Abstract
Objective
We aimed to determine in relapsing multiple sclerosis (MS) whether intrathecal synthesis of immunoglobulin (Ig) M and IgG is associated with outcomes reflecting inflammatory activity and chronic worsening.
Methods
We compared cerebrospinal fluid analysis, clinical and magnetic resonance imaging data, and serum neurofilament light chain (sNfL) levels at baseline and follow‐up in 530 patients with relapsing MS. Patients were categorized by the presence of oligoclonal IgG bands (OCGB) and intrathecal synthesis of IgG and IgM (intrathecal fraction [IF]: IgGIF and IgMIF). Relationships with the time to first relapse, sNfL concentrations, T2‐weighted (T2w) lesions, MS Severity Score (MSSS), and time to initiation of high‐efficacy therapy were analyzed in covariate‐adjusted statistical models.
Results
By categorical analysis, in patients with IgMIF the median time to first relapse was 28 months shorter and MSSS on average higher by 1.11 steps compared with patients without intrathecal immunoglobulin synthesis. Moreover, patients with IgMIF had higher sNfL concentrations, more new/enlarging T2w lesions, and higher total T2w lesion counts (all p ≤ 0.01). These associations were absent or equally smaller in patients who were positive for only OCGB or OCGB/IgGIF. Furthermore, quantitative analyses revealed that in patients with IgMIF ≥ median, the time to first relapse and to initiation of high‐efficacy therapy was shorter by 32 and by 203 months, respectively (both p < 0.01), in comparison to patients with IgMIF < median. Dose‐dependent associations were also found for IgMIF but not for IgGIF with magnetic resonance imaging‐defined disease activity and sNfL.
Interpretation
This large study supports the value of intrathecal IgM synthesis as an independent biomarker of disease activity and severity in relapsing MS. ANN NEUROL 2021;90:477–489
The clinical course of multiple sclerosis is heterogeneous and unpredictable on individual grounds at the time of diagnosis. Biomarkers bridging this prognostic gap are urgently needed for personalized therapeutic decision making. The presence of oligoclonal immunoglobulin (Ig) G bands (OCGB) in cerebrospinal fluid (CSF) is a diagnostic hallmark of multiple sclerosis and was recently reintroduced in the early diagnostic algorithm for multiple sclerosis to satisfy the criterion of dissemination in time. 1 Besides this qualitative measure, intrathecal accumulation of B cells and plasma cells can also be detected by intrathecal production of IgG (intrathecal fraction of IgG [IgGIF]),2 which has been found in 70 to 86% 2 , 3 of patients with multiple sclerosis. In a large clinically isolated syndrome (CIS) cohort, OCGB and the presence of intrathecal IgGIF were associated with 1.4‐ and 1.6‐fold increased 4 likelihoods of conversion to multiple sclerosis. Unlike the adaptive immune response in lymphatic tissue, where the initial production of IgM is typically supplanted by IgG owing to an isotype switch of B cells, intrathecal production of IgM persists as a characteristic feature of multiple sclerosis. 5 The intrathecal fraction of IgM (IgMIF) according to Reiber's formula 2 is present in up to 23% of multiple sclerosis patients4, 6, 7
The presence of OCMB has been reported to be associated with a more active inflammatory disease phenotype, both in relapsing and in primary progressive multiple sclerosis. 8 , 9 , 10 , 11
Moreover, some studies have found that quantitated intrathecal IgM synthesis is associated with a higher likelihood of conversion from CIS to clinically definite multiple sclerosis and a more severe disease course, 4 , 7 , 12 , 13 but other studies did not confirm this finding. 6 , 14 , 15 Little is known how intrathecal IgM and IgG synthesis are associated independently with magnetic resonance imaging (MRI) and body fluid markers related to progression and, eventually, the choice of therapy, and whether this relation is dose‐dependent.
The aim of this study was to investigate the added value of quantitative estimates of IgMIF and IgGIF to prognosticate the long‐term disease activity and severity based on the time interval between first symptoms and a first relapse, the Multiple Sclerosis Severity Score (MSSS), respectively, and the level of longitudinal development of neuroaxonal damage and disease burden as measured by serum neurofilament light chain (sNfL) and MRI lesions. Lastly, we explored how these measures correlated with the physician's decision to initiate or to switch to high‐efficacy disease‐modifying therapies (heDMT).
Patients and Methods
Patients
We included participants in the Swiss Multiple Sclerosis Cohort Study (SMSC) diagnosed as either CIS or multiple sclerosis1, 16, 17 who had a lumbar puncture (LP) with complete analysis of OCGB, IgGIF, IgMIF, and the intrathecal fraction of IgA (IgAIF). The initial clinical event and first relapse were defined as new, worsening (in the case of first relapse), or recurrent neurologic symptoms that lasted for ≥24 hours without fever, infection, or adverse reaction to a prescribed medication and that were preceded by a stable or improving neurologic status of ≥30 days (in the case of first relapse). Institutional review boards at the respective SMSC centers approved the study, and written informed consent was obtained from all participants.
Baseline and Follow‐Up Clinical Data, Multiple Sclerosis Data, and Serum Collection
Demographic and clinical variables collected at the baseline visit included sex, date of birth, date of onset of multiple sclerosis symptoms, and first relapse (2 patients experienced their first relapse with unknown date) where applicable, and current and previous disease‐modifying therapies (DMTs). Therapies were categorized into “heDMT” (ocrelizumab, rituximab, alemtuzumab, and natalizumab), “oral” (teriflunomide, dimethyl fumarate, fingolimod, and siponimod), “platform” (interferon beta and glatiramer acetate), and “untreated”. Standardized clinical assessments based on Expanded Disability Status Scale (EDSS) scores were performed by certified raters (http://www.neurostatus.net) at baseline and each follow‐up visit every 6 or 12 months. 16 , 18 Blood samples were collected within 8 days from the clinical visit and processed and stored at −80°C following standardized procedures. The median (interquartile range [IQR]) follow‐up from baseline was 5.1 (3.0–7.0) years.
Cerebrospinal Fluid Analysis
OCGBs were assessed by isoelectric focusing followed by immunoblotting, immunofixation, or silver staining (depending on the study center). Testing of OCGBs was considered positive if pattern 2 or 3 (local synthesis of IgG within the central nervous system) was present. 19 CSF and serum concentrations of IgA, IgG, IgM, and albumin were measured nephelometrically. We used the derived CSF‐to‐serum quotients for Igs and albumin (Q IgG, Q IgM, Q IgA, and Q alb) for calculations of the intrathecal Ig synthesis according to the formulae proposed by Reiber and colleagues. 2
The amount of intrathecal IgG, IgM, or IgA synthesis was expressed as the intrathecal fraction as a percentage of the total measured isotype concentration in CSF (IgGIF, IgMIF, and IgAIF). 2 The integrity of the blood–CSF barrier was determined by calculating the Q alb. 20
Serum Neurofilament Light Chain Measurements
sNfL was measured (at baseline and every 6 or 12 months) with the NF‐light® assay (Quanterix, Billerica, MA, USA).
Intra‐ and interassay variability were evaluated with 3 native serum samples in each of the runs on independent days. The mean coefficients of variation (CVs) of duplicate determinations for concentration were 5.5% (6.2pg/ml, sample 1), 3.3% (18.9pg/ml, sample 2), and 3.1% (37.3pg/ml, sample 3). Interassay CVs were 7.0% (sample 1), 5.6% (sample 2), and 5.6% (sample 3). For the generation of age‐dependent sNfL Z‐scores, we used sNfL measurements in 8,865 samples of 4,209 healthy controls (median age = 44.8 years). 21
Magnetic Resonance Imaging
Brain MRI scans were performed annually in 1.5 (n = 940) or 3T (n = 978) scanners. The imaging protocol was standardized across centers and included a 3‐dimensional magnetization prepared – rapid gradient echo (MPRAGE) and a 3‐dimensional fluid attenuated inversion recovery (FLAIR) sequence, which were acquired at a spatial resolution of 1mm. 3 The number and occurrence of new/enlarging T2‐weighted (T2w) lesions were automatically assessed annually by using a deep learning‐based approach 22 and a longitudinal evaluation method, 23 respectively, followed by manual quality assessment and correction.
Statistical Analysis
Data are presented as the median and interquartile range (IQR) or mean and standard deviation (SD), and as absolute and relative frequencies in the case of categorical data.
Categorical Analysis
Given that the presence of intrathecal Ig subtypes is not distributed evenly and independently, in a first step the patients were categorized in ascending order for the presence or absence of OCGB, IgGIF (>0% vs 0%) and IgMIF (>0% vs 0%; Fig 1): (1) OCGB−/IgGIF −/IgMIF − (ie, absence of OCGB and IgGIF and IgMIF), n = 46; (2) OCGB+/IgGIF −/IgMIF −, n = 114; (3) OCGB+/IgGIF +/IgMIF −, n = 229; and (4) OCGB+/IgGIF +/IgMIF + (ie, presence of OCGB and IgGIF and IgMIF), n = 111. Eleven of 530 patients (2.1%) were IgGIF +, IgMIF +, or IgA IF + in combination with OCGB−, whereas 19 of 530 (3.6%) had an OCGB+/IgGIF −/IgMIF + combination; all these were excluded from this categorization.
FIGURE 1.
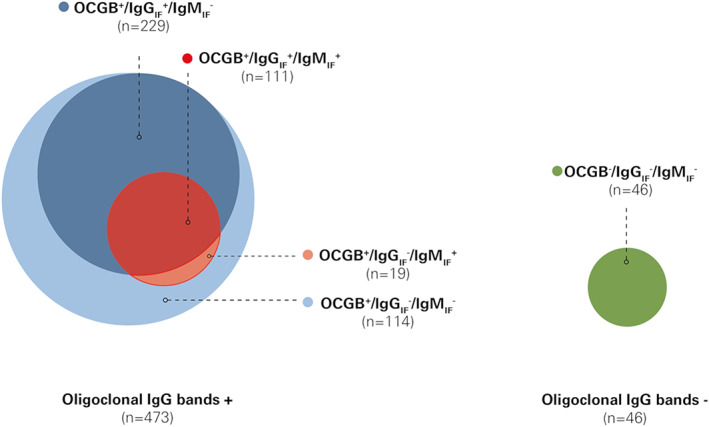
Combination of CSF immunoglobulin synthesis and frequencies in patients with and without presence of oligoclonal IgG bands. OCGB were present (+) in 473 patients, and 340 of them had additional IgGIF and 130 showed presence of IgMIF, whereas 46 had no (−) OCGB, IgGIF, and IgMIF. Patients were categorized into the following 4 groups according to the presence or absence of OCGB, IgGIF, and IgMIF: (1) OCGB−/IgGIF −/IgMIF −, n = 46 (green); (2) OCGB+/IgGIF −/IgMIF −, n = 114 (light blue); (3) OCGB+/IgGIF +/IgMIF −, n = 229 (blue); and (4) OCGB+/IgGIF +/IgMIF +, n = 111 (red). The presence of IgMIF coincided with that of IgGIF in 85.4% (111 of 130) samples (only 19 of 130 patients [15.6%] had an OCGB+/IgGIF −/IgMIF + pattern). In OCGB−, 11 of 530 patients (2.1%) showed IgGIF +, IgMIF +, or IgAIF + and were excluded from this categorization (not shown in figure). CSF = cerebrospinal fluid; IgG = immunoglobulin G; IgGIF/MIF/AIF = immunoglobulin G/M/A intrathecal fraction; IgM = immunoglobulin M; OCGB = oligoclonal IgG bands; + = presence of OCGB or IgG/IgM by Reiber formula; − = absence of OCGB or IgG/IgM by Reiber formula.
In a second step, we investigated associations between demographic (sex and age) or CSF parameters (OCGB+ vs OCGB−; IgGIF + vs IgGIF −; IgMIF + vs IgMIF −; IgAIF + vs IgAIF −; above categories 2–4 vs category 1) with described endpoints (time to a first relapse, longitudinal sNfL levels, MRI lesions, MSSS, and time to heDMT) by univariable (Cox proportional hazards, linear mixed, negative binominal mixed, and linear) models, respectively.
Next, associations between these 4 categories and time to first relapse and time to first initiation of an heDMT were investigated by multivariable Cox proportional hazards models, adjusted for age at first symptoms, sex, and DMT category (heDMT, oral, platform, or untreated; DMT category was a time‐varying covariate). To analyze associations between the CSF categories and longitudinal sNfL concentrations, age‐dependent sNfL Z‐scores were modeled in healthy controls using a generalized additive model for location, scale, and shape (GAMLSS) to reflect the deviation of a patient's sNfL value from the mean value of same‐age healthy controls. 21 Serum NfL Z‐scores (dependent variable) were analyzed by multivariable linear mixed models adjusted for sex and DMT category at the respective visits for blood sampling.
T2w hyperintense lesion counts and the occurrence of new/enlarging T2w hyperintense lesions were analyzed by multivariable mixed negative binomial models including disease duration or time between MRI scans, respectively as offset, and adjusted for age at first symptoms, sex, and dominant DMT category (DMT category with longest treatment duration) or DMT category at visit, respectively. MSSS (calculated at the last follow‐up visit) was analyzed by a multivariable linear model, adjusted for age, sex, and dominant treatment category.
Quantitative Analysis
In a fourth step, we investigated in OCGB+/IgGIF +/IgMIF + patients whether there was an association between the outcomes above and the quantitative amount of IgGIF/IgMIF by (1) splitting at median IgGIF/IgMIF, and (2) including IgGIF and IgMIF as continuous variables in the same (ie, “combined”) model in order to assess their potential individual and independent contributions. For all endpoints, the same model structure described above was used and adjusted for the same covariables.
For time to first relapse and time to start of an heDMT, IgMIF was additionally assessed by splitting at the median in OCGB+/IgGIF +/IgMIF +, and IgGIF by splitting at the median in OCGB+/IgGIF +/IgMIF − patients.
For each model, the estimated effect of the Ig parameter is presented together with its 95% confidence intervals (CIs) and p‐value. In addition, the estimates (marginal effects) are presented graphically by extracting the predicted values together with their 95% CIs using the R package sjPlot. 24
Time to first relapse and time to first initiation of an heDMT are presented in Kaplan–Meier curves.
Model assumptions were assessed using graphical methods. For the Cox proportional hazard models, we inspected Schoenfeld's residuals. Linear models were assessed by checking residuals and leverages. The residuals were checked by visual inspection using a plot of the residuals versus the fitted values, in addition to a normal Q‐Q plot. All analyses were conducted using the statistical software R (version 3.6.3) (R is an open‐source, interpreted (statistical) programming language available at www.r‐project.org).
Results
Demographic, Clinical, and CSF Characteristics
Five hundred and thirty patients were included in this study (Table 1). OCGBs were detected in 473 (89.2%); 340 (71.9%) of those had IgGIF, 27.5% IgMIF, and 12.3% IgAIF (65.1, 26.0, and 11.7%, respectively, of the entire cohort; see Fig 1).
TABLE 1.
Demographic, Clinical and CSF Characteristics at Lumbar Puncture
| Characteristic | All a | OCGB− IgGIF − IgMIF − | OCGB+ IgGIF − IgMIF − | OCGB+ IgGIF + IgMIF − | OCGB+ IgGIF + IgMIF + |
|---|---|---|---|---|---|
| Demographics | |||||
| n (%) | 530 (100) | 46 (8.7) | 114 (21.5) | 229 (43.2) | 111 (20.9) |
| Female, n (%) | 355 (67.0) | 31 (67.4) | 56 (49.1) | 169 (73.8) | 78 (70.3) |
| Age at first symptoms, mean (SD), yr | 33.5 (10.7) | 36.0 (11.8) | 35.3 (11.3) | 33.4 (10.0) | 31.0 (10.9) |
| Characteristics at LP | |||||
| 1 clinical event, n (%) | 319 (60.2) | 34 (73.9) | 69 (60.5) | 133 (58.1) | 65 (58.6) |
| ≥2 clinical events, n (%) | 211 (39.8) | 12 (26.1) | 45 (39.5) | 96 (41.9) | 46 (41.4) |
| Age, mean (SD), yr | 35.7 (11.0) | 38.3 (13.8) | 37.8 (11.5) | 35.9 (10.0) | 32.7 (11.1) |
| First symptoms to LP, median (IQR), mo | 2.8 (0.4, 20.7) | 1.5 (0.3, 10.5) | 3.9 (0.4, 18.0) | 3.1 (0.4, 25.4) | 2.0 (0.3, 19.8) |
| DMT before LP | 18 (3.4) | 3 (6.5) | 3 (2.6) | 10 (4.4) | 2 (1.8) |
| HeDMT before LP | 4 (0.7) | 1 (2.2) | 0 (0) | 2 (0.9) | 0 (0) |
| CSF characteristics | |||||
| OCGB+, n (%) | 473 (89.2) | 0 (0) | 114 (100) | 229 (100) | 111 (100) |
| IgGIF +, n (%) | 345 (65.1) | 0 (0) | 0 (0) | 229 (100) | 111 (100) |
| IgMIF +, n (%) | 138 (26.0) | 0 (0) | 0 (0) | 0 (0) | 111 (100) |
| IgAIF + (n, %) | 62 (11.7) | 0 (0) | 7 (6.1) | 25 (10.9) | 22 (19.8) |
| Albumin quotient, median (IQR) | 4.9 (3.9, 6.5) | 5.1 (4.4, 6.7) | 6.0 (4.6, 8.3) | 5.0 (3.9, 6.4) | 4.2 (3.4, 5.0) |
| White cell count, median (IQR), n/μl | 5.0 (2.3, 10.3) | 2.0 (1.0, 4.0) | 4.0 (2.0, 6.7) | 5.6 (3.0, 10.7) | 10.0 (5.0, 19.0) |
Eleven of 530 patients were OCGB− and IgGIF + and/or IgMIF + and/or IgAIF +, and 19 of 530 patients had an OCGB+/IgGIF −/IgMIF + pattern; these were excluded from the categorization in columns 3–6 (OCGB−/IgGIF −/IgMIF − or OCGB+/IgGIF −/IgMIF − or OCGB+/IgGIF +/IgMIF − or OCGB+/IgGIF +/IgMIF +).
CSF = cerebrospinal fluid; heDMT = high‐efficacy disease‐modifying treatment; IgG/M/AIF = immunoglobulin G/M/A intrathecal fraction; IQR = interquartile range; LP = lumbar puncture; OCGB = oligoclonal IgG bands.
At the time of LP, 319 (60.2%) patients had experienced 1 clinical attack and 211 (39.8%) ≥2 clinical attacks (see Table 1). CSF variables between patients with LP before versus after first relapse were similar (Table S1).
The following results were based on multivariable analyses; all corresponding univariable analyses showed congruent results (Table S2).
Time Interval between First Symptoms and First Relapse
Three hundred and ninety‐two (74.0%) patients had a first relapse (second clinical attack), whereas 138 (26.0%) patients remained relapse free during a median disease duration of 9.0 years at last follow‐up (IQR 5.3–13.3).
OCGB+/IgGIF +/IgMIF + patients had a 94% higher risk of experiencing a first relapse (hazard ratio [HR] 1.94 [CI: 1.24, 3.05], p < 0.01) compared with patients without intrathecally produced Ig (OCGB−/IgGIF −/IgMIF −). In contrast, the IgGIF status was not associated with time to first relapse, showing similar, smaller hazard ratios for OCGB+/IgGIF + and OCGB+/IgGIF − patients (Table 2A). Accordingly, the median time to first relapse was shorter in OCGB+/IgGIF +/IgMIF + versus OCGB+/IgGIF +/IgMIF − and OCGB+/IgGIF −/IgMIF − patients (median time to first relapse 18 vs 36 vs 30 months, respectively; 46 months in OCGB−/IgGIF −/IgMIF − patients; Fig 2A).
TABLE 2.
Multivariable Models for the Following Outcomes: (A) Time to First Relapse, (B) sNfL Z‐scores, (C) sNfL Z‐scores in Untreated Patients, (D) Total T2w Lesion Count, (E) New/Enlarging T2w Lesion Count, (F) Multiple Sclerosis Severity Score and (G) Time to First Initiation of a heDM
| A. Time to First Relapse | n | HR | CI | p |
|---|---|---|---|---|
| Male versus female | 498 | 0.93 | 0.74, 1.17 | 0.528 |
| Age | 498 | 0.99 | 0.98, 1.00 | 0.237 |
| OCGB+/IgGIF −/IgMIF − a | 114 | 1.45 | 0.93, 2.27 | 0.102 |
| OCGB+/IgGIF +/IgMIF − a | 228 | 1.40 | 0.92, 2.14 | 0.115 |
| OCGB+/IgGIF +/IgMIF + a | 110 | 1.94 | 1.24, 3.05 | <0.01 |
| B. sNfL Z‐scores | n | Est. | CI | p |
|---|---|---|---|---|
| Male versus female | 3045 | 0.03 | −0.18, 0.23 | 0.782 |
| OCGB+/IgGIF −/IgMIF − a | 667 | 0.10 | −0.27, 0.47 | 0.594 |
| OCGB+/IgGIF +/IgMIF − a | 1394 | 0.12 | −0.22, 0.46 | 0.502 |
| OCGB+/IgGIF +/IgMIF + a | 716 | 0.41 | 0.04, 0.78 | 0.032 |
| C. sNfL Z‐scores in Untreated Patients | n | Est. | CI | p |
|---|---|---|---|---|
| Male versus female | 456 | 0.51 | 0.10, 0.92 | 0.016 |
| OCGB+/IgGIF −/IgMIF − a | 100 | 0.71 | 0.06, 1.36 | 0.033 |
| OCGB+/IgGIF +/IgMIF − a | 181 | 0.79 | 0.21, 1.38 | <0.01 |
| OCGB+/IgGIF +/IgMIF + a | 88 | 1.18 | 0.53, 1.83 | <0.01 |
| D. T2w Lesion Count | n | IRR | CI | p |
|---|---|---|---|---|
| Male versus female | 1795 | 1.16 | 0.91, 1.48 | 0.226 |
| Age | 1795 | 1.02 | 1.01, 1.03 | <0.01 |
| OCGB+/IgGIF −/IgMIF − a | 389 | 1.66 | 1.08, 2.57 | 0.021 |
| OCGB+/IgGIF +/IgMIF − a | 809 | 1.59 | 1.06, 2.37 | 0.023 |
| OCGB+/IgGIF +/IgMIF + a | 414 | 2.53 | 1.63, 3.93 | <0.01 |
| E. New/Enlarging T2w Lesion Count | n | IRR | CI | p |
|---|---|---|---|---|
| Male versus female | 838 | 0.93 | 0.55, 1.58 | 0.789 |
| Age | 838 | 0.98 | 0.96, 1.00 | 0.072 |
| OCGB+/IgGIF −/IgMIF − a | 164 | 1.84 | 0.73, 4.62 | 0.195 |
| OCGB+/IgGIF +/IgMIF − a | 387 | 1.61 | 0.70, 3.69 | 0.258 |
| OCGB+/IgGIF +/IgMIF + a | 195 | 3.13 | 1.29, 7.58 | 0.011 |
| F. Multiple Sclerosis Severity Score | n | Est. | CI | p |
|---|---|---|---|---|
| Male versus female | 499 | 0.70 | 0.30, 1.10 | <0.01 |
| Age | 499 | 0.06 | 0.04, 0.08 | <0.01 |
| OCGB+/IgGIF −/IgMIF − a | 114 | 0.73 | 0.01, 1.45 | 0.047 |
| OCGB+/IgGIF +/IgMIF − a | 228 | 0.86 | 0.19, 1.52 | 0.012 |
| OCGB+/IgGIF +/IgMIF + a | 111 | 1.11 | 0.38, 1.84 | <0.01 |
| G. Time to First Initiation of an heDMT | n | HR | CI | p |
|---|---|---|---|---|
| Male versus female | 500 | 1.06 | 0.79, 1.42 | 0.715 |
| Age | 500 | 0.99 | 0.97,1.00 | 0.037 |
| OCGB+/IgGIF −/IgMIF − a | 114 | 2.02 | 0.99, 4.14 | 0.055 |
| OCGB+/IgGIF +/IgMIF − a | 229 | 2.09 | 1.04, 4.16 | 0.037 |
| OCGB+/IgGIF +/IgMIF + a | 111 | 2.95 | 1.44, 6.02 | <0.01 |
Comparator group: OCGB−/IgGIF −/IgMIF −: n = 46 patients.
CI = 95% confidence interval; heDMT = high‐efficacy disease‐modifying treatment; HR = hazard ratio; IgGIF/MIF = immunoglobulin G/M intrathecal fraction; OCGB+ = presence of oligoclonal IgG bands.
FIGURE 2.
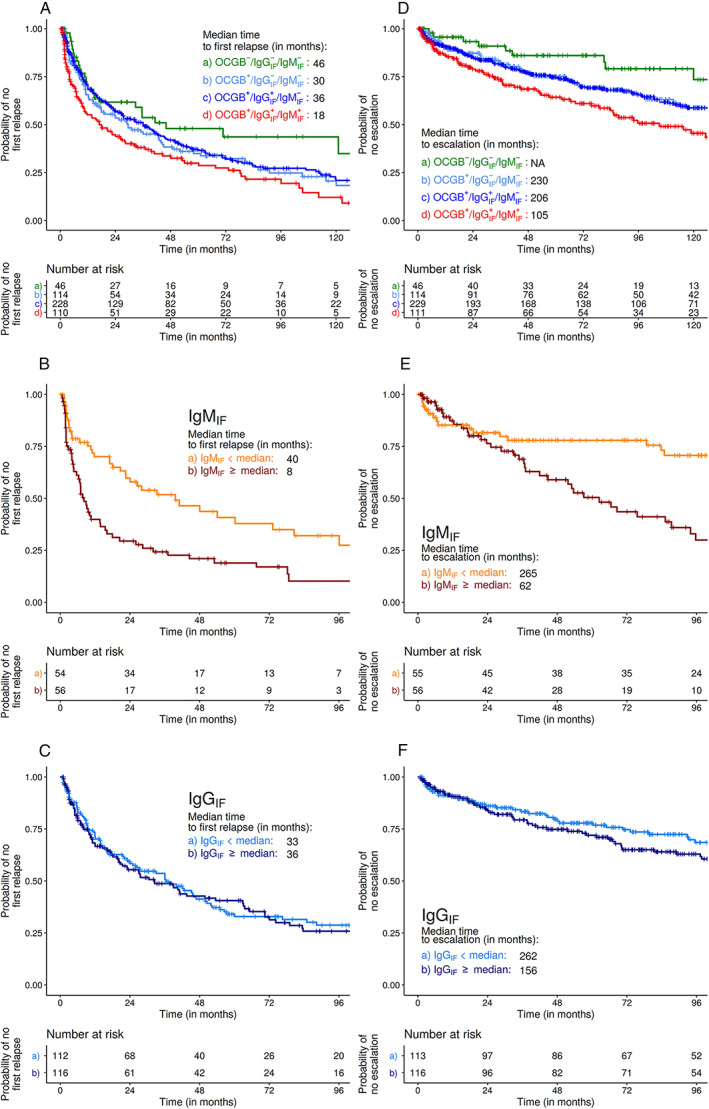
Kaplan–Meier analyses for probability of a first relapse (A–C) and escalation to a high efficacy treatment (D–F). (A) Median time to first relapse was shorter in OCBG+/IgGIF +/IgMIF + (red line) than in OCGB−/IgGIF −/IgMIF − patients (green line; 18 vs 46 months, HR 1.94 [CI 1.24, 3.05], p < 0.01). Time to first relapse in OCGB+/IgMIF − patients was not impacted by the IgGIF status (light blue line = OCGB+/IgGIF −/IgMIF −, median time 30 months; dark blue line = OCGB+/IgGIF +/IgMIF −, 36 months). (B) In OCGB+/IgGIF +/IgMIF + patients (n = 110), we saw a dose effect of the amount of IgMIF: patients with higher IgMIF experienced a first relapse earlier than those with lower IgMIF (above [red line] vs below [orange line] median IgMIF: HR 1.86 [CI 1.18, 2.93], p < 0.01, n = 110). (C) For OCGB+/IgGIF +/IgMIF − patients, no such dose effect for IgGIF was found (HR 1.12 [CI 0.82, 1.52]; p = 0.492, n = 228). (D) Time to switch to heDMT was shortest in the OCGB+/IgGIF +/IgMIF + group (red line; median 105 months, HR 2.95 [CI 1.44, 6.02], p < 0.01; n = 111) versus OCGB−/IgGIF −/IgMIF − (green line), whereas in OCGB+/IgGIF −/IgMIF − (light blue line) it was 230 months, and in OCGB+/IgGIF +/IgMIF − (dark blue line) it was 206 months. (E) Patients with increasing IgMIF levels showed an increased hazard for escalation to heDMT (above [red line] vs below [orange line] median IgMIF: HR 2.28 [CI 1.30, 3.99]; p < 0.01; n = 111). (F) No increased hazard of switching to an heDMT was found for IgGIF + (above [dark blue line] vs below [light blue line] median: HR 1.38 [CI 0.92, 2.09]; p = 0.124; n = 229). CI = 95% confidence interval; heDMT = high‐efficacy disease‐modifying treatment; HR = hazard ratio; IgGIF/MIF = immunoglobulin G/M intrathecal fraction; OCGB = oligoclonal IgG bands; + = presence of OCGB or IgGIF/IgMIF; − = absence of OCGB or IgGIF/IgMIF.
In the quantitative analysis, patients with IgMIF levels above the median had their first relapse earlier than those with values below the median (HR 1.86 [CI 1.18, 2.93], p < 0.01, n = 110; see Fig 2B), whereas the quantity of IgGIF in OCGB+/IgGIF +/IgMIF − patients was not related to the time to the first relapse (HR 1.12 [CI 0.82, 1.52], p = 0.492, n = 228; see Fig 2C). In the model combining IgMIF and IgGIF, patients with IgMIF above versus below the median showed an increased hazard of a first relapse, which was not seen for IgGIF (Table 3A).
TABLE 3.
Multivariable Models for Dose‐dependent Effects of IgMIF and IgGIF in OCGB+/IgGIF +/IgMIF + Patients for the Following Outcomes: (A) Time to First Relapse, (B) sNfL Z‐scores, (C) sNfL Z‐scores in Untreated Patients, (D) Total T2w Lesion Count, (E) New/Enlarging T2w lesion count, (F) Multiple Sclerosis Severity Score and (G) Time to First Initiation of a heDMT
| A. Time to First Relapse | HR | CI | p |
|---|---|---|---|
| 1. IgMIF (> vs <median) | 1.85 | 1.17, 2.92 | <0.01 |
| 2. IgGIF (> vs <median) | 1.13 | 0.73,1.75 | 0.578 |
| 3. IgMIF (per 1%) | 1.01 | 1.00,1.02 | 0.064 |
| 4. IgGIF (per 1%) | 1.01 | 1.00,1.02 | 0.180 |
| B. sNfL Z‐scores (n = 716) | Est. | CI | p |
|---|---|---|---|
| 1. IgMIF (> vs <median) | 0.43 | 0.10,0.77 | 0.013 |
| 2. IgGIF (> vs <median) | 0.01 | −0.32, 0.34 | 0.951 |
| 3. IgMIF (per 1%) | 0.01 | 0.00, 0.02 | 0.034 |
| 4. IgGIF (per 1%) | 0.00 | −0.01, 0.01 | 0.909 |
| C. sNfL Z‐scores in untreated patients (n = 88) | Est. | CI | p |
|---|---|---|---|
| 1. IgMIF (> vs <median) | 0.92 | 0.18, 1.66 | 0.019 |
| 2. IgGIF (> vs <median) | −0.15 | −0.88, 0.59 | 0.696 |
| 3. IgMIF (per 1%) | 0.02 | 0.00, 0.04 | 0.032 |
| 4. IgGIF (per 1%) | 0.00 | −0.02, 0.02 | 0.855 |
| D. T2w Lesion Count (n = 414) | HR | CI | p |
|---|---|---|---|
| 1. IgMIF (> vs <median) | 1.63 | 0.96, 2.75 | 0.071 |
| 2. IgGIF (> vs <median) | 1.02 | 0.61, 1.72 | 0.940 |
| 3. IgMIF (per 1%) | 1.01 | 1.00, 1.02 | 0.271 |
| 4. IgGIF (per 1%) | 1.01 | 0.99, 1.02 | 0.385 |
| E. New/Enlarging T2w Lesion Count (n = 195) | HR | CI | p |
|---|---|---|---|
| 1. IgMIF (> vs <median) | 2.86 | 1.25, 6.54 | 0.013 |
| 2. IgGIF (> vs <median) | 1.04 | 0.49, 2.20 | 0.913 |
| 3. IgMIF (per 1%) | 1.02 | 1.01, 1.02 | <0.01 |
| 4. IgGIF (per 1%) | 1.00 | 1.00, 1.01 | 0.417 |
| F. Multiple Sclerosis Severity Score (n = 111) | HR | CI | p |
|---|---|---|---|
| 1. IgMIF (> vs <median) | 0.59 | −0.23, 1.40 | 0.156 |
| 2. IgGIF (> vs <median) | −0.08 | −0.87, 0.71 | 0.843 |
| 3. IgMIF (per 1%) | 0.01 | −0.01, 0.03 | 0.201 |
| 4. IgGIF (per 1%) | 0.00 | −0.01, 0.02 | 0.680 |
| G. Time to First Initiation of an heDMT (n = 111) | HR | CI | p |
|---|---|---|---|
| 1. IgMIF (> vs <median) | 2.35 | 1.33, 4.16 | <0.01 |
| 2. IgGIF (> vs <median) | 0.87 | 0.51, 1.48 | 0.603 |
| 3. IgMIF (per 1%) | 1.02 | 1.01, 1.03 | <0.01 |
| 4. IgGIF (per 1%) | 1.000 | 0.99, 1.01 | 0.944 |
Lines 1 and 2: Model 1: IgMIF and IgGIF split by median. Lines 3 and 4: Model 2: IgMIF and IgGIF as continuous variables.
CI = 95% confidence interval; heDMT = high‐efficacy disease‐modifying treatment; HR = hazard ratio; IgGIF/MIF = immunoglobulin G/M intrathecal fraction; OCGB+ = presence of oligoclonal IgG bands.
Serum Neurofilament Light Chain Concentrations
sNfL was measured in 520 patients with 3,260 longitudinal samples (median [IQR] number of samples per patient: 6 [4–8]).
OCGB+/IgGIF +/IgMIF + patients had higher sNfL Z‐scores when compared with patients without intrathecal Ig synthesis (OCGB−/IgGIF −/IgMIF −; estimate 0.41 [CI 0.04, 0.78], p = 0.032; ie, OCGB+/IgGIF +/IgMIF + patients had on average 0.41 standard deviations higher sNfL concentrations). In those patients with presence of only OCGB+ or OCGB+/IgGIF +, sNfL Z‐scores were not elevated (Fig 3A; Table 2B).
FIGURE 3.
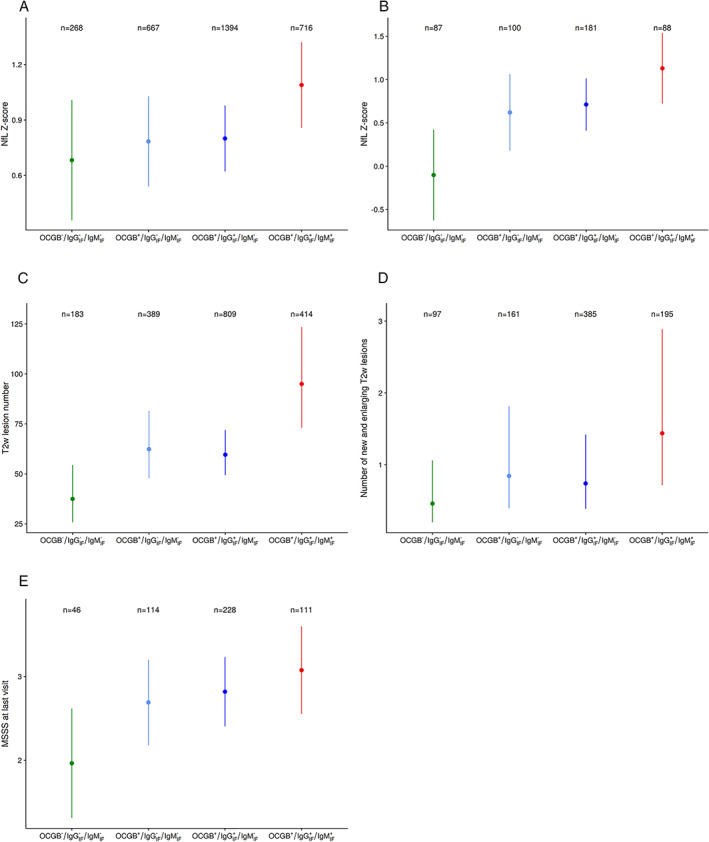
Estimates (marginal effects) as derived from the multivariable analyses for serum neurofilament light chain Z‐scores (A, all patients; B, untreated patients), MRI disease activity (C, number of T2w hyperintense lesions; D, number of new/enlarging lesions) and Multiple Sclerosis Severity Score (E) stratified by CSF immunoglobulin profiles. (A) sNfL Z‐scores in all patients (n = 491 with 3,045 longitudinal samples). After adjustment for covariates, OCGB+/IgGIF +/IgMIF + patients had higher sNfL Z‐scores when compared with patients without any intrathecal Ig synthesis (Est. 0.41 [CI 0.04; 0.78], p = 0.032). In patients with presence of only OCGB+ or OCGB+/IgGIF +, sNfL Z‐scores were not significantly elevated (Est. 0.10 [CI −0.27, 0.47], p = 0.594 and Est. 0.12 [CI −0.22, 0.46], p = 0.502). (B) sNfL Z‐scores in untreated patients (n = 186 with 456 longitudinal samples). The highest sNfL Z‐scores were found in OCGB+/IgGIF +/IgMIF + patients (Est. 1.18 [CI 0.53, 1.83], p < 0.01), and weaker effects on a similar level for those patients with only OCGB+ or OCGB+/IgGIF + (Est. 0.71 [CI 0.06, 1.36], p = 0.033 and Est. 0.79 [CI 0.21, 1.38], p < 0.01) were found. (C) Total T2w lesion count (n = 458 patients with 1,795 longitudinal MRIs). OCGB+/IgGIF +/IgMIF + patients had the highest T2 lesion counts (IRR = 2.53 [CI 1.63, 3.93], p < 0.01). Patients with only OCGB+ or OCGB+/IgGIF + showed smaller but similar effect sizes (IRR = 1.66 [CI 1.08, 2.57], p = 0.021 and IRR = 1.59 [CI 1.06, 2.37], p = 0.023, respectively). (D) New/enlarging T2w lesion count (n = 282 patients with 838 longitudinal MRIs). OCGB+/IgGIF +/IgMIF + patients had the highest counts of new/enlarging T2 lesions (IRR = 3.13 [CI 1.29, 7.58], p = 0.011), with no effects in the other 2 categories (OCGB+ and OCGB+/IgGIF +: IRR = 1.84 [CI 0.73, 4.62], p = 0.195 and IRR = 1.61 [CI 0.70, 3.69], p = 0.258, respectively). (E) Multiple Sclerosis Severity Score (MSSS) (n = 499 patients). The MSSS was on average 1.11 points [CI 0.382, 1.843] higher in OCGB+/IgGIF +/IgMIF + patients (Est. 1.112 [CI 0.382, 1.843], p < 0.01), followed by those with OCGB+/IgGIF +/IgMIF − (Est. 0.855 [CI 0.190, 1.520], p = 0.012) and OCGB+/IgGIF −/IgMIF − (Est 0.727 [CI 0.009, 1.445], p = 0.047), compared with those patients without intrathecal immunoglobulin synthesis. Plots show the estimates (marginal effects) and median together with their 95% CI. CI = 95% confidence interval; CSF = cerebrospinal fluid; MRI = magnetic resonance imaging; Est. = estimate; IgGIF/MIF = immunoglobulin G/M intrathecal fraction; IRR = incidence rate ratio; OCGB = oligoclonal IgG bands; sNfL Z‐score = serum neurofilament light chain Z‐score; T2w = T2‐weighted; + = presence of OCGB or IgGIF/IgMIF; − = absence of OCGB or IgGIF/IgMIF.
In the combined model, sNfL Z‐scores were higher in patients with IgMIF above versus below the median, whereas for IgGIF this was not the case. Similar results were seen when using IgMIF and IgGIF as continuous variables (Table 3B).
In untreated patients (494 samples in 197 patients), similar results were seen. The highest sNfL Z‐scores were found in OCGB+/IgGIF +/IgMIF + patients (estimate 1.18 [CI 0.53, 1.83], p < 0.01), followed by only OCGB+ or OCGB+/IgGIF + patients (see Fig 3B; Table 2C).
Again, in the combined model the patients with IgMIF above versus below the median or with higher IgMIF when analyzed as a continuous variable had higher sNfL Z‐scores, which was not seen for IgGIF (Table 3C).
MRI: Total and New/Enlarging T2w Lesions
T2w lesion count data were available from 488 patients and 1,918 MRIs (number of MRIs per patient: 4 [2–5]).
OCGB+/IgGIF +/IgMIF + patients had on average 2.5 times more T2w lesions compared with patients without intrathecal Ig (incidence rate ratio [IRR] 2.53 [CI 1.63, 3.93], p < 0.01, Table 2D; see Fig 3C). In the combined model, we found numerically stronger effects for IgM than for IgG (Table 3D).
Information on new/enlarging T2w lesions from annual scans was available from 305 patients and 905 MRIs. OCGB+/IgGIF +/IgMIF + patients had the highest number of new/enlarging T2w lesions (IRR 3.13 [CI 1.29, 7.58], p = 0.011), with no such associations seen in OCGB+ only and in OCGB+/IgGIF + patients (Table 2E; see Fig 3D).
Patients with IgMIF above versus below the median or analyzed as a continuous variable had a higher incidence of new/enlarging T2w lesions when analyzed in the same model with IgGIF (Table 3E). A similar effect was not seen for IgGIF (see Table 3E).
Disability (Multiple Sclerosis Severity Score)
The MSSS was on average higher by 1.11 points in OCGB+/IgGIF +/IgMIF + patients (estimate 1.11 [CI 0.38, 1.84], p < 0.01), followed by those with OCGB+/IgGIF +/IgMIF − (estimate 0.86 [CI 0.19, 1.52], p = 0.012) and OCGB+/IgGIF −/IgMIF − (estimate 0.73 [CI 0.01, 1.45], p = 0.047), when compared with those without intrathecal Ig synthesis (Table 2F; see Fig 3E). No dose‐dependent effects of IgMIF + (nor of IgGIF +) were found in relation to MSSS (Table 3F).
Initiation of High‐Efficacy Disease‐Modifying Treatments
HeDMT was started in 223 of 530 patients (42.1%; no previous DMT: 66 [29.6%]; escalation from platform therapies: 81 [36.3%]; escalation from oral treatments: 76 [34.1%]). OCGB+/IgGIF +/IgMIF + patients featured the highest probability of escalation to heDMT (HR 2.95 [CI 1.44, 6.02], p < 0.01) compared with patients without intrathecal Ig synthesis. In those with either only OCGB+ or OCGB+/IgGIF +, these effects were weaker (Table 2G). Accordingly, the time to initiation of heDMT was shortest in the OCGB+/IgGIF +/IgMIF + (105 months) versus OCGB−/IgGIF −/IgMIF − patients (given that only 26.8% were treated with heDMT in this group, the median time to switch could not be determined), whereas in OCGB+/IgGIF −/IgMIF − and in OCGB+/IgGIF +/IgMIF − patients it was 230 and 206 months, respectively (see Fig 2D); similar to the analysis of the time interval to first relapse, the time to heDMT in OCGB+/IgMIF − patients was not impacted by the IgGIF status. Again, this association was dosedependent for IgMIF, but not for IgGIF. In OCGB+/IgGIF +/IgMIF + patients, IgMIF + levels above the median were associated with a >2‐fold increased hazard for escalation to heDMT (HR 2.28 [CI 1.30, 3.99], p < 0.01, n = 111) versus those below the median (see Fig 2E), whereas this was not the case for IgGIF + in patients with only OCGB+/IgGIF + (HR 1.38 [CI 0.92, 2.09], p = 0.12, n = 229; see Fig 2F). In the combined model, similar results were found (Table 3G).
Discussion
The prediction of the individual course of multiple sclerosis based on current clinical and MRI measures provides limited accuracy for therapeutic decision making. OCGB are present in up to 98% of multiple sclerosis patients and have been reinstated as a diagnostic criterion in the 2017 MS diagnostic criteria. 1 However, the presence of OCGB as a prognostic biomarker is relevant only in the minority of multiple sclerosis patients where they are absent, because this might go along with a milder disease course. 25
OCMB have shown prognostic value, 9 , 11 , 26 , 27 , 28 but owing to the difficulties in standardizing the methodology, this measure is not widely used. In contrast, the quantitation of intrathecal Ig synthesis using the Reiber formulae is a fully standardized analytical method.
Here, we have identified that the presence and, furthermore, the quantity of IgMIF is a stronger and more independent prognostic biomarker than OCGB and IgGIF for acute disease activity (time interval from disease onset to first relapse) and the chronic course of disability (MSSS), and for biological and radiological measures (sNfL Z‐scores and MRI activity).
Additionally, we demonstrate that the presence of intrathecal IgM production is associated with a first choice of heDMT or an earlier switch to heDMT, as the physician's consequence for the need for a more aggressive therapy. Moreover, this association holds true also when the quantity of IgMIF, is considered; in this case, we observed that the quantity of IgMIF is related to a shorter time to DMT escalation and to “time to first relapse,” in addition to increased sNfL concentrations and disease activity as determined by MRI measures. In contrast, the presence of IgGIF, or the quantity of IgGIF in addition to OCGB has not shown significant additional prognostic value (see Tables 2, 3; Figs 2 and 3). In line with prior results, 6 intrathecal IgAIF was relatively rare in the present study cohort (present in 62 [11.7%] of patients; see Table 1) and was not associated with any of the outcomes (see Table S2), suggesting that its presence might lack a relevant pathogenic role in multiple sclerosis.
Our results are somewhat discordant with recent data from the German multiple sclerosis cohort, in which IgGIF but not IgMIF was associated with risk and shorter time to EDSS worsening within 4 years. 6 A potential explanation for this discrepancy might be the different statistical approaches: unconfirmed EDSS worsening was analyzed for individual Ig isotypes, whereas we adjusted for the presence of OCGB, IgGIF, and IgMIF in combined models. Furthermore, a longer observation time (median 9.0 vs 4 years since disease onset), a larger patient cohort with disease worsening data (530 vs 330 patients) in conjunction with different statistical approaches (no imputation of data vs imputation of 27–41% of EDSS data at year 4, additionally shortening observation interval) in our study versus the German multiple sclerosis cohort study might be additional factors. 6
The contribution of antibodies and their capacity to activate complement during the initial development of a multiple sclerosis plaque has been demonstrated in autopsy studies. Complement C3b colocalizes with IgG and IgM deposits on oligodendrocytes and axons, leading to demyelination and axonal injury. 29 , 30 Intrathecal synthesis of IgM (but not IgG or IgA) was shown to be associated with early activation of the complement cascade, with intrathecal activation of complement component C3, which is in line with IgM being the most efficient isotype for complement activation. 31
An indication of a pathogenic role of intrathecal Ig synthesis could be its longitudinal decrease under some heDMTs in parallel to clinical improvement or stabilization of disease. Continuous treatment with the B cell‐targeting drugs rituximab and ocrelizumab can lead to hypogammaglobulinemia32, 33, 34 an effect that is more prominent and occurs faster for IgM than for IgG. Only small case series describe the course of intrathecal Ig synthesis under rituximab or natalizumab therapy in multiple sclerosis: IgGIF decreased transiently under rituximab and returned to levels prior to therapy during months 6 to 9. 35 Accordingly, intrathecal IgG synthesis (OCGB 36 , 37 ; IgGIF [Reiber formula] 38 ) under natalizumab therapy decreased in some patients, but returned after treatment cessation to initial levels. 37 In contrast, intrathecal IgM synthesis and its relation to treatment effects have not been evaluated; an aspect we are now actively evaluating.
Limitations
Given that most patients entered the SMSC after the lumbar puncture had been performed, a correlation of intrathecal Ig synthesis with complete longitudinal EDSS scores from disease start was not possible in our study. In addition, our results are restricted mainly to CSF analysis in an early phase of multiple sclerosis; an extension of the CSF profiling into later stages of relapsing multiple sclerosis to gain a better understanding of the role of intrathecal IgM synthesis and its value as biomarker is warranted.
Conclusions
Our study strongly supports the value of a quantitative analysis of IgM levels in CSF as a prognostic biomarker, independent of other Ig measures, which might be useful for therapeutic decision making in the early phases of disease.
Author Contributions
Conception and design of study: J.O., S.S., P.B., D.L., and J.K.
Acquisition and analysis of data: J.O., S.S., P.B., S.M., L.A., J.V., G.D., O.F., B.F., A.O., A.C., C.P., M.B., R.R., R.G., I.H., P.L., J.W., S.S., S.A., D.C., Y.N., A.M., S.M., K.B., H.W., T.L., J.L., O.Y., T.S., T.D., A.R., C.Z., C.G., L.K., C.G., D.L., and J.K.
Drafting of the manuscript: J.O., D.L., and J.K.
Potential Conflicts of Interest
Nothing to report.
Supporting information
Table S1 Distribution of cerebrospinal fluid parameters in lumbar punctures performed before and after a first relapse (second clinical event).
Table S2 Univariable models for the following outcomes: (A) time to first relapse; (B) sNfL Z‐scores; (C) sNfL Z‐scores in untreated patients; (D) total T2w lesion count; (E) new/enlarging T2w lesion count; (F) Multiple Sclerosis Severity Score; and (G) time of initiation of a high‐efficacy DMT.
Acknowledgment
The Swiss MS Cohort study received funding from the Swiss MS Society and unrestricted grant funding from Biogen, Celgene, Merck, Novartis, Roche, and Sanofi. The research is supported by the Swiss National Science Foundation (grant 320030_189140 / 1, J.K.).
We express our deep thankfulness to patients and relatives for their participation and support, study nurses in participating centers for their motivated collaboration and recruitment efforts, and the administrative personnel of the Swiss Multiple Sclerosis Cohort study.
References
- 1. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–173. [DOI] [PubMed] [Google Scholar]
- 2. Reiber H, Ungefehr S, Jacobi C. The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. Mult Scler 1998;4:111–117. [DOI] [PubMed] [Google Scholar]
- 3. Link H, Tibbling G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within the central nervous system in multiple sclerosis. Scand J Clin Lab Invest 1977;37:397–401. [DOI] [PubMed] [Google Scholar]
- 4. Huss A, Abdelhak A, Halbgebauer S, et al. Intrathecal immunoglobulin M production: a promising high‐risk marker in clinically isolated syndrome patients. Ann Neurol 2018;83:1032–1036. [DOI] [PubMed] [Google Scholar]
- 5. Beltran E, Obermeier B, Moser M, et al. Intrathecal somatic hypermutation of IgM in multiple sclerosis and neuroinflammation. Brain 2014;137:2703–2714. [DOI] [PubMed] [Google Scholar]
- 6. Gasperi C, Salmen A, Antony G, et al. Association of Intrathecal Immunoglobulin G Synthesis with Disability Worsening in multiple sclerosis. JAMA Neurol 2019;76:841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pfuhl C, Grittner U, Giess RM, et al. Intrathecal IgM production is a strong risk factor for early conversion to multiple sclerosis. Neurology 2019;93:e1439–e1451. [DOI] [PubMed] [Google Scholar]
- 8. Villar LM, Casanova B, Ouamara N, et al. Immunoglobulin M oligoclonal bands: biomarker of targetable inflammation in primary progressive multiple sclerosis. Ann Neurol 2014;76:231–240. [DOI] [PubMed] [Google Scholar]
- 9. Villar LM, Masjuan J, Gonzalez‐Porque P, et al. Intrathecal IgM synthesis predicts the onset of new relapses and a worse disease course in MS. Neurology 2002;59:555–559. [DOI] [PubMed] [Google Scholar]
- 10. Villar LM, Masjuan J, Gonzalez‐Porque P, et al. Intrathecal IgM synthesis is a prognostic factor in multiple sclerosis. Ann Neurol 2003;53:222–226. [DOI] [PubMed] [Google Scholar]
- 11. Villar LM, Sadaba MC, Roldan E, et al. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J Clin Invest 2005;115:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ozakbas S, Cinar BP, Ozcelik P, et al. Intrathecal IgM index correlates with a severe disease course in multiple sclerosis: clinical and MRI results. Clin Neurol Neurosurg 2017;160:27–29. [DOI] [PubMed] [Google Scholar]
- 13. Perini P, Ranzato F, Calabrese M, et al. Intrathecal IgM production at clinical onset correlates with a more severe disease course in multiple sclerosis. J Neurol Neurosurg Psychiatry 2006;77:953–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schneider R, Euler B, Rauer S. Intrathecal IgM‐synthesis does not correlate with the risk of relapse in patients with a primary demyelinating event. Eur J Neurol 2007;14:907–911. [DOI] [PubMed] [Google Scholar]
- 15. Stauch C, Reiber H, Rauchenzauner M, et al. Intrathecal IgM synthesis in pediatric MS is not a negative prognostic marker of disease progression: quantitative versus qualitative IgM analysis. Mult Scler 2011;17:327–334. [DOI] [PubMed] [Google Scholar]
- 16. Disanto G, Benkert P, Lorscheider J, et al. The Swiss multiple sclerosis cohort‐study (SMSC): a prospective Swiss wide investigation of key phases in disease evolution and new treatment options. PLoS One 2016;11:e0152347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 19. Andersson M, Alvarez‐Cermeno J, Bernardi G, et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol Neurosurg Psychiatry 1994;57:897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Felgenhauer KSG, Rapic N. Evaluation of the blood‐CSF barrier by protein gradients and the humoral immune response within the central nervous system. J Neurol Sci 1976;30:113–111. [DOI] [PubMed] [Google Scholar]
- 21. Benkert P, Schaedelin S, Maceski A, et al. Serum NfL Z‐scores derived from a large healthy control group reflect different levels of treatment effect in a real‐ world setting. MSVirtual2020; 8th Joint ACTRIMS‐ECTRIMS Meeting September 11–13 2020; Poster 0160.
- 22. International MICCAI Brainlesion Workshop . Automated Segmentation of Multiple Sclerosis Lesions Using Multi‐dimensional Gated Recurrent Units. Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries: BrainLes 2017; 14th September. pp. 31–42.
- 23. Fartaria MJ, Kober T, Granziera C, et al. Longitudinal analysis of white matter and cortical lesions in multiple sclerosis. Neuroimage Clin 2019;23:101938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lüdecke D, Bartel A, Schwemmer C, et al. sjPlot: Data Visualization for Statistics in Social Science. R package version 2.8.6. 2020.
- 25. Joseph FG, Hirst CL, Pickersgill TP, et al. CSF oligoclonal band status informs prognosis in multiple sclerosis: a case control study of 100 patients. J Neurol Neurosurg Psychiatry 2009;80:292–296. [DOI] [PubMed] [Google Scholar]
- 26. Ferraro D, Simone AM, Bedin R, et al. Cerebrospinal fluid oligoclonal IgM bands predict early conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome. J Neuroimmunol 2013;257:76–81. [DOI] [PubMed] [Google Scholar]
- 27. Magraner MJ, Bosca I, Simo‐Castello M, et al. Brain atrophy and lesion load are related to CSF lipid‐specific IgM oligoclonal bands in clinically isolated syndromes. Neuroradiology 2012;54:5–12. [DOI] [PubMed] [Google Scholar]
- 28. Villar LM, Espino M, Cavanillas ML, et al. Immunological mechanisms that associate with oligoclonal IgM band synthesis in multiple sclerosis. Clin Immunol 2010;137:51–59. [DOI] [PubMed] [Google Scholar]
- 29. Mead RJ, Singhrao SK, Neal JW, et al. The membrane attack complex of complement causes severe demyelination associated with acute axonal injury. J Immunol 2002;168:458–465. [DOI] [PubMed] [Google Scholar]
- 30. Piddlesden SJ, Lassmann H, Zimprich F, et al. The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am J Pathol 1993;143:555–564. [PMC free article] [PubMed] [Google Scholar]
- 31. Sellebjerg F, Christiansen M, Garred P. MBP, anti‐MBP and anti‐PLP antibodies, and intrathecal complement activation in multiple sclerosis. Mult Scler 1998;4:127–131. [DOI] [PubMed] [Google Scholar]
- 32. Evertsson BFK, Finn A, Piehl F, Nimer F. Low dose rituximab depletes B cells and lowers IgM in blood in MS patients: a study on possible biomarkers to predict treatment response and adverse event profile. Berlin: ECTRIMS, 2018. [Google Scholar]
- 33. Hallberg SBM, Evertsson B, Lillvall E, et al. Risk of hypogammaglobuinaemia in long‐term treatment with rituximab in multiple sclerosis. Stockholm: ECTRIMS, 2019. [Google Scholar]
- 34. Tallantyre ECWD, Jolles S. Secondary antibody deficiency: a complication of anti‐CD20 therapy for neuroinflammation. J Neurol 2017;265:1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rommer PS, Patejdl R, Winkelmann A, et al. Rituximab for secondary progressive multiple sclerosis: a case series. CNS Drugs 2011;25:607–613. [DOI] [PubMed] [Google Scholar]
- 36. Mancuso R, Franciotta D, Rovaris M, et al. Effects of natalizumab on oligoclonal bands in the cerebrospinal fluid of multiple sclerosis patients: a longitudinal study. Mult Scler 2014;20:1900–1903. [DOI] [PubMed] [Google Scholar]
- 37. Von Glehn F, Farias AS, de Oliveira AC, et al. Disappearance of cerebrospinal fluid oligoclonal bands after natalizumab treatment of multiple sclerosis patients. Mult Scler 2012;18:1038–1041. [DOI] [PubMed] [Google Scholar]
- 38. Harrer A, Wipfler P, Einhaeupl M, et al. Natalizumab therapy decreases surface expression of both VLA‐heterodimer subunits on peripheral blood mononuclear cells. J Neuroimmunol 2011;234:148–154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Distribution of cerebrospinal fluid parameters in lumbar punctures performed before and after a first relapse (second clinical event).
Table S2 Univariable models for the following outcomes: (A) time to first relapse; (B) sNfL Z‐scores; (C) sNfL Z‐scores in untreated patients; (D) total T2w lesion count; (E) new/enlarging T2w lesion count; (F) Multiple Sclerosis Severity Score; and (G) time of initiation of a high‐efficacy DMT.


