Abstract
Aim
To evaluate the impact of dulaglutide 3.0 and 4.5 mg versus 1.5 mg on body weight in patients with type 2 diabetes (T2D) based on exploratory analyses of the AWARD‐11 trial.
Materials and Methods
Patients were randomized to once‐weekly dulaglutide 1.5 (n = 612), 3.0 (n = 616) or 4.5 mg (n = 614) for 52 weeks. The primary objective was superiority of dulaglutide 3.0 and/or 4.5 mg over 1.5 mg in HbA1c reduction at 36 weeks. Secondary and exploratory assessments included weight reduction in the overall trial population and baseline body mass index (BMI) and HbA1c subgroups.
Results
At baseline, patients had a mean age of 57.1 years, HbA1c 8.6% (70 mmol/mol), weight 95.7 kg and BMI 34.2 kg/m2. At 36 weeks, dulaglutide 3.0 and 4.5 mg were superior to 1.5 mg for weight change from baseline (1.5 mg, −3.1 kg; 3.0 mg, −4.0 kg [P = .001]; 4.5 mg, −4.7 kg [P < .001]). Higher dulaglutide doses were associated with numerically greater weight reduction compared with 1.5 mg in each baseline BMI and HbA1c subgroup. Absolute weight reduction increased with increasing BMI category, but percentage weight loss was similar between subgroups. Weight reductions with dulaglutide were greater in patients with lower versus higher baseline HbA1c.
Conclusions
In patients with T2D, inadequately controlled by metformin, incremental weight loss was observed with dulaglutide 1.5, 3.0 and 4.5 mg doses regardless of baseline BMI or HbA1c. Although absolute weight loss was numerically greater in patients with higher baseline BMI, percentage of weight loss was similar between BMI subgroups.
Keywords: dulaglutide, type 2 diabetes, weight
1. INTRODUCTION
Excess weight is a major risk factor for diabetes, and 60% of patients with type 2 diabetes (T2D) have obesity (body mass index [BMI] ≥ 30 kg/m2) and probably have insulin resistance. 1 , 2 , 3 , 4 People with T2D who are also overweight or have obesity are at a greater risk of cardiovascular (CV) disease, early mortality and other complications. 1 Weight reduction may help improve insulin resistance, HbA1c and outcomes in patients with T2D. 4 , 5 Clinical guidelines advocate for weight management for the treatment of T2D, including the recommendation to consider the effect on weight when selecting a glucose‐lowering agent. 6 , 7 , 8 , 9
The glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) class has proven to be effective at lowering blood glucose, decreasing weight and, in some CV outcomes trials, providing a CV benefit for people with T2D. 10 , 11 , 12 Dulaglutide is a once‐weekly GLP‐1 RA that improves glycaemic control as an adjunct to diet and exercise in patients with T2D, has a low risk of hypoglycaemia, 13 , 14 and reduces the risk of major adverse CV events in adults with T2D who have established CV disease or multiple CV risk factors. 13 , 15 The AWARD‐11 trial showed superior HbA1c reduction of up to 1.9% and weight loss of up to 5.0 kg in patients with T2D inadequately controlled on metformin by using higher doses of dulaglutide (3.0 and 4.5 mg) compared with 1.5 mg. 16 The following exploratory analyses further examined the effect of dulaglutide on weight in patients with T2D.
2. MATERIALS AND METHODS
2.1. Study design and participants
AWARD‐11 was a randomized, phase 3, double‐blind, multicentre, parallel‐arm study conducted at 203 sites in 15 countries. The study included three periods: a 2‐week lead‐in period, followed by a 52‐week treatment period (36‐week primary efficacy time point) and a 4‐week safety follow‐up period. The study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. Patients provided written informed consent, and protocols were approved by local ethical review boards. The study design and patient inclusion/exclusion criteria were previously published 16 (ClinicalTrials.gov identifier NCT03495102). Eligible patients were adults with T2D for 6 months or longer, HbA1c of 7.5% or higher (≥58 mmol/mol) and 11.0% or less (≤97 mmol/mol), BMI of 25 kg/m2 or higher, insulin‐ and GLP‐1 RA‐naive, and taking metformin (≥1500 mg/d) for 3 months or longer.
Patients were randomized 1:1:1 to once‐weekly dulaglutide 1.5, 3.0 or 4.5 mg administered via subcutaneous injection with a single‐dose pen. Patients initiated treatment with dulaglutide 0.75 mg for 4 weeks, followed by stepwise dose escalation every 4 weeks to the randomized dose of 1.5, 3.0 or 4.5 mg.
2.2. Outcomes
The primary and secondary objectives of AWARD‐11 were previously reported. 16 This report describes additional analyses of the secondary outcome measure of overall weight reduction and prespecified exploratory efficacy measures of the proportion of patients achieving weight‐loss thresholds (≥5% and ≥10%), and the proportion of patients achieving prespecified composite endpoints of (a) HbA1c less than 7.0% (<53 mmol/mol), no weight gain (≤0%) and no documented symptomatic (plasma glucose ≤70 mg/dL) or severe hypoglycaemia as defined by the American Diabetes Association (ADA), 17 and (b) HbA1c less than 7.0% (<53 mmol/mol), weight loss of 5% or higher, and no documented symptomatic or severe hypoglycaemia. In addition, post hoc analyses of change in weight as a percentage of baseline weight were performed. The primary time point for all efficacy measures was 36 weeks, with efficacy analyses through 52 weeks considered exploratory.
Patient‐reported outcome (PRO) measures included Impact of Weight on Self‐Perception (IW‐SP) and Ability to Perform Physical Activities of Daily Living (APPADL) questionnaires. Patients' self‐perception related to their weight was assessed using the IW‐SP, a three‐item questionnaire that has shown validity, reliability and responsiveness in individuals with T2D. 18 The APPADL questionnaire contains seven items that assess how difficult it is for patients to engage in various physical activities considered integral to normal daily life. 19 , 20 Two other PRO questionnaires were administered: the generic health‐related quality of life tool EQ‐5D‐5L and the Diabetes Injection Device Experience Questionnaire (DID‐EQ). The DID‐EQ was administered at week 12 only (or at the early termination visit if it occurred prior to week 12); all other PRO measures were assessed at baseline, 36 and 52 weeks. Additional details regarding the EQ‐5D and DID‐EQ are provided in Appendix S1.
Key safety outcomes by randomized treatment were previously published. 16 Here, common treatment‐emergent adverse events (TEAEs) among patients in baseline BMI subgroups along with common TEAEs in the highest quartile of weight loss compared with the overall study population are reported, and change from baseline in weight is compared between patients with and without reported nausea, vomiting and diarrhoea during the 52‐week study period.
2.3. Statistical analysis
Efficacy analyses for the current report were conducted in the intent‐to‐treat population, defined as all patients randomized who received at least one dose of study drug. For the prespecified analyses of the primary and secondary study objectives at 36 weeks reported previously, two different estimands were defined: an efficacy estimand and a treatment‐regimen estimand. 16 In addition, the efficacy estimand was prespecified for use across all other efficacy analyses, including exploratory endpoints, subgroup analyses and efficacy analyses through 52 weeks. Thus, the results reported herein use the efficacy estimand only, which included data for all patients up to the point of either initiation of any new antihyperglycaemic medication for more than 14 days or premature treatment discontinuation. Safety analyses were conducted in the safety population, defined as all patients randomized who received at least one dose of study drug.
The primary analysis model was a mixed model for repeated measures (MMRM), including pooled country, treatment, visit and treatment‐by‐visit interaction as fixed effects and baseline value as a covariate. Baseline HbA1c stratum (≥8.5% and <8.5% [69 mmol/mol]) was added in the MMRM model as a fixed effect for weight and PRO analyses except for DID‐EQ. For DID‐EQ analysis, an analysis of covariance model was used with pooled country, baseline HbA1c stratum and treatment as fixed effects.
The proportions of patients achieving weight targets were analysed using a longitudinal logistic regression model for repeated measures with pooled country, baseline HbA1c stratum, treatment, visit and treatment‐by‐visit interaction as fixed effects, and baseline weight as a covariate. The proportions of patients achieving composite endpoints were analysed using a logistic regression model with pooled country and treatment as fixed effects, and baseline HbA1c and baseline weight as covariates.
Prespecified analyses assessed weight loss in baseline HbA1c subgroups (<8.5% [69 mmol/mol] or ≥8.5%) and post hoc analyses assessed weight loss in baseline BMI subgroups as defined by clinical practice guidelines (overweight [BMI <30 kg/m2], class I obesity [30 to <35 kg/m2], class II obesity [35 to <40 kg/m2] or class III obesity [≥40 kg/m2]). Prespecified analyses assessing weight loss in additional baseline BMI subgroups (<median [33.2 kg/m2] or ≥ median) are reported in Appendix S1. Analyses were conducted within each subgroup using the same MMRM model as for the overall population. The treatment‐by‐subgroup interaction was analysed using the same MMRM model plus fixed effects of subgroup, treatment‐by‐subgroup, visit‐by‐subgroup and visit‐by‐treatment‐by‐subgroup, and the interaction P value of treatment‐by‐subgroup at 36 or 52 weeks was used to evaluate the subgroup interaction effect using a significance level of .10, unadjusted.
3. RESULTS
3.1. Patient baseline characteristics
A total of 1842 patients were randomized to a dulaglutide dose (1.5 mg, 612; 3.0 mg, 616; 4.5 mg, 614). Baseline characteristics in the overall population have been previously reported and were comparable across treatment arms. 16 A summary of key baseline characteristics by clinically relevant BMI subgroup is provided in Table S1 and by HbA1c subgroup and additional BMI subgroup in Table S2. Baseline HbA1c differs between HbA1c subgroups, while baseline weight and BMI are similar. Between BMI subgroups, BMI and weight are different, while baseline HbA1c is similar. The mean baseline weight for each BMI subgroup category was 77.1 kg (overweight), 91.3 kg (class I obesity), 105.3 kg (class II obesity) and 123.0 kg (class III obesity).
3.2. Weight change in the overall study population
All three dulaglutide doses resulted in significant least‐squares mean (LSM) reductions in weight from baseline at the 36‐week primary time point (1.5 mg, −3.1 ± 0.19 kg; 3.0 mg, −4.0 ± 0.19 kg; 4.5 mg, −4.7 ± 0.19 kg; P < .001, all) (Figure 1A). Both dulaglutide 3.0 mg (estimated treatment difference [ETD] = −0.9 kg; P < .001) and 4.5 mg (ETD = −1.6 kg; P < .001) were superior to 1.5 mg. Weight decreased further through 52 weeks in all dose groups, with significantly greater changes from baseline in both the 3.0 mg (ETD = −0.8 kg; P = .006) and 4.5 mg (ETD = −1.6 kg; P < .001) groups compared with 1.5 mg (Figure 1A).
FIGURE 1.
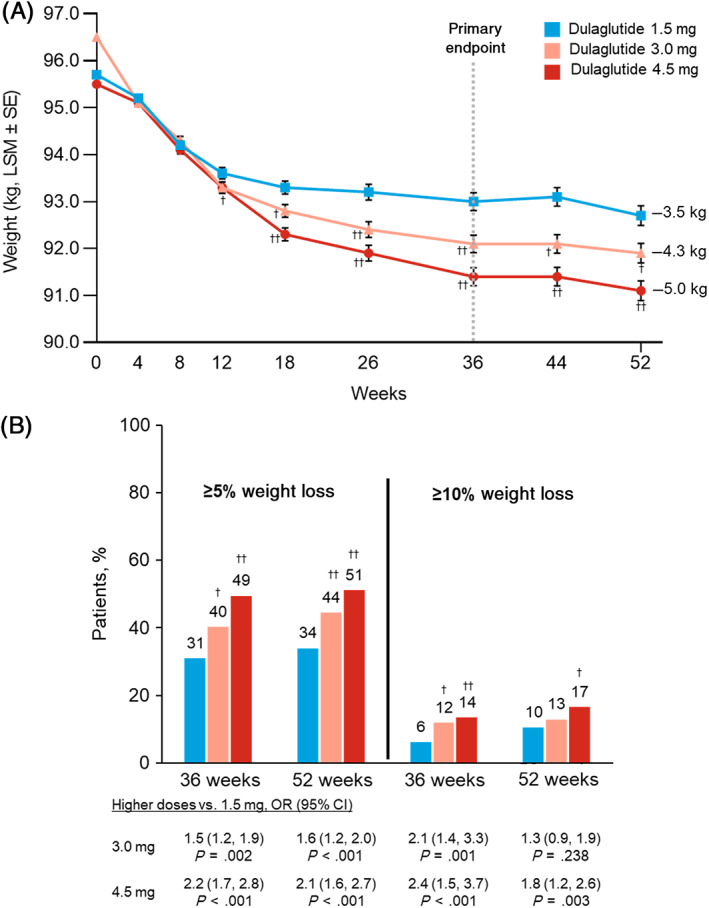
Change in weight over time and proportion of patients achieving weight‐loss thresholds through 52 weeks. A, Change in weight over time, MMRM. B, Proportion of patients achieving weight‐loss thresholds, longitudinal logistic regression. †,†† P < .05 or P < .001 versus dulaglutide 1.5 mg, respectively. All analyses included data collected up to either initiation of any new antihyperglycaemic medication for more than 14 days (regardless of whether the investigator indicated that it was for severe persistent hyperglycaemia) or premature treatment discontinuation, whichever occurred first. CI, confidence interval; LSM, least‐squares mean; MMRM, mixed model for repeated measures; OR, odds ratio; SE, standard error
A significantly larger proportion of patients treated with dulaglutide 3.0 and 4.5 mg achieved 5% or higher weight reduction compared with 1.5 mg at both 36 and 52 weeks (Figure 1B). The proportion of patients achieving 10% or higher weight reduction at 36 and 52 weeks was also significantly greater in the 4.5 mg group versus the 1.5 mg group, whereas the difference between 3.0 and 1.5 mg was only significant at 36 weeks (Figure 1B).
3.3. Weight change by subgroups
3.3.1. Baseline BMI
Treatment with all three dulaglutide doses resulted in significant dose‐related LSM reductions in weight from baseline in all baseline BMI categories at both the primary 36‐week time point and at 52 weeks (P < .001, all) (Figure 2A). At both 36 and 52 weeks, the absolute weight change from baseline was numerically greater for patients with higher baseline BMI for all three dulaglutide doses. There was no significant treatment‐by‐BMI‐subgroup interaction (36 weeks, P interaction = .905; 52 weeks, P interaction = .767). The percentage weight change from baseline was generally similar for each dulaglutide dose regardless of baseline BMI at both 36 and 52 weeks (Figure 2B) with no significant treatment‐by‐BMI‐subgroup interaction (36 weeks, P interaction = .473; 52 weeks, P interaction = .149).
FIGURE 2.
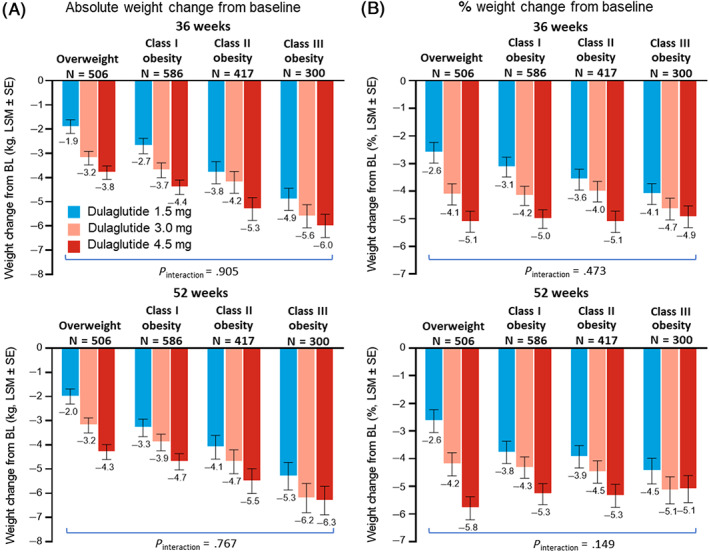
Change in weight by clinically relevant baseline body mass index (BMI) subgroup. A, Absolute change in weight by baseline BMI subgroup at 36 weeks (top) and 52 weeks (bottom), MMRM. B, Percentage change in weight by baseline BMI subgroup at 36 weeks (top) and 52 weeks (bottom), MMRM. All analyses included data collected up to either initiation of any new antihyperglycaemic medication for more than 14 days (regardless of whether the investigator indicated that it was for severe persistent hyperglycaemia) or premature treatment discontinuation, whichever occurred first. N, patients with non‐missing baseline value and at least one non‐missing postbaseline value of the response variable. P values are for treatment‐by‐subgroup interaction evaluated using a significance level of .10, unadjusted. BL, baseline; ETD, estimated treatment difference versus dulaglutide 1.5 mg; LSM, least‐squares mean; MMRM, mixed model for repeated measures; SE, standard error
The proportion of patients achieving 5% or higher weight reduction was comparable across all baseline BMI subgroups for each dulaglutide dose at 36 and 52 weeks (Figure S1). The treatment‐by‐BMI‐subgroup interaction was not significant (36 weeks, P interaction = .890; 52 weeks, P interaction = .983). The proportion of patients who achieved 10% or higher weight loss was also generally comparable although more variable across baseline BMI subgroups (Figure S2).
3.3.2. Baseline HbA1c
Treatment with all three dulaglutide doses resulted in significant LSM reductions from baseline in weight, with greater weight loss in each of the higher dulaglutide dose groups compared with the 1.5 mg group in each of the baseline HbA1c subgroups (Figure S3C). Mean changes in weight from baseline through 52 weeks were numerically larger across all dulaglutide groups in patients with baseline HbA1c less than 8.5% (<69 mmol/mol; mean baseline weight = 96.6 kg) compared with those with baseline HbA1c of 8.5% or higher (≥69 mmol/mol; mean baseline weight = 95.1 kg) (Figure S3D). The treatment‐by‐HbA1c‐subgroup interaction was significant (P interaction = .064) for change in weight at the primary 36‐week time point, largely driven by the greater LSM change from baseline in weight in each of the higher dulaglutide dose groups versus the 1.5 mg dose in patients with baseline HbA1c of less than 8.5% (<69 mmol/mol).
Other prespecified exploratory analyses on weight included subgroups by baseline HbA1c of less than 8% (<64 mmol/mol) versus 8% or higher, country and region. The same pattern of results was observed for HbA1c subgroups of less than 8% (<64 mmol/mol) versus 8% or higher at the primary 36‐week time point (P interaction = .050; data not shown) compared with HbA1c subgroups of less than 8.5% (<69 mmol/mol) versus 8.5% or higher. The treatment‐by‐subgroup interaction was not significant for either of the following prespecified subgroup analyses on weight at the primary 36‐week time point: country (P interaction = .510) and region defined as United States versus European Union versus other (P interaction = .461). Therefore, there is no evidence that the treatment effect on weight varied between countries or regions.
3.4. Composite endpoints
At both 36 and 52 weeks, a significantly greater proportion of patients treated with dulaglutide 3.0 and 4.5 mg achieved the composite endpoints of HbA1c less than 7.0% (<53 mmol/mol), no documented symptomatic or severe hypoglycaemia, and either no weight gain (Figure 3A) or weight loss of 5% or higher (Figure 3B) compared with dulaglutide 1.5 mg.
FIGURE 3.
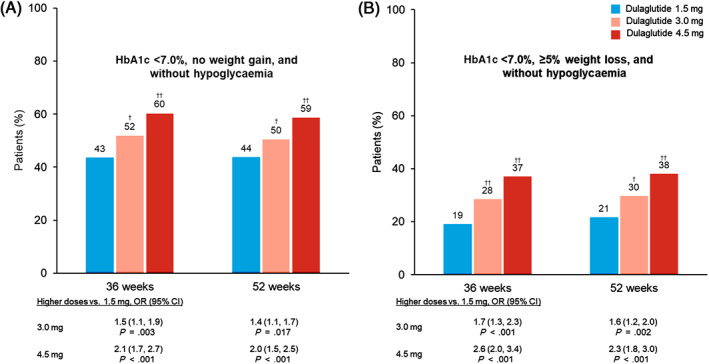
Proportion of patients achieving composite weight and glycaemic control endpoints. A, Proportion of patients achieving no weight gain (≤0%) and HbA1c <7% (53 mmol/mol) without hypoglycaemia (plasma glucose ≤70 mg/dL or severe hypoglycaemia), logistic regression. B, Proportion of patients achieving ≥5% weight loss and HbA1c <7.0% (53 mmol/mol) without hypoglycaemia, logistic regression. †,†† P < .05 or P < .001 versus dulaglutide 1.5 mg, respectively. All analyses included data collected up to either initiation of any new antihyperglycaemic medication for more than 14 days (regardless of whether the investigator indicated that it was for severe persistent hyperglycaemia) or premature treatment discontinuation, whichever occurred first. CI, confidence interval; OR, odds ratio
3.5. Patient‐reported outcomes
At the primary 36‐week time point, all three dulaglutide groups showed significant improvements from baseline on IW‐SP total score, indicating better self‐perception pertaining to weight, and on the APPADL total score, indicating an improvement in ability to perform activities of daily living (Figure 4). The improvements in IW‐SP at 36 weeks were larger in patients escalated to dulaglutide 3.0 mg (P = .026) compared with those maintained on 1.5 mg, while the difference between 4.5 and 1.5 mg was not statistically significant (P = .053). Compared with patients in the 1.5 mg group, the improvement in APPADL score at 36 weeks was larger in patients escalated to dulaglutide 4.5 mg (P = .018) but not 3.0 mg (P = .111). Improvements from baseline in IW‐SP and APPADL total scores were maintained at 52 weeks, but there were no significant differences between dulaglutide dose groups at this final time point. The DID‐EQ device characteristics score showed high positive perception of the injection device used in the study with no significant difference between the three dulaglutide doses at 12 weeks. Generic health‐related quality of life was assessed using the EQ‐5D. EQ‐5D‐5L visual analogue scale scores showed significant improvements from baseline for all three dulaglutide dose groups with no significant differences between groups at both 36 and 52 weeks. EQ‐5D‐5L UK showed significant improvements from baseline in all three dulaglutide dose groups at 36 weeks. Significant improvements were maintained at 52 weeks for the 3.0 and 4.5 mg dose groups with a significantly greater change from baseline compared with the 1.5 mg group. A summary of PRO scores is included in Table S3.
FIGURE 4.
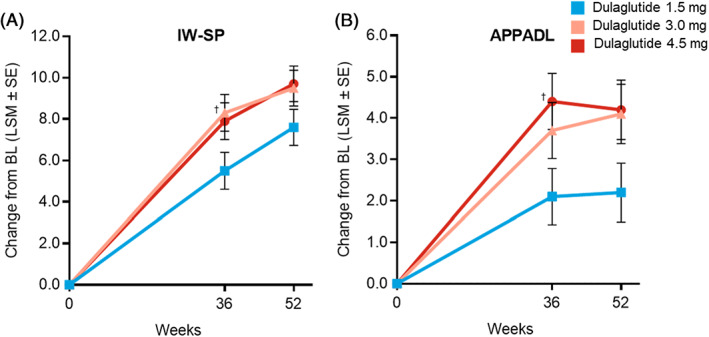
Change in weight‐related PRO measures (total transformed scores). A, Change over time in IW‐SP total transformed score, MMRM. B, Change over time in APPADL total transformed score, MMRM. † P < .05 versus dulaglutide 1.5 mg. All analyses included data collected up to either initiation of any new antihyperglycaemic medication for more than 14 days (regardless of whether the investigator indicated that it was for severe persistent hyperglycaemia) or premature treatment discontinuation, whichever occurred first. APPADL, Ability to Perform Physical Activities of Daily Living; BL, baseline; IW‐SP, Impact of Weight on Self‐Perception; LSM, least‐squares mean; MMRM, mixed model for repeated measures; PRO, patient‐reported outcome; SE, standard error
3.6. Adverse events by BMI subgroup
The occurrence of common TEAEs (reported in ≥5% of patients in any arm) among patients in each baseline BMI subgroup was assessed and compared qualitatively. The pattern and frequency of common TEAEs were found to be similar in each BMI subgroup and versus the overall population (Table S4).
3.7. Relationship between weight loss and adverse events
Two analyses were performed to evaluate whether weight loss with dulaglutide treatment was associated with the reporting of gastrointestinal (GI)‐related adverse events. The occurrence of TEAEs (reported in ≥5% of patients in any arm) was assessed among those patients in the top quartile of weight loss at 52 weeks and compared qualitatively with the corresponding TEAEs in the overall study population. The LSM change in weight from baseline to 52 weeks in this highest quartile of weight loss was −6.8 kg. The pattern and frequency of GI‐related TEAEs were similar between patients in the top quartile of weight loss versus the overall population. Notably, the incidence of nausea, vomiting and diarrhoea among patients who lost the most weight was similar to incidence in the overall study population (Table S5).
Additional analyses included comparing the LSM changes in weight from baseline among patients who did or did not report at least one TEAE of nausea, vomiting or diarrhoea through 52 weeks. A total of 1361 patients (73.9%) reported no TEAE of nausea, vomiting or diarrhoea and had at least one postbaseline weight measurement for inclusion in the analysis. Excluding patients who reported at least one of these GI‐related TEAEs had no notable impact on the magnitude of LSM change in weight compared with the overall study population. The change in weight from baseline at 52 weeks was similar between patients with or without reported TEAEs of nausea, vomiting or diarrhoea, and was similar to that of the overall study population (Table 1).
TABLE 1.
Change in body weight at 52 weeks overall and among patients reporting or not reporting ≥1 TEAE of nausea, vomiting or diarrhoea
| Dulaglutide dose (mg) | Change in weight from baseline (kg) | ||
|---|---|---|---|
| Total | Patients reporting no GI TEAEs a | Patients reporting ≥1 GI TEAEs a | |
| N = 1809 | N = 1361 | N = 448 | |
| 1.5 | −3.5 (0.21) | −3.6 (0.24) | −3.1 (0.47) |
| 3.0 | −4.3 (0.21) | −4.1 (0.24) | −5.0 (0.44) |
| 4.5 | −5.0 (0.21) | −5.0 (0.24) | −5.0 (0.42) |
Abbreviations: GI, gastrointestinal; LSM, least‐squares mean; MMRM, mixed model for repeated measures; TEAE, treatment‐emergent adverse event; SE, standard error.
Nausea, vomiting and/or diarrhoea. All values presented as LSM (SE), MMRM. N, patients with baseline and at least one postbaseline weight measurement.
4. DISCUSSION
Dulaglutide is an effective glucose‐lowering agent that is also associated with weight loss in patients with T2D. Prior studies in patients with T2D on metformin monotherapy treated with dulaglutide 1.5 mg once‐weekly resulted in a mean weight loss of approximately 3.0 kg through 26 weeks or more of therapy. 21 In the current AWARD‐11 study, mean weight loss with dulaglutide 1.5 mg at the primary 36‐week time point was similar to these prior studies (−3.1 kg), while significantly greater weight loss was observed in patients escalated to dulaglutide doses of 3.0 mg (−4.0 kg) or 4.5 mg (−4.7 kg), with further weight loss across all dulaglutide groups through 52 weeks of treatment.
Weight loss has favourable effects on insulin sensitivity 22 and glycaemic control 7 and is an important component of the overall clinical management of T2D. 6 , 7 Although a weight reduction of 5% or more is a recommended target according to standards of care 6 and regulatory guidance, 23 , 24 a weight reduction of 2%‐5% can produce clinically meaningful reductions in fasting blood glucose. 25 Larger weight reductions (≥10%) are associated with further improvement in markers of glycaemic control and cardiometabolic risk. 26 , 27 In this study, the odds of achieving these clinically relevant thresholds were increased by nearly 2‐fold in patients escalated to the 4.5 mg dose compared with those maintained on 1.5 mg. Consistent with the weight‐loss findings, there were significant improvements for all three dulaglutide groups in patients' self‐perception related to weight and ability to engage in activities of daily living as measured by the IW‐SP and APPADL, respectively. The clinical relevance of the change in IW‐SP and APPADL scores within groups is difficult to establish, as published minimally important changes for these instruments are sample‐specific and were obtained from a study conducted with a comparatively small (n = 40), predominantly African American (54%) sample with higher baseline weight than the AWARD‐11 study or general population with T2D. 18 , 20 Improvements in EQ‐5D‐5L scores from baseline showed a positive impact on generic health‐related quality of life. DID‐EQ scores assessing patient satisfaction and experience using the injection device were high and consistent with DID‐EQ scores for the dulaglutide device reported in a previous observational study. 28
The current analysis has shown that, regardless of baseline BMI, dulaglutide‐treated patients can achieve dose‐dependent weight reduction, which is important for patients with T2D who are overweight or obese. Patients with higher BMI at baseline had greater absolute weight loss compared with those with lower baseline BMI across all dose groups. However, patients had a similar mean percentage weight loss across the BMI subgroups, with those escalated to the 4.5 mg dose having an average weight loss of around 5% in the overall study population and in each BMI subgroup.
Additional weight reduction with dulaglutide dose escalation was observed regardless of baseline HbA1c category. Changes in weight across all dulaglutide dose groups, as well as dose‐related treatment differences in weight loss, were larger among patients with lower (<8.5% [69 mmol/mol]) versus higher (≥8.5%) baseline HbA1c. This is consistent with prior studies suggesting an inverse relationship between the magnitude of GLP‐1 RA‐mediated weight loss and baseline HbA1c. 21 , 29 , 30 The physiological basis for this phenomenon is not clear, but could be related to greater reductions in energy loss through glucosuria following greater improvements in glycaemic control observed in the higher baseline HbA1c subgroup (and thus poorer baseline glycaemic control) compared with the lower baseline HbA1c subgroup. 30 , 31 , 32 In other words, weight loss may be partially mitigated by greater calorie (energy) retention dictated by the diminished glucosuria occurring in subjects with more pronounced baseline hyperglycaemia. Importantly, clinically relevant weight loss is observed with GLP‐1 RA treatment regardless of baseline HbA1c, but these subgroup findings suggest that the magnitude of weight loss after initiation of these medications may be larger among patients with better baseline glycaemic control.
The ADA suggests multiple goals of therapy for the treatment of T2D, such as HbA1c less than 7% (<53 mmol/mol), no incidence of hypoglycaemia and weight reduction. 33 Composite endpoints allow assessment of both the clinical benefit and potential risks of a therapy. Importantly, a greater proportion of patients randomized to the higher dulaglutide doses were able to achieve glycaemic control with weight loss or no weight gain without hypoglycaemia as measured by the prespecified composite endpoints evaluated in this study. These endpoints may be important to patients and providers, as they provide an estimate of the proportion of patients that can reach both their glycaemic and weight‐management goals.
Weight loss with dulaglutide treatment was not driven by the occurrence of nausea, vomiting or diarrhoea. Similar weight reduction was observed between patients who did not experience these GI‐related adverse events and the overall study population, and patients in the highest quartile of weight loss did not report a higher frequency of GI‐related events. These findings are consistent with prior studies showing little, if any, contribution of GI adverse events to the overall weight‐loss effect mediated by dulaglutide or other GLP‐1 RAs. 26 , 34 GLP‐1 RA‐mediated weight loss is probably multifactorial, including delayed gastric emptying and increased satiety to reduce food intake. 35
The study population included patients with chronic hyperglycaemia who required intensification of antihyperglycaemic therapy; thus, the results may not be generalizable to patients who do not meet these criteria. Although patients were asked to not initiate an organized diet and/or exercise weight reduction programme during the study and were provided with standard of care lifestyle and dietary counselling for T2D, the study was not designed to specifically assess the effect of intervention on weight loss. Other than the analysis of body weight at the primary 36‐week time point, all of the endpoints and analyses presented in this report were defined in the protocol as exploratory and not controlled for multiple testing under the prespecified graphical testing method. Although all but one of the subgroup analyses presented in this report were prespecified, they are still considered exploratory and hypothesis‐generating. Additional study limitations were previously published. 16
In conclusion, compared with patients treated with once‐weekly dulaglutide 1.5 mg, patients escalated to either dulaglutide 3.0 or 4.5 mg had further incremental reductions in weight regardless of baseline BMI or HbA1c, and were significantly more probable to achieve clinically important weight‐loss thresholds and composite glycaemic and weight‐control endpoints. Weight loss is a critical component of the overall management of T2D for most patients, because it helps improve glycaemic control and other outcomes associated with the disease. Because T2D is a progressive disease, treatment intensification over time is required to enable patients to achieve their treatment goals, generally requiring treatment with multiple agents. The availability of higher dulaglutide doses beyond 1.5 mg may provide additional benefits on glycaemic control and weight without increasing the complexity of diabetes treatment.
CONFLICT OF INTEREST
EB declares advisory boards and consulting: Abbott, AstraZeneca, Becton Dickinson, Boehringer Ingelheim, Bristol Myers Squibb, Bruno Farmaceutici, Daiichi‐Sankyo, Eli Lilly and Company, Janssen, Johnson & Johnson, Merck Sharp and Dohme, Mundipharma, Novartis, Novo Nordisk, Roche, Sanofi, Servier and Takeda. JPF declares research support: Allergan, AstraZeneca, Boehringer Ingelheim, BMS, Eli Lilly and Company, Intercept, Janssen, Madrigal, Metacrine, Merck, NorthSea Therapeutics, Novartis, Novo Nordisk, Oramed, Pfizer, Poxil, Sanofi and Theracos; advisory boards and consulting: Akero, Altimmune, Axcella Health, Boehringer Ingelheim, Coherus Therapeutics, Echosens, 89bio, Eli Lilly and Company, Gilead, Intercept, Merck, Novo Nordisk and Sanofi; and speaker bureau: Merck and Sanofi. FJT has received speaker's bureau and consultant/advisory board fees from AstraZeneca, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, MSD (Merck & Co.), Novartis Pharmaceuticals Co., Novo Nordisk and Sanofi Aventis. JV declares research support: Eli Lilly and Company, Pfizer and Sanofi. REM, RM, MAB, AYMK and DAC are employees of Eli Lilly and Company and own stock in the company. ZY is a former employee of Eli Lilly and Company and is currently an employee of Bristol Myers Squibb.
AUTHOR CONTRIBUTIONS
DAC contributed to the trial design. ZY was responsible for the statistical considerations in the analysis and trial design. MAB and DAC are the guarantors of this work and, as such, take responsibility for the integrity of the data and the accuracy of the data analysis. All authors participated in critical review and interpretation of the data for the manuscript. All authors had full access to the data related to these studies and approved the final version submitted for publication. Some of the data were presented at the Endocrine Society annual meeting held virtually 8‐22 June 2020; 80th annual meeting for the American Diabetes Association held virtually 12‐16 June 2020; and 81st annual meeting for the American Diabetes Association held virtually 25‐29 June 2021.
Supporting information
Appendix S1: Supporting information.
ACKNOWLEDGEMENTS
This trial was sponsored by Eli Lilly and Company. We thank the trial investigators, trial staff and trial participants for their contributions; Sohini Raha, PhD, for additional statistical analysis; Luis‐Emilio Garcia‐Perez, MD, PhD, MBA, for critically reviewing the manuscript; and Alastair Knights, PhD, and Oralee Varnado, PhD, for writing support.
Bonora E, Frias JP, Tinahones FJ, et al. Effect of dulaglutide 3.0 and 4.5 mg on weight in patients with type 2 diabetes: Exploratory analyses of AWARD‐11. Diabetes Obes Metab. 2021;23(10):2242‐2250. 10.1111/dom.14465
DATA AVAILABILITY STATEMENT
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance (which will be June 2021). No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment for up to 2 years per proposal. For details on submitting a request, see the instructions provided at vivli.org.
REFERENCES
- 1. Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an Endocrine Society Scientific Statement. Endocr Rev. 2018;39(2):79‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Services UDoHaH . Management of Overweight and Obesity in Adults: Systematic Evidence Review from the Expert Panel; 2013. https://www.nhlbi.nih.gov/sites/default/files/media/docs/obesity-evidence-review.pdf. Accessed May 28, 2020.
- 4. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239‐2251. [DOI] [PubMed] [Google Scholar]
- 5. Salvia MG. The look AHEAD trial: translating lessons learned into clinical practice and further study. Diabetes Spectr. 2017;30(3):166‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association . 8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes‐2020. Diabetes Care. 2020;43(suppl 1):S89‐S97. [DOI] [PubMed] [Google Scholar]
- 7. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scheen AJ, Van Gaal LF. Combating the dual burden: therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):911‐922. [DOI] [PubMed] [Google Scholar]
- 10. Giugliano D, Maiorino MI, Bellastella G, Longo M, Chiodini P, Esposito K. GLP‐1 receptor agonists for prevention of cardiorenal outcomes in type 2 diabetes: an updated meta‐analysis including the REWIND and PIONEER 6 trials. Diabetes Obes Metab. 2019;21(11):2576‐2580. [DOI] [PubMed] [Google Scholar]
- 11. Nolen‐Doerr E, Stockman MC, Rizo I. Mechanism of glucagon‐like peptide 1 improvements in type 2 diabetes mellitus and obesity. Curr Obes Rep. 2019;8(3):284‐291. [DOI] [PubMed] [Google Scholar]
- 12. Trujillo JM, Nuffer W, Ellis SL. GLP‐1 receptor agonists: a review of head‐to‐head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eli Lilly and Company . Trulicity (dulaglutide) prescribing information. 2021. http://pi.lilly.com/us/trulicity-uspi.pdf. Accessed June 25, 2021.
- 14. Jendle J, Grunberger G, Blevins T, Giorgino F, Hietpas RT, Botros FT. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes Metab Res Rev. 2016;32(8):776‐790. [DOI] [PubMed] [Google Scholar]
- 15. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394(10193):121‐130. [DOI] [PubMed] [Google Scholar]
- 16. Frias JP, Bonora E, Nevarez Ruiz L, et al. Efficacy and safety of dulaglutide 3.0 mg and 4.5 mg versus dulaglutide 1.5 mg in metformin‐treated patients with type 2 diabetes in a randomized controlled trial (AWARD‐11). Diabetes Care. 2021;44(3):765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayes RP, DeLozier AM. Reliability, validity, and responsiveness of the Impact of Weight on Self‐Perceptions Questionnaire (IW‐SP) in individuals with type 2 diabetes and obesity. Diabetes Technol Ther. 2015;17(3):210‐214. [DOI] [PubMed] [Google Scholar]
- 19. Hayes RP, Nelson DR, Meldahl ML, Curtis BH. Ability to perform daily physical activities in individuals with type 2 diabetes and moderate obesity: a preliminary validation of the impact of weight on activities of daily living questionnaire. Diabetes Technol Ther. 2011;13(7):705‐712. [DOI] [PubMed] [Google Scholar]
- 20. Hayes RP, Schultz EM, Naegeli AN, Curtis BH. Test‐retest, responsiveness, and minimal important change of the ability to perform physical activities of daily living questionnaire in individuals with type 2 diabetes and obesity. Diabetes Technol Ther. 2012;14(12):1118‐1125. [DOI] [PubMed] [Google Scholar]
- 21. Gallwitz B, Dagogo‐Jack S, Thieu V, et al. Effect of once‐weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes Metab. 2018;20(2):409‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reaven G, Abbasi F, McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res. 2004;59:207‐223. [DOI] [PubMed] [Google Scholar]
- 23. European Medicines Agency . Guideline on clinical evaluation of medicinal products used in weight management; 2016. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-evaluation-medicinal-products-used-weight-management-revision-1_en.pdf. Accessed June 19, 2020.
- 24. Food and Drug Administration . Guidance for industry developing products for weight management; 2007. https://www.fda.gov/media/71252/download. Accessed June 19, 2020.
- 25. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985‐3023. [DOI] [PubMed] [Google Scholar]
- 26. Knell G, Li Q, Pettee Gabriel K, Shuval K. Long‐term weight loss and metabolic health in adults concerned with maintaining or losing weight: findings from NHANES. Mayo Clin Proc. 2018;93(11):1611‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matza LS, Boye KS, Currie BM, et al. Patient perceptions of injection devices used with dulaglutide and liraglutide for treatment of type 2 diabetes. Curr Med Res Opin. 2018;34(8):1457‐1464. [DOI] [PubMed] [Google Scholar]
- 29. Aroda VR, Capehorn MS, Chaykin L, et al. Impact of baseline characteristics and beta‐cell function on the efficacy and safety of subcutaneous once‐weekly semaglutide: a patient‐level, pooled analysis of the SUSTAIN 1‐5 trials. Diabetes Obes Metab. 2020;22(3):303‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dahlqvist S, Ahlen E, Filipsson K, et al. Variables associated with HbA1c and weight reductions when adding liraglutide to multiple daily insulin injections in persons with type 2 diabetes (MDI Liraglutide trial 3). BMJ Open Diabetes Res Care. 2018;6(1):e000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724‐1732. [DOI] [PubMed] [Google Scholar]
- 32. Pi‐Sunyer FX. Weight loss in type 2 diabetic patients. Diabetes Care. 2005;28(6):1526‐1527. [DOI] [PubMed] [Google Scholar]
- 33. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes‐2020. Diabetes Care. 2020;43(suppl 1):S98‐S110. [DOI] [PubMed] [Google Scholar]
- 34. Umpierrez GE, Pantalone KM, Kwan AYM, Zimmermann AG, Zhang N, Fernández LL. Relationship between weight change and glycaemic control in patients with type 2 diabetes receiving once‐weekly dulaglutide treatment. Diabetes Obes Metab. 2016;18(6):615‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shah M, Vella A. Effects of GLP‐1 on appetite and weight. Rev Endocr Metab Disord. 2014;15(3):181‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information.
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance (which will be June 2021). No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment for up to 2 years per proposal. For details on submitting a request, see the instructions provided at vivli.org.


