. (2002). Domoic acid neurotoxicity in cultured cerebellar granule neurons is mediated predominantly by NMDA receptors that are activated as a consequence of excitatory amino acid release. Journal of Neurochemistry, 69(2), 693–703. 10.1046/j.1471-4159.1997.69020693.x [DOI] [PubMed] [Google Scholar]
- Bintanja, R. , & van der Linden, E. C. (2013). The changing seasonal climate in the Arctic. Scientific Reports, 3, 1556. 10.1038/srep01556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braund, S. R. , & Associates . (2018) Description of Alaskan Eskimo bowhead whale subsistence sharing practices. Final report submitted to the Alaska Eskimo Whaling Commission, May 2018. [Google Scholar]
- Bukhtiyarov, Y. A. , Frost, K. J. , & Lowry, L. E. (1984). New information on foods of the spotted seal, Phoca largha, in the Bering Sea in spring. In Fay F. H. & Fedoseev G. A. (Eds.), Soviet‐America cooperative research on marine mammals, Pinnipeds (NOAA Technical Report NMFS 12; Vol. 1, pp. 55–59). U.S. Department of Commerce. [Google Scholar]
- Capotondi, A. , Alexander, M. A. , Bond, N. A. , Curchitser, E. N. , & Scott, J. D. (2012). Enhanced upper ocean stratification with climate change in the CMIP3 models. Journal of Geophysical Research: Oceans, 117, C04031. 10.1029/2011JC007409 [DOI] [Google Scholar]
- Cembella, A. , & Desbiens, M. (1994). Fate of paralytic shellfish toxins in the American lobster Homarus americanus . Journal of Shellfish Research, 13, 302. [Google Scholar]
- Cook, P. F. , Reichmuth, C. , Rouse, A. A. , Libby, L. A. , Dennison, S. E. , Carmichael, O. T. , Kruse‐Elliott, K. T. , Bloom, J. , Singh, B. , Fravel, V. A. , Barbosa, L. , Stuppino, J. J. , van Bonn, W. G. , Gulland, F. , & Ranganath, C. (2015). Algal toxin impairs sea lion memory and hippocampal connectivity, with implications for strandings. Science, 350(6267), 1545–1547. 10.1126/science.aac5675 [DOI] [PubMed] [Google Scholar]
- Crawford, J. A. , Quakenbush, L. T. , & Citta, J. J. (2015). A comparison of ringed and bearded seal diet, condition and productivity between historical (1975–1984) and recent (2003‐2012) periods in the Alaskan Bering and Chukchi seas. Progress in Oceanography, 136, 133–150. 10.1016/j.pocean.2015.05.011 [DOI] [Google Scholar]
- Danielson, S. L. , Ahkinga, O. , Ashjian, C. , Basyuk, E. , Cooper, L. W. , Eisner, L. , Farley, E. , Iken, K. B. , Grebmeier, J. M. , Juranek, L. , Khen, G. , Jayne, S. R. , Kikuchi, T. , Ladd, C. , Lu, K. , McCabe, R. M. , Moore, G. W. K. , Nishino, S. , Ozenna, F. , … Weingartner, T. J. (2020). Manifestation and consequences of warming and altered heat fluxes over the Bering and Chukchi Sea continental shelves. Deep‐Sea Research Part II: Topical Studies in Oceanography, 177, 104781. 10.1016/j.dsr2.2020.104781 [DOI] [Google Scholar]
- DeGange, A. R. , & Vacca, M. M. (1989). Sea otter mortality at Kodiak Island, Alaska, during summer 1987. Journal of Mammalogy, 70(4), 836–838. 10.2307/1381723 [DOI] [Google Scholar]
- Dehn, L.‐A. , Sheffield, G. G. , Follmann, E. H. , Duffy, L. K. , Thomas, D. L. , & O'Hara, T. M. (2007). Feeding ecology of phocid seals and some walrus in the Alaskan and Canadian Arctic as determined by stomach contents and stable isotope analysis. Polar Biology, 30, 167–181. 10.1007/s00300-006-0171-0 [DOI] [Google Scholar]
- Etheridge, S. M. (2010). Paralytic shellfish poisoning: Seafood safety and human health perspectives. Toxicon, 56(2), 108–122. 10.1016/j.toxicon.2009.12.013 [DOI] [PubMed] [Google Scholar]
- Flewelling, L. J. , Naar, J. P. , Abbott, J. P. , Baden, D. G. , Barros, N. B. , Bossart, G. D. , Bottein, M. Y. D. , Hammond, D. G. , Haubold, E. M. , Heil, C. A. , Henry, M. S. , Jacocks, H. M. , Leighfield, T. A. , Pierce, R. H. , Pitchford, T. D. , Rommel, S. A. , Scott, P. S. , Steidinger, K. A. , Truby, E. W. , van Dolah F. M. Landsberg, J. H. (2005). Brevetoxicosis: Red tides and marine mammal mortalities. Nature, 435(7043), 755–756. 10.1038/nature435755a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame, E. , & Lefebvre, K. A. (2013). ELISA methods for domoic acid quantification in multiple marine mammal species and sample matrices (NOAA Technical Memorandum NMFS‐NWFSC‐122). U.S. Department of Commerce. [Google Scholar]
- Fritz, L. , Quilliam, M. A. , Wright, J. L. C. , Beale, A. M. , & Work, T. M. (1992). An outbreak of domoic acid poisoning attributed to the pennate diatom Pseudonitzschia australis . Journal of Phycology, 28(4), 439–442. 10.1111/j.0022-3646.1992.00439.x [DOI] [Google Scholar]
- Frost, K. J. , & Lowry, L. F. (1980). Feeding of ribbon seals (Phoca fasciata) in the Bering Sea in spring. Canadian Journal of Zoology, 58(9), 1601–1607. 10.1139/z80-219 [DOI] [Google Scholar]
- Garlich‐Miller, J. , & Burn, D. (1999). Estimating the harvest of Pacific walrus, Odobenus rosmarus divergens, in Alaska. Fishery Bulletin, 97(4), 1043–1046. [Google Scholar]
- Geraci, J. R. , Anderson, D. M. , Timperi, R. J. , St. Aubin, D. J. , Early, G. A. , Prescott, J. H. , & Mayo, C. A. (1989). Humpback whales (Megaptera novaeangliae) fatally poisoned by dinoflagellate toxin. Canadian Journal of Fisheries and Aquatic Sciences, 46(11), 1895–1898. 10.1139/f89-238 [DOI] [Google Scholar]
- Goldstein, T. , Mazet, J. A. K. , Zabka, T. S. , Langlois, G. , Colegrove, K. M. , Silver, M. , Bargu, S. , van Dolah, F. , Leighfield, T. , Conrad, P. A. , Barakos, J. , Williams, D. C. , Dennison, S. , Haulena, M. , & Gulland, F. M. D. (2008). Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): An increasing risk to marine mammal health. Proceedings of the Royal Society B: Biological Sciences, 275(1632), 267–276. 10.1098/rspb.2007.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulland, F. (2000). Domoic acid toxicity in California sea lions (Zalophus californianus) stranded along the central California coast, May–October 1998 (NOAA Technical Memorandum NMFS‐OPR‐17). U.S. Department of Commerce. [Google Scholar]
- Gulland, F. , & Hall, A. J. (2007). Is marine mammal health deteriorating? Trends in the global reporting of marine mammal disease. EcoHealth, 4(2), 135–150. 10.1007/s10393-007-0097-1 [DOI] [Google Scholar]
- Gulland, F. , Pérez‐Cortés, H. , Urbán, J. , Rojas‐Bracho, L. , Ylitalo, G. , Weir, J. , Norman, S. A. , Muto, M. M. , Rugh, D. J. , Kreuder, C. , & Rowles, T. (2005). Eastern North Pacific gray whale (Eschrichtius robustus) unusual mortality event, 1999–2000 (NOAA Technical Memorandum NMFS‐AFSC‐150). U.S. Department of Commerce. [Google Scholar]
- Hu, A. , Meehl, G. A. , Han, W. , Timmermann, A. , Otto‐Bliesner, B. , Liu, Z. , Washington, W. M. , Large, W. , Abe‐Ouchi, A. , Kimoto, M. , Lambeck, K. , & Wu, B. (2012). Role of the Bering Strait on the hysteresis of the ocean conveyor belt circulation and glacial climate stability. Proceedings of the National Academy of Sciences of the United States of America, 109(17), 6417–6422. 10.1073/pnas.1116014109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen, O. M. , Bengtsson, L. , Miles, M. W. , Kuzmina, S. I. , Semenov, V. A. , Alekseev, G. v. , Nagurnyi, A. P. , Zakharov, V. F. , Bobylev, L. P. , Pettersson, L. H. , Hasselmann, K. , & Cattle, H. P. (2004). Arctic climate change: observed and modelled temperature and sea‐ice variability. Tellus A: Dynamic Meteorology and Oceanography, 56(4), 328–341. 10.3402/tellusa.v56i4.14418 [DOI] [Google Scholar]
- Johnson, M. L. , Fiscus, C. H. , Ostenson, B. T. , & Barbour, M. L. (1966). Marine mammals. In Wilimovsky N. J. & Wolfe J. N. (Eds.), Environment of the Cape Thompson region, Alaska (pp. 877–924). U.S. Atomic Energy Commission. [Google Scholar]
- Kvitek, R. , Goldberg, J. , Smith, G. , Doucette, G. , & Silver, M. (2008). Domoic acid contamination within eight representative species from the benthic food web of Monterey Bay, California, USA. Marine Ecology Progress Series, 367, 35–47. 10.3354/meps07569 [DOI] [Google Scholar]
- Laidre, K. L. , Stern, H. , Kovacs, K. M. , Lowry, L. , Moore, S. E. , Regehr, E. v. , Ferguson, S. H. , Wiig, Ø. , Boveng, P. , Angliss, R. P. , Born, E. W. , Litovka, D. , Quakenbush, L. , Lydersen, C. , Vongraven, D. , & Ugarte, F. (2015). Arctic marine mammal population status, sea ice habitat loss, and conservation recommendations for the 21st century. Conservation Biology, 29(3), 724–737. 10.1111/cobi.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg, J. , Lefebvre, K. A. , & Flewelling, L. (2014). Effects of toxic microalgae on marine organisms. In Rossini G. P. (Ed.), Toxins and biologically active compounds from microalgae (Vol. 2, pp. 379–449). CRC Press. 10.1201/b16806-17 [DOI] [Google Scholar]
- Lefebvre, K. A. , Bargu, S. , Kieckhefer, T. , & Silver, M. W. (2002). From sanddabs to blue whales: The pervasiveness of domoic acid. Toxicon, 40(7), 971–977. 10.1016/S0041-0101(02)00093‐4 [DOI] [PubMed] [Google Scholar]
- Lefebvre, K. A. , Powell, C. L. , Busman, M. , Doucette, G. J. , Moeller, P. D. R. , Silver, J. B. , Miller, P. E. , Hughes, M. P. , Singaram, S. , Silver, M. W. , & Tjeerdema, R. S. (1999). Detection of domoic acid in northern anchovies and California sea lions associated with an unusual mortality event. Natural Toxins, 7(3), 85–92. 10.1002/(SICI)1522-7189(199905/06)7:385::AID-NT393.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- Lefebvre, K. A. , Quakenbush, L. , Frame, E. , Huntington, K. B. , Sheffield, G. , Stimmelmayr, R. , Bryan, A. , Kendrick, P. , Ziel, H. , Goldstein, T. , Snyder, J. A. , Gelatt, T. , Gulland, F. , Dickerson, B. , & Gill, V. (2016). Prevalence of algal toxins in Alaskan marine mammals foraging in a changing arctic and subarctic environment. Harmful Algae, 55, 13–24. 10.1016/j.hal.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre, K. A. , Robertson, A. , Frame, E. R. , Colegrove, K. M. , Nance, S. , Baugh, K. A. , Wiedenhoft, H. , & Gulland, F. (2010). Clinical signs and histopathology associated with domoic acid poisoning in northern fur seals (Callorhinus ursinus) and comparison of toxin detection methods. Harmful Algae, 9(4), 374–383. 10.1016/j.hal.2010.01.007 [DOI] [Google Scholar]
- Lefebvre, K. A. , Silver, M. W. , Coale, S. L. , & Tjeerdema, R. S. (2002). Domoic acid in planktivorous fish in relation to toxic Pseudo‐nitzschia cell densities. Marine Biology, 140(3), 625–631. 10.1007/s00227-001-0713-5 [DOI] [Google Scholar]
- Logerwell, E. , Rand, K. , Danielson, S. , & Sousa, L. (2018). Environmental drivers of benthic fish distribution in and around Barrow Canyon in the northeastern Chukchi Sea and western Beaufort Sea. Deep‐Sea Research Part II, 152, 170–181. [Google Scholar]
- Logerwell, E. , Rand, K. , & Weingartner, T. J. (2011). Oceanographic characteristics of the habitat of benthic fish and invertebrates in the Beaufort Sea. Polar Biology, 34, 1783–1796. [Google Scholar]
- Loukashkin, A. S. (1970). On the diet and feeding behavior of the northern anchovy Engraulis mordax (Girard). Proceedings of the California Academy of Sciences, 4th Series, 37, 419–458. [Google Scholar]
- Lowry, L. F. , & Frost, K. (1981). Feeding and trophic relationships of phocid seals and walrus in the eastern Bering Sea. In Hood D. & Calder J. (Eds.), The eastern Bering Sea shelf: oceanography and resources (Vol. 2, pp. 813–824). U.S. Department of Commerce. [Google Scholar]
- Lowry, L. F. , Frost, K. J. , & Burns, J. J. (1980a). Feeding of bearded seals in the Bering and Chukchi Seas and trophic interaction with Pacific walruses. Arctic, 33, 330–342. 10.14430/arctic2566 [DOI] [Google Scholar]
- Lowry, L. F. , Frost, K. J. , & Burns, J. J. (1980b). Variability in the diet of ringed seals, Phoca hispida . Canadian Journal of Fisheries and Aquatic Sciences, 37, 2254–2261. 10.1139/f80-270 [DOI] [Google Scholar]
- MacCracken, J. G. , Beatty, W. S. , Garlich‐Miller, J. L. , Kissling, M. L. , & Snyder, J. A. (2017). Final species status assessment for the Pacific walrus (Odobenus rosmarus divergens), May 2017 (Version 1.0). Marine Mammals Management, U.S. Fish and Wildlife Service. 10.13140/RG.2.2.29363.12325 [DOI] [Google Scholar]
- McCabe, R. M. , Hickey, B. M. , Kudela, R. M. , Lefebvre, K. A. , Adams, N. G. , Bill, B. D. , Gulland, F. , Thomson, R. E. , Cochlan, W. P. , & Trainer, V. L. (2016). An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophysical Research Letters, 43(19), 10,366–10,376. 10.1002/2016GL070023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie, C. H. , Bates, S. S. , Martin, J. L. , Haigh, N. , Howland, K. L. , Lewis, N. I. , Locke, A. , Peña, A. , Poulin, M. , Rochon, A. , Rourke, W. A. , Scarratt, M. G. , Starr, M. , & Wells, T. (2021). Three decades of Canadian marine harmful algal events: Phytoplankton and phycotoxins of concern to human and ecosystem health. Harmful Algae, 102, 101852. 10.1016/j.hal.2020.101852 [DOI] [PubMed] [Google Scholar]
- Moore, S. E. , & Stabeno, P. J. (2015). Synthesis of Arctic Research (SOAR) in marine ecosystems of the Pacific Arctic. Progress in Oceanography, 136, 1–11. [Google Scholar]
- Natsuike, M. , Nagai, S. , Matsuno, K. , Saito, R. , Tsukazaki, C. , Yamaguchi, A. , & Imai, I. (2013). Abundance and distribution of toxic Alexandrium tamarense resting cysts in the sediments of the Chukchi Sea and the eastern Bering Sea. Harmful Algae, 27, 52–59. 10.1016/j.hal.2013.04.006 [DOI] [Google Scholar]
- Nelson, M. , Quakenbush, L. , Taras, B. , & Ice Seal, C. (2019). Subsistence harvest of ringed, bearded, spotted, and ribbon seals in Alaska is sustainable. Endangered Species Research, 40, 1–16. 10.3354/esr00973 [DOI] [Google Scholar]
- Peery, M. Z. , Beissinger, S. R. , Burkett, E. , & Newman, S. H. (2006). Local survival of marbled murrelets in central California: Roles of oceanographic processes, sex, and radiotagging. Journal of Wildlife Management, 70(1), 78–88. 10.2193/0022-541x(2006)70[78:lsommi]2.0.co;2 [DOI] [Google Scholar]
- Perl, T. M. , Bédard, L. , Kosatsky, T. , Hockin, J. C. , Todd, E. C. D. , & Remis, R. S. (1990). An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. New England Journal of Medicine, 322(25), 1775–1780. 10.1056/NEJM199006213222504 [DOI] [PubMed] [Google Scholar]
- Persson, A. , Smith, B. C. , Wikfors, G. H. , & Quilliam, M. (2006). Grazing on toxic Alexandrium fundyense resting cysts and vegetative cells by the eastern oyster (Crassostrea virginica). Harmful Algae, 5(6), 678–684. 10.1016/j.hal.2006.02.004 [DOI] [Google Scholar]
- Pickart, R. S. , Schulze, L. M. , Moore, G. W. K. , Charette, M. A. , Arrigo, K. R. , van Dijken, G. , & Danielson, S. L. (2013). Long‐term trends of upwelling and impacts on primary productivity in the Alaskan Beaufort Sea. Deep‐Sea Research Part I: Oceanographic Research Papers, 79, 106–121. 10.1016/j.dsr.2013.05.003 [DOI] [Google Scholar]
- Radovich, J. (1952). Food of the Pacific sardine, Sardinops caerulea, from central Baja California and southern California. California Fish and Game Bulletin, 3(48), 575–585. [Google Scholar]
- R Core Team . (2018). R: A language and environment for statistical computing [Computer software]. R Foundation for Statistical Computing. [Google Scholar]
- Scholin, C. A. , Gulland, F. , Doucette, G. J. , Benson, S. , Busman, M. , Chavez, F. P. , Cordaro, J. , DeLong, R. , de Vogelaere, A. , Harvey, J. , Haulena, M. , Lefebvre, K. , Lipscomb, T. , Loscutoff, S. , Lowenstine, L. J. , Marin, R. , Miller, P. E. , McLellan, W. A. , Moeller, P. D. R. , … van Dolah, F. M. (2000). Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature, 403(6765), 80–84. 10.1038/47481 [DOI] [PubMed] [Google Scholar]
- Shumway, S. E. (1990). A review of the effects of algal blooms on shellfish and aquaculture. Journal of the World Aquaculture Society, 21(2), 65–104. 10.1111/j.1749-7345.1990.tb00529.x [DOI] [Google Scholar]
- Stevenson, D. E. , & Lauth, R. R. (2019). Bottom trawl surveys in the northern Bering Sea indicate recent shifts in the distribution of marine species. Polar Biology, 42(2), 407–421. 10.1007/s00300-018-2431-1 [DOI] [Google Scholar]
- Stone, R. S. , Dutton, E. G. , Harris, J. M. , & Longenecker, D. (2002). Earlier spring snowmelt in northern Alaska as an indicator of climate change. Journal of Geophysical Research: Atmospheres, 107(9–10), ACL 10–1. 10.1029/2000jd000286 [DOI] [Google Scholar]
- Todd, E. C. D. (1993). Domoic acid and amnesic shellfish poisoning ‐ A review. Journal of Food Protection, 56(1), 69–83. 10.4315/0362-028x-56.1.69 [DOI] [PubMed] [Google Scholar]
- Trainer, V. L. , Cochlan, W. P. , Erickson, A. , Bill, B. D. , Cox, F. H. , Borchert, J. A. , & Lefebvre, K. A. (2007). Recent domoic acid closures of shellfish harvest areas in Washington State inland waterways. Harmful Algae, 6(3), 449–459. 10.1016/j.hal.2006.12.001 [DOI] [Google Scholar]
- Tremblay, J.‐É. , & Gagnon, J. (2009). The effects of irradiance and nutrient supply on the productivity of Arctic waters: a perspective on climate change. In Nihoul J. C. J. & Kostianoy A. G. (Eds.), Influence of climate change on the changing Arctic and sub‐arctic conditions (pp. 73–93). Springer Netherlands. 10.1007/978-1-4020-9460-6_7 [DOI] [Google Scholar]
- Turner, J. , & Overland, J. (2009). Contrasting climate change in the two polar regions. Polar Research, 28(2), 146–164. 10.1111/j.1751-8369.2009.00128.x [DOI] [Google Scholar]
- U.S. Federal Register . 2012a. Threatened status for the Arctic, Okhotsk, and Baltic subspecies of the ringed seal and endangered status for the Ladoga subspecies of the ringed seal . FR 77(249), 76706–76738 (28 December 2012). National Oceanic and Atmospheric Administration, U.S. Department of Commerce. [Google Scholar]
- U.S. Federal Register . 2012b. Threatened status for the Beringia and Okhotsk distinct population segments of the Erignathus barbatus nauticus subspecies of the bearded seal . FR 77(249), 76740–76768 (28 December 2012). National Oceanic and Atmospheric Administration, U.S. Department of Commerce. [Google Scholar]
- Usup, G. , Kulis, D. M. , & Anderson, D. M. (1994). Growth and toxin production of the toxic dinoflagellate Pyrodinium bahamense var. compressum in laboratory cultures. Natural Toxins, 2(5), 254–262. 10.1002/nt.2620020503 [DOI] [PubMed] [Google Scholar]
- Wekell, J. C. , Hurst, J. , & Lefebvre, K. A. (2004). The origin of the regulatory limits for PSP and ASP toxins in shellfish. Article in Journal of Shellfish Research, 23(3), 927–930. [Google Scholar]
- White, A. W. (1980). Recurrence of kills of Atlantic herring (Clupea harengus harengus) caused by dinoflagellate toxins transferred through herbivorous zooplankton. Canadian Journal of Fisheries and Aquatic Sciences, 37(12), 2262–2265. 10.1139/f80-271 [DOI] [Google Scholar]
- White, A. W. (1981). Marine zooplankton can accumulate and retain dinoflagellate toxins and cause fish kills. Limnology and Oceanography, 26(1), 103–109. 10.4319/lo.1981.26.1.0103 [DOI] [Google Scholar]
- Woodgate, R. A. , Stafford, K. M. , & Prahl, F. G. (2015). A synthesis of year‐round interdisciplinary mooring measurements in the Bering Strait (1990–2014) and the RUSALCA years (2004–2011). Oceanography 28(3), 46–67. 10.5670/oceanog.2015.57 [DOI] [Google Scholar]
- Work, T. M. , Barr, B. , Beale, A. M. , Fritz, L. , Quilliam, M. A. , & Wright, J. L. C. (1993). Epidemiology of domoic acid poisoning in brown pelicans (Pelecanus occidentalis) and Brandt's cormorants (Phalacrocorax penicillatus) in California. Journal of Zoo and Wildlife Medicine, 24(1), 54–62. [Google Scholar]
- Zhu, Z. , Qu, P. , Fu, F. , Tennenbaum, N. , Tatters, A. O. , & Hutchins, D. A. (2017). Understanding the blob bloom: Warming increases toxicity and abundance of the harmful bloom diatom Pseudo‐nitzschia in California coastal waters. Harmful Algae, 67, 36–43. 10.1016/j.hal.2017.06.004 [DOI] [PubMed] [Google Scholar]


