Abstract
Objective
The optimal time to start biologics in polyarticular juvenile idiopathic arthritis (JIA) remains uncertain. The Childhood Arthritis and Rheumatology Research Alliance (CARRA) developed 3 consensus treatment plans (CTPs) for untreated polyarticular JIA to compare strategies for starting biologics.
Methods
Start Time Optimization of Biologics in Polyarticular JIA (STOP‐JIA) was a prospective, observational, CARRA Registry study comparing the effectiveness of 3 CTPs: 1) the step‐up plan (initial nonbiologic disease‐modifying antirheumatic drug [DMARD] monotherapy, adding a biologic if needed, 2) the early combination plan (DMARD and biologic started together), and 3) the biologic first plan (biologic monotherapy). The primary outcome measure was clinically inactive disease according to the provisional American College of Rheumatology (ACR) criteria, without glucocorticoids, at 12 months. Secondary outcome measures included Patient‐Reported Outcomes Measurement Information System (PROMIS) pain interference and mobility scores, inactive disease as defined by the clinical Juvenile Arthritis Disease Activity Score in 10 joints (JADAS‐10), and the ACR Pediatric 70 criteria (Pedi 70).
Results
Of 400 patients enrolled, 257 (64%) began the step‐up plan, 100 (25%) the early combination plan, and 43 (11%) the biologic first plan. After propensity score weighting and multiple imputation, clinically inactive disease according to the ACR criteria was achieved in 37% of those on the early combination plan, 32% on the step‐up plan, and 24% on the biologic first plan (P = 0.17). Inactive disease according to the clinical JADAS‐10 (score ≤2.5) was also achieved in more patients on the early combination plan than the step‐up plan (59% versus 43%; P = 0.03), as was ACR Pedi 70 (81% versus 62%; P = 0.008), but generalizability was limited by missing data. PROMIS measures improved in all groups, but without significant differences. Twenty serious adverse events were reported (mostly infections).
Conclusion
Achievement of clinically inactive disease without glucocorticoids did not significantly differ between groups at 12 months. While there was a significantly higher likelihood of early combination therapy achieving inactive disease according to the clinical JADAS‐10 and ACR Pedi 70, these results require further exploration.
INTRODUCTION
Juvenile idiopathic arthritis (JIA) is the most common pediatric rheumatic disease, with prevalence estimates ranging from 1–4 per 1,000 (1, 2, 3, 4, 5). The term “JIA” describes a clinically heterogeneous group of diseases, including a polyarticular form of JIA defined by involvement of ≥5 joints (6). Children with polyarticular JIA often have long periods of active disease that increase the risk of joint damage and result in impaired quality of life and worsened functional outcomes (7, 8). Therefore, a major treatment goal is timely attainment of inactive disease to prevent long‐term morbidities (9). Nearly half of patients in longitudinal observational cohorts report recurrent or ongoing disease activity in adulthood (10, 11, 12, 13, 14, 15). Although disease‐modifying antirheumatic drugs (DMARDs) and biologic agents have vastly improved polyarticular JIA outcomes, questions remain regarding the ideal timing of biologic initiation. Prior clinical trials have attempted to address this question without a definitive answer (16, 17). As a result, wide variations in clinical practice continue, negatively impacting health outcomes (18, 19) despite the availability of multiple effective therapies for polyarticular JIA with regulatory approval (20, 21, 22).
The optimal time to start biologics in children with untreated polyarticular JIA has been the focus of active research. Two prior randomized trials of initial biologic therapy in polyarticular JIA reached different conclusions about early biologic use, possibly reflecting different designs and study populations (16, 17). Recent American College of Rheumatology (ACR)/Arthritis Foundation guidelines for the treatment of polyarticular JIA, derived from the systematic review of published data and expert consensus, supported initial DMARD treatment with rapid escalation to biologics for poor or limited response (23). The recommendations suggest that children who are at high risk for more severe disease (e.g., those who are rheumatoid factor [RF] positive, have joint damage, or have high‐risk joints involved) may benefit from initial biologic treatment.
While large multicenter randomized controlled trials (RCTs) are frequently considered the gold standard for determining treatment efficacy, such studies in polyarticular JIA have limited feasibility because of relatively low disease prevalence and the financial and logistical constraints associated with traditional RCTs. In addition, patients and families have become more reluctant to participate in randomized studies when approved treatments are available. Observational study design approaches, and comparative effectiveness research methodologies in particular, are more feasible and acceptable to patients, families, and providers. The Childhood Arthritis and Rheumatology Research Alliance (CARRA) developed standardized consensus treatment plans (CTPs) using formal consensus methodology for children and adolescents newly diagnosed as having polyarticular JIA, as well as other pediatric rheumatic diseases, as an innovative approach to studying treatment outcomes in these diseases (24).
The objective of the Start Time Optimization of Biologics in Polyarticular JIA (STOP‐JIA) study was to compare the 3 CARRA CTPs for untreated polyarticular JIA, which differ in the timing of starting biologics: the step‐up plan (nonbiologic DMARD monotherapy, with a biologic added later if needed), the early combination plan (nonbiologic and biologic DMARDs started together), and the biologic first plan (biologic monotherapy) (25). The STOP‐JIA study is the first large‐scale study to use this novel approach to conducting comparative effectiveness research, implementing standardized CTPs within the observational CARRA patient registry to reduce treatment variability and allow for comparisons of effectiveness of the 3 CTPs in untreated polyarticular JIA (26). Understanding the optimal time to start biologic treatment is of critical importance to patients and families, as well as clinicians.
PATIENTS AND METHODS
Patients
Patients with untreated polyarticular JIA who were ≤19 years old at diagnosis and presented to one of 56 CARRA Registry sites participating in the STOP‐JIA study were approached to enroll in the CARRA Registry. (See Supplementary Table 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41888/abstract, for full inclusion/exclusion criteria.) See Appendix A for a list of the CARRA STOP‐JIA investigators. The CARRA Registry began recruitment in July 2015 and serves as a platform for comparative effectiveness research, clinical trials, translational research, and pharmacosurveillance studies (6). Enrollment occurred between December 2015 and August 2018. Follow‐up was completed September 2019.
Registry data, including disease activity assessments, medication start and stop dates, and severe adverse event (SAE)/event of special interest reporting were collected for STOP‐JIA study participants at 3, 6, 9, and 12 months. Specific questions about CTP use and patient‐reported outcomes were added. A Stakeholder Advisory Committee led by a parent of a patient with JIA and a young adult with JIA (VDG and KLM) was formed during the development of the funding proposal, and met regularly throughout the study to ensure study outcomes were relevant to patients, and to assist with enrollment strategies and the dissemination of interim and final study results. The study was approved by the Duke University Institutional Review Board (Pro00054616) and used the same consent form as the CARRA Registry.
Treatment strategies
The polyarticular JIA CTPs used in the STOP‐JIA study were developed based on an initial CARRA‐wide survey about current treatment practices, followed by face‐to‐face consensus conferences at CARRA meetings, and refined by a core workgroup of JIA experts through regular teleconferences. The final CTPs were endorsed by 96% of the CARRA JIA workgroup at the 2013 CARRA meeting and published (24). The 3 CTPs used in the STOP‐JIA study (the step‐up, early combination, and biologic first plans) differed with regard to the timing of biologic treatment initiation (see Supplementary Table 2, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41888/abstract, for CTP details). As recommended by the CTPs, the clinical Juvenile Arthritis Disease Activity Score in 10 joints (JADAS‐10) was used as a guide to disease activity status and shared decision‐making, with treatment escalation recommended every 3 months if values were >2.5 at the clinical visit (27).
Outcome measures
The primary outcome measure was the ACR provisional criteria for clinically inactive disease without glucocorticoids at 12 months after initiation of therapy (9). Clinically inactive disease was chosen because it was the only validated measure of disease state in JIA and is strongly related to disease remission (sustained clinically inactive disease)—the first step toward cure, the ultimate goal of JIA treatment (9, 17). Limiting glucocorticoid treatment is a critical part of the outcome, because while they are able to reduce disease activity, glucocorticoids are unacceptable as ongoing treatment due to side effects and long‐term toxicity.
Secondary outcome measures included Patient‐Reported Outcomes Measurement Information System (PROMIS) pain interference score and PROMIS mobility score. Pain was highly rated as an outcome of importance in our patient/parent survey, as was the ability to participate in activities (28). One question from the Juvenile Arthritis Multidimensional Assessment Report was used to capture patient‐reported medication side effects (29). Additional outcome measures included disease activity at each study visit (clinical JADAS‐10), and percentages of children who achieved inactive disease according to the clinical JADAS‐10 (defined as a clinical JADAS‐10 of ≤2.5) while not receiving glucocorticoids. The clinical JADAS‐10 is a simple sum (maximum score 30) derived by adding the physician global assessment of disease activity (on a 10‐cm visual analog scale [VAS]), the patient/parent assessment of overall well‐being (on a 10‐cm VAS), and the number of joints with active disease (maximum 10), making it a straightforward assessment for use at point of care. Published cutoffs for clinical JADAS‐10 define levels of inactive, low, moderate, and high disease activity (25). The ACR Pediatric 70 (ACR Pedi 70) response level while not receiving glucocorticoids was also assessed (30). Comparisons of glucocorticoid use, SAEs/events of special interest, and medication side effects between CTP groups were also performed. Medication safety was assessed through adverse event reporting mechanisms in place for the Registry.
Statistical analysis
The primary analyses were intent‐to‐treat, comparing the percentage of patients with clinically inactive disease without glucocorticoids at 1 year in each CTP. The treating physician and family selected the CTP at baseline. There were 2 major stages to the analysis. First, a generalized boosted model was constructed from potential confounders to produce propensity scores (PS) for each participant to be on his or her assigned CTP (31). The goal of this first stage was to find a PS model yielding satisfactory balance between CTP groups on the potential confounders. Second, inverse PS‐weighted pairwise comparisons of outcomes between CTP groups were performed to estimate average treatment effects; these results were checked for sensitivity to inclusion of a small number of covariates with residual imbalance. For PS details, see Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41888/abstract.
To account for missing outcomes, PS‐weighted comparisons for clinically inactive disease, inactive disease according to the clinical JADAS‐10, and ACR Pedi 70 outcomes were pooled across 30 imputed data sets. Missing clinically inactive disease values during follow‐up were imputed from a model that for each participant included available components of clinically inactive disease at that time, clinically inactive disease at other months, CTP group, and baseline values of the physician global assessment of disease activity score, the patient/parent assessment of overall well‐being score, and the number of joints with active disease. For details, see Methods for Handling Missing Data in the Supplementary Methods, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41888/abstract.
A similar approach was used for inactive disease according to the clinical JADAS‐10 and for the ACR Pedi 70. For these 3 binary outcomes, the analyses compute inverse PS‐weighted differences in percentages, their 95% confidence intervals (95% CIs), and a Wald test of equality of the percentages in the 3 CTP groups, all pooled across imputations. Because some participants were declared to have started one CTP at baseline, but for various reasons (e.g., insurance coverage, changes in family preferences) ended up following a different CTP, the primary analysis was repeated with the actual CTP used. Two physicians (YK and PFW) assigned the actual CTP after reviewing medication timing, and adjudication occurred (SR) if there was disagreement regarding the treatment assignment in patients not clearly adhering to a CTP.
T scores for PROMIS pain interference and mobility were analyzed using linear mixed‐effects models, with inverse PS weighting. For each patient‐reported outcome, the model included random intercepts for each participant and fixed effects for time of assessment, CTP, and the interaction between time and CTP, which represents a differential response to treatment. If the test of the differential response to treatment hypothesis had a P value greater than 0.05, a second model was fitted without the interaction to estimate the average change over time for all CTPs. The time variable was parameterized so that estimates represent the mean difference in T scores between adjacent assessment times (0–3 months, 3–6 months, etc.).
CTPs were also compared with regard to the percentage of patients who were not receiving glucocorticoids at various time points. Time to first visit with clinically inactive disease was analyzed with a Weibull proportional hazards model, using interval censoring, since the exact date of clinically inactive disease occurrence was unknown, again weighting by PS.
Analyses were performed in R 3.6.1 software using the packages twang for PS analysis and mice for imputation (32, 33, 34).
RESULTS
Patient characteristics
A total of 444 participants were assessed for eligibility, and 401 were enrolled (Figure 1). One patient was determined not to have polyarticular JIA and was excluded from the analysis. Of the 400 analyzable participants, 257 (64%) were started on the step‐up CTP, 100 (25%) were started on the early combination CTP, and 43 (11%) were started on the biologic first CTP at baseline. Eighteen participants were lost to follow‐up before 12 months: 2 withdrew consent and 16 moved to a non‐participating clinical site, leaving 382 participants who had at least 12 months of follow‐up (250 for the step‐up plan, 94 for the early combination plan, and 38 for the biologic first plan). Of these 382 participants, 44 missed the 12‐month primary end point visit, leaving a total of 338 evaluable participants for the primary end point at 12 months, including 222 participants on the step‐up plan, 81 on the early combination plan, and 35 on the biologic first plan.
Figure 1.
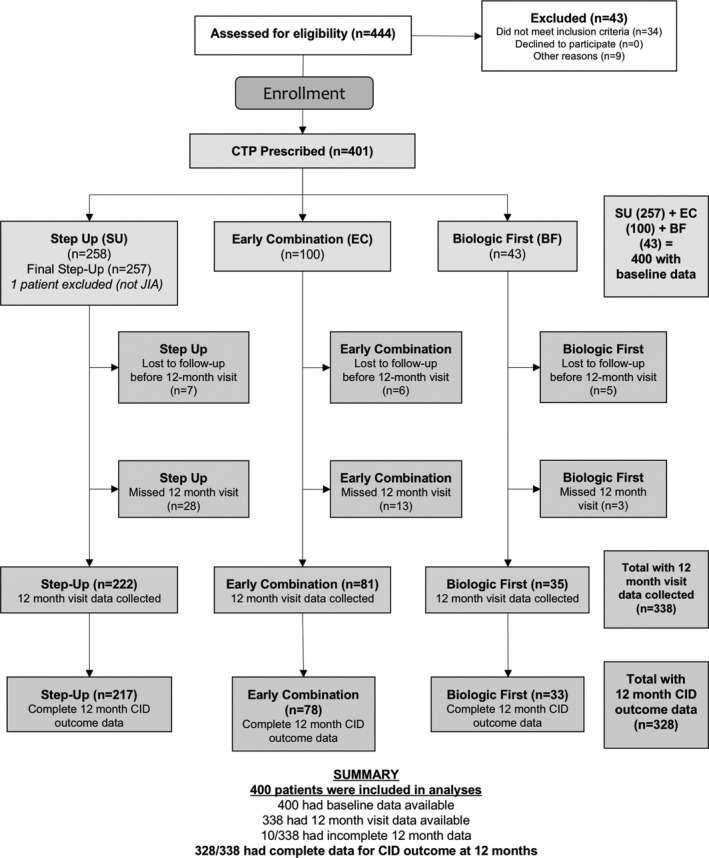
Disposition of the study patients. A total of 444 participants were screened, and 401 were enrolled. One patient was determined not to have polyarticular juvenile idiopathic arthritis (JIA) and was excluded from the analysis. Of the 400 analyzable participants at baseline, 257 (64%) were started on the step‐up consensus treatment plan (CTP), 100 (25%) on the early combination CTP, and 43 (11%) on the biologic first CTP. Eighteen participants were lost to follow‐up: 2 withdrew consent and 16 moved to a non‐participating clinical site. Of the patients lost to follow‐up, 2 patients were lost to follow‐up after the baseline visit, 2 patients after the 3 month visit, 2 patients after the 6 month visit, and 12 patients after the 9 month visit, leaving 382 participants with at least 12 months of follow‐up data available (250 in the step‐up CTP group, 94 in the early combination CTP group, and 38 in the biologic first CTP group). Of these 382 participants, 44 missed the 12‐month primary end point visit, leaving a total of 338 evaluable CTP participants for the primary end point (222 in the step‐up CTP group, 81 in the early combination CTP group, and 35 in the biologic first CTP group). CID = clinically inactive disease.
While there were few demographic differences between CTP groups, there were clinically important differences in baseline disease characteristics, including JIA category, clinical JADAS‐10 score, number of joints with active disease, physician global assessment of disease activity, patient/parent assessment of overall well‐being, and the Childhood Health Assessment Questionnaire score (35) (Table 1). In general, participants on the early combination and biologic first CTPs had higher baseline disease activity and severity measurements, as might be expected, since initial treatment with a biologic is considered more aggressive. See Supplementary Table 3, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41888/abstract, for the baseline characteristics for each group after PS reweighting.
Table 1.
Baseline characteristics of the patients with JIA in each CTP group*
|
Overall (n = 400) |
Step‐up CTP (n = 257) |
Early combination CTP (n = 100) |
Biologic first CTP (n = 43) |
P | |
|---|---|---|---|---|---|
| Age, mean ± SD years | 10.40 ± 4.94 | 10.03 ± 5.03 | 11.12 ± 4.54 | 10.89 ± 5.17 | 0.139 |
| Sex, no. (%) male | 106 (26.5) | 65 (25.3) | 25 (25.0) | 16 (37.2) | 0.242 |
| Race, no. (%) | 0.347 | ||||
| Black | 30 (7.5) | 17 (6.6) | 7 (7.0) | 6 (14.0) | |
| Other | 79 (19.8) | 47 (18.3) | 24 (24.0) | 8 (18.6) | |
| White | 291 (72.8) | 193 (75.1) | 69 (69.0) | 29 (67.4) | |
| Time since symptom onset, median (IQR) months | 6.10 (2.90–16.11) | 5.60 (2.76–14.09) | 7.31 (3.51–17.16) | 5.16 (2.10–30.93) | 0.420 |
| Time since diagnosis, median (IQR) months | 0.00 (0.00–0.83) | 0.00 (0.00–0.80) | 0.00 (0.00–0.47) | 0.47 (0.00–2.12) | 0.034 |
| Disease course, no. (%) | 0.001 | ||||
| Enthesitis related | 33 (8.2) | 15 (5.8) | 10 (10.0) | 8 (18.6) | |
| Extended oligoarticular | 14 (3.5) | 12 (4.7) | 0 (0.0) | 2 (4.7) | |
| RF‐negative polyarticular | 242 (60.5) | 171 (66.5) | 54 (54.0) | 17 (39.5) | |
| RF‐positive polyarticular | 78 (19.5) | 42 (16.3) | 28 (28.0) | 8 (18.6) | |
| Psoriatic | 23 (5.8) | 12 (4.7) | 5 (5.0) | 6 (14.0) | |
| Undifferentiated | 10 (2.5) | 5 (1.9) | 3 (3.0) | 2 (4.7) | |
| Previous NSAID use, no. (%)† | 155 (83.3) | 94 (80.3) | 41 (91.1) | 20 (83.3) | 0.257 |
| PGA, mean ± SD (10‐cm VAS)‡ | 5.52 ± 2.12 | 5.07 ± 1.99 | 6.41 ± 2.14 | 6.14 ± 2.02 | <0.001 |
| PtGA, mean ± SD (10‐cm VAS)§ | 4.33 ± 2.68 | 3.94 ± 2.70 | 4.88 ± 2.51 | 5.32 ± 2.51 | 0.001 |
| Clinical JADAS‐10, mean ± SD¶ | 18.08 ± 4.67 | 17.08 ± 4.55 | 20.18 ± 4.37 | 19.05 ± 4.29 | <0.001 |
| No. of joints with active disease, mean ± SD | 12.79 ± 8.58 | 11.89 ± 8.06 | 15.96 ± 9.42 | 10.79 ± 7.86 | <0.001 |
| Duration of morning stiffness, no. (%) | 0.031 | ||||
| None | 64 (16.0) | 50 (19.5) | 7 (7.0) | 7 (16.3) | |
| ≤15 minutes | 43 (10.8) | 29 (11.3) | 7 (7.0) | 7 (16.3) | |
| 16–60 minutes | 123 (30.8) | 73 (28.4) | 33 (33.0) | 17 (39.5) | |
| >60 minutes | 130 (32.5) | 80 (31.1) | 42 (42.0) | 8 (18.6) | |
| Unknown | 40 (10.0) | 25 (9.7) | 11 (11.0) | 4 (9.3) | |
| No. of joints with a limited range of motion, mean ± SD# | 8.89 ± 8.38 | 7.70 ± 7.23 | 12.00 ± 9.92 | 7.91 ± 8.64 | <0.001 |
| Abnormal ESR, no. (%)** | 129 (43.1) | 74 (39.6) | 40 (49.4) | 15 (48.4) | 0.272 |
| Abnormal CRP, no. (%)** | 99 (33.1) | 57 (30.5) | 31 (38.3) | 11 (35.5) | 0.441 |
| C‐HAQ, mean ± SD†† | 0.90 ± 0.72 | 0.80 ± 0.70 | 1.05 ± 0.68 | 1.14 ± 0.85 | 0.002 |
JIA = juvenile idiopathic arthritis; CTP = consensus treatment plan; IQR = interquartile range; RF = rheumatoid factor; NSAID = nonsteroidal antiinflammatory drug; PGA = physician global assessment of disease activity; VAS = visual analog scale; PtGA = patient/parent assessment of overall well‐being; JADAS‐10 = Juvenile Arthritis Disease Activity Score in 10 joints; ESR = erythrocyte sedimentation rate; CRP = C‐reactive protein; C‐HAQ = Childhood Health Assessment Questionnaire.
Data were missing for 214 patients.
Data were missing for 5 patients.
Data were missing for 37 patients.
Data were missing for 40 patients.
Data were missing for 78 patients.
Data were missing for 101 patients.
Data were missing for 36 patients.
As stated in the methods, all reported analyses are intent‐to‐treat, but we assessed the impact of reassigning CTP groups to match the received treatments. Reclassification resulted in 5 patients whose treatment patterns did not match any CTP. Thirty‐nine of the remaining patients were reclassified to a different CTP from the one reported at study outset, as follows: 1) from the early combination plan, 18 were reclassified to the step‐up plan and 2 to the biologic first plan; 2) from the step‐up plan, 15 were reclassified to the early combination plan and 1 to the biologic first plan; and 3) from the biologic first plan, 2 were reclassified to the early combination plan and 1 to the step‐up plan. There were no differences between the analyses of the reclassified CTPs and the intent‐to‐treat analyses. Of note, 148 of the 257 patients (58%) who chose the step‐up CTP at baseline later started a biologic, with a median time to biologic start of 114 days (interquartile range 70–170 days).
Clinically inactive disease without glucocorticoids at 12 months
Complete data for the assessment of the primary end point of clinically inactive disease at 12 months were available for 328 participants. After PS weighting and multiple imputation, an estimated 38% of participants on the step‐up plan, 47% of participants on the early combination plan, and 34% of participants on the biologic first plan achieved clinically inactive disease while not receiving glucocorticoids at 12 months (P = 0.39 by the Wald test) (Table 2). The baseline characteristics of those who achieved the primary outcome (n = 328) and those who did not achieve the primary outcome (n = 72) were similar.
Table 2.
Analysis of the primary end point of clinically inactive disease at 12 months in each CTP group*
| Estimated % (95% CI) | Estimated difference (95% CI) | ||
|---|---|---|---|
| Compared to step‐up plan | Compared to biologic first plan | ||
| Unadjusted model† | |||
| Step‐up CTP | 32.3 (26.2, 39.0) (70/217) | – | 8.0 (−9.6, 25.7) |
| Early combination CTP | 37.2 (26.7, 48.9) (29/78) | 4.9 (−8.3, 18.2) | 12.9 (−7.4, 33.2) |
| Biologic first CTP | 24.2 (11.7, 42.6) (8/33) | – | – |
| Model with PS weighting and multiple imputation | |||
| Step‐up CTP | 37.8 (29.4, 46.2) | – | 4.2 (−14.8, 23.3) |
| Early combination CTP | 47.3 (32.6, 62.0) | 9.5 (−4.1, 23.2) | 13.7 (−8.2, 35.7) |
| Biologic first CTP | 33.6 (14.5, 52.6) | – | – |
P = 0.39 for the comparison of propensity score (PS)–weighted percentages between groups, by the Wald test, accounting for multiple imputation. There were no significant differences between any of the consensus treatment plans (CTPs).
Observed data were analyzed in the unadjusted model. Values are the estimated percentages of patients in whom clinically inactive disease was achieved (95% confidence interval [95% CI]) (no. of patients with clinically inactive disease/no. of patients assessed).
Clinical JADAS‐10 and ACR Pedi 70 outcomes
Clinical JADAS‐10 scores improved over time, with all participants in a state of moderate or severe disease activity at baseline (mean ± SD 18.1 ± 4.7) and the majority (70%) achieving low or moderate disease activity at 12 months (mean ± SD 4.7 ± 5.5) (Figure 2 and Supplementary Table 4, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41888/abstract). Clinical JADAS‐10 scores were available for 90% of the participants at baseline, 71% at 6 months, and 66% at 12 months. After multiple imputation and PS weighting, inactive disease according to the clinical JADAS‐10 while not receiving glucocorticoids was achieved at 12 months by an estimated 43% of participants on the step‐up CTP, 59% of participants on the early combination CTP, and 47% of participants on the biologic first CTP (P = 0.05 by the Wald test) (Table 3). The percentage with inactive disease according to the clinical JADAS‐10 was significantly higher in the early combination CTP group compared to the step‐up CTP group (95% CI 2, 30%; P = 0.03). Low participant numbers limited conclusions about comparisons involving the biologic first CTP group.
Figure 2.
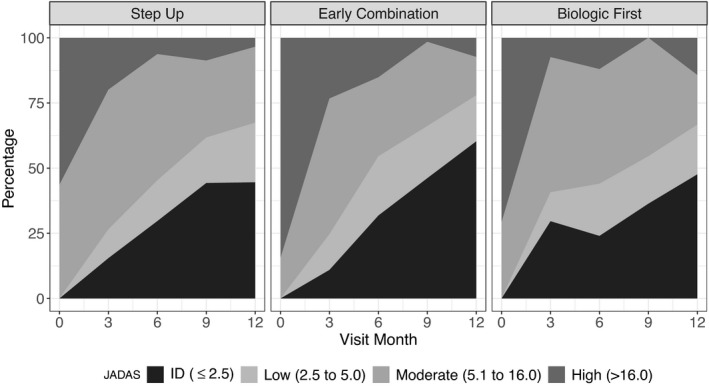
Percentage of patients with polyarticular juvenile idiopathic arthritis in the step‐up consensus treatment plan (CTP) group, early combination CTP group, and biologic first CTP group with inactive disease (ID), low disease activity, moderate disease activity, and high disease activity, according to the clinical Juvenile Arthritis Disease Activity Score in 10 joints (JADAS‐10), throughout the study period.
Table 3.
Comparisons of outcomes for the ACR Pedi 70 criteria and inactive disease according to the clinical JADAS‐10 at 12 months in each CTP group*
|
ACR Pedi 70 (PS‐weighted, imputed) |
Inactive disease according to the clinical JADAS‐10 (PS‐weighted, imputed) |
|
|---|---|---|
| Percentage with outcome in each group (95% CI) | ||
| Step‐up CTP | 61.5 (53.5, 69.5) | 42.8 (35.7, 49.9) |
| Early combination CTP | 80.7 (69.5, 91.9) | 58.8 (46.6, 71.1) |
| Biologic first CTP | 63.6 (37.7, 89.5) | 47.1 (25.0, 69.3) |
| Difference in percentage between groups (95% CI) | ||
| Biologic first CTP versus step‐up CTP | 2.1 (−25.2, 29.4) | 4.3 (−18.8, 27.5) |
| Early combination CTP versus step‐up CTP | 19.2 (5.0, 33.4)† | 16.0 (1.8, 30.2)‡ |
| Biologic first CTP versus early combination CTP | −17.1 (−45.3, 11.1) | −11.7 (−36.7, 13.3) |
For the comparison of propensity score (PS)–weighted percentages between groups, accounting for multiple imputation, P = 0.02 for the American College of Rheumatology (ACR) Pediatric 70 (Pedi 70) criteria; P = 0.05 for inactive disease according to the clinical Juvenile Arthritis Disease Activity Score in 10 joints (JADAS‐10), by the Wald test. CTP = consensus treatment plan; 95% CI = 95% confidence interval.
P = 0.0082.
P = 0.0270.
Supplementary Figure 2, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41888/abstract, shows the distribution of participants who attained ACR Pedi 70 at 6 and 12 months. ACR Pedi 70 scores could be calculated for 65% of the participants at 6 months and 60% at 12 months. At 12 months, with PS weighting and multiple imputation, 81% of the participants on the early combination CTP had achieved an ACR Pedi 70, as opposed to 62% of those on the step‐up CTP and 64% of those on the biologic first CTP (P = 0.02 by the Wald test); the percentage for the early combination CTP was significantly higher than that for the step‐up CTP (95% CI 5, 33%; P = 0.008).
Table 3 also compares the secondary disease activity measures (ACR Pedi 70 and inactive disease according to the clinical JADAS‐10). Overall, the percentages achieving ACR Pedi 70 and inactive disease according to the clinical JADAS‐10 in the early combination CPT group were significantly higher than the percentages in the other CTP groups, despite no significant differences in the primary outcome of clinically inactive disease.
Patient‐reported outcomes
Figure 3 shows the results for the PROMIS pain interference and mobility scores. Each CTP group improved toward the reference population mean by the 12‐month visit, except for the biologic first CTP group, but that group was exceedingly small. The differences in time trends between the groups were not significant for pain interference (P = 0.21) or mobility (P = 0.35). Completion rates for all patient‐reported outcomes were low and decreased over time. For example, 75% of 400 participants completed the pain interference measure at baseline, but only 49% at 12 months. Numbers of completed measures for each treatment group at a given time became exceedingly small, especially in the biologic first CTP group (17 of 44 for pain interference and 14 of 44 for mobility). There were no notable differences in baseline characteristics between participant groups that had 0, 1, 2, and ≥3 visits with a completed patient‐reported outcome measure.
Figure 3.
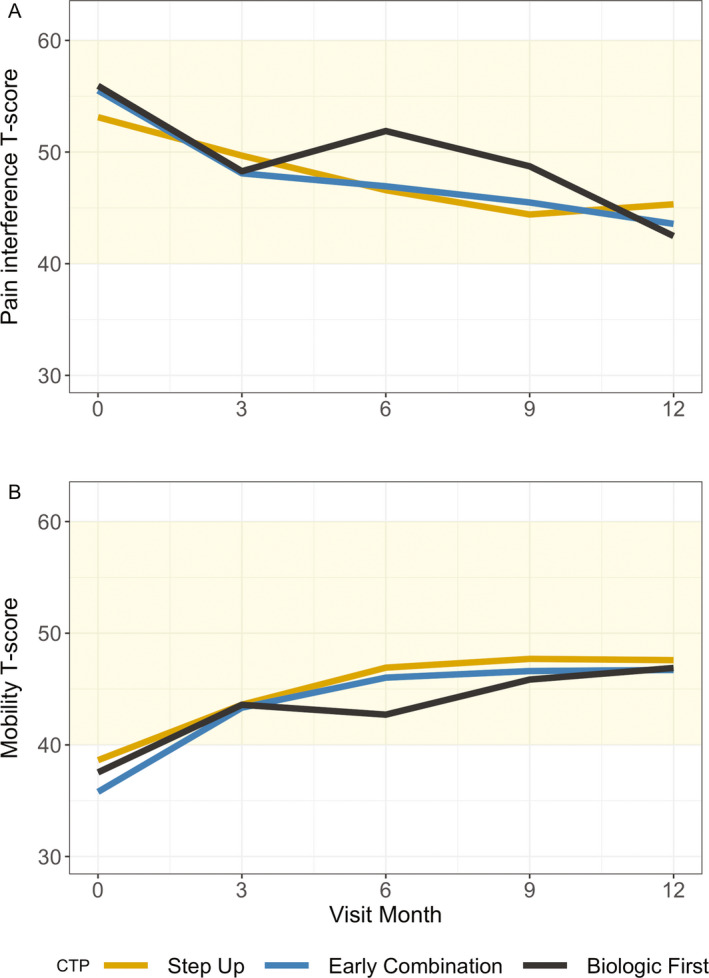
Patient‐Reported Outcomes Measurement Information System pain interference (A) and mobility (B) T scores over time in patients with juvenile idiopathic arthritis in the step‐up consensus treatment plan (CTP) group, early combination CTP group, and biologic first CTP group. Shaded areas indicate the mean and expected SD (50 ± 10) in the healthy population. Higher T scores indicate more pain or improved mobility. For both measures, all groups improved over time. There were no significant differences between the CTP groups.
Glucocorticoid use
The PS‐weighted percentage of participants in the early combination CTP group who were continuing to receive glucocorticoids at each follow‐up visit was lower than in the other groups at every time point except 9 months (Supplementary Figure 3, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41888/abstract). For example, at 3 months, 7% of the participants on the early combination CPT were receiving glucocorticoids compared to 16% and 17% of the participants on the biologic first CTP and step‐up CTP, respectively. The difference between the early combination and step‐up CTP groups was significant at 3 months (P = 0.012) and 6 months (P = 0.003) but not 9 months, when there was a small increase in the number of glucocorticoid users in the early combination CTP group (P = 0.40). At 12 months, few patients were continuing to receive glucocorticoids, so no adjusted analysis was performed, but no early combination CTP participants were continuing to receive glucocorticoids, while 3.2% of the participants on the step‐up CTP and 5.7% of the participants on the biologic first CTP continued to receive glucocorticoids.
Adverse events and side effects
Forty‐four participants experienced 20 SAEs and 25 events of special interest (Supplementary Table 5, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41888/abstract). No deaths were reported. Three patients were diagnosed as having inflammatory bowel disease (1 SAE). Nine patients developed infections (all SAE), including influenza (n = 2), infections requiring intravenous antibiotics (n = 5), shingles (n = 1), and cellulitis (n = 1). Two patients experienced fractures (both SAEs), 3 had hip pain and effusion (all SAEs), 1 developed drug‐induced lupus (SAE), and 1 had macrophage activation syndrome (SAE). Two patients had psychiatric disorders (both SAEs), 1 had vertigo (SAE), 2 had leukopenia (no SAE), 12 developed new‐onset uveitis (no SAE), 6 had hepatitis (no SAE), 1 had a hypersensitivity reaction (no SAE), and 3 had psoriasis (no SAE). The numbers were too small to compare differences between groups. Compared to other safety registries, this cohort reported similar rates of AEs and events of special interest. A recent report describing event rates for 3 large registries (Pharmachild, Germany, and Sweden) included >15,000 children and reported SAEs in 6.9–7.4% of children (36), comparable to the percentages of children with SAEs in this cohort (5.3%).
Supplementary Table 6, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41888/abstract, shows the number of reported medication side effects at each visit and the number of patients reporting them. Although higher numbers of side effects were reported in both the step‐up CTP group (60%) and early combination CTP group (56.6%) compared to the biologic first CTP group (34.4%) (after PS adjustment, P = 0.006 for the biologic first CTP versus the step‐up CTP and P = 0.06 for the early combination CTP versus the biologic first CTP), there were no significant differences between groups for specific side effects. The most commonly reported side effects were nausea (26%), mood disturbance (21%), headache (20%), sleep disturbance (13%), injection site reaction (13%), stomachache and vomiting (12% each), and rash, mouth sores, and weight gain (11% each).
DISCUSSION
The STOP‐JIA study is the first multicenter, prospective observational study to assess the optimal timing of biologic initiation in polyarticular JIA. Using CTPs to assess comparative effectiveness within the CARRA Registry facilitated the successful enrollment of 400 children with untreated polyarticular JIA, one of the world’s largest prospectively followed up inception cohorts of children with polyarticular JIA. The STOP‐JIA study adds important real‐world outcomes for a large group of children seen in routine clinical care. Overall, there were no significant differences between CTPs in achievement of clinically inactive disease without glucocorticoids at 12 months. The tendency toward a higher percentage of patients in the early combination CTP achieving clinically inactive disease without glucocorticoids at 12 months was more pronounced after statistical adjustments. However, the confidence intervals were wide, and the differences between groups were not significant (P = 0.17 for the step‐up CTP versus the early combination CTP). Patient‐reported outcomes improved throughout the study but did not differ between CTPs.
The achievement of more durable outcomes, such as clinical remission while receiving medications (inactive disease while receiving treatment maintained for ≥6 months) and clinical remission without medications (inactive disease without treatment maintained for ≥12 months), will be assessed in the future, since STOP‐JIA study participants are also enrolled in the CARRA Registry, ensuring longer follow‐up. CARRA Registry follow‐up will allow continued prospective evaluation of the participants and add invaluable information about longer‐term outcomes in this cohort.
Analyses of inactive disease according to the clinical JADAS‐10, a less stringent categorization of disease inactivity, suggested a potential benefit of the early combination CTP as compared to the other approaches, a result that merits additional evaluation in focused future studies. Inactive disease according to the clinical JADAS‐10 may be a better target outcome than clinically inactive disease according to the ACR criteria, which reflects disease inactivity at only one point in time, may be transient, and may not be the most important target outcome. The clinical significance of clinically inactive disease at 12 months, and whether this predicts longer‐term outcomes is unknown. An analysis of the UK Childhood Arthritis Prospective Study showed that achievement of inactive disease according to the clinical JADAS‐10 was associated with better functional ability, better psychosocial health, and fewer joints with a limited range of motion in the short‐term and long‐term (5 years) compared to achievement of clinically inactive disease according to the ACR criteria (9) at 1 year (37). In the STOP‐JIA cohort, analysis of both the ACR Pedi 70 and the clinical JADAS‐10 scores indicated that participants in the early combination CTP group had significantly higher rates of achieving both outcomes than those in the step‐up CTP group after PS weighting and multiple imputation.
The early combination CTP group also had significantly lower rates of glucocorticoid use at 3 and 6 months, which may reflect earlier disease control. Adjunctive glucocorticoid treatment is common in polyarticular JIA (almost 40% of CARRA Registry JIA patients have been exposed to glucocorticoids), so rapid reduction and discontinuation of glucocorticoid treatment remains an important treatment goal (38).
Safety events (SAEs and events of special interest) were reported for STOP‐JIA study participants, but event numbers were too low to detect group differences. The percentage of children experiencing an SAE was comparable to percentages of children with SAEs in other large, observational safety registries of JIA patients.
The STOP‐JIA study was the first large‐scale study to utilize CARRA CTPs and the CARRA Registry to perform an observational comparative effectiveness study—an approach specifically developed by CARRA for research in rare diseases (39). The results suggest that the CTP development process was successful in distilling highly variable treatment practices into standardized treatment strategies acceptable to pediatric rheumatologists. In this study, the overall rate of clinically inactive disease achieved at 12 months was low in all 3 polyarticular JIA CTPs. Future research should address how to increase clinically inactive disease rates and disease inactivity/low disease activity states in children with JIA, including identification of JIA subgroups that may particularly benefit from early initiation of biologics and whether stricter treat‐to‐target approaches than were used for the STOP‐JIA study could lead to sustained disease control and better long‐term outcomes.
While the CTPs facilitated enrollment of 400 children into the study, several limitations of the observational study design arose, particularly problems associated with missing data, missed visits, and confounding by indication. The baseline differences between CTP groups are of particular concern for confounding. For example, RF‐positive polyarticular JIA and enthesitis‐related arthritis were relatively overrepresented in the early biologic CTPs (early combination and biologic first), and these groups had higher disease activity measures at baseline than the step‐up CTP group. Statistical methods, including propensity weight adjustment, were used to reduce bias; however, potential bias may not have been eliminated. Additionally, patient numbers in the study arms were imbalanced, with lower than expected enrollment in the early biologic groups. In combination with missing data, this resulted in few analyzable patients for some outcomes. Multiple imputation can reduce bias resulting from omission of patients with missing outcomes but relies on the assumption that the probability that a value is missing depends only on observed data and not on unobserved or missing data—the “missing at random” assumption.
Although a total of 72 participants (18%) did not have complete data for the 12‐month clinically inactive disease outcome, most had partial data at 12 months or complete clinically inactive disease data at earlier time points, so imputation was based on variables strongly associated with clinically inactive disease at 12 months. Table 2 shows that estimated clinically inactive disease was higher in the imputed data for all groups, suggesting that those missing the 12‐month assessment or with incomplete 12‐month data were more likely to have achieved clinically inactive disease than those with complete data. Further analyses are underway to assess treatment effectiveness based on the actual use and timing of medication, without reference to CTPs.
This study evaluated 1 primary outcome measure, 2 secondary outcome measures, and several tertiary outcome measures; each outcome measure involved 3 pairwise comparisons between CTPs, so many P values and confidence intervals appear in the results. Neither the P values nor the widths of confidence intervals were adjusted for multiple comparisons.
Since families and physicians together selected the CTP, it is possible that they wanted the chosen CTP to appear to be the right choice. This could mean that subjectively reported outcomes would appear better than if judged by an impartial observer. There is evidence that this is not generally the case since the incidence of clinically inactive disease is far below what was anticipated when the study began. Furthermore, the tendency to overstate benefit should occur in each group and not favor one group over another. We have included reasons given for CTP choice in Supplementary Table 7, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41888/abstract.
This study identified important opportunities to optimize data collection in the CARRA Registry. In particular, efforts are underway to improve longitudinal outcomes data and develop new capabilities to capture patient‐reported outcomes between CARRA Registry visits. As additional longitudinal data sources become available to the CARRA Registry, we anticipate greater capability to understand and account for the effects of missing data and confounding variables, particularly those that are time varying. We believe these enhancements will increase the Registry’s ability to support comparative effectiveness research, including use and analysis of CTPs developed for other childhood‐onset rheumatic diseases.
The CARRA STOP‐JIA comparative effectiveness study addressed the optimal timing of initial biologic therapy in polyarticular JIA, finding no clear differences between initial/early biologic versus delayed biologic treatment approaches in the attainment of clinically inactive disease without glucocorticoids at 1 year. However, the early combination CTP showed increased benefits in secondary analyses assessing key outcomes such as the clinical JADAS‐10, ACR Pedi 70, and earlier discontinuation of glucocorticoids, although these results require additional validation. Lastly, a separate study applying latent class trajectory analysis to STOP‐JIA data, also published in this issue of Arthritis & Rheumatology (40), showed that early use of biologics was associated with more rapid achievement of inactive disease. These results further underscore that for many patients with polyarticular JIA, earlier biologic treatment may result in more immediate improvement, but the impact on long‐term outcomes remains unproven.
In conclusion, STOP‐JIA study results will help inform shared decision‐making discussions between families and physicians as they weigh the risks and benefits of initial treatment approaches. The STOP‐JIA data set represents a unique and rich resource of highly curated data on a large cohort of patients with new‐onset polyarticular JIA that will address additional questions through further data analyses and longer‐term follow up through the CARRA Registry.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Kimura had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Kimura, Schanberg, Tomlinson, Riordan, Dennos, Del Gaizo, Murphy, Weiss, Feldman, Ringold.
Acquisition of data
Kimura, Tomlinson, Dennos, Weiss, Feldman, Ringold.
Analysis and interpretation of data
Kimura, Schanberg, Tomlinson, Riordan, Dennos, Del Gaizo, Murphy, Weiss, Natter, Feldman, Ringold.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This work could not have been accomplished without the aid of the following organizations: the Childhood Arthritis and Rheumatology Research Alliance (CARRA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the NIH, and the Arthritis Foundation. We would also like to thank all participants and hospital sites that recruited patients for the CARRA Registry. We also acknowledge the STOP‐JIA Stakeholder Advisory Panel: Charla Cury, Uday Deshmukh, Janet Hobble, Nick (Yongjay) Kim, Melanie Kohlheim, Kate Kuhns, Lauren Revis, and Suzanne Schrandt. We acknowledge the Rheumatology Research Foundation’s support of the original development of the CARRA polyarticular JIA consensus treatment plans.
APPENDIX A. THE CARRA STOP‐JIA INVESTIGATORS
The CARRA STOP‐JIA Investigators are as follows: A. Adams, R. Agbayani, S. Akoghlanian, E. Allenspach, W. Ambler, E. Anderson, S. Ardoin, S. Armendariz, I. Balboni, S. Balevic, L. Ballenger, S. Ballinger, N. Balmuri, F. Barbar‐Smiley, M. Basiaga, K. Baszis, M. Becker, H. Bell‐Brunson, H. Benham, S. Benseler, W. Bernal, T. Beukelman, T. Bigley, B. Binstadt, M. Blakley, J. Bohnsack, A. Brown, H. Brunner, M. Buckley, D. Bullock, B. Cameron, S. Canna, L. Cannon, V. Cartwright, E. Cassidy, E. Chalom, I. Chang, J. Chang, V. Chauhan, T. Chinn, P. Chira, D. Co, A. Cooper, J. Cooper, C. Correll, R. Cron, L. Curiel‐Duran, M. Curry, A. Dalrymple, A. Davis, T. Davis, D. De Ranieri, J. Dean, F. Dedeoglu, M. DeGuzman, V. Dempsey, E. DeSantis, J. Dingle, J. Dowling, J. Drew, K. Driest, Q. Du, D. Durkee, J. Dvergsten, A. Eberhard, M. Eckert, C. Edens, M. Elder, S. Fadrhonc, L. Favier, B. Feldman, J. Fennell, P. Ferguson, K. Fields, C. Fleming, L. Fogel, E. Fox, R. Fuhlbrigge, J. Fuller, D. Gerstbacher, M. Gillispie‐Taylor, I. Goh, A. Gotte, B. Gottlieb, T. Graham, S. Grevich, T. Griffin, A. Grom, M. Guevara, P. Guittar, M. Guzman, M. Hager, O. Halyabar, M. Hance, S. Haro, J. Harris, J. Hausmann, K. Hayward, J. Heiart, L. Henderson, M. Henrickson, A. Hersh, L. Hiraki, M. Hiskey, P. Hobday, C. Hoffart, M. Holland, M. Hollander, S. Hong, M. Horwitz, J. Hsu, A. Huber, J. Huggins, J. Hui‐Yuen, A. Huttenlocher, M. Ibarra, C. Inman, H. Jackson, S. Jackson, K. James, G. Janow, J. Jaquith, S. Jared, N. Johnson, J. Jones, K. Jones, S. Jones, S. Joshi, C. Justice, K. Kaufman, U. Khalsa, B. Kienzle, S. Kim, Y. Kimura, D. Kingsbury, M. Kitcharoensakkul, T. Klausmeier, K. Klein, M. Klein‐Gitelman, S. Kramer, C. Kremer, J. Lai, B. Lang, S. Lapidus, A. Lasky, E. Lawson, R. Laxer, P. Lee, T. Lee, M. Lerman, D. Levy, S. Li, S. Lieberman, C. Lin, N. Ling, M. Lo, D. Lovell, N. Luca, B. Malla, J. Maller, M. Mannion, A. Martyniuk, T. Mason, K. Mcallister, L. McAllister, K. McConnell, I. McHale, E. Meidan, E. Mellins, P. Miettunen, M. Miller, M. Mitchell, R. Modica, K. Moore, E. Morgan Dewitt, T. Moussa, V. Mruk, E. Muscal, K. Nanda, L. Nassi, S. Nativ, J. Neely, B. Nelson, L. Newhall, P. Nigrovic, B. Nolan, E. Oberle, O. Okeke, M. Oliver, K. O'Neil, K. Onel, A. Orandi, M. Orlando, R. Oz, E. Pagano, A. Paller, N. Pan, J. Patel, P. Pepmueller, R. Pooni, S. Protopapas, B. Puplava, J. Quach, C. Rabinovich, S. Radhakrishna, S. Ramsey, R. Randell, A. Reed, H. Reid, A. Richmond, S. Ringold, M. Riordan, M. Riskalla, M. Ritter, M. Rodriquez, K. Rojas, M. Rosenkranz, T. Rubinstein, N. Saad, R. Sadun, C. Sandborg, L. Schanberg, K. Schikler, H. Schmeling, K. Schmidt, E. Schmitt, R. Schneider, G. Schulert, T. Seay, C. Seper, J. Shalen, R. Sheets, S. Shenoi, J. Shirley, E. Silverman, V. Sivaraman, C. Smith, J. Smith, E. Smitherman, J. Soep, M. Son, S. Spence, L. Spiegel, J. Spitznagle, H. Stapp, K. Steigerwald, S. Stern, A. Stevens, B. Stevens, R. Stevenson, K. Stewart, C. Stingl, M. Stoll, E. Stringer, J. Sumner, R. Sundel, M. Sutter, R. Syed, S. Taber, T. Tanner, G. Tarshish, S. Tarvin, J. Taylor, M. Tesher, A. Thatayatikom, B. Thomas, T. Ting, K. Torok, C. Toruner, S. Tse, M. Twilt, T. Valcarcel, H. Van Mater, N. Vasquez, R. Vehe, K. Veiga, J. Velez, N. Volpe, E. von Scheven, S. Vora, L. Wagner‐Weiner, D. Wahezi, H. Walters, M. Waterfield, A. Watts, P. Weiser, J. Weiss, P. Weiss, A. White, L. Woolnough, T. Wright, M. Yee, R. Yeung, K. Yomogida, Y. Zhang, Y. Zhao, and A. Zhu.
ClinicalTrials.gov identifier: NCT02593006.
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient‐Centered Outcomes Research Institute.
Supported in part by Patient‐Centered Outcomes Research Institute award CER‐1408‐20534.
Dr. Kimura has received research support from CARRA and Genentech and royalties from UpToDate. Dr. Schanberg has received consulting fees, speaking fees, and/or honoraria from Sobi, UCB, and Sanofi (less than $10,000 each) and research support from CARRA and Bristol Myers Squibb. Dr. Riordan has received research support from CARRA. Dr. Weiss has received consulting fees from Lilly and Pfizer (less than $10,000 each). Dr. Natter has received research support from CARRA. Dr. Feldman has received consulting fees, speaking fees, and/or honoraria from Pfizer and AB2 Bio (less than $10,000 each). Dr. Ringold has received research support from CARRA and Bristol Myers Squibb and royalties from UpToDate. No other disclosures relevant to this article were reported.
Contributor Information
Yukiko Kimura, Email: Yukiko.Kimura@HMHN.org.
the CARRA STOP‐JIA Investigators:
Rosabel Agbayani, Shoghik Akoghlanian, Edwin Anderson, Margaret Andrew, Kevin Baszis, Mara Becker, Heather Bell‐Brunson, Heather Benham, Susanne Benseler, Timothy Beukelman, Hermine Brunner, Annica Bryson, Hulya Bukulmez, Elizabeth Chalom, Johanna Chang, Nick Charron, Vibha Chauhan, Nazma Chowdhury, Sydney Cooper, Trevor Davis, Joni Dean, Fatma Dedeoglu, Victoria Dempsey, Marija Dionizovik‐Dimanovski, Jameson Dowling, Joanne Drew, Kayla Evans, Martha Falcon, Brian Feldman, Polly Ferguson, Bianca Ferreira, Ca’Lecia Fleming, Lourdes Franco, Ingrid Goh, Donald Goldsmith, Beth Gottlieb, Thomas Graham, Thomas Griffin, Monica Guevara, Melissa Hance, Arielle Hay, Sherry Hillyer, Matthew Hollander, Joyce Hsu, Adam Huber, Ching Hung, Anna Huttenlocher, Lisa Imundo, Christi Inman, Jane Jaquith, Sharon Jared, Kim Jennings, Rita Jerath, Jordan Jones, Suzy Jones, Philip Kahn, Kristin Klein, Marisa Klein‐Gitelman, Daniel Kingsbury, Sara Kramer, Sivia Lapidus, Sean Linehan, Bipin Malla, Thomas Mason, Alexandra Martyniuk, Brooke McCallum, Karen McConnell, Deborah McCurdy, Kieran McKibben, Emilie Misajon, Smirti Moahn, Katharine Moore, Eyal Muscal, Bhupinder Nahal, Kathleen O’Neil, Karen Onel, Ashlee Parsons, Kate Phillippi, Lori Ponder, Sampath Prahalad, Consuelo Rabinovich, Sarah Ringold, Mary Ellen Riordan, Michelle Ritter, Angela Robinson, Margalit Rosenkranz, Bonnie Rosolowski, Natasha Ruth, Kenneth Schikler, Ana Sepulveda, Chelsey Smith, Heidi Stapp, Katie Stewart, Jacob Strelow, Justine Sumner, Reema Syed, Alysha Taxter, Marcelle Terry, Melissa Tesher, Akaluck Thatayatikom, Lauren Vannoy, Richard Vehe, Emily von Scheven, Dawn Wahezi, Cindy Wang, Marla Watson, Allen Watts, Jennifer Weiss, Pamela Weiss, Autumn Wolverton, Jennifer Woo, Andrew Zeft, Lawrence Zemel, Aihua Zhu, A. Adams, R. Agbayani, S. Akoghlanian, E. Allenspach, W. Ambler, E. Anderson, S. Ardoin, S. Armendariz, I. Balboni, S. Balevic, L. Ballenger, S. Ballinger, N. Balmuri, F. Barbar‐Smiley, M. Basiaga, K. Baszis, M. Becker, H. Bell‐Brunson, H. Benham, S. Benseler, W. Bernal, T. Beukelman, T. Bigley, B. Binstadt, M. Blakley, J. Bohnsack, A. Brown, H. Brunner, M. Buckley, D. Bullock, B. Cameron, S. Canna, L. Cannon, V. Cartwright, E. Cassidy, E. Chalom, I. Chang, J. Chang, V. Chauhan, T. Chinn, P. Chira, D. Co, A. Cooper, J. Cooper, C. Correll, R. Cron, L. Curiel‐Duran, M. Curry, A. Dalrymple, A. Davis, T. Davis, D. De Ranieri, J. Dean, F. Dedeoglu, M. DeGuzman, V. Dempsey, E. DeSantis, J. Dingle, J. Dowling, J. Drew, K. Driest, Q. Du, D. Durkee, J. Dvergsten, A. Eberhard, M. Eckert, C. Edens, M. Elder, S. Fadrhonc, L. Favier, B. Feldman, J. Fennell, P. Ferguson, K. Fields, C. Fleming, L. Fogel, E. Fox, R. Fuhlbrigge, J. Fuller, D. Gerstbacher, M. Gillispie‐Taylor, I. Goh, A. Gotte, B. Gottlieb, T. Graham, S. Grevich, T. Griffin, A. Grom, M. Guevara, P. Guittar, M. Guzman, M. Hager, O. Halyabar, M. Hance, S. Haro, J. Harris, J. Hausmann, K. Hayward, J. Heiart, L. Henderson, M. Henrickson, A. Hersh, L. Hiraki, M. Hiskey, P. Hobday, C. Hoffart, M. Holland, M. Hollander, S. Hong, M. Horwitz, J. Hsu, A. Huber, J. Huggins, J. Hui‐Yuen, A. Huttenlocher, M. Ibarra, C. Inman, H. Jackson, S. Jackson, K. James, G. Janow, J. Jaquith, S. Jared, N. Johnson, J. Jones, K. Jones, S. Jones, S. Joshi, C. Justice, K. Kaufman, U. Khalsa, B. Kienzle, S. Kim, Y. Kimura, D. Kingsbury, M. Kitcharoensakkul, T. Klausmeier, K. Klein, M. Klein‐Gitelman, S. Kramer, C. Kremer, J. Lai, B. Lang, S. Lapidus, A. Lasky, E. Lawson, R. Laxer, P. Lee, P. Lee, T. Lee, M. Lerman, D. Levy, S. Li, S. Lieberman, C. Lin, N. Ling, M. Lo, D. Lovell, N. Luca, B. Malla, J. Maller, M. Mannion, A. Martyniuk, T. Mason, K. Mcallister, L. McAllister, K. McConnell, I. McHale, E. Meidan, E. Mellins, P. Miettunen, M. Miller, M. Mitchell, R. Modica, K. Moore, E. Morgan Dewitt, T. Moussa, V. Mruk, E. Muscal, K. Nanda, L. Nassi, S. Nativ, J. Neely, B. Nelson, L. Newhall, P. Nigrovic, B. Nolan, E. Oberle, O. Okeke, M. Oliver, K. O’Neil, K. Onel, A. Orandi, M. Orlando, R. Oz, E. Pagano, A. Paller, N. Pan, J. Patel, P. Pepmueller, R. Pooni, S. Protopapas, B. Puplava, J. Quach, C. Rabinovich, S. Radhakrishna, S. Ramsey, R. Randell, A. Reed, A. Reed, H. Reid, A. Richmond, S. Ringold, M. Riordan, M. Riskalla, M. Ritter, M. Rodriquez, K. Rojas, M. Rosenkranz, T. Rubinstein, N. Saad, R. Sadun, C. Sandborg, L. Schanberg, K. Schikler, H. Schmeling, K. Schmidt, E. Schmitt, R. Schneider, G. Schulert, T. Seay, C. Seper, J. Shalen, R. Sheets, S. Shenoi, J. Shirley, E. Silverman, V. Sivaraman, C. Smith, J. Smith, E. Smitherman, J. Soep, M. Son, S. Spence, L. Spiegel, J. Spitznagle, H. Stapp, K. Steigerwald, S. Stern, A. Stevens, B. Stevens, R. Stevenson, K. Stewart, C. Stingl, M. Stoll, E. Stringer, J. Sumner, R. Sundel, M. Sutter, R. Syed, R. Syed, S. Taber, T. Tanner, G. Tarshish, S. Tarvin, J. Taylor, M. Tesher, A. Thatayatikom, B. Thomas, T. Ting, K. Torok, C. Toruner, S. Tse, M. Twilt, T. Valcarcel, H. Van Mater, N. Vasquez, R. Vehe, K. Veiga, J. Velez, N. Volpe, E. von Scheven, S. Vora, L. Wagner‐Weiner, D. Wahezi, H. Walters, M. Waterfield, A. Watts, P. Weiser, J. Weiss, P. Weiss, A. White, L. Woolnough, T. Wright, M. Yee, R. Yeung, K. Yomogida, Y. Zhang, Y. Zhao, and A. Zhu
References
- 1. Sacks JJ, Helmick CG, Luo YH, Ilowite NT, Bowyer S. Prevalence of and annual ambulatory health care visits for pediatric arthritis and other rheumatologic conditions in the United States in 2001–2004. Arthritis Rheum 2007;57:1439–45. [DOI] [PubMed] [Google Scholar]
- 2. Denardo BA, Tucker LB, Miller LC, Szer IS, Schaller JG. Demography of a regional pediatric rheumatology patient population: affiliated children's arthritis centers of New England. J Rheumatol 1994;21:1553–61. [PubMed] [Google Scholar]
- 3. Malleson PN, Fung MY, Rosenberg AM. The incidence of pediatric rheumatic diseases: results from the Canadian Pediatric Rheumatology Association Disease Registry. J Rheumatol 1996;23:1981–7. [PubMed] [Google Scholar]
- 4. Oen KG, Cheang M. Epidemiology of chronic arthritis in childhood. Semin Arthritis Rheum 1996;26:575–91. [DOI] [PubMed] [Google Scholar]
- 5. Krause ML, Crowson CS, Michet CJ, Mason T, Muskardin TW, Matteson EL. Juvenile idiopathic arthritis in Olmsted County, Minnesota, 1960–2013. Arthritis Rheumatol 2016;68:247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beukelman T, Kimura Y, Ilowite NT, Mieszkalski K, Natter MD, Burrell G, et al. The new Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry: design, rationale, and characteristics of patients enrolled in the first 12 months. Pediatr Rheumatol Online J 2017;15:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wallace CA, Huang B, Bandeira M, Ravelli A, Giannini EH. Patterns of clinical remission in select categories of juvenile idiopathic arthritis. Arthritis Rheum 2005;52:3554–62. [DOI] [PubMed] [Google Scholar]
- 8. Ringold S, Seidel KD, Koepsell TD, Wallace CA. Inactive disease in polyarticular juvenile idiopathic arthritis: current patterns and associations. Rheumatology (Oxford) 2009;48:972–7. [DOI] [PubMed] [Google Scholar]
- 9. Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N, for the Childhood Arthritis and Rheumatology Research Alliance (CARRA), the Pediatric Rheumatology Collaborative Study Group (PRCSG), and the Paediatric Rheumatology International Trials Organisation (PRINTO) . American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011;63:929–36. [DOI] [PubMed] [Google Scholar]
- 10. Minden K, Niewerth M, Listing J, Biedermann T, Bollow M, Schöntube M, et al. Long‐term outcome in patients with juvenile idiopathic arthritis. Arthritis Rheum 2002;46:2392–401. [DOI] [PubMed] [Google Scholar]
- 11. Oen K, Malleson PN, Cabral DA, Rosenberg AM, Petty RE, Cheang M. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol 2002;29:1989–99. [PubMed] [Google Scholar]
- 12. Packham JC, Hall MA, Pimm TJ. Long‐term follow‐up of 246 adults with juvenile idiopathic arthritis: predictive factors for mood and pain. Rheumatology (Oxford) 2002;41:1444–9. [DOI] [PubMed] [Google Scholar]
- 13. Zak M, Pedersen FK. Juvenile chronic arthritis into adulthood: a long‐term follow‐up study. Rheumatology (Oxford) 2000;39:198–204. [DOI] [PubMed] [Google Scholar]
- 14. Seid M, Opipari L, Huang B, Brunner HI, Lovell DJ. Disease control and health‐related quality of life in juvenile idiopathic arthritis. Arthritis Rheum 2009;61:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glerup M, Rypdal V, Arnstad ED, Ekelund M, Peltoniemi S, Aalto K, et al. Long‐term outcomes in juvenile idiopathic arthritis: eighteen years of follow‐up in the population‐based Nordic Juvenile Idiopathic Arthritis cohort. Arthritis Care Res (Hoboken) 2020;72:507–16. [DOI] [PubMed] [Google Scholar]
- 16. Tynjala P, Vahasalo P, Tarkiainen M, Kröger L, Aalto K, Malin M, et al. Aggressive combination drug therapy in very early polyarticular juvenile idiopathic arthritis (ACUTE‐JIA): a multicentre randomised open‐label clinical trial. Ann Rheum Dis 2011;70:1605–12. [DOI] [PubMed] [Google Scholar]
- 17. Wallace CA, Giannini EH, Spalding SJ, Hashkes PJ, O'Neil KM, Zeft AS, et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum 2012;64:2012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beukelman T, Guevara JP, Albert DA, Sherry DD, Burnham JM. Variation in the initial treatment of knee monoarthritis in juvenile idiopathic arthritis: a survey of pediatric rheumatologists in the United States and Canada. J Rheumatol 2007;34:1918–24. [PubMed] [Google Scholar]
- 19. Cron RQ, Sharma S, Sherry DD. Current treatment by United States and Canadian pediatric rheumatologists. J Rheumatol 1999;26:2036–8. [PubMed] [Google Scholar]
- 20. Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al, for the Pediatric Rheumatology Collaborative Study Group . Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med 2000;342:763–9. [DOI] [PubMed] [Google Scholar]
- 21. Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med 2008;359:810–20. [DOI] [PubMed] [Google Scholar]
- 22. Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio‐Pérez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double‐blind, placebo‐controlled withdrawal trial. Lancet 2008;372:383–91. [DOI] [PubMed] [Google Scholar]
- 23. Ringold S, Angeles‐Han ST, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for non‐systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Rheumatol 2019;71:846–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ringold S, Weiss PF, Colbert RA, DeWitt EM, Lee T, Onel K, et al. Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans for new‐onset polyarticular juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2014;66:1063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Consolaro A, Negro G, Gallo MC, Bracciolini G, Ferrari C, Schiappapietra B, et al. Defining criteria for disease activity states in nonsystemic juvenile idiopathic arthritis based on a three‐variable juvenile arthritis disease activity score. Arthritis Care Res (Hoboken) 2014;66:1703–9. [DOI] [PubMed] [Google Scholar]
- 26. Sox HC, Greenfield S. Comparative effectiveness research: a report from the Institute of Medicine. Ann Intern Med 2010;151:203–5. [DOI] [PubMed] [Google Scholar]
- 27. Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni‐Manzoni S, Filocamo G, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 2009;61:658–66. [DOI] [PubMed] [Google Scholar]
- 28. Morgan EM, Mara CA, Huang B, Barnett K, Carle AC, Farrell JE, et al. Establishing clinical meaning and defining important differences for Patient‐Reported Outcomes Measurement Information System (PROMIS) measures in juvenile idiopathic arthritis using standard setting with patients, parents, and providers. Qual Life Res 2017;26:565–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Filocamo G, Consolaro A, Schiappapietra B, Dalprà S, Lattanzi B, Magni‐Manzoni S, et al. A new approach to clinical care of juvenile idiopathic arthritis: the Juvenile Arthritis Multidimensional Assessment Report. J Rheumatol 2011;38:938–53. [DOI] [PubMed] [Google Scholar]
- 30. Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997;40:1202–9. [DOI] [PubMed] [Google Scholar]
- 31. McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med 2013;32:3388–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ridgeway G, McCaffrey D, Morral A, Griffin BA, Burgette L. twang: Toolkit for Weighting and Analysis of Nonequivalent Groups. July 2021. URL: https://CRAN.R‐project.org/package=twang.
- 33. Van Buuren S, Groothuis‐Oushoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 34. The R project for statistical computing. URL: http://www.r‐project.org.
- 35. Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum 1994;37:1761–9. [DOI] [PubMed] [Google Scholar]
- 36. Swart J, Giancane G, Horneff G, Magnusson B, Hofer M, Alexeeva E, et al. Pharmacovigilance in juvenile idiopathic arthritis patients treated with biologic or synthetic drugs: combined data of more than 15,000 patients from Pharmachild and national registries. Arthritis Res Ther 2018;20:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shoop‐Worrall SJ, Verstappen SM, McDonagh JE, Baildam E, Chieng A, Davidson J, et al. Long‐term outcomes following achievement of clinically inactive disease in juvenile idiopathic arthritis: the importance of definition. Arthritis Rheumatol 2018;70:1519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beukelman T, Ringold S, Davis TE, DeWitt EM, Pelajo CF, Weiss PF, et al. Disease‐modifying antirheumatic drug use in the treatment of juvenile idiopathic arthritis: a cross‐sectional analysis of the CARRA Registry. J Rheumatol 2012;39:1867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ringold S, Nigrovic PA, Feldman BM, Tomlinson GA, von Scheven E, Wallace CA, et al. The Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans: toward comparative effectiveness in the pediatric rheumatic diseases. Arthritis Rheumatol 2018;70:669–78. [DOI] [PubMed] [Google Scholar]
- 40. Ong MS, Ringold S, Kimura Y, Schanberg LE, Tomlinson GA, Natter MD, and the CARRA Registry Investigators . Improved disease course associated with early initiation of biologics in polyarticular juvenile idiopathic arthritis: trajectory analysis of a Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans study. Arthritis Rheumatol 2021. doi: http://onlinelibrary.wiley.com/doi/ 10.1002/art.41892/abstract. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


