Abstract
This guideline has been initiated by the task force Autoimmune Blistering Diseases of the European Academy of Dermatology and Venereology, including physicians from all relevant disciplines and patient organizations. It is a S3 consensus‐based guideline that systematically reviewed the literature on mucous membrane pemphigoid (MMP) in the MEDLINE and EMBASE databases until June 2019, with no limitations on language. While the first part of this guideline addressed methodology, as well as epidemiology, terminology, aetiology, clinical presentation and outcome measures in MMP, the second part presents the diagnostics and management of MMP. MMP should be suspected in cases with predominant mucosal lesions. Direct immunofluorescence microscopy to detect tissue‐bound IgG, IgA and/or complement C3, combined with serological testing for circulating autoantibodies are recommended. In most patients, serum autoantibodies are present only in low levels and in variable proportions, depending on the clinical sites involved. Circulating autoantibodies are determined by indirect IF assays using tissue substrates, or ELISA using different recombinant forms of the target antigens or immunoblotting using different substrates. The major target antigen in MMP is type XVII collagen (BP180), although in 10–25% of patients laminin 332 is recognized. In 25–30% of MMP patients with anti‐laminin 332 reactivity, malignancies have been associated. As first‐line treatment of mild/moderate MMP, dapsone, methotrexate or tetracyclines and/or topical corticosteroids are recommended. For severe MMP, dapsone and oral or intravenous cyclophosphamide and/or oral corticosteroids are recommended as first‐line regimens. Additional recommendations are given, tailored to treatment of single‐site MMP such as oral, ocular, laryngeal, oesophageal and genital MMP, as well as the diagnosis of ocular MMP. Treatment recommendations are limited by the complete lack of high‐quality randomized controlled trials.
Diagnostics
Diagnosis of mucous membrane pemphigoid (MMP) is based on clinical findings (see part I) together with detection of anti‐basement membrane zone (BMZ) autoantibodies. These autoantibodies are tissue‐bound, detected by direct immunofluorescence (DIF) microscopy and/or direct immunoelectron microscopy, or circulating when detected either by indirect IF (IIF), ELISA or immunoblotting. Histopathology may be helpful in some cases when MMP, or another autoimmune blistering disease (AIBD), cannot be detected using these methods. About 50% of cases of ocular MMP cannot be confirmed by BMZ autoantibody detection tests. To exclude other cicatrizing conjunctival disorders with a similar disease course, this subset of ocular MMP cases requires an additional panel of investigations before a diagnosis of ocular MMP can be confirmed (see section on Diagnosis of ocular MMP).
Direct immunofluorescence microscopy
Direct immunofluorescence visualizes in vivo bound immunoreactants in skin or mucosa and shows linear deposition of IgG and/or IgA and complement C3 along the BMZ in MMP. DIF of a perilesional biopsy is considered the reference standard for diagnosis of MMP. 1 Sensitivities have been reported in a wide range, between 41 and 100%, depending on biopsy site. Mainly retrospective studies have been performed to assess the diagnostic accuracy of DIF, reporting high sensitivities when DIF is used as the reference standard for diagnosis, and lower sensitivities when clinical criteria have been used. Highest sensitivity has been found in MMP whereby both mucosa and skin were affected. 2 DIF biopsies of mucosa have been reported to have sensitivities between 41 and 100%, 2 , 3 , 4 , 5 , 6 , 7 and of skin between 44 and 100%. 2 , 3 , 6 , 7 , 8 , 9 , 10 , 11
Conclusions

Recommendations

Immunoreactants can also be detected by DIF in non‐affected asymptomatic sites. 9 , 10 , 12 A recent retrospective study in 251 oral MMP patients compared DIF performed on normal buccal mucosa with a perilesional punch mucosal biopsy, and detected no significant difference between the two approaches in sensitivity for oral MMP (93.7% vs. 89.6%). 7 Immunoreactants can be detected in a skin biopsy by DIF of affected or non‐affected skin (in 44–48%), and may confirm diagnosis of MMP. 2 , 9 , 11 A minimum biopsy size of 3–4 mm of skin, and for mucosa, is recommended. 9 , 13 , 14 , 15 Saline transportation can be used for skin or mucosal biopsies (within 24 h), but is not suitable for conjunctival biopsies. 16 , 17 Routine testing should be performed for IgG, IgA and complement C3. 2 , 4 , 11 IgM and fibrin depositions can also be found in conjunction with other immunoreactants, and as solitary findings in oral lichen planus. 2
Recommendations


Negativity of DIF may possibly depend on biopsy site or technical difficulties in cases of conjunctival biopsies, and multiple simultaneous and sequential biopsies may increase the diagnostic yield. 6 Similarly, IgG4 staining may increase the yield of DIF. 18 Positive DIF findings in MMP do not distinguish predominant cutaneous variants of pemphigoid, which should be determined on clinical grounds. 1 , 12
Conclusions

Recommendations

Serration pattern analysis allows for identification of tissue‐bound antibodies against type VII collagen. 17 , 19 , 20 Serration pattern analysis is often not determinable in mucosal biopsies, but can be performed in biopsies taken from the skin. 17 , 19 , 20
Conclusions

Recommendations
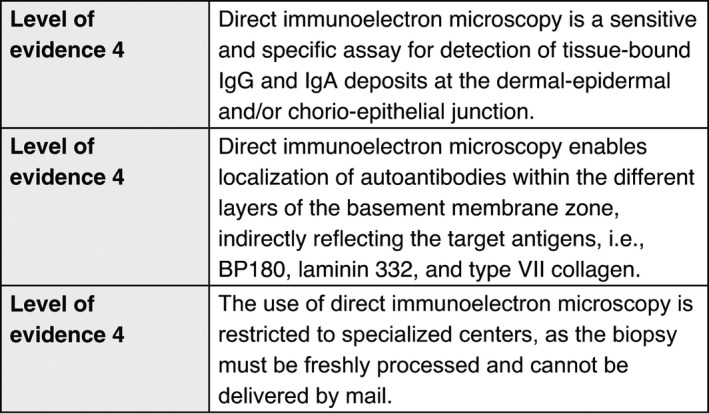
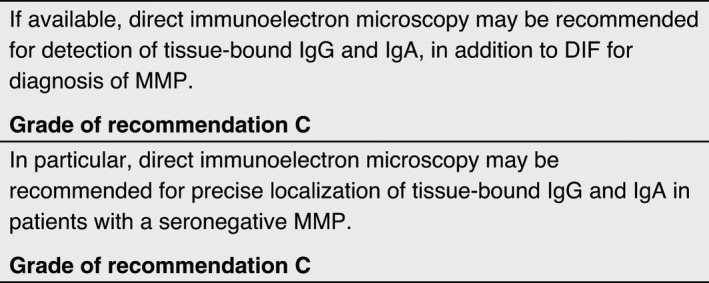
Direct immunoelectron microscopy
Electron microscopy studies allow the analysis of BMZ, including hemidesmosomes, anchoring filaments and anchoring fibrils; these structures cannot be seen by light microscopy. Two techniques of electron microscopy are available: standard transmission electron microscopy and immunoelectron microscopy. Transmission electron microscopy enables precise identification of the level of blister formation, and of structural abnormalities of junction systems which lead to this cleavage. Direct immunoelectron microscopy, like DIF, allows detection of in vivo bound IgA, IgG, IgM and/or C3. While DIF gives only linear staining of the BMZ at the dermo‐epidermal or chorio‐epithelial junction, direct immunoelectron microscopy demonstrates more precise ultrastructural in vivo location of antibodies within the dermo‐epidermal or chorio‐epithelial junctions.
For immunoelectron microscopy, a biopsy must be obtained from clinically normal‐appearing skin or mucous membrane adjacent to a lesion within 1–2 cm of the lesions. The minimal diameter size of the biopsy is 6 mm. The sample must be immersed immediately in the appropriate medium 21 and transported within one hour to the laboratory under proper conditions, as any delay will cause it to dry out and result in irreversible damage, making it unsuitable for analysis. Avoid anaesthesia with adrenalin, and taking biopsies of blistered skin, because this often results in artefacts or false‐negative results. Details on the detection of binding sites of immune deposits are provided in the Appendix S1.
Conclusions

Recommendations

Immunoserological tests
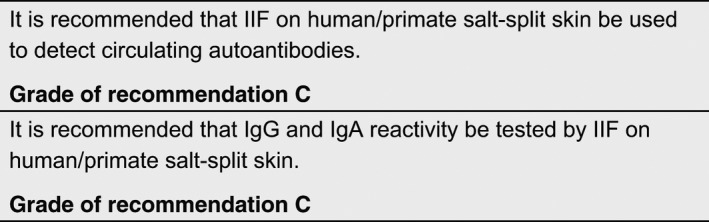
Indirect immunofluorescence on tissue substrates
IIF detects circulating autoantibodies in the patient’s sera through an isotype‐specific fluorescent‐labelled secondary antibody. 22 In case of MMP, positivity is defined by the detection of linear IgG or IgA along the BMZ of different tissue substrates. 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 Compared to bullous pemphigoid, IgG positivity by IIF in MMP occurs less frequently and usually with lower titres. 25 , 27 , 31 , 32 This may be due to the heterogeneity of the target antigens, or the lower amount of autoantibodies in MMP sera. IIF reactivity is largely accounted for by IgG class autoantibodies, but IgA autoantibodies can be detected in about 60% of MMP sera. 27 , 32 , 33 Combined IgA and IgG reactivity was associated with more severe disease, compared to the presence IgG autoantibodies alone 32 , 33 whereas other studies failed to reveal a similar relation. 29 , 34 One study has reported the presence of circulating anti‐BMZ IgE in 24% of 29 MMP patients, 35 but this study awaits confirmation. The sensitivity of IIF depends on the substrate used. Circulating antibodies have been detected in a small percentage of patients on monkey oesophagus substrate, ranging from 2.6% to 8%. 28 , 29 , 36 Normal human skin had previously demonstrated higher values (17–35%). 25 , 31 , 37 In the salt‐split skin technique, normal human or primate skin is incubated in 1 mol/L NaCl until splitting occurs within the lamina lucida of the BMZ. 38 This procedure showed positivity in a significantly greater proportion of MMP patients (36% to 84%). 23 , 25 , 27 , 29 , 32 , 39 , 40 IIF on normal human oral mucosa showed a sensitivity of 85% in a recent study 37 , whereas in another study, the same substrate tested negative in all patients. 40 Further investigations are needed to clarify the diagnostic value of oral mucosa as substrate for IIF in MMP. Further details are given in the Appendix S1 and in Table S1.
Conclusions
Recommendations

Target antigen‐specific detection of autoantibodies
The target antigens of MMP autoantibodies are components of the epidermal BMZ. Currently, five different target antigens have been identified at the molecular level: BP180 (type XVII collagen), BP230, all three laminin 332 subunits, both subunits of integrin α6β4 and type VII collagen. 1 , 41 A pathogenic role of MMP IgG autoantibodies against laminin 332 and α6β4 integrin has been described. 42 , 43 , 44 , 45 , 46 Different methods have been established that enable target antigen‐specific detection of serum autoantibodies in MMP sera, including ELISA, immunoblotting, immunoprecipitation and indirect IF microscopy (detailed in the Appendix S1). Five assays (ELISA and IIF) applying four target antigens are highly standardized and widely available; they allow the detection of (i) IgG autoantibodies against the 16th non‐collagenous domain of BP180 (NC16A), (ii) C‐ (and N‐terminal) part(s) of BP230 and (iii) the laminin 332 heterotrimer. Other serological test systems are available only in specialized laboratories.
It is noteworthy that the reported sensitivities and specificities of these serological tests, discussed in more detail below, are mainly based on studies of selected and well‐characterized patients. In addition, some studies have applied cut‐off values for these serological tests in MMP that have been established in bullous pemphigoid, e.g. BP180 NC16A‐ and BP230‐specific ELISA.
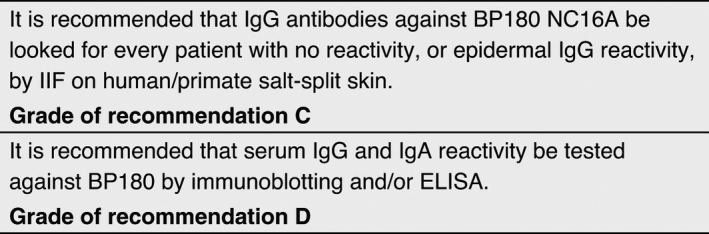
Detection of antibodies against BP180
BP180 (also termed type XVII collagen) is the most frequent target antigen in MMP and is recognized by approximately 70% of MMP sera. 14 , 25 , 27 , 29 , 31 , 33 , 34 , 39 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 Immunoblotting has been performed using various substrates, including extracts of human cultured keratinocytes from skin and oral mucosa, epidermis or amniotic membrane; keratinocyte hemidesmosome‐rich fraction; enriched preparations of the soluble ectodomain of BP180 in medium of cultured keratinocytes (LAD‐1); and various recombinant fragments. With these approaches, IgG autoantibodies to BP180 were found in 30–78% of MMP patients, while IgA were detected in 11–51% of MMP sera. 25 , 27 , 31 , 33 , 34 , 39 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 56 , 59 In a cohort of non‐scarring oral MMP cases, 75% showed antibodies against BP180. 54 A different cohort of oral MMP cases showed BP180 reactivity in 46% of the cases and found no significant differences in antibody recognition pattern in patients with restricted oral lesions and patients with also other affected sites. 34
Several studies described the reactivity of MMP sera with the C‐terminal portions of the molecule, whether or not combined with a reactivity to the NC16A portion immunodominant in bullous pemphigoid. 1 , 25 , 27 , 33 , 48 , 49 , 52 , 54 , 60 , 61 A large majority of bullous pemphigoid patients also showed reactivity with the C‐terminal portion in addition to reactivity with the NC16A domain of BP180. 62 In addition, direct binding of BP180 to type IV collagen, and the capability of antibodies targeting the C‐terminus of BP180 to hinder this binding in oral mucosa keratinocytes, has been recently reported. 59 Importantly, anti‐BP180 autoAbs are not limited to the IgG isotype, and testing for IgA reactivity increased the detection rate. 25 , 27 , 52 , 61 , 63 The soluble ectodomain of BP180, originally described as target antigens of linear IgA disease, LAD‐1 or a 97 kDa fragment of LAD‐1, is recognized by a portion of MMP sera by immunoblotting. 27 , 33 , 51 , 56 , 60 , 64 , 65 Serum levels of autoantibodies to BP180 did not correlate with disease severity in MMP patients. 29 , 55 , 66 In line with this, while the pathogenic relevance of IgG antibodies against the NC16A domain is unclear, its murine homologue has been clearly demonstrated in several mouse models, resulting in predominant skin lesions 67 , 68 , 69 ; no mouse model has been reported that mimics human MMP based on antibodies against a C‐terminal stretch of BP180. However, the presence of autoantibodies recognizing different target antigens, or multiple BP180 epitopes, or belonging to IgG and IgA isotypes, has been observed in MMP patients with more severe clinical features. 33
Conclusions
Recommendations

Detection of antibodies against BP230
Autoreactivity against BP230 is detected occasionally in MMP sera, with a frequency ranging from 0% to 40%. 25 , 29 , 33 , 34 , 47 , 48 , 49 , 50 , 51 , 54 , 55 , 57 , 64 , 65 , 66 , 70 , 71 , 72 Anti‐BP230 IgA are absent or less represented than IgG. 34 , 49 While injection of anti‐BP230 antibodies in neonatal mice has resulted in skin lesions, the pathophysiological relevance of antibodies against BP230 in MMP has not yet been demonstrated. 73 In line with this, antibodies against BP230 have not been found to correlate with MMP severity. 29 , 55 , 66
Conclusions

Recommendations
Detection of antibodies against laminin 332
Laminin 332 is the second most frequent target antigen of autoantibodies in MMP. 74 Although IIF on salt‐split human/primate skin is a sensitive serological test for detection of circulating autoantibodies in MMP, a portion of MMP sera reactive to laminin 332 are negative when tested by IIF; this emphasizes the relevance of using additional techniques for serological diagnosis. 25 , 39 , 55 Until very recently, detection of anti‐laminin 332 antibodies was limited to specialized laboratories and performed using different inhouse assays, including immunoblotting, immunoprecipitation and ELISA. After comparison of different methods for the detection of anti‐laminin 332 antibodies, immunoprecipitation with radiolabelled keratinocyte extracts was found to be the most sensitive technique, followed by immunoblotting with extracellular matrix of cultured human keratinocytes. 50 , 75 , 76 In unselected MMP patients, detection in tested sera of antibodies to laminin 332 by immunoblotting or immunoprecipitation ranged from 4% to 31%. 25 , 27 , 29 , 39 , 49 , 50 The α3 subunit of laminin 332 was the most frequently targeted chain, followed by the γ2 subunit, 29 , 56 , 71 , 75 , 76 , 77 , 78 , 79 , 80 and IgG4 was the most strongly represented subclass. 76 , 80 , 81 Also serum IgE and IgA were reactive with laminin 332 in small subsets of patients. 56 , 82
Several ELISAs for detection of anti‐laminin 332 IgG have been established. 55 , 70 , 76 , 81 , 83 When tested on laminin 332 positive sera from MMP patients, this approach showed high sensitivity but limited specificity, ranging from 75% to 94% and from 60% to 98%, respectively. 70 , 76 , 83 In a large group of unselected MMP patients, Bernard and coworkers detected laminin 332 antibodies in 20% of sera, with a specificity of 91% (3/32 of healthy controls). 55 Further, a sensitive (100%, n = 16) and specific assay (96.9%, n = 127), based on detection by IIF of IgG binding to laminin 332 secreted by human keratinocytes, named the keratinocyte footprint assay, has been reported. 84 Moreover, a sensitive and specific assay based on IIF on HEK293 cells expressing the laminin 332 heterotrimer on the cell surface (biochip mosaic), has recently been developed. When a large cohort of 93 laminin 332 positive MMP patients was assayed, a sensitivity of 84% and specificity of 99.6% were obtained. 80 This assay is highly standardized and widely available.
Another elegant but non‐routine method for the detection of anti‐laminin 332 serum antibodies is indirect IF microscopy on the skin of patients with inherited junctional epidermolysis bullosa deficient of laminin 332. 85 However, this method requires reactivity on human/primate skin, and the absence of reactivity with any other BMZ antigen. Furthermore, the availability of laminin 332‐deficient skin is limited.
Conclusions

Recommendations
Detection of antibodies against α6β4 integrin
Reactivity of MMP sera with α6β4 integrin was originally described by Ahmed’s group. 86 By employing immunoblotting and immunoprecipitation with α6β4 integrin‐rich tumour cell lysates (e.g. DU145 prostate cancer cells) and different tissue lysates (bovine and human epidermis, gingiva, conjunctiva), they showed that sera of patients with different clinical phenotypes react specifically with one of the two subunits of the integrin. 87 , 88 , 89 They reported that ocular MMP sera, and MMP sera from patients with at least two involved mucosal sites, recognized the β4 integrin subunit, while oral MMP sera reacted with the α6 integrin subunit. 87 , 88 , 90 In addition, anti‐β4 and anti‐α6 IgG serum levels correlated with disease activity and response to therapy. 66 , 91 , 92 A limited number of studies from different laboratories have confirmed the results obtained by Ahmed’s group, while other authors failed to detect any α6β4 integrin reactivity in MMP patient sera. 14 , 54 , 57 Oyama and coworkers reported that 26 of 124 (21%) of MMP patient sera recognized the β4 integrin subunit; they used immunoblotting on placental amnion proteins, of which 23/26 (88%) had ocular involvement, suggesting that the β4 integrin might be a site‐specific antigenic determinant in MMP with ocular involvement. 33 More recently, analysis of 43 ocular MMP sera by immunoblotting on hemidesmosome‐rich fraction showed IgG reactivity to the cytoplasmic domain of β4 integrin in 42% of sera and to the α6 ectodomain in 19%. 56
Conclusions

Recommendations
Detection of antibodies against type VII collagen
Type VII collagen (Col7) is the major component of anchoring fibrils and the autoantigen of epidermolysis bullosa acquisita (EBA). The serological diagnosis of EBA has previously been discussed in detail in a consensus paper by a group of international experts. 93 Reactivity with Col7 in MMP is rare and may account for fewer than 5% of cases. 29 , 94 Several assays for the serological detection of anti‐Col7 antibodies have been described, including (i) several ELISA systems that apply recombinant forms of Col7; (ii) immunoblotting of recombinant or forms of Col7; (iii) immunoblotting of cell‐derived forms of Col7, e.g. in human dermis or an amnion epithelial cell line; (iv) an IIF‐based test which uses a human cell line that expresses the recombinant NC1 domain on the cell surface; and (v) indirect IF on Col7‐deficient skin. 93 Two of these assays are highly standardized and widely available: an ELISA that employs the recombinant NC1 domain (sensitivity and specificity for EBA, 92.9% and 100%), and an indirect IF‐based biochip mosaic, where recombinant NC1 domain is present on the cell surface (sensitivity and specificity for EBA, 87.5% and 100%). 95 , 96 , 97 , 98 , 99
Conclusions

Recommendations

Further details about the detection of autoantibodies against the individual target antigens are provided in the Appendix S1.
Histopathology
Histopathology is less sensitive and specific in diagnosing MMP compared to DIF, and in a recent study reached a sensitivity of 69.4% in 134 patients. 7 Its main role in MMP is to rule out other diseases, e.g. lichen planus, infectious diseases, pemphigus vulgaris and erythema multiforme. The characteristic histopathological picture shows subepithelial splitting, with a non‐specific mixed infiltrate consisting of lymphocytes, histiocytes, plasma cells, neutrophils and eosinophils. 7 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 However, less eosinophilic granulocytes than in bullous pemphigoid have been observed. 108 Epithelial changes reminiscent of lichen planus with acanthosis, hypergranulosis, as well as vacuolar degeneration with fibrosis and a band‐like infiltrate have also been described. 112 However, in many cases, only a non‐specific ulcerative inflammation can be seen, with granulation tissue and scarring. In these cases, one cannot differentiate between MMP and aforementioned differential diagnoses. Scarring is commonly seen in late or recurrent lesions. Conjunctival biopsies often lack a subepithelial split, and instead show epithelial metaplasia, a reduced number of goblet cells, fibrosis and a non‐specific chronic infiltrate. 15 , 100 , 113 , 114
Conclusions

Recommendations
Diagnosis of ocular MMP
Up to 50% of ocular MMP cases do not meet the immunopathological criteria recommended in the 2002 Consensus for a diagnosis of MMP. 1 Because the current standard of care for the causes of cicatrizing conjunctivitis other than MMP is topical therapy, and not the systemic immunomodulatory therapy required for ocular MMP, 36 , 115 , 116 implementation of the Consensus guideline has resulted either in delayed diagnosis in individual patients with ocular MMP, or a diagnosis of a non‐MMP severe chronic cicatrizing conjunctivitis. 10 , 117 , 118 In both situations, inappropriate treatment with topical therapy has resulted in poor outcomes for individual patients. The background to the opposing recommendations regarding the definition and diagnosis of ocular MMP is described in two recent case series, in which 26/55 (47.3%) 117 and 20/73 (27.4%) patients 10 with ocular MMP did not meet current immunopathological criteria for diagnosis, but in whom the clinical phenotype, disease severity and disease course were identical to that in immunopathology positive ocular MMP cases.
The subset of ocular MMP cases with undetectable autoantibodies requires an additional panel of investigations before a diagnosis of ocular MMP can be confirmed, to exclude other cicatrizing conjunctival disorders with a similar disease course. These investigations include both conventional histopathology and a careful clinical history, and systemic examination outlined below. 10 , 117 , 118 , 119 , 120 , 121
-
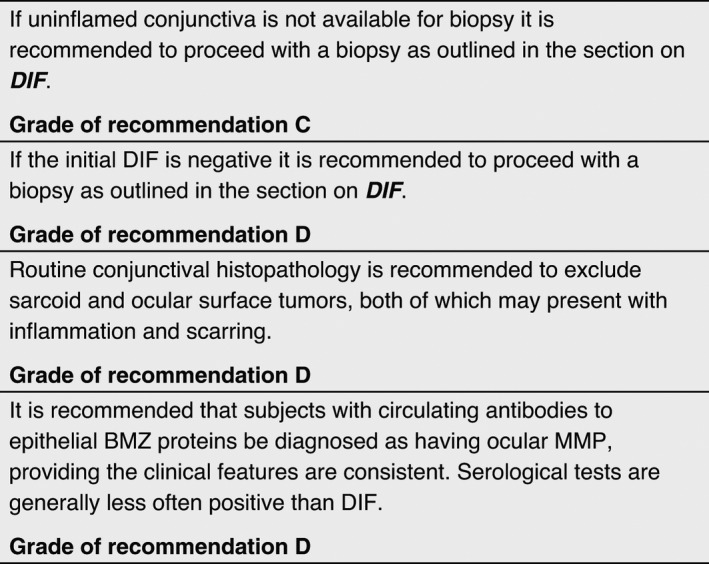
DIF on the conjunctiva and/or tissue from other sites. Patients with DIF showing IgG, IgA, and/or C3, either in the conjunctiva or from another site, meet the currently widely adopted 2002 Consensus criteria. Biopsy of normal skin or oral mucosa may be positive when a conjunctival biopsy is DIF negative in ocular MMP. 117
-
a
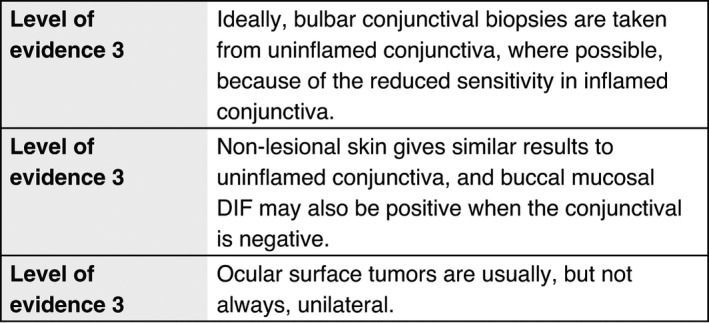
Where possible, bulbar conjunctival biopsies should be taken from uninflamed conjunctiva because of the reduced sensitivity in inflamed conjunctiva. 9 , 122 When they are taken from inflamed conjunctiva, this should be recorded.
-
b
Biopsies should be taken from another non‐lesional site if the conjunctiva is inflamed, and because multiple biopsies improve the detection of a positive DIF. 6 Non‐lesional skin gives results similar to those of uninflamed conjunctiva, 9 and buccal mucosal DIF may also be positive when the conjunctival is negative. More data are needed regarding the numbers of biopsies that are optimal to provide good DIF sensitivity.
-
c
Conjunctival DIF should also include staining for fibrinogen to identify lichen planus, which shows shaggy discontinuous fibrinogen deposits at the BMZ. 1
-
a
Routine conjunctival histopathology is needed to exclude sarcoid and ocular surface tumours, both of which may present with inflammation and scarring. Ocular surface tumours are usually, but not always, unilateral.
Serology tests: Patients with positive IIF, or the presence of antibodies to epithelial BMZ proteins, can be diagnosed as having ocular MMP providing the clinical features are consistent. These tests are generally less often positive than DIF 1 , and it is important to be aware that a variable proportion of age‐ and sex‐matched healthy controls have positive serology findings (see Table S1).
When both DIF and serology are negative, and the other diseases that may cause cicatricial conjunctivitis have been excluded, this immunopathology negative subset of patients can be diagnosed as having ocular MMP. However, if the disease course or response to therapy is not as expected, all tests should be repeated.
Conclusions
Recommendations

Algorithm for the diagnosis of MMP
The recommended algorithm for the diagnosis of MMP is shown in Fig. 1.
Figure 1.

Diagnostic algorithm and work‐up, and diagnostic criteria for mucous membrane pemphigoid. 1Alternatively or in addition, direct immunoelectron microcopy can be performed. 2A positive DIF from any site is diagnostic for MMP, providing the clinical phenotype at the site that has not been biopsied is consistent with MMP. 3If ocular MMP is suspected, take biopsies from the least inflamed bulbar conjunctiva of both eyes together with another site (buccal mucosa or skin). Also take an additional lesional biopsy for routine histopathology to exclude both ocular surface neoplasia and sarcoid (which may present in the conjunctiva). 4Patients with predominant or exclusive IgA deposition could also be classified as having linear IgA disease. 5Patients with reactivity with type VII collagen could also be classified as having Epidermolysis Bullosa Acquisita. 6On human/primate salt‐split skin. 7Commercially available (for IgG antibodies). 8Only available in specialized diagnostic centers. 9Associated with a malignancy in 25–30% of patients; a tumor search is indicated. 10A diagnosis of immunopathology unconfirmed ocular monosite MMP can be made by exclusion of the more than 25 other causes of cicatrising conjunctivitis (CC). MMP is the most common cause of CC in most developed countries. Causes of CC, except for sarcoid & surface neoplasia: (i) have a history consistent with another cause of conjunctival disease; (ii) are positive on routine histopathology for neoplasia or sarcoid; or (iii) are DIF+ for another immuno‐bullous disease. If, after initiating appropriate therapy for immunopathology negative ocular monosite MMP, the disease course or response to therapy is not as expected, then this algorithm (both for DIF in ocular cases and serology) should be repeated and alternative diagnoses considered (e.g., severe ocular rosacea which can be difficult to differentiate from ocular MMP. DIF, direct immunofluorescence microscopy; ELISA, enzyme‐linked immuno sorbent assay; IIF, indirect immunofluorescence microscopy; MMP, mucous membrane pemphigoid.
Differential diagnoses of MMP
When repeated DIF and serology are negative, the diagnosis of MMP cannot be made, with the exception of individual cases of ocular MMP. In these rare cases, differential diagnoses need to be considered by an experienced ophthalmologist.
If multiple sites are involved, in particular the eyes, only few differential diagnoses remain, including pemphigus vulgaris (intraepithelial splitting by histopathology, antibodies against desmoglein 3, intercellular binding of autoantibodies in the epithelium by DIF), erythema multiforme, Steven Johnson syndrome and toxic epidermal necrolysis.
In single‐site MMP, the following differential diagnoses may be addressed:
Oral MMP: Herpes simplex virus infection, Candida infection, lichen planus, aphthous stomatitis, systemic lupus erythematosus, erythema multiforme, Steven Johnson syndrome, toxic epidermal necrolysis, leukoplakia, Crohn’s disease, malnutrition, radiation mucositis and chemotherapy‐induced mucositis.
Ocular MMP: Rosacea, viral and bacterial infections, atopic keratoconjunctivitis, trauma, malignant tumours, Sjögren's syndrome, systemic lupus erythematosus, sarcoidosis.
Genital MMP: Lichen sclerosus et atrophicus, erosive lichen planus, pemphigus and sexual abuse.
Laryngeal MMP: Pemphigus, epidermolysis bullosa and malignancy.
Conclusions
Recommendations

Management
Aim of therapy and multidisciplinary care
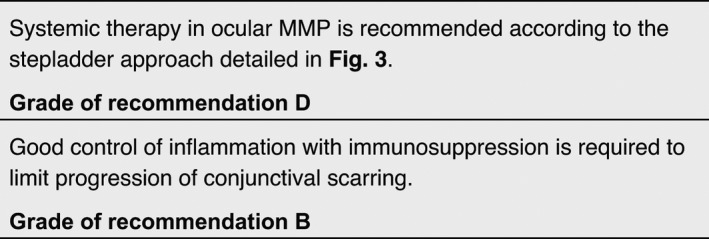
The aim of treatment is to stop the inflammation, and hereby the progression of scarring, especially of conjunctivae, larynx, oesophagus and genital mucous membranes. Surgical release of scarring and strictures is indicated only after the inflammatory phase of MMP has been fully controlled for several months.
Management of MMP requires a multidisciplinary team, involving specialists from dermatology, ophthalmology, otorhinolaryngology, gastroenterology and gynaecology/urology. Systemic treatment is ideally co‐ordinated by a specialist, collaborating with the clinicians treating the complications of disease at other sites of involvement. In centres focusing on the management of MMP, often an established multidisciplinary team (dermatologist, ophthalmologist, stomatologist, otorhinolaryngology, etc.) is involved in the diagnostics and follow‐up of the patients, including management of complications, and depending on the affected sites.
Mild MMP (moderate) and severe MMP are defined according to Chan et al.: patients with mild MMP have disease occurring only in oral mucosa, or in oral mucosa and skin. Patients with severe MMP have disease occurring in any of the following sites: ocular, genital, nasopharyngeal, oesophageal and/or laryngeal mucosae. 1
Conclusion

Recommendations

Topical medications
Oral MMP
Topical therapies available for use in oral MMP include a broad range of corticosteroids, or the calcineurin inhibitor tacrolimus. There are no randomized placebo‐controlled trials to support efficacy of topical therapies in MMP. Evidence is based largely upon small case series or RCTs conducted to study mixed oral vesiculoerosive disease. Nevertheless, the findings of these studies are frequently used in clinical practice.
Topical corticosteroid therapy was advocated in the First Consensus statement on MMP 1 for mild to moderate MMP as a first‐line approach, and more recently, the available evidence was evaluated in a systematic review. 123 This therapy is often used in clinical practice for mild or moderate disease oral MMP as first‐line therapy, and in more severe disease, it is used in addition to systemic therapy for patients with multisite or single‐site disease. Topical steroids, particularly the superpotent clobetasol propionate, can lead to remission. 124 , 125 The latter corticosteroid is the most frequently used topical ointment, while betamethasone sodium phosphate tablets 0.5mg may be diluted in water and used for rinsing for 2–3 min before discarding, between one and four times per day. Fluticasone propionate 400 micrograms (1 mg/mL) may also be used twice daily as a mouthwash. Corticosteroid metered‐dosed inhalers may be sprayed directly onto active lesions. The frequency of use is tailored to the severity of the disease, with one application ideally before sleep, as saliva flow is reduced overnight and the length of contact is therefore optimized; applications can be tapered as lesions improve.
For gingival lesions, use of a custom‐made, soft drug‐delivery tray covering the gingivae to extend drug contact time and absorption, has been described. 126 This is a method sometimes used in routine clinical practice, though no study has compared its efficacy with other methods of application. Adjuvant analgesic, anti‐inflammatory and anti‐infectious therapy can be additionally used, e.g. chlorhexidine 0.12–0.20%.
There are case reports demonstrating efficacy of topical tacrolimus in localized oral MMP, and reporting complete remission within 2–3 months. However, the cost is greater, and tolerance may be lower due to oral burning upon application. 127 , 128 , 129 No good evidence supports the use of topical cyclosporine for oral MMP. Further details on the topical treatment of oral MMP are provided in the Appendix S1.
Conclusions

Recommendations

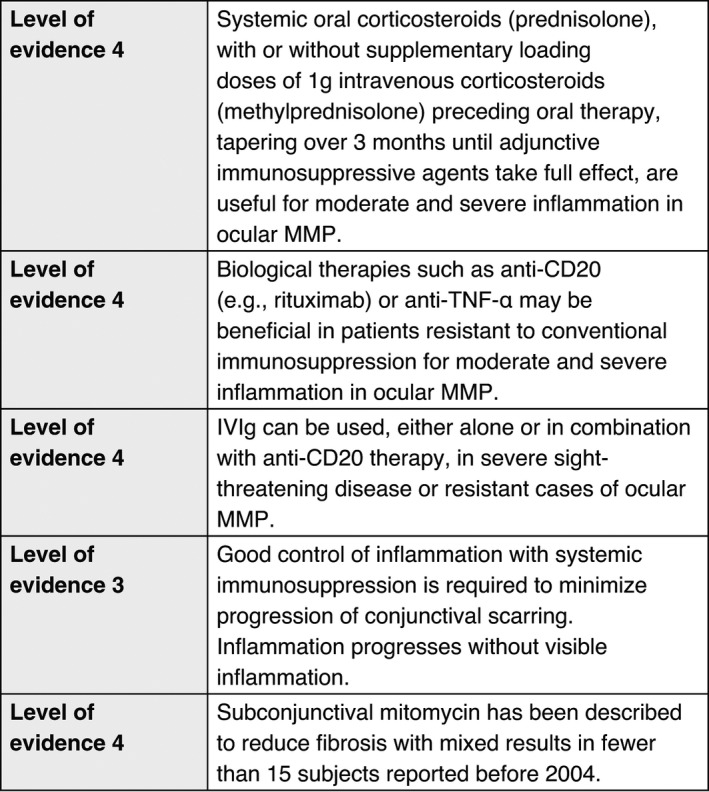
Ocular MMP
Historical evidence suggests that topical therapy does not alter the natural history of the disease, and offers only variable symptomatic relief. 130 , 131 , 132 , 133 But in patients intolerant to immunosuppression, or where it is not safe to administer immunosuppression, then topical steroids, combined with systemic matrix‐metallo proteinase inhibitors (tetracyclines), are a useful alternative for treating mild disease. Subconjunctival steroids, such as triamcinolone, may provide temporary benefit, but relapses may occur, together with complications such as cataract, glaucoma or localized scleral thinning. Topical tacrolimus and ciclosporin have been used in isolated cases with limited response. 134 , 135 , 136 , 137 Topical treatment in the form of lubricant drops, gels and ointments should be used to reduce trauma. These lubricants should preferably be free of preservatives to avoid iatrogenic toxicity. Serum eye drops may be used as alternative, or in addition, to provide nutrients to severely dry ocular surfaces.
Conclusions

Recommendations

Genital MMP
Only case reports on genital MMP have been presented in the literature. Topical corticosteroids, particularly clobetasol propionate, can lead to remission in juvenile MMP. 138 , 139 Topical tacrolimus was reported to be effective as monotherapy in one case report of juvenile MMP. 140 In two case reports, topical corticosteroids were ineffective in controlling the progression of juvenile MMP, and dapsone was added. 141 , 142 Farrell et al. reported remission of three patients with juvenile genital MMP treated with topical clobetasol propionate cream and tetracycline combined cream. Two patients required systemic corticosteroids, sulphones, azathioprine and dapsone. 143 Topical therapy in adult MMP is often not sufficient to achieve remission of genital lesions. 144 , 145
Recommendations

Systemic medications
Disease control has previously been defined as the point at which new inflammatory lesions cease to form and established lesions begin to heal. 146 Immunosuppressive agents need to be chosen with a ‘stepladder’ approach, beginning with drugs that have the fewest side effects.
Recommendations

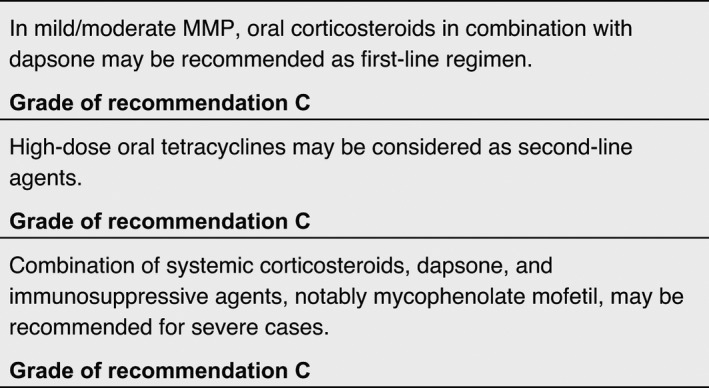
Tetracyclines
Tetracyclines are generally used as antibiotics due to their efficacy in controlling bacterial proliferation and growth. In addition to these effects, tetracyclines have been shown to have also anti‐inflammatory and collagenolytic properties. In light of their anti‐inflammatory action, tetracycline has been proposed as first‐line agent in mild/moderate MMP, also due to its better side‐effect profile as compared to corticosteroids and other conventional immunosuppressive agents. 147 , 148 Most patients included in the studies taken into consideration have been switched to tetracyclines due to adverse effects with previous treatments. On the other hand, minocycline has been stopped in five out of nine patients included in a case series of predominantly oral MMP due to its side effects, mainly vertigo and gastric upset. 149 In this case series with a follow‐up of 2 years, only one patient achieved persistent remission with no relapse.
Conclusion(s)

Recommendations

Dapsone
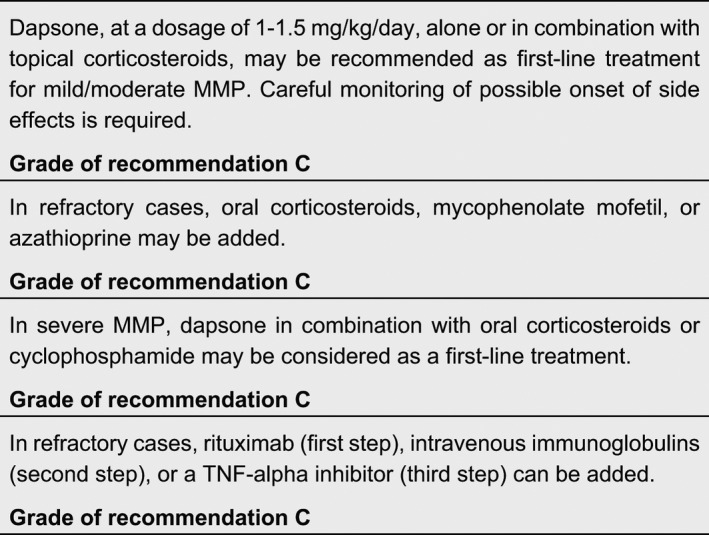
Dapsone, a well‐known anti‐leprosy drug, is effective in several dermatologic diseases due to its anti‐inflammatory properties. The corticosteroid‐sparing effect of dapsone could be explained by several mechanisms, including oxygen‐radical scavenging, reduction in tumour necrosis factor (TNF)‐α and dysregulation of lymphocyte function. Dapsone is used to treat both mild/moderate and severe cases of different autoimmune bullous diseases, usually in association with corticosteroids. Prior to initiation of therapy, the patient’s glucose‐6‐phosphate dehydrogenase (G6PD) level should be checked to be normal, since low levels are associated with a higher incidence of haemolytic anaemia.
Due to its anti‐inflammatory properties, dapsone is regarded as a first‐choice treatment for mild/moderate MMP. In a cross‐sectional retrospective review, seven out of 20 patients with oral MMP were maintained successfully on dapsone 50–150 mg/day. 150 However, possible side effects caused by dapsone: haemolytic anaemia, skin rash, malaise and gastrointestinal problems, have led to high discontinuation rates in different trials. 151 , 152 , 153 , 154
Conclusions

Recommendations

Mycophenolate mofetil
Mycophenolate mofetil (MMF) is a prodrug of mycophenolic acid and inhibits the de novo pathway of guanosine nucleotide synthesis. T‐ and B‐lymphocytes are critically dependent on this pathway for their proliferation, but the potent cytostatic effects of MMF inhibit proliferative responses of T‐ and B‐lymphocytes to both mitogenic and allospecific stimulation. Mycophenolic acid also suppresses antibody formation by B‐lymphocytes. The use of MMF for the treatment of mild/moderate or severe MMP has been investigated in few clinical trials. 153 , 155 , 156 , 157 Its efficacy in controlling inflammatory lesions and its safety, either in monotherapy or in association with corticosteroids, have been confirmed in all these studies.
Conclusions
Recommendations

Cyclophosphamide
Cyclophosphamide is an oxazaphosphorine‐substituted nitrogen mustard alkylating agent, with powerful cytotoxic and immunosuppressive effects. It is used to treat haematological and solid cancers as well as autoimmune diseases, including refractory and/or severe autoimmune bullous diseases. Main side effects of cyclophosphamide are haemorrhagic cystitis, infertility and bladder cancer.
Different clinical studies on cyclophosphamide, administered orally or intravenously, demonstrated its effectiveness in severe MMP, 158 , 159 , 160 , 161 , 162 , 163 and it has been shown to be effective for many years. 164 Both oral and intravenous pulsed cyclophosphamide showed a high rate of efficacy, preventing relapses in ocular MMP and allowing to taper corticosteroids. It induced sustained clinical remission both as monotherapy 160 and in combination with corticosteroids 158 , 161 , 163 or pentoxyfilline. 163
Conclusions

Recommendations

Corticosteroids
Systemic corticosteroids are widely used for their excellent anti‐inflammatory and immunosuppressive effects. A wide range of dermatoses, including autoimmune bullous diseases, are successfully treated with systemic steroids. However, the chronic courses of treatment required favour the onset of side effects, such as osteoporosis, adrenal suppression, hyperglycaemia, dyslipidaemia, cardiovascular disease, Cushing’s syndrome and psychiatric disturbances. Steroids may be administered orally, intravenously or through intramuscular injections.
Although systemic corticosteroids (initial oral prednisone 0.5–1.5 mg/kg/day) are effective in achieving rapid control in cases of acute, severe disease, the adverse effects associated with long‐term use limit their value. Systemic corticosteroids are usually associated in combination with MMF as second‐line treatment in mild/moderate MMP 153 , 157 and in combination with cyclophosphamide in severe MMP. 162 , 163 The use of systemic corticosteroids has also been investigated in combination with rituximab. 165 Studies focusing on systemic corticosteroids in monotherapy have not been found, but in clinical practice, they are widely used, even at high dosages, for controlling flare‐ups.
Conclusions

Recommendations

Methotrexate
Methotrexate is an antifolic and antimetabolic drug widely used for autoimmune and haematological diseases. It is used in dermatology as a steroid‐sparing immunomodulating agent. Its mechanism of action is based on its interference with DNA synthesis and replication, as well as the inhibition of rapidly dividing cells.
McCluskey et al. reported that an approximately 15‐month course of methotrexate therapy led to complete control and/or suppression of conjunctival inflammation in 10 out of 12 (83%) patients with ocular MMP. 166 Moreover, only 24% of patients developed side effects requiring cessation of methotrexate therapy, and these were reversible. In a retrospective, non‐controlled, case series study involving 11 patients with severe ocular MMP, Shi et al. demonstrated that low‐dose methotrexate improved visual acuity in three patients. 167
Conclusions

Recommendations

Azathioprine
Azathioprine is a synthetic purine analog derived from 6‐mercaptopurine, which is thought to act by disrupting nucleic acid synthesis, and has recently been found to interfere with T‐cell activation. Albeit originally developed for its anticancer properties, azathioprine is nowadays more widely used for its immunosuppressant properties. One of the most recognized uses of azathioprine in dermatology is as treatment for autoimmune bullous disorders, including MMP.
Azathioprine showed a low success rate as compared to methotrexate and dapsone, and its discontinuation due to adverse effects (gastrointestinal, headache, malaise, dizziness, elevated liver function tests and myelosuppression) was higher than in patients treated with other immunosuppressants. In fact, successful treatment was achieved in 43% and 47% of MMP patients treated with azathioprine by Pasadhika et al. and Saw et al., respectively. 153 , 168 In MMP with ocular involvement, an evaluation of 115 patients on a variety of therapies found that azathioprine had a success rate (no conjunctival inflammation) of 38/80 (47%) and qualified success (partial inflammation control) in 19/80 (24%), with failure in 23/80 (29%). However, the side‐effect profile was poor, resulting in discontinuations in 24/60 (40%). For the latter reason, mycophenolate was recommended for use in this study, instead of azathioprine (except as a second‐line agent for patients not tolerating mycophenolate), because of the higher success rate in 27/46 (59%) and improved tolerance, resulting in discontinuations of 15% (5/34). 153
Conclusions

Recommendations

Rituximab
Rituximab is a chimeric monoclonal antibody directed against CD20, which is a cell surface marker expressed by B cells. Recently licensed for moderate/severe pemphigus, 169 it has a long record in refractory autoimmune blistering diseases, including MMP. 170 , 171 , 172 Le‐Roux‐Villet et al. found that 17 out of the 25 patients with severe refractory MMP included in their study showed a complete response after one cycle, and five additional patients after a second cycle, yielding a 88% response rate. Three patients (all receiving concomitant high‐dose immunosuppressants) developed severe infectious complications. 173 In this study, immunosuppressant regimens were discontinued at the initiation of rituximab and 88% of patients were continued on maintenance dapsone or sulfasalazine. You et al. found that 46 eyes (77.0%) in 26 MMP patients treated with rituximab alone, or in combination with other immunomodulatory treatments, achieved clinical remission, with an average sustained remission time of 24.5 months. 174
Maley et al. studied 24 patients treated with rituximab added to conventional immunosuppression, and 25 treated only with conventional immunosuppression. They found that 100% of patients in the rituximab group achieved disease control compared with 40% in the conventional group (P < 0.01), with a mean time to disease control of 10.17 and 37.7 months (P = 0.02). 165 Recently, Lamberts et al. observed in a cohort of 14 MMP patients treated with rituximab 1 g at day 1 and at day 14, disease control in 85.7%, partial response in 64.3%, and complete response in 28.6% patients, with a relapse rate of 75% during follow‐up. 175 Since 2018, an open‐label, phase 3 clinical trial comparing the safety and effectiveness of RTX vs oral cyclophosphamide is ongoing (NCT: 03295383).
Conclusions

Recommendations

Intravenous human immunoglobulins
Intravenous immunoglobulins (IVIg) are a purified IgG preparation derived from pooled human plasma, and contain more than 95% of unmodified IgG, which has functionally intact Fc‐dependent effector functions. IVIg may be a therapeutic option in several dermatologic diseases, including autoimmune bullous diseases, and is usually applied at a dose of 2 g/kg body weight over 2–4 days at monthly intervals. 176 IVIg are used when conventional therapies are contraindicated, or when the disease is progressive despite conventional systemic therapies. Adverse events are usually mild, self‐limiting and apparently predominantly infusion‐related. The most frequent are headache, back pain, chills, flushing, fever, hypertension, myalgia, nausea and vomiting. The major limitation of IVIg is their cost.
IVIg have been reported as an effective and safe treatment for progressive MMP unresponsive to conventional therapies. Letko et al. 177 compared two groups of ocular MMP – one treated with IVIg and the second with conventional therapies – revealing a faster, more effective, and safer response in the first group. Leuci et al. 178 showed that the efficacy of IVIGs is persistent for a long time. Foster et al. and Steger et al. confirmed the favourable response of severe and progressive ocular MMP to combination therapy with rituximab and IVIg. 179 , 180 Despite evidence of progressive scarring in some of their patients, blindness was prevented. Adverse events reported were limited (headache and nausea), and the authors did not induce discontinuation of the therapy in almost all but two patients described by Segura et al. 181
Conclusions

Recommendations

Anti‐TNFα drugs
Increased levels of TNFα have been observed in the sera of MMP patients, compared with controls. The use of anti‐TNFα drugs in MMP is supported only by case reports or case series, such as that by Canizares et al. reporting on the effectiveness of etanercept in three patients with ocular MMP. 182 Etanercept is a recombinant human dimeric fusion protein consisting of the extracellular ligand‐binding domain of the TNFα receptor fused to the Fc portion of the human IgG1.
Conclusions

Recommendations

Algorithm for the treatment of MMP
The recommended algorithm for the treatment of MMP is shown in Fig. 2.
Figure 2.
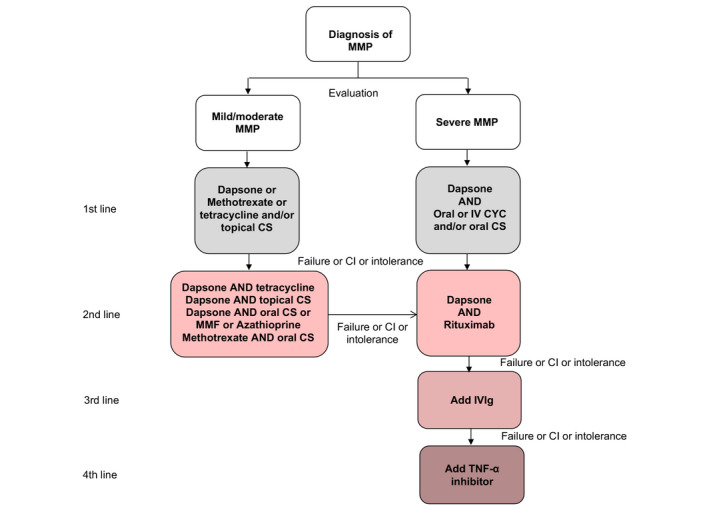
Algorithm for treatment of mucous membrane pemphigoid. CI, contraindication; CS, corticosteroid; CYC, cyclophosphamide; IV, intravenous; IVIg, Intravenous Immunoglobulin; MMF, mycophenolate mofetil; MMP, mucous membrane pemphigoid; TNF, tumour necrosis factor.
Systemic medical management of ocular, oral, genital, laryngeal and oesophageal MMP
Ocular MMP
Conclusions

Recommendations
Figure 3.
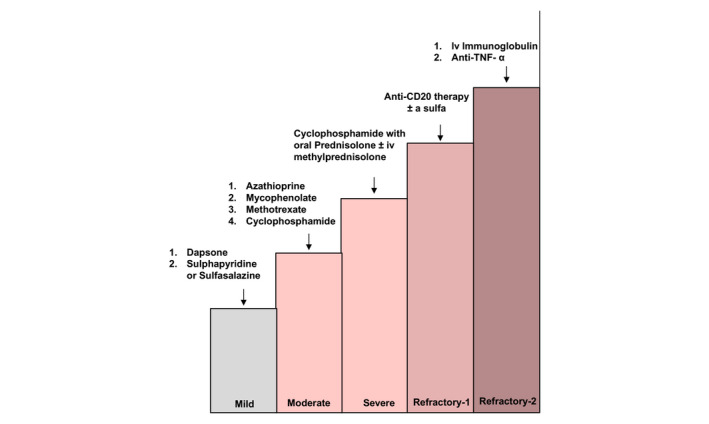
Algorithm for systemic treatment of ocular mucous membrane pemphigoid. The complete legend is shown in the Appendix S1.
Oral MMP
Conclusions
Recommendations

See also above recommendations for topical treatment in ocular disease.
Laryngeal MMP
Conclusions
Recommendations

Oesophageal MMP
Conclusions

Recommendations

Genital MMP
Conclusions

Recommendations

See also above recommendations for topical treatment in genital disease.
Details on systemic treatment of single‐site MMP are shown in the Appendix S1.
Rescue procedures in ocular involvement
Conclusions
Recommendations
Details on rescue procedures and additional local measures in ocular involvement in MMP are shown in the Appendix S1.
Oral hygiene advice
MMP, among other disorders causing desquamative gingivitis, may potentially intensify the development and progression of plaque‐related periodontal disease. A number of studies have described the gingival status in patients with MMP. 183 , 184 , 185 , 186 A systematic review showed an increased incidence of periodontitis in patients with desquamative gingivitis (MMP, n = 65) compared to healthy individuals. 187 This review showed that patients had worse periodontal parameters, including bleeding upon probing clinical attachment level of the periodontal ligament, probing depth, plaque index and/or gingival index/recession. Patients with a diagnosis of MMP >5 years were also shown to have more recession and furcation involvement. 183 Desquamative gingivitis may indirectly increase the long‐term risk for developing periodontal disease via plaque accumulation when pain associated with such lesions impairs capacity to perform efficient oral hygiene practices. In addition, discomfort associated with gingival lesions could predispose patients to less frequent dental visits. A direct effect of MMP on periodontitis may also be plausible based on the possible shared pathogenic mechanisms between antibody and bacterial‐induced inflammatory tissue damage.
Improving oral hygiene is prudent, as this may reduce the chronicity of the disease and the need for complex treatments. In conjunction with medical therapy, the avoidance of trauma and elimination of infection is beneficial. There is evidence for the beneficial effect of conservative treatment in improving the clinical parameters and severity of MMP lesions or symptoms. Non‐surgical periodontal therapy, consisting of scaling and root planing, and effective bacterial plaque control can be effective in reducing the gingival manifestations, representing a complementary treatment to the use of corticosteroids. 188 , 189 , 190 , 191 , 192 A recent systematic review evaluated the efficacy of daily hygiene and professional prophylaxis for treatment of desquamative gingivitis, regardless of its aetiology. 192 This review concluded that the combination of appropriate daily gingival hygiene techniques at home, and the performance of periodontal treatment, including scaling and root planning, decreased pain‐perception, disease activity, dental plaque and gingival bleeding. General dentists, hygienists and periodontists therefore play a key role in controlling the oral manifestations of MMP. Patients should be instructed in the maintenance of good oral hygiene, using toothbrushes with soft or extra‐soft bristles, applying the modified Bass brushing technique, and using dental floss. In their review, Garcia‐Pola et al. also recommended rinsing with chlorhexidine twice daily, initially with a concentration of 0.2%, and a maintenance concentration of 0.12% for one to four weeks.
Information for patients
Written information is provided by the EADV webpage and the patient support groups. The purpose of these associations is to promote knowledge about the disease, to furnish comfort and share the experience of patients regarding daily life, and to disseminate information. Such information may contribute to a better overall management of the disease by promoting cooperation between patients, patient associations and health professionals. Patients are also informed about referral centres
Recommendations
List of support groups for patients with MMP:
International Pemphigus and Pemphigoid foundation: www.pemphigus.org
Pemphigus und Pemphigoid Selbsthilfegruppe e.V.: www.pemphigus‐pemphigoid‐selbsthilfe.de
Association Pemphigus Pemphigoïde‐France: www.pemphigus.asso.fr
Pemfriends: www.pemfriends.co.uk
Associazione Nazionale Pemfigo/Pemfigoide:
Netwerk voor Blaarziekten: www.netwerkblaarziekten.nl
Pemfigus Hastaları Yardımlaşma ve Dayanışma Dernegi: www.pemfigus.org.tr
Future perspective and gaps in knowledge
Several important gaps in knowledge that exist were formulated by the guideline working group:
-
‐
Effectiveness and sequence of the different drugs used in MMP
-
‐
Ocular MMP: laser therapy and plugging eyelashes
-
‐
Validation of outcome measurements
-
‐
Scoring system for multisite MMP
Supporting information
Appendix S1. Supplementary material.
Acknowledgements
We thank the late prof. Dr. Marcel Jonkman for his contribution to this guideline and Dr. John Dart for providing the contribution to the sections on ocular mucous membrane pemphigoid.
Conflict of interest
M. Carrozzo received a grant from AFYX. F. Caux has been advisor, speaker or investigator for Principia Biopharma, Roche Laboratories, Pierre Fabre Dermatologie, LEO, Abbvie and Novartis. G. Geerling has been advisor, speaker or investigator for, and received grants from, Dompé, Chiesi, Novartis, Alcon, Allergan, Santen, Oculus, Tearlab, Tearscience, Theapharma and Visumed. B. Horvath has been advisor, speaker or investigator for, and received grants from, Abbvie, Janssen‐Cilag, Solenne B.V, Amgen, Akari Pharmaceutics, Roche, Novartis, UCB Pharma. P. Joly has been consultant for Roche, Amgen, Principia Biopharma, Argenx, AstraZeneca, Regeneron and Thermofisher. D.F. Murrell has been consultant, investigator or speaker for Abbvie, Argenx, AstraZeneca, Dermira, Janssen, Lilly, Novartis, Principia Biopharma, Regeneron, Sanofi and UCB. A. Patsatsi has been advisor, speaker or investigator for Abbvie, Jannsen‐Cilag, Lilly, Novartis, LEO, UCB, Principia Biopharma, L’Oreal and Genesis Pharma. M. Roth has been speaker for Theapharma and Bayer. E. Schmidt has been consultant for, and received grants and honoraria from, UCB, Biotest, Incyte, Euroimmun, Novartis, ArgenX, AstraZeneca, Fresenius Medical Care, Dompe, Synthon/byondis, Admirx, Topas, Thermo Fisher and Roche. G. Zambruno has been consultant for Argenx. D. Zillikens has been consultant, speaker or investigator for Euroimmun, Almirall, UCB, ArgenX, Biotest, Fresenius, Miltenyi, Roche, Biogen, Abbvie and Janssen.
Funding source
None.
References
- 1. Chan LS, Ahmed AR, Anhalt GJ et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol 2002; 138: 370–379. [DOI] [PubMed] [Google Scholar]
- 2. Chan LS. Immune‐mediated subepithelial blistering diseases of mucous membranes. Arch Dermatol 1993; 129: 448. [DOI] [PubMed] [Google Scholar]
- 3. Alexandre M, Brette MD, Pascal F et al. A prospective study of upper aerodigestive tract manifestations of mucous membrane pemphigoid. Medicine (Baltimore) 2006; 85: 239–252. [DOI] [PubMed] [Google Scholar]
- 4. Arduino PG, Broccoletti R, Carbone M et al. Describing the gingival involvement in a sample of 182 Italian predominantly oral mucous membrane pemphigoid patients: a retrospective series. Med Oral Patol Oral Cir Bucal 2017; 22: e149–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fine J, Neises GR, Katz SI. Immunofluorescence and immunoelecton microscopic studies in cicatricial pemphigoid. J Invest Dermatol 1984; 82: 39–43. [DOI] [PubMed] [Google Scholar]
- 6. Shimanovich I, Nitz JM, Zillikens D. Multiple and repeated sampling increases the sensitivity of direct immunofluorescence testing for the diagnosis of mucous membrane pemphigoid. J Am Acad Dermatol 2017; 77: 700–705.e3. [DOI] [PubMed] [Google Scholar]
- 7. Carey B, Joshi S, Abdelghani A, Mee J, Andiappan M, Setterfield J. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol 2020; 182: 747–753. [DOI] [PubMed] [Google Scholar]
- 8. Laskaris G, Nicolis G. Immunopathology of oral mucosa in bullous pemphigoid. Oral Surg Oral Med Oral Pathol 1980; 50: 340–345. [DOI] [PubMed] [Google Scholar]
- 9. Mehra T, Guenova E, Dechent F et al. Diagnostic relevance of direct immunofluorescence in ocular mucous membrane pemphigoid. J Dtsch Dermatol Ges 2015; 13: 1268–1274. [DOI] [PubMed] [Google Scholar]
- 10. Ong HS, Setterfield JF, Minassian DC et al. Mucous membrane pemphigoid with Ocular Involvement: the clinical phenotype and its relationship to direct immunofluorescence findings. Ophthalmology 2018; 125: 496–504. [DOI] [PubMed] [Google Scholar]
- 11. Fj D, Neises GR, Katz SI. Immunofluorescence and immunoelecton microscopic studies in cicatricial pemphigoid. J Invest Dermatol 1984; 82: 39–43. [DOI] [PubMed] [Google Scholar]
- 12. Firth NA, Rich AM, Radden BG, Reade PC. Direct immunofluorescence of oral mucosal biopsies: a comparison of fresh‐frozen tissue and formalin‐fixed, paraffin‐embedded tissue. J Oral Pathol Med 1992; 21: 358–363. [DOI] [PubMed] [Google Scholar]
- 13. Hoang‐Xuan T, Robin H, Demers PE et al. Pure ocular cicatricial pemphigoid. A distinct immunopathologic subset of cicatricial pemphigoid. Ophthalmology 1999; 106: 355–361. [DOI] [PubMed] [Google Scholar]
- 14. Jonkman MF, De Groot AC, Slegers TJ, De Jong MCJM, Pas HH. Immune diagnosis of pure ocular mucous membrane pemphigoid: indirect immunofluorescence versus immunoblot. Eur J Dermatology 2009; 19: 456–460. 10.1684/ejd.2009.0740. [DOI] [PubMed] [Google Scholar]
- 15. Mudhar HS. Biopsies of cicatricial conjunctivitis cases reveal highly variable sampling practice among ophthalmologists: time for national and international standardisation. Br J Ophthalmol 2016; 100: 736–744. [DOI] [PubMed] [Google Scholar]
- 16. Vodegel RM, de Jong MCJM , Meijer HJ, Weytingh MB, Pas HH, Jonkman MF. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol 2004; 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Terra JB, Pas HH, Hertl M, Dikkers FG, Kamminga N, Jonkman MF. Immunofluorescence serration pattern analysis as a diagnostic criterion in antilaminin‐332 mucous membrane pemphigoid: immunopathological findings and clinical experience in 10 Dutch patients. Br J Dermatol 2011; 165: 815–822. [DOI] [PubMed] [Google Scholar]
- 18. Suresh L, Kumar V. Significance of IgG4 in the diagnosis of mucous membrane pemphigoid. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 104: 359–362. [DOI] [PubMed] [Google Scholar]
- 19. Vodegel RM, Jonkman MF, Pas HH, De Jong MCJM. U‐serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol 2004; 151: 112–118. [DOI] [PubMed] [Google Scholar]
- 20. Meijer JM, Atefi I, Diercks GFH et al. Serration pattern analysis for differentiating epidermolysis bullosa acquisita from other pemphigoid diseases. J Am Acad Dermatol 2018; 78: 754–759.e6. [DOI] [PubMed] [Google Scholar]
- 21. Bhogal BS, Black MM. Diagnosis, diagnostic and research techniques. In: Briggaman RA, Wojnarowska FBR, eds. Management of Blistering Diseases. Springer, Boston, MA, 1990: 15–34. [Google Scholar]
- 22. Pohla‐Gubo G, Hintner H. Direct and indirect immunofluorescence for the diagnosis of bullous autoimmune diseases. Dermatol Clin 2011; 29: 365–372, vii. [DOI] [PubMed] [Google Scholar]
- 23. Jindal A, Rao R, Bhogal BS. Advanced diagnostic techniques in autoimmune bullous diseases. Indian J Dermatol 2017; 62: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kamaguchi M, Iwata H, Ujiie H, Ohga N, Kitagawa Y, Shimizu H. Mucosal substrates successfully identify the autoantigen in a case of mucous membrane pemphigoid. J Dtsch Dermatol Ges 2018; 16: 1032–1034. [DOI] [PubMed] [Google Scholar]
- 25. Hayakawa T, Furumura M, Fukano H et al. Diagnosis of oral mucous membrane pemphigoid by means of combined serologic testing. Oral Surg Oral Med Oral Pathol Oral Radiol 2014; 117: 483–496. [DOI] [PubMed] [Google Scholar]
- 26. Tsuruta D, Dainichi T, Hamada T, Ishii N, Hashimoto T. Molecular diagnosis of autoimmune blistering diseases. Methods Mol Biol 2013; 961: 17–32. [DOI] [PubMed] [Google Scholar]
- 27. Schmidt E, Skrobek C, Kromminga A et al. Cicatricial pemphigoid: IgA and IgG autoantibodies target epitopes on both intra‐ and extracellular domains of bullous pemphigoid antigen 180. Br J Dermatol 2001; 145: 778–783. [DOI] [PubMed] [Google Scholar]
- 28. Hecht E, Pitz S, Renieri G. In‐vivo confocal microscopy for the diagnosis of mucous membrane pemphigoid. Klin Monbl Augenheilkd 2015; 232: 1077–1081. In German. [DOI] [PubMed] [Google Scholar]
- 29. Cozzani E, Di Zenzo G, Calabresi V et al. Autoantibody profile of a cohort of 78 Italian patients with mucous membrane pemphigoid: correlation between reactivity profile and clinical involvement. Acta Derm Venereol 2016; 96: 768–773. [DOI] [PubMed] [Google Scholar]
- 30. van Beek N , Zillikens D, Schmidt E. Diagnosis of autoimmune bullous diseases. J Dtsch Dermatol Ges 2018; 16: 1077–1091. [DOI] [PubMed] [Google Scholar]
- 31. Bernard P, Prost C, Lecerf V et al. Studies of cicatricial pemphigoid autoantibodies using direct immunoelectron microscopy and immunoblot analysis.pdf. J Invest Dermatol 1990; 94: 630–635. [DOI] [PubMed] [Google Scholar]
- 32. Setterfield J, Shirlaw PJ, Kerr‐Muir M et al. Mucous membrane pemphigoid: a dual circulating antibody response with IgG and IgA signifies a more severe and persistent disease. Br J Dermatol 1998; 138: 602–610. [DOI] [PubMed] [Google Scholar]
- 33. Oyama N, Setterfield JF, Powell AM et al. Bullous pemphigoid antigen II (BP180) and its soluble extracellular domains are major autoantigens in mucous membrane pemphigoid: the pathogenic relevance to HLA class II alleles and disease severity. Br J Dermatol 2006; 154: 90–98. [DOI] [PubMed] [Google Scholar]
- 34. Carrozzo M, Cozzani E, Broccoletti R et al. Analysis of antigens targeted by circulating IgG and IgA antibodies in patients with mucous membrane pemphigoid predominantly affecting the oral cavity. J Periodontol 2004; 75: 1302–1308. [DOI] [PubMed] [Google Scholar]
- 35. Corti L, Fanoni D, Venegoni L, Muratori S, Recalcati S, Berti E. Detection of IgE autoantibodies in mucous membrane pemphigoid and their association with disease severity. G Ital Dermatol Venereol 2020; 155: 754–759. [DOI] [PubMed] [Google Scholar]
- 36. Dart JK. The 2016 Bowman lecture conjunctival curses: scarring conjunctivitis 30 years on. Eye (Lond) 2017; 31: 301–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamaguchi M, Iwata H, Ujiie H et al. Oral mucosa is a useful substrate for detecting autoantibodies of mucous membrane pemphigoid. Br J Dermatol 2018; 178: e119–e121. [DOI] [PubMed] [Google Scholar]
- 38. Mustafa MB, Porter SR, Smoller BR, Sitaru C. Oral mucosal manifestations of autoimmune skin diseases. Autoimmun Rev 2015; 14: 930–951. 10.1016/j.autrev.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 39. Grootenboer‐Mignot S, Descamps V, Picard‐Dahan C et al. Place of human amniotic membrane immunoblotting in the diagnosis of autoimmune bullous dermatoses. Br J Dermatol 2010; 162: 743–750. [DOI] [PubMed] [Google Scholar]
- 40. Maglie R, Borgi A, Caproni M, Antiga E. Indirect immunofluorescence in mucous membrane pemphigoid: which substrate should be used? Br J Dermatol 2019; 180: 1266–1267. [DOI] [PubMed] [Google Scholar]
- 41. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet (London, England) 2013; 381: 320–332. [DOI] [PubMed] [Google Scholar]
- 42. Lazarova Z, Yee C, Darling T, Briggaman RA, Yancey KB. Passive transfer of anti‐laminin 5 antibodies induces subepidermal blisters in neonatal mice. J Clin Invest 1996; 98: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Colón JE, Bhol KC, Razzaque MS, Ahmed AR. In vitro organ culture model for mucous membrane pemphigoid. Clin Immunol 2001; 98: 229–234. [DOI] [PubMed] [Google Scholar]
- 44. Kumari S, Bhol KC, Simmons RK et al. Identification of ocular cicatricial pemphigoid antibody binding site(s) in human beta4 integrin. Invest Ophthalmol Vis Sci 2001; 42: 379–385. [PubMed] [Google Scholar]
- 45. Bhol KC, Colon JE, Ahmed AR. Autoantibody in mucous membrane pemphigoid binds to an intracellular epitope on human beta4 integrin and causes basement membrane zone separation in oral mucosa in an organ culture model. J Invest Dermatol 2003; 120: 701–702. [DOI] [PubMed] [Google Scholar]
- 46. Heppe EN, Tofern S, Schulze FS et al. Experimental laminin 332 mucous membrane pemphigoid critically involves C5aR1 and reflects clinical and immunopathological characteristics of the human disease. J Invest Dermatol 2017; 137: 1709–1718. [DOI] [PubMed] [Google Scholar]
- 47. Bernard P, Prost C, Durepaire N, Basset‐Seguin N, Didierjean L, Saurat JH. The major cicatricial pemphigoid antigen is a 180kD protein that shows immunologic crossreactivities with the bullous pemphigoid antigen. J Invest Dermatol 1992; 99: 174–179. 10.1111/1523-1747.ep12616797. [DOI] [PubMed] [Google Scholar]
- 48. Balding SD, Prost C, Diaz LA et al. Cicatricial pemphigoid auto antibodies react with multiple sites on the BP180 extracellular domain. J Invest Dermatol 1996; 106: 141–146. [DOI] [PubMed] [Google Scholar]
- 49. Murakami H, Nishioka S, Setterfield J et al. Analysis of antigens targeted by circulating IgG and IgA autoantibodies in 50 patients with cicatricial pemphigoid. J Dermatol Sci 1998; 17: 39–44. [DOI] [PubMed] [Google Scholar]
- 50. Leverkus M, Schmidt E, Lazarova Z, Bröcker EB, Yancey KB, Zillikens D. Antiepiligrin cicatricial pemphigoid: an underdiagnosed entity within the spectrum of scarring autoimmune subepidermal bullous diseases? Arch Dermatol 1999; 135: 1091–1098. [DOI] [PubMed] [Google Scholar]
- 51. Egan CA, Taylor TB, Meyer LJ, Petersen MJ, Zone JJ. Bullous pemphigoid sera that contain antibodies to BPAg2 also contain antibodies to LABD97 that recognize epitopes distal to the NC16A domain. J Invest Dermatol 1999; 112: 148–152. [DOI] [PubMed] [Google Scholar]
- 52. Christophoridis S, Büdinger L, Borradori L, Hunziker T, Merk HF, Hertl M. IgG, IgA and IgE autoantibodies against the ectodomain of BP180 in patients with bullous and cicatricial pemphigoid and linear IgA bullous dermatosis. Br J Dermatol 2000; 143: 349–355. [DOI] [PubMed] [Google Scholar]
- 53. Oyama N, Bhogal BS, Carrington P, Gratian MJ, Black MM. Human placental amnion is a novel substrate for detecting autoantibodies in autoimmune bullous diseases by immunoblotting. Br J Dermatol 2003; 148: 939–944. [DOI] [PubMed] [Google Scholar]
- 54. Calabresi V, Carrozzo M, Cozzani E et al. Oral pemphigoid autoantibodies preferentially target BP180 ectodomain. Clin Immunol 2006; 122: 207–213. [DOI] [PubMed] [Google Scholar]
- 55. Bernard P, Antonicelli F, Bedane C et al. Prevalence and clinical significance of anti‐laminin 332 autoantibodies detected by a novel enzyme‐linked immunosorbent assay in mucous membrane pemphigoid. JAMA Dermatology 2013; 149: 533–540. [DOI] [PubMed] [Google Scholar]
- 56. Li X, Qian H, Sogame R et al. Integrin β4 is a major target antigen in pure ocular mucous membrane pemphigoid. Eur J Dermatol 2016; 26: 247–253. [DOI] [PubMed] [Google Scholar]
- 57. Gaudin O, Seta V, Alexandre M et al. Gliptin accountability in mucous membrane pemphigoid induction in 24 out of 313 patients. Front Immunol 2018; 9: 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kamaguchi M, Iwata H, Miyauchi T et al. The identification of autoantigens in mucous membrane pemphigoid using immortalized oral mucosal keratinocytes. J Oral Pathol Med 2019; 48: 60–67. [DOI] [PubMed] [Google Scholar]
- 59. Kamaguchi M, Iwata H, Miyauchi T et al. The identification of autoantigens in mucous membrane pemphigoid using immortalized oral mucosal keratinocytes. J oral Pathol Med 2019; 48: 60–67. [DOI] [PubMed] [Google Scholar]
- 60. Yasukochi A, Teye K, Ishii N, Hashimoto T. Clinical and immunological studies of 332 japanese patients tentatively diagnosed as anti‐BP180‐type mucous membrane pemphigoid: a novel BP180 C‐terminal domain enzyme‐linked immunosorbent assay. Acta Derm Venereol 2016; 96: 762–767. [DOI] [PubMed] [Google Scholar]
- 61. Lee JB, Liu Y, Hashimoto T. Cicatricial pemphigoid sera specifically react with the most C‐terminal portion of BP180. J Dermatol Sci 2003; 32: 59–64. [DOI] [PubMed] [Google Scholar]
- 62. Di Zenzo G, Thoma‐Uszynski S, Fontao L et al. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clin Immunol 2008; 128: 415–426. [DOI] [PubMed] [Google Scholar]
- 63. Horváth B, Niedermeier A, Podstawa E et al. IgA autoantibodies in the pemphigoids and linear IgA bullous dermatosis. Exp Dermatol 2010; 19: 648–653. [DOI] [PubMed] [Google Scholar]
- 64. Egan CA, Hanif N, Taylor TB, Meyer LJ, Petersen MJ, Zone JJ. Characterization of the antibody response in oesophageal cicatricial pemphigoid. Br J Dermatol 1999; 140: 859–864. [DOI] [PubMed] [Google Scholar]
- 65. Roh JY, Yee C, Lazarova Z, Hall RP, Yancey KB. The 120‐kDa soluble ectodomain of type XVII collagen is recognized by autoantibodies in patients with pemphigoid and linear IgA dermatosis. Br J Dermatol 2000; 143: 104–111. [DOI] [PubMed] [Google Scholar]
- 66. Yeh SW, Usman AQ, Ahmed AR. Profile of autoantibody to basement membrane zone proteins in patients with mucous membrane pemphigoid: long‐term follow up and influence of therapy. Clin Immunol 2004; 112: 268–272. [DOI] [PubMed] [Google Scholar]
- 67. Liu AY, Valenzuela R, Helm TN, Camisa C, Melton AL, Bergfeld WF. Indirect immunofluorescence on rat bladder transitional epithelium: a test with high specificity for paraneoplastic pemphigus. J Am Acad Dermatol 1993; 28(5 Pt 1): 696–699. [DOI] [PubMed] [Google Scholar]
- 68. Nishie W, Sawamura D, Goto M et al. Humanization of autoantigen. Nat Med 2007; 13: 378–383. [DOI] [PubMed] [Google Scholar]
- 69. Schulze FS, Beckmann T, Nimmerjahn F et al. Fcγ receptors III and IV mediate tissue destruction in a novel adult mouse model of bullous pemphigoid. Am J Pathol 2014; 184: 2185–2196. [DOI] [PubMed] [Google Scholar]
- 70. Bekou V, Thoma‐Uszynski S, Wendler O et al. Detection of laminin 5‐specific auto‐antibodies in mucous membrane and bullous pemphigoid sera by ELISA. J Invest Dermatol 2005; 124: 732–740. [DOI] [PubMed] [Google Scholar]
- 71. Amber KT, Bloom R, Hertl M. A systematic review with pooled analysis of clinical presentation and immunodiagnostic testing in mucous membrane pemphigoid: association of anti‐laminin‐332 IgG with oropharyngeal involvement and the usefulness of ELISA. J Eur Acad Dermatology Venereol 2016; 30: 72–77. [DOI] [PubMed] [Google Scholar]
- 72. Ghohestani RF, Nicolas JF, Rousselle P, Claudy AL. Diagnostic value of indirect immunofluorescence on sodium chloride‐split skin in differential diagnosis of subepidermal autoimmune bullous dermatoses. Arch Dermatol 1997; 133: 1102–1107. [PubMed] [Google Scholar]
- 73. Haeberle S, Wei X, Bieber K et al. Regulatory T‐cell deficiency leads to pathogenic bullous pemphigoid antigen 230 autoantibody and autoimmune bullous disease. J Allergy Clin Immunol 2018; 142: 1831–1842.e7. [DOI] [PubMed] [Google Scholar]
- 74. Domloge‐Hultsch N, Gammon WR, Briggaman RA, Gil SG, Carter WG, Yancey KB. Epiligrin, the major human keratinocyte integrin ligand, is a target in both an acquired autoimmune and an inherited subepidermal blistering skin disease. J Clin Invest 1992; 90: 1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Egan CA, Lazarova Z, Darling TN, Yee C, Yancey KB. Anti‐epiligrin cicatricial pemphigoid: clinical findings, immunopathogenesis, and significant associations. Medicine (Baltimore) 2003; 82: 177–186. [DOI] [PubMed] [Google Scholar]
- 76. Lazarova Z, Salato VK, Lanschuetzer CM, Janson M, Fairley JA, Yancey KB. IgG anti‐laminin‐332 autoantibodies are present in a subset of patients with mucous membrane, but not bullous, pemphigoid. J Am Acad Dermatol 2008; 58: 951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hisamatsu Y, Nishiyama T, Amano S, Matsui C, Ghohestani R, Hashimoto T. Usefulness of immunoblotting using purified laminin 5 in the diagnosis of anti‐laminin 5 cicatricial pemphigoid. J Dermatol Sci 2003; 33: 113–119. [DOI] [PubMed] [Google Scholar]
- 78. Lazarova Z, Hsu R, Yee C, Yancey KB. Antiepiligrin cicatricial pemphigoid represents an autoimmune response to subunits present in laminin 5 (alpha3beta3gamma2). Br J Dermatol 1998; 139: 791–797. [DOI] [PubMed] [Google Scholar]
- 79. Lazarova Z, Yee C, Lazar J, Yancey KB. IgG autoantibodies in patients with anti‐epiligrin cicatricial pemphigoid recognize the G domain of the laminin 5 alpha‐subunit. Clin Immunol 2001; 101: 100–105. [DOI] [PubMed] [Google Scholar]
- 80. Goletz S, Probst C, Komorowski L et al. A sensitive and specific assay for the serological diagnosis of antilaminin 332 mucous membrane pemphigoid. Br J Dermatol 2019; 180: 149–156. [DOI] [PubMed] [Google Scholar]
- 81. Hsu R, Lazarova Z, Yee C, Yancey KB. Noncomplement fixing, IgG4 autoantibodies predominate in patients with anti‐epiligrin cicatricial pemphigoid. J Invest Dermatol 1997; 109: 557–561. [DOI] [PubMed] [Google Scholar]
- 82. Natsuga K, Nishie W, Shinkuma S et al. Circulating IgA and IgE autoantibodies in antilaminin‐332 mucous membrane pemphigoid. Br J Dermatol 2010; 162: 513–517. [DOI] [PubMed] [Google Scholar]
- 83. Chiorean R, Danescu S, Virtic O et al. Molecular diagnosis of anti‐laminin 332 (epiligrin) mucous membrane pemphigoid. Orphanet J Rare Dis 2018; 13: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Giurdanella F, Nijenhuis AM, Diercks GFH, Jonkman MF, Pas HH. Keratinocyte footprint assay discriminates anti‐laminin‐332 pemphigoid from all other forms of pemphigoid diseases. Br J Dermatol 2019; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vodegel RM, Kiss M, Cjm De Jong M et al. The use of skin substrates deficient in basement membrane molecules for the diagnosis of subepidermal autoimmune bullous disease. Eur J Dermatol 1998; 8: 83–85. [PubMed] [Google Scholar]
- 86. Tyagi S, Bhol K, Natarajan K, Livir‐Rallatos C, Foster CS, Ahmed AR. Ocular cicatricial pemphigoid antigen: partial sequence and biochemical characterization. Proc Natl Acad Sci U S A 1996; 93: 14714–14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bhol KC, Dans MJ, Simmons RK, Foster CS, Giancotti FG, Ahmed AR. The autoantibodies to alpha 6 beta 4 integrin of patients affected by ocular cicatricial pemphigoid recognize predominantly epitopes within the large cytoplasmic domain of human beta 4. J Immunol 2000; 165: 2824–2829. [DOI] [PubMed] [Google Scholar]
- 88. Bhol KC, Goss L, Kumari S et al. Autoantibodies to human alpha6 integrin in patients with oral pemphigoid. J Dent Res 2001; 80: 1711–1715. [DOI] [PubMed] [Google Scholar]
- 89. Rashid KA, Gurcan HM, Ahmed AR. Antigen specificity in subsets of mucous membrane pemphigoid. J Invest Dermatol 2006; 126: 2631–2636. [DOI] [PubMed] [Google Scholar]
- 90. Rashid KA, Stern JNH, Ahmed AR. Identification of an epitope within human integrin alpha 6 subunit for the binding of autoantibody and its role in basement membrane separation in oral pemphigoid. J Immunol 2006; 176: 1968–1977. [DOI] [PubMed] [Google Scholar]
- 91. Letko E, Bhol K, Foster SC, Ahmed RA. Influence of intravenous immunoglobulin therapy on serum levels of anti‐beta 4 antibodies in ocular cicatricial pemphigoid. A correlation with disease activity. A preliminary study. Curr Eye Res 2000; 21: 646–654. [PubMed] [Google Scholar]
- 92. Sami N, Bhol KC, Ahmed AR. Treatment of oral pemphigoid with intravenous immunoglobulin as monotherapy. Long‐term follow‐up: influence of treatment on antibody titres to human alpha6 integrin. Clin Exp Immunol 2002; 129: 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Prost‐Squarcioni C, Caux F, Schmidt E et al. International Bullous Diseases Group: consensus on diagnostic criteria for epidermolysis bullosa acquisita. Br J Dermatol 2018; 179: 30–41. [DOI] [PubMed] [Google Scholar]
- 94. Buijsrogge JJA, Diercks GFH, Pas HH, Jonkman MF. The many faces of epidermolysis bullosa acquisita after serration pattern analysis by direct immunofluorescence microscopy. Br J Dermatol 2011; 165: 92–98. [DOI] [PubMed] [Google Scholar]
- 95. Saleh MA, Ishii K, Kim Y‐J et al. Development of NC1 and NC2 domains of type VII collagen ELISA for the diagnosis and analysis of the time course of epidermolysis bullosa acquisita patients. J Dermatol Sci 2011; 62: 169–175. [DOI] [PubMed] [Google Scholar]
- 96. Marzano AV, Cozzani E, Fanoni D et al. Diagnosis and disease severity assessment of epidermolysis bullosa acquisita by ELISA for anti‐type VII collagen autoantibodies: an Italian multicentre study. Br J Dermatol 2013; 168: 80–84. [DOI] [PubMed] [Google Scholar]
- 97. Komorowski L, Müller R, Vorobyev A et al. Sensitive and specific assays for routine serological diagnosis of epidermolysis bullosa acquisita. J Am Acad Dermatol 2013; 68: e89–95. [DOI] [PubMed] [Google Scholar]
- 98. van Beek N , Dähnrich C, Johannsen N et al. Prospective studies on the routine use of a novel multivariant enzyme‐linked immunosorbent assay for the diagnosis of autoimmune bullous diseases. J Am Acad Dermatol 2017; 76: 889–894.e5. [DOI] [PubMed] [Google Scholar]
- 99. Schmidt T, Hoch M, Lotfi Jad SS et al. Serological diagnostics in the detection of IgG autoantibodies against human collagen VII in epidermolysis bullosa acquisita: a multicentre analysis. Br J Dermatol 2017; 177: 1683–1692. [DOI] [PubMed] [Google Scholar]
- 100. Bean SF. Cicatricial pemphigoid. Arch Dermatol 1974; 110: 552–555. [PubMed] [Google Scholar]
- 101. Buonavoglia A, Leone P, Dammacco R et al. Pemphigus and mucous membrane pemphigoid: an update from diagnosis to therapy. Autoimmun Rev 2019; 18: 349–358. [DOI] [PubMed] [Google Scholar]
- 102. Fleming TE, Korman NJ. Cicatricial pemphigoid. J Am Acad Dermatol 2000; 43: 571–594. [DOI] [PubMed] [Google Scholar]
- 103. Gilvetti C, Collyer J, Gulati A, Barrett AW. What is the optimal site and biopsy technique for the diagnosis of oral mucosal autoimmune blistering disease? J Oral Pathol Med 2019; 48: 239–243. [DOI] [PubMed] [Google Scholar]
- 104. Rameshkumar A, Varghese AK, Dineshkumar T, Ahmed S, Venkatramani J, Sugirtharaj G. Oral mucocutaneous lesions ‐ a comparative clinicopathological and immunofluorescence study. J Int Oral Heal 2015; 7: 59–63. [PMC free article] [PubMed] [Google Scholar]
- 105. Rinaggio J, Crossland DM, Zeid MY. A determination of the range of oral conditions submitted for microscopic and direct immunofluorescence analysis. J Periodontol 2007; 78: 1904–1910. [DOI] [PubMed] [Google Scholar]
- 106. Rogers RS, 3rd , Sheridan PJ, Nightingale SH. Desquamative gingivitis: clinical, histopathologic, immunopathologic, and therapeutic observations. J Am Acad Dermatol 1982; 7: 729–735. [DOI] [PubMed] [Google Scholar]
- 107. Yih WY, Maier T, Kratochvil FJ, Zieper MB. Analysis of desquamative gingivitis using direct immunofluorescence in conjunction with histology. J Periodontol 1998; 69: 678–685. [DOI] [PubMed] [Google Scholar]
- 108. Ahmed AR, Hombal SM. Cicatricial pemphigoid. Int J Dermatol 1986; 25: 90–96. [DOI] [PubMed] [Google Scholar]
- 109. Bagan J, Lo Muzio L, Scully C. Mucosal disease series. Number III. Mucous membrane pemphigoid. Oral Dis 2005; 11: 197–218. [DOI] [PubMed] [Google Scholar]
- 110. Lamey PJ, Rees TD, Binnie WH, Rankin KV. Mucous membrane pemphigoid. Treatment experience at two institutions. Oral Surg Oral Med Oral Pathol 1992; 74: 50–53. [DOI] [PubMed] [Google Scholar]
- 111. Silverman SJ, Gorsky M, Lozada‐Nur F, Liu A. Oral mucous membrane pemphigoid. A study of sixty‐five patients. Oral Surg Oral Med Oral Pathol 1986; 61: 233–237. [DOI] [PubMed] [Google Scholar]
- 112. Megahed M. Histopathology of Blistering Diseases, Springer‐Verlag, Berlin Heidelberg, 2004. [Google Scholar]
- 113. Butt Z, Kaufman D, McNab A, McKelvie P. Drug‐induced ocular cicatricial pemphigoid: a series of clinico‐pathological reports. Eye (Lond) 1998; 12(Pt 2): 285–290. [DOI] [PubMed] [Google Scholar]
- 114. Thomas E, Fleming NJK. Cicatricial pemphigoid. J Am Acad Dermatol 2000; 43: 571–594. [DOI] [PubMed] [Google Scholar]
- 115. Chan LS. Ocular and oral mucous membrane pemphigoid (cicatricial pemphigoid). Clin Dermatol 2012; 30: 34–37. [DOI] [PubMed] [Google Scholar]
- 116. Sobolewska B, Deuter C, Zierhut M. Current medical treatment of ocular mucous membrane pemphigoid. Ocul Surf 2013; 11: 259–266. [DOI] [PubMed] [Google Scholar]
- 117. Labowsky MT, Stinnett SS, Liss J et al. Clinical implications of direct immunofluorescence findings in patients with ocular mucous membrane pemphigoid. Am J Ophthalmol 2017; 183: 48–55. [DOI] [PubMed] [Google Scholar]
- 118. Margolis T. Evidence‐based insights into the utility of conjunctival biopsy in mucous membrane pemphigoid. Ophthalmology 2018; 125: 474–475. [DOI] [PubMed] [Google Scholar]
- 119. Saw VPJ, Dart JKG. Ocular mucous membrane pemphigoid: diagnosis and management strategies. Ocul Surf 2008; 6: 128–142. [DOI] [PubMed] [Google Scholar]
- 120. Tauber J. Ocular cicatricial pemphigoid. Ophthalmology 2008; 115: 1631–1639. [DOI] [PubMed] [Google Scholar]
- 121. Radford CF, Rauz S, Williams GP, Saw VPJ, Dart JKG. Incidence, presenting features, and diagnosis of cicatrising conjunctivitis in the United Kingdom. Eye (Lond) 2012; 26: 1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bernauer W, Elder MJ, Leonard JN, Wright P, Dart JK. The value of biopsies in the evaluation of chronic progressive conjunctival cicatrisation. Graefe’s Arch Clin Exp Ophthalmol 1994; 232: 533–537. [DOI] [PubMed] [Google Scholar]
- 123. Taylor J, McMillan R, Shephard M et al. World workshop on oral medicine VI: a systematic review of the treatment of mucous membrane pemphigoid. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 120: 161–171.e20. [DOI] [PubMed] [Google Scholar]
- 124. Carrozzo M, Carbone M, Broccoletti R, Garzino‐Demo P, Gandolfo S. [Therapeutic management of mucous membrane pemphigoid. Report of 11 cases]. Minerva Stomatol 1997; 46: 553–559 (in Italian). [PubMed] [Google Scholar]
- 125. Gonzalez‐Moles MA, Ruiz‐Avila I, Rodriguez‐Archilla A et al. Treatment of severe erosive gingival lesions by topical application of clobetasol propionate in custom trays. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 95: 688–692. [DOI] [PubMed] [Google Scholar]
- 126. Lee MS, Wakefield PE, Konzelman JLJ, James WD. Oral insertable prosthetic device as an aid in treating oral ulcers. Arch Dermatol 1991; 127: 479–480. [PubMed] [Google Scholar]
- 127. Assmann T, Becker J, Ruzicka T, Megahed M. Topical tacrolimus for oral cicatricial pemphigoid. Clin Exp Dermatol 2004; 29: 674–676. [DOI] [PubMed] [Google Scholar]
- 128. Suresh L, Martinez Calixto LE, Radfar L. Successful treatment of mucous membrane pemphigoid with tacrolimus. Spec Care Dent 2006; 26: 66–70. [DOI] [PubMed] [Google Scholar]
- 129. Lee HY, Blazek C, Beltraminelli H, Borradori L. Oral mucous membrane pemphigoid: complete response to topical tacrolimus. Acta Derm Venereol 2011; 91: 604–605. [DOI] [PubMed] [Google Scholar]
- 130. Tseng SC. Topical tretinoin treatment for severe dry‐eye disorders. J Am Acad Dermatol 1986; 15(4 Pt 2): 860–866. [DOI] [PubMed] [Google Scholar]
- 131. McCluskey P, Wakefield D, York L. Topical fibronectin therapy in persistent corneal ulceration. Aust N Z J Ophthalmol 1987; 15: 257–262. [DOI] [PubMed] [Google Scholar]
- 132. Sharon Y, Chu DS. Adrenocorticotropic hormone analogue as novel treatment regimen in ocular cicatricial pemphigoid. Am J Ophthalmol case reports 2018; 10: 264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. You C, Ma L, Anesi SD, Stephen FC. Long‐term remission of ocular cicatricial pemphigoid off immunomodulatory therapy. Eur J Ophthalmol 2018; 28: 157–162. [DOI] [PubMed] [Google Scholar]
- 134. Tseng SCG, Maumenee AE, Stark WJ et al. Topical retinoid treatment for various dry‐eye disorders. Ophthalmology 1985; 92: 717–727. [DOI] [PubMed] [Google Scholar]
- 135. Reinhard T, Reis A, Mayweg S, Oberhuber H, Mathis G, Sundmacher R. [Topical Fk506 in inflammatory corneal and conjunctival diseases. A pilot study]. Klin Monbl Augenheilkd 2002; 219: 125‐131 (in German). [DOI] [PubMed] [Google Scholar]
- 136. Alonso A, Bignone ML, Brunzini M, Brunzini R. Ocular autoimmune pemphigoid and cyclosporin A. Allergol Immunopathol (Madr) 2006; 34: 113–115. [DOI] [PubMed] [Google Scholar]
- 137. Lee YJ, Kim SW, Seo KY. Application for tacrolimus ointment in treating refractory inflammatory ocular surface diseases. Am J Ophthalmol 2013; 155: 804–813. [DOI] [PubMed] [Google Scholar]
- 138. Hoque SR, Patel M, Farrell AM. Childhood cicatricial pemphigoid confined to the vulva. Clin Exp Dermatol 2006; 31: 63–64. [DOI] [PubMed] [Google Scholar]
- 139. DeCastro P, Jorizzo JL, Rajaraman S, Solomon AR, Briggaman RA, Raimer SS. Localized vulvar pemphigoid in a Child. Pediatr Dermatol 1985; 2: 302–307. [DOI] [PubMed] [Google Scholar]
- 140. Lebeau S, Mainetti C, Masouyé I, Saurat JH, Borradori L. Localized childhood vulval pemphigoid treated with tacrolimus ointment. Dermatology 2004; 208: 273–275. [DOI] [PubMed] [Google Scholar]
- 141. Schoeffler A, Roth B, Causeret A, Kanitakis J, Faure M, Claudy A. Vulvar cicatricial pemphigoid of childhood. Pediatr Dermatol 2004; 21: 51–53. [DOI] [PubMed] [Google Scholar]
- 142. Gürbüz O, Khalilazar R, Ergun T, Yücelten D, Demirçay Z. Vulvar cicatricial pemphigoid in a child. J Eur Acad Dermatology Venereol 1994; 3: 418–420. [Google Scholar]
- 143. Farrell AM, Kirtschig G, Dalziel KL et al. Childhood vulval pemphigoid: a clinical and immunopathological study of five patients. Br J Dermatol 1999; 140: 308–312. [DOI] [PubMed] [Google Scholar]
- 144. Goldstein AT, Anhalt GJ, Klingman D, Burrows LJ. Mucous membrane pemphigoid of the vulva. Obstet Gynecol 2005; 105 5 II: 1188–1190. [DOI] [PubMed] [Google Scholar]
- 145. Marren P, Walkden V, Mallon E, Wojnarowska F. Vulval cicatricial pemphigoid may mimic lichen sclerosus. Br J Dermatol 1996; 134: 522–524. [PubMed] [Google Scholar]
- 146. Murrell DF, Marinovic B, Caux F et al. Definitions and outcome measures for mucous membrane pemphigoid: recommendations of an international panel of experts. J Am Acad Dermatol 2015; 72: 168–174. [DOI] [PubMed] [Google Scholar]
- 147. Reiche L, Wojnarowska F, Mallon E. Combination therapy with nicotinamide and tetracyclines for cicatricial pemphigoid: further support for its efficacy. Clin Exp Dermatol 1998; 23: 254–257. [DOI] [PubMed] [Google Scholar]
- 148. Poskitt L, Wojnarowska F. Treatment of cicatricial pemphigoid with tetracycline and nicotinamide. Clin Exp Dermatol 1995; 20: 258–259. [DOI] [PubMed] [Google Scholar]
- 149. Carrozzo M, Arduino P, Bertolusso G, Cozzani E, Parodi A. Systemic minocycline as a therapeutic option in predominantly oral mucous membrane pemphigoid: a cautionary report. Int J Oral Maxillofac Surg 2009; 38: 1071–1076. [DOI] [PubMed] [Google Scholar]
- 150. Hegarty AM, Ormond M, Sweeney M, Hodgson T. Dapsone efficacy and adverse events in the management of mucous membrane pemphigoid. Eur J Dermatol 2010; 20: 223–224. [DOI] [PubMed] [Google Scholar]
- 151. Doan S, Lerouic JF, Robin H, Prost C, Savoldelli M, Hoang‐Xuan T. Treatment of ocular cicatricial pemphigoid with sulfasalazine. Ophthalmology 2001; 108: 1565–1568. [DOI] [PubMed] [Google Scholar]
- 152. Miserocchi E, Baltatzis S, Roque MR, Ahmed AR, Foster CS. The effect of treatment and its related side effects in patients with severe ocular cicatricial pemphigoid. Ophthalmology 2002; 109: 111–118. [DOI] [PubMed] [Google Scholar]
- 153. Saw VPJ, Dart JKG, Rauz S et al. Immunosuppressive therapy for ocular mucous membrane pemphigoid strategies and outcomes. Ophthalmology 2008; 115: 253–261.e1. [DOI] [PubMed] [Google Scholar]
- 154. Hegarty AM, Ormond M, Sweeney M, Hodgson T. Dapsone efficacy and adverse events in the management of mucous membrane pemphigoid. Eur J Dermatol 2010; 20: 223–224. [DOI] [PubMed] [Google Scholar]
- 155. Nottage JM, Hammersmith KM, Murchison AP, Felipe AF, Penne R, Raber I. Treatment of mucous membrane pemphigoid with mycophenolate mofetil. Cornea 2013; 32: 810–815. [DOI] [PubMed] [Google Scholar]
- 156. Doycheva D, Deuter C, Blumenstock G, Stuebiger N, Zierhut M. Long‐term results of therapy with mycophenolate mofetil in ocular mucous membrane pemphigoid. Ocul Immunol Inflamm 2011; 19: 431–438. [DOI] [PubMed] [Google Scholar]
- 157. Staines K, Hampton PJ. Treatment of mucous membrane pemphigoid with the combination of mycophenolate mofetil, dapsone, and prednisolone: a case series. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114: e49–56. [DOI] [PubMed] [Google Scholar]
- 158. Thorne JE, Woreta FA, Jabs DA et al. Treatment of ocular mucous membrane pemphigoid with immunosuppressive drug therapy. Ophthalmology 2008; 115: 2146–2152.e1. [DOI] [PubMed] [Google Scholar]
- 159. Friedman J, Marcovich AL, Kleinmann G, Schattner A. Low‐dose pulsed intravenous cyclophosphamide for severe ocular cicatricial pemphigoid in elderly patients. Cornea 2014; 33: 1066–1070. [DOI] [PubMed] [Google Scholar]
- 160. Munyangango EM, Le Roux‐Villet C, Doan S et al. Oral cyclophosphamide without corticosteroids to treat mucous membrane pemphigoid. Br J Dermatol 2013; 168: 381–390. [DOI] [PubMed] [Google Scholar]
- 161. Elder MJ, Lightman S, Dart JK. Role of cyclophosphamide and high dose steroid in ocular cicatricial pemphigoid. Br J Ophthalmol 1995; 79: 264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Suelves AM, Arcinue CA, González‐Martín JM, Kruh JN, Foster CS. Analysis of a novel protocol of pulsed intravenous cyclophosphamide for recalcitrant or severe ocular inflammatory disease. Ophthalmology 2013; 120: 1201–1209. [DOI] [PubMed] [Google Scholar]
- 163. El Darouti MA, Fakhry Khattab MA, Hegazy RA, Hafez DA, Gawdat HI. Pentoxifylline (anti‐tumor necrosis factor drug): effective adjuvant therapy in the control of ocular cicatricial pemphigoid. Eur J Ophthalmol 2011; 21: 529–537. [DOI] [PubMed] [Google Scholar]
- 164. Foster CS. Cicatricial pemphigoid. Trans Am Ophthalmol Soc 1986; 84: 527–663. [PMC free article] [PubMed] [Google Scholar]
- 165. Maley A, Warren M, Haberman I, Swerlick R, Kharod‐Dholakia B, Feldman R. Rituximab combined with conventional therapy versus conventional therapy alone for the treatment of mucous membrane pemphigoid (MMP). J Am Acad Dermatol 2016; 74: 835–840. [DOI] [PubMed] [Google Scholar]
- 166. McCluskey P, Chang JH, Singh R, Wakefield D. Methotrexate therapy for ocular cicatricial pemphigoid. Ophthalmology 2004; 111: 796–801. [DOI] [PubMed] [Google Scholar]
- 167. Shi Y, Xie C, He Y, Liu H, Zhu B, Zhu J. Efficacy and adverse reactions of methotrexate in the treatment of ocular cicatricial pemphigoid: a case series study. Medicine (Baltimore) 2018; 97: e12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Pasadhika S, Kempen JH, Newcomb CW et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol 2009; 148: 500–509.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Joly P, Maho‐Vaillant M, Prost‐Squarcioni C et al. First‐line rituximab combined with short‐term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel‐group, open‐label randomised trial. Lancet 2017; 389: 2031–2040. [DOI] [PubMed] [Google Scholar]
- 170. Schmidt E, Goebeler M. CD20‐directed therapy in autoimmune diseases involving the skin: role of rituximab. Expert Rev Dermatol 2008; 3: 259–278. [Google Scholar]
- 171. Schmidt E, Seitz CS, Benoit S, Bröcker EB, Goebeler M. Rituximab in autoimmune bullous diseases: mixed responses and adverse effects. Br J Dermatol 2007; 156: 352–356. [DOI] [PubMed] [Google Scholar]
- 172. Kasperkiewicz M, Shimanovich I, Ludwig RJ, Rose C, Zillikens D, Schmidt E. Rituximab for treatment‐refractory pemphigus and pemphigoid: a case series of 17 patients. J Am Acad Dermatol 2011; 65: 552–558. [DOI] [PubMed] [Google Scholar]
- 173. Le Roux‐Villet C, Prost‐Squarcioni C, Alexandre M et al. Rituximab for patients with refractory mucous membrane pemphigoid. Arch Dermatol 2011; 147: 843–849. [DOI] [PubMed] [Google Scholar]
- 174. You C, Lamba N, Lasave AF, Ma L, Diaz MH, Foster CS. Rituximab in the treatment of ocular cicatricial pemphigoid: a retrospective cohort study. Graefe’s Arch Clin Exp Ophthalmol 2017; 255: 1221–1228. [DOI] [PubMed] [Google Scholar]
- 175. Lamberts A, Euverman HI, Terra JB, Jonkman MF, Horváth B. Effectiveness and safety of rituximab in recalcitrant pemphigoid diseases. Front Immunol 2018; 9: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Hadaschik E, Eming R, French LE et al. European Guidelines (S1) on the use of high‐dose intravenous immunoglobulin in dermatology. Hautarzt 2020; 71: 542–552. [DOI] [PubMed] [Google Scholar]
- 177. Letko E, Miserocchi E, Daoud YJ, Christen W, Foster CS, Ahmed AR. A nonrandomized comparison of the clinical outcome of ocular involvement in patients with mucous membrane (cicatricial) pemphigoid between conventional immunosuppressive and intravenous immunoglobulin therapies. Clin Immunol 2004; 111: 303–310. [DOI] [PubMed] [Google Scholar]
- 178. Leuci S, Amato M, Calabria E et al. Long‐term follow‐up after intravenous immunoglobulin therapy in patients with severe ocular mucous membrane pemphigoid unresponsive to conventional therapy. J Ophthalmol 2018; 2018: 8372146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Foster CS, Chang PY, Ahmed AR. Combination of rituximab and intravenous immunoglobulin for recalcitrant ocular cicatricial pemphigoid: a preliminary report. Ophthalmology 2010; 117: 861–869. [DOI] [PubMed] [Google Scholar]
- 180. Steger B, Madhusudan S, Kaye SB et al. Combined use of rituximab and intravenous immunoglobulin for severe autoimmune cicatricial conjunctivitis‐an interventional case series. Cornea 2016; 35: 1611–1614. [DOI] [PubMed] [Google Scholar]
- 181. Segura S, Iranzo P, de Pablo IM et al. High‐dose intravenous immunoglobulins for the treatment of autoimmune mucocutaneous blistering diseases: evaluation of its use in 19 cases. J Am Acad Dermatol 2007; 56: 960–967. [DOI] [PubMed] [Google Scholar]
- 182. Canizares MJ, Smith DI, Conners MS, Maverick KJ, Heffernan MP. Successful treatment of mucous membrane pemphigoid with etanercept in 3 patients. Arch Dermatol 2006; 142: 1457–1461. [DOI] [PubMed] [Google Scholar]
- 183. Tricamo MB, Rees TD, Hallmon WW, Wright JM, Cueva MA, Plemons JM. Periodontal status in patients with gingival mucous membrane pemphigoid. J Periodontol 2006; 77: 398–405. [DOI] [PubMed] [Google Scholar]
- 184. Schellinck AE, Rees TD, Plemons JM, Kessler HP, Rivera‐Hidalgo F, Solomon ES. A comparison of the periodontal status in patients with mucous membrane pemphigoid: a 5‐year follow‐up. J Periodontol 2009; 80: 1765–1773. [DOI] [PubMed] [Google Scholar]
- 185. Lo Russo L, Guiglia R, Pizzo G et al. Effect of desquamative gingivitis on periodontal status: a pilot study. Oral Dis 2010; 16: 102–107. [DOI] [PubMed] [Google Scholar]
- 186. Arduino PG, Farci V, D’Aiuto F et al. Periodontal status in oral mucous membrane pemphigoid: initial results of a case‐control study. Oral Dis 2011; 17: 90–94. [DOI] [PubMed] [Google Scholar]
- 187. Jascholt I, Lai O, Zillikens D, Kasperkiewicz M. Periodontitis in oral pemphigus and pemphigoid: a systematic review of published studies. J Am Acad Dermatol 2017; 76: 975–978.e3. 10.1016/j.jaad.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 188. Damoulis PD, Gagari E. Combined treatment of periodontal disease and benign mucous membrane pemphigoid. Case report with 8 years maintenance. J Periodontol 2000; 71: 1620–1629. [DOI] [PubMed] [Google Scholar]
- 189. Orrico SRP, Navarro CM, Rosa FP, Reis FAC, Salgado DS, Onofre MA. Periodontal treatment of benign mucous membrane pemphigoid. Dent Today 2010; 29: 100–103. [PubMed] [Google Scholar]
- 190. Arduino PG, Lopetuso E, Carcieri P et al. Professional oral hygiene treatment and detailed oral hygiene instructions in patients affected by mucous membrane pemphigoid with specific gingival localization: a pilot study in 12 patients. Int J Dent Hyg 2012; 10: 138–141. [DOI] [PubMed] [Google Scholar]
- 191. Carcieri P, Broccoletti R, Giacometti S et al. Favourably effective formulation of sodium iodide and salicylic acid plus professional hygiene in patients affected by desquamative gingivitis. J Biol Regul Homeost Agents 2016; 30: 1141–1145. [PubMed] [Google Scholar]
- 192. Garcia‐Pola M‐J, Rodriguez‐López S, Fernánz‐Vigil A, Bagán L, Garcia‐Martín J‐M. Oral hygiene instructions and professional control as part of the treatment of desquamative gingivitis. Systematic review. Med Oral Patol Oral Cir Bucal 2019; 24: e136–e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary material.


