ABSTRACT
Explanations of floral adaptation to diverse pollinator faunas have often invoked visitor‐mediated trade‐offs in which no intermediate, generalized floral phenotype is optimal for pollination success, i.e. fitness valleys are created. In such cases, plant species are expected to specialize on particular groups of flower visitors. Contrary to this expectation, it is commonly observed that flowers interact with various groups of visitors, while at the same time maintaining distinct phenotypes among ecotypes, subspecies, or congeners. This apparent paradox may be due to a gap in our understanding of how visitor‐mediated trade‐offs could affect floral adaptation. Here we provide a conceptual framework for analysing visitor‐mediated trade‐offs with the hope of stimulating empirical and theoretical studies to fill this gap. We propose two types of visitor‐mediated trade‐offs to address negative correlations among fitness contributions of different visitors: visitor‐mediated phenotypic trade‐offs (phenotypic trade‐offs) and visitor‐mediated opportunity trade‐offs (opportunity trade‐offs). Phenotypic trade‐offs occur when different groups of visitors impose conflicting selection pressures on a floral trait. By contrast, opportunity trade‐offs emerge only when some visitors’ actions (e.g. pollen collection) remove opportunities for fitness contribution by more beneficial visitors. Previous studies have observed disruptive selection due to phenotypic trade‐offs less often than expected. In addition to existing explanations, we propose that some flowers have achieved ‘adaptive generalization’ by evolving features to avoid or eliminate the fitness valleys that phenotypic trade‐offs tend to produce. The literature suggests a variety of pathways to such ‘trade‐off mitigation’. Trade‐off mitigation may also evolve as an adaptation to opportunity trade‐offs. We argue that active exclusion, or floral specialization, can be viewed as a trade‐off mitigation, occurring only when flowers cannot otherwise avoid strong opportunity trade‐offs. These considerations suggest that an evolutionary strategy for trade‐off mitigation is achieved often by acquiring novel combinations of traits. Thus, phenotypic diversification of flowers through convergent evolution of certain trait combinations may have been enhanced not only through adaptive specialization for particular visitors, but also through adaptive generalization for particular visitor communities. Explorations of how visitor‐mediated trade‐offs explain the recurrent patterns of floral phenotypes may help reconcile the long‐lasting controversy on the validity of pollination syndromes.
Keywords: adaptive generalization, adaptive specialization, floral evolution, opportunity trade‐offs, phenotypic trade‐offs, pollination syndrome, trade‐off mitigation
I. INTRODUCTION
The diversity of floral phenotypes and their correlated evolution with flower visitors has been one of the most enduring topics in floral biology (Faegri & van der Pijl, 1979; Fenster et al., 2004; McCall & Irwin, 2006; Armbruster, 2014, 2017). The central paradigm is that flower visitors – both mutualistic and antagonistic – differ in sensory systems, morphology, physiology, or behaviour, and therefore exert conflicting selection pressures on floral traits (Lange & Scott, 1999; Kessler et al., 2015). Such relationships among the functional adaptation of a floral trait (or suite of covarying traits) to different interaction partners, hereafter, visitor‐mediated trade‐offs, have been considered to promote specialization towards the most beneficial interactions and, in turn, diversification of floral phenotypes (Stebbins, 1970; Schemske & Horvitz, 1984; Johnson & Steiner, 2000; Armbruster, 2014).
If the above argument is correct, then each flower species would be expected to cater mostly to one primary pollinator group at the evolutionary equilibrium, while discouraging its major antagonists. However, contrary to this expectation, it is quite common that flowers harbour multiple groups of visitors at the same time (Herrera, 1996; Ollerton, 1996; Waser et al., 1996; Gómez & Zamora, 2006; McCall & Irwin, 2006; Kessler, Diezel & Baldwin, 2010). Such diffuse interactions appear to contradict the view that visitor‐mediated trade‐offs limit the potential of flowers to adapt simultaneously to multiple groups of visitors.
Does this mean that most flowers have failed to adapt to the biotic environment and therefore suffer from fitness losses due to visitor‐mediated trade‐offs? If so, then how has the enormous floral diversity with distinct phenotypes evolved, and how is it maintained? Under which conditions are floral adaptations to specific visitors to be expected? Such questions have been repeatedly raised during recent decades, with little agreement, integration, or reconciliation. Some relevant propositions include: (i) that floral traits adapt to the most effective pollinators even in situations with seemingly diffuse interactions (Stebbins, 1970; Rosas‐Guerrero et al., 2014; Ashworth et al., 2015; Johnson & Wester, 2017); (ii) that the most effective pollinators often disagree with the visitors inferred from suites of floral traits (Fishbein & Venable, 1996; Ollerton et al., 2009; Guzmán, Gómez & Vargas, 2017); (iii) that diffuse interactions could favour distinct floral traits that discourage specific pollinators, because the presence of highly efficient pollinators turns the other less‐efficient pollinators into ‘conditional parasites’ (Thomson & Thomson, 1992; Thomson et al., 2000); and (iv) that it is questionable how frequently floral adaptations to specific pollinators occur in reality, because the most effective pollinators are often hard to identify and variable in space and time (Herrera, 1988, 1996; Price et al., 2005).
As has been suggested by Aigner (2001, 2006), the discrepancy between floral appearance and observed flower visitors may not be contradictory if visitor‐mediated trade‐offs are absent. For example, a particular phenotype may sometimes optimize contributions from different visitors at the same time (Gómez & Zamora, 2006). However, we know very little about how often and why this would happen in nature (Armbruster, 2014, 2017). Moreover, we have no explanation as to how phenotypic diversity of flowers through convergent evolution of certain trait combinations could be generated and be maintained in the absence of constraints imposed by visitor‐mediated trade‐offs. Apparently, we need a more thorough consideration of visitor‐mediated trade‐offs and their relation to floral evolution.
Our goal here is to provide a conceptual framework for analysing floral visitor‐mediated trade‐offs and their consequences for floral adaptation. We start our review with a new classification of visitor‐mediated trade‐offs based on how fitness conflicts emerge. We then present graphical models to identify possible evolutionary outcomes of visitor‐mediated trade‐offs, while developing a literature overview to validate our proposal. Based on these considerations, we suggest that a better understanding of visitor‐mediated trade‐offs will help us explain the diversity of floral phenotypes as evolutionary responses to varying assemblages of flower visitors.
Throughout this paper, we use the term ‘visitor‐mediated trade‐offs’, to include all types of flower visitors that can potentially incur fitness trade‐offs with one another, e.g. pollinators, nectar robbers, pollen thieves, florivores, seed predators, predators of other visitors. We divide these visitors into two categories, i.e. mutualists and antagonists, depending on whether their net effect on plant fitness is positive or negative (Bronstein, 1994). In this view, flower visitors can be mutualists only when they provide greater benefits than costs. For example, most nectar robbers are antagonists even if they provide some pollination services (Maloof & Inouye, 2000). Furthermore, the same pollinators could function as mutualists in some ecological circumstances but as antagonists in the presence of better pollinators (Thomson, 2003). We adopt this inclusive term ‘visitor’ to review traditional studies that have only focused on groups of mutualists [pollinator‐mediated fitness trade‐offs (Horvitz & Schemske, 1990; Aigner, 2001, 2004; Gómez & Zamora, 2006; Muchhala, 2007; Smith, Ané & Baum, 2008; Gómez & Perfectti, 2010)] or on contrasts between mutualists and antagonists [defence–attraction trade‐offs (Herrera et al., 2002; Irwin, Adler & Brody, 2004; Kessler et al., 2015; Sletvold, Moritz & Ågren, 2015)], and to take all the relevant interactions into consideration of trade‐offs in flowers. Accordingly, we use the term ‘fitness contribution’ as the net effect of a particular visitor group on the fitness of a plant. This could be rephrased as ‘pollination effectiveness’ in the context of mutualistic interactions, or ‘damage caused’ in the context of antagonistic interactions.
II. CATEGORIZING VISITOR‐MEDIATED TRADE‐OFFS IN FLOWERS
The concept of trade‐offs in biology refers to situations in which a trait, function, or realized fitness component, etc., cannot increase without a decrease in another, and vice versa (Garland, 2014). Trade‐offs have played a pivotal role in evolutionary studies for various reasons, most of which are directly related to the factors that limit the adaptive potential of organisms (Futuyma & Moreno, 1988; Robinson, Wilson & Shea, 1996; Roff & Fairbairn, 2007; Agrawal, Conner & Rasmann, 2010).
We recognize two types of potential trade‐offs in flowers caused by multiple groups of visitors: (i) visitor‐mediated phenotypic trade‐offs (hereafter, we use phenotypic trade‐offs in italics as an abbreviation for ‘visitor‐mediated phenotypic trade‐offs’) and (ii) visitor‐mediated opportunity trade‐offs (hereafter, we use opportunity trade‐offs in italics as an abbreviation for ‘visitor‐mediated opportunity trade‐offs’). A phenotypic trade‐off in flowers occurs when different groups of visitors impose conflicting selection pressures on a single aspect of a flower (Fig. 1A–C). It will arise when a phenotypic adaptation to a certain type of visitor reduces visits and/or pollination by other mutualistic visitors, or increases visits and/or damages by other antagonistic visitors. Examples that fall into this category would include conflicting requirements for corolla width to be effectively pollinated by bats and hummingbirds (Muchhala, 2007), or conflicts between floral fragrance to attract pollinators and deter florivores (Theis & Adler, 2012). Conflicting requirements could also be placed on a trait by multiple antagonists, just as those for gall size to defend against different kinds of predators in gall‐making insects (Weis, Abrahamson & Andersen, 1992), although we are not aware of floral examples.
Fig 1.
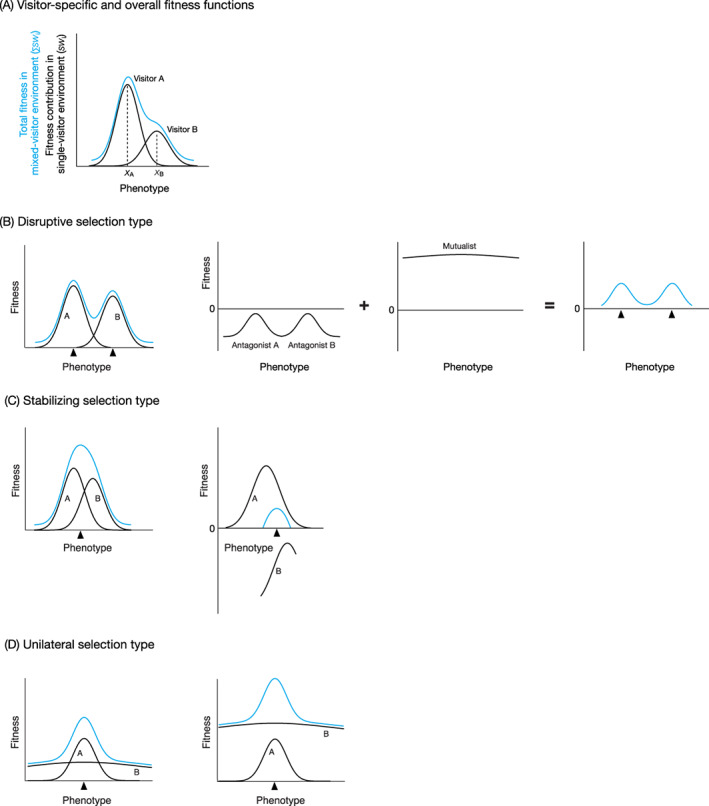
Graphical representations of visitor‐mediated phenotypic trade‐offs between visitor groups A and B and their possible evolutionary consequences. (A) Fitness function graph showing the relationships between floral phenotype (trait value or status) and the visitor‐specific fitness contributions (black curves) as well as the total fitness (blue curve). We refer to the former as visitor‐specific fitness functions, and the latter as overall fitness function. (B) Disruptive selection type which produces a distinct phenotype as a singular adaptation to either A or B. Filled triangles indicate optimal floral phenotypes. (C) Stabilizing selection type which produces an intermediate phenotype as a compromise between adaptation to A and B. (D) Unilateral selection type which produces a distinct phenotype as a dual adaptation to both A and B.
By contrast, an opportunity trade‐off occurs when different visitor groups with different profitability for flowers share the limited opportunity for affecting plant fitness and exhibit negative correlations in their contributions. This concept was highlighted by Thomson & Thomson (1992), who demonstrated how pollen wastage during transport by certain types of pollinators could make them detrimental to plants in the presence of better pollen deliverers. We illustrate a hypothetical example in Fig. 2A. Pollinator A and B bring the greatest fitness contribution at flower tubes of the same length, while pollinator B is lower in their quality in terms of pollen transfer efficiency (Fig. 2A). This could happen in systems where the likelihood of contacting anthers and stigmas varies among different groups of pollinators (Castellanos, Wilson & Thomson, 2004; Sakamoto et al., 2012). In this condition, the plant may face a dilemma in harbouring pollinator A and B; pollinator B becomes antagonistic in the presence of pollinator A, in the sense that B's net effect on plant fitness is negative (Fig. 2B) (Thomson, 2003). This occurs because the opportunity for more efficient pollen transfer by A is lost due to visits by pollinator B. In other words, pollinator B is inferior to pollinator A in terms of opportunity use efficiency and wastes pollen that, if left behind, would have been delivered by pollinator A.
Fig 2.
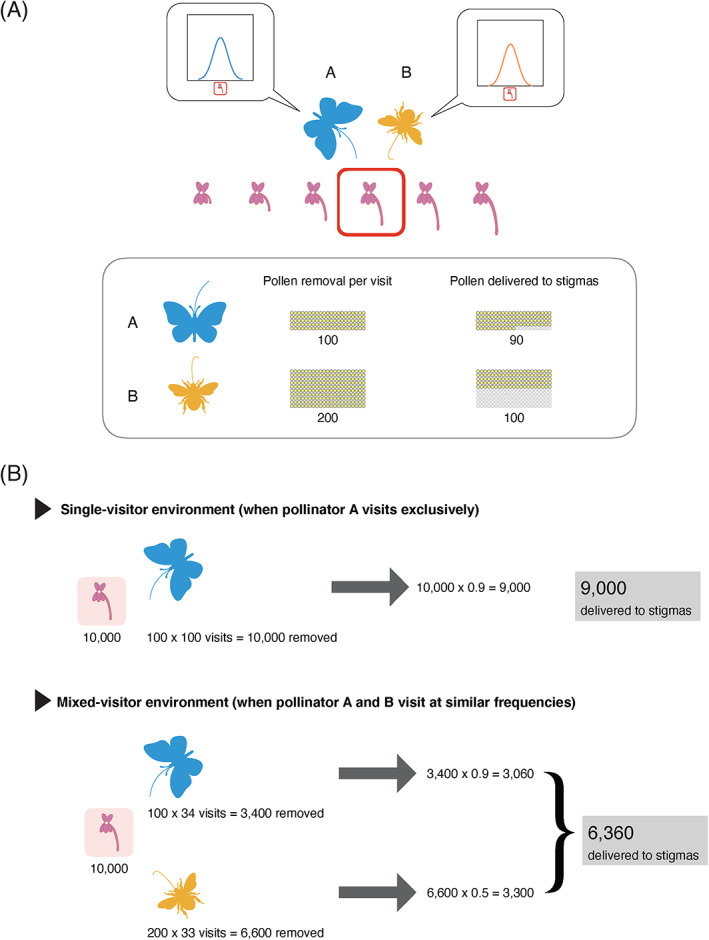
Examples showing how differences in opportunity use efficiency between different pollinator groups result in fitness losses through opportunity trade‐offs. (A) Pollinators A and B confer the same fitness peak for floral tube length. But they differ in pollen transfer efficiency, i.e. how much pollen they remove from anthers, and how much of the removed pollen they deliver to stigmas. (B) The total fitness becomes lower in a mixed‐visitor environment (bottom panel) than in a single‐visitor environment (top panel) because the opportunity for more efficient pollen transfer by A is lost due to visits by B.
Similar opportunity losses will occur whenever distinct groups of visitors compete over the limited number of pollen grains, ovules or stigmatic surface, and also vary in the degree of pollen loss during dispersal. Various processes contribute: grooming, collecting, or passive loss of pollen (Wilson & Thomson, 1991; Miyake & Yahara, 1998; Castellanos, Wilson & Thomson, 2003; Muchhala & Thomson, 2010); spatial precision in pollen placement and pickup (Armbruster, 2006; Culbert & Forrest, 2016; Funamoto, 2019); geitonogamy or biparental inbreeding caused by area‐restricted movement (Waser, 1982; Matsuki et al., 2008; Ohashi & Thomson, 2009; Hasegawa, Suyama & Seiwa, 2015); or heterospecific pollen transfer (Funamoto, 2019). An opportunity trade‐off may also occur when nectar consumption by one pollinator group leads to a reduction in visits by the other pollinator group (Carpenter, 1979; Laverty & Plowright, 1985), although such conditions will be rather limited. In addition to such antagonistic pollinators, obligate antagonists such as pre‐dispersal seed predators could also cause opportunity trade‐offs by depriving mutualists of their contribution opportunities (Irwin & Brody, 2011). Unlike phenotypic trade‐offs, on the other hand, opportunity trade‐offs cannot occur between antagonists. Because no antagonist benefits plants (by definition), attacks by multiple groups of antagonists will pose no dilemma for plants in terms of opportunity use.
This type of conflict has not been frequently described as a ‘trade‐off’ in the literature, because the opportunity loss largely reflects the influence of extrinsic factors (e.g. the abundance of different animal groups) and seems more like an ecological conflict rather than an evolutionary one. It is, however, important to regard a conflict over opportunity use as a distinct type of trade‐off, because it creates negative correlations among realized fitness contributions from different visitor groups, similarly to phenotypic trade‐offs causing negative correlations among functional adaptations to – or fundamental fitness contributions from – different visitor groups. And as we will see, opportunity losses to the other visitor groups could cause evolutionary changes in traits that are unique and different from any evolutionary consequences of phenotypic trade‐offs. To reach a comprehensive view of visitor‐mediated trade‐offs and their effects on phenotypic evolution of flowers, it is critical to extend the concept to encompass this component of negative correlations in fitness in addition to phenotypic trade‐offs. We thus regard opportunity trade‐offs as a particular type of visitor‐mediated trade‐off, rather than assigning an unrelated term such as ‘negative interactions’ (Aigner, 2001).
It is important to note that a single floral trait can be subject to either or both types of visitor‐mediated trade‐offs involving a particular pair of visitor groups. For example, Fig. 2 illustrates a situation where only an opportunity trade‐off is caused by two groups of nectar foragers, but in other situations where these foragers differ in tongue lengths, a phenotypic trade‐off on floral tube length may additionally be imposed. In another situation where these foragers are constantly scarce, only a phenotypic trade‐off may be observed. We suggest that one should not overlook the possibility that different types of visitor‐mediated trade‐offs may be involved behind the observed negative correlation in visitor‐specific fitness contributions.
III. THE EVOLUTIONARY CONSEQUENCES OF VISITOR‐MEDIATED PHENOTYPIC TRADE‐OFFS
(1). Earlier views on floral evolution under phenotypic trade‐offs
Conflicts among functional adaptations to different flower visitors have long been one of the main explanations for divergent floral phenotypes among ecotypes, subspecies, or congeners (Grant & Grant, 1965; Stebbins, 1970; Johnson & Steiner, 2000; van der Niet et al., 2014). For example, evolutionary transitions in flower colour are often attributed to selection exerted by different groups of pollinators with distinct colour preferences (Faegri & van der Pijl, 1979; Fenster et al., 2004; Whittall & Hodges, 2007; but see Muchhala, Johnsen & Smith, 2014). The same may be true for floral scents associated with different pollinator preferences (Pellmyr, 1986; Gross, Sun & Schiestl, 2016) or shared preferences between pollinators and herbivores (Schiestl & Johnson, 2013; Kessler et al., 2015). Divergences in floral morphology such as tube length (Anderson et al., 2014; Boberg et al., 2014; Newman, Manning & Anderson, 2014), corolla width (Inoue & Amano, 1986; Muchhala, 2007) and corolla flare (Galen, Zimmer & Newport, 1987; Galen & Cuba, 2001; Aigner, 2004) have also been considered as consequences of phenotypic trade‐offs caused by different visitor groups.
These examples are commonly based on the notion that a phenotypic trade‐off would exert disruptive selection pressure on a floral trait. However, disruptive selection may not be the only possible outcome of a phenotypic trade‐off (Strauss & Whittall, 2006; Sletvold, 2019). For a more comprehensive approach, phenotypic selection models should be used (Lande & Arnold, 1983). Aigner (2001, 2006) employed optimality modelling to predict the evolutionary consequences of pollinator‐mediated trade‐offs for a floral phenotype, assuming that the total fitness of a plant equals the sum of gains from different pollinator groups with which it interacts during its lifetime (i.e. in a fine‐grained pollination environment; see also Levins, 1968). Here, we follow this approach. Although Aigner (2001, 2006) used examples of phenotypic trade‐offs over quantitative traits between two pollinators, such as those over corolla length entailed by long‐ and short‐tongued pollinators, similar arguments could also apply to qualitative or signal traits such as colour and odour, and also to situations where flower visitors include both mutualists and antagonists (Strauss & Whittall, 2006).
Consider a plant population expressing a range of phenotypes with respect to some trait, x. For simplicity, let us assume that the trait x has high heritability, and that all values of the trait within a phenotypic range are genetically possible and equally easy to achieve. The fitness contribution (positive or negative) from each group of flower visitors can be expressed as a function over the range of the considered phenotypes. Assuming that the fitness contribution from any type of flower visitors is related to the lifetime reproductive success of the plant, hereafter we call this relationship between fitness contribution and the trait ‘visitor‐specific fitness function’. By contrast, we call the relationship between total fitness and the trait – the sum of individual visitor‐specific fitness functions – ‘overall fitness function’ (Fig. 1A).
When the ith group of animals are visiting the plant population alone, the relationship between their fitness contribution and the trait can be expressed as the visitor‐specific fitness function in single‐visitor environment, sw i(x). The visitor‐specific fitness function sw i(x) may also depend on the frequency of the focal phenotype relative to other phenotypes in a population (Smithson & Macnair, 1996; Gigord, Macnair & Smithson, 2001), but here we do not consider such effects. In a mixed‐visitor environment with two visitor groups A and B, the phenotype producing the maximum fitness contribution may differ between the two groups. Within such a discrepancy region of optimal phenotypes, a phenotypic trade‐off between A and B can be defined as a negative correlation between the fitness contributions of A and B across the trait. This can be expressed as:
| (1) |
where x A and x B are the optimal phenotypes that maximize the fitness contributions of A and B, respectively.
Phenotypic trade‐offs could occur among various types of visitors, whether these animals are mutualistic (sw i > 0) or antagonistic (sw i < 0) for the plants. For example, phenotypic trade‐offs occur between mutualists and antagonists within the range of effective pollination, i.e. ∑sw i(x) >0 (Fig. 1C, right). In the presence of effective pollination over a wide range of phenotypes with respect to the trait x, phenotypic trade‐offs could occur even among different groups of antagonists (Fig. 1B, right).
The evolutionary consequences of a phenotypic trade‐off will depend on the difference between phenotypic adaptations to the groups A and B, which is determined by the proximity between x A and x B, the concave and convex shape of sw i(x), and the relative abundance of A and B. First, when adaptations to A and B would result in very different flower phenotypes (Fig. 1B), there would be no intermediate optimum between phenotypes x A and x B, and a fitness valley occurs in the overall fitness function, ∑sw i(x). This is the condition where disruptive selection will operate on a floral trait. A few empirical studies have suggested that such disruptive selection may have produced morphological diversification between congeneric species specialized for distinct pollinator groups (Muchhala, 2007; Miller, Raguso & Kay, 2014).
Second, when adaptations to A and B are expected to yield different but similar optimal phenotypes (Fig. 1C), there will be no valley in the overall fitness function, and an intermediate phenotype between x A and x B gives a broad peak in total fitness. This is the condition where stabilizing selection will operate. This scenario may apply in cases where different pollinator groups provide more or less equally good pollination for a given plant species (Herrera, 1988, 1996; Gómez & Zamora, 1999; Suzuki, Dohzono & Hiei, 2007; Fig. 1C, left) or in cases where fitness contributions of mutualists and antagonists give similar peaks with respect to a floral trait (Galen & Cuba, 2001; Schiestl et al., 2014; Kessler et al., 2015; Fig. 1C, right). The peak of the overall fitness function may become even broader in reality, because these visitor‐specific fitness functions often vary depending on the extrinsic context (reviewed by Sletvold, 2019).
In addition to the above two well‐established evolutionary scenarios, Aigner (2001, 2006) identified a third potential condition in multispecies interactions: the fitness contribution varies with the focal floral trait only for a particular visitor group, while not for the others, and no phenotypic trade‐off is imposed on a floral trait (Fig. 1D). In the absence of phenotypic trade‐offs, the shape of the overall fitness function, ∑sw i(x), is largely determined by the visitor group whose fitness contribution varies significantly with the phenotype (group A in Fig. 1D). Hence, a plant can optimize the floral trait by tuning its value or state towards such visitors, x A. We refer to this as ‘unilateral selection’, in order to distinguish it from the above stabilizing selection scenario where the optimum is between x A and x B. Note that the visitor groups whose fitness contribution varies little over the range of feasible phenotypes of the trait – ‘relaxed’ visitors – would have little or no impact on the evolution of the trait, regardless of whether or not they make a significant contribution to total fitness (group B in Fig. 1D). For example, honeybees and bumble bees can learn to associate a wide range of colour stimuli with rewards (Gould & Gould, 1988; Gumbert, 2000), potentially making them relaxed visitors with respect to floral coloration, in contrast to the dronefly, Eristalis tenax L., which exhibits a persistent preference for yellow (An et al., 2018). When these relaxed visitors are mutualistic, their inclusion into the visitor community will always provide marginal benefits to plant fitness without sacrificing adaptation to other visitors, unless they significantly reduce the contribution opportunities of more efficient pollinators and thereby become antagonists (opportunity trade‐off; see Section IV).
At least three examples of unilateral selection on floral traits have been reported in the literature. First, stigma exsertion of wild radish, Raphanus raphanistrum L., hardly affected pollen deposition by honeybees, while an intermediate exsertion received maximum deposition by butterflies (Conner, Davis & Rush, 1995). Second, flexible pedicels of orange jewelweed, Impatiens capensis Meerb., had little effect on the pollination service of the primary visitor, e.g. apid bees, while increasing pollen transfer by infrequent visitors, e.g. hummingbirds, via increased movement of flowers (Hurlbert et al., 1996). Third, corolla width of Dudleya greenei Rose had little effect on pollination by bumble bees, while narrow flowers performed best in terms of both visitation and pollination effectiveness by hummingbirds (Aigner, 2004).
(2). Extending earlier views
Research over recent decades has demonstrated that multispecies interactions can produce not only disruptive selection but also stabilizing and unilateral selection in flowers (Herrera, 1988, 1996; Conner et al., 1995; Hurlbert et al., 1996; Gómez & Zamora, 1999; Aigner, 2004; Castellanos et al., 2004; Suzuki et al., 2007; Sletvold, 2019). On the other hand, these insights leave open the question of why we have so little empirical evidence that phenotypic trade‐offs exert disruptive selection on floral traits. Even though the data are still too few to draw any conclusion (Fenster et al., 2004; Aigner, 2006; Gómez & Zamora, 2006; Armbruster, 2014, 2017), a phenotypic trade‐off with a fitness valley has been demonstrated so far only between flowers highly specialized for pollination by divergent groups of animals (Muchhala, 2007; Miller et al., 2014). This may simply mean that adaptive flowers harbour different groups of visitors only when their traits are not under disruptive selection, or these flowers represent non‐adaptive generalization in non‐equilibrium conditions (Gómez & Zamora, 2006). Alternatively, selection imposed by certain visitor groups may vary across extrinsic contexts such as surrounding communities and resource availability (Sletvold, 2019), which may preclude consistent disruptive selection. At the same time, the paucity of reports on visitor‐mediated disruptive selection on floral traits still seems counterintuitive, given the great variation among different groups of flower visitors in sensory systems, morphology, physiology and behaviour (Proctor, Yeo & Lack, 1996; Willmer, 2011).
To probe more deeply, we need to consider one more possibility. Examples of disruptive selection due to phenotypic trade‐offs may be rare because flowers have adaptively avoided being trapped in fitness valleys, even in multispecies interactions that we would expect to produce valleys. Generalization in flowers has often been attributed to compromises that sacrifice pollination effectiveness in return for reproductive assurance, or to ecological forces that constrain adaptive specialization (Waser et al., 1996; Johnson & Steiner, 2000; Gómez & Zamora, 2006). This may not always be the case, however. Flowers may have evolved to reduce disruptive selection due to phenotypic trade‐offs and exploit a variety of visitors. Here we draw attention to this possibility by proposing the term ‘adaptive generalization’ to describe any situation where flowers are visited by diverse groups of animals and reproduce successfully, without suffering fitness valley due to phenotypic trade‐offs. Some authors have used the term ‘dual specialization’ or ‘specialized bimodal pollination’ to describe flowers that have simultaneously adapted to multiple groups of pollinators (Liu et al., 2002; Dellinger et al., 2019b ; Hargreaves, Langston & Johnson, 2019). Although we understand that such terminology would help to avoid the non‐adaptive connotations of the term generalization, we prefer ‘adaptive generalization’ because it directly invokes a fundamental question: if trade‐offs are the rule, how can an organism adaptively generalize for divergent animals? To facilitate further discussion, we also propose the term ‘trade‐off mitigation’ to refer to any evolutionary changes in flowers that reduce the fitness costs derived from visitor‐mediated trade‐offs. It seems possible that such evolution has shaped ecologically generalized systems where plants need visits from diverse groups of pollinators to ensure reproduction, or where plants cannot avoid visitor groups that potentially impose disruptive selection. We suggest two types of trade‐off mitigation that a plant could employ (Fig. 3). One is the adoption of multiple phenotypes in time or space to gain benefits from multiple fitness peaks (multi‐phenotypic strategies), and the other is a change of scenario from disruptive to stabilizing or unilateral selection, through combinations of multiple traits that modify the visitor‐specific fitness functions (combinational strategies). Possible examples of these strategies will be reviewed below and are summarized in Table 1.
Fig 3.
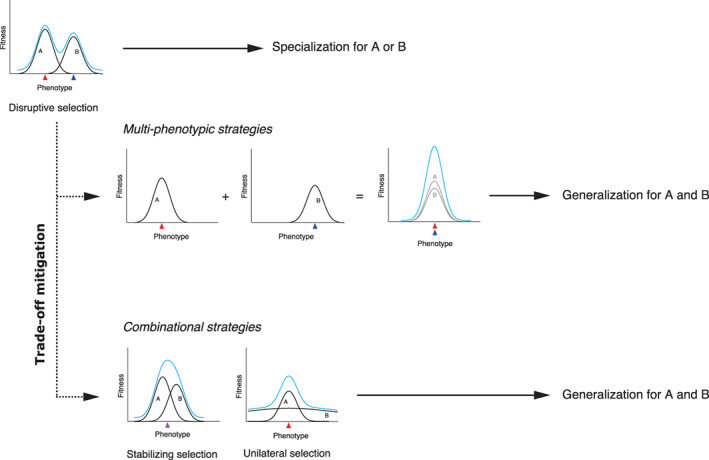
Graphical definition of trade‐off mitigation under disruptive selection due to phenotypic trade‐offs. Trade‐off mitigation refers to any evolutionary changes in flowers that allow them to harbour multiple visitor groups without being dragged down to fitness valleys due to phenotypic trade‐offs, as represented by the dashed arrows. The resultant floral generalization would be more advantageous than the original situation in the top left corner, as long as it provides an optimum in total fitness higher than that obtained by specialization for either of the visitor groups. Two types of mitigation strategies against a phenotypic trade‐off are possible: (1) multi‐phenotypic strategies, where plants avoid a fitness valley by producing distinct phenotypes either in time (temporal switch) or space (intra‐individual polymorphism) and (2) combinational strategies, where the visitor‐specific fitness functions over a trait are modified through the evolution of another trait (modifier), which changes the visitor‐mediated disruptive selection into stabilizing (optima convergence) or unilateral (peak blunting).
Table 1.
A summary of proposed floral strategies to mitigate visitor‐mediated trade‐offs with empirical examples in the literature providing supportive or suggestive evidence
| Floral strategy to mitigate visitor‐mediated trade‐offs | Description | Specific example | References | ||
|---|---|---|---|---|---|
| (A) Mitigation of visitor‐mediated phenotypic trade‐offs | |||||
| Multi‐phenotypic strategies | |||||
| Temporal switch | |||||
| Individual flowers change phenotypes of a trait either periodically or ontogenetically, to avoid fitness valleys produced by phenotypic trade‐offs | Change in scent profile between day and night (Fig. 4A) | Jürgens et al. (2014); Chapurlat et al. (2018) | |||
| Change in scent emission rate between day and night | Morinaga et al. (2009); Chapurlat et al. (2018) | ||||
| Change in nectar productivity between day and night | Stephenson & Thomas (1977); Liu et al. (2002) | ||||
| Change in exposed petal colour between high‐ and low‐temperature conditions | Kemp & Ellis (2019) | ||||
| Ontogenetic change in nectar composition | Amorim et al. (2013) | ||||
| Ontogenetic change in flower angle | Eisikowitch & Rotem (1987) | ||||
| Ontogenetic change in flower tube length | Macior (1986); Dohzono & Suzuki (2002) | ||||
| Intra‐individual polymorphism | |||||
| One plant individual simultaneously produces multiple phenotypes or morphs of a trait in a number of modules, to avoid fitness valleys produced by phenotypic trade‐offs | Within‐anther pollen size dimorphism (Fig. 4B) | Jürgens et al. (2012); Wang et al. (2017) | |||
| Combinational strategies | |||||
| Peak blunting | |||||
| Flowers relax a close link between a trait and fitness contribution of underused visitor groups by changing modifier traits and reducing phenotypic trade‐offs, which increases total fitness they can obtain from multiple groups of visitors | Floral colour change in old, rewardless flowers (Fig. 4C) | Suzuki & Ohashi (2014); Makino & Ohashi (2017) | |||
| Narrow entrance of short‐tubed flowers | Hargreaves et al. (2012, 2019) | ||||
| Optima convergence | |||||
| Flowers make the peaks of fitness functions for different visitor groups closer to one another by changing modifier traits and eliminate fitness valleys due to phenotypic trade‐offs, which increases total fitness they can obtain from multiple groups of visitors | Only hypothetical at present; shallow flowers in compact inflorescence may fall into this category because they can be successfully pollinated by both short‐tongued and long‐tongued insects (Fig. 4D). | — | |||
| (B) Mitigation of visitor‐mediated opportunity trade‐offs | |||||
| Active exclusion | |||||
| Flowers actively exclude antagonists or inefficient pollinators to avoid net fitness loss due to strong opportunity trade‐offs | Chemical, morphological, or phenological defence against florivores, nectar robbers, or pathogens | Irwin et al. (2004) | |||
| Cryptic coloration (Fig. 4E) | Shuttleworth & Johnson (2009); Lunau et al. (2011); Shuttleworth & Johnson (2012); Bergamo et al. (2016); Gegear et al. (2017); Camargo et al. (2019) | ||||
| Pollinator‐specific odour attractants | Shuttleworth & Johnson (2009) | ||||
| Temporary closure | Martén‐Rodríguez et al. (2009) | ||||
| Downward‐facing aperture (Fig. 4E) | Castellanos et al. (2004); Gegear et al. (2017) | ||||
| Unpalatable nectar | Johnson et al. (2006); Shuttleworth & Johnson (2009) | ||||
| Dilute nectar (Fig. 4E) | Gegear et al. (2017) | ||||
| Low nectar accumulation | Fleming et al. (2009) | ||||
| Schedule optimization | |||||
| Flowers temporally postpone visits by inefficient pollinator groups until they are first visited by efficient pollinator groups, increasing total fitness they can obtain from multiple groups of visitors | Timing of anthesis (Fig. 4F) | Miyake & Yahara (1998, 1999); Muchhala (2003); Prieto‐Benítez et al. (2016); Funamoto & Ohashi (2017); Funamoto (2019) | |||
(3). Multi‐phenotypic strategies
Phenotypic trade‐offs occur when a plant cannot adopt different phenotypes at the same time and there is no intermediate optimum. However, Lloyd (1984) pointed out that some plants avoid this dilemma by producing distinct structures, either conditionally (conditional strategy) or stochastically (mixed strategy). For example, dichogamy (temporal shift from male to female phase in a flower, or vice versa) can be viewed as a conditional strategy, while monoecy (proportional production of distinct floral sex morphs by the same individual) as a mixed strategy. Such strategies could enable a plant to avoid fitness valleys among different functional adaptations and gain benefits from multiple fitness peaks. Similarly, we suggest that plant individuals could avoid fitness valleys due to phenotypic trade‐offs by producing multiple floral phenotypes, either temporally (temporal switch) or spatially (intra‐individual polymorphism).
Temporal switching refers to a conditional strategy where a plant changes the phenotype of a trait within each flower either periodically or ontogenetically to avoid fitness valleys among adaptations to different flower visitor groups. This has been reported for floral scent profiles of goat willow, Salix caprea L., that change between day and night corresponding to olfactory preferences of diurnal bees and nocturnal moths (Jürgens et al., 2014; Fig. 4A). Similar changes have been reported for the orchid Gymnadenia conopsea (L.) R.Br., although their correspondence to diurnal and nocturnal pollinators remains speculative (Chapurlat et al., 2018). Temporal switches between day and night have also been reported for scent emission rates (Morinaga et al., 2009; Chapurlat et al., 2018) and nectar production rates (Stephenson & Thomas, 1977; Liu et al., 2002). However, possible effects of the abiotic environment have not been entirely ruled out in any of these cases. In other cases, temporal switches help flowers avoid fitness valleys due to phenotypic trade‐offs between pollinators and florivores. For example, South African daisies reduce florivory by closing flowers and exposing the cryptic‐coloured undersides of petals when pollinators are inactive at low temperatures. In accordance with this relaxation of phenotypic trade‐offs, the upper surface of petals in flower‐closing species exhibits more conspicuous colour than in non‐closing ones (Kemp & Ellis, 2019). Temporal switches may also occur in morphological traits. For example, changes in flower angle between day (downward) and night (upward) in wild tobacco, Nicotiana attenuata Steud., have been considered to protect pollen from direct sunlight and heat during the day, while attracting hawkmoth pollinators during the night (Haverkamp et al., 2019). Although this particular trade‐off is not visitor mediated, the study illustrates how temporal changes in flower angle, or any other morphological trait, could potentially avoid fitness valleys due to phenotypic trade‐offs.
Fig 4.

Examples of floral strategies for mitigating visitor‐mediated trade‐offs. (A) Temporal switch: flowers of goat willow (Salix caprea) switch scent profiles between day and night to attract diurnal bees (left; Andrena fulva Schrk) and nocturnal moths (right; Orthosia incerta Hufnagl). (B) Intra‐individual polymorphism: flowers of a monkshood (left, Aconitum gymnandrum; red arrow indicates lower sepal, yellow arrow indicates lateral sepal) producing two distinct pollen phenotypes (right). Large (round) and small (fusiform) grains suit bee and wind pollination, respectively. (C) Peak blunting: two Weigela congeners retain old, rewardless flowers to attract opportunistic pollinators such as flies or inexperienced bees. W. coraeensis (right) changes the colour of old flowers from white to red, by which it can include experienced bees in its pollinator assemblage. By contrast, W. hortensis (left) retaining old, rewardless flowers in the same colour cannot make use of experienced bees that can quickly learn to avoid such deceptive displays. (D) Optima convergence: spatially separated, shallow flowers of Sicyos angulatus L. (left) cannot make use of long‐tongued rice‐plant skippers (Parnara guttata Bremer & Grey) as effective pollinators. In combination with the compact inflorescence, shallow flowers of Allium tuberosum Rottler ex Spreng. (right) successfully deposit pollen grains on the ventral side of foraging skipper's body; thus the plants can converge optimal corolla depths for short‐ and long‐tongued insects. (E) Active exclusion: purple, laterally facing flowers of Penstemon strictus Benth. (left) primarily attract bumble bees (here, Bombus flavifrons Cresson). UV‐absorbing red, downward‐facing flowers of P. cardinalis Wooton & Standl. (right) only attract hummingbirds (here, Selasphorus rufus Gmelin) while keeping bumble bees away. (F) Schedule optimization: flowers of Lonicera japonica are pollinated by both diurnal bees (left; Tetralonia nipponensis Pérez) and nocturnal hawkmoths (right; Theretra japonica de L'Orza), but invariably open at dusk, postponing visits by pollen‐wasteful bees. Photographs A, C and D by Kazuharu Ohashi, E by James Thomson, F by Takashi Miyake and B from Wang et al. (2017) licensed under the Creative Commons Attribution International License (CC BY).
In other cases, temporal switches that occur ontogenetically can mitigate fitness valleys through temporal partitioning of flower use among different visitor groups. For example, age‐related changes in floral nectar composition (Amorim, Galetto & Sazima, 2013), flower angle (Eisikowitch & Rotem, 1987) and tube length (Macior, 1986; Dohzono & Suzuki, 2002) correspond to preferences of different pollinator groups.
Intra‐individual polymorphism refers to a mixed strategy where one plant individual simultaneously produces multiple phenotypes or morphs of a floral trait to avoid fitness valleys due to phenotypic trade‐offs. Theory suggests that such a mixed strategy can be advantageous only when total fitness is calculated as the geometric mean of fitness components (the so‐called ‘coarse‐grained environment’ sensu Levins, 1968) and the environment fluctuates to a great extent in unpredictable ways (Levins, 1968; Venable, 1985). In this sense, it is a form of bet‐hedging to avoid complete reproductive failure in particular conditions where certain mutualists are unavailable or damages by certain antagonists are catastrophic.
One suspected case of intra‐individual polymorphism associated with a phenotypic trade‐off is within‐anther pollen size dimorphism in many species of Caryophylloideae (Jürgens, Witt & Gottsberger, 2012). Larger grains are often associated with greater pollen‐tube competition (Lord & Eckard, 1984; McCallum & Chang, 2016), although their production will reduce the total pollen amount (Vonhof & Harder, 1995; Sarkissian & Harder, 2001). In addition, experimental data suggest that nocturnal settling moths deposit pollen grains more distally on the extended stigmatic surface, whereas diurnal bumble bees deposit them nearer to the base (Jürgens et al., 2012). Therefore, it could be argued that this pollen size dimorphism is a mixed strategy to use multiple visitor groups for pollination, i.e. the large pollen morph is suited for pollination by nocturnal settling moths where a greater competitive ability is needed, while small grains are better suited for pollination by diurnal bumble bees where more pollen grains are necessary to outweigh losses due to grooming (Harder & Thomson, 1989). A similar pollen dimorphism has been discovered in a monkshood, Aconitum gymnandrum Maxim., that is pollinated by both bumble bees and wind (Wang et al., 2017; Fig. 4B).
(4). Combinational strategies
Whether or not a phenotypic trade‐off creates a fitness valley in total fitness depends on the shape and relative locations of the visitor‐specific fitness functions (Fig. 1). Hence, any changes in visitor‐specific functions that turn the overall fitness function from disruptive to stabilizing or unilateral could become strategies for trade‐off mitigation (Fig. 3). Visitor‐specific fitness functions often vary with extrinsic factors such as community context or resource availability (Sletvold, 2019), which could lead to a reduction of phenotypic trade‐offs in certain contexts. However, here we focus on the effects of more intrinsic factors that could modify visitor‐specific fitness functions: the expression pattern of other traits of a plant, i.e. an intrinsic context (Sletvold, 2019). For example, an increased handling cost associated with floral complexity decreases the number of flowers probed sequentially on a plant by bumble bees, leading to a reduction of geitonogamous self‐pollination (Ohashi, 2002). Hummingbirds that usually prefer narrow floral tubes increase a preference for wider corolla tubes when the flowers have larger petals (Fenster et al., 2006) and taller floral displays (Fenster et al., 2015). Female swallowtail butterflies have innate colour preference for blue, but their tendency to select red increases when the scent of orange flowers is presented simultaneously (Yoshida et al., 2015). Castellanos et al. (2004) have also discussed that stamen/stigma exsertion in Penstemon flowers may enforce contact with the hummingbird's forehead only in situations where narrow corolla tubes restrict angles at which the birds enter the flower.
Such intrinsic context dependence in visitor‐specific fitness functions may provide opportunities for flowers to generalize adaptively for multiple visitors by eliminating fitness valleys caused by phenotypic trade‐offs. In other words, the depth of a fitness valley caused by a phenotypic trade‐off may be more than a prerequisite for the evolution of floral traits: it could be the target of natural selection in itself. For example, imagine a plant population where a certain trait has adapted toward one mutualistic pollinator group. Another potential pollinator group is also available, but their visitation is infrequent because functional adaptation to this group would require a crossing of the fitness valley over the trait. Here, let us assume that the depth of the fitness valley varies among plants due to genetic variation in another trait that could modify the visitor‐specific function for this underused pollinator. If the current visitors do not ensure adequate and robust pollination, or when the population is linked by gene flow with other populations with different pollinator compositions, then plants with eligible phenotypes of such modifier traits are more likely to spread through the population by filling in the fitness valley and receiving greater pollination services in total. Note that this type of trade‐off mitigation would be driven by directional selection imposed on modifier traits, not on the focal trait. Similar processes could occur in generalization for mutualists and antagonists or multiple antagonists whose selection on the focal trait is disruptive. Depending on the type of selection to which the reduction of a fitness valley leads, we recognize two combinational strategies, ‘peak blunting’ and ‘optima convergence’ (Fig. 5, Table 1).
Fig 5.
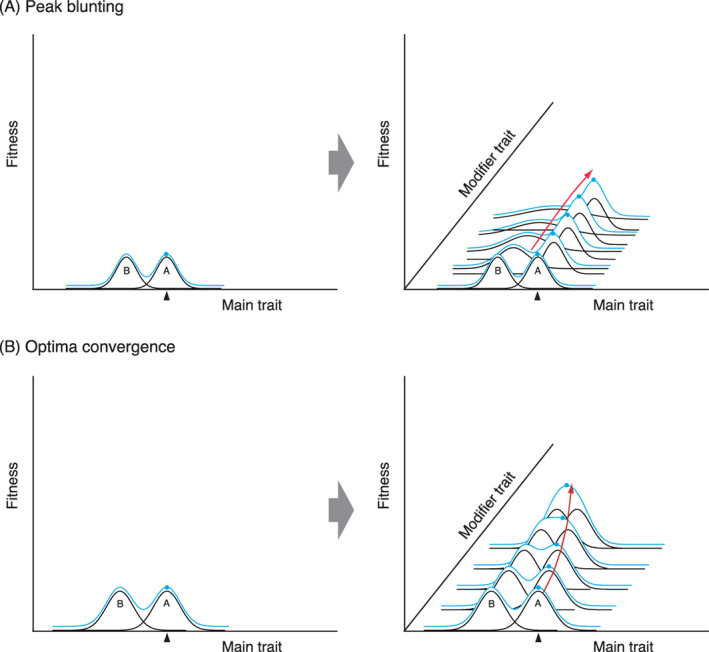
Hypothetical representations of how flowers could adaptively generalize for pollinators through the evolution of two combinational strategies for trade‐off mitigation: (A) peak blunting, and (B) optima convergence. Both strategies start from the state where the main trait is adapted to pollinator A (left); because of disruptive selection due to a phenotypic trade‐off, these flowers cannot receive contribution from pollinator B. Filled triangles indicate optimal phenotypes. In peak blunting (A; right), as the modifier trait changes, the fitness function of the trait for B is gradually flattened, while that for A hardly changes. This would increase total fitness (blue curves) because flowers can receive marginal benefits from B. In optima convergence (B; right), fitness functions of the trait for A or B (or both) gradually approach each other as the modifier trait changes. This would increase total fitness (blue curves) because they can receive benefits from both pollinators. In both cases, flowers will climb up the fitness ridge (red arrows) through natural selection imposed on the modifier trait.
Peak blunting refers to a situation where flowers evolve in the direction of relaxing a close link between a trait and fitness contribution of some visitor groups and changing the overall fitness function from disruptive (Fig. 1B) to unilateral (Fig. 1D) with respect to the trait. In a hypothetical example where a plant species that has adapted a certain trait (‘main trait’) to a mutualistic pollinator group A (Fig. 5A), changes in value or status of a modifier trait could blunt the visitor‐specific fitness function for underused pollinator group B, like ironing the wrinkles out of a shirt (Fig. 5A, right). This blunting or ironing out of the lumpiness of the visitor‐specific fitness function for B would undergo positive directional selection, as long as it increases the total fitness of the plant due to a marginal gain from B. In terms of a fitness landscape, this could be described as climbing up the fitness ridge through natural selection acting on the modifier trait (red arrow in Fig. 5A, right).
An empirical example for this is floral colour change, i.e. the retention of old, rewardless flowers in an altered colour (Weiss, 1995; Ohashi, Makino & Arikawa, 2015; Fig. 4C, right). It has been suggested that the retention of old flowers increases the size of floral display, thereby luring opportunistic foragers such as flies and enhancing pollination (Ishii & Sakai, 2001; Teixido et al., 2019). However, such false advertisement may not work for more economically efficient and/or cognitively sophisticated foragers such as bumble bees, because they could develop learned preferences towards individual plants and can be deceived only when they lack experience (Makino, Ohashi & Sakai, 2007; Makino & Sakai, 2007). In other words, the number of old flowers (equivalent to the main trait in Fig. 5A) is subject to disruptive selection due to a phenotypic trade‐off between experienced, sophisticated foragers and opportunistic or inexperienced foragers; retention of old flowers is favoured by the latter but not the former (Fig. 6A, left). The aversion to displays with old flowers in sophisticated foragers, however, may decrease as plants increase the level of honest signals, such as the amount of colour change in old flowers (equivalent to the modifier trait in Fig. 5A). As the honest signal becomes more detectable, sophisticated foragers may learn to avoid old flowers individually instead of avoiding the entire display with old flowers (Fig. 6A, right). This hypothesis has been supported in a field observation where one species of Weigela retaining old flowers in the same colour (Fig. 4C, left) primarily attracted fly pollinators, while its floral colour‐changing congener (Fig. 4C, right) attracted both bee and fly pollinators (Suzuki & Ohashi, 2014). Moreover, a laboratory experiment with bumble bees and artificial flowers showed that an inexperienced forager initially prefers to visit larger displays of any kind but develops a preference for displays presenting nectar and nectarless flowers in different colours over displays presenting them in the same colour (Makino & Ohashi, 2017). Thus, in combination with colour change as a modifier, large displays with old flowers can adaptively generalize for pollinator groups with different foraging preferences.
Fig 6.
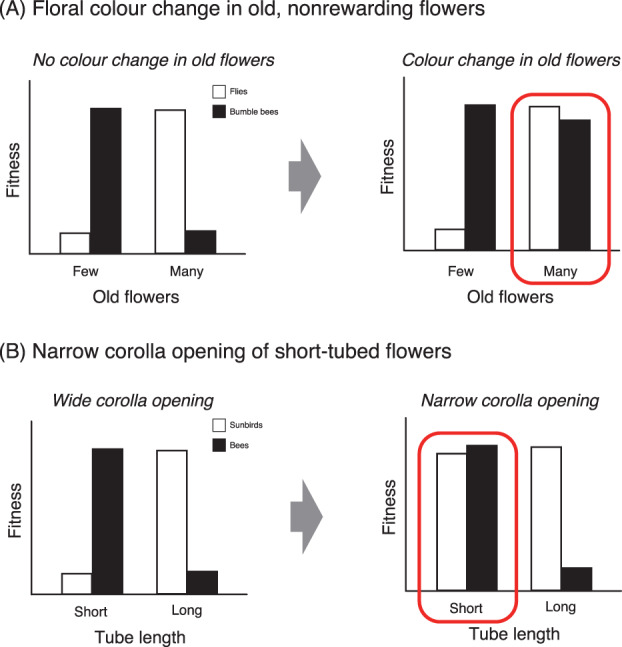
Examples of adaptive generalization by peak blunting. (A) Plants retaining many old flowers cause a phenotypic trade‐off between fly and bee pollinators but can generalize to both pollinators when old flowers are presented in an altered colour (Suzuki & Ohashi, 2014). (B) Plants with short‐tubed flowers cause a phenotypic trade‐off between bees and sunbirds but can generalize to both pollinators when short tubes are combined with a narrow corolla opening (Hargreaves et al., 2012, 2019). Red line enclosures indicate the state of adaptive generalization, which has been observed in nature.
The mitigation of a phenotypic trade‐off by peak blunting may also be represented by the flowers of Aloe kraussii Baker, which use both bees and sunbirds as pollinators (Hargreaves, Harder & Johnson, 2012; Hargreaves et al., 2019). Here, tube length is the main trait, while the width of corolla opening is the modifier trait. The short‐tubed flowers of this species conform to the bee‐pollination syndrome; the short tubes would prevent the long‐billed sunbirds from touching anthers and stigmas with their head feathers – the normal location of pollen transfer by birds (Fig. 6B, left; Hargreaves et al., 2012). However, tube length becomes less influential on sunbird pollination as the corolla openings of A. kraussii flowers become narrower, because narrow openings facilitate pollen transfer via sunbird bills in place of heads (Fig. 6B, right; Hargreaves et al., 2019). In other words, by combining a short tube with a narrow corolla opening as a modifier trait, these flowers may turn sunbirds into relaxed pollinators having no significant fitness‐trait covariance with respect to tube length and, in turn, increase their total fitness.
Optima convergence is another possibility for how modifier traits can eliminate fitness valleys due to phenotypic trade‐offs. It refers to cases where modifiers move the peaks of multiple visitor‐specific fitness functions closer to one another, or change their shape from convex downward to convex upward, and consequently alter the overall fitness function from disruptive (Fig. 1B) to stabilizing (Fig. 1C) with respect to the trait. Figure 5B shows a hypothetical example where a plant species has adapted the main trait to a mutualistic pollinator group A. Changes in the modifier trait could bring the visitor‐specific fitness function for underused pollinator group B closer to that of group A. If such convergence increases the total fitness of the plant due to a marginal gain from group B, this would undergo positive directional selection. In terms of fitness landscape, this means that a combination of a certain trait and its modifier can create a ‘fitness ridge’ for one visitor group, along which a population climbs up to the confluence with the ridge for the other visitor group (red arrow in Fig. 5B, right).
Different groups of visitors often provide more or less equally good pollination services within the range of natural trait variation, producing a broad peak of total fitness around the intermediate phenotype (Herrera, 1988, 1996; Gómez & Zamora, 1999; Suzuki et al., 2007). These patterns have often been interpreted as the result of functional equivalence among different visitor groups (Gómez & Zamora, 1999, 2006; Corbet, 2006). We go one step further and point out that such stabilizing selection may not always be the result of inherent similarity among visitors in their selective roles. Rather, the situation itself may be an outcome of evolution in modifier traits that induce convergence of fitness functions for different visitors and eliminate fitness valleys in total fitness.
Optima convergence may explain how shallow flowers in compact inflorescences have successfully catered to a diverse range of visitors in many plant taxa such as Asteraceae and Apiaceae (Corbet, 2006; Gómez & Zamora, 2006). Here, flower depth can be seen as the main trait, and the formation of compact inflorescences as the modifier trait. Flower depths seem to undergo disruptive selection in mixed pollination systems because deep flowers would exclude short‐tongued or ‐billed visitors (Borrell, 2005) or encourage them to rob nectar (Maloof & Inouye, 2000; Rojas‐Nossa, Sánchez & Navarro, 2016), while shallow flowers would allow long‐tongued or ‐billed animals to steal nectar without providing pollination services (Inouye, 1980; Haran, Izhaki & Dafni, 2018; Fig. 4D, left). If shallow flowers are tightly packed on a plane, however, even long‐tongued or ‐billed animals may successfully transfer pollen on the ventral side of their body, unless they hover to feed (Fig. 4D, right). This of course increases selfing and interference, but those costs could be avoided by dichogamy. In this way, flowers could achieve convergence to optimal depths for different pollinator groups by combining shallow corollas with compact inflorescences. This proposition is testable.
Previous studies have often stressed the importance of trait combinations in the context of adaptive specialization, in that synergistic effects among traits improve pollination (Reynolds, Dudash & Fenster, 2010; Fenster et al., 2015) or exclude inefficient pollinators (Gegear, Burns & Swoboda‐Bhattarai, 2017). We urge future studies to broaden the view and look more into the role of trait combinations in adaptive generalization. Experimental approaches using manipulations of floral phenotype or artificial flowers with various trait combinations will be required. There are some difficulties in designing such studies, however. First, detecting the potential modifier traits may be tricky, especially if their effects are not readily predictable, as exemplified in Fenster et al. (2015). Statistical tools such as the morphospace approach (Raup & Michelson, 1965; Sidlauskas, 2008; Chartier et al., 2014) might help identify such unexplored functional coordination based on the repeated evolution of particular trait combinations across angiosperms. Second, the visitor‐specific fitness function needs to be examined in a controlled environment where visits by other groups are prevented (e.g. Muchhala, 2007). If this condition is not met, the realized fitness contribution of the focal group may decrease irrespective of its potential contribution (Carpenter, 1979; Laverty & Plowright, 1985; Miyake & Yahara, 1999; Thomson et al., 2000). In the next section, we define such fitness conflicts emerging in ecological contexts as visitor‐mediated opportunity trade‐offs and discuss their evolutionary consequences.
IV. THE EVOLUTIONARY CONSEQUENCES OF VISITOR‐MEDIATED OPPORTUNITY TRADE‐OFFS
(1). Model description of opportunity trade‐offs
An opportunity trade‐off refers to a conflict between opportunities of a flower to receive fitness contributions from different visitor groups; at least one of the visitor groups involved needs to be mutualistic (see Section II). To study how opportunity trade‐offs could affect floral evolution, we adopt a graphical approach inspired by those employed in previous studies to summarize the results of numerical simulations (Thomson & Thomson, 1992; Thomson et al., 2000). Let us consider the floral phenotype x o with respect to some heritable trait, x, and its realized fitness in the presence or absence of other flower visitor groups. For simplification purposes, we assume that the phenotype x o is invariable. The fitness contribution (positive or negative) of the ith visitor group to the plant in a single‐visitor environment is expressed as sw i(x o). In a mixed‐visitor environment with two visitor groups A and B, the increased visits by group A may reduce the fitness contribution from B, and vice versa. This can be expressed as:
| (2) |
where mw A(x o), mw B(x o) and V A represent the realized fitness contributions of A and B and the frequency of visits by group A, respectively. Positive relationships between visits or contributions by different visitor groups have been suggested (e.g. Soper Gorden & Adler, 2018), but we do not consider such cases here.
Equation (2) is equivalent to a condition where the two groups A and B are in a competitive relationship over the limited amount of structure or space that provides opportunities for these visitors to contribute to the plant's fitness. Therefore, the sum of the realized fitness contributions from A and B is smaller than the sum of the fitness contributions from A and B at phenotype x o in a single‐visitor environment (Fig. 7A):
| (3) |
The relationship between the fitness contributions sw i(x o) and mw i(x o) may be compared to that between the fundamental niche and the realized niche in classical niche theory for a system of multiple consumers limited by one resource (Hutchinson, 1957).
Fig 7.
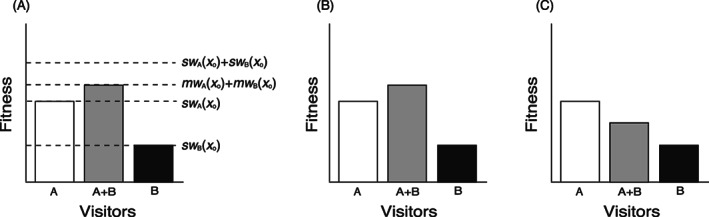
Graphical representations of visitor‐mediated opportunity trade‐offs (opportunity trade‐offs). (A) Context‐dependent fitness graph showing a plant's fitness when only one group A or B is visiting [sw A(x o) or sw B(x o)] or both groups A and B are visiting [mw A(x o) + mw B(x o)], where sw A(x o) is the fitness contribution of visitor A in the single‐visitor environment, and mw A(x o) is the realized fitness contribution of A in the two‐visitor environment. (B) A weak opportunity trade‐off. (C) A strong opportunity trade‐off.
The strength of opportunity trade‐offs depends on how the two groups A and B differ in opportunity use efficiency, i.e. how much they contribute to fitness relative to the amount by which they reduce the opportunity for the other group's fitness contribution. The total fitness in a mixed‐visitor environment, ∑mw i(x o) = mw A(x o) + mw B(x o), becomes smaller as the difference between A and B in opportunity use efficiency increases. Two different types of opportunity trade‐offs can be distinguished accordingly: weak opportunity trade‐offs and strong opportunity trade‐offs. Weak opportunity trade‐offs refer to the situation where ∑mw i(x o) is still larger than any of the fundamental fitness contributions in single‐visitor environment, i.e. sw A(x o) and sw B(x o) (Fig. 7B). Strong opportunity trade‐offs refer to the situation where ∑mw i(x o) becomes smaller than either sw A(x o) or sw B(x o) (Fig. 7C). Flowers would face a dilemma in harbouring both visitors A and B only in strong opportunity trade‐offs. The difference between sw A(x o) and ∑mw i(x o) in Fig. 7C is an opportunity cost incurred from visits by B. Based on these considerations, we suggest two strategies that plants could employ to mitigate opportunity trade‐offs. One is an elimination of certain groups from the visitor assemblage (active exclusion), and the other is temporal prioritization of visits by more efficient mutualist groups (schedule optimization). Examples of these strategies are reviewed below and listed in Table 1.
(2). Active exclusion
When an opportunity trade‐off is strong, the visitation of flowers by one group of animals reduces the total fitness for the plant by diminishing opportunities for the contribution of other visitors. In such situations, the plant should selectively filter out the detrimental group, unless it could prioritize the arrival of more beneficial visitors (see Section IV.3). Thus, it should be emphasized that the evolutionary consequences of opportunity trade‐offs are distinctly different from those of phenotypic trade‐offs in that they involve trade‐off mitigation by actively excluding particular visitors. Phenotypic trade‐offs could also lead to an apparent restriction of some visitors, but only as a passive result of floral adaptation to other visitor groups.
The obvious situation where active exclusions are adaptive is when flowers are subject to antagonistic visitors whose net contribution to fitness is negative. If exclusion or deterrence against one group of antagonists has no fitness cost in terms of other biotic interactions, plants would simply evolve traits that maximize such defences. When defence traits impede or enhance interactions with other animals, however, phenotypic trade‐offs may occur between defence and functional adaptations to pollinators, or to other antagonists with different characteristics. In this way, the evolution of defence against floral antagonists may often involve phenotypic trade‐offs, but the selective force driving the evolution of defence is primarily opportunity trade‐offs or opportunity loss to antagonists.
An opportunity trade‐off, strong enough to drive the evolution of active exclusion, could also occur between pollinator groups, in which inefficient groups become functional parasites, i.e. antagonists, in the presence of more efficient groups (Thomson & Thomson, 1992; Thomson et al., 2000). This occurs when inclusion of inefficient pollinator groups deprives flowers of the chance to be pollinated more efficiently by other groups and, as a result, decreases the total fitness. In this situation, floral traits actively excluding less‐efficient pollinator groups – often referred to as floral filters (Johnson, Hargreaves & Brown, 2006) – would benefit plants unless they simultaneously deter efficient pollinators to the point that the total fitness is reduced. In some specialized systems, floral traits such as cryptic coloration (Shuttleworth & Johnson, 2009, 2012; Lunau et al., 2011; Bergamo et al., 2016; Gegear et al., 2017; Camargo et al., 2019; Fig. 4E), pollinator‐specific odour attractants (Shuttleworth & Johnson, 2009), temporary closure (Martén‐Rodríguez, Almarales‐Castro & Fenster, 2009), downward‐facing aperture (Castellanos et al., 2004; Gegear et al., 2017), unpalatable nectar (Johnson et al., 2006; Shuttleworth & Johnson, 2009), dilute nectar (Gegear et al., 2017), or low nectar accumulation (Fleming, Geiselman & Kress, 2009), have been suggested to function as filters that deter or repel pollinators with lower efficiencies of opportunity use, i.e. antagonists, while attracting or having little impact on better pollinators, i.e. mutualists.
Pollinator filtering becomes advantageous only when opportunity trade‐offs are strong enough to offset the marginal fitness gain from having the additional visitors. This assumption is difficult to test, because it requires a quantitative comparison of total fitness between single‐visitor and mixed‐visitor environments, as exemplified by the context‐dependent fitness graphs in Fig. 7. This is particularly difficult to demonstrate for the male fitness component, i.e. pollen donated to compatible stigmas. Only a few simulation models have demonstrated so far that strong opportunity trade‐offs over the opportunity for pollen transfer could solely drive specialization to the most efficient pollinator group (Thomson & Thomson, 1992; Muchhala et al., 2010).
(3). Schedule optimization
Although obligate antagonists are always the target of active exclusion, pollinator filtering may not always be the best strategy, because it increases the risk of reproductive failure when efficient pollinators are scarce. On other occasions, inclusion of inefficient pollinator groups may result in a total fitness lower than the potential maximum but higher than fitness obtained in a single‐visitor environment (Fig. 7B). In such situations, the plant should only mitigate the opportunity trade‐off instead of excluding the inefficient pollinators. One possible way to achieve this is to adjust the timing of anthesis to prioritize visits by efficient pollinators in time and minimize the detrimental effect of inefficient pollinators (Thomson & Thomson, 1992). For instance, flowers of Japanese honeysuckle, Lonicera japonica Thunb., last longer than a day, and are visited by both diurnal and nocturnal pollinators (Fig. 4F). Miyake & Yahara (1998) suggested that diurnal bees are less efficient pollinators than nocturnal hawkmoths because the bees waste pollen by harvesting or grooming it off. Nevertheless, L. japonica seems to have no filtering mechanism to discourage bees, probably because visitation by hawkmoths is quite infrequent. Instead, this species opens its buds almost invariably at dusk, as if it temporally postpones visits by diurnal bees until it first has an opportunity to be visited by nocturnal hawkmoths. Using a simulation model, Miyake & Yahara (1999) showed that nocturnal anthesis maximizes total pollen transfer from these flowers, by increasing the chances for successful, nocturnal transfer of pollen that would otherwise be removed from circulation by wasteful bees. Interestingly, their model predicts that the selective advantage of diurnal anthesis decreases as diurnal bees are more abundant, because the more frequently bees visit, the more quickly they deplete pollen from day‐opening flowers, which would take away the opportunity for pollination by hawkmoths. By contrast, dusk‐opening flowers can benefit both from diurnal and nocturnal pollination by first allowing pollen removal by hawkmoths alone and later offering the leftover pollen for a ‘clearance sale’ as bees start foraging.
Similar explanations may also apply to nocturnal anthesis and nectar secretion only at night in some Burmeistera and Adenophora species, where observations suggest that nocturnal visitors are more efficient pollinators than diurnal visitors (Muchhala, 2003; Funamoto & Ohashi, 2017; Funamoto, 2019). Another example is the flowers of Silene colorata Poir. The flowers exhibit repeated daytime closures before wilting, but they remain open in the morning for a while at the time when they have some nectar and pollen left (Prieto‐Benítez, Dötterl & Giménez‐Benavides, 2016). All these species have apparently adapted to nocturnal pollination, but they have not excluded the possibility of receiving compensatory pollination from diurnal visitors when nocturnal pollinators are scarce – they are conditional generalists in that sense.
Schedule optimization could also mitigate opportunity trade‐offs among different groups of diurnal pollinators. Diurnal pollinators often vary in daily patterns of foraging activity, primarily as a result of species‐specific responses to temperature and radiation (Tepedino, 1981; Willmer, 1983; Herrera, 1990). Given the likelihood that these pollinators vary in their pollination quality (Herrera, 1987), the patterns of their sequential arrival may be important for plants in terms of not wasting gametes and rewards (Thomson & Thomson, 1992). For example, Tepedino (1981) showed that the flowers of summer squash, Cucurbita pepo L., open early in the morning, prioritizing visits by a particular bee species and rendering the later‐arriving bee species inconsequential. In this case, the early bees were not necessarily superior, at least in terms of per‐visit female fitness contribution (neither efficiency nor male fitness contribution was investigated). Further explorations of the succession of visitors to long‐lived flowers would be worthwhile.
Schedule optimization may be the easiest strategy for utilizing multiple pollinator groups with different foraging timetables, regardless of whether the opportunity trade‐off is strong or weak. We expect active exclusions to evolve in only two conditions: (i) when obligate antagonists are involved; or (ii) when the arrivals of different pollinators overlap in time, and inefficient pollinators become antagonistic in the presence of better pollinators.
Thus, opportunity trade‐offs are distinctively different from phenotypic trade‐offs both in causes and evolutionary consequences. Whereas a phenotypic trade‐off is caused by conflicting selection pressures exerted on a floral trait and determines the evolutionary consequences of the trait, an opportunity trade‐off is caused by competition over the opportunity for fitness contribution and imposes selection on the traits that determine how interactions occur, with whom, and in which order. It is crucial that only an opportunity trade‐off could facilitate the evolution of active exclusion. When one finds a negative correlation between fitness contributions of two different flower visitors in field conditions, therefore, a clear distinction needs to be made as to whether the trade‐off occurs purely as a phenotypic trade‐off or to what extent it involves an opportunity trade‐off. Although such a distinction requires careful investigation, it would help to comprehend the direction and intensity of natural selection on floral traits and their combinations, as well as the evolutionary significance of floral phenotypes.
V. FUTURE PERSPECTIVES FOR INVESTIGATING TRADE‐OFFS IN FLOWER–VISITOR RELATIONSHIPS
(1). Possibilities for adaptive specialization and generalization
There is a long‐standing notion that most angiosperm flowers are specialized for pollination by particular groups of animals (Grant & Grant, 1965; Stebbins, 1970), with the implicit assumption that floral traits are generally subject to disruptive selection caused by visitor‐mediated phenotypic trade‐offs among different groups of animals. However, this does not always seem to be the case. Flowers presumably have evolved from specialization to generalization in more than a few taxa (Armbruster & Baldwin, 1998; Tripp & Manos, 2008; Martén‐Rodríguez et al., 2010; Vereecken et al., 2012; Brito et al., 2016; Dellinger et al., 2019a ). Most members of Asteraceae, one of the families with highest species diversity, exhibit highly generalized pollination systems (Proctor et al., 1996). Interactions with different animal groups appear unavoidable for many flowers. Given these considerations, it may be fair to assume that some flowers have adapted to diffuse or generalized interactions.
Even if we accept such an argument, a couple of questions remain. During the course of adaptation to multiple visitors, how do flowers prevent the reduction in effectiveness due to visitor‐mediated trade‐offs from offsetting the advantage of reproductive assurance (Waser et al., 1996)? In particular, how do they avoid being trapped in fitness valleys while generalizing for diverse visitors who would potentially exert disruptive selection? Moreover, some flowers seem to filter out not only obligate antagonists but also a subset of pollinators (Gegear et al., 2017). If plants have the potential to generalize adaptively for multiple partners, how could such choosiness be favoured?
Based on our conceptual framework, we suggest two answers. First, the potential fitness reduction due to visitor‐mediated trade‐offs may be countered by floral evolution (Fig. 3). We have hypothesized possible floral strategies to cope with visitor‐mediated trade‐offs entailed by different groups of visitors, and offered examples from the current literature (Table 1). With the introduction of such a view, existing notions about the evolution of flowers under disruptive selection, e.g. that flowers inevitably adapt their traits to either of the divergent visitor groups or remain in a state of evolutionary non‐equilibrium, will be relaxed.
Second, active exclusion of particular pollinator groups could only be advantageous when there are strong opportunity trade‐offs among groups with temporally overlapped daily activities. The opportunity cost of having less‐efficient pollinators in the presence of more efficient alternatives has been proposed as a potential factor driving pollination specialization (Thomson & Thomson, 1992; Thomson et al., 2000; Thomson, 2003; Muchhala et al., 2010). To gain a comprehensive understanding of floral adaptation under diffuse interactions, we reemphasize the importance of such ecological conflicts, separately from the phenotypic trade‐offs that have been the focus of previous studies.
For the understanding of floral phenotypes, it is important to consider whether and how flowers have adapted to include or exclude different flower visitor groups. Given that a visitor's value for a flower is often influenced by the context of other available visitors (Thomson & Thomson, 1992; Fig. 7), simple observations of flower visitation or single‐visit fitness contribution would not distinguish whether flowers have adapted to include or exclude a certain visitor group. Separate measurements of phenotypic trade‐offs and opportunity trade‐offs are essential, although they may not be easy if the original trade‐offs have already been mitigated and translated into another form during the course of adaptation to diffuse interactions.
(2). Floral adaptations to flower visitor communities
During recent decades, pollination biologists have argued about the apparent discrepancy between suites of floral traits indicating adaptations to specific types of pollinators and the observed wide spectrum of visitors (Ollerton, 1996; Waser et al., 1996; Waser, 2006; Ollerton et al., 2009; Rosas‐Guerrero et al., 2014). Our conceptual framework may also help reconcile this long‐lasting controversy over the validity of pollination syndromes.
As summarized in Table 1, all the proposed strategies for trade‐off mitigation are made possible by acquiring new combinations of traits. This suggests that adaptive responses to mitigate visitor‐mediated trade‐offs could have repeatedly generated characteristic trait combinations or ‘floral syndromes’ in angiosperms. This may be best exemplified by the convergent evolution of floral colour change producing a combination of extended flower life and colour change in old, rewardless flowers (Weiss, 1995; Ohashi et al., 2015; Figs 4C, 6A). In particular, a theoretical study has confirmed that the trait combination in floral colour change becomes evolutionarily stable only in generalized systems where both opportunistic and well‐informed pollinators visit (Ito, Suzuki & Mochizuki, 2021). Recent studies have also revealed that species in Melastomataceae have undergone convergent and correlated evolution of multiple floral characters in association with generalized pollination systems (Dellinger et al., 2019a ; Gavrutenko et al., 2020). Thus, floral syndromes in angiosperms might represent not only adaptive specializations for particular visitor groups, but also adaptive generalizations for particular visitor communities; they will be characterized by modifier traits for trade‐off mitigation, in addition to refining traits for mutualistic visitors and filter traits against antagonistic visitors.
It is also important to note that floral phenotypes, even at evolutionary equilibrium, do not always represent adaptations to flower visitors that have the greatest influence on the overall level of reproduction. Rather, phenotypic adaptation to a particular group of visitors would occur only when its fitness contribution varies significantly with the focal trait (Aigner, 2001, 2006). This is most evident in cases of unilateral selection, where floral traits adapt to visitors whose fitness contribution is relatively small (Fig. 1D, right). This means that one cannot easily determine only from floral traits which visitors have the greatest impact on the plant's reproduction. With these viewpoints, we suggest that the real question is not whether a floral phenotype reflects its most influential visitors (Ollerton et al., 2009; Rosas‐Guerrero et al., 2014), but rather whether and how it has adapted to multiple selection pressures (Caruso et al., 2019) and visitor‐mediated trade‐offs imposed by the whole visitor community.
Constraints imposed by visitor‐mediated trade‐offs have been suggested to promote phenotypic diversity of flowers via the evolution of adaptive trait combinations for distinct groups of pollinators (Thomson et al., 2000; Aigner, 2001; Muchhala et al., 2010; Armbruster, 2017). We expect that flowers will have further avenues through which to achieve phenotypic diversity, i.e. evolutionary mitigation of visitor‐mediated trade‐offs. This seems especially relevant for phenotypic diversity in diffuse interaction systems where possible combinations of visitors are almost infinite. For example, bee‐dominated visitor communities may further be classified by less‐dominant groups, such as flies, butterflies, birds, or the lack of them (Waser, 1983; Herrera, 1996). Such compositionally diverse visitor communities may explain why each pollination syndrome includes two or more (instead of one) typical combinations of floral traits (Ollerton et al., 2009), why the boundaries between syndromes are often obscured by shared floral characteristics (van der Pijl, 1961; Willmer, 2011), and why complex flowers often host as diverse visitors as simple flowers (Minckley & Roulston, 2006). Moreover, when visitor communities vary geographically, separate populations of generalized flower species may diverge phenotypically as a result of evolutionary adjustment of trait combinations to selection regimes caused by the different sets of visitor‐mediated phenotypic trade‐offs and opportunity trade‐offs. Such geographic diversification of floral phenotypes may fit into concepts such as ‘adaptive wandering’ (Wilson & Thomson, 1996; Thomson et al., 2000; Thomson & Wilson, 2008; Zych et al., 2019) or the ‘geographic mosaic theory of coevolution’ (Thompson, 1994). We predict that future studies will find that the structure of flower–visitor communities and the associated trade‐offs have played critical roles in generating the recurrent patterns of trait combinations in floral adaptation. Identifying convergent adaptations to particular community contexts may help us to understand better the evolutionary link between diverse floral phenotypes and their visitors.
VI. CONCLUSIONS
Conflicting selection pressures imposed on flowers by various animals – visitor‐mediated trade‐offs – have been considered to promote floral diversity as a result of phenotypic specialization. However, it is commonly observed that flowers interact with various groups of visitors, while at the same time maintaining distinct phenotypes among ecotypes, subspecies and congeners. Discussions on this contradictory pattern have been repeatedly made, with little agreement. We provide a conceptual framework for analysing visitor‐mediated trade‐offs to stimulate future research.
In addition to the conventionally recognized trade‐off in flowers, i.e. a visitor‐mediated phenotypic trade‐off, another source of negative correlations of fitness contributions among different visitors needs to be distinguished, i.e. a visitor‐mediated opportunity trade‐off. The former occurs when phenotypic adaptation to one visitor reduces visits and/or pollination effectiveness by other animals, or increases the visits and/or damage caused by antagonists. The latter emerges only when antagonists or less‐efficient pollinators negatively affect floral features that would otherwise provide opportunities for fitness contribution by more efficient pollinators.
Researchers have implicitly assumed that floral generalization represents a non‐equilibrium state or a suboptimal solution to conflicting selection pressures. Considering that most flowers in nature are ecologically generalized, however, it may also be fair to consider possibilities that some flowers have evolved features to mitigate visitor‐mediated trade‐offs and achieved adaptive generalization as a stable endpoint. Evolutionary considerations and our literature survey suggest that there are a variety of pathways to such trade‐off mitigation, which has largely been overlooked in earlier discussions. This may explain why we rarely observe visitor‐mediated disruptive selection in ecologically generalized flowers.
Active exclusion of particular visitors could evolve only when a strong visitor‐mediated opportunity trade‐off is imposed on flowers. Active exclusion occurs even against pollinators, when the presence of efficient and reliable pollinators turns other inefficient pollinators into antagonists, and visits by the better pollinators cannot be prioritized by adjusting the timing of anthesis. Our conceptual framework thus clarifies that the evolution of floral filters, or floral specialization in a narrow sense, is a special case of trade‐off mitigation in which generalization can never be adaptive.
Regardless of how it is achieved, and regardless of whether it is directed to specialization or generalization, any strategy for trade‐off mitigation comes from the acquisition of novel combinations of traits. This suggests that diffuse interactions with various animals may have enriched, rather than limited, the diversity of floral phenotypes with distinct trait associations. Explorations of how visitor‐mediated trade‐offs explain the recurrent patterns of floral phenotypes may help reconcile the long‐lasting controversy over the validity of pollination syndromes, i.e. the discrepancy between observed flower visitors and those predicted based on floral phenotypes.
VII. ACKNOWLEDGEMENTS
K.O. and A.J. conceived the fundamental ideas and wrote the manuscript; J.D.T. contributed to idea development and editing. We thank Taina Witt and Chieko Koshida for their invariable support throughout the study, and Hana Ohashi for accompanying K.O. every morning for a walk in the woods to refine the ideas. We acknowledge Takashi Miyake, who provided photos of L. japonica flowers and their pollinators. Finally, we thank the anonymous reviewers for their careful and insightful reviews. This work was supported by a JSPS Grants‐in‐Aid for Scientific Research (KAKENHI no. 15KK0249) to K.O.
REFERENCES
- Agrawal, A. A. , Conner, J. K. & Rasmann, S. (2010). Trade‐offs and negative correlations in evolutionary ecology. In Evolution Since Darwin: The First 150Years (eds Bell M. A., Futuyma D. J., Eanes W. F. and Levinton J. S.), pp. 243–268. Sinauer Associates, Sunderland, MA. [Google Scholar]
- Aigner, P. A. (2001). Optimality modeling and fitness trade‐offs: when should plants become pollinator specialists? Oikos 95, 177–184. [Google Scholar]
- Aigner, P. A. (2004). Floral specialization without trade‐offs: optimal corolla flare in contrasting pollination environments. Ecology 85, 2560–2569. [Google Scholar]
- Aigner, P. A. (2006). The evolution of specialized floral phenotypes in a fine‐grained pollination environment. In Plant‐Pollinator Interactions: From Specialization to Generalization (eds Waser N. M. and Ollerton J.), pp. 23–46. University of Chicago Press, Chicago. [Google Scholar]
- Amorim, F. W. , Galetto, L. & Sazima, M. (2013). Beyond the pollination syndrome: nectar ecology and the role of diurnal and nocturnal pollinators in the reproductive success of Inga sessilis (Fabaceae). Plant Biology 15, 317–327. [DOI] [PubMed] [Google Scholar]
- An, L. , Neimann, A. , Eberling, E. , Algora, H. , Brings, S. & Lunau, K. (2018). The yellow specialist: dronefly Eristalis tenax prefers different yellow colours for landing and proboscis extension. Journal of Experimental Biology 221, jeb184788. 10.1242/jeb.184788. [DOI] [PubMed] [Google Scholar]
- Anderson, B. , Ros, P. , Wiese, T. J. & Ellis, A. G. (2014). Intraspecific divergence and convergence of floral tube length in specialized pollination interactions. Proceedings of the Royal Society B: Biological Sciences 281, 20141420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster, W. S. (2006). Evolutionary and ecological aspects of specialized pollination: views from the arctic to the tropics. In Plant‐Pollinator Interactions: From Specialization to Generalization, pp. 260–282. University of Chicago Press, Chicago. [Google Scholar]
- Armbruster, W. S. (2014). Floral specialization and angiosperm diversity: phenotypic divergence, fitness trade‐offs and realized pollination accuracy. AoB Plants 6, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster, W. S. (2017). The specialization continuum in pollination systems: diversity of concepts and implications for ecology, evolution and conservation. Functional Ecology 31, 88–100. [Google Scholar]
- Armbruster, W. S. & Baldwin, B. G. (1998). Switch from specialized to generalized pollination. Nature 394, 632. [Google Scholar]
- Ashworth, L. , Aguilar, R. , Martén‐Rodríguez, S. , Lopezaraiza‐Mikel, M. , Avila‐Sakar, G. , Rosas‐Guerrero, V. & Quesada, M. (2015). Pollination syndromes: a global pattern of convergent evolution driven by the most effective pollinator. In Evolutionary Biology: Biodiversification from Genotype to Phenotype (ed. Pontarotti P.), pp. 203–224. Springer International Publishing, Switzerland. [Google Scholar]
- Bergamo, P. J. , Rech, A. R. , Brito, V. L. G. & Sazima, M. (2016). Flower colour and visitation rates of Costus arabicus support the ‘bee avoidance’ hypothesis for red‐reflecting hummingbird‐pollinated flowers. Functional Ecology 30, 710–720. [Google Scholar]
- Boberg, E. , Alexandersson, R. , Jonsson, M. , Maad, J. , Ågren, J. & Nilsson, L. A. (2014). Pollinator shifts and the evolution of spur length in the moth‐pollinated orchid Platanthera bifolia . Annals of Botany 113, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell, B. J. (2005). Long tongues and loose niches: evolution of euglossine bees and their nectar flowers. Biotropica 37, 664–669. [Google Scholar]
- Brito, V. L. G. , Fendrich, T. G. , Smidt, E. C. , Varassin, I. G. & Goldenberg, R. (2016). Shifts from specialised to generalised pollination systems in Miconieae (Melastomataceae) and their relation with anther morphology and seed number. Plant Biology 18, 585–593. [DOI] [PubMed] [Google Scholar]
- Bronstein, J. L. (1994). Our current understanding of mutualism. Quarterly Review of Biology 69, 31–51. [Google Scholar]
- Camargo, M. G. G. , Lunau, K. , Batalha, M. A. , Brings, S. , de Brito, V. L. G. & Morellato, L. P. C. (2019). How flower colour signals allure bees and hummingbirds: a community‐level test of the bee avoidance hypothesis. New Phytologist 222, 1112–1122. [DOI] [PubMed] [Google Scholar]
- Carpenter, F. L. (1979). Competition between hummingbirds and insects for nectar. Integrative and Comparative Biology 19, 1105–1114. [Google Scholar]
- Caruso, C. M. , Eisen, K. E. , Martin, R. A. & Sletvold, N. (2019). A meta‐analysis of the agents of selection on floral traits. Evolution 73, 4–14. [DOI] [PubMed] [Google Scholar]
- Castellanos, M. C. , Wilson, P. & Thomson, J. D. (2003). Pollen transfer by hummingbirds and bumblebees, and the divergence of pollination modes in Penstemon . Evolution 57, 2742–2752. [DOI] [PubMed] [Google Scholar]
- Castellanos, M. C. , Wilson, P. & Thomson, J. D. (2004). ‘Anti‐bee’ and ‘pro‐bird’ changes during the evolution of hummingbird pollination in Penstemon flowers. Journal of Evolutionary Biology 17, 876–885. [DOI] [PubMed] [Google Scholar]
- Chapurlat, E. , Anderson, J. , Ågren, J. , Friberg, M. & Sletvold, N. (2018). Diel pattern of floral scent emission matches the relative importance of diurnal and nocturnal pollinators in populations of Gymnadenia conopsea . Annals of Botany 121, 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier, M. , Jabbour, F. , Gerber, S. , Mitteroecker, P. , Sauquet, H. , Von Balthazar, M. , Staedler, Y. , Crane, P. R. & Schönenberger, J. (2014). The floral morphospace ‐ a modern comparative approach to study angiosperm evolution. New Phytologist 204, 841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner, J. K. , Davis, R. & Rush, S. (1995). The effect of wild radish floral morphology on pollination efficiency by four taxa of pollinators. Oecologia 104, 234–245. [DOI] [PubMed] [Google Scholar]
- Corbet, S. (2006). A typology of pollination systems: implications for crop management and the conservation of wild plants. In Plant‐Pollinator Interactions: From Specialization to Generalization (eds Waser N. M. and Ollerton J.), pp. 315–340. University of Chicago Press, Chicago. [Google Scholar]
- Culbert, B. M. & Forrest, J. R. K. (2016). Floral symmetry affects bumblebee approach consistency in artificial flowers. Journal of Pollination Ecology 18, 1–6. [Google Scholar]
- Dellinger, A. S. , Chartier, M. , Fernández‐Fernández, D. , Penneys, D. S. , Alvear, M. , Almeda, F. , Michelangeli, F. A. , Staedler, Y. , Armbruster, W. S. & Schönenberger, J. (2019a). Beyond buzz‐pollination – departures from an adaptive plateau lead to new pollination syndromes. New Phytologist 221, 1136–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger, A. S. , Scheer, L. M. , Artuso, S. , Fernández‐Fernández, D. , Sornoza, F. , Penneys, D. S. , Tenhaken, R. , Dötterl, S. & Schönenberger, J. (2019b). Bimodal pollination systems in Andean Melastomataceae involving birds, bats, and rodents. American Naturalist 194, 104–116. [DOI] [PubMed] [Google Scholar]
- Dohzono, I. & Suzuki, K. (2002). Bumblebee‐pollination and temporal change of the calyx tube length in Clematis stans (Ranunculaceae). Journal of Plant Research 115, 355–359. [DOI] [PubMed] [Google Scholar]
- Eisikowitch, D. & Rotem, R. (1987). Flower orientation and color change in Quisqualis indica and their possible role in pollinator partitioning. Botanical Gazette 148, 175–179. [Google Scholar]
- Faegri, K. & van der Pijl, L. (1979). The Principles of Pollination Ecology. Pergamon Press, Oxford. [Google Scholar]
- Fenster, C. B. , Armbruster, W. S. , Wilson, P. , Dudash, M. R. & Thomson, J. D. (2004). Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics. 35, 375–403. [Google Scholar]
- Fenster, C. B. , Cheely, G. , Dudash, M. R. & Reynolds, R. J. (2006). Nectar reward and advertisement in hummingbird‐pollinated Silene virginica (Caryophyllaceae). American Journal of Botany 93, 1800–1807. [DOI] [PubMed] [Google Scholar]
- Fenster, C. B. , Reynolds, R. J. , Williams, C. W. , Makowsky, R. & Dudash, M. R. (2015). Quantifying hummingbird preference for floral trait combinations: the role of selection on trait interactions in the evolution of pollination syndromes. Evolution 69, 1113–1127. [DOI] [PubMed] [Google Scholar]
- Fishbein, M. & Venable, D. L. (1996). Diversity and temporal change in the effective pollinators of Asclepias tuberosa . Ecology 77, 1061–1073. [Google Scholar]
- Fleming, T. H. , Geiselman, C. & Kress, W. J. (2009). The evolution of bat pollination: a phylogenetic perspective. Annals of Botany 104, 1017–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto, D. (2019). Precise sternotribic pollination by settling moths in Adenophora maximowicziana (Campanulaceae). International Journal of Plant Sciences 180, 200–208. [Google Scholar]
- Funamoto, D. & Ohashi, K. (2017). Hidden floral adaptation to nocturnal moths in an apparently bee‐pollinated flower, Adenophora triphylla var. japonica (Campanulaceae). Plant Biology 19, 767–774. [DOI] [PubMed] [Google Scholar]
- Futuyma, D. J. & Moreno, G. (1988). The evolution of ecological specialization. Annual Review of Ecology and Systematics 19, 207–233. [Google Scholar]
- Galen, C. & Cuba, J. (2001). Down the tube: pollinators, predators, and the evolution of flower shape in the alpine skypilot, Polemonium viscosum . Evolution 55, 1963–1971. [DOI] [PubMed] [Google Scholar]
- Galen, C. , Zimmer, K. A. & Newport, M. E. (1987). Pollination in floral scent morphs of Polemonium viscosum: a mechanism for disruptive selection on flower size. Evolution 41, 599. [DOI] [PubMed] [Google Scholar]
- Garland, T. (2014). Trade‐offs. Current Biology 24, R60–R61. [DOI] [PubMed] [Google Scholar]
- Gavrutenko, M. , Reginato, M. , Kriebel, R. , Nicolas, A. N. & Michelangeli, F. A. (2020). Evolution of floral morphology and symmetry in the Miconieae (Melastomataceae): multiple generalization trends within a specialized family. International Journal of Plant Sciences 181, 732–747. [Google Scholar]
- Gegear, R. J. , Burns, R. & Swoboda‐Bhattarai, K. A. (2017). Hummingbird floral traits interact synergistically to discourage visitation by bumble bee foragers. Ecology 98, 489–499. [DOI] [PubMed] [Google Scholar]
- Gigord, L. D. B. , Macnair, M. R. & Smithson, A. (2001). Negative frequency‐dependent selection maintains a dramatic flower color polymorphism in the rewardless orchid Dactylorhiza sambucina (L.) Soò. Symposium A Quarterly Journal In Modern Foreign Literatures 98, 6253–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez, J. M. & Perfectti, F. (2010). Evolution of complex traits: the case of Erysimum corolla shape. International Journal of Plant Sciences 171, 987–998. [Google Scholar]
- Gómez, J. M. & Zamora, R. (1999). Generalization vs. specialization in the pollination system of Hormathophylla spinosa (Cruciferae). Ecology 80, 796–805. [Google Scholar]
- Gómez, J. M. & Zamora, R. (2006). Ecological factors that promote the evolution of generalization in pollination systems. In Plant‐Pollinator Interactions: From Specialization to Generalization (eds Waser N. M. and Ollerton J.), pp. 145–166. University of Chicago Press, Chicago. [Google Scholar]
- Gould, J. L. & Gould, C. G. (1988). The Honey Bee. Scientific American Library, New York. [Google Scholar]
- Grant, V. & Grant, K. A. (1965). Flower Pollination in the Phlox Family. Columbia University Press, New York. [Google Scholar]
- Gross, K. , Sun, M. & Schiestl, F. P. (2016). Why do floral perfumes become different? Region‐specific selection on floral scent in a terrestrial orchid. PLoS One 11, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbert, A. (2000). Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behavioral Ecology and Sociobiology 48, 36–43. [Google Scholar]
- Guzmán, B. , Gómez, J. M. & Vargas, P. (2017). Is floral morphology a good predictor of floral visitors to Antirrhineae (snapdragons and relatives)? Plant Biology 19, 515–524. [DOI] [PubMed] [Google Scholar]
- Haran, R. , Izhaki, I. & Dafni, A. (2018). Specialists nectarivorous birds (Cinnyris osea) steal nectar whereas omnivourous birds are pollen transfer vectors of Anagyris foetida . Journal of Pollination Ecology 23, 82–89. [Google Scholar]
- Harder, L. D. & Thomson, J. D. (1989). Evolutionary options for maximizing pollen dispersal of animal‐pollinated plants. American Naturalist 133, 323–344. [Google Scholar]
- Hargreaves, A. L. , Harder, L. D. & Johnson, S. D. (2012). Floral traits mediate the vulnerability of aloes to pollen theft and inefficient pollination by bees. Annals of Botany 109, 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves, A. L. , Langston, G. T. & Johnson, S. D. (2019). Narrow entrance of short‐tubed Aloe flowers facilitates pollen transfer on long sunbird bills. South African Journal of Botany 124, 23–28. [Google Scholar]
- Hasegawa, Y. , Suyama, Y. & Seiwa, K. (2015). Variation in pollen‐donor composition among pollinators in an entomophilous tree species, Castanea crenata, revealed by single‐pollen genotyping. PLoS One 10, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp, A. , Li, X. , Hansson, B. S. , Baldwin, I. T. , Knaden, M. & Yon, F. (2019). Flower movement balances pollinator needs and pollen protection. Ecology 100, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera, C. M. (1987). Components of pollinator ‘quality’: comparative analysis of a diverse insect assemblage. Oikos 50, 79. [Google Scholar]
- Herrera, C. M. (1988). Variation in mutualisms: the spatiotemporal mosaic of a pollinator assemblage. Biological Journal of the Linnean Society 35, 95–125. [Google Scholar]
- Herrera, C. M. (1990). Daily patterns of pollinator activity, differential pollinating effectiveness, and floral resource availability, in a summer‐flowering Mediterranean shrub. Oikos 58, 277. [Google Scholar]
- Herrera, C. M. (1996). Floral traits and plant adaptation to insect pollinators: a devil's advocate approach. In Floral Biology, pp. 65–87. Springer, Boston. [Google Scholar]
- Herrera, C. M. , Medrano, M. , Rey, P. J. , Sánchez‐Lafuente, A. M. , Garcia, M. B. , Guitián, J. & Manzaneda, A. J. (2002). Interaction of pollinators and herbivores on plant fitness suggests a pathway for correlated evolution of mutualism‐ and antagonism‐related traits. Proceedings of the National Academy of Sciences of the United States of America 99, 16823–16828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz, C. C. & Schemske, D. W. (1990). Spatiotemporal variation in insect mutualists of a neotropical herb. Ecology 71, 1085–1097. [Google Scholar]
- Hurlbert, A. H. , Hosoi, S. A. , Temeles, E. J. & Ewald, P. W. (1996). Mobility of Impatiens capensis flowers: effect on pollen deposition and hummingbird foraging. Oecologia 105, 243–246. [DOI] [PubMed] [Google Scholar]
- Hutchinson, G. E. (1957). Concluding remarks: cold Sprig Harbor symposia on quantitative biology. In Cold Sprig Harbor Symposia on Quantitative Biology, pp. 66–77. Yale University, New Haven. [Google Scholar]
- Inoue, K. & Amano, M. (1986). Evolution of Campanula punctata lam. In the Izu Islands: changes of pollinators and evolution of breeding systems. Plant Species Biology 1, 89–97. [Google Scholar]
- Inouye, D. W. (1980). The terminology of floral larceny. Ecology 61, 1251–1253. [Google Scholar]
- Irwin, R. E. , Adler, L. S. & Brody, A. K. (2004). The dual role of floral traits: pollinator attraction and plant defense. Ecology 85, 1503–1511. [Google Scholar]
- Irwin, R. E. & Brody, A. K. (2011). Additive effects of herbivory, nectar robbing and seed predation on male and female fitness estimates of the host plant Ipomopsis aggregata . Oecologia 166, 681–692. [DOI] [PubMed] [Google Scholar]
- Ishii, H. S. & Sakai, S. (2001). Effects of display size and position on individual floral longevity in racemes of Narthecium asiaticum (Liliaceae). Functional Ecology 15, 396–405. [Google Scholar]
- Ito, K. , Suzuki, M. F. & Mochizuki, K. (2021). Evolution of honest reward signal in flowers. Proceedings of the Royal Society B: Biological Sciences 288, 20202848. 10.1098/rspb.2020.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, S. D. , Hargreaves, A. L. & Brown, M. (2006). Dark, bitter‐tasting nectar functions as a filter of flower visitors in a bird‐pollinated plant. Ecology 87, 2709–2716. [DOI] [PubMed] [Google Scholar]
- Johnson, S. D. & Steiner, K. E. (2000). Generalization versus specialization in plant pollination systems. Trends in Ecology & Evolution 15, 140–143. [DOI] [PubMed] [Google Scholar]
- Johnson, S. D. & Wester, P. (2017). Stefan Vogel's analysis of floral syndromes in the south African flora: an appraisal based on 60 years of pollination studies. Flora 232, 200–206. [Google Scholar]
- Jürgens, A. , Glück, U. , Aas, G. & Dötterl, S. (2014). Diel fragrance pattern correlates with olfactory preferences of diurnal and nocturnal flower visitors in Salix caprea (Salicaceae). Botanical Journal of the Linnean Society 175, 624–640. [Google Scholar]
- Jürgens, A. , Witt, T. & Gottsberger, G. (2012). Pollen grain size variation in Caryophylloideae: a mixed strategy for pollen deposition along styles with long stigmatic areas? Plant Systematics and Evolution 298, 9–24. [Google Scholar]
- Kemp, J. E. & Ellis, A. G. (2019). Cryptic petal coloration decreases floral apparency and herbivory in nocturnally closing daisies. Functional Ecology 33, 2130–2141. [Google Scholar]
- Kessler, D. , Diezel, C. & Baldwin, I. T. (2010). Changing pollinators as a means of escaping herbivores. Current Biology 20, 237–242. [DOI] [PubMed] [Google Scholar]
- Kessler, D. , Kallenbach, M. , Diezel, C. , Rothe, E. , Murdock, M. & Baldwin, I. T. (2015). How scent and nectar influence floral antagonists and mutualists. eLife 4, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande, R. & Arnold, S. J. (1983). The measurement of selection on correlated characters. Evolution 37, 1210–1226. [DOI] [PubMed] [Google Scholar]
- Lange, R. S. & Scott, P. E. (1999). Hummingbird and bee pollination of Penstemon pseudospectabilis . Journal of the Torrey Botanical Society 126, 99. [Google Scholar]
- Laverty, T. M. & Plowright, R. C. (1985). Competition between hummingbirds and bumble bees for nectar in flowers of Impatiens biflora . Oecologia 66, 25–32. [DOI] [PubMed] [Google Scholar]
- Levins, R. (1968). Evolution in Changing Environments. Princeton University Press, Princeton. [Google Scholar]
- Liu, A. , Li, D.‐Z. , Wang, H. & Kress, W. J. (2002). Ornithophilous and chiropterophilous pollination in Musa itinerans (Musaceae), a pioneer species in tropical rain forests of Yunnan, southwestern China. Biotropica 34, 254–260. [Google Scholar]
- Lloyd, D. G. (1984). Variation strategies of plants in heterogeneous environments. Biological Journal of the Linnean Society 21, 357–385. [Google Scholar]
- Lord, E. M. & Eckard, K. J. (1984). Incompatibility between the dimorphic flowers of Collomia grandiflora, a cleistogamous species. Science 223, 695–696. [DOI] [PubMed] [Google Scholar]
- Lunau, K. , Papiorek, S. , Eltz, T. & Sazima, M. (2011). Avoidance of achromatic colours by bees provides a private niche for hummingbirds. Journal of Experimental Biology 214, 1607–1612. [DOI] [PubMed] [Google Scholar]
- Macior, L. W. (1986). Floral resource sharing by bumblebees and hummingbirds in Pedicularis (Scrophulariaceae) pollination. Bulletin of the Torrey Botanical Club 113, 101–109. [Google Scholar]
- Makino, T. T. & Ohashi, K. (2017). Honest signals to maintain a long‐lasting relationship: floral colour change prevents plant‐level avoidance by experienced pollinators. Functional Ecology 31, 831–837. [Google Scholar]
- Makino, T. T. , Ohashi, K. & Sakai, S. (2007). How do floral display size and the density of surrounding flowers influence the likelihood of bumble bee revisitation to a plant? Functional Ecology 21, 87–95. [Google Scholar]
- Makino, T. T. & Sakai, S. (2007). Experience changes pollinator responses to floral display size: from size‐based to reward‐based foraging. Functional Ecology 21, 854–863. [Google Scholar]
- Maloof, J. E. & Inouye, D. W. (2000). Are nectar robbers cheaters or mutualists? Ecology 81, 2651–2661. [Google Scholar]
- Martén‐Rodríguez, S. , Almarales‐Castro, A. & Fenster, C. B. (2009). Evaluation of pollination syndromes in Antillean Gesneriaceae: evidence for bat, hummingbird and generalized flowers. Journal of Ecology 97, 348–359. [Google Scholar]
- Martén‐Rodríguez, S. , Fenster, C. B. , Agnarsson, I. , Skog, L. E. & Zimmer, E. A. (2010). Evolutionary breakdown of pollination specialization in a Caribbean plant radiation. New Phytologist 188, 403–417. [DOI] [PubMed] [Google Scholar]
- Matsuki, Y. , Tateno, R. , Shibata, M. & Isagi, Y. (2008). Pollination efficiencies of flower‐visiting insects as determined by direct genetic analysis of pollen origin. American Journal of Botany 95, 925–930. [DOI] [PubMed] [Google Scholar]
- McCall, A. C. & Irwin, R. E. (2006). Florivory: the intersection of pollination and herbivory. Ecology Letters 9, 1351–1365. [DOI] [PubMed] [Google Scholar]
- McCallum, B. & Chang, S. M. (2016). Pollen competition in style: effects of pollen size on siring success in the hermaphroditic common morning glory, Ipomoea purpurea . American Journal of Botany 103, 460–470. [DOI] [PubMed] [Google Scholar]
- Miller, T. J. , Raguso, R. A. & Kay, K. M. (2014). Novel adaptation to hawkmoth pollinators in Clarkia reduces efficiency, not attraction of diurnal visitors. Annals of Botany 113, 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minckley, R. L. & Roulston, T. H. (2006). Incidental mutualisms and pollen specialization among bees. In Plant‐Pollinator Interactions: From Specialization to Generalization (eds Waser N. M. and Ollerton J.), pp. 69–98. University of Chicago Press, Chicago. [Google Scholar]
- Miyake, T. & Yahara, T. (1998). Why does the flower of Lonicera japonica open at dusk? Canadian Journal of Botany 76, 1806–1811. [Google Scholar]
- Miyake, T. & Yahara, T. (1999). Theoretical evolution of pollen transfer and diurnal evaluation by nocturnal: when should a flower open ? Oikos 2, 233–240. [Google Scholar]
- Morinaga, S. I. , Kumano, Y. , Ota, A. , Yamaoka, R. & Sakai, S. (2009). Day‐night fluctuations in floral scent and their effects on reproductive success in Lilium auratum . Population Ecology 51, 187–195. [Google Scholar]
- Muchhala, N. (2003). Exploring the boundary between pollination syndromes: bats and hummingbirds as pollinators of Burmeistera cyclostigmata and B. tenuiflora (Campanulaceae). Oecologia 134, 373–380. [DOI] [PubMed] [Google Scholar]
- Muchhala, N. (2007). Adaptive trade‐off in floral morphology mediates specialization for flowers pollinated by bats and hummingbirds. American Naturalist 169, 494–504. [DOI] [PubMed] [Google Scholar]
- Muchhala, N. , Brown, Z. , Armbruster, W. S. & Potts, M. D. (2010). Competition drives specialization in pollination systems through costs to male fitness. American Naturalist 176, 732–743. [DOI] [PubMed] [Google Scholar]
- Muchhala, N. , Johnsen, S. & Smith, S. D. (2014). Competition for hummingbird pollination shapes flower color variation in Andean Solanaceae. Evolution 68, 2275–2286. [DOI] [PubMed] [Google Scholar]
- Muchhala, N. & Thomson, J. D. (2010). Fur versus feathers: pollen delivery by bats and hummingbirds and consequences for pollen production. American Naturalist 175, 717–726. [DOI] [PubMed] [Google Scholar]
- Newman, E. , Manning, J. & Anderson, B. (2014). Matching floral and pollinator traits through guild convergence and pollinator ecotype formation. Annals of Botany 113, 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi, K. (2002). Consequences of floral complexity for bumblebee‐mediated geitonogamous self‐pollination in Salvia nipponica miq. (Labiatae). Evolution 56, 2414–2423. [DOI] [PubMed] [Google Scholar]
- Ohashi, K. , Makino, T. T. & Arikawa, K. (2015). Floral colour change in the eyes of pollinators: testing possible constraints and correlated evolution. Functional Ecology 29, 1144–1155. [Google Scholar]
- Ohashi, K. & Thomson, J. D. (2009). Trapline foraging by pollinators: its ontogeny, economics and possible consequences for plants. Annals of Botany 103, 1365–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerton, J. (1996). Reconciling ecological processes with phylogenetic patterns: the apparent paradox of plant‐pollinator systems. The Journal of Ecology 84, 767. [Google Scholar]
- Ollerton, J. , Alarcon, R. , Waser, N. M. , Price, M. V. , Watts, S. , Cranmer, L. , Hingston, A. , Peter, C. I. & Rotenberry, J. (2009). A global test of the pollination syndrome hypothesis. Annals of Botany 103, 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellmyr, O. (1986). Three pollination morphs in Cimicifuga simplex; incipient speciation due to inferiority in competition. Oecologia 68, 304–307. [DOI] [PubMed] [Google Scholar]
- Price, M. V. , Waser, N. M. , Irwin, R. E. , Campbell, D. R. & Brody, A. K. (2005). Temporal and spatial variation in pollination of a montane herb: a seven‐year study. Ecology 86, 2106–2116. [Google Scholar]
- Prieto‐Benítez, S. , Dötterl, S. & Giménez‐Benavides, L. (2016). Diel variation in flower scent reveals poor consistency of diurnal and nocturnal pollination syndromes in Sileneae. Journal of Chemical Ecology 41, 1095–1104. [DOI] [PubMed] [Google Scholar]
- Proctor, M. , Yeo, P. & Lack, A. (1996). The Natural History of Pollination. HarperCollins Publishers, New York. [Google Scholar]
- Raup, D. M. & Michelson, A. (1965). Theoretical morphology of the coiled shell. Science 147, 1294–1295. [DOI] [PubMed] [Google Scholar]
- Reynolds, R. J. , Dudash, M. R. & Fenster, C. B. (2010). Multiyear study of multivariate linear and nonlinear phenotypic selection on floral traits of hummingbird‐pollinated Silene virginica . Evolution 64, 358–369. [DOI] [PubMed] [Google Scholar]
- Robinson, B. W. , Wilson, D. S. & Shea, G. (1996). Trade‐offs of ecological specialization: an intraspecific comparison of pumpkinseed sunfish phenotypes. Ecology 77, 170–178. [Google Scholar]
- Roff, D. A. & Fairbairn, D. J. (2007). The evolution of trade‐offs: where are we? Journal of Evolutionary Biology 20, 433–447. [DOI] [PubMed] [Google Scholar]
- Rojas‐Nossa, S. V. , Sánchez, J. M. & Navarro, L. (2016). Nectar robbing: a common phenomenon mainly determined by accessibility constraints, nectar volume and density of energy rewards. Oikos 125, 1044–1055. [Google Scholar]
- Rosas‐Guerrero, V. , Aguilar, R. , Martén‐Rodríguez, S. , Ashworth, L. , Lopezaraiza‐Mikel, M. , Bastida, J. M. & Quesada, M. (2014). A quantitative review of pollination syndromes: do floral traits predict effective pollinators? Ecology Letters 17, 388–400. [DOI] [PubMed] [Google Scholar]
- Sakamoto, R. L. , Morinaga, S. I. , Ito, M. & Kawakubo, N. (2012). Fine‐scale flower‐visiting behavior revealed by using a high‐speed camera. Behavioral Ecology and Sociobiology 66, 669–674. [Google Scholar]
- Sarkissian, T. S. & Harder, L. D. (2001). Direct and indirect responses to selection on pollen size in Brassica rapa L. Journal of Evolutionary Biology 14, 456–468. [Google Scholar]
- Schemske, D. W. & Horvitz, C. C. (1984). Variation among floral visitors in pollination ability: a precondition for mutualism specialization. Science 225, 519–521. [DOI] [PubMed] [Google Scholar]
- Schiestl, F. P. , Kirk, H. , Bigler, L. , Cozzolino, S. & Desurmont, G. A. (2014). Herbivory and floral signaling: phenotypic plasticity and trade‐offs between reproduction and indirect defense. New Phytologist 203, 257–266. [DOI] [PubMed] [Google Scholar]
- Schiestl, F. P. & Johnson, S. D. (2013). Pollinator‐mediated evolution of floral signals. Trends in Ecology and Evolution 28, 307–315. [DOI] [PubMed] [Google Scholar]
- Shuttleworth, A. & Johnson, S. D. (2009). The importance of scent and nectar filters in a specialized wasp‐pollination system. Functional Ecology 23, 931–940. [Google Scholar]
- Shuttleworth, A. & Johnson, S. D. (2012). The Hemipepsis wasp‐pollination system in South Africa: a comparative analysis of trait convergence in a highly specialized plant guild. Botanical Journal of the Linnean Society 168, 278–299. [Google Scholar]
- Sidlauskas, B. (2008). Continuous and arrested morphological diversification in sister clades of characiform fishes: a phylomorphospace approach. Evolution 62, 3135–3156. [DOI] [PubMed] [Google Scholar]
- Sletvold, N. (2019). The context dependence of pollinator‐mediated selection in natural populations. International Journal of Plant Sciences 180, 934–943. [Google Scholar]
- Sletvold, N. , Moritz, K. K. & Ågren, J. (2015). Additive effects of pollinators and herbivores result in both conflicting and reinforcing selection on floral traits. Ecology 96, 214–221. [DOI] [PubMed] [Google Scholar]
- Smith, S. D. , Ané, C. & Baum, D. A. (2008). The role of pollinator shifts in the floral diversification of Iochroma (Solanaceae). Evolution 62, 793–806. [DOI] [PubMed] [Google Scholar]
- Smithson, A. & Macnair, M. R. (1996). Frequency‐dependent selection by pollinators: mechanisms and consequences with regard to behaviour of bumblebees Bombus terrestris (L.) (hymenoptera: Apidae). Journal of Evolutionary Biology 9, 571–588. [Google Scholar]
- Soper Gorden, N. L. & Adler, L. S. (2018). Consequences of multiple flower–insect interactions for subsequent plant–insect interactions and plant reproduction. American Journal of Botany 105, 1835–1846. [DOI] [PubMed] [Google Scholar]
- Stebbins, G. L. (1970). Adaptive radiation of reproductive characteristics in angiosperms, I: pollination mechanisms. Annual Review of Ecology and Systematics 1, 307–326. [Google Scholar]
- Stephenson, A. G. & Thomas, W. W. (1977). Diurnal and nocturnal pollination of Catalpa speciosa (Bignoniaceae). Systematic Botany 2, 191. [Google Scholar]
- Strauss, S. Y. & Whittall, J. B. (2006). Non‐pollinator agents of selection on floral traits. In Ecology and Evolution of Flowers (eds Harder L. D. and Barrett S. C. H.), pp. 120–138. Oxford University Press, New York. [Google Scholar]
- Suzuki, K. , Dohzono, I. & Hiei, K. (2007). Evolution of pollinator generalization in bumblebee‐pollinated plants. Plant Species Biology 22, 141–159. [Google Scholar]
- Suzuki, M. F. & Ohashi, K. (2014). How does a floral colour‐changing species differ from its non‐colour‐changing congener? ‐ A comparison of trait combinations and their effects on pollination. Functional Ecology 28, 549–560. [Google Scholar]
- Teixido, A. L. , Duarte, M. O. , Ballego‐Campos, I. , Sanín, D. , Cunha, J. S. , Oliveira, C. S. & Silveira, F. A. O. (2019). One for all and all for one: retention of colour‐unchanged old flowers increases pollinator attraction in a hermaphroditic plant. Plant Biology 21, 167–175. [DOI] [PubMed] [Google Scholar]
- Tepedino, J. (1981). The pollination efficiency of the squash bee (Peponapis pruinosa) and the honey bee (Apis mellifera) on summer squash (Cucurbita pepo). Journal of the Kansas Entomological Society 54, 359–377. [Google Scholar]
- Theis, N. & Adler, L. S. (2012). Advertising to the enemy: enhanced floral fragrance increases beetle attraction and reduces plant reproduction. Ecology 93, 430–435. [DOI] [PubMed] [Google Scholar]
- Thomson, J. (2003). When is it mutualism? American Naturalist 162, S1–S9. [DOI] [PubMed] [Google Scholar]
- Thomson, J. D. & Thomson, B. A. (1992). Pollen presentation and viability schedules in animal‐pollinated plants: consequences for reproductive success. In Ecology and Evolution of Plant Reproduction (ed. Wyatt R.), pp. 1–24. Chapman & Hall, New York. [Google Scholar]
- Thomson, J. D. & Wilson, P. (2008). Explaining evolutionary shifts between bee and hummingbird pollination: convergence, divergence, and directionality. International Journal of Plant Sciences 169, 23–38. [Google Scholar]
- Thomson, J. D. , Wilson, P. , Valenzuela, M. & Malzone, M. (2000). Pollen presentation and pollination syndromes, with special reference to Penstemon. Plant Species Biology 15, 11–29. [Google Scholar]
- Thompson, J. N. (1994). The Coevolutionary Process. University of Chicago Press, Chicago. [Google Scholar]
- Tripp, E. A. & Manos, P. S. (2008). Is floral specialization an evolutionary dead‐end? Pollination system transitions in Ruellia (Acanthaceae). Evolution 62, 1712–1737. [DOI] [PubMed] [Google Scholar]
- van der Niet, T. , Pirie, M. D. , Shuttleworth, A. , Johnson, S. D. & Midgley, J. J. (2014). Do pollinator distributions underlie the evolution of pollination ecotypes in the cape shrub Erica plukenetii? Annals of Botany 113, 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pijl, L. (1961). Ecological aspects of flower evolution. II. Zoophylous flower classes. Evolution 15, 44–59. [Google Scholar]
- Venable, D. L. (1985). The evolutionary ecology of seed heteromorphism. American Naturalist 126, 577–595. [Google Scholar]
- Vereecken, N. J. , Wilson, C. A. , Hötling, S. , Schulz, S. , Banketov, S. A. & Mardulyn, P. (2012). Pre‐adaptations and the evolution of pollination by sexual deception: Cope's rule of specialization revisited. Proceedings of the Royal Society B: Biological Sciences 279, 4786–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonhof, M. J. & Harder, L. D. (1995). Size‐number trade‐offs and pollen production by papilionaceous legumes. American Journal of Botany 82, 230–238. [Google Scholar]
- Wang, L. L. , Zhang, C. , Yang, M. L. , Zhang, G. P. , Zhang, Z. Q. , Yang, Y. P. & Duan, Y. W. (2017). Intensified wind pollination mediated by pollen dimorphism after range expansion in an ambophilous biennial Aconitum gymnandrum . Ecology and Evolution 7, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser, N. M. (1982). A comparison of distances flown by different visitors to flowers of the same species. Oecologia 55, 251–257. [DOI] [PubMed] [Google Scholar]
- Waser, N. M. (1983). Competition for pollination and floral character differences among sympatric plant species: a review of evidence. In Handbook of Experimental Pollination Biology (eds Jones C. E. and Little R. J.), pp. 277–293. Van Nostrand Reinhold, New York. [Google Scholar]
- Waser, N. M. (2006). Specialization and generalization in plant‐pollinator interactions: a historical perspective. In Plant‐Pollinator Interactions: From Specialization to Generalization (eds Waser N. M. and Ollerton J.), pp. 3–18. University of Chicago Press. [Google Scholar]
- Waser, N. M. , Chittka, L. , Price, M. V. , Williams, N. M. & Ollerton, J. (1996). Generalization in pollination systems, and why it matters. Ecology 77, 1043–1060. [Google Scholar]
- Weis, A. E. , Abrahamson, W. G. & Andersen, M. C. (1992). Variable selection on Eurosta's gall size, I: the extent and nature of variation in phenotypic selection. Evolution 46, 1674. [DOI] [PubMed] [Google Scholar]
- Weiss, M. R. (1995). Floral color change: a widespread functional convergence. American Journal of Botany 82, 167–185. [Google Scholar]
- Whittall, J. B. & Hodges, S. A. (2007). Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 447, 706–709. [DOI] [PubMed] [Google Scholar]
- Willmer, P. G. (1983). Thermal constraints on activity patterns in nectar‐feeding insects. Ecological Entomology 8, 455–469. [Google Scholar]
- Willmer, P. G. (2011). Pollination and Floral Ecology. Princeton University Press, Princeton. [Google Scholar]
- Wilson, P. & Thomson, J. D. (1991). Heterogeneity among floral visitors leads to discordance between removal and deposition of pollen. Ecology 72, 1503–1507. [Google Scholar]
- Wilson, P. & Thomson, J. D. (1996). How do flowers diverge? In Floral Biology (eds Lloyd D. G. and Barrett S. C. H.), pp. 88–111. Chapman & Hall, New York. [Google Scholar]
- Yoshida, M. , Itoh, Y. , Ômura, H. , Arikawa, K. & Kinoshita, M. (2015). Plant scents modify innate colour preference in foraging swallowtail butterflies. Biology Letters 11, 20150390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zych, M. , Junker, R. R. , Nepi, M. , Stpiczyńska, M. , Stolarska, B. & Roguz, K. (2019). Spatiotemporal variation in the pollination systems of a supergeneralist plant: is Angelica sylvestris (Apiaceae) locally adapted to its most effective pollinators? Annals of Botany 123, 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]


