Abstract
Aim
To assess if sodium‐glucose co‐transporter‐2 inhibitors (SGLT2is) reduce the risk of all‐cause mortality, cardiovascular death and hospitalization for heart failure (HF) or chronic kidney disease (CKD) to a greater extent than dipeptidyl peptidase‐4 inhibitors (DPP4is) in people with type 2 diabetes (T2D) with or without established cardiovascular and/or renal disease (CVRD).
Methods
This retrospective cohort study propensity‐matched 24 438 patients receiving an SGLT2i 1:1 to a patient receiving a DDP4i, stratified based on the presence of CVRD. The primary outcomes were the time to each of the following: all‐cause mortality, cardiovascular death or hospitalization for HF, myocardial infarction, stroke and CKD.
Results
Overall, SGLT2is were associated with reductions in all‐cause mortality, cardiovascular mortality, hospitalization for HF and hospitalization for CKD compared with DPP4is. In patients with no CVRD history, SGLT2is were associated with reductions in all‐cause mortality (HR 0.71, 95% CI 0.57‐0.88; P = .002), hospitalization for HF (HR 0.76, 95% CI 0.59‐0.98; P = .035) and hospitalization for CKD (HR 0.75, 95% CI 0.63‐0.88; P < .001). In patients with established cardiovascular disease (CVD) or at high risk, SGLT2is were associated with reductions in all‐cause mortality (HR 0.69, 95% CI 0.59‐0.82; P < .001), cardiovascular mortality (HR 0.76, 95% CI 0.62‐0.95; P = .014), hospitalization for HF (HR 0.73, 95% CI 0.63‐0.85; P < .001), hospitalization for stroke (HR 0.75, 95% CI 0.59‐0.94; P = .013) and hospitalization for CKD (HR 0.49, 95% CI 0.43‐0.54; P < .001).
Conclusion
There was consistency across subgroups and sensitivity analyses. SGLT2is were associated with a reduced risk of all‐cause mortality and hospitalization for HF and CKD compared with DPP4‐is, highlighting the need to introduce SGLT2is early in the management of patients with T2D.
Keywords: cardiovascular disease, clinical trial, dapagliflozin, diabetes complications, dipeptidyl peptidase‐4 inhibitor, heart failure
1. INTRODUCTION
Most people with type 2 diabetes (T2D) eventually develop heart failure (HF), chronic kidney disease (CKD) or both, which increases the probability of hospitalization and premature death. 1 , 2 , 3 , 4 , 5
Indeed, T2D is associated with a 2.5‐fold higher risk of developing HF than in people without diabetes. 1 In people with HF, T2D is associated with the probability of first hospitalization (adjusted odds ratio [aOR]: 1.29; 95% confidence interval [CI]: 1.24‐1.34) and mortality (aOR: 1.24; 95% CI: 1.29‐1.40). 6 Another retrospective study of 55 959 patients aged 45 years and older with a new diagnosis of HF reported survival rates of 75.9% (95% CI 75.5%‐76.3%) after 1 year, 45.5% (95% CI 45.1%‐46.0%) after 5 years, 24.5% (95% CI 23.9%‐25.0%) after 10 years and 12.7% (95% CI 11.9%‐13.5%) after 15 years. 5 A cross‐sectional study reported that 58% of patients with T2D without known albuminuria developed CKD. 2 Crucially, HF and CKD alone doubled cardiovascular and all‐cause mortality (hazard ratio [HR] 2.02, 95% CI 1.75‐2.33 and 2.05, 95% CI 1.82‐2.32, respectively). In combination, the two diseases more than trebled cardiovascular and all‐cause mortality (HR 3.91, 95% CI 3.02‐5.07 and 3.14, 95% CI 2.90‐3.40, respectively). 7 Increasing evidence shows that sodium‐glucose co‐transporter‐2 inhibitors (SGLT2is) reduce the risk of HF and CKD, 8 , 9 , 10 , 11 regardless of whether the patient has diabetes 8 and background HF treatment. 9 Dipeptidyl peptidase‐4 inhibitors (DPP4is), one of the most widely prescribed second‐line treatment options for glucose‐lowering in people with T2D, have not shown similar benefits. Head‐to‐head trials comparing SGLT2is and DPP4is, however, are unlikely. Large‐scale real‐world evidence studies are, therefore, required to compare SGLT2is and DPP4is. In tandem with evidence from randomized controlled clinical trials (RCTs), several real‐world studies have shown that SGLT2is are associated with improved cardiorenal outcomes compared with other glucose‐lowering therapies across a wide range of patients, including those ineligible for RCTs. 10 , 11 , 12 , 13 , 14 A recent meta‐analysis reported that glucagon‐like peptide‐1 receptor agonists (GLP1‐RAs) and SGLT2is reduced the composite endpoint of myocardial infarction (MI), stroke and cardiovascular death by 12% and 11%, respectively. 13 SGLT2is also reduced hospitalizations for HF by 31% and the risk of a composite of worsening estimated glomerular filtration rate, end‐stage kidney disease or renal death by 45%. GLP1‐RAs did not significantly reduce these outcomes. 13
Recently, evidence of risk reduction in people without established cardiovascular co‐morbidities has started to emerge. In a multinational propensity‐matched observational study, cardiovascular and/or renal disease (CVRD)‐free new users of SGLT2is or DPP4is were propensity score‐matched 1:1. SGLT2is were associated with a 44% lower risk of cardiorenal disease (95% CI 0.42‐0.74) and a 29% (95% CI 0.59‐0.86) and 56% (95% CI 0.28‐0.69) reduced risk of HF and CKD, respectively. All‐cause and cardiovascular death were 33% (95% CI 0.59‐0.77) and 39% (95% CI 0.44‐0.85) lower, respectively, among those receiving SGLT2is. 15
The value of this study is that for the first time we have been able to map the DECLARE‐like cohorts to a single health service, which is the NHS in England. In addition, while the multinational paper only studied the CVRD‐free population, this paper shows the similarities (and differences) of the effects of SGLT2is in both those with and without CVRD.
DECLARE‐TIMI 58, which enrolled participants with T2D who had or were at risk of atherosclerotic cardiovascular disease (CVD), showed that the SGLT2i dapagliflozin reduced the composite outcome of cardiovascular death and hospitalization for HF. 16 However, DPP4is and sulphonylureas are the most commonly used antidiabetic drugs after metformin. 17 This is despite international management guidelines that now recommend SGLT2is for patients with atherosclerotic CVD in whom HF and/or CKD co‐exists or is of special concern. 18
Despite this evidence, globally SGLT2is are prescribed less commonly than DPP4is. 17 , 19 Against this background, we hypothesized that SGLT2is will reduce the risk of all‐cause mortality, cardiovascular death and hospitalization for HF or CKD to a greater extent than DPP4is in people with T2D with or without established CVRD. The population in this real‐world study mimics that of DECLARE‐TIMI 58, one of the largest studies assessing this group of patients.
2. MATERIALS AND METHODS
This retrospective cohort study used the Clinical Practice Research Datalink (CPRD) Aurum. This database contains routinely collected data from primary care practices in England about diagnoses, symptoms, prescriptions, referrals and tests for more than 19 million patients, and shows high levels (>90%) of correctness and completeness for data about T2D. 20 , 21
CPRD Aurum data were linked to Hospital Episode Statistics (HES), with detailed hospitalization admission information in England, as well as death registration (Office for National Statistics) data, which provide information on all‐cause and cause‐specific mortality. Patients with T2D were identified by diagnostic codes and confirmed by records of drug issue for glucose‐lowering drugs, as defined previously in Birkeland et al. 15 The new user index date refers to the initial prescription for an SGLT2i or DPP4i. Patients initiating an SGLT2i and a DPP4i on the same date and those with a prior prescription of the same drug class 12 months before initiation were excluded. The analysis followed patients with 2TD aged at least 18 years from the day after the index date (1 January 2013) until the earliest study outcome, death, moved out of the practice or study end date (30 November 2018).
People with type 1 or gestational diabetes were excluded. Enrolled patients were further classified into ‘CVRD‐free’ and ‘high risk or established CVD’ groups (equivalent to DECLARE‐TIMI). The CVRD‐free group had no known history of angina pectoris, myocardial infarction, HF, stroke, transient ischaemic attack, peripheral artery disease (PAD), CKD or prescription for nitrates. The high risk or established CVD groups included people aged 40 years or older with ischaemic heart disease or stroke or PAD, or men aged 55 years or older or women aged 60 years or older with hypertension or dyslipidaemia or who were current smokers. Diagnosis history for both groups was defined using both primary care (CPRD) and secondary care (HES) diagnoses. Co‐morbidities, and diagnostic and drug issues in primary care were defined from the presence of relevant SNOMED–CT codes obtained from clinical codes repositories (CALIBER, UCL Institute of Health Informatics and Data Compass, London School of Hygiene and Tropical Medicine). Co‐morbidities in secondary care as well as outcomes of specific hospitalizations reported were defined by the presence of ICD‐10 codes outlined previously in Birkeland et al. 7
The primary outcomes were the time to each of the following considered individually: all‐cause death, cardiovascular death or first recorded hospital diagnoses of HF, MI, stroke and CKD. Each patient receiving an SGLT2i was propensity score‐matched 1:1 to a patient using a DPP4i. The propensity score included age, sex, presence of microvascular complications, frailty and cardiovascular co‐morbidities (see the supporting information), following the method described by Birkeland et al., 15 and was based on intention to treat (1 January 2013 to 30 October 2018). To avoid immortal time bias, only the first episode of either SGLT2i or DPP4i treatment during the inclusion period was eligible.
Baseline characteristics are described using standard statistical measures including mean and standard deviations for numerical variables and frequencies and percentages for categorical variables. An imbalance in baseline characteristics was considered when a standardized difference of more than 10% occurred between the two groups. The time to first event was compared between groups using Cox proportional hazards models.
Results are presented as relative risk reductions (RRRs) or HR and 95% CI. Subgroup analyses investigated outcomes between treatment groups by baseline characteristics. Sensitivity analyses were performed 1) without saxagliptin, which is associated with an increased risk of hospitalization for HF; 2) in an on‐treatment, instead of intention‐to‐treat, analysis; 3) using outcomes recorded in the primary diagnostic position only of hospitalisations, and 4) with extra characteristics included in the propensity score matching (including CKD), extending the list of variables used in Birkeland et al. 15
In the multivariate Cox proportional hazard models, we adjusted for the following: age, sex, frailty (at least one hospitalization of three consecutive days within 1 year prior to index), use of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, statins, beta blockers, calcium channel blockers and aldosterone antagonists, loop diuretics and low ceiling diuretics.
3. RESULTS
Overall, 131 824 people with T2D were identified (Figure 1 and supporting information). Of these, 76 804 (58.3%) had no known history of previous CVRD and 80 172 (60.8%) had a documented history of CVD or were at high risk, as defined in the DECLARE‐TIMI 58 study. 16 Overall, 117 089 (88.8%) patients had a history of using metformin, 59 300 (50.0%) had used sulphonylureas and 16 964 (12.9%) were using insulin. After propensity matching, 24 438 new users of SGLT2is and the same number of new users of DPP4is were included in the ‘all patients’ group. Baseline characteristics were well balanced after propensity matching (Table 1). Patients who received SGLT2is tended to be 10 years younger and were more probable to have a history of receiving loop diuretics, GLP1‐RAs and insulin than those in the DPP4i group. The CVRD‐free group included 76 804 people, with 17 353 (22.6%) in each propensity‐matched group for SGLT2is and DPP4is. Both groups were followed for approximately 1.9 years (limited by the available duration of drugs in the UK). The high risk or established CVD group included 80 172 patients, with 11 175 (13.9%) in each matched group, who were followed for approximately 1.8 years. Initiation of an SGLT2i, compared with initiation of a DPP4i, was associated with a significantly lower risk of each of the following considered individually: all‐cause mortality, cardiovascular death and hospitalization for HF or CKD. In all patients, the RRR observed was about 27% for death from any cause (HR 0.73, 95% CI 0.63‐0.84), 20% for cardiovascular death (HR 0.80, 95% CI 0.66‐0.97), 26% for hospitalization for HF (HR 0.74, 95% CI 0.65‐0.84) and 49% for hospitalization for CKD (HR 0.51, 95% CI 0.47‐0.56, all P < .001; Figure 2).
FIGURE 1.
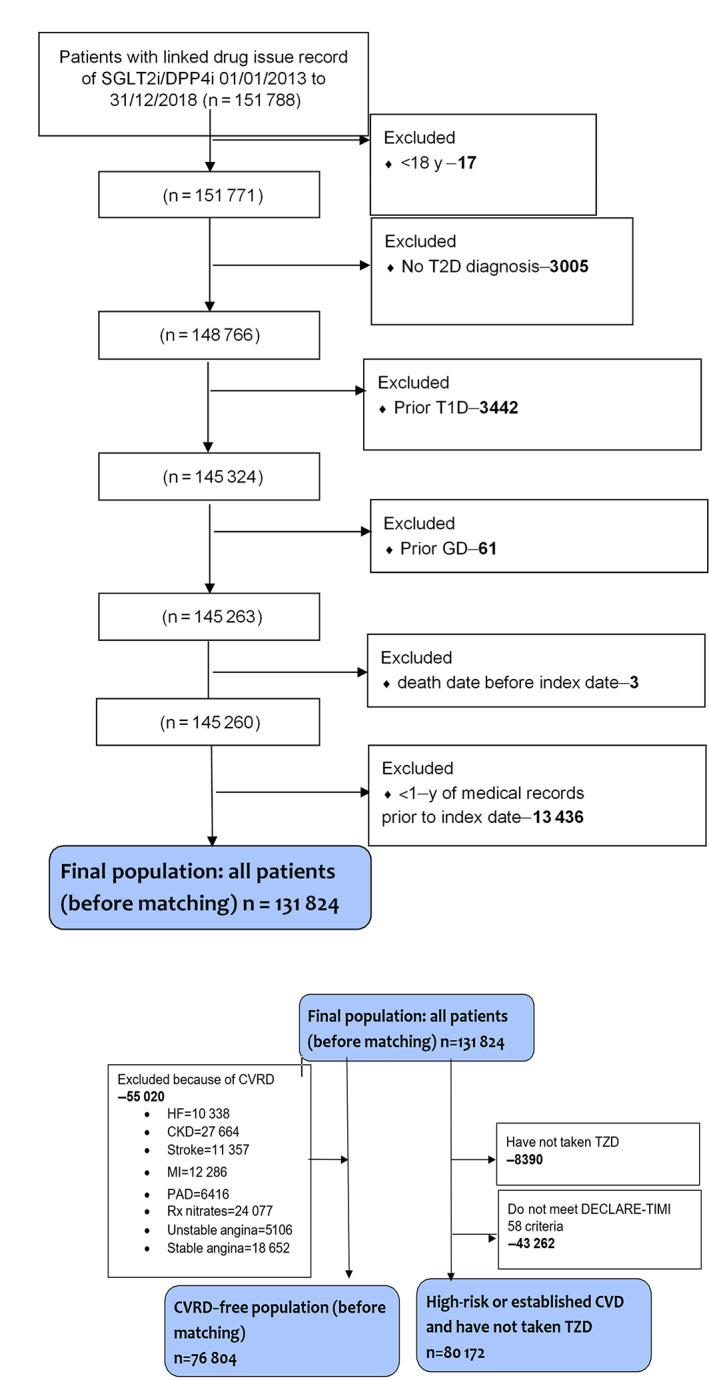
Patient disposition. CKD, chronic kidney disease; CVD, cardiovascular disease; CVRD, cardiovascular and/or renal disease; DPP4i, dipeptidyl peptidase‐4 inhibitor; GD, gestational diabetes; HF, heart failure; MI, myocardial infarction; PAD, peripheral artery disease; Rx, prescribed; SGLT2i, sodium‐glucose co‐transporter‐2 inhibitor; TZD, thiazolidinedione; T1D, type 1 diabetes; T2D, type 2 diabetes
TABLE 1.
Baseline characteristics of patients with type 2 diabetes without a history of cardiovascular and/or renal disease (CVRD) and those at high risk of cardiorenal disease: postmatching
| Variable | All patients | CVRD‐free | DECLARE‐like | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SGLT2i | DPP4i | StdD (%) a | SGLT2i | DPP4i | StdD (%) a | SGLT2i | DPP4i | StdD (%) a | |
| Number of patients | 24 438 | 24 438 | 17 353 | 17 353 | 11 367 | 11 367 | |||
| Age, y (SD) | 56.7 (10.94) | 56.1 (12.70) | −4.8 | 54.8 (10.89) | 54.3 (12.03) | −4.3 | 64.3 (7.24) | 64.1 (8.19) | −2.5 |
| Females, n (%) | 10 422 (42.6) | 10 352 (42.4) | −0.6 | 7759 (44.7) | 7741 (44.6) | −0.2 | 3695 (32.5) | 3815 (33.6) | 2.2 |
| Microvascular complications, n (%) | 5626 (23.0) | 5485 (22.4) | −1.4 | 3791 (21.8) | 3743 (21.6) | −0.7 | 2818 (24.8) | 2762 (24.3) | −1.1 |
| Frailty, n (%) | 5386 (22.0) | 5430 (22.2) | 0.4 | 5316 (30.6) | 5229 (30.1) | −1.1 | 1746 (15.4) | 1692 (14.9) | −1.3 |
| CVD prevention | |||||||||
| Statins, n (%) | 18 196 (74.5) | 18 107 (74.1) | −0.8 | 12 119 (69.8) | 12 039 (69.4) | −1.0 | 9773 (86.0) | 9772 (86.0) | 0.0 |
| Antihypertensives, n (%) | 15 633 (64.0) | 15 497 (63.4) | −1.2 | 9934 (57.2) | 9817 (56.6) | −1.4 | 8956 (78.8) | 8964 (78.9) | 0.2 |
| ACEi, n (%) | 10 929 (44.7) | 10 819 (44.3) | −0.9 | 7022 (40.5) | 6902 (39.8) | −1.4 | 6056 (53.3) | 5985 (52.7) | −1.3 |
| ARBs, n (%) | 3892 (15.9) | 3878 (15.9) | −0.2 | 2363 (13.6) | 2313 (13.3) | −0.8 | 2390 (21.0) | 2370 (20.8) | −0.4 |
| Beta blockers, n (%) | 4926 (20.2) | 4668 (19.1) | −2.7 | 1788 (10.3) | 1749 (10.1) | −0.7 | 3413 (30.0) | 3370 (29.6) | −0.8 |
| LOOP diuretics, n (%) | 1673 (6.8) | 1440 (5.9) | −3.9 | 545 (3.1) | 510 (2.9) | −1.2 | 1190 (10.5) | 1117 (9.8) | −2.1 |
| Aldosterone antagonists, n (%) | 477 (2.0) | 454 (1.9) | −0.7 | 121 (0.7) | 103 (0.6) | −1.3 | 356 (3.1) | 348 (3.1) | −0.4 |
| Glucose‐lowering drugs | |||||||||
| Metformin, n (%) | 22 780 (93.2) | 22 981 (94.0) | 3.4 | 16 325 (94.1) | 16 373 (94.4) | 1.2 | 10 543 (92.8) | 10 602 (93.3) | 2.0 |
| Sulphonylurea, n (%) | 9608 (39.3) | 9570 (39.2) | −0.3 | 6685 (38.5) | 6691 (38.6) | 0.1 | 4642 (40.8) | 4618 (40.6) | −0.4 |
| GLP1‐RA, n (%) | 2043 (8.4) | 1517 (6.2) | −8.3 | 1155 (6.7) | 829 (4.8) | −8.1 | 1084 (9.5) | 843 (7.4) | −7.6 |
| Thiazolidinediones, n (%) | 1730 (7.1) | 1682 (6.9) | −0.8 | 1268 (7.3) | 1231 (7.1) | −0.8 | – | – | 0.0 |
| Insulin, n (%) | 5238 (21.4) | 4652 (19.0) | −6.0 | 2835 (16.3) | 2614 (15.1) | −3.5 | 3100 (27.3) | 2930 (25.8) | −3.4 |
| Index year | |||||||||
| 2013 | 678 (2.8) | 678 (2.8) | 0.0 | 473 (2.7) | 473 (2.7) | 0.0 | 272 (2.4) | 272 (2.4) | 0.0 |
| 2014 | 2502 (10.2) | 2502 (10.2) | 0.0 | 1750 (10.1) | 1750 (10.1) | 0.0 | 1162 (10.2) | 1162 (10.2) | 0.0 |
| 2015 | 4393 (18.0) | 4393 (18.0) | 0.0 | 3032 (17.5) | 3032 (17.5) | 0.0 | 2057 (18.1) | 2057 (18.1) | 0.0 |
| 2016 | 4783 (19.6) | 4783 (19.6) | 0.0 | 3397 (19.6) | 3397 (19.6) | 0.0 | 2182 (19.2) | 2182 (19.2) | 0.0 |
| 2017 | 5531 (22.6) | 5531 (22.6) | 0.0 | 3997 (23.0) | 3997 (23.0) | 0.0 | 2570 (22.6) | 2570 (22.6) | 0.0 |
| 2018 | 6551 (26.8) | 6551 (26.8) | 0.0 | 4704 (27.1) | 4704 (27.1) | 0.0 | 3124 (27.5) | 3124 (27.5) | 0.0 |
Note: All numbers in parentheses are percentages if not stated otherwise. Frailty, three or more consecutive days in hospital within the year prior to index.
Abbreviations: ACEi, angiotensin‐converting enzyme inhibitor; ARBs, angiotensin receptor blockers; CVD, cardiovascular disease; DPP4i, dipeptidyl peptidase‐4 inhibitor; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; SD, standard deviation; SGLT2i, sodium‐glucose co‐transporter‐2 inhibitor; StdD, standardized difference.
An imbalance in baseline characteristics was considered when the standardized difference was >10%.
FIGURE 2.
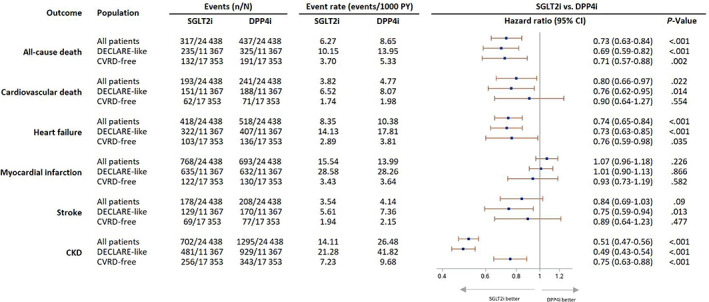
Risk of cardiovascular and renal disease in patients with type 2 diabetes (T2D) without a history of cardiovascular or renal disease and those at high risk of cardiorenal disease (DECLARE‐like). Adjusted Cox regression model with estimates of relative risk reduction. P value is from the test statistic for testing for difference in sodium‐glucose co‐transporter‐2 inhibitors (SGLT2is) versus dipeptidyl peptidase‐4 inhibitors (DPP4is). CKD, chronic kidney disease; CVRD, cardiovascular or renal disease; PY, person years
In patients with no previous CVRD history, initiation of a SGLT2i was associated with an RRR of 29% for death from any cause (HR 0.71, 95% CI 0.57‐0.88, P = .002), 24% for hospitalization for HF (HR 0.76, 95% CI 0.59‐0.98, P = .035) and 25% for hospitalization for CKD (HR 0.75, 95% CI 0.63‐0.88, P < .001; Figure 2). In patients with established CVD or at high risk, the RRR was 31% for death from any cause (HR 0.69, 95% CI 0.59‐0.82, P < .001), 24% for cardiovascular death (HR 0.76, 95% CI 0.62‐0.95, P = .014), 27% for hospitalization for HF (HR 0.73, 95% CI 0.63‐0.85, P < .001), 25% for hospitalization for stroke (HR 0.75, 95% CI 0.59‐0.94, P = .013) and 51% for hospitalization for CKD (HR 0.49, 95% CI 0.43‐0.54, P < .001; Figure 2).
Differences shown in all‐cause death, HF and CKD between the two groups are both statistically different and scientifically meaningful (all more than 20% reduction favouring the SGLT2i group). The results were consistent across a large number of subgroups, including sex, age (<65 and ≥65 years), history of cardiovascular and diabetes medication, and CKD (supporting information). A potential difference emerged among people with a history of cancer. For example, all‐cause mortality in the SGLT2i group was 61% lower (HR 0.39, 95% CI 0.28‐0.54) than among those receiving a DPP4i. The findings were consistent across subgroup analyses of all‐cause mortality, cardiovascular death, HF and CKD and in the sensitivity analyses where there was sufficient statistical power. In the subgroup analysis, there were no significant differences in outcomes by baseline HbA1c level with all CVD and renal outcomes, except with all‐cause mortality. For all‐cause mortality the results favour DPP4i (supporting information).
To help check for residual bias from unmeasured confounding, we repeated the analysis using (a) external injuries, and (b) diseases of the skin and subcutaneous tissue as negative control outcomes under the assumption that any sources of uncontrolled confounding in the main analysis would similarly lead to lower incidence of the negative control outcomes in the SGLT2i group. The finding of no association between treatment groups and the negative control outcomes provided additional support for the conclusion from the primary analysis (Figure S1 ).
4. DISCUSSION
Increasing evidence shows that SGLT2is reduce the risk of HF and CKD, 8 , 9 , 22 , 23 regardless of whether the patient has diabetes 8 and background HF treatment. 10 Neither natriuretic actions nor hypoglycaemia account for these cardiorenal benefits. 24 SGLT2is have only limited effects on the plasma volume and blood levels of natriuretic peptides. Although not included in the initial focus of the study, these benefits appear to be independent of the Hba1c effect, which was similar in both study groups. While further studies need to characterize the mechanisms of the reported cardiorenal benefits, it is probable, however, that the beneficial effects arise from the interaction of several direct and indirect actions. 25 However, it is unlikely that glucose lowering alone is responsible: other antidiabetic therapies that produce a greater hypoglycaemic effect do not show similar cardiorenal benefits of SGLT2is. 24 In this real‐word study, SGLT2is were associated with significant reductions in the RRR of all‐cause mortality (up to 31%), hospitalization for HF (up to 27%) and hospitalization for CKD (up to 51%) in comparison with DPP4is. The benefits emerged regardless of the presence or absence of established CVD and across a large number of subgroups and sensitivity analyses (supporting information data). This consistency suggests that the results are robust, clinically relevant and additional to the glycaemic benefits associated with SGLT2is (i.e. HbA1c values that were similar between the two groups; Table S1 ). Crucially, our analysis stratified patients based on the presence or absence of established CVRD, which is, to the best of the authors' knowledge, the first time this analysis has been performed in a real‐world cohort mimicking that of DECLARE‐TIMI 58.
The results are also consistent with findings from RCTs across the drug class. 11 , 22 , 26 , 27 , 28 The high risk or established CVD group in this real‐world study mimics that of DECLARE‐TIMI 58, which enrolled participants with T2D who had or were at risk of atherosclerotic CVD. 16 During a median follow‐up of 4.2 years, dapagliflozin (n = 8582) reduced cardiovascular death or hospitalization for HF by 17% (95% CI 0.73‐0.95), which reflected the 27% lower rate of hospitalization for HF (95% CI 0.61‐0.88) compared with placebo (n = 8578). The risk of renal events was 24% lower among those receiving dapagliflozin (95% CI 0.67‐0.87). 18 A meta‐analysis of three RCTs reported that SGLT2is reduced the risk of cardiovascular death or hospitalization for HF by 23% and the risk of renal disease progression by 45%. 22 An observational real‐world study propensity‐matched 209 867 new users of an SGLT2i 1:1 to new users of a DPP4i. During a mean of 0.9 years and compared with DPP4is, SGLT2is were associated with decreased risks of major adverse cardiovascular events (MACE; 16.5 and 11.4 per 1000 person years, respectively; HR 0.76, 95% CI 0.69‐0.84), MI (5.1 and 6.4 per 1000 person years, respectively; HR 0.82, 95% CI 0.70‐0.96), cardiovascular death (7.7 and 3.9 per 1000 person years, respectively; HR 0.60, 95% CI 0.54‐0.67), HF (7.7 and 3.1 per 1000 person years, respectively; HR 0.43, 95% CI 0.37‐0.51), all‐cause mortality (17.3 and 8.7 per 1000 person years, respectively; HR 0.60, 95% CI 0.54‐0.67) and a trend with ischaemic stroke (3.5 and 2.6 per 1000 person years, respectively; HR 0.85, 95% CI 0.72‐1.01). 29
In our analysis, an unexpected finding emerged among people with a history of cancer. For example, all‐cause mortality in the SGLT2i group was 61% lower (95% CI 0.28‐0.54) than in those receiving a DPP4i. A type 1 (false positive) statistical error cannot be excluded, especially as the analysis was not corrected for multiple comparisons. The finding was, however, apparent in subgroup analyses of all‐cause mortality, cardiovascular death, HF and CKD. Whether the reduction in all‐cause mortality in the cancer subgroup was driven by chance, cancer deaths or unmeasured confounding is unclear. The association should be investigated further in prospective studies.
This was a retrospective cohort study and, therefore, cannot establish causality. Nevertheless, the findings are in line with RCTs and real‐world evidence. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 26 , 27 The use of propensity matching meant that most patients were not included in the analysis. For example, 37% of the 131 824 patients identified were included in the propensity‐matched SGLT2i and DPP4i groups. The possibility that residual confounding by covariates not included in the propensity scores may have influenced the results cannot be excluded. The consistent results from subgroup and sensitivity analyses suggest, however, that the findings are robust and clinically relevant. Finally, the analysis was not able to distinguish potential differences between drugs within the same pharmacological class. A recent large real‐world observational study found similar benefits for MACE for canagliflozin, dapagliflozin and empagliflozin compared with DPP4is over a mean follow‐up of 0.9 years. 30 With respect to the weaker benefits of SGLT2is seen in the CVRD‐free population compared to the other two sub‐populations, this is not unexpected as the population initiated treatment without co‐morbid CVRD at baseline and are probable to be a ‘healthier’ population with a lower baseline risk of CVRD events.
In conclusion, SGLT2is are associated with significant reductions in all‐cause mortality, hospitalizations for HF and hospitalizations for CKD compared with DPP4is. Crucially, our analysis stratified patients based on the presence or absence of established CVRD, which is the first time this analysis has been performed in a real‐world cohort mimicking that of DECLARE‐TIMI 58 and reporting a wide range of outcomes, including mortality, cardiovascular and renal outcomes. These findings, taken together with the results of prospective RCTs and previous real‐world evidence, highlight the need to introduce SGLT2is early in the management of people with T2D to reduce the incidence of new onset HF and CKD and to reduce the risk of premature mortality.
Further analysis of these results should explore the impact on healthcare resources and the overall cost benefits arising from the cardiorenal actions of SGLT2is, continuing evaluation of the study over a longer time frame to observe any additional findings and matching of HbA1c as a predefined variable.
CONFLICT OF INTEREST
KK has acted as a consultant, speaker or received grants for investigator‐initiated studies for AstraZeneca, Novartis, Novo Nordisk, Sanofi–Aventis, Lilly and Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin–Chemie AG/Menarini Group, Janssen and Napp. II has acted as an advisory board member, speaker or received grants for Eli Lilly, Novo Nordisk, Merck Sharp & Dohme, AstraZeneca, Abbot Diabetes Care, Sanofi and Boehringer. RZ, JBM, MF and TM are employed by AstraZeneca UK Ltd, a biopharmaceutical company that develops, manufactures and markets medicines in the cardiovascular, renal and metabolic disease area. AB has received research grants from AstraZeneca.
AUTHOR CONTRIBUTIONS
All authors participated in the research design. RZ performed the data management and statistical analyses after discussion with all authors. All authors participated in data interpretation and contributed to the scientific discussion. All authors took final responsibility for the decision to submit for publication.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14437.
Supporting information
Appendix S1 Supplementary Information.
ACKNOWLEDGEMENTS
Medical writing and manuscript support: Jonathan Tulip and Mark Greener (Omega Scientific UK Ltd). Mark Greener of Omega Scientific provided medical writing and editorial assistance funded by AstraZeneca. KK is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM) and the NIHR Leicester Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NIHR, NHS or the Department of Health and Social Care. II is supported by the NIHR Nottingham Biomedical Research Centre and carried out work at/was supported by the NIHR Nottingham Clinical Research Facilities. AB is supported by research funding from NIHR, British Medical Association, AstraZeneca, UK Research and Innovation, and the Innovative Medicines Initiative‐2 (BigData@Heart Consortium, under grant agreement No. 116074, supported by the European Union's Horizon 2020 research and innovation programme and EFPIA; chaired by DE Grobbee and SD Anker, partnering with 20 academic and industry partners and ESC). We would like to thank Johan Bodegård (AstraZeneca, Norway) and Marcus Thuresson (Statisticon AB, Sweden) for their clinical and statistical input, respectively. This study is based in part on data from the CPRD obtained under licence from the UK Medicines and Healthcare Products Regulatory Agency. The data are provided by patients and collected by the NHS as part of their care and support. The ONS is also acknowledged as the provider of the ONS Data contained within the CPRD Data. The interpretation and conclusions contained in this study are those of the author/s alone. Copyright © (2020), reused with the permission of NHS Digital. All rights reserved. The study protocol was approved by the Independent Scientific Advisory Committee (ISAC) of CPRD; protocol reference number: 19_231. This research was funded by AstraZeneca.
Idris I, Zhang R, Mamza JB, et al. Lower risk of hospitalization for heart failure, kidney disease and death with sodium‐glucose co‐transporter‐2 inhibitors compared with dipeptidyl peptidase‐4 inhibitors in type 2 diabetes regardless of prior cardiovascular or kidney disease: A retrospective cohort study in UK primary care. Diabetes Obes Metab. 2021;23(10):2207‐2214. 10.1111/dom.14437
DATA AVAILABILITY STATEMENT
This work uses data provided by patients and collected by the NHS as part of their care and support. https://www.cprd.com
REFERENCES
- 1. Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27(8):1879‐1884. [DOI] [PubMed] [Google Scholar]
- 2. Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG. Prevalence and risk factors formicroalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69(11):2057‐2063. [DOI] [PubMed] [Google Scholar]
- 3. Thrainsdottir IS, Aspelund T, Thorgeirsson G, et al. The association between glucose abnormalities and heart failure in the population‐based Reykjavik study. Diabetes Care. 2005;28(3):612‐616. [DOI] [PubMed] [Google Scholar]
- 4. Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis. 2019;62(4):298‐302. [DOI] [PubMed] [Google Scholar]
- 5. Taylor CJ, Ordóñez‐Mena JM, Roalfe AK, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: population based cohort study. BMJ. 2019;364:l223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lawson CA, Jones PW, Teece L, et al. Association between type 2 diabetes and all‐cause hospitalization and mortality in the UK general heart failure population: stratification by diabetic glycemic control and medication intensification. JACC Heart Fail. 2018;6(1):18‐26. [DOI] [PubMed] [Google Scholar]
- 7. Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22(9):1607‐1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413‐1424. [DOI] [PubMed] [Google Scholar]
- 9. Docherty KF, Jhund PS, Inzucchi SE, et al. Effects of dapagliflozin in DAPA–HF according to background heart failure therapy. Eur Heart J. 2020;41(25):2379‐2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Birkeland KI, Jørgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs (CVD–REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5(9):709‐717. [DOI] [PubMed] [Google Scholar]
- 11. Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real‐world clinical practice (CVD–REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8(1):27‐35. [DOI] [PubMed] [Google Scholar]
- 12. Kosiborod M, CSP L, Kohsaka S, et al. Cardiovascular events associated with SGLT‐2 inhibitors versus other glucose‐lowering drugs: the CVD–REAL 2 study. J Am Coll Cardiol. 2018;71(23):2628‐2639. [DOI] [PubMed] [Google Scholar]
- 13. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon‐like peptide receptor agonists and sodium‐glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139(17):2022‐2031. [DOI] [PubMed] [Google Scholar]
- 14. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose cotransporter‐2 inhibitors versus other glucose‐lowering drugs. Circulation. 2017;136(3):249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Birkeland KI, Bodegard J, Banerjee A, et al. Lower cardiorenal risk with sodium‐glucose cotransporter‐2 inhibitors versus dipeptidyl peptidase‐4 inhibitors in type 2 diabetes patients without cardiovascular and renal diseases: a large multinational observational study. Diabetes Obes Metab. 2021;23:75‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 17. Nicolucci A, Charbonnel B, Gomes MB, et al. Treatment patterns and associated factors in 14 668 people with type 2 diabetes initiating a second‐line therapy: results from the global DISCOVER study programme. Diabetes Obes Metab. 2019;21(11):2474‐2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chaplin S. DPP‐4/SGLT2 inhibitor combined therapy for type 2 diabetes. Prescriber. 2017;28(11):32‐38. [Google Scholar]
- 20. Wolf A, Dedman D, Campbell J, et al. Data resource profile: clinical practice research Datalink (CPRD) Aurum. Int J Epidemiol. 2019;48(6):1740‐1740g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Persson R, Vasilakis‐Scaramozza C, Hagberg KW, et al. CPRD Aurum database: assessment of data quality and completeness of three important comorbidities. Pharmacoepidemiol Drug Saf. 2020;29:1456‐1464. [DOI] [PubMed] [Google Scholar]
- 22. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31‐39. [DOI] [PubMed] [Google Scholar]
- 23. Docherty KF, Jhund PS, Anand I, et al. Effect of dapagliflozin on outpatient worsening of patients with heart failure and reduced ejection fraction: a prespecified analysis of DAPA–HF. Circulation. 2020;142(17):1623‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care. 2020;43(3):508‐511. [DOI] [PubMed] [Google Scholar]
- 25. Margonato D, Galati G, Mazzetti S, et al. Renal protection: a leading mechanism for cardiovascular benefit in patients treated with SGLT2 inhibitors. Heart Fail Rev. 2021;26:337‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 27. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 28. Kohsaka S, CSP L, Kim DJ, et al. Risk of cardiovascular events and death associated with initiation of SGLT2 inhibitors compared with DPP‐4 inhibitors: an analysis from the CVD–REAL 2 multinational cohort study. Lancet Diabetes Endocrinol. 2020;8(7):606‐615. [DOI] [PubMed] [Google Scholar]
- 29. Filion KB, Lix LM, Yu OH, et al. Sodium glucose cotransporter 2 inhibitors and risk of major adverse cardiovascular events: multi‐database retrospective cohort study. BMJ. 2020;370:m3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type diabetes mellitus. N Engl J Med. 2013;369(14):1317‐1326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary Information.
Data Availability Statement
This work uses data provided by patients and collected by the NHS as part of their care and support. https://www.cprd.com


