Abstract
The carboxyl terminus of p53 is a target of a variety of signals for regulation of p53 DNA binding. Growth suppressor c-Abl interacts with p53 in response to DNA damage and overexpression of c-Abl leads to G1 growth arrest in a p53-dependent manner. Here, we show that c-Abl binds directly to the carboxyl-terminal regulatory domain of p53 and that this interaction requires tetramerization of p53. Importantly, we demonstrate that c-Abl stimulates the DNA-binding activity of wild-type p53 but not of a carboxyl-terminally truncated p53 (p53Δ363C). A deletion mutant of c-Abl that does not bind to p53 is also incapable of activating p53 DNA binding. These data suggest that the binding to the p53 carboxyl terminus is necessary for c-Abl stimulation. To investigate the mechanism for this activation, we have also shown that c-Abl stabilizes the p53-DNA complex. These results led us to hypothesize that the interaction of c-Abl with the C terminus of p53 may stabilize the p53 tetrameric conformation, resulting in a more stable p53-DNA complex. Interestingly, the stimulation of p53 DNA-binding by c-Abl does not require its tyrosine kinase activity, indicating a kinase-independent function for c-Abl. Together, these results suggest a detailed mechanism by which c-Abl activates p53 DNA-binding via the carboxyl-terminal regulatory domain and tetramerization.
p53 exerts its tumor suppression function by inducing growth arrest and apoptosis (11, 13). The biochemical activity of p53 that is required for this relies on its ability to bind to specific DNA sequences and to function as a transcription factor (22). The importance of the activation of transcription by p53 is underscored by the fact that the majority of p53 mutations found in tumors are located within the domain required for sequence-specific DNA binding (11, 13). Therefore, it is clear that this activity is critical to the role of p53 in tumor suppression.
A contiguous stretch of 30 amino acid residues at the carboxyl terminus of p53 (C terminus; amino acids 363 to 393) constitutes a domain required for regulation of p53 DNA binding. Interference with this domain by modification, including phosphorylation (23) and acetylation (4, 17), or by deletion (5) has been shown to enhance p53 DNA-binding activity. Moreover, several proteins, including Ref-1 (8) and 14-3-3 (31), have been shown to bind to this region of p53 and enhance the DNA-binding activity of p53. A model for this activation has been proposed in which the C terminus of p53 interacts with the core of the molecule and in which this interaction locks the core into a conformation that is inactive for DNA binding (6). When this interaction is disrupted by modification, deletion, or protein-protein interaction, the core is able to adopt an active conformation. Despite compelling evidences for such a model, the motif on core domain that interacts with the C terminus remains to be identified. Nevertheless, these studies defined the C-terminal domain as a negative regulatory domain that normally results in a latent, low-affinity DNA-binding form of p53. Therefore, it follows that, upon DNA damage, signals which regulate cell growth may also function through this domain to stimulate p53 DNA binding.
Recently, the c-Abl tyrosine kinase has been shown to interact with p53 in response to DNA damage (10, 32, 33), and overexpression of c-Abl leads to G1 growth arrest in a p53-dependent manner (3). In addition, c-Abl was also found to enhance the ability of p53 to activate transcription from a promoter containing a p53 DNA-binding site in transient-transfection assays (3) and to stimulate the expression of p21 (33). Deletion of the p53-binding domain in c-Abl (ΔProl, a deletion of a proline-rich domain; amino acids 793 to 1044) impairs the ability of c-Abl to stimulate p53 transcriptional activity and to suppress growth (3). These results suggest that the p53-Abl interaction plays an important role in regulation of p53-mediated transcription and growth suppression. It is important to note, however, that a kinase-inactive form of c-Abl [c-Abl(K-R)], which is defective in its ability to suppress growth, was also found to enhance the ability of p53 to activate transcription (3, 33), suggesting that the tyrosine kinase activity of c-Abl is required for growth suppression but not for transcriptional activation. These data argue that c-Abl may stimulate p53-dependent transcription in a kinase-independent manner. Consequently, a detailed understanding of how c-Abl stimulates p53-dependent transcription is of significance.
In the present study, we show that c-Abl binds directly to the C terminus of p53 and that this interaction requires a tetrameric conformation of p53. Furthermore, we demonstrate that c-Abl significantly stimulates the DNA binding of p53 but not of a C-terminally truncated form of p53 (p53Δ363C), suggesting that the interaction with the p53 C terminus is necessary for c-Abl stimulation. To characterize the mechanism for this activation, we have shown that c-Abl stabilizes the p53-DNA complex. On the basis of these results, we hypothesize that the interaction of c-Abl with the C terminus of p53 may stabilize the p53 tetrameric conformation, resulting in a more stable p53-DNA complex. In addition, as discussed above, the tyrosine kinase activity of c-Abl is not required for p53 transcriptional activation. Consistent with this, we show that the stimulation of p53 DNA binding by c-Abl does not require its tyrosine kinase activity, indicating a kinase-independent function of c-Abl. Together, these observations suggest a detailed mechanism of activation via the p53 C-terminal regulatory domain and tetramerization by the growth suppressor protein c-Abl.
MATERIALS AND METHODS
Plasmid construction.
The p53Δ292 deletion mutant was constructed by PCR amplification of amino acids 1 to 292 of p53 from pcDNA-p53 (14) by using primers that introduce HindIII sites at both the 5′ and 3′ ends. The amplified DNA fragments were then cloned into the HindIII site of pcDNA (Invitrogen). The p53Δ363 deletion mutant was constructed by PCR amplification of amino acids 1 to 363 of p53 from pcDNA-p53 by using primers that introduce a HindIII site at the 5′ end and an EcoRI site and a stop codon at the 3′ end. The amplified DNA fragments were then cloned into the HindIII and EcoRI sites of pcDNA. The internal deletion mutants of p53, p53Δ325-356 and p53Δ316-322, were generated from pcDNA-p53 by PCR with a pair of inverted primers that would delete the base pairs coding for the corresponding amino acids. PCR products were then phosphorylated with T4 kinase and ligated. Similarly, the p53 tetramerization mutant (341K344E348E355K, Tet Mut; Stürzbecher et al. [28]), was constructed by using primers that contained mutations at corresponding amino acid positions. All mutant constructions were confirmed by sequencing analysis. The luciferase reporter plasmid, pRGCE4Luc, was constructed by cloning the BamHI-Asp718 fragment containing RGCE4TATA from pRGCE4CAT (3) into pZLuc (27).
Purification of the c-Abl and p53 proteins.
Sf21 cells were infected with recombinant baculoviruses expressing GST-Abl, GST-Abl-ΔSH3 (a deletion construct of c-Abl lacking the SH3 domain [19]) and GST-Abl-ΔC (a deletion construct of c-Abl truncated after the catalytic domain, amino acid 532; a gift from B. Mayer, Harvard University) and lysed as described by Pendergast et al. (21). The glutathione S-transferase (GST) fusion proteins were then bound to glutathione-Sepharose (Pharmacia) and eluted with buffer containing 10 mM glutathione, and purified proteins were dialyzed in 0.1 M KCl D buffer (20 mM HEPES [pH 7.9], 20% glycerol, 0.2 mM EDTA, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride). To purify p53, HeLa cells were infected with recombinant vaccinia virus expressing a hemagglutinin epitope-tagged p53 (HA-p53), and p53 was either purified from the nuclear extract of infected cells by binding to a matrix of monoclonal antibody (12CA5) specific for the epitope tag, followed by elution with the epitope peptide as described by Liu and Berk (15), or purified with a matrix of monoclonal anti-p53 antibody (421) as described by Sheppard et al. (24). The purified proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). To purify p53Δ363, Sf21 cells were infected with recombinant baculovirus expressing HA-p53Δ363 (a gift from C. Prives, Columbia University), and p53Δ363 was purified with 12CA5 antibody as described above.
GST pulldown assay.
Wild-type and mutant p53 RNAs were synthesized under conditions recommended by the manufacturer (Promega). The mRNAs were translated in vitro for 1.5 h at 30°C in rabbit reticulocyte lysate in the presence of [35S]methionine. Bacterially expressed GST-Abl-C (a portion of the c-Abl carboxyl terminus starting at amino acid 711) which binds to p53 (unpublished data) was incubated for 60 min with glutathione-Sepharose (Pharmacia) in lysis buffer (10% glycerol, 1% Triton X-100, 10 mM EDTA in phosphate-buffered saline). Beads were then incubated for 60 min with the [35S]methionine-labeled p53 mutants in incubation buffer (20 mM HEPES [pH 7.4], 150 mM NaCl, 0.1% Triton X-100, 10% glycerol, 1 mM PMSF, and 50 μg of ethidium bromide, 4 μg of aprotinin, and 4 μg of leupeptin per ml) with constant mixing. After incubation, the beads were washed three times with incubation buffer and boiled in 15 μl of 2× SDS sample buffer. The bound proteins were analyzed by SDS-PAGE, and 35S-labeled proteins were visualized by autoradiography.
Immunoprecipitation assay.
Baculovirus-expressed GST-Abl was incubated with in vitro-labeled full-length and mutant p53 in incubation buffer (50 mM Tris-HCl [pH 8.0], 120 mM NaCl, 0.5% NP-40, 1 mM DTT, 4 μg of aprotinin per ml, 4 μg of leupeptin per ml) at 4°C for 2 h. Immunoprecipitation was performed by using anti-Abl antibody, pEX5 (a gift from O. Witte, University of California at Los Angeles) as described earlier (14).
EMSA.
Electrophoretic mobility shift assay (EMSA) was carried out as described elsewhere (24). Briefly, the ribosomal gene cluster (RGC) p53-binding site probe (5′-AGCTTGCCTCGAGCTTGCCTGGACTTGCCTGGTCGACGC-3′) was labeled with the Klenow fragment of Escherichia coli DNA polymerase. Binding reactions contained 60 mM KCl, 12% glycerol, 5 mM MgCl2, 1 mM EDTA, 0.2 μg of bovine serum albumin (BSA), 0.1 μg of poly(dG-dC), 200 cpm of 32P-labeled probe, and proteins as indicated in a total volume of 12.5 μl. Reactions were incubated at 30°C for 45 min or as indicated when association experiments were performed and then analyzed on a 5% polyacrylamide gel containing 0.5× TBE (0.045 mM Tris-borate, 0.045 mM sodium borate, 0.001 mM EDTA [pH 8.0]). DNA-protein complexes were visualized with a phosphorimager by using Adobe Photoshop software. When required, reactions were incubated in the presence of 2 mM ATPγS, a nonhydrolyzable ATP analog, to inhibit kinase activity. When dissociation experiments were performed, reactions were incubated for 30 min, which was immediately followed by the addition of 20× excess of unlabeled RGC oligonucleotide to challenge the DNA-protein complex for the indicated times.
c-Abl in vitro phosphorylation assay.
c-Abl in vitro phosphorylation assay was performed essentially as described elsewhere (12). Briefly, GST-Abl was incubated with glutathione-Sepharose, and phosphorylation was carried out at 30°C in kinase buffer (20 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid); pH 7.0], 20 mM MnCl2, and 0.25 mg of BSA per ml) in the presence of [32P]ATP. When required, reactions were incubated in the presence of 2 mM ATPγS, a nonhydrolyzable ATP analog, to inhibit kinase activity.
Transcriptional activation assay.
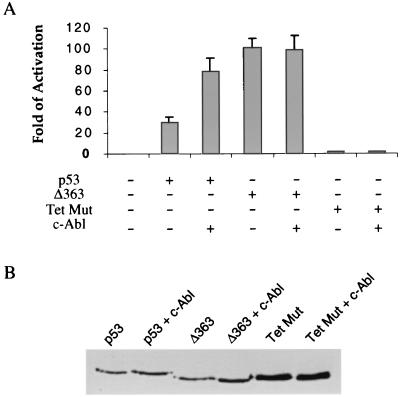
The transcription activity of p53 was measured by using pRGCE4Luc, which contains one copy of the RGC p53 binding site cloned upstream of the adenovirus E4 TATA box and luciferase gene. Various combinations of plasmid DNAs (see Fig. 6) were transfected into Saos-2 cells by using calcium phosphate. The amounts of plasmids transfected for 60-mm plates were as follows: 0.5 μg of pRGCE4Luc, 0.2 μg of pcDNA-p53, 0.2 μg of pcDNA-p53Δ363, 0.2 μg of pcDNA-p53TetMut, and 0.5 μg of pSRαMSVtkNeo-Abl (3). All samples for luciferase assays were normalized for β-galactosidase activity from a cotransfected control expression vector as described earlier (14). The protein levels were determined by Western blot analysis with the anti-p53 antibody DO-1 (Santa Cruz, Calif.).
FIG. 6.
Stimulation of p53 transcriptional activity by c-Abl requires the C terminus and tetramerization of p53. (A) Saos-2 cells were transfected with the plasmid combination listed below the figure. At 40 h after transfection, the luciferase activity was measured after normalization to β-galactosidase activity and expressed as the fold activation relative to the level seen with the reporter alone (lane 1). The mean and standard deviations from three independent experiments are presented. (B) The transfected cells were lysed, and the p53 protein levels were determined by Western blot analysis with DO-1 anti-p53 antibody.
RESULTS
c-Abl interacts with the C-terminal regulatory domain of p53.
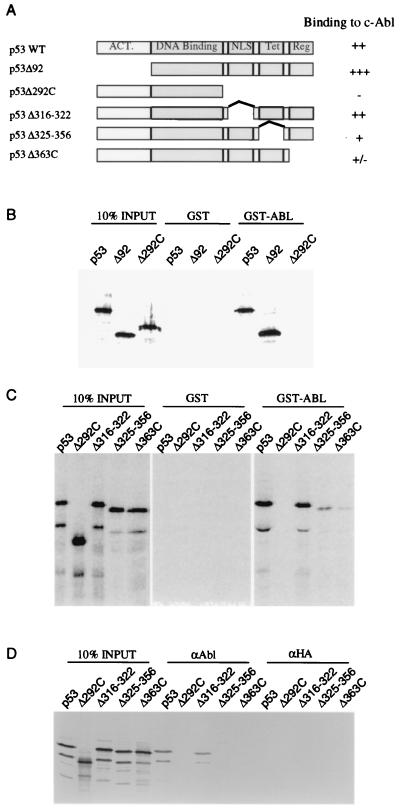
It has been shown previously that c-Abl binds to p53 and activates p53-dependent transcription. To investigate the mechanism by which c-Abl stimulates transcription, we mapped the domains on p53 that are required for c-Abl binding, as p53 can be regulated via different mechanisms through protein-protein interaction at different functional domains. A panel of N- or C-terminal truncated p53 mutants (Fig. 1A) were in vitro translated in the presence of [35S]methionine and incubated with immobilized GST and GST-Abl-C which contains p53 binding domain as reported by Goga et al. (3). After incubation, the beads were washed, and proteins bound to the beads were analyzed by SDS-PAGE (Fig. 1B). Deletion of the p53 transactivation domain (p53Δ92) had no effect on binding to GST-Abl. In contrast, deletion of the p53 carboxyl terminus (p53Δ292C) completely abrogated binding to GST-Abl-C, suggesting the carboxyl-terminal region is required for c-Abl binding.
FIG. 1.
The C-terminal region of p53 is required for association with c-Abl. (A) p53 proteins containing N-terminal and C-terminal deletions used in this study. (B and C) The p53 proteins shown in panel A were translated in vitro and tested for binding to GST-Abl and GST. Binding of the p53 proteins to c-Abl was measured by incubated with immobilized GST-Abl protein, washing, SDS-PAGE, and autoradiography of proteins retained on the beads. Binding of p53 protein to GST was measured by incubation with immobilized GST protein in the same condition. (D) Immunoprecipitations were carried out by using antibody against c-Abl, pEX5, from extracts of baculovirus-infected insect cells and the in vitro-labeled p53 proteins. Identical immunoprecipitations were carried out by using a control anti-HA antibody, 12CA5. The immunoprecipitates were fractionated by SDS-PAGE and analyzed by fluorography.
The C terminus (amino acids 293 to 393) harbors functional domains responsible for nuclear localization (amino acids 316 to 322), tetramerization (amino acids 325 to 356), and regulation of p53 DNA binding (amino acids 363 to 393). To further localize the c-Abl binding domain within the C terminus of p53, we next conducted GST-Abl binding assays with a series of p53 C-terminal small deletion mutants (p53Δ316-322, p53Δ325-356, and p53Δ363C [Fig. 1A]) in which each of these three functional domains was individually removed. Because c-Abl and p53 are both DNA binding proteins, we included 50 μg of ethidium bromide per ml to the binding buffer to disrupt possible interactions mediated through protein-DNA interactions. Figure 1C shows the GST binding results. Deletion of the p53 nuclear localization signal (p53Δ316-322) had no effect on binding to c-Abl. However, deletion of the regulatory domain in p53, p53Δ363C significantly disrupted its ability to bind to c-Abl. This region has been previously identified as an inhibitory domain for p53 DNA binding. Furthermore, deletion of the tetramerization domain, p53Δ325-356, also greatly reduced binding to c-Abl. These findings show that the interaction between c-Abl and p53 requires the C-terminal regulatory domain and tetramerization domain of p53.
To confirm the GST pulldown results, we also performed coimmunoprecipitation experiments. As shown in Fig. 1D, binding of baculovirus-expressed c-Abl protein to full-length p53 and p53Δ316-322 was clearly observed, while binding to p53Δ363C and p53Δ325-356 was not detected. Control immunoprecipitations with the anti-HA antibody, 12CA5, failed to precipitate labeled p53. These coimmunoprecipitation results further demonstrate that the interaction between c-Abl and p53 requires the C-terminal regulatory domain and tetramerization domain of p53.
Tetrameric conformation is necessary for the p53-cAbl interaction.
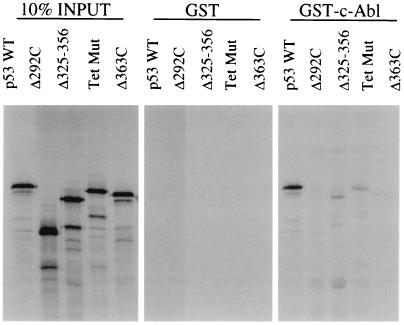
The tetramerization domain is important for higher-order p53 complex formation, DNA binding (20), phosphorylation at Ser15, Ser20, and Ser33 (25), and degradation by Mdm2 (18), as well as for the dominant-negative effect of mutant p53 molecules over wild-type p53 (29). The results from the GST binding experiments in Fig. 1C and the immunoprecipitation experiments in Fig. 1D led us to hypothesize that c-Abl interacts with the regulatory domain of p53 and that this interaction may require the tetrameric conformation of p53. It is also possible, however, that c-Abl may interact with residues in the tetramer domain directly. To test this, we constructed a tetramerization impaired mutant, 341K344E348E355K (Tet Mut), which contains four mutated residues at positions 341, 344, 348, and 355 as reported by Stürzbecher et al. (28). The ability of the mutant to interact with c-Abl was tested by using the GST pulldown assay as described above (Fig. 2). The results showed that this mutant, like Δ325-356, fails to bind to c-Abl, revealing the requirement of the tetrameric conformation of p53 for c-Abl interaction.
FIG. 2.
The tetrameric conformation of p53 is necessary for the p53-Abl interaction. Radiolabeled p53 proteins were prepared by in vitro translation and were incubated with either GST or GST-Abl. After being washed, proteins were subjected to SDS-PAGE and analyzed by fluorography. The tetramerization impaired mutant 341K344E348E355K (Tet Mut) was deficient in binding to c-Abl.
Activation of p53-DNA binding by c-Abl requires p53 C terminus.
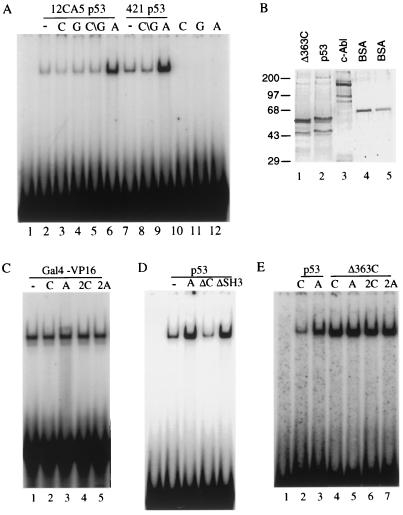
If the interaction of c-Abl with the regulatory domain of p53 is of functional significance, we reasoned that c-Abl should alter the negative regulatory effect of the C terminus on p53 DNA binding. To test this possibility, we examined the effect of c-Abl on p53 DNA binding in an EMSA with a probe containing the p53 cis element identified in the RGC as described by Sheppard et al. (24). As expected, 50 ng of vaccinia virus-expressed epitope-tagged human p53 (one-half the amount as shown in Fig. 3B, lane 2) purified from HeLa cells with 12CA5 antibody bound to this probe and produced a retarded p53-DNA complex (Fig. 3A, lane 2). Addition of 30 ng of baculovirus-expressed GST-Abl (an amount of protein that corresponds roughly to 1:1 molar ratio to p53 tetramer; Fig. 3B) resulted in a marked stimulation of the p53 DNA binding (5- to 10-fold activation, Fig. 3A, lanes 2 and 6). In contrast to c-Abl, the same amount of control extract purified from mock-infected cells (C; Fig. 3A, lane 3), purified GST protein (G; lane 4), or a combination of both (C∖G; lane 5) were incapable of stimulating p53 DNA binding. Each of the extracts used in this experiment have been tested over a range of concentrations corresponding from one-half to four times the amount used in Fig. 3A. At each of the concentrations tested, stimulation by c-Abl was observed (data not shown). This c-Abl-stimulated p53-DNA complex could be supershifted by the addition of anti-p53 antibody but not by the addition of anti-Abl antibody, suggesting that c-Abl may not be part of the p53-DNA complex (data not shown). Similar results were also reported with other proteins, such as 421 antibody and Ref-1, which interact with the C terminus of p53 and enhance p53 DNA binding. Two explanations may account for this result. First, c-Abl may dissociate from the p53-DNA complex during the electrophoresis. Second, c-Abl may induce a conformational change of latent p53 and dissociate from p53 once bound to DNA. Nevertheless, the results suggest that c-Abl interacts with the p53 regulatory domain to relieve its negative effect on p53 DNA binding. The fact that stoichiometric quantities of c-Abl stimulate p53 DNA binding supports the view that tetrameric conformation of p53 is necessary for the stimulation.
FIG. 3.
The C-terminal domain is required for c-Abl activation. (A) A radiolabeled probe containing the p53-binding site from RGC was incubated with either 50 ng of p53 purified with 12CA5 or 25 ng of p53 purified with 421 in the presence of 30 ng of control extract (“C”), GST (“G”), or GST-Abl (“A”) as indicated. (B) Results of a silver-stained SDS-PAGE are shown. Lane 1, 150 ng of p53Δ363C eluted with HA peptide from a monoclonal antibody 12CA5 affinity column; lane 2, 100 ng of p53; lane 3, 300 ng of GST-Abl; lanes 4 and 5, 100 and 50 ng of BSA, respectively. The sizes of molecular mass standards are indicated on the left in kilodaltons. (C) A radiolabeled probe containing the Gal4 site was incubated with either 10 ng of purified Gal4-VP16 in the presence of 10 or 20 ng of control extract (C or 2C) or GST-Abl (A or 2A) as indicated. (D) The RGC probe was incubated with 50 ng of 12CA5 purified p53 in the absence (−) or presence (+) of 30 ng of GST-Abl (A), GST-Abl-ΔC (ΔC), or GST-Abl-ΔSH3 (ΔSH3) as indicated. (E) The RGC probe was incubated with 50 ng of 12CA5 purified p53 or 10 ng of 12CA5 purified p53Δ363C in the presence of GST-Abl (A, 30 ng; 2A, 60 ng) or control extract (C and 2C) as indicated.
To test whether c-Abl can specifically stimulate p53 DNA-binding, we performed a gel shift assay with an unrelated transcription factor, Gal4-VP16, in the presence of c-Abl or a control GST extract. As shown in Fig. 3C, Gal4-VP16 resulted in a slowly migrating band, the Gal4-VP16-DNA complex (lane 1). Addition of c-Abl (lanes 3 and 5) or the control GST extract (lanes 2 and 4) did not have any effect on DNA binding, suggesting that the c-Abl interaction plays a role in the activation of p53 DNA binding.
If this result is correct, a construct of c-Abl, that lacks the p53 binding domain (c-Abl-ΔC), should not activate p53 DNA binding, whereas another construct of c-Abl that binds to p53 but lacks SH3 domain (c-Abl-ΔSH3) should activate p53 DNA binding under our assay condition. We tested this hypothesis by comparing the abilities of wild-type c-Abl, c-Abl-ΔSH3, and c-Abl-ΔC to stimulate p53 DNA binding (Fig. 3D). The c-Abl-ΔSH3 mutant continued to activate p53 DNA binding, whereas c-Abl-ΔC was significantly impaired in its ability to stimulate p53 DNA binding. These findings demonstrate a correlation between the ability to bind p53 and to activate p53 DNA binding. Consequently, c-Abl interaction is required for activation of p53 DNA binding.
To further test this hypothesis, we performed a gel shift assay with the C-terminal truncated form of p53 (Δ363C) in the presence of c-Abl or a control GST extract (Fig. 3E) since c-Abl should not affect the DNA binding of Δ363C. As expected, p53 resulted in a slowly migrating band, the p53-DNA complex (lane 2), and addition of c-Abl significantly activated the p53 DNA binding (lane 3). In contrast, Δ363C bound to DNA more efficiently than p53 (compare lane 4 to lane 2). Addition of c-Abl to Δ363C, however, did not have any effect on DNA binding (lanes 5 and 7). The striking difference in activation of p53 DNA binding by c-Abl supports our conclusion that c-Abl interacts with the regulatory domain to diminish its negative regulatory effect on p53 DNA binding.
It has been demonstrated previously that 421 antibody binds to the regulatory domain (amino acids 372 to 382) and activates p53 in a manner similar to that observed with c-Abl. Therefore, we tested whether c-Abl can stimulate p53 purified with 421 antibody. Surprisingly, 421-purified p53 can be activated by c-Abl to a similar extent as 12CA5-purified p53 (Fig. 3A, lanes 7 to 9). Several explanations may account for this result. c-Abl and 421 antibody may interact differently with the negative regulatory domain and affect p53 DNA binding independently. Support for this assumption comes from the interaction data in that c-Abl binding, unlike 421 antibody, requires the tetrameric conformation of p53 (Fig. 2). Alternatively, c-Abl may alter the conformation of the regulatory domain more efficiently, resulting in a further stimulation of DNA binding of 421-purified p53.
c-Abl activates p53 DNA binding in a kinase-independent manner.
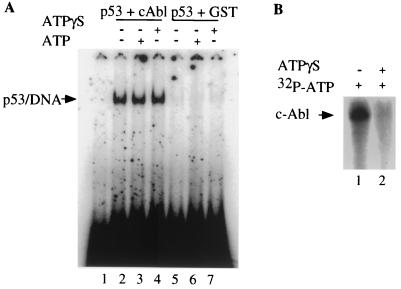
Because a kinase-inactive form of c-Abl [c-Abl(K-R)] has been reported to bind p53 and to enhance the ability of p53 to activate transcription (3, 33), we considered that the kinase activity of c-Abl might not be required for the activation of p53 DNA binding. We therefore examined the effect of ATPγS, a nonhydrolyzable ATP analog on the ability of c-Abl to stimulate p53 DNA binding. Under our assay condition, ATPγS can inhibit c-Abl kinase activity in both the autophosphorylation assay (Fig. 4B) and the GST-Crk phosphorylation assay (data not shown). Figure 4A shows that addition of 2 mM ATPγS did not have any effect on the activation of p53 DNA binding by c-Abl (compare lane 4 to lane 2). As a control, ATPγS was also added to p53 DNA binding reaction without c-Abl, and no effect on p53 DNA binding was observed (compare lane 7 to lane 5). These results suggest that the activation of p53 DNA binding by c-Abl is kinase independent. These results are consistent with the previous observation that c-Abl enhances the ability of p53 to activate transcription in a kinase-independent manner and suggest that c-Abl activation of p53 DNA binding occurs independent of its function as a protein tyrosine kinase.
FIG. 4.
c-Abl stimulates p53 DNA binding in an ATP-independent manner. (A) A radiolabeled probe containing the p53-binding site from RGC was incubated with 50 ng of 12CA5 purified p53 with (+) or without (−) 30 ng of GST-Abl and with or without 2 mM ATPγS or 2 mMATP as indicated. (B) c-Abl phosphorylation was carried out with or without 2 mM ATPγS or 2 mMATP as shown. The phosphorylated proteins were fractionated by SDS-PAGE and analyzed by autoradiography.
Interaction with c-Abl stabilizes the p53-DNA complex.
The activation of p53 DNA binding by c-Abl could result from increasing the rate of p53-DNA complex formation or by decreasing the rate at which p53 dissociates from the DNA.
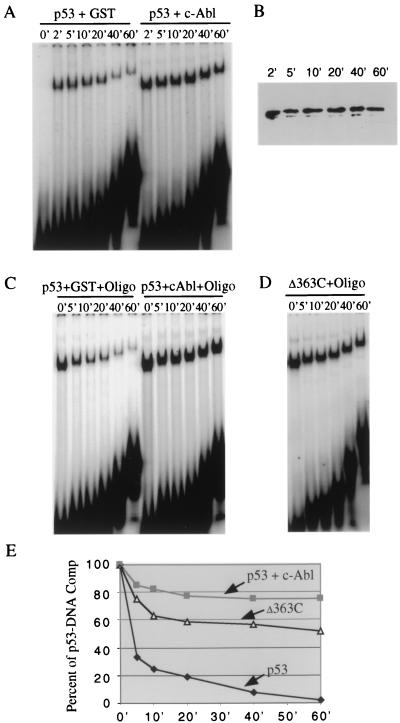
To determine whether c-Abl affects the rate of p53-DNA complex formation, p53 was incubated with the RGC probe in the presence of either c-Abl or the GST control. At different time points (0, 2, 5, 10, 20, 40, and 60 min) after mixing, aliquots of the reaction mixture were loaded on a running gel (Fig. 5A). The results show that p53 gel shift bands were observed at maximal levels 2 min after incubation, in the presence or absence of c-Abl, suggesting that formation of the p53-DNA complex may not be affected by the presence of c-Abl. To exclude the possibility that a small difference in the association rate of p53-DNA, in the presence or absence of c-Abl, may exist, we performed the same gel shift experiments at 4°C or with a decreasing amount of p53. In either case, no difference in the association rate of p53-DNA complex could be detected (data not shown). Of note, a decreased level of p53-DNA complex was observed after 40 min of incubation without c-Abl, but not with c-Abl, indicating that c-Abl may stabilize p53-DNA complex. Western blot analysis was performed (Fig. 5B) to ensure that p53 is equally stable during the time course.
FIG. 5.
c-Abl prevents the dissociation of the p53-DNA complex. (A) Determination of the association rate of the p53-DNA complex in the presence or absence of c-Abl. Binding reactions were performed as described, and samples were loaded on a running gel at different time points. (B) Western blot analysis of p53 protein levels at different time points. (C) Determination of the dissociation rate of the p53-DNA complex in the presence or absence of c-Abl. At equilibrum, DNA-binding reaction mixtures were challenged by addition of a 20× excess of unlabeled RGC competitor, and samples were removed and loaded on a running gel at various time points. (D) Determination of the dissociation rate of the Δ363-DNA complex. (E) The intensity of the bands representing the p53-DNA complex in panels C and D was quantitated with a phosphorimager and plotted as a percentage of the intensity at equilibrum.
To test the hypothesis that c-Abl may stabilize p53-DNA complex, we next determined whether c-Abl decreased the dissociation rate of a preformed p53-DNA complex. For this experiment, purified p53, in the presence of either c-Abl or the GST control, was incubated with the RGC probe for 30 min, and then the formed complexes were challenged with a 20-fold molar excess of unlabeled RGC oligonucleotide as a competitor. Aliquots of the reaction mixture were loaded on a running gel at 0, 5, 10, 20, 40, and 60 min after the addition of the competitor DNA (Fig. 5C). At the end of the electrophoresis, the amount of DNA shifted was quantitated by phosphorimager analysis. Figure 5E shows a plot of the data from four independent experiments, where 100% represented the amount of p53-DNA complex formed before the addition of unlabeled competitor (0 min). The data clearly show that c-Abl stabilizes the p53 DNA binding.
If this result is correct, we reasoned that p53Δ363C should also stabilize the p53-DNA complex by decreasing the dissociation rate of the p53-DNA complex. For this experiment, purified Δ363C was incubated with the RGC probe for 30 min, and then the formed complexes were challenged with unlabeled RGC oligonucleotide. The results demonstrated that p53Δ363C also stabilizes the p53 DNA-binding (Fig. 5D and E).
The C terminus and tetramerization of p53 are responsible for activation of p53-dependent transcription by c-Abl.
Our results showing that c-Abl interacts with the C-terminal regulatory domain in the tetrameric form of p53 to activate DNA binding prompted us to determine the effect of c-Abl on the ability of the tetramerization impaired p53 (Tet Mut) and the C-terminally truncated p53 (Δ363C) to activate transcription in vivo. Toward this end, we performed transient-transfection experiments from a minimal promoter containing one p53 binding site (RGC) and the E4 TATA box (RGCE4) in Saos-2 cells. Figure 6A summarizes the results from three independent experiments in which RGCE4 was cotransfected with expression vectors for p53, Δ363C, or Tet Mut in the presence or absence of c-Abl, and luciferase was measured after adjustments were made for the difference in transfection efficiencies. As expected, addition of c-Abl to full-length p53 resulted in nearly threefold enhancement of activation. In contrast, addition of c-Abl to Δ363C and Tet Mut had no detectable effect on the transcription. The enhancement by c-Abl is p53 dependent since c-Abl alone over a wide range had no effect on the RGCE4 promoter (data not shown). Western blot analysis was performed to ensure that Δ363C mutant is expressed in equal levels compared to wild-type p53, and c-Abl has no effect on the expression of the transfected p53 (Fig. 6B). Tet Mut was expressed at higher levels, a finding which is consistent with the observation that tetramerization is required for p53 to be efficiently degradated (18). These findings support our conclusion that the C terminus and tetramerization of p53 are responsible for stimulation of p53-dependent transcription by c-Abl.
DISCUSSION
A mechanism for c-Abl activation of p53-dependent transcription.
Although it is known that c-Abl stimulates p53-dependent transcription, a function required for c-Abl growth suppressor activity (3), the molecular mechanisms by which this occurs remain elusive. The results reported here show that c-Abl interacts with the C-terminal regulatory domain of tetrameric form of p53 and functions to activate the p53 DNA binding. In an effort to assess the mechanism of c-Abl activation, we also show that c-Abl activates p53 DNA binding by stabilizing the p53-DNA complex. Collectively, these results suggest a model for c-Abl activation. In this model c-Abl activates latent p53 by relieving the C-terminal inhibitory domain of p53 and enhances p53 DNA binding by forming a stable p53-DNA complex. Support for this mechanism also comes from the correlation between the effect of c-Abl mutations on the interaction with p53 and on the activation of p53 DNA binding. These results indicate that c-Abl contributes to p53 transactivation by functioning as a stimulator of p53 DNA binding.
c-Abl functions as a p53 DNA-binding stimulator in a manner different from several other stimulator proteins which also activate DNA binding by relieving the C-terminal inhibitory effect. Examples include p300, which acetylates lysine residues at the C terminus and activates latent p53 (4), and Ref-1, which activates p53 DNA binding via the C-terminal domain in a redox-dependent manner (8). c-Abl is distinct from these proteins in that it did not appear to covalently modify p53 or to rely on the redox state of p53. It may be similar in this regard to 421 antibody activation, wherein the antibody binds to the C-terminal domain and relieves its negative effect on p53 DNA binding. However, 421 antibody recognizes both tetrameric and monomeric forms of p53, indicating a conformation-independent binding. In the case of c-Abl, the specific binding of c-Abl requires p53 to be in a tetrameric form. What remains to be determined is the significance of c-Abl binding the p53 tetramer but not the monomer.
Our data suggest that c-Abl stimulates p53-mediated transcription, at least in part, by activation of p53 DNA binding. Of note, two recent studies have shown that overexpression of c-Abl also induces p53 accumulation (33), probably via the neutralization of the inhibitory effect of Mdm2 by c-Abl (26). These data suggest that the c-Abl–p53 interaction induces a conformational change which may dissociate p53 from Mdm2. p300 has been shown to activate p53 via two different mechanisms, activation of p53 DNA binding (4, 17) and stabilization of the p53 protein (34). Therefore, as with the activation of p53 by p300, our data together with the studies cited above suggest that c-Abl may stimulate p53-mediated transcription by more than one mechanism, i.e., enhanced DNA binding as well as protein accumulation.
Proposed model for the stabilization of the p53-DNA complex by c-Abl.
We have shown that c-Abl stabilizes the p53-DNA complex. In order to explain this increased stability, we speculate that the interaction of c-Abl with the C terminus of p53 may stabilize the p53 tetrameric conformation. There are several reasons that led us to believe this is the case. Our results show that c-Abl interacts with the tetrameric form of p53 but not with the monomeric form, suggesting that multiple contacts between c-Abl and p53 may be required for the interaction. These multiple contacts, in principle, could induce a stable tetrameric form of p53, resulting in a more stable protein-DNA complex (20). Of note, it has been shown that the p53 tetramer could bind DNA via one dimer interacting with one half-site, but binding was stabilized significantly if the second dimer of a tetramer simultaneously bound DNA beside the first dimer, suggesting that cooperative interdimer interactions stabilize tetramer binding to DNA (20). Similar studies were also done with nuclear receptors and showed that dimerization of nuclear receptors stabilizes the binding of the receptors to DNA (2). In the absence of the dimerization domain, DNA-binding domain (DBD) monomers dissociate from the DNA very rapidly. In contrast, a dimer of the full-length receptor was found to dissociated from the DNA very slowly. These findings were explained in a one-step–two-step model by Jiang et al. (9) in which the DBD monomers dissociated from the DNA one at a time in two energetically favorable steps, whereas the full-length receptor dissociated from the DNA in a single step process. This one-step dissociation was considered to be energetically unfavorable, since the contacts between two DBD monomers and DNA had to be broken at the same time. This model could also apply to other DNA-binding proteins such as p53. In this case, the stabilization of DNA binding would be more effective since p53 exists as a tetramer.
It is tempting to speculate that our hypothesis may also explain why the C-terminal domain inhibits p53 DNA binding. The C-terminal regulatory domain was proposed to interact with a motif in the core of the p53 tetramer, thereby forming a conformationally inactive complex (6). Despite compelling evidences for such a model, the motif on core domain that interacts with the C terminus remains to be identified. In addition, the increased association rate of p53 and DNA after disruption of the C-terminal inhibition has not been observed. An alternative explanation, therefore, is that the C-terminal domain may interfere with the tetramerization of p53, resulting in a less-stable p53-DNA complex. Supporting this assumption are the experimental evidences that, first, the association rate of p53 and DNA is unaffected by c-Abl (Fig. 5A) and, second, p53Δ363C also stabilizes the p53-DNA complex by decreasing the dissociation rate of the p53-DNA complex (Fig. 5D and E) but not by increasing the association rate (data not shown). The fact that the C-terminal domain is closely located next to the tetramerization domain also makes this alternative model physically possible. Further studies of the role of the C-terminal regulatory domain in tetramerization will be required to distinguish between these possibilities.
A kinase-independent activity for c-Abl.
The c-Abl protein is a nuclear tyrosine kinase. However, c-Abl-p53 complexes are detected in cells expressing either wild-type or the kinase-inactive c-Abl(K-R) in response to ionizing radiation (33). Furthermore, the kinase activity of c-Abl is not required for transcriptional activation by p53 in transient-transfection assays from a promoter containing p53 DNA-binding sites (3). Consistent with these results, our data reveal that the c-Abl kinase activity is not required for the activation of p53 DNA binding. On the basis of these observations, we propose a kinase-independent activity for c-Abl: activation of p53 DNA binding. Several lines of evidence have lent support to this activity. First, although a deletion of the c-Abl SH3 domain increases Abl-mediated tyrosine phosphorylation in vivo (1, 7, 30), similar effects on transactivation (3) by wild-type Abl and Abl-ΔSH3 (a deletion of c-Abl lacking SH3 domain) were observed. Second, the amounts by which the kinase-inactive c-Abl(K-R) stabilizes p53 were similar to the stabilization by wild-type c-Abl (26), suggesting that c-Abl functions to induce a possible conformation change in p53 in a non-kinase-dependent manner. Finally, the overexpression of both wild-type c-Abl and c-Abl(K-R) enhances the expression of endogenous p21 (33).
By comparison to p53, c-Abl has been shown to interact and phosphorylate p73, a structural and functional homologue of p53. Importantly, the c-Abl tyrosine kinase activity is required for the stimulation of p73-mediated transactivation and apoptosis (35). Therefore, together with our data, these findings suggest that c-Abl regulates p53 family in response to DNA damage through different mechanisms.
A link between DNA damage and activation of p53 via the C-terminal domain.
c-Abl contributes to radiation-induced G1 arrest via a p53-dependent mechanism (33), indicating that p53 lies in a pathway downstream from c-Abl. We demonstrate that c-Abl binds to the C terminus of p53 and stimulates p53 DNA binding. These findings directly link c-Abl to activation of p53 DNA binding via the C-terminal domain in response to DNA damage. Interestingly, a recent study has shown that irradiation leads to dephosphorylation of Ser376, resulting in an association of 14-3-3 proteins with p53 via the C-terminal domain which, in turn, enhanced the affinity of p53 for sequence-specific DNA (31). This observation suggests that p53 lies in a pathway downstream from the 14-3-3 protein in response to DNA damage. Our data, together with this finding, support the view that there are multiple molecular pathways that signal DNA damage (16) and activate p53 via the C-terminal domain. Although it is clear that the interaction of p53 with c-Abl is DNA damage inducible, it remains to be determined whether Ser376 dephosphorylation contributes to such a c-Abl–p53 association.
Transient-transfection assays showed a significant stimulatory effect of c-Abl on the ability of cotransfected p53 to activate transactivation. The observation that c-Abl did not stimulate p53Δ363C and Tet Mut in these assays supports the assumption that the C-terminal domain and tetramerization of p53 are likely targeted by DNA damage signaling pathways in vivo. Definitive evidence for a loss of c-Abl response in cells expressing p53Δ363C and Tet Mut will be required to validate such a model. In addition, further analysis of the effects of c-Abl on the promoters of natural p53 response genes, such as p21, in such cells should help to clarify this issue.
The function of p53 in the DNA damage response is clearly important to the proper functioning of many cell types. In this study, we have provided an example of activation of p53 DNA binding via the C-terminal regulatory domain and tetramerization by a growth suppressor protein, c-Abl. Our finding further supports the view that the C terminus of p53 is a target for stimulation of p53 DNA binding in response to DNA damage and suggests for the first time that tetramerization is required for this stimulation.
ACKNOWLEDGMENTS
We are very grateful to B. Mayer (Harvard University) for providing baculoviruses expressing GST-cAbl, GST-cAbl-ΔSH3, and in particular GST-cAbl-ΔC prior to publication, to C. Prives (Columbia University) for baculovirus expressing p53Δ363C, and to O. N. Witte (UCLA) for anti-Abl antibody. We thank F. Sladek, A. Merino, and S. Corneillie for many helpful discussions and valuable comments on the manuscript.
This work was supported by grants CA75180 (X.L.) from the National Cancer Institute and DAMD17-96-6076 (X.L.) from the U.S. Army Breast Cancer Research Program.
REFERENCES
- 1.Franz W M, Berger P, Wang J Y J. Deletion of an N-terminal regulatory domain of the c-abl tyrosine kinase activates its oncogenic potential. EMBO J. 1989;8:137–147. doi: 10.1002/j.1460-2075.1989.tb03358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass C K. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr Rev. 1994;15:391–407. doi: 10.1210/edrv-15-3-391. [DOI] [PubMed] [Google Scholar]
- 3.Goga A, Liu X, Hambuch T M, Senechal K, Mayor E, Berk A J, Witte O N, Sawyers C L. p53-dependent growth suppression by the c-Abl nuclear tyrosine kinase. Oncogene. 1995;11:791–799. [PubMed] [Google Scholar]
- 4.Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 5.Hupp T R, Meek D W, Midgley C A, Lane D P. Regulation of the specific DNA binding function of p53. Cell. 1992;71:875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- 6.Hupp T R, Sparks A, Lane D P. Small peptide activate the latent sequence-specific DNA binding function of p53. Cell. 1995;83:237–245. doi: 10.1016/0092-8674(95)90165-5. [DOI] [PubMed] [Google Scholar]
- 7.Jackson P, Baltimore D. N-terminal mutations activate the leukemogenic potential of the myristoylated form of c-abl. EMBO J. 1989;8:449–456. doi: 10.1002/j.1460-2075.1989.tb03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayaraman L, Murthy K G K, Zhu C, Curran T, Xanthoudakis S, Prives C. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 1997;11:558–570. doi: 10.1101/gad.11.5.558. [DOI] [PubMed] [Google Scholar]
- 9.Jiang G, Lee U, Sladek F M. Proposed mechanism for the stabilization of nuclear receptor DNA binding via protein dimerization. Mol Cell Biol. 1997;17:6546–6554. doi: 10.1128/mcb.17.11.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kharbanda S, Yuan Z M, Weichselbaum R, Kufe D. Determination of cell fate by c-Abl activation in the response to DNA damage. Oncogene. 1998;17:3309–3318. doi: 10.1038/sj.onc.1202571. [DOI] [PubMed] [Google Scholar]
- 11.Ko L J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 12.Konopka J B, Witte O N. Detection of c-Abl tyrosine kinase activity in vitro permits direct comparison of normal and altered abl gene products. Mol Cell Biol. 1985;5:3116–3123. doi: 10.1128/mcb.5.11.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine A J. p53, the cellular gatekeeper for the growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Miller C W, Koeffler H P, Berk A J. The p53 activation domain binds the TATA box-binding polypeptide in holo-TFIID, and a neighboring p53 domain inhibits transcription. Mol Cell Biol. 1993;13:3291–3300. doi: 10.1128/mcb.13.6.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Berk A J. Reversal of in vitro p53 squelching by both TFIIB and TFIID. Mol Cell Biol. 1995;15:6474–6478. doi: 10.1128/mcb.15.11.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z G, Baskaran R, Lea-Chou E T, Wood L D, Chen Y, Karin M, Wang J Y J. Three distinct signalling responses by murine fibroblasts to genotoxic stress. Nature. 1996;384:273–276. doi: 10.1038/384273a0. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Scolnick D M, Trievel R C, Zhang H B, Marmorstein R, Halazonetis T D, Berger S L. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol Cell Biol. 1999;19:1202–1209. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maki C G. Oligomerization is required for p53 to be efficiently ubiquitinated by Mdm2. J Biol Chem. 1999;274:16531–16535. doi: 10.1074/jbc.274.23.16531. [DOI] [PubMed] [Google Scholar]
- 19.Mayer B J, Baltimore D. Mutagenic analysis of the roles of SH2 and SH3 domains in regulation of the Abl tyrosine kinase. Mol Cell Biol. 1994;14:2883–2894. doi: 10.1128/mcb.14.5.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLure K G, Lee P W. How p53 binds DNA as a tetramer. EMBO J. 1998;17:3342–3350. doi: 10.1093/emboj/17.12.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pendergast A M, Muller A J, Havlik M H, Maru Y, Witte O N. BCR sequences essential for transformation by the BCR-ABL oncogene bind to the ABL Sh2 regulatory domain in a non-phosphotyrosine-dependent manner. Cell. 1991;66:161–171. doi: 10.1016/0092-8674(91)90148-r. [DOI] [PubMed] [Google Scholar]
- 22.Pietenpol J A, Tokino T, Thiagalingam S, Ei-Deiry W S, Kinzler K W, Vogelstein B S. Sequence-specific transcriptional activation is essential for growth suppression by p53. Proc Natl Acad Sci USA. 1994;91:1998–2002. doi: 10.1073/pnas.91.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prives C. Signaling to p53: breaking the Mdm2-p53 circuit. Cell. 1998;95:5–8. doi: 10.1016/s0092-8674(00)81774-2. [DOI] [PubMed] [Google Scholar]
- 24.Sheppard H M, Corneillie S I, Espiritu C, Liu X. New insight into the mechanism of inhibition of p53 by simian virus 40 large T antigen. Mol Cell Biol. 1999;19:2746–2753. doi: 10.1128/mcb.19.4.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shieh S-Y, Taya Y, Prives C. DNA damage-inducible phosphorylation of p53 at N-terminal sites including a novel site, Ser20, requires tetramerization. EMBO J. 1999;18:1815–1823. doi: 10.1093/emboj/18.7.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sionov R V, Moallem E, Berger M, Kazaz A, Gerlitz O, Ben-Neriah Y, Oren M, Haupt Y. C-Abl neutralizes the inhibitory effect of Mdm2 on p53. J Biol Chem. 1999;274:8371–8374. doi: 10.1074/jbc.274.13.8371. [DOI] [PubMed] [Google Scholar]
- 27.Sladek F M, Qing D-Y, Nepomuceno L. MODY1 mutation Q268X in hepatocyte nuclear factor 4a allows for dimerization in solution but cause abnormal subcellular localization. Diabetes. 1998;47:985–990. doi: 10.2337/diabetes.47.6.985. [DOI] [PubMed] [Google Scholar]
- 28.Stürzbecher H W, Addison B R, Rudge C, Remm K, Grimaldi M, Keenan M, Jenkins E., Jr A C-terminal alpha-helix plus basic region motif is the major structural determinant of p53 tetramerization. Oncogene. 1992;7:1513–1523. [PubMed] [Google Scholar]
- 29.Unger T, Mietz J A, Scheffner M, Yee C L, Howley P. Functional domains of wild-type and mutant p53 proteins involved in transcriptional regulation, transdominant inhibition, and transformation suppression. Mol Cell Biol. 1993;13:5186–5194. doi: 10.1128/mcb.13.9.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Etten R A, Debnath J, Zhou H, Casasnovas J M. Introduction of a loss-of-function point mutation from the SH3 region of the Caenorhabditis elegans sem-5 gene activates the transforming ability of c-abl in vivo and abolishes binding of proline-rich ligands in vitro. Oncogene. 1995;10:1977–1988. [PubMed] [Google Scholar]
- 31.Waterman M J F, Stavridi E S, Waterman J L F, Halazonetis T D. ATM-dependent activation of p53 involves dephosphorylation and association with 14-3-3 proteins. Nat Genet. 1998;19:175–178. doi: 10.1038/542. [DOI] [PubMed] [Google Scholar]
- 32.Yuan Z M, Huang Y, Fan M M, Sawyers C L, Kharbanda S, Kufe D. Genotoxic drugs induce interaction of the c-Abl tyrosine kinase and the tumor suppressor protein p53. J Biol Chem. 1996;271:26457–26460. doi: 10.1074/jbc.271.43.26457. [DOI] [PubMed] [Google Scholar]
- 33.Yuan Z M, Huang Y, Whang Y, Sawyers C L, Weichselbaum R, Kharbanda S, Kufe D. Role for c-Abl tyrosine kinase in growth arrest response to DNA damage. Nature. 1996;382:272–274. doi: 10.1038/382272a0. [DOI] [PubMed] [Google Scholar]
- 34.Yuan Z M, Huang Y, Ishiko T, Nakada S, Utsugisawa T, Shioya H, Weichselbaum R, Shi Y, Kufe D. Role for p300 in stabilization of p53 in the response to DNA damage. J Biol Chem. 1999;274:1883–1886. doi: 10.1074/jbc.274.4.1883. [DOI] [PubMed] [Google Scholar]
- 35.Yuan Z M, Shioya H, Ishiko T, Sun X, Gu J, Huang Y Y, Lu H, Kharbanda S, Weichselbaum R, Kufe D. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature. 1999;399:814–817. doi: 10.1038/21704. [DOI] [PubMed] [Google Scholar]